Abstract
Corneal Epithelial Stem Cells (CESCs) and their proliferative progeny, the Transit Amplifying Cells (TACs), are responsible for homeostasis and maintaining corneal transparency. Owing to our limited knowledge of cell fates and gene activity within the cornea, the search for unique markers to identify and isolate these cells remains crucial for ocular surface reconstruction. We performed single-cell RNA sequencing of corneal cells from larval and adult stages of Xenopus. Our results indicate that as the cornea develops and matures, there is an increase in cellular diversity which is accompanied by a substantial shift in transcriptional profile, gene regulatory network and cell-cell communication dynamics. Our data also reveals several novel genes expressed in corneal cells and changes in gene expression during corneal differentiation at both developmental time-points. Importantly, we identify specific basal cell clusters in both the larval and adult cornea that comprise a relatively undifferentiated cell type and express distinct stem cell markers, which we propose are the putative larval and adult CESCs, respectively. This study offers a detailed atlas of single-cell transcriptomes in the frog cornea. In the future, this work will be useful to elucidate the function of novel genes in corneal epithelial homeostasis, wound healing and regeneration.
Keywords: Cornea, Single-cell RNA-sequencing, Xenopus, Corneal Epithelial Stem Cells, Biomarkers
1. Introduction
The corneal epithelium is the outermost layer of the eye, and is essential for focusing light, maintaining normal vision and protecting deeper eye tissues. As it is exposed to the external environment, the corneal epithelial tissue has a remarkable capacity for self-renewal and repair, which is attributed to a population of somatic stem cells, called Corneal Epithelial Stem Cells (CESCs), and their proliferative progeny, the Transit Amplifying Cells (TACs). Therefore, the self-renewing corneal epithelium serves as an outstanding model to study the biology of epithelial stem cells, and their contributions to a highly specialized differentiated tissue.
The cellular composition and homeostatic maintenance of the corneal epithelium have been studied extensively (Cotsarelis et al., 1989; Lavker et al., 2004; Schermer et al., 1986; Thoft and Friend, 1983). In adult vertebrates, the stratified corneal epithelium consists of an innermost layer of undifferentiated basal cells, suprabasal cells, and multiple layers of differentiated, superficial (apical) squamous cells. Some studies in humans and mice (Amitai-Lange et al., 2015; Davanger and Evensen, 1971; Di Girolamo, 2011; Zhao et al., 2009) have indicated that CESCs reside in the basal epithelium (in a specialized niche located in the peripheral “limbus”). In contrast, some other studies suggest that CESCs are distributed throughout the basal corneal epithelial layer (Chang et al., 2008; Majo et al., 2008). Despite some ambiguity about their precise location, it is known that these cells divide either symmetrically for stem cell renewal, or asymmetrically to produce more highly proliferative TACs that migrate centripetally to populate the corneal epithelium (Kinoshita et al., 1981; Tseng, 1989). As these TACs divide and move superficially, their progeny progressively become more differentiated and give rise to post-mitotic, terminally differentiated cells (TDCs), which eventually undergo senescence and are sloughed off from the ocular surface. Despite the long known existence of CESCs and TACs among vertebrates, we are limited in our understanding of the comprehensive genetic profiles of these cornea cells. This has largely affected the search for definitive biomarkers to identify and isolate corneal stem cells that can be used for clinical applications.
Several studies on transcriptional profiling of corneal epithelial cells isolated from humans, rats and mice have relied on bulk-sampling techniques and cell enrichment using pre-defined markers (Adachi et al., 2006; Bath et al., 2013; Ma and Lwigale, 2019; Sartaj et al., 2017; Zhou et al., 2006). As these studies were restricted to specific subpopulations or regions of the corneal epithelium, it has been difficult to compare findings across these studies to systematically analyze corneal epithelial heterogeneity. Therefore, to facilitate the search for CESC biomarkers, a comprehensive and unbiased characterization of the transcriptome of many individual corneal epithelial cells representing progressive stages of vertebrate corneal maturation is required.
The South African clawed frog, Xenopus laevis, has been a valuable research model, particularly for studying eye tissue development, repair and regeneration, which includes both the cornea and the lens (Barbosa-Sabanero et al., 2012; Henry et al., 2008; Kha et al., 2018). The anatomy and development of the frog cornea are nearly identical to that of humans (Hu et al., 2013) making it an excellent, low-cost vertebrate model to study cornea biology and determine the dynamics of CESCs. Early during development (e.g., larval stages 48-51) (Nieuwkoop and Faber, 1956), the corneal epithelium consists of a distinct apical layer of differentiated cells, a single basal layer, and relatively few keratocytes underlying this epithelium. As the larva undergoes metamorphosis and matures into an adult (stage 66 and beyond), the cornea undergoes dramatic changes; the stromal space thickens and is filled with collagen lamellae and keratocytes, new epithelial layers are added, and limbal crypt-like palisades are observed (similar to those seen in mammals) (Goldberg and Bron, 1982; Hamilton and Henry, 2016). The presence of oligopotent epithelial stem cells in the frog cornea has been demonstrated in previous studies. For example, cells undergoing S phase DNA replication and mitosis (examined by EdU labeling and anti-phospho-Histone H3 (S10)) are scattered throughout the basal corneal epithelium of tadpoles (Perry et al., 2013; Thomas and Henry, 2014). Whereas in adult frogs, long-term BrdU or EdU label-retaining cells, that likely identify CESCs, become concentrated in the limbal area (Hamilton and Henry, 2016). Furthermore, molecular characterization of corneal epithelia of larval and adult frogs reveals that putative stem cell markers are expressed at different stages in different epithelial layers (Sonam et al., 2019). Although these markers label different cellular layers and regions of the corneal epithelium during maturation, none was found to be unique to CESCs or TACs. This largely agrees with similar conclusions reached by immunolocalization studies using corneas of other vertebrates (Kammergruber et al., 2019; Morita et al., 2015; Mort et al., 2012; Schlotzer-Schrehardt and Kruse, 2005). However, these studies provide only a glimpse into the genes expressed by these cells. Single-cell resolution molecular data is needed to provide a comprehensive, unbiased picture of tissue heterogeneity and to better understand cellular states and transcriptional profiles during corneal development and differentiation.
Single-cell RNA sequencing (scRNA-seq) is a revolutionary technique that has enabled the identification of cell subpopulations, analysis of rare cell types, reconstruction of cell lineage trajectories and the building of gene regulatory networks for tissues and organs including the skin, heart, kidney, uterine epithelium and intestinal epithelium (Combes et al., 2019; Griffiths et al., 2018; Haber et al., 2017; Haensel et al., 2020; Lescroart et al., 2018; Wu et al., 2017). It has provided key insights into the biology and transcriptional states of different types of stem cells, including hematopoietic progenitors, neural progenitors, embryonic stem cells, muscle stem cells and others (Dell'Orso et al., 2019; Klein et al., 2015; Kumar et al., 2017; Llorens-Bobadilla et al., 2015; Zhou et al., 2016). Recent scRNA-seq studies have even provided insights into the molecular and cellular heterogeneity of eye tissues, including human and mouse retinas (Macosko et al., 2015; Voigt et al., 2019), as well as human and mouse corneas (Kaplan et al., 2019; Li et al., 2021a; Li et al., 2021b). However, these studies focused on adult eye tissues, making it difficult to understand the developmental progression of gene expression changes at the single-cell level as the ocular tissue undergoes development and maturation.
Here, we use scRNA-seq to expand our understanding of the vertebrate corneal epithelium in an amphibian model at two distinct stages of development. By examining 22,481 cells, we identified eight distinct cell clusters in larvae and thirteen clusters in adult frog corneas, each having discrete transcriptional signatures. In search of novel biomarkers for corneal stem cells, we identified the distinctly enriched expression of marker genes in subsets of basal corneal cells – e.g., tspan1, gpha2 in larvae, and tgm2 in adults. Furthermore, the reconstruction of pseudotemporal trajectories of epithelial cells allowed us to better understand the gene expression changes that take place during the process of corneal differentiation in larval and adult frogs. In addition, we identified key gene regulatory networks of the corneal basal epithelium that are both developmentally conserved and unique, as well as examined the cell-cell communication dynamics that characterize the two developmental time-points. Together, this is the first study to uncover the transcriptional profile of the amphibian cornea, and this data may be extrapolated to understand the biology of cornea development in other vertebrates.
2. Materials and Methods
Animals
Adult Xenopus laevis were obtained from Nasco (Fort Atkinson, WI). Fertilized eggs were collected, and larvae were raised to Stage 49-51 based on developmental staging by Nieuwkoop and Faber (1956). All animal care and experiments performed in this study were approved and monitored by the Institutional Animal Care and Use Committee (IACUC) and the Division of Animal Resources (DAR) at the University of Illinois.
Single-cell dissociation
Larvae:
Tadpoles were anesthetized at room temperature (22°C) by incubation for 1-2 min in a 1:2000 dilution of MS222 (ethyl 3-aminobenzoate methanesulfonate, Sigma, St. Louis, MO) diluted in 1/20X Normal Amphibian Media, NAM (Slack, 1984), while adult frogs were incubated for 30-35 mins in 2g/L MS222. Anesthetized tadpoles were transferred to clay-lined dishes containing Ca++- Mg++ free Dulbecco’s Phosphate Buffered Saline (1X DPBS; Corning, NY, #21-031-CV). The corneal epithelial tissue was dissected using fine microscissors (Henry et al., 2018), taking care not to include any surrounding skin. The skin is demarcated by the outer edge of the orbit, a lack of transparency, and the obvious presence of pigmented melanophores. To limit the amount of time needed to collect these tissues, three operators simultaneously collected a total of 80 corneas in three separate glass depression dishes containing 400 μL 1X DPBS, on ice. Care was taken to ensure that corneas were carefully removed from the tip of the forceps and remained submerged in the 1X DPBS solution. Using a 200 μL pipette tip, most of the DPBS was gently removed without disturbing the corneas at the bottom of each glass dish. To dissociate the corneal epithelial tissue, ~350 μL of Accutase (CellnTec Advanced Cell Systems, Bern, Switzerland) was added to each dish, and the dishes were covered with plastic petri dish lids (35 x 10 mm, Corning, #351008), flat side down, and wrapped tightly with lab tape to secure them and prevent desiccation. Next, the dishes were incubated at 37°C for 35 min with intermittent trituration using a 200 μL pipette tip. To stop the reaction, 200 μL of media containing 61% Leibovitz's L-15 (Invitrogen, Carlsbad, CA), 0.5 μM EDTA (Invitrogen) and 10% Fetal Bovine Serum (Invitrogen) was added to each dish. For mechanical dissociation, the suspension was finally triturated 8-10 times using a 200 μL pipette tip. The three cell suspensions were pooled in a 2mL LoBind microcentrifuge tube (Eppendorf, Enfield, CT; #022431048), spun at 325g for 5 min in a fixed angle centrifuge, and resuspended in 200 μL L-15 with 10% FBS and 0.5 μM EDTA. Finally, the cells were passed through a 40 μm FlowMi cell strainer (Bel-Art, Wayne, NJ; #136800045) and collected in a 2mL LoBind tube. Cell viability was assessed using Acridine Orange/Propidium Iodide (AO/PI) staining solution (Nexcelom Bioscience, Lawrence, MA, #CS2-0106) that labels live and dead cells respectively, and counted using an automated cell counter (Cellometer K2, Nexcelom Bioscience).
Adults:
Similarly, for experiments with adult animals, 7-8 mature corneas were dissected out of the animals for each sample replicate. For harvested tissues collected in a glass dish, dissociation was performed using ~600 μL of Accutase for 60 min at 37°C. While holding the tissue submerged in dissociation media, the stroma was carefully peeled from the epidermis, and the epithelial tissue was gently trimmed into small fragments using microscissors. Further dissociation was done by trituration, and the reaction was quenched by adding 61% L-15 + 0.5 μM EDTA + 10% FBS. The suspension was pooled, centrifuged at 325g for 5 min, resuspended in 400 μL 61% L-15 + 0.5 μM EDTA + 10% FBS media and strained through 40 μm FlowMi cell strainers. Finally, the cell viability (as mentioned above) was determined before proceeding with library preparation.
