Summary
The interest in vaccination efficacy and toxicity has surged following the COVID-19 pandemic. Immune responses to several vaccines have been shown to be suboptimal in patients with chronic liver disease (CLD) and liver transplant (LT) recipients, as a consequence of cirrhosis-associated immune dysfunction or post-LT immunosuppression, respectively. Accordingly, vaccine-preventable infections may be more common or severe in those with liver disease than in the general population. The COVID-19 pandemic has greatly accelerated research and development into vaccination technology and platforms, which will have spillover benefits for patients with liver disease. The aims of this review are: (i) to discuss the impact of vaccine-preventable infections on patients with CLD and LT recipients, (ii) to appraise current evidence supporting vaccination strategies, and (iii) to provide some insight into recent developments relevant for patients with liver disease.
Keywords: Vaccination, COVID-19, hepatitis, cirrhosis, liver transplant
Key points.
-
•
Vaccine-preventable infections may be more common or severe in patients with chronic liver disease and liver transplant recipients than in the general population.
-
•
Immune responses to several vaccines may be suboptimal in these populations.
-
•
Vaccine hesitancy is a growing concern, with vaccine acceptance declining since the onset of the pandemic.
-
•
Vaccination rates for vaccine preventable infections are generally low in patients with chronic liver disease.
-
•
Recent advances in vaccination technology and platforms may improve vaccine efficacy and positively influence outcomes for patients with cirrhosis.
Introduction
Broadly, the interest in vaccination efficacy and toxicity has surged following the COVID-19 pandemic, and consequently the need for evidence-based reviews in this area has escalated. Vaccine hesitancy is a growing concern, with vaccine acceptance declining since the onset of the pandemic.1 Even amongst patients with liver disease and solid organ transplant recipients, vaccine hesitancy remains an issue affecting a minority.2 Moreover, less than 70% of the global population have completed initial COVID-19 vaccination, with this figure dropping to 35% in Africa, demonstrating that vaccination remains an important topic for the global hepatology community.3
This subject is of importance in the context of chronic liver disease (CLD) and liver transplant(ation) (LT), as vaccine-preventable infections may have particularly deleterious consequences in patients with CLD (especially at advanced stages) or LT recipients under immunosuppressive therapy.4 Furthermore, individuals with CLD often share risk factors that can result in dual or superimposed infections that are associated with higher morbidity and mortality than in the general population.5 Superimposed infections also tend to present in a different manner in patients with advanced liver disease than in those without liver disease; for example, SARS-CoV-2 presents with decompensation of CLD in one-third of cases.6
The aims of this review are to discuss the impact of vaccine-preventable infections on patients with CLD and LT recipients, to review evidence supporting vaccination strategies for these patients, and finally to provide some insight into technological developments that are relevant for patients with liver disease.
General principles of vaccination in patients with CLD and LT recipients
Immune responses have been shown to be sub-optimal in patients with CLD, and to decline with the stage of the disease.7 Cirrhosis-associated immune dysfunction (CAID) comprises a distinctive spectrum of immune alterations, ranging from systemic inflammation to immune paralysis.8 Both the innate and adaptive immune compartments are affected, as well as liver-resident immune cells which play a key role in regulating responses to gut-derived antigens. A consequence of the immune paralysis is an increased risk of infection (bacterial or other pathogens), which in turn cause a greater degree of systemic inflammation and organ failure; severe bacterial infections are associated with an overall mortality rate of 38% in patients with cirrhosis compared to 10% in healthy individuals.9,10 Thus, broad vaccination schedules are recommended by most societies, but CAID is also characterised by abnormalities in B- and T-lymphocyte populations and decreased vaccine responses are frequently observed.8 The low-level systemic inflammation that underpins CAID is thought to be a consequence of chronic antigenic stimulation from gut-derived microbial products. This has some parallels with other areas, such as ageing, where chronic inflammation can lead to impaired vaccine responses.[11], [12], [13]
LT recipients have a clearly increased risk of infection due to immunosuppression, although the risk is greatest in the first 6 months when immunosuppression is highest.14 The risk of infection may be increased and prolonged if the patient is treated for acute rejection. Thus, the situation is similar to CLD, with patients being caught between Scylla and Charybdis – being at an increased risk of infection while also showing a decreased response to vaccination owing to immunosuppression.