Library preparation and sequencing
Single-cell sequencing libraries were prepared individually from each replicate/sample using the 10X Genomics Chromium Single Cell 3' Kit v3 and v3.1 (10X Genomics, San Francisco, CA) and sequenced with Illumina NovaSeq 6000 on a SP/SP4 flow cell to obtain 150bp paired reads. Library preparation and sequencing was conducted at the High-Throughput Sequencing and Genotyping Unit of the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign.
scRNA-seq data processing
The RNA sequencing reads were processed using the CellRanger v3.1.0 pipeline by 10X Genomics. For generating the CellRanger reference, the Xenopus laevis v9.2 reference genome and gene models were downloaded from Xenbase (http://ftp.xenbase.org/pub/Genomics/JGI/Xenla9.2/XENLA_9.2_Xenbase.gff3) (Karimi et al., 2018). Further downstream processing was performed in R Studio v3.6.1. The output from CellRanger was uploaded into an R Studio session, and processed using the Seurat v3.1 pipeline (Butler et al., 2018; Satija et al., 2015), wherein a standard filtering and preprocessing pipeline was employed. Batch-effects across samples were removed through a harmony algorithm using sample source (2 time points X 2 replicates -> 4 samples) as the different batches (Korsunsky et al., 2019). Genes expressed in less than 3 cells, and cells expressing < 200 or > 3,000 genes were removed from downstream analysis. We used a 5% cutoff for mitochondrial reads to remove dead/apoptotic cells. Post-normalization, we scaled data using the ScaleData function and performed this for all features/genes. We used FindNeighbours and FindClusters functions at parameter values of dims 1:20 and resolution 0.2, respectively. Significantly enriched genes within clusters were identified using the FindMarkers functionality in Seurat, that uses the MAST algorithm and the ratio of number of genes expressed/detected in a cell as a variable to regress. Cell type identities were assigned using expression patterns of previously established marker genes from the available literature (Castro-Muñozledo, 2015; Guo et al., 2018) and our previous study (Sonam et al., 2019). The frog X. laevis is allotetraploid, and gene expression data can be accessed for each allele from both the Large (Gene.L) and/or Short (Gene.S) chromosomes (Session et al., 2016). Taking into consideration that these alleles may have different functions, we reported cluster-specific allele expression patterns for each gene.
Trajectory Analysis
To infer the trajectory of single cells, we used Monocle2 (Trapnell et al., 2014) and applied the DDRtree algorithm for dimensionality reduction. Differentially expressed genes were calculated using comparison across the different clusters found by Seurat v3.1 above. We used the Top 3,000 genes to order cells and determine the pseudo-time trajectory. Relevant functions within Monocle2 were used to plot a heatmap for genes varying along the pseudo timeline. To define Gene Ontology (GO) for the regulated genes along the trajectory, we humanized the X. laevis gene names and converted them to human Ensembl GeneIDs, and used DAVID 6.8 for GO analysis (Dennis et al., 2003). GO option GOTERM_BP_ALL was selected, and the terms with a P-value < 0.05 were chosen as significant categories.
Cell Cycle Analysis
For cell cycle analysis, we used a previously defined core set of 84 G1/S and 98 G2/M cell cycle genes (Aztekin et al., 2019). We identified the approximate cell cycle state of each cell with the average expression (called the “cycle score”) for the two gene sets using the Cell cycle scoring function in Seurat v3.1, and used default classification by Seurat.
Gene Regulatory Network (GRN) analysis
We used the single-cell regulatory network inference and clustering (SCENIC) pipeline (Aibar et al., 2017), to estimate the AUCell Score activity matrix from the scRNA-seq dataset (Chembazhi et al., 2021). Briefly, since current relevant databases do not hold gene regulatory information specific to X. laevis, we collapsed raw counts from .L and .S gene forms in our data. This collapsed data was processed in the Seurat v3.1 pipeline, as explained above. A normalized count table was extracted from the Seurat object, and Xenopus genes with a known human ortholog were retained for downstream analysis, and the gene names were humanized. Unlike the standard SCENIC workflow where this AUCell score activity matrix is binarized by thresholding to generate a binary regulon-activity matrix, we retained the full AUCell score for all further analysis. The matrix of AUCell score containing regulons (rows) x cells (columns) was used as input to Seurat object and used to cluster and identify important regulons for each previously identified cluster.
Cell-cell communication analysis
To determine the cell-cell communication network, we first collapsed raw counts from .L and .S gene forms, and the Xenopus genes with a known mouse ortholog were retained for further analysis. To systematically construct the cell-cell communication networks and perform statistics of interactions, we utilized methods described in detail by Farbehi et al. (2019). Briefly, we used a directed and weighted network with four layers of nodes: source cell populations expressing the ligands, the ligands that are expressed by the source populations, the receptors directed by the ligands, and the target cell populations. Weights of edges that link ‘source to ligand’ and ‘receptors to target’ were calculated as log2 (fold change) in expression of ligand/receptor in source/target in comparison to other cells. We determined the ligand-receptor interactions using a mouse-specific ligand-receptor interaction dataset published earlier by Farbehi et al. (2019). The sum of weights along the path was used to determine path weights connecting a source to target through a ligand:receptor interaction. We chose all ligand:receptor connections with a minimum path weight of 1.5 and calculated the overall weight, ws:t, as the sum of all path weights between the corresponding source and target. Only edges with Benjamini-Hochberg adjusted P-values, Pw<0.01 were regarded as significant. Next, we constructed ligand:receptor interaction dot plots using ggplot2.
Immunofluorescence staining and imaging
Immunostaining of larval corneas was performed using a previously published protocol (Sonam et al., 2019). Briefly, the larval specimens were fixed in Dent’s fixative (80% methanol, 20% dimethyl sulfoxide, DMSO), or 3.7% Paraformaldehyde (PFA). Next, the eyes were excised from each sample, taking care to keep the corneas intact. The excised eyes were washed six times for 10 min each in 1X PBST (Phosphate buffered saline; 1.86 mM NaH2PO4, 8.41 mM Na2HPO4, 175 mM NaCl, pH7.4, containing 0.5% Tween-20). Blocking was done using 5% Bovine Serum Albumin/10% normal goat serum/1X PBST for 2 h at room temperature. Finally, the eyes were incubated with primary antibodies diluted in the blocking solution overnight at 4°C. Following primary incubation, six more washes were conducted with 1X PBST prior to incubating the eyes with secondary antibodies. Goat anti-mouse Alexa-Fluor 488 or 546, and goat anti-rabbit Alexa-Fluor 488 or 546 (Invitrogen, Rockford, IL) were used as secondary antibodies at a concentration of 1:300 diluted in blocking solution for 2 h at room temperature. Finally, eyes were washed twice with 1X PBST and incubated with 1μg/mL solution of Hoechst 33342 (Molecular Probes, Eugene, OR) in 1X PBS for nuclear counter-staining. RainX (ITW Global Brands, Houston, TX) treated slides were used for mounting the corneas, and SlowFade antifade reagent (Life Technologies, Eugene, OR) was used for the mounting media. To flat mount corneas on slides, labeled corneas were carefully removed from the whole eyes, and small, peripheral, radial incisions were made to facilitate flattening during the mounting process. Confocal imaging of immunolabeled larval corneas was performed using an inverted LSM 700 microscope (Carl Zeiss, Munich, Germany). Z-stacks were processed with Zen software (Carl Zeiss, Munich, Germany), and images were compiled using Adobe Photoshop.
3. Results
Single-cell RNA sequencing identifies cellular diversity in developing Xenopus cornea
To study cellular and transcriptional heterogeneity during amphibian corneal development, we isolated corneal cells from frog larvae and adults. We developed an Accutase enzyme-based digestion method for dissociating viable single cells from corneal tissue (Fig. 1A). Using the 10X Genomics-based scRNA-seq platform, we sequenced 24,000 cells in total, with ~139,000 mean reads per cell, ~1,700 median genes per cell, and a median of ~6,900 unique transcript molecules per cell. After quality assessment and filtering, a total of 22,481 high-quality cells were retained for downstream computational analysis. To allow cross-sample comparisons, we merged datasets from all samples and corrected the batch effects using the harmony algorithm (Fig. 1B and Fig. S1).
Figure 1. Overview depicting workflow for isolation of corneal cells from Xenopus for single-cell RNA sequencing (scRNA-seq).
(A) Isolation of corneal epithelial cells from Xenopus larvae and adults. Corneal epithelia were dissected, pooled and dissociated to isolate single cells. After determining high cell viability, a single-cell library was prepared using the 10X Chromium Single Cell 3' Reagent Kit (V3). (B) Computational workflow demonstrating data processing and analysis pipeline for scRNA-seq data. Cell Ranger was used to align raw reads and generate feature-barcode matrices. Seurat v3.1 was used to perform basic quality check (QC) and normalization, followed by use of Harmony to remove batch-specific effects.
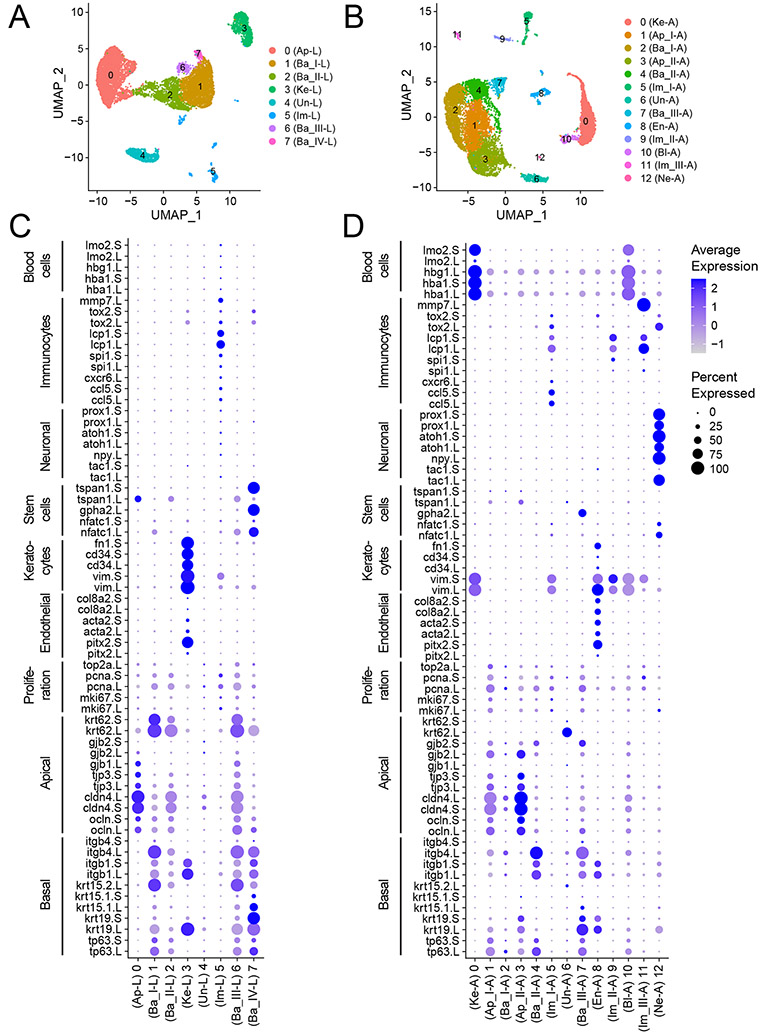
Next, an unbiased, graph-based clustering algorithm of the Seurat software was used to group cells according to their gene expression profiles. We identified eight and thirteen distinct cell clusters in larval and adult frog corneas, respectively, and these clusters were visualized in two-dimensional space using Uniform Manifold Approximation and Projection (UMAP) (Becht et al., 2018) (Fig. 2A). Furthermore, we projected harmony-corrected data using tSNE (van der Maaten and Hinton, 2008) and PHATE embedding (Moon et al., 2019), and found cells annotated as a cell type to cluster together in these projections (Fig. S2).
Figure 2. Identification of cell types in scRNA-seq dataset of corneas from Xenopus larvae and adults.
(A) Unbiased clustering of 10,659 high-quality cells from larval corneas after QC cutoffs, visualized by Uniform Manifold Approximation and Projection (UMAP). (B) Clustering of 12,155 filtered cells from adult frog corneas, visualized by UMAP. Each dot represents a single cell, and cells from the same cluster are similarly colored. Color codes in A, B represent unique clusters in each data set, and are not homologous. (C, D) Dot plot showing expression of known cell-type marker genes for each cluster. Dot diameter depicts the percentage of cluster cells expressing that marker and intensity encodes average expression of a gene among cells within that cluster. Abbreviations: Ap-L: Apical Larval; Ba-L: Basal Larval; Im-L: Immunocytes Larval; Ke-L: Keratocytes Larval; Un-L: Unknown Larval, and Ap-A: Apical Adult; Ba-A: Basal Adult; En-A: Endothelial Adult; Im-A: Immunocytes Adult; Ke-A: Keratocytes Adult; Ne-A: Neuronal Adult.
Cell-type identity was assigned based on a wide panel of cell-type-specific marker genes previously reported in various vertebrates, including Xenopus laevis (Castro-Muñozledo, 2015; Chen et al., 2004; Davies et al., 2009; Sonam et al., 2019; Yam et al., 2020; Yi et al., 2000). This included marker genes tp63.L, tp63.S, krt19.L, krt19.S, krt15.2.L, itgb1.L, itgb1.S and itgb4.L for basal epithelial cells; tjp3.L, tjp3.S, ocln.L, ocln.S, gjb1.L and gjb2.S for differentiated apical epithelial cells; nfatc1.L, nfatc1.S, gpha2.L for stem cells; col8a2.L, col8a2.S, pitx2.L, pitx2.S, acta2.L, acta2.S for endothelial cells; vim.L, vim.S, cd34.L, cd34.S, fn1.S for stromal cells/keratocytes; ccl5.L, ccl5.S, cxcr6.L, spi1.L, spi1.S, lcp1.L, lcp1.S, mmp7.L for immunocytes; tac1.L, tac1.S, npy.L, atoh1.L, atoh1.S, prox1.L, prox1.S for neuronal cells, and hba1.L, hba1.S, hbg1.L, lmo2.L, lmo2.S for blood cells.
Based on the expression pattern of these known markers, we were able to assign clusters (C) 0 as apical epithelial cells (Ap-L), C1/C2/C6/C7 as basal epithelial cells (Ba_I-L, Ba_II-L, Ba_III-L and Ba_IV-L, respectively), C3 as stromal keratocytes (Ke-L), C4 as unknown (Un-L), and C5 as immunocytes (Im-L) in the larval cornea. In adult corneas, C1/C3 are apical cells (Ap_I-A, Ap_II-A), C2/C4/C7 are basal cells (Ba_I-A, Ba_II-A and Ba_III-A, respectively), C8 are endothelial cells (En-A), C5/C9/C11 are immune cells (Im_I-A, Im_II-A, Im_III-A), C0 are stromal keratocytes (Ke-A), C12 are neuronal cells (Ne-A), C10 are blood cells (Bl-A), while identity of cluster C6 was unknown (Un-A). Given that we excluded the loose endothelial layer during cornea dissections in larvae, we did not identify a discrete cluster representative of the endothelial cell population. Despite our attempt to peel off the stroma and endothelial layer located beneath the adult cornea epithelium, we detected cell clusters expressing markers of stromal keratocytes and endothelium (C0 and C8, respectively).
This cluster analysis demonstrated that cellular heterogeneity increases as the frog cornea undergoes development and maturation from larval to adult stages. In addition, very low to no expression of mlana.L, tyr.S, mitf.L, mitf.S, tyrp1.L, dct.L, helped us rule out the presence of any skin melanophores in our data. Likewise, we did not detect the presence of cryaa.S, cryab.L, cryab.S marker genes, that confirmed the absence of any contaminating lens cells (Brahma and McDevitt, 1974; Henry et al., 2002) (Fig. S3).
Proliferative cells characterize the corneal epithelium in frogs
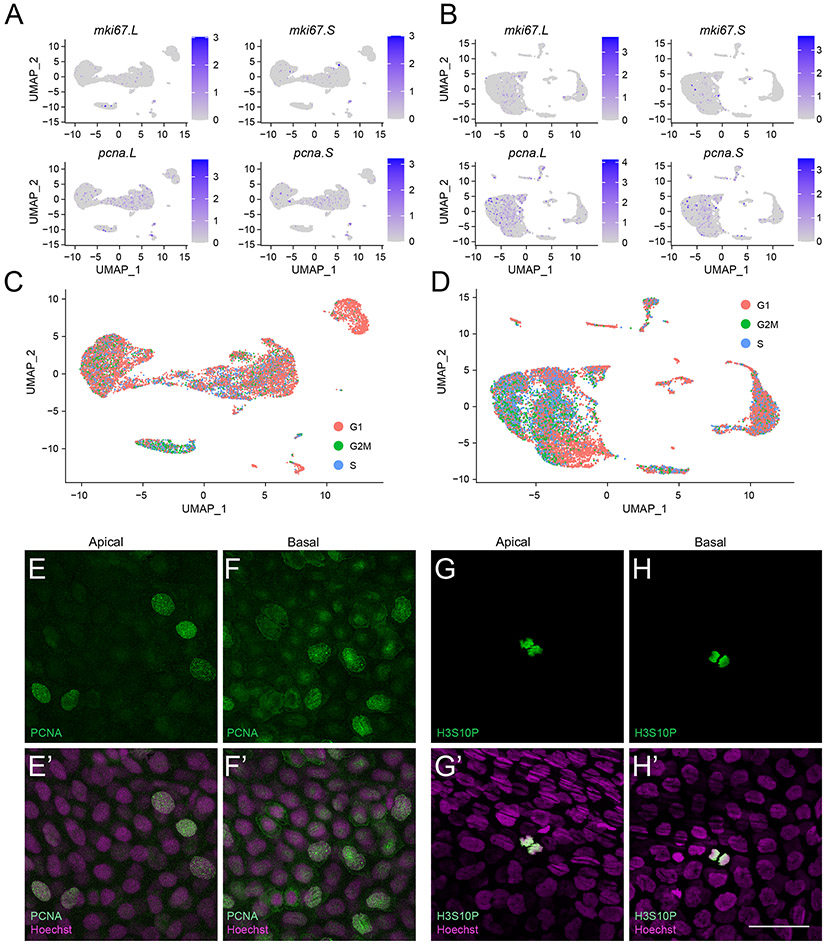
To distinguish the presence of transit amplifying cells (TACs) in our dataset, we looked for the expression of proliferation marker genes. However, these genes including mki67.L, mki67.S, pcna.L, and pcna.S, showed distributed expression across all clusters at both developmental time-points (Fig. 3A, B). Cell cycle analysis corroborated these findings (Fig. 3C, D), demonstrating that a considerable percentage of cells exist in the S and/or G2/M phases. To test the presence of proliferative cells in the bi-layered larval epithelium, we immunostained the cornea for the ubiquitous cell cycle marker PCNA. Using a Xenopus specific antibody (Agathocleous et al., 2009), we detected PCNA labeled nuclei in both basal and apical epithelia (Fig. 3E, F) of larval cornea. The presence of proliferative cells in both epithelial layers differs from previous Xenopus corneal studies (Hu et al., 2013; Perry et al., 2013), that reported the presence of dividing cells primarily in the larval basal epithelium. The proliferative cells in the corneal epithelium appear to be those involved in continuous growth and renewal of the larval cornea. To support our observation, we immunostained the corneal tissue with an anti-phospho-histone-H3 (S10) antibody and detected the presence of labeled nuclei in both epithelial layers (Fig. 3G, H). As phosphorylated Histone H3 (S10) is a marker of mitotic cells (Hans and Dimitrov, 2001), the positive staining further confirms cell division activity throughout this bi-layered epithelium.
Figure 3. Proliferative cells are distributed throughout the corneal epithelium in Xenopus.
(A, B) Gene expression UMAP overlay with proliferation genes mki67.L, mki67.S, pcna.L, and pcna.S in larval (A) and adult (B) corneas. Color intensity correlates with the relative transcript level for the gene. (C, D) UMAP plot showing the cell cycle status of each cell in (C) larval, and (D) adult clusters, determined using the “CellCycleScoring” module in Seurat v3.1. Color key indicates the cell cycle state. (E-F) Confocal images showing immunofluorescent staining for Proliferating Cell Nuclear Antigen (PCNA) (green) in the tadpole corneal epithelium. (E’-F’) Merged images for E-F with Hoechst labeled nuclei (magenta). (E, E’) Nuclear staining for PCNA is detected in some apical epithelial cells. (F, F’) PCNA labeled nuclei are detected in the basal epithelium. (G-H) Confocal images showing anti-phospho-Histone H3 (H3S10P) (green) in the tadpole corneal epithelium. (G’-H’) Merged images for G-H with Hoechst labeled nuclei (magenta). (G, G’) Mitotic nuclei (H3S10P) are present in a few apical epithelial cells. (H, H’) H3S10P labeled nuclei are detected in the basal epithelium. Scale bar in H’ equals 50 μm for E-H’.
In addition, the presence of proliferative cells along the entire length of sectioned corneal epithelium in adult frogs was previously shown by EdU labeling studies carried out by Hu and colleagues (2013). However, we were unable to successfully immunostain the adult corneal cross-sections to examine the presence of PCNA-positive or phospho-histone-H3 (S10)-positive cells in the multilayered adult epithelium. Altogether, our observations indicate that unlike recent scRNA-seq corneal studies in humans and mice (Kaplan et al., 2019; Li et al., 2021a; Li et al., 2021b; Ligocki et al., 2021), a single, discrete proliferative cell cluster (TAC population) could not be clearly identified in frog corneas.
Basal epithelial cells subcluster into distinct subtypes with unique transcriptional signatures, providing novel markers to identify corneal cell types
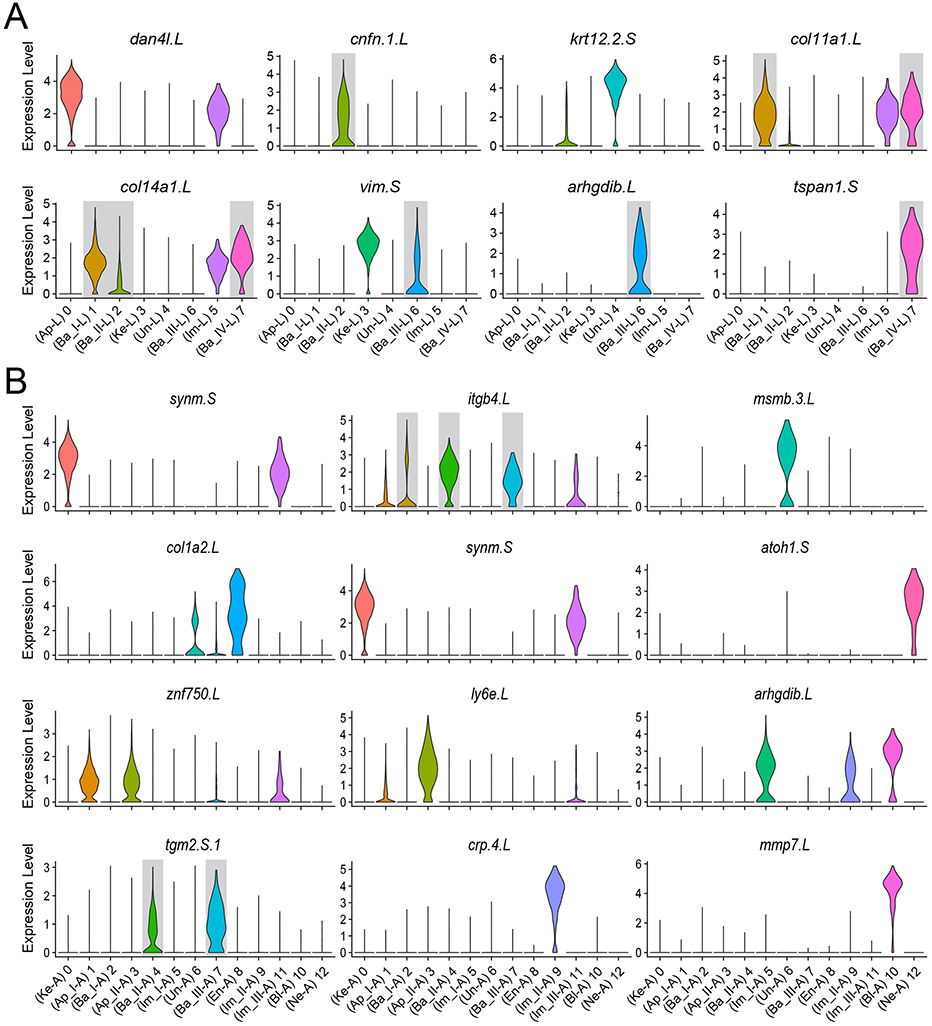
In organisms where detailed reference datasets are limited for select tissues and developmental stages, a notable advantage of scRNA-seq analysis is the unbiased identification of new marker genes for various cell types. Genes with Differential Expression (DE) in a given cluster, compared to all other clusters (average log fold change > 0.25; adjusted P-value <0.01), were identified using Seurat’s “FindMarkers” function. Next, we filtered DE genes using the difference between pct.1 and pct.2, along with a higher overall expression fold change value. For our analysis, we focused on genes expressed in more than 50% of the cells within that cluster. Fig. 4A, B depicts differentially expressed genes in individual corneal cell clusters of larval and adult frogs (the top 5 differentially expressed genes from each cluster is visualized by a heatmap in Fig. S4).
Figure 4. Identification of new marker genes for different cell types at two developmental time-points.
(A) Violin plots showing the cluster-specific expression of the top-ranking candidate marker genes for each identified cell type in larvae. (B) Violin plots with cluster-specific expression of the top-ranking candidate marker genes for each identified cell type in the adult frog. The highlighted (grey) marker genes are differentially enriched in basal cell clusters of larvae and adults.