A further concern for LT recipients is the theoretical risk of vaccine-induced graft-rejection. A small number of such cases have been reported following SARS-CoV-2 vaccination (see below) but it remains difficult to ascribe causality given the large-scale vaccine roll-out.15 Moreover, no other examples of vaccine-induced rejection exist in the post-LT literature, so patients can, in general, be reassured.
Vaccine-preventable infections: Clinical impact, vaccine efficacy, safety and effectiveness
Recommended vaccination schedules for patients with CLD or LT are summarised in Table 1. In general, live attenuated vaccines are not recommended due to the theoretical risk of disseminated infection, although very few cases have been reported and their use is currently debated within transplant societies.16 Additionally, post-vaccination testing of antibody titres has not been recommended, although, again, this is a topic of debate in the post-pandemic era.17 Specifically, a translatable ‘correlate of protection’ is highlighted as a major unmet need for immunosuppressed patients, perhaps over and above serological titres (see below).
Table 1.
Recommended immunization schedule in patients with CLD or LT recipients.
| Vaccine | Liver transplant recipients | Patients with chronic liver disease |
|---|---|---|
| Hepatitis B | All patients should receive:
|
|
| Hepatitis A | All patients in Europe and United States should receive:
|
|
| Pneumococcal | All patients should receive 1 dose of PCV13 followed by 3 doses of PPSV23 at:
|
Patients between 19-64 years should receive 1 dose of PPSV23. Patients >64 years should receive 1 dose of PPSV23 at least 1 or 5 years after PCV13 or PPSV23, respectively |
| Influenza inactivated | Adult patients should receive 1 dose annually | |
| Zoster live attenuated (Zostavax) | Not recommended | Vaccination might be indicated if benefit of protection outweighs risk of adverse reaction in specific patient |
| Zoster recombinant (Shingrix) | Not recommended. If given, it should be administered before LT | Patients ≥50 years should receive 2 doses 2-6 months apart, regardless of previous herpes zoster or history of zoster live vaccine |
| Tetanus, diphtheria and pertussis | All patients should receive 1 dose of Tdap, then Td or Tdap booster every 10 years | |
| Measles, mumps and rubella | Not recommended. It should be given before LT | Patients with no evidence of immunity and born in 1957 or later should receive 1 or 2 dose(s) depending on indication |
| Human papillomavirus | All patients should receive 3 doses through age 26 | Adult patients should receive 2 or 3 doses through age 26 depending on age at initial vaccination |
| Meningococcal ACWY and B | Recommended for adults with an additional risk factor/indication, e.g. anatomical or functional asplenia, haematopoietic stem cell transplant or other additional factors | |
| Haemophilus influenzae | Recommended for adults with an additional risk factor/indication, e.g. anatomical or functional asplenia, haematopoietic stem cell transplant or other additional factors | |
| COVID-19 vaccine | Adult patients should receive:
|
Adult patients should receive:
|
Modified from: Advisory Committee on Immunization Practices. Recommended Adult Immunization Schedule for ages 19 years or older, United States, 2022. Centers for Disease Control and Prevention. Available at:https://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html (Accessed on November 1st, 2022) and American Association for the Study of the Liver Disease Expert Consensus Statement: COVID-19 clinical best practice advice for hepatology and liver transplant providers. Available at: https://www.aasld.org/sites/default/files/2022-10/AASLD%20COVID-19%20Guidance%20Document%2010.06.2022F.pdf
Hepatitis B vaccination
Impact of acute hepatitis B in CLD and post-LT
It is recognised that superimposed acute viral hepatitis may precipitate acute decompensation or acute-on-chronic liver failure (ACLF) in patients with cirrhosis, with high associated morbidity and mortality.18 Most reported cases of acute hepatitis B virus (HBV) in CLD are on the background of chronic hepatitis C virus (HCV), with a severe clinical course reported in almost 30% of patients.19,20 Additionally, the risk of hepatocellular carcinoma (HCC) is increased in patients with concurrent HBV and HCV infection.[21], [22], [23] Acute HBV is also of relevance in LT recipients; Crespo et al. demonstrated that post-transplant de novo HBV infection can lead to graft loss24 (Table 2).
Table 2.
Impact of vaccine-preventable infections in patients with CLD or LT.
| Study | Patients included | Underlying condition | Main outcomes |
|---|---|---|---|
| Hepatitis B | |||
| Benvegnù L, 199422 | 290 patient with cirrhosis | 25% HBsAg positive 69% anti-HCV 26% alcohol abuse |
By multivariate analysis, age (p <0.01), positivity for HBsAg and HCV antibodies (p <0.05), male sex (p <0.05), and previous alcohol abuse (p <0.08) were independently related to HCC. |
| Tsai JF, 199723 | 400 patients with cirrhosis | 21% anti-HCV 59% HBsAg positive 10% positive HBsAg and anti-HCV 11% both negative |
HCV/HBV co-infection (HR 6.41; 95% CI 1.80-22.80), anti-HCV alone (HR, 3.74; 95% CI 1.07-13.07) and HBsAg alone (HR, 4.06; 95% CI 1.23-13.34) were independent risk factors for HCC. |
| Zarski JP, 199821 | 23 patients and 69 age- and sex-matched HBsAg negative patients with chronic HCV | Chronic HCV and HBV subdivided according to HBV DNA replication | Prevalence of cirrhosis was greater in HBV and HCV patients than in patients with HCV alone (p = 0.01). Among HBV and HCV, HCV RNA level was significantly lower in HBV DNA positive than in HBV DNA negative (p = 0.01) patients. |
| Crespo J, 199924 | 136 HBsAg negative | LT recipients | 6 patients (4.4%) became HBsAg positive. Two developed acute liver failure, 4 had severe chronic hepatitis related to HBV. |
| Liaw YF, 200019 | 2 HBV superinfection | Chronic HCV | 1 death from hepatic failure. The other recovered with seroclearance of HBsAg, and antibodies to HCV. |
| Sagnelli E, 200220 |
44 HBV acute infection |
21 anti-HCV positive 20 anti-HCV negative 3 HBV/HCV concurrent infection |
Severe acute hepatitis was more frequent in the chronic HCV carriers than in the control group (28.6% vs. 0%, p <0.05). HBV superinfection strongly and persistently depresses HCV. |
|
Hepatitis A | |||
| Vento S, 199847 | 432 patients with HCV | Chronic HCV | 17 cases of HAV superinfection. 7 cases of acute liver failure and 6 deaths. |
| Pramoolsinsap C, 1999 |
32 cases of HAV superinfection and 100 cases of isolated HAV |
20 HBsAg carrier 8 HBV 4 HCV |
All cases with isolated HAV recovered. Fulminant or submassive hepatitis occurred in 11 (55%) of the HBsAg carriers and 4 (33%) of the 12 patients with CLD related to either HBV or HCV. |
|
Pneumococcal disease | |||
| Viasus D, 201159 | 3,420 cases of community-acquired pneumonia | 90 cases in patients with cirrhosis compared with non-cirrhotic cases | Impaired consciousness at admission (p <0.001), septic shock (p = 0.011), high-risk Pneumonia Severity Index classes (p = 0.002), bacteraemia (p = 0.023), and early (p = 0.048) and overall (p <0.024) mortality rates were higher in patients with cirrhosis than in patients without cirrhosis. |
| Kim T, 201660 | 50 SBP cases due to Streptococcus pneumoniae 100 SBP cases due to other organisms |
Cirrhosis | Patients with SPP were more likely to present concurrent bacteraemia (p = 0.002), to present with variceal bleeding (p = 0.02) and the 30-day mortality was significantly lower (p = 0.04). |
| Baxter R, 201657 | 1,549 IPD cases from 15,102,047 person-years in the Kaiser Permanente Northern California | Several medical conditions | Highest adjusted RR for IPD were chronic liver disease (RR 2.1, 95% CI 1.5-2.8) and chronic obstructive pulmonary disease (RR 2.1, 95% CI 1.8-2.5). |
| Imai K, 201858 |
10.4 million individuals, representing 9.3 million person-years of follow-up in Japan |
Eleven medical conditions |
Adults aged 50–64 years with an underlying medical condition (rate: 39-212 per 100,000 person-years) had a higher rate of infection than those aged ≥65 years without any condition (rate: 13-93 per 100,000 person-years). |
|
Influenza virus | |||
| Duchini A, 200063 | 3 cases of influenza A with hepatic decompensation | 1 Wilson and 2 alcohol-related liver disease | Two patients had hepatic decompensation and the third had acute hepatocellular damage. All recovered within 1 month. |
| Vilchez RA, 200068 | 1 case of influenza A myocarditis | LT recipient | Global hypokinesis and severe impairment of left ventricular function, circulatory compromise, severe liver damage and AKI. |