As the basal epithelium is believed to harbor the corneal stem cells and highly proliferative TAC population in vertebrates (Gonzalez et al., 2017), we focused our investigation on the heterogeneity in basal corneal clusters. A closer look at the tadpole corneal basal cells revealed that some marker genes, including col14a1.L, col11a1.L, col17a1.L, col5a3.L, itgb4.L and krt19.L, were highly expressed and shared among the basal clusters, while other genes, including cnfn. 1.L, arhgdib.L and tspan1.S, were exclusively expressed in individual basal clusters (Fig. 4A). Many of these biomarker genes are known to play important roles in stem cells of individual organs and tissues in different organisms (Deng et al., 2021; Michel et al., 1996; Shcherbina et al., 2020; Zeng et al., 2018), and can be further investigated for their potential function as CESC markers at early stages of frog development. For instance, the gene Col17a1 encodes a hemidesmosomal transmembrane collagen protein that is expressed in basal cells of the epidermis. A higher level of Col17a1 expression is considered to be a marker of long-term epidermal stem cells and is crucial for skin homeostasis (Liu et al., 2019).
Among the four clusters annotated as basal epithelial cells in our larval dataset, cluster Ba_IV-L comprises a small percentage of basal corneal cells (~1.3% in our study) (Fig. S5A). Notably, cluster Ba_IV-L was also differentially enriched in tspan1.S and gpha2.L genes. Gpha2 (glycoprotein hormone subunit alpha 2) has been recently identified as a novel marker for limbal stem cells in both mouse and humans (Altshuler et al., 2021; Collin et al., 2021). Likewise, the cell-surface protein-coding gene, tspan1 (tetraspanin 1) was shown to be a marker of adult pluripotent stem cells (neoblasts) that underlie regeneration in planarians (Zeng et al., 2018). In addition, another member of tetraspanin family, TSPAN7, was identified as a novel marker of limbal stem cells in humans (Li et al., 2021a). Cumulatively, these observations suggest that cluster Ba_IV-L represents a putative CESC population in larval frogs.
Likewise, we investigated the three basal clusters in adult frog corneas. Among them, cells of cluster Ba_III-A constitute approximately 2.9% of cells in total (Fig. S5B). While cluster Ba_III-A expresses many of the stem-cell related marker genes such as collagens (col14a1, col17a1), integrins (itgb4, itga6), and keratins (krt19), cluster Ba_I-A was differentially enriched for adult-type keratins (krt12.6.L, krt78.2.S, krt57.L) (Suzuki et al., 2017). Based on this, we postulate that Ba_III-A identifies the “least differentiated basal cells” population, and potentially characterizes the putative CESC population in mature frogs. On the other hand, cluster Ba_I-A is most advanced developmentally and represents the “most differentiated basal cells”. As one of the most enriched and novel marker genes, we detected the transcript of tgm2 (transglutaminase 2) in cluster Ba_III-A. This is a protein-coding gene related to the maintenance of stemness (Kang et al., 2018), and an increased expression of tgm2 points towards its possible role as a unique marker and key regulator of the CESC population in adult frogs. A third group of basal cells, namely cluster Ba_II-A, co-express genes of the later stage (e.g., krt12.6.S, cldn1.L, cldn1.S) while still retaining expression of stem cell-related marker genes (e.g., itgb4, col17a1, itga6), suggesting that these cells are transitioning from Ba_III-A to Ba_I-A clusters. Therefore, we posit this cluster as “transitional basal cells”.
Pseudo-temporal analysis reveals the differentiation trajectory of larval corneal epithelium and associated changes in gene expression
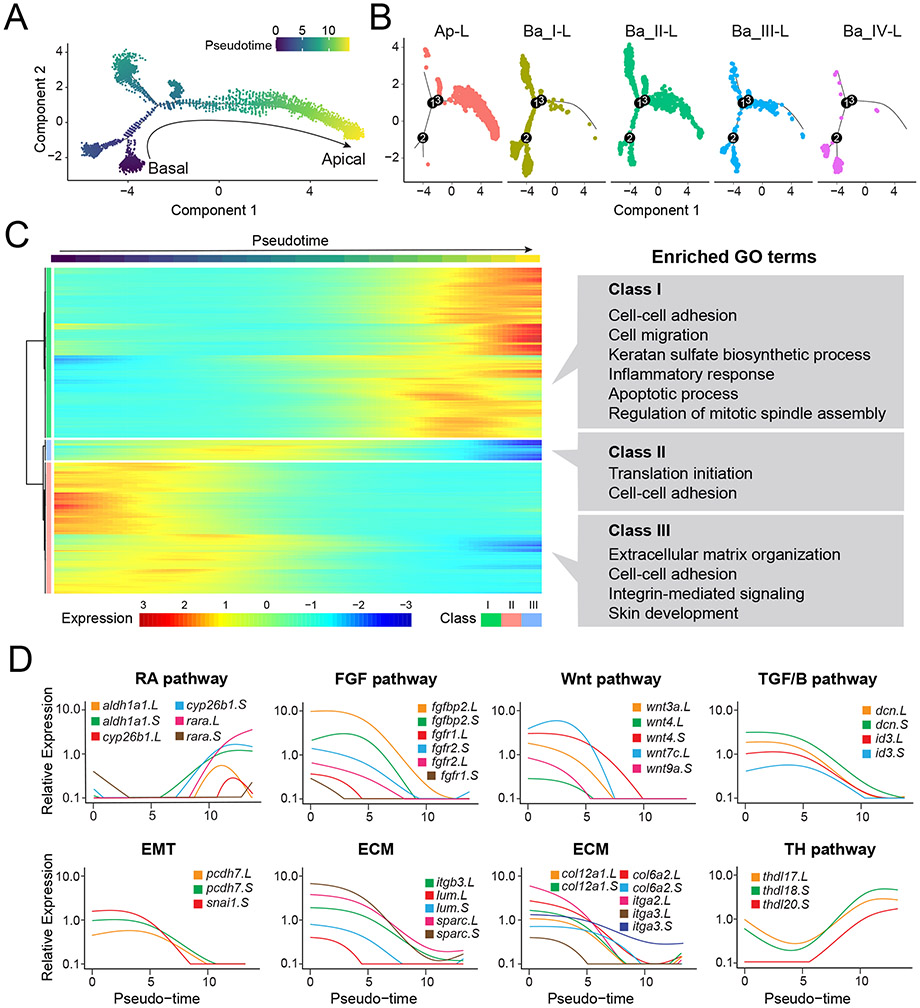
To investigate the differentiation trajectory of corneal epithelial cells, we leveraged one of the most powerful features of scRNA-seq datasets — single cells can be ordered in an unbiased way along paths to resolve their progressions. Taking into consideration the bi-layered organization of larval corneas, we expected our data to follow a rather linear trajectory (basal → apical) with some possible branching; therefore, we utilized the trajectory inference tools best suited for such analyses. Using Monocle2 (Trapnell et al., 2014), we performed pseudo-time-based ordering of single cells from Ap-L, Ba_I-L, Ba_II-L, Ba_III-L and Ba_IV-L clusters (Fig. 5A). The additional clusters — corneal keratocytes and immune cells — were omitted from this analysis, as they were transcriptionally disconnected and presumably lineage restricted from the epithelial cell types.
Figure 5. Pseudo-temporal ordering of epithelial cells reconstructs the larval corneal differentiation process.
(A) Pseudo-time plot indicating the cellular trajectory of all larval corneal basal and apical cells (Clusters Ap-L, Ba_I-L, Ba_II-L, Ba_III-L and Ba_IV-L). Single-cell trajectories were constructed, and pseudo-time values were calculated using Monocle 2. (B) Trajectories are colored by cluster identity that corresponds to the key in Figure 2A. (C) Heatmap depicting the classes of genes that vary along the pseudo-time plot during larval corneal differentiation. Pseudo-time is indicated by the color key similar to that shown in (A). Gene Ontology (GO) analysis was performed using DAVID and the enriched GO terms (P < 0.05) in each class are listed. Relative expression is indicated by the color key. (D) Gene expression kinetics along the pseudo-time progression of representative genes belonging to different pathways and processes, as indicated. Genes shown belong to the Retinoic Acid pathway (RA pathway), the Fibroblast Growth Factor pathway (FGF pathway), the Wnt pathway, Transforming Growth Factor (TGF) beta pathway, Epithelial- to-Mesenchymal Transition (EMT), Extracellular Matrix (ECM) deposition, and the Thyroid Hormone pathway (TH pathway).
In the pseudo-temporal trajectory derived for these epithelial cells, we found that while Ba_IV-L cells were located at the beginning, the cluster of apical cells (Ap-L) was concentrated primarily towards the trajectory terminus. Cells of Ba_I-L and Ba-III-L reside mostly on the left arm of the trajectory, while Ba_II-L cluster cells are distributed throughout the trajectory (see Fig. 5B), and likely represent the population of basal cells that are undergoing a transition to the apical layer. This ordering of cells along the axis originating from basal cells towards the terminally differentiated apical state is in agreement with the known corneal stratification in tadpoles (Hu et al., 2013; Sonam et al., 2019).
Next, we identified expression dynamics of the top 2500 genes that change as a function of progress through pseudo-time. These genes can be categorized into three distinct gene classes (namely Class I, II and III; Fig. 5C). To better understand the biological significance of these classes, we performed Gene Ontology (GO) analysis. Genes whose expression peaks late along this trajectory (Class I) are enriched in GO terms associated with cell adhesion, cell migration, keratan sulfate biosynthesis, inflammatory response and apoptotic process. On the other hand, genes predominantly expressed at the beginning of the developmental trajectory show an abundance of GO terms associated with ECM organization, integrin signaling and skin development (Class III). In addition, the smallest class (Class II) with genes that peak transiently along the pseudo-time was enriched for translational initiation and cell adhesion.
The pseudo-time trajectory can also be used to gain insight into the temporal expression changes of individual genes. As signaling mechanisms (such as Retinoic Acid, FGF and Wnt/β-catenin), processes involved in ECM deposition and EMT-related processes are crucial for corneal formation and differentiation (Dhouailly et al., 2014; Nakatsu et al., 2011), we closely investigated the expression changes of genes implicated in these pathways/processes along the pseudo-time (Fig. 5D). Retinoic acid signaling genes (cyb26b1, aldh1a1, rara), show an overall increasing trend along this pseudo-time, while genes in the FGF (fgfbp2, fgfr2, fgfr1), Wnt/β-catenin (wnt3a, wnt4 and others), and TGF/β pathway (dcn, id3), show a reduction in expression. We also noted a reduction in expression of genes that are involved in Epithelial-to-Mesenchymal (EMT) transition process (pcdh7, snai), and those constituting the corneal ECM (lum, sparc, itga2, col12a1 and others), along the trajectory. Furthermore, we noted a gradual increase in expression levels of thyroid hormone-controlled genes (thdl17, thdl18 and thdl20), along the pseudo-time axis. Changes in thyroid hormone gene expression dynamics represent a novel observation in the context of Xenopus eye development, and these changes appear to be similar to those observed in frog skin (Suzuki et al., 2009; Yoshizato, 2007). Furthermore, the corneal epithelium in anurans has been described to undergo changes during the process of metamorphosis (Hu et al., 2013; Slansky et al., 1970).
Differentiation trajectory of adult corneal epithelium involves key cell path decisions
The adult cornea in frogs has higher cellular complexity compared to pre-metamorphic stages, with a well-formed multilayered, stratified corneal epithelium, stroma and endothelium. The epithelium is composed of approximately 13 cellular layers at the center and 10 layers of cells on the periphery, and contains cuboidal cells at the basal side cells and flat squamous epithelial cells at the apical side (Hu et al., 2013). To examine the developmental relationship among basal cells, we initially focused on clusters Ba_I-A, Ba_II-A and BA_III-A and performed pseudo-time ordering based on a Monocle2 algorithm. We observed one branch point in the trajectory (Fig. 6A), with cells of cluster Ba_III-A concentrated at the start of the trajectory. Cells of cluster Ba_I-A largely constituted cell path 1, while Ba_II-A cells exist along both cell paths 1 and 2 (Fig. 6B). This pseudo-temporal trajectory deciphers major cell fate decisions of basal cells along the adult corneal differentiation process. We can conclude two key points here: (i) the data supports our hypothesis that the Ba_I-A cluster is representative of a “mature basal cell state”, and (ii) the cells of cluster Ba_II-A cluster exhibit an “transitional basal cell state,” between the immature/undifferentiated basal cell type and the mature basal cell type.
Figure 6. Differentiation trajectory in adult corneal epithelium involves key cell path decisions.
(A) Pseudo-temporal ordering of all cells from the basal epithelium present in the adult cornea (Ba_I-A, Ba_II-A and Ba_III-A). The trajectory has two different paths distributed around branch point 1, namely, cell paths 1 and 2. The arrows indicate the developmental directionality of the cell clusters, based on our inference. (B) Distribution of cells from three basal clusters along the pseudo-time shown in A. While cells of Ba_I-A are present towards the end of path 1, cells of Ba_II-A are distributed throughout the trajectory, and cells of Ba_III-A are located at start of trajectory. (C) Heat map showing bifurcation of gene expression programs executed along the pseudo-time after branching. Three distinct classes of genes (with top GO terms enriched in each class) were identified. (D) Pseudo-time plot to further resolve the cellular trajectories from basal clusters (Ba_I-A and Ba_II-A) to apical clusters (Ap_I-A and Ap_II-A). The branch point 3, which is under evaluation has two different paths, namely cell paths 3 and 4, around it. (E) Pseudo-time plots showing distribution of each of the four clusters along combined cellular trajectories shown in D. (F) Heatmap of gene expression along the pseudo-time plot in D, with the top GO terms enriched in each class of genes, as indicated.
To further examine how cellular gene expression changes while traversing through branchpoint 1 into either cell path 1 or 2, we used BEAM analysis within the Monocle2 pipeline. This led to identification of ~2500 genes that could largely be distinguished into three main classes based on their behavior across the branchpoint (Fig. 6C). Class I and II contained genes that had low expression in the pre-branch state, but post-branching, they were reciprocally up- or down-regulated along cell paths 1 and 2, respectively. These classes were enriched for genes representing cell adhesion, FGFR pathway, Notch pathway, cell proliferation (Class I), and ECM organization, cell migration, integrin signaling, and Wnt signaling (Class II). Alternatively, Class III represented genes that were relatively highly expressed pre-branching followed by an increased or decreased expression in cell paths 2 and 1, respectively. These were enriched for GO terms including cell migration, cell proliferation, BMP signaling and activated MAPK signaling.
Having explored the course of progression among different basal cell types, we sought to understand the cellular transitions involved in further differentiation of basal cells into the outermost epithelial cells, including acquisition of positional cell states (i.e. basal or apical). At first, we included cells belonging to apical epithelial clusters (Ap_I-A and Ap_II-A), and basal clusters, Ba_I-A and Ba_II-A (excluding the cells of cluster Ba_III-A) for constructing the pseudo-time trajectory. The ordering analysis revealed bifurcation into two distinct cell paths (namely, cell path 3 and 4) around branch point 3 in the trajectory (see Fig. 6D). The cells of cluster Ba_II-A, mainly concentrated at the start of the trajectory, can convert into either Ba_I-A or Ap_I-A/Ap_II-A (traveling right or left around branch point 3, respectively). The right arm of the trajectory (denoted by cell path 4), indicates that a subset of “transitional basal cells” (Ba_II-A), transform to “mature basal cells” along the differentiation course. On the other hand, cells of apical clusters Ap_I-A and Ap_II-A primarily constitute cell path 3 (the trajectory’s left arm around branch point 3). This path represents the progression of “transition basal cells” to terminally differentiated cell states, which potentially involves intermediate cell states and can be experimentally tested in future studies (Fig. 6E). Furthermore, we investigated the differentiation course of Ba_I-A cluster cells. Our Monocle pseudo-time analysis (including clusters Ba_I-A, Ap_I-A and Ap_II-A), shows that the mature basal cells (cluster Ba_I-A) ultimately differentiate to become cells of the apical epithelia (see Fig. S6). To further identify signature genes between the different cell populations we performed differential gene expression analysis between the two branches (Fig. 6F). For cell path 3, the differentially expressed genes (DEGs) enriched in the GO terms of cell-cell adhesion, negative regulation of apoptotic process, inflammatory response, positive regulation of I-kappaB kinase/NF-kappaB signaling (Class VI), and mitochondrial electron transport, mRNA splicing, response to cAMP (Class V), while for cell path 4, DEGs were enriched for GO terms of extracellular matrix organization, cell proliferation, integrin signaling, and Notch signaling (Class IV).
Gene regulatory network analysis reveals key differences in the regulation of corneal basal epithelial cells during development
To characterize gene regulatory networks (GRNs) that govern the developmental trajectory of basal corneal cells in vertebrates, we used the SCENIC pipeline to create a gene regulatory framework (Aibar et al., 2017). As transcription factors (TFs) are known to be highly conserved despite the allotetraploidization event in Xenopus laevis (Watanabe et al., 2017), we converted the X. laevis gene names to their human orthologs (see Materials and Methods). SCENIC calculates the activity of TFs from individual cells by integrating co-expression data with transcription factor motif enrichment analysis to generate a “regulon.” A regulon is comprised of an expressed transcription factor and all of its co-expressed target genes. We determined the regulon activities using Area Under Cell (AUCell), which ranks targets of an individual regulon among the expressed genes, thereby generating a regulon-by-cell activity matrix.
To explore regulon activities distinguishing the cellular features of larval and adult corneal basal cells, we analyzed the AUCell score activity matrix of basal clusters obtained from the two developmental time-points (i.e., clusters Ba_I-L, Ba_II-L, Ba_III-L, Ba _IV-L, and B_I-A, Ba_II-A, Ba_III-A). Upon 2D UMAP projection — constructed from the AUCell scores — we noted that basal cells from larval and adult stages formed distinct nonoverlapping clusters, representing clear differences in their GRNs (Fig. 7A). Cells from Ba_III-L and Ba_IV-L overlapped with Ba_I-L, demonstrating that mostly similar regulons are active in a majority of larval basal cells. However, few basal cells from Ba_II-L overlapped with the Ba_I-L cluster. Interestingly, for basal cells of Ba_II-A and Ba_III-A origin, the clusters overlapped and were located at the far end of the UMAP plot, indicating their GRNs are substantially discrete from those active in larvae; however, the cells from the Ba_I-A grouped more closely to larval basal cells (Fig. 7B).
Figure 7. Distinct gene regulatory networks are active in basal epithelial cells of the larval and adult frog corneas.
(A) UMAP projection of all basal cells based on the AUC scores for each regulon calculated using SCENIC. Cells belonging to the same regulon are colored, according to the key. (B) AUC score based UMAP projection, grouped according to the origin of basal cells. Regulons of basal cells (Ba_I-L, Ba_II-L, Ba_III-L and Ba_IV-L) belonging to larval frogs group together and overlap, whereas regulons from adult basal cells (Ba_I-A, Ba_II-A and Ba_III-A) are separated from larval regulons. (C, D) Dot plot showing unique and shared regulons that are active in different basal cell states. Dot diameter depicts the percentage of cluster cells expressing that regulon and color intensity encodes average expression of a regulon among cells within that cluster, as indicated in the keys.
Furthermore, we explored the regulons characterizing each of the basal cell clusters to identify the main TFs and their target genes that drive those cell states (Fig. 7C, D). To our interest, we found that distinct developmental regulons are activated in basal cells of tadpole and adult corneas. For the tadpole basal clusters, MEIS1, NFATC1, ARID3A, XBP1, and CREB3L1 regulons were active while these were muted in adult clusters. Whereas, for adult basal clusters exclusively activated regulons included E2F4, CEBPA, NFIA, NRF1, NFE2L2 and JUND (Fig. 7C). On the other hand, certain regulons associated with ETS-like factors (e.g., ELF1, ELF3), Kruppel-like factors (e.g., KLF6), EGR (e.g., EGR1), MYC, IRF (e.g., IRF8, IRF9), and CEBP (e.g., CEBPD, CEBPG) were detected in basal corneal clusters of both the larvae and adults (Fig. 7D), indicating significant conservation of regulons through development. Among these, many of the proteins and target genes belonging to TF families such as Nuclear Factor of Activated T Cells (e.g., NFATC1), homeobox (e.g., MEIS1), activator protein-1 (e.g., JUND), and basic region-leucine zipper (e.g., CEBPA, ATF4) proteins are known to play critical roles in stem cell maintenance of different tissues (Horsley et al., 2008; Unnisa et al., 2012; Zhao et al., 2015). For instance, a regulon comprising the CCAAT enhancer binding protein delta (CEBPD) transcription factor, detected in all corneal basal cells, is known to be instrumental in regulating self-renewal and cell cycle length of limbal stem cells in humans (Barbaro et al., 2007). Likewise, FOS which is a key factor in regulating cell cycle entry, proliferative expansion, and adult muscle stem activation (Almada et al., 2021), has an enriched expression among all basal cells. The identification of these regulons, both well-established and novel, will open new windows to explore TF markers and pathways for stem cell maintenance and differentiation at key developmental time-points.
Variable cell-cell signaling dynamics dictate cornea development in frog larvae and adult
Next, we examined the intracellular crosstalk that exist among different cell types in larval and adult frog cornea by constructing potential cell-cell communication networks (Farbehi et al., 2019) from our dataset. The edges of the network point from source to target cells, which express specific ligands and their corresponding receptors, respectively. The thickness of edges denote weights representing fold-changes in the expression of ligand-receptor pairs (see Material and Methods). Using this we generated a weighted and directed network of potential cell-cell interactions within the frog cornea. In larval frogs (Fig. 8A), the keratocytes presented a unique interaction landscape with strong inbound and outbound connections with most other cell types, whereas apical epithelial cells were refractory to significant crosstalk. The basal cluster, representing the immature putative CESC population (Ba_IV-L), actively communicates with keratocytes, as the ligands and receptors identified in these epithelial cells display a significant number of interactions (Fig. 8A, C and Fig. S7). Previous studies in humans have demonstrated that the communication between limbal epithelial stem cells and stromal cells is crucial for maintaining the stem cell phenotype and thereby regulates corneal homeostasis (Notara et al., 2010; Wong et al., 2021; Xie et al., 2011). Our current findings in the Xenopus larval cornea supports these observations of epithelial-stromal interactions in the vertebrate cornea. Interestingly, the immunocytes appear to constitute another prominent communicating cell population, although it possessed low cell number. On the other hand, in corneas of adult frogs (Fig. 8B) we noticed an overall increase in interactions that accompanies the rise in cellular diversity. The endothelial cell population have the highest outbound interactions with other cell types, while the apical epithelial cells showing minimal interactions exhibit a pattern similar to that seen in larvae. Surprisingly, we did not detect the stromal keratocytes as an actively communicating cell population within corneas of the adult frog. Of note, keratocytes (in larvae) and endothelial cells (in adults) also seemed to target the respective cell types themselves, suggesting an autocrine mode of regulation.
Figure 8. Dynamics of cell-cell communication networks active in the larval and adult frog corneas.
(A, B) Network diagrams depicting significant cell-cell interactions marked by arrows (edges) pointing in the source-to-target direction. Thickness of arrows shows the sum of weighted paths between cell types, and the color of arrows corresponds to the source. Network diagrams for (A) larval and (B) adult frog corneas are shown. (C, D) Dot plot of representative outbound signals to different cell types in (C) larval and (D) adult frog corneas. Size of each dot signifies the weight of the corresponding ligand-receptor interaction, and the color indicates negative log10 P-value of the source-to-target interaction, as indicated in the keys. The ligands of the source cell are in black and the receptor present on the target cell follows in red. Empirical P-values were calculated and Benjamini-Hochberg correction was performed. The detailed ligand-receptor interactions are shown in Figure S7.
Furthermore, we analyzed the individual ligand-receptor interactions among various cell types. We created dot plots for corneas from both developmental time points demonstrating all ligand-receptor interactions with a minimum path weight of 1.5, for all significant cell-cell relationships (Padj < 0.01) (Fig. 8C, D and Fig. S7). This helped resolve a comprehensive network of potential cell-cell interactions, revealing prominent ligand-receptor interactions that characterize the frog cornea. In line with reports from humans and other vertebrates (Robertson et al., 2021; Zhang et al., 2015), we detected the expression of ligands and receptors of key signaling pathways, such as the Wnt/β-catenin, TGF/β, FGF and Notch pathways in the frog cornea. For instance, Fibroblast Growth Factor 7 (FGF7), has been implicated in promoting limbal stem cell proliferation and modulating renewal and homeostasis of the corneal epithelium (Hayashi et al., 2005). In our study, Fgf7 was detected in basal epithelial cells, apical epithelial cells, as well as immune cells, while their corresponding receptors (Fgfr1, Fgfr2, Fgfr3) were mainly identified in keratocytes (in larvae) and endothelial cells (in adults). Here, we detected Tenascin C (Tnc) expression exclusively in basal cells (Ba_IV-L), and its corresponding receptors, including Itgb1, Itga9, Itga5 and Egfr were present both in basal cells and keratocytes of larvae, indicating intra-and inter-cellular communications. Similarly, in adult corneas Tnc ligands were mostly expressed in endothelial cells, while their respective receptors were heterogeneously detected in apical, basal and endothelial cells. Similar to recent single-cell reports for the human limbus (Collin et al., 2021), we observed multiple chemokine ligands, such as Ccl5, Cxcl12, Cxcl16 and their receptors in the frog cornea, indicative of potential regulatory interactions exhibited by immune cells.
4. Discussion
In this study, we characterized over 22,000 single-cell transcriptomes to generate the first comprehensive cellular atlas of the cornea in Xenopus larvae and adults. Recent single-cell transcriptomic studies in humans (Collin et al., 2021; Dou et al., 2021) and mouse (Kaplan et al., 2019) focused on fully formed mature corneas; however, both early and adult developmental stages of corneal transcriptional heterogeneity have not been analyzed in any single species. We leveraged the transition from the simple bi-layered corneal tissue organization of larvae, to the anatomically more advanced organization of adult frog corneas, which is similar to that of humans, to understand the gene profile dynamics, differentiation program, and transcription factor regulatory networks for in vivo corneal development and maturation.