| Marzano A, 201365 | 48 inpatients with A/H1N1/09 infections 44 outpatients with mild influenza-like illness |
Inpatients: 21 and 27 with and without cirrhosis Outpatients: without cirrhosis |
A/H1N1/09 infection rate did not differ in patients with and without cirrhosis (19% and 15%), but three patients with cirrhosis died while none of patients without cirrhosis died. |
| Schütte A, 201964 | 45 inpatients with influenza infection | 11 patients with cirrhosis and 34 without | Cirrhotic patients presented higher organ failure scores, lower blood pressure, higher proportion of secondary bacterial infections, ACLF and deaths. |
| Premkumar M, 201967 | 110 patient with cirrhosis admitted to ICU with suspected A/H1N1/09 infection | 22 A/H1N1/09 positive and 88 influenza-like pneumonia | Death occurred in 82% of patients with A/H1N1/09 compared with 40% of the control group. PaO2/FiO2ratio <200 and serum creatinine >1.8 mg/dl were predictors of mortality. |
| Liu WD, 202066 |
73 patients with influenza A and 23 with influenza B |
Adult patients with several medical conditions |
11% and 44% deaths occurred within 30 days in each group. Factors associated with mortality were CLD (HR: 3.94; 95% CI 1.07-14.45) rheumatologic diseases (HR: 7.45; 95% CI 2.34-23.69) and influenza B (HR: 4.33; 95% CI 1.68-11.13). |
|
Tuberculosis | |||
| Thulstrup AM, 200090 | Cohort of 22,675 patients in Denmark | Cirrhosis | The incidence was 169 per 100,000 person-years of risk. The highest incidence was among men >65 years (246 per 100000 person-years of risk). The 30-day and 1-year case-fatality were 27% and 48%. |
Summary of impact of vaccine-preventable infections (excluding COVID-19) in patients with CLD and LT recipients.
AKI, acute kidney injury; CLD, chronic liver disease; HAV, hepatitis A virus; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; ICU, intensive care unit; ICU; IPD, invasive pneumococcal disease; LT, liver transplant; RR, relative risk; SBP, spontaneous bacterial peritonitis; SPP, spontaneous pneumococcal peritonitis.
Immunological efficacy of hepatitis B vaccination in patients with CLD and LT recipients
Currently, there are single antigen vaccines (Engerix-B, Recombivax-HB and Heplisav-B) and a combined hepatitis A and B vaccine (Twinrix) that have received FDA approval. These vaccines induce specific humoral antibodies against the surface antigen of hepatitis B. The vaccines differ in terms of the concentration of hepatitis B surface antigen and the nature of aluminum adjuvants (see below). General recommendations are to administer two doses of Heplisav-B 4 weeks apart, or three doses of Engerix-B, Recombivax-HB or Twinrix at 0, 1 and 6 months. Although HBV vaccination (monovalent, recombinant) is highly effective in immunocompetent adults,25 rates of response are markedly lower in those with CLD.26 Serological response rates decline with increasing severity of disease, from 88% in patients with Child-Pugh A cirrhosis to 33% for Child-Pugh B,27,28 and only 16-20% for Child-Pugh C.26 Even with increased doses of monovalent vaccine, or accelerated regimens, response rates in advanced liver disease remain poor.29,30
Alcohol-related liver disease has also been associated with lower immunogenicity (44%-75%)28,31,32 than other causes of CLD such as HCV (69-100%).[33], [34], [35] In one study evaluating response to vaccination in patients with fatty liver disease without cirrhosis, response was unimpaired (94%).36
Enhancing response rates to HBV vaccination is an active area of translational research. Vaccine adjuvants, as described above, enhance the adaptive immune response to vaccines, largely through non-specific innate immune effects (e.g. Toll-like receptor stimulation).37 Variations in adjuvants are the major difference between available HBV vaccines; Engerix-B uses aluminium hydroxide and trace amounts of thimerosal from the manufacturing process, whereas Recombivax-HB contains aluminium hydrophosphate sulphate, and Heplisav-B combines hepatitis B surface antigen with a proprietary Toll-like receptor 9 agonist.