Cluster analysis revealed increasing cellular complexity with the progression of corneal development. This includes 4 clusters of basal epithelia, 1 cluster of apical epithelia, 1 keratocyte cluster, and 1 immunocyte cluster found in larvae (cells of the loose larval endothelial cell layer were not captured here). Whereas in the adult, 3 clusters of basal cells, 2 clusters of apical cells, 1 keratocyte cluster, 1 endothelial cluster, 1 neuronal cluster, 3 immunocyte clusters and 1 blood cell cluster were identified (Fig. 2). Traditionally, studies have considered proliferation marker genes such as Mki67 and PCNA, to characterize TACs (Guzman et al., 2013; Lehrer et al., 1998). In addition, a more recent study by Li et al. (2021b) described additional cell cycle-dependent genes (e.g., RRM2, TK1 and CENPF, CDC20) as signature markers for identifying TACs. Surprisingly, none of the basal subclusters (either in larvae or adult) were differentially enriched for these marker genes. The presence of a TAC cell type has been challenged using the mouse tail epidermis model (Jones et al., 2007), and our observations could align with arguments in the field that TACs are not a real entity; rather simply a functional concept. On the other hand, the lack of a unique TAC cluster in the frog corneas could be a species-specific feature. The heterogeneous distribution of proliferation marker genes, and cells existing in S and G2/M phases bolster the observation that a specific TAC cell type may not exist in frogs (Lavker et al., 2020; Mort et al., 2012).
The heterogeneity present among limbal basal epithelial cells has been previously postulated (Nowell and Radtke, 2017), and was recently characterized at single-cell level in humans. While work by Li et al. (2021a) highlighted five major cell types spanning stages of limbal SC differentiation in the basal epithelium of humans, another study by Dou et al. (2021) reported four cell states in limbal epithelial stem cells that vary in properties of stemness and differentiation. In the present study, we identified four distinct transcriptional states in basal cells of larval frogs (Ba_I-L, Ba_II-L, Ba_III-L and Ba_IV-L) and three in adult frogs (Ba_I-A, Ba_II-A and Ba_III-A). In our attempt to identify genes that specifically characterize basal populations, we detected a high transcript level expression of corneal marker genes (e.g., tp63, krt19, krt15.2, itga6, itgb4) known from studies in other vertebrates. Of significance, the smallest basal clusters found in larvae (Ba_IV-L) and adults (Ba_III-A) exhibited differential expression of known stem-cell related genes (e.g., col17a1, col14a1, nfatc1, gpha2), leading us to postulate that these clusters represent the putative stem cell pool in frogs.
To discover the gene expression patterns along the differentiation axis, we modeled a pseudo-temporal trajectory of epithelial cells at both developmental stages. As illustrated by the pseudo-temporal plot in larvae (Fig. 5), we propose a trajectory of epithelial cells originating from the least differentiated cluster, Ba_IV-L, transitioning through three discrete basal cell states, Ba_I-L, Ba_II-L and Ba_III-L, and then finally to the terminally differentiated epithelial cluster, Ap-L. The underlying cellular and molecular transitions represent a complex differentiation and migratory process of epithelial cells in early-stage frog corneas. Although it cannot be ruled out that the temporal progressions of individual cells during differentiation might be more dynamic than captured in this analysis, the data adds to our current understanding of earlier stages of corneal development and differentiation. We are particularly intrigued by our new finding of the role of thyroid hormone genes in frog corneal differentiation, and will seek to explore thyroid hormone function in future studies. Furthermore, a number of signaling pathways emerged as being involved in regulating cornea differentiation (e.g., the Retinoic Acid, FGF, and Wnt/β-catenin pathways), that have also been shown to play crucial roles in supporting cornea-lens regeneration in larvae (Day and Beck, 2011; Fukui and Henry, 2011; Hamilton et al., 2016; Thomas and Henry, 2014).
Compared to larvae, the adult frogs have a significantly complex cornea with a multilayered epithelia. Therefore, to uncover gene expression dynamics that regulate differentiation of adult corneal epithelial cells, we conducted independent pseudotime analyses involving only basal cells, followed by basal to apical cells. We inferred cellular trajectories going from the least differentiated cell type (Ba_III-A) to a transitional basal cell type (Ba_II-A) or directly leading to a more mature basal cell type (Ba_I-A). This is followed by cells of cluster Ba_II-A committing along either apical cell paths (i.e., cell path 3; terminally differentiated superficial cell type, Ap_I-A and Ap_II-A) or the mature basal cell path (i.e., cell path 4; Ba_I-A) (Fig. 6), which in turn convert to Ap_I-A and Ap_II-A cells (Fig. S6). However, to comprehensively understand the precise progression of cells through various layers in the adult cornea, we need to integrate spatial information with scRNA-seq data. In the future, lineage tracing along with scRNA-seq (Kester and van Oudenaarden, 2018) will help reconstruct a more refined hierarchical trajectory of corneal cells in these anurans — similar to that reported in adult mice corneal epithelium and skin epithelium (Altshuler et al., 2021; Guerrero-Juarez et al., 2019; Haensel et al., 2020). Moreover, live imaging of these fate transitions would help shed light on their behaviors.
At the transcriptomic level, the expression patterns and functions of several key genes involved in corneal epithelial development are known, but regulators of these genes are still being identified (Stephens et al., 2013; Swamynathan, 2013). In addition, a recent single-cell transcriptomic study investigating the inter-species (humans, pigs, mouse and others) conservation of TFs and target genes (regulons) in different compartments of the eye, including the cornea, reported that key regulons are highly conserved (Gautam et al., 2021). Therefore, the study of expressed TFs in frog corneal epithelium may provide insight into the machinery that maintains the various cell types during the vertebrate cornea developmental window. Here, we leveraged our scRNA-seq data to comparatively analyze the dynamic transcriptional regulons active in different cell types (i.e., basal and apical epithelial cells, etc.) at discrete stages. In our analysis, we identified various regulons to be conserved between larvae and adult basal cells, indicating that certain TFs and target genes in these modules globally regulate these basal cell states. Based on the identification of regulons that are unique and enriched in basal cells of larval vs. adult corneas (Fig. 7), we propose that biomarkers of basal CESCs may be developmental stage specific. This is in line with conclusions from our previous study where we demonstrated that protein level expression of various biomarkers, including TFs (e.g., p63, Pax6, Tcf7l2) varies through the developmental time course in Xenopus (Sonam et al., 2019). Similar conclusions were drawn by earlier studies examining anatomy and stem cell activity in developing corneas in humans and rats (Chung et al., 1992; Davies et al., 2009). Taking this into account, it is imperative that future studies on the regulation of corneal cell types should particularly focus on multiple, distinct stages of corneal morphogenesis rather than only the mature adult corneas. This will facilitate identification of many previously uncharacterized TFs and other genes that can be potentially explored as excellent CESC biomarker candidates for a given developmental stage.
How do cell-cell interactions change as frog corneas undergo development and maturation? To comprehensively probe underlying cell communication that drives heterogeneity and cell state transitions, we first constructed the communication map between all corneal cell types and dissected all putative interactional links across corneal cell types. During stages 48-50, keratocytes are actively migrating to form the stromal layer beneath the epithelium (Hu et al., 2013). We found that during the larval stage, the stromal keratocytes constitute the cell type with the highest number of interactions. However, in adult frogs with a fully mature cornea, the endothelial cells exhibit the greatest number of communication links. Recent single-cell studies have unraveled the communication dynamics of limbal stem cells with surrounding niche cells and surveyed the ligand-receptors that regulate the human limbus (Dou et al., 2021). Our work to identify multiple ligand-receptor pairs in the basal cells and other cell types of larval and adult frog corneas provides important clues about the regulation of the corneal epithelia in yet another vertebrate model and adds to current knowledge in the field of corneal biology.
One of the key features of Xenopus development is the metamorphosis process of tadpoles into juvenile adults (Dodd and Dodd, 1976), which involves an extensive remodeling process of various tissues and organs (such as the skin, digestive tract and nervous system), and this is accompanied by a dramatic loss of regenerative competence of organs, including the lens (Filoni et al., 1997). It is fascinating that while larval stages of Xenopus can regenerate many of their body parts, including the lens (e.g., tail, spinal cord, limb bud) (Beck et al., 2009), adult frogs dramatically lose this ability. A fundamental and rather unresolved question is why the same tissue from different stage of development exhibits such markedly different abilities for regenerative growth. Earlier work on Xenopus lens regeneration had identified some candidate genes, including pax6 and fgfr2 (bek isoform), that may contribute to the cornea’s competence to regrow a lens (Arresta et al., 2005; Gargioli et al., 2008). Understanding regenerative processes at an unprecedented single-cell resolution has been possible in various vertebrates, including spinal cord and tail regeneration in Xenopus (Aztekin et al., 2019; Kakebeen et al., 2020), as well as limb regeneration in axolotls (Leigh et al., 2018). Future single-cell transcriptomic profiling encompassing the complete time course of Xenopus cornea development, leading up to the adult frog cornea, will help us comprehensively understand the molecular and transcriptional heterogeneity that contributes to the cornea’s capacity to regenerate a lens. Furthermore, a time course-based single-cell analyses at different stages of lens reformation will shed light on gene expression profiles and the precise developmental trajectory of individual corneal cells and discern their spatio-temporal progression, as the cornea reforms a lens.
In summary, the transcriptional atlas of the Xenopus cornea described here provides detailed insights into corneal epithelium development and differentiation. One can extrapolate the findings of this study to test a number of newly identified enriched basal corneal epithelial genes for their potential application as specific markers in other vertebrates, including mice and humans. An extensive molecular and cellular characterization of corneal epithelia — with a key emphasis on transcriptional composition of basal cells and their regulation — will serve as a baseline for future studies in regenerating corneal tissues in vertebrates. This will also be crucial for designing and improving therapeutic treatments for diseases and dystrophies that affect corneal epithelial integrity.
Supplementary Material
Figure S1. Quality control (QC) metrics of Xenopus cornea scRNA-seq data. (A) Table showing the QC cutoffs applied on raw reads from scRNA-seq data prior to analysis. (B) Violin plots showing the distribution of gene counts, UMI counts and % mitochondrial content in larval and adult samples, after applying QC cutoffs.
Figure S2. (A, B) tSNE and PHATE projections of all larval corneal cells. (C, D) tSNE and PHATE projections of all adult corneal cells. Cells are colored by annotated cell types, as shown in Figure 2.
Figure S3. The absence of skin and lens cell types in (A) larval and (B) adult frog cornea was verified based on the expression of marker genes. Feature plots of cell-type-specific marker genes. Skin cell marker genes (highlighted in blue): melanocyte inducing transcription factor (mitf.L and mitf.S), tyrosinase (tyr.S), melan-A (mlana.L), dopachrome tautomerase (dct.L). Lens cell marker genes (highlighted in red): crystallin alpha A (cryaa.S), crystallin alpha B (cryab.L and cryab.S).
Figure S4. Heatmap demonstrating the cluster-specific expression of the top 5 ranking novel candidate marker genes in each cluster of (A) larvae and (B) adult cornea cells identified in the scRNA-seq datasets. Cells are colored by annotated cell types, as shown in Figure 2.
Figure S5. Proportion of cell populations in each cluster for (A) larval and (B) adult scRNA-seq datasets. Numbers on each bar indicate the number of cells.
Figure S6. (A) Pseudo-temporal ordering of all cells from basal cluster (Ba_I-A) and apical clusters (Ap_I-A and Ap_II-A) in adult cornea to resolve the path of transition basal cells (Ba_I-A) to apical layers. (B) Distribution of clusters along the cellular trajectory identified in (A).
Figure S7. Dot plot representing all outbound signals to different cell types in (A) larval and (B) adult frog corneas. Size of each dot signifies the weight of the corresponding ligand-receptor interactions, and the color indicates negative log10 P-value of the source-to-target interaction, as indicated in the keys.
Acknowledgements
We acknowledge support from the High-Throughput Sequencing Center, UIUC. We appreciate Dr. Ratnakar Singh for critical reading of the manuscript.
Funding
This research was supported by the National Institute of Health grant EY023979 (J.J.H) and R01HL126845, R01AA010154, R21HD104039 (A.K). S.B was supported by the NIH Tissue microenvironment training program (T32-EB019944), and the Scott Dissertation Completion Fellowship. U.V.C. was supported by the Herbert E. Carter Fellowship in Biochemistry.
Footnotes
Competing Interests
No competing interests declared.
Data Availability
All raw RNA-seq data files are available on the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE154896.