Most recent developments in this area relate to adjuvants; indeed, around a quarter of all FDA and EMA licensed vaccine products containing adjuvants are for HBV. However, relatively few have been tested in CLD or post-LT settings. Fendrix is a double-adjuvanted HBV vaccine, approved by the EMA but not the FDA, that has been shown, in patients with decompensated cirrhosis, to significantly increase the proportion of patients with seroprotective anti-HBs levels compared to three doses of monovalent HBV vaccine (Engerix B) (60% vs. 32%).
Safety and effectiveness of hepatitis B vaccination in CLD and post-LT
Several studies have reported that hepatitis B vaccination is safe in patients with CLD, with no differences compared to healthy individuals.[38], [39], [40] Despite this, vaccination rates in patients with CLD do not exceed 23-32% in US populations.41,42 Similarly, a recent study performed in Germany demonstrated that only 9 out of 37 (24%) patients with cirrhosis evaluated for LT were vaccinated against HBV.43 Reasons for low vaccination rates include primary care physicians’ inadequate knowledge and unjustified concerns,44 and a seeming lack of motivation among hepatologists.45
Hepatitis a vaccination
Impact of acute hepatitis a in CLD
As noted above, acute viral hepatitis on the background of cirrhosis can lead to decompensation or ACLF. Relatively few studies have specifically evaluated the impact of hepatitis A virus (HAV) superinfection in CLD, and most have focused on underlying chronic viral hepatitis. Vento S et al. reported an incidence of HAV superinfection of 4% in a cohort of patients with chronic hepatitis C. Of these, 40% developed acute liver failure and 35% died.46 Similarly, another study of 20 cases of acute hepatitis A superimposed on hepatitis B surface antigen carriers showed that 55% of cases developed fulminant or submassive hepatitis47 (Table 2).
Immunological efficacy of hepatitis a vaccination in CLD and post-LT
There are two vaccines currently available for HAV, Havrix and Vaqta. Seroconversion rates of two-dose inactivated hepatitis A vaccine seem to be adequate (94-98%) in patients with non-advanced stages of CLD;48 however, antibody titres are lower in patients with CLD than in controls.48,49 Moreover, responses are decreased in decompensated cirrhosis, with rates falling to 71% for Child-Pugh B and 57% for Child-Pugh C cirrhosis.50
In LT recipients, seroconversion is low (8%, 19% and 26% at 1, 6 and 7 months). Longer time from transplant seems to be associated with higher seroconversion rates, as well as other immunological factors such as higher total white blood cell and lymphocyte count.51 In another study of LT recipients, following a booster dose given 6 months after the first dose, seroconversion changed from 41% to 97%, suggesting that patients with LT may benefit from additional doses.52
Safety and effectiveness of hepatitis a vaccination in CLD and post-LT
No serious adverse events requiring special monitoring have been described following vaccination in patients with CLD.48,52 Real-world data from the US Veterans Affairs database confirms efficacy amongst individuals with HCV infection. The overall vaccination rate for HAV was 21%; the incidence of superinfection with acute HAV was low, but it was significantly lower in patients who received vaccination than in those who did not (0.16% vs. 0.01%, p <0.001)..53
Pneumococcal vaccine
Impact of pneumococcal disease in CLD
Infection with Streptococcus pneumoniae (S. pneumoniae) is more prevalent in older patients, cigarette smokers, and those with severe underlying conditions including CLD and alcoholism.54,55 The risk of invasive pneumococcal disease has been shown to be 2- to 13-fold higher in patients with CLD compared to the general population, depending on age.56,57 The most common pneumonia-causing organism in patients with cirrhosis is S. pneumoniae, and the risk of complications is significantly higher in cirrhosis: risk of severe infection (74% vs. 58%; p = 0.002), bacteraemia (22% vs. 13%; p = 0.023) and death (14.4% vs. 7.4%; p <0.024, all compared with hospitalised adult patients without cirrhosis).58 S. pneumoniae is also a common cause of spontaneous bacterial peritonitis in patients with cirrhosis59 (Table 2).
Immunological efficacy of pneumococcal vaccination in CLD and post-LT
Two vaccines are currently available, the pneumococcal conjugate vaccine (PCV13 or Prevnar 13) and the pneumococcal polysaccharide vaccine (PPSV23 or Pneumovax 23). No large prospective studies evaluating the immunogenicity of these vaccines in patients with CLD are available and the few reports in the literature show varying results. Four decades ago, Pirovino et al. studied antibody responses to Pneumovax-14 (14-valent pneumococcal polysaccharide vaccine, no longer available), and found no difference in immunogenicity between alcohol-related cirrhosis, chronic obstructive pulmonary disease and healthy volunteer groups.60 More recently, McCashland et al. compared immune responses to PPSV23 in 45 patients with cirrhosis being evaluated for LT with 13 age-matched controls at 1 and 6 months after vaccination, and before and after transplantation. Controls had higher IgG responses; the cirrhosis group had higher initial IgM and IgA levels, but these levels declined faster. At 3 months after LT, antibody levels were at, or below, pre-vaccination baselines, suggesting that PPSV23 may not be effective for patients during and after LT.7
Safety and effectiveness of pneumococcal vaccination in CLD
No specific adverse events have been described in patients with CLD undergoing pneumococcal vaccination. In terms of vaccine uptake, in a prospective cohort of patients with cirrhosis from the Mayo clinic followed for 8 years, of all available vaccines, the rate of uptake of the pneumococcal vaccine was the highest at the end of the study period (63%, 180 of 285 patients).53 A protocol for a systematic review of the effectiveness of pneumococcal and influenza vaccines in preventing serious health complications in adults with CLD has been published, but results are not yet available.61
Influenza vaccination
Impact of influenza virus in CLD and post-LT
Influenza remains one of the major communicable diseases worldwide; influenza has been known to infect up to 20% of the global population (depending on seasonal strain), and approximately 650,000 deaths are linked to influenza each year. In patients with cirrhosis, influenza may present with acute decompensation and ACLF.62,63 Mortality from influenza is also elevated in cirrhosis, with two studies demonstrating that the risk of death is 3-4-fold greater than in patients without cirrhosis.64,65 Mortality rates may also be higher depending on viral strain; Premkumar et al. reported that 82% of patients with cirrhosis and confirmed A/H1N1/09 infection died as a result of respiratory failure, whereas the mortality rate was 40% in a control group of patients with cirrhosis and influenza-like pneumonia, who tested negative for A/H1N1/09.66 In LT recipients, non-pulmonary complications such as influenza A myocarditis, despite prophylactic vaccination, have also been reported67 (Table 2).
Immunological efficacy of influenza vaccination in CLD and post-LT
The production of influenza vaccines is a unique process, since dominant strains are selected several months before ‘flu season’ based on data from the WHO Global Influenza Surveillance and Response System. Consequently, each batch of vaccine may have a slightly different epitope. Currently, two inactivated vaccines are available (trivalent and quadrivalent). A further live attenuated vaccine is available, but this is not recommended for patients with CLD or LT recipients. A recent systematic review and meta-analysis evaluated the serological effect of influenza vaccination in patients with CLD and reported robust responses. Although of low quality, it included six studies with a total of 262 patients, with seroconversion rates of 80% for A/H1N1 and 87% for the B strain in patients with CLD.68 Another study performed in 20 patients with chronic HBV/HCV-related cirrhosis, and eight age-matched controls, showed a seroconversion rate of 75-85% in patients and 100% in controls.69 Regarding LT recipients, the findings are less clear-cut. A study of 62 LT recipients and 59 adult controls showed response rates of 92-95% and 97-100%, respectively, 21 days after a single vaccination,70 while another study showed a 68% response rate after the first vaccination and more than 80% after a second dose.71 Response may be influenced by time since transplantation, with reported response rates of 14% at <4 months, 67% at 4-12 months and 86% at >12 months post-LT.72
Safety, clinical efficacy and effectiveness of influenza vaccination in CLD
In terms of safety, mild and transient erythema at the inoculation site has been documented with the inactivated vaccines in patients with CLD, with no differences compared to controls. No other safety concerns have been described.69 Unusually, the clinical efficacy of influenza vaccination in CLD has been addressed in a prospective clinical trial from Korea comparing 198 vaccinated patients with CLD (trivalent vaccine) with 113 unvaccinated controls, demonstrating a higher incidence of influenza-like illness and complications (p = 0.064) and culture positivity (p = 0.009) in non-vaccinated patients.73 Additionally, effectiveness has been addressed in the aforementioned systematic review and meta-analysis. The risk of hospitalisation from influenza, following vaccination, decreased from 205/1,000 to 149/1,000 (risk difference −0.06, 95% CI −0.07 to 0.04); vaccinated patients were 27% less likely to be admitted to hospital than unvaccinated patients (risk ratio 0.73; 95% CI 0.66 to 0.80), without a significant impact on mortality.68
Other vaccines
Herpes zoster vaccine
Herpes zoster (HZ), also known as shingles, represents reactivation of varicella zoster virus (VZV) following primary infection (chickenpox); it causes a characteristic painful, blistering dermatomal rash. Complications, although rare, can lead to significant morbidity such as persistent neuralgic pain, disability and occasional neurological issues, e.g. HZ ophthalmicus. The incidence of HZ in CLD is low but may be slightly higher than in the general population. Lai et al. demonstrated that episodes of HZ were slightly increased in patients with cirrhosis (adjusted hazard ratio [aHR] 1.11; 95% CI 1.004-1.24) compared to sex- and age-matched individuals without cirrhosis, and further increased in decompensated stages of the disease (aHR 1.33; 95% CI 1.02-1.74).74 By contrast, Wu et al. did not note any difference in incidence in patients with CLD compared to the general population.75 In LT recipients, HZV is more common, with an incidence ranging from 11 to 18 per 1,000 person-years.[76], [77], [78] Older age (≥50 years) at LT and mycophenolate mofetil were independent risk factors for infection in a retrospective Korean study of 993 LT recipients.76
Limited data is available on the use of VZV vaccines in patients with CLD.79 In non-liver patients, most data exist for the live attenuated VZV vaccine (Zostavax) although this is not recommended for patients with CLD or LT recipients. A recombinant, inactive, adjuvanted vaccine is also available (Shingrix). A recent systematic review and meta-analysis showed that the live attenuated HZV vaccine was effective in preventing the infection in people with comorbidities, including two low quality studies that included patients with CLD (vaccine effectiveness 42-53%).80 Aside from vaccination, post-exposure prophylaxis is recommended for seronegative LT recipients, and HZV immunoglobulin should be administered as soon as possible following exposure.
Diphtheria, tetanus and pertussis vaccine
There are no data available on the impact of tetanus, diphtheria or Bordetella pertussis infection in patients with CLD or LT recipients, and data on the effectiveness of the vaccine is also limited. The DTP (diphtheria, tetanus and pertussis) vaccine is recommended in early childhood in most countries, and consequently the only available data are in this population. A paediatric study comparing vaccine effectiveness in patients with chronic hepatic failure, LT recipients and controls found that LT recipients achieved similar rates of protection as controls, and patients with chronic hepatic failure actually had higher antibody titres to diphtheria and polio than the control group.81 Therefore, recommendations in CLD and LT are similar to those in the general population.
Measles, mumps and rubella vaccine
Similarly, limited data are available on the risk of measles, mumps or rubella infection in patients with CLD or LT recipients. Two studies evaluated the prevalence of immunity in paediatric LT recipients, showing that immunity is low (22-54% of patients are measles non-immune, and 63% are varicella non-immune after LT).82,83 Risk factors for measles seronegativity were younger age at transplant (p = 0.02) and greater time from transplant to testing (p = 0.04).83 The MMR (measles, mumps and rubella) vaccine is typically recommended in childhood, hence recommendations in adults with liver disease are similar to those in the general population.
Human papillomavirus vaccine
Human papillomaviruses (HPVs) have been causally linked to certain human cancers, such as cervical carcinoma. Additionally, anecdotal reports suggest differing effects on the risk of HCC in patients with HPV infection.84,85 The HPV vaccine (Gardasil, a recombinant, subunit, adjuvanted vaccine) has been in clinical use for around a decade, although policies regarding administration vary between countries, and it is not usually administered beyond early adulthood. No specific literature regarding the immunogenicity or effectiveness of the HPV vaccine in patients with CLD or LT recipients is available. However, Gardasil is recommended in organ transplant recipients, owing to the increased risk of HPV-associated malignancy.86
Meningococcal and haemophilus influenzae vaccines
As noted above, bacterial infections are more common, and more severe, in patients with CLD. There are few data concerning the incidence of meningococcal infection in CLD per se, and cirrhosis does not appear as an important underlying factor in larger cohorts of patients with meningitis. However, case series in cirrhosis suggest that bacterial meningitis is associated with high morbidity and mortality, and may present in an atypical fashion.87,88 By contrast, the incidence of meningococcal infection does not seem to be elevated in LT recipients. Vaccination guidance from the ACIP (Advisory Committee on Immunization Practices) recommends that patients with CLD, or LT recipients undergoing splenectomy for other medical conditions, should undergo meningococcal (both types) and haemophilus influenzae vaccination.
Tuberculosis vaccine
Tuberculosis remains the leading, fatal infectious disease worldwide. A number of studies have demonstrated a markedly increased risk of tuberculosis in patients with cirrhosis compared to the general population (14-15-fold)89,90 (Table 2). In addition, the prognosis of these patients is poor, with a case-fatality rate of 27% at 30 days and 48% at 1 year according to a Danish nationwide population-based study.89 In addition, the greatest challenge in patients with CLD is managing tuberculosis therapy since most first-line therapies are hepatotoxic and baseline liver function is often deranged.91 BCG (Bacille Calmette-Guérin) is the only licensed vaccine against Mycobacterium tuberculosis. This vaccine is not widely used in the United States or Europe and, where it is currently used, it is usually administered to infants with the aim of preventing childhood tuberculosis (there is no evidence for protection in adulthood). Consequently there are no data supporting the use of BCG in patients with cirrhosis or LT recipients, thus it is not recommended for adults with CLD or LT recipients.
COVID-19 vaccination
Impact of COVID-19 in CLD and post-LT
The COVID-19 pandemic has posed the largest public health challenge in living memory. As with many other infections, patients with liver disease have been disproportionately affected by COVID-19, with an ∼3-fold higher risk of COVID-19-related mortality in patients with CLD than in those without CLD, and a further increased risk in patients with established cirrhosis (∼5-fold).[92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104] Between 20% and 50% of patients with cirrhosis present with acute decompensation or ACLF.6,93 Alcohol-related liver disease, non-alcoholic fatty liver disease and infection with HCV have also been associated with severe COVID-19 and higher mortality rates, while autoimmune liver disease (AILD) or LT were not associated with an increase incidence of infection or worse clinical outcomes. The mechanism of disease progression is likely related to the exaggerated release of inflammatory cytokines and the activation of inflammasome pathways in target cells which trigger pro-inflammatory cell death following SARS-CoV-2 infection.105 In patients with cirrhosis, hepatocytes are predisposed to pro-inflammatory cell death while in patients under immunosuppressive therapy, this inflammatory response may be decreased.[106], [107], [108], [109], [110]
Hepatocellular carcinoma is a common complication of CLD, and these patients may be at particularly increased risk of severe COVID-19. However, data in this population are variable. On the one hand, data from an international registry demonstrated that the presence of HCC was not independently associated with mortality, however, in a multicentre US-based cohort, HCC was an independent predictor of death from COVID-19.6,111
In addition, antiviral drugs for SARS-CoV-2 infection can induce liver injury. Administration of lopinavir-ritonavir has been associated with hepatotoxicity, even in low-dose boost regimens, and should be used with caution in patients with CLD. Both remdesivir and tocilizumab also contributed to raised liver enzyme levels in 10% of patients. Most clinical trials of SARS-CoV-2-directed therapies excluded patients with CLD, and thus clinical experience in CLD is limited to case reports.112,113
Immunological efficacy of COVID-19 vaccination in CLD and post-LT
As is widely known, several COVID-19 vaccines were approved with emergency use authorizations between 2020 and 2022. These include mRNA vaccines (BNT162b2 Pfizer-BioNTech and mRNA-1273 Moderna), an adjuvanted recombinant protein vaccine (NVX-CoV2372 Novavax) and vaccines that use a replication-incompetent adenovirus vector (Ad26.COV2.S Janssen/Johnson & Johnson and AZD1222 Oxford-AstraZeneca). However, almost all of the pivotal studies which led to the emergency use authorization for these vaccines excluded patients with CLD and LT recipients, hence most available data are from investigator-initiated studies from the last 2 years. Data are accumulating and suggest impaired vaccine responses in certain subgroups of patients with CLD. Two studies from the US and Italy demonstrate significantly lower anti-spike IgG responses in patients with cirrhosis compared to controls after two doses of mRNA vaccine.114,115 Similarly, a prospective multicentre