References
- Adachi W, Ulanovsky H, Li Y, Norman B, Davis J, Piatigorsky J, 2006. Serial analysis of gene expression (SAGE) in the rat limbal and central corneal epithelium. Invest Ophthalmol Vis Sci 47, 3801–3810. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB, 2009. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136, 3289–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC, Geurts P, Aerts J, van den Oord J, Atak ZK, Wouters J, Aerts S, 2017. SCENIC: single-cell regulatory network inference and clustering. Nature methods 14, 1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almada AE, Horwitz N, Price FD, Gonzalez AE, Ko M, Bolukbasi OV, Messemer KA, Chen S, Sinha M, Rubin LL, Wagers AJ, 2021. FOS licenses early events in stem cell activation driving skeletal muscle regeneration. Cell reports 34, 108656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler A, Amitai-Lange A, Tarazi N, Dey S, Strinkovsky L, Hadad-Porat S, Bhattacharya S, Nasser W, Imeri J, Ben-David G, Abboud-Jarrous G, Tiosano B, Berkowitz E, Karin N, Savir Y, Shalom-Feuerstein R, 2021. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R, 2015. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 33, 230–239. [DOI] [PubMed] [Google Scholar]
- Arresta E, Bernardini S, Gargioli C, Filoni S, Cannata SM, 2005. Lens-forming competence in the epidermis of Xenopus laevis during development. J Exp Zool A Comp Exp Biol 303, 1–12. [DOI] [PubMed] [Google Scholar]
- Aztekin C, Hiscock TW, Marioni JC, Gurdon JB, Simons BD, Jullien J, 2019. Identification of a regeneration-organizing cell in the Xenopus tail. Science 364, 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M, 2007. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol 177, 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, Del Rio-Tsonis K, 2012. Lens and retina regeneration: new perspectives from model organisms. Biochem J 447, 321–334. [DOI] [PubMed] [Google Scholar]
- Bath C, Muttuvelu D, Emmersen J, Vorum H, Hjortdal J, Zachar V, 2013. Transcriptional dissection of human limbal niche compartments by massive parallel sequencing. PLoS One 8, e64244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW, 2018. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. [DOI] [PubMed] [Google Scholar]
- Beck CW, Izpisua Belmonte JC, Christen B, 2009. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn 238, 1226–1248. [DOI] [PubMed] [Google Scholar]
- Brahma SK, McDevitt DS, 1974. Ontogeny and localization of the lens crystallins in Xenopus laevis lens regeneration. J Embryol Exp Morphol 32, 783–794. [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R, 2018. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Muñozledo F, 2015. The Mammalian Limbal Stem Cell Niche: A Complex Interaction Between Cells, Growth Factors and Extracellular Matrix. 23–56. [Google Scholar]
- Chang CY, Green CR, McGhee CN, Sherwin T, 2008. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci 49, 5279–5286. [DOI] [PubMed] [Google Scholar]
- Chembazhi UV, Bangru S, Hernaez M, Kalsotra A, 2021. Cellular plasticity balances the metabolic and proliferation dynamics of a regenerating liver. Genome Res 31, 576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ, 2004. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 22, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EH, Bukusoglu G, Zieske JD, 1992. Localization of corneal epithelial stem cells in the developing rat. Invest Ophthalmol Vis Sci 33, 2199–2206. [PubMed] [Google Scholar]
- Collin J, Queen R, Zerti D, Bojic S, Dorgau B, Moyse N, Molina MM, Yang C, Dey S, Reynolds G, Hussain R, Coxhead JM, Lisgo S, Henderson D, Joseph A, Rooney P, Ghosh S, Clarke L, Connon C, Haniffa M, Figueiredo F, Armstrong L, Lako M, 2021. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul Surf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes AN, Phipson B, Lawlor KT, Dorison A, Patrick R, Zappia L, Harvey RP, Oshlack A, Little MH, 2019. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development 146. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM, 1989. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209. [DOI] [PubMed] [Google Scholar]
- Davanger M, Evensen A, 1971. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229, 560–561. [DOI] [PubMed] [Google Scholar]
- Davies SB, Chui J, Madigan MC, Provis JM, Wakefield D, Di Girolamo N, 2009. Stem cell activity in the developing human cornea. Stem Cells 27, 2781–2792. [DOI] [PubMed] [Google Scholar]
- Day RC, Beck CW, 2011. Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC developmental biology 11, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orso S, Juan AH, Ko KD, Naz F, Perovanovic J, Gutierrez-Cruz G, Feng X, Sartorelli V, 2019. Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CC, Hu YF, Zhu DH, Cheng Q, Gu JJ, Feng QL, Zhang LX, Xu YP, Wang D, Rong Z, Yang B, 2021. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nature communications 12, 3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA, 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4, P3. [PubMed] [Google Scholar]
- Dhouailly D, Pearton DJ, Michon F, 2014. The vertebrate corneal epithelium: from early specification to constant renewal. Dev Dyn 243, 1226–1241. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, 2011. Stem cells of the human cornea. Br Med Bull 100, 191–207. [DOI] [PubMed] [Google Scholar]
- Dodd M, Dodd J, 1976. The biology of metamorphosis. Physiology of the Amphibia 3, 467–599. [Google Scholar]
- Dou S, Wang Q, Zhang B, Jiang H, Chen S, Qi X, Duan H, Lu Y, Dong J, Cao Y, Xie L, Zhou Q, Shi W, 2021. Molecular identity of human limbal heterogeneity involved in corneal homeostasis and privilege. Ocul Surf. [DOI] [PubMed] [Google Scholar]
- Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub-Lis K, Ho JW, Nordon RE, Harvey RP, 2019. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM, D'Alessio A, 1997. Lens regeneration in larval Xenopus laevis: experimental analysis of the decline in the regenerative capacity during development. Dev Biol 187, 13–24. [DOI] [PubMed] [Google Scholar]
- Fukui L, Henry JJ, 2011. FGF signaling is required for lens regeneration in Xenopus laevis. Biol Bull 221, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Giambra V, Santoni S, Bernardini S, Frezza D, Filoni S, Cannata SM, 2008. The lens-regenerating competence in the outer cornea and epidermis of larval Xenopus laevis is related to pax6 expression. Journal of anatomy 212, 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Hamashima K, Chen Y, Zeng Y, Makovoz B, Parikh BH, Lee HY, Lau KA, Su X, Wong RCB, Chan WK, Li H, Blenkinsop TA, Loh YH, 2021. Multi-species single-cell transcriptomic analysis of ocular compartment regulons. Nature communications 12, 5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MF, Bron AJ, 1982. Limbal palisades of Vogt. Transactions of the American Ophthalmological Society 80, 155–171. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY, 2017. Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley interdisciplinary reviews. Developmental biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JA, Scialdone A, Marioni JC, 2018. Using single-cell genomics to understand developmental processes and cell fate decisions. Mol Syst Biol 14, e8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, Ma D, Liu Y, Yamaga K, Shestova O, Gay DL, Yang Z, Kessenbrock K, Nie Q, Pear WS, Cotsarelis G, Plikus MV, 2019. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nature communications 10, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZH, Zhang W, Jia YYS, Liu QX, Li ZF, Lin JS, 2018. An Insight into the Difficulties in the Discovery of Specific Biomarkers of Limbal Stem Cells. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman A, Ramos-Balderas JL, Carrillo-Rosas S, Maldonado E, 2013. A stem cell proliferation burst forms new layers of P63 expressing suprabasal cells during zebrafish postembryonic epidermal development. Biology open 2, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A, 2017. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel D, Jin S, Sun P, Cinco R, Dragan M, Nguyen Q, Cang Z, Gong Y, Vu R, MacLean AL, Kessenbrock K, Gratton E, Nie Q, Dai X, 2020. Defining Epidermal Basal Cell States during Skin Homeostasis and Wound Healing Using Single-Cell Transcriptomics. Cell reports 30, 3932–3947 e3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PW, Henry JJ, 2016. The lens regenerative competency of limbal vs. central regions of mature Xenopus cornea epithelium. Exp Eye Res 152, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PW, Sun Y, Henry JJ, 2016. Lens regeneration from the cornea requires suppression of Wnt/beta-catenin signaling. Exp Eye Res 145, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans F, Dimitrov S, 2001. Histone H3 phosphorylation and cell division. Oncogene 20, 3021–3027. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Hayashi Y, Liu CY, Tichelaar JW, Kao WW, 2005. Over expression of FGF7 enhances cell proliferation but fails to cause pathology in corneal epithelium of Kerapr-rtTA/FGF7 bitransgenic mice. Mol Vis 11, 201–207. [PubMed] [Google Scholar]
- Henry JJ, Carinato ME, Schaefer JJ, Wolfe AD, Walter BE, Perry KJ, Elbl TN, 2002. Characterizing gene expression during lens formation in Xenopus laevis: Evaluating the model for embryonic lens induction. Developmental Dynamics 224, 168–185. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Perry KJ, Hamilton PW, 2018. Ex Vivo Eye Tissue Culture Methods for Xenopus. Cold Spring Harb Protoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Wever JM, Natalia Vergara M, Fukui L, 2008. Chapter 6 - Xenopus, an Ideal Vertebrate System for Studies of Eye Development and Regeneration A2 - Tsonis, Panagiotis A, Animal Models in Eye Research. Academic Press, London, pp. 57–92. [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E, 2008. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Haamedi N, Lee J, Kinoshita T, Ohnuma S, 2013. The structure and development of Xenopus laevis cornea. Exp Eye Res 116, 109–128. [DOI] [PubMed] [Google Scholar]
- Jones PH, Simons BD, Watt FM, 2007. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell 1, 371–381. [DOI] [PubMed] [Google Scholar]
- Kakebeen AD, Chitsazan AD, Williams MC, Saunders LM, Wills AE, 2020. Chromatin accessibility dynamics and single cell RNA-Seq reveal new regulators of regeneration in neural progenitors. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammergruber E, Rahn C, Nell B, Gabner S, Egerbacher M, 2019. Morphological and immunohistochemical characteristics of the equine corneal epithelium. Vet Ophthalmol 22, 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Oh SC, Min BW, Lee DH, 2018. Transglutaminase 2 Regulates Self-renewal and Stem Cell Marker of Human Colorectal Cancer Stem Cells. Anticancer Res 38, 787–794. [DOI] [PubMed] [Google Scholar]
- Kaplan N, Wang J, Wray B, Patel P, Yang W, Peng H, Lavker RM, 2019. Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Invest Ophthalmol Vis Sci 60, 3570–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi K, Fortriede JD, Lotay VS, Burns KA, Wang DZ, Fisher ME, Pells TJ, James-Zorn C, Wang Y, Ponferrada VG, Chu S, Chaturvedi P, Zorn AM, Vize PD, 2018. Xenbase: a genomic, epigenomic and transcriptomic model organism database. Nucleic Acids Res 46, D861–D868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester L, van Oudenaarden A, 2018. Single-Cell Transcriptomics Meets Lineage Tracing. Cell Stem Cell 23, 166–179. [DOI] [PubMed] [Google Scholar]
- Kha CX, Son PH, Lauper J, Tseng KA, 2018. A model for investigating developmental eye repair in Xenopus laevis. Exp Eye Res 169, 38–47. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Friend J, Thoft RA, 1981. Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci 21, 434–441. [PubMed] [Google Scholar]
- Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW, 2015. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S, 2019. Fast, sensitive and accurate integration of single-cell data with Harmony. Nature methods 16, 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Tan Y, Cahan P, 2017. Understanding development and stem cells using single cell-based analyses of gene expression. Development 144, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavker RM, Kaplan N, Wang J, Peng H, 2020. Corneal epithelial biology: Lessons stemming from old to new. Exp Eye Res, 108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavker RM, Tseng SC, Sun TT, 2004. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res 78, 433–446. [DOI] [PubMed] [Google Scholar]
- Lehrer MS, Sun TT, Lavker RM, 1998. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. Journal of cell science 111 ( Pt 19), 2867–2875. [DOI] [PubMed] [Google Scholar]
- Leigh ND, Dunlap GS, Johnson K, Mariano R, Oshiro R, Wong AY, Bryant DM, Miller BM, Ratner A, Chen A, Ye WW, Haas BJ, Whited JL, 2018. Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nature communications 9, 5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F, Wang X, Lin X, Swedlund B, Gargouri S, Sanchez-Danes A, Moignard V, Dubois C, Paulissen C, Kinston S, Gottgens B, Blanpain C, 2018. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 359, 1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Kim S, Li JM, Gao Q, Choi J, Bian F, Hu J, Zhang Y, Li J, Lu R, Li Y, Pflugfelder SC, Miao H, Chen R, 2021a. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul Surf 20, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Kim S, Zhang Y, Bian F, Hu J, Lu R, Pflugfelder SC, Chen R, Li DQ, 2021b. Single-Cell Transcriptomics Identifies a Unique Entity and Signature Markers of Transit-Amplifying Cells in Human Corneal Limbus. Invest Ophthalmol Vis Sci 62, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligocki AJ, Fury W, Gutierrez C, Adler C, Yang T, Ni M, Bai Y, Wei Y, Lehmann GL, Romano C, 2021. Molecular characteristics and spatial distribution of adult human corneal cell subtypes. Sci Rep 11, 16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Matsumura H, Kato T, Ichinose S, Takada A, Namiki T, Asakawa K, Morinaga H, Mohri Y, De Arcangelis A, Geroges-Labouesse E, Nanba D, Nishimura EK, 2019. Stem cell competition orchestrates skin homeostasis and ageing. Nature 568, 344–350. [DOI] [PubMed] [Google Scholar]
- Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A, 2015. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 17, 329–340. [DOI] [PubMed] [Google Scholar]
- Ma J, Lwigale P, 2019. Transformation of the Transcriptomic Profile of Mouse Periocular Mesenchyme During Formation of the Embryonic Cornea. Invest Ophthalmol Vis Sci 60, 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, 2015. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y, 2008. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456, 250–254. [DOI] [PubMed] [Google Scholar]
- Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L, 1996. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. Journal of cell science 109 ( Pt 5), 1017–1028. [DOI] [PubMed] [Google Scholar]
- Moon KR, van Dijk D, Wang Z, Gigante S, Burkhardt DB, Chen WS, Yim K, Elzen AVD, Hirn MJ, Coifman RR, Ivanova NB, Wolf G, Krishnaswamy S, 2019. Visualizing structure and transitions in high-dimensional biological data. Nat Biotechnol 37, 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Fujita N, Takahashi A, Nam ER, Yui S, Chung CS, Kawahara N, Lin HY, Tsuzuki K, Nakagawa T, Nishimura R, 2015. Evaluation of ABCG2 and p63 expression in canine cornea and cultivated corneal epithelial cells. Vet Ophthalmol 18, 59–68. [DOI] [PubMed] [Google Scholar]
- Mort RL, Douvaras P, Morley SD, Dora N, Hill RE, Collinson JM, West JD, 2012. Stem cells and corneal epithelial maintenance: insights from the mouse and other animal models. Results Probl Cell Differ 55, 357–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX, 2011. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci 52, 4734–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J, 1956. Normal table of Xenopus laevis (Daudin: ). 162–203. [Google Scholar]
- Notara M, Shortt AJ, Galatowicz G, Calder V, Daniels JT, 2010. IL6 and the human limbal stem cell niche: a mediator of epithelial-stromal interaction. Stem Cell Res 5, 188–200. [DOI] [PubMed] [Google Scholar]
- Nowell CS, Radtke F, 2017. Corneal epithelial stem cells and their niche at a glance. Journal of cell science 130, 1021–1025. [DOI] [PubMed] [Google Scholar]
- Perry KJ, Thomas AG, Henry JJ, 2013. Expression of pluripotency factors in larval epithelia of the frog Xenopus: evidence for the presence of cornea epithelial stem cells. Dev Biol 374, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SYT, Roberts JS, Deng SX, 2021. Regulation of Limbal Epithelial Stem Cells: Importance of the Niche. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartaj R, Zhang C, Wan P, Pasha Z, Guaiquil V, Liu A, Liu J, Luo Y, Fuchs E, Rosenblatt MI, 2017. Characterization of slow cycling corneal limbal epithelial cells identifies putative stem cell markers. Sci Rep 7, 3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija R, Farrell JA, Gennert D, Schier AF, Regev A, 2015. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol 33, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT, 1986. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. The Journal of Cell Biology 103, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE, 2005. Identification and characterization of limbal stem cells. Exp Eye Res 81, 247–264. [DOI] [PubMed] [Google Scholar]
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, van Heeringen SJ, Quigley I, Heinz S, Ogino H, Ochi H, Hellsten U, Lyons JB, Simakov O, Putnam N, Stites J, Kuroki Y, Tanaka T, Michiue T, Watanabe M, Bogdanovic O, Lister R, Georgiou G, Paranjpe SS, van Kruijsbergen I, Shu S, Carlson J, Kinoshita T, Ohta Y, Mawaribuchi S, Jenkins J, Grimwood J, Schmutz J, Mitros T, Mozaffari SV, Suzuki Y, Haramoto Y, Yamamoto TS, Takagi C, Heald R, Miller K, Haudenschild C, Kitzman J, Nakayama T, Izutsu Y, Robert J, Fortriede J, Burns K, Lotay V, Karimi K, Yasuoka Y, Dichmann DS, Flajnik MF, Houston DW, Shendure J, DuPasquier L, Vize PD, Zorn AM, Ito M, Marcotte EM, Wallingford JB, Ito Y, Asashima M, Ueno N, Matsuda Y, Veenstra GJ, Fujiyama A, Harland RM, Taira M, Rokhsar DS, 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbina A, Larouche J, Fraczek P, Yang BA, Brown LA, Markworth JF, Chung CH, Khaliq M, de Silva K, Choi JJ, Fallahi-Sichani M, Chandrasekaran S, Jang YC, Brooks SV, Aguilar CA, 2020. Dissecting Murine Muscle Stem Cell Aging through Regeneration Using Integrative Genomic Analysis. Cell reports 32, 107964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM, 1984. Regional biosynthetic markers in the early amphibian embryo. J Embryol Exp Morphol 80, 289–319. [PubMed] [Google Scholar]
- Slansky HH, Tolpin DW, Webster RG Jr., 1970. Collagenolytic activity in the cornea of the metamorphosing tadpole. Archives of ophthalmology (Chicago, Ill. : 1960) 83, 760–764. [DOI] [PubMed] [Google Scholar]
- Sonam S, Srnak JA, Perry KJ, Henry JJ, 2019. Molecular markers for corneal epithelial cells in larval vs. adult Xenopus frogs. Exp Eye Res 184, 107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Klein RH, Salmans ML, Gordon W, Ho H, Andersen B, 2013. The Ets transcription factor EHF as a regulator of cornea epithelial cell identity. J Biol Chem 288, 34304–34324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Machiyama F, Nishino S, Watanabe Y, Kashiwagi K, Kashiwagi A, Yoshizato K, 2009. Molecular features of thyroid hormone-regulated skin remodeling in Xenopus laevis during metamorphosis. Development, growth & differentiation 51, 411–427. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Suzuki M, Shigeta M, Fortriede JD, Takahashi S, Mawaribuchi S, Yamamoto T, Taira M, Fukui A, 2017. Clustered Xenopus keratin genes: A genomic, transcriptomic, and proteomic analysis. Dev Biol 426, 384–392. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, 2013. Ocular surface development and gene expression. J Ophthalmol 2013, 103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoft RA, Friend J, 1983. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 24, 1442–1443. [PubMed] [Google Scholar]
- Thomas AG, Henry JJ, 2014. Retinoic acid regulation by CYP26 in vertebrate lens regeneration. Dev Biol 386, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL, 2014. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SC, 1989. Concept and application of limbal stem cells. Eye (London, England) 3 ( Pt 2), 141–157. [DOI] [PubMed] [Google Scholar]
- Unnisa Z, Clark JP, Roychoudhury J, Thomas E, Tessarollo L, Copeland NG, Jenkins NA, Grimes HL, Kumar AR, 2012. Meis1 preserves hematopoietic stem cells in mice by limiting oxidative stress. Blood 120, 4973–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Maaten L, Hinton G, 2008. Visualizing high-dimensional data using t-sne. 4. [Google Scholar]
- Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2019. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc Natl Acad Sci U S A 116, 24100–24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Yasuoka Y, Mawaribuchi S, Kuretani A, Ito M, Kondo M, Ochi H, Ogino H, Fukui A, Taira M, Kinoshita T, 2017. Conservatism and variability of gene expression profiles among homeologous transcription factors in Xenopus laevis. Dev Biol 426, 301–324. [DOI] [PubMed] [Google Scholar]
- Wong HL, Poon SHL, Bu Y, Lo ACY, Jhanji V, Chan YK, Shih KC, 2021. A Systematic Review on Cornea Epithelial-Stromal Homeostasis. Ophthalmic Res 64, 178–191. [DOI] [PubMed] [Google Scholar]
- Wu B, An C, Li Y, Yin Z, Gong L, Li Z, Liu Y, Heng BC, Zhang D, Ouyang H, Zou X, 2017. Reconstructing Lineage Hierarchies of Mouse Uterus Epithelial Development Using Single-Cell Analysis. Stem cell reports 9, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HT, Chen SY, Li GG, Tseng SC, 2011. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells 29, 1874–1885. [DOI] [PubMed] [Google Scholar]
- Yam GHF, Riau AK, Funderburgh ML, Mehta JS, Jhanji V, 2020. Keratocyte biology. Exp Eye Res, 108062. [DOI] [PubMed] [Google Scholar]
- Yi X, Wang Y, Yu FS, 2000. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci 41, 4093–4100. [PubMed] [Google Scholar]
- Yoshizato K, 2007. Molecular mechanism and evolutional significance of epithelial-mesenchymal interactions in the body- and tail-dependent metamorphic transformation of anuran larval skin. Int Rev Cytol 260, 213–260. [DOI] [PubMed] [Google Scholar]
- Zeng A, Li H, Guo L, Gao X, McKinney S, Wang Y, Yu Z, Park J, Semerad C, Ross E, Cheng LC, Davies E, Lei K, Wang W, Perera A, Hall K, Peak A, Box A, Sanchez Alvarado A, 2018. Prospectively Isolated Tetraspanin(+) Neoblasts Are Adult Pluripotent Stem Cells Underlying Planaria Regeneration. Cell 173, 1593–1608 e1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh LK, Zhang S, Call M, Yuan Y, Yasunaga M, Kao WW, Liu CY, 2015. Wnt/beta-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 142, 3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Mo V, Nagasaki T, 2009. Distribution of Label-retaining Cells in the Limbal Epithelium of a Mouse Eye. Journal of Histochemistry & Cytochemistry 57, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhou J, Liu D, Dong F, Cheng H, Wang W, Pang Y, Wang Y, Mu X, Ni Y, Li Z, Xu H, Hao S, Wang X, Ma S, Wang QF, Xiao G, Yuan W, Liu B, Cheng T, 2015. ATF4 plays a pivotal role in the development of functional hematopoietic stem cells in mouse fetal liver. Blood 126, 2383–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Li X, Wang W, Zhu P, Zhou J, He W, Ding M, Xiong F, Zheng X, Li Z, Ni Y, Mu X, Wen L, Cheng T, Lan Y, Yuan W, Tang F, Liu B, 2016. Tracing haematopoietic stem cell formation at single-cell resolution. Nature 533, 487–492. [DOI] [PubMed] [Google Scholar]
- Zhou M, Li XM, Lavker RM, 2006. Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells. A comparison of limbal and corneal epithelial basal cells. J Biol Chem 281, 19600–19609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Quality control (QC) metrics of Xenopus cornea scRNA-seq data. (A) Table showing the QC cutoffs applied on raw reads from scRNA-seq data prior to analysis. (B) Violin plots showing the distribution of gene counts, UMI counts and % mitochondrial content in larval and adult samples, after applying QC cutoffs.
Figure S2. (A, B) tSNE and PHATE projections of all larval corneal cells. (C, D) tSNE and PHATE projections of all adult corneal cells. Cells are colored by annotated cell types, as shown in Figure 2.
Figure S3. The absence of skin and lens cell types in (A) larval and (B) adult frog cornea was verified based on the expression of marker genes. Feature plots of cell-type-specific marker genes. Skin cell marker genes (highlighted in blue): melanocyte inducing transcription factor (mitf.L and mitf.S), tyrosinase (tyr.S), melan-A (mlana.L), dopachrome tautomerase (dct.L). Lens cell marker genes (highlighted in red): crystallin alpha A (cryaa.S), crystallin alpha B (cryab.L and cryab.S).
Figure S4. Heatmap demonstrating the cluster-specific expression of the top 5 ranking novel candidate marker genes in each cluster of (A) larvae and (B) adult cornea cells identified in the scRNA-seq datasets. Cells are colored by annotated cell types, as shown in Figure 2.
Figure S5. Proportion of cell populations in each cluster for (A) larval and (B) adult scRNA-seq datasets. Numbers on each bar indicate the number of cells.
Figure S6. (A) Pseudo-temporal ordering of all cells from basal cluster (Ba_I-A) and apical clusters (Ap_I-A and Ap_II-A) in adult cornea to resolve the path of transition basal cells (Ba_I-A) to apical layers. (B) Distribution of clusters along the cellular trajectory identified in (A).
Figure S7. Dot plot representing all outbound signals to different cell types in (A) larval and (B) adult frog corneas. Size of each dot signifies the weight of the corresponding ligand-receptor interactions, and the color indicates negative log10 P-value of the source-to-target interaction, as indicated in the keys.
Data Availability Statement
All raw RNA-seq data files are available on the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE154896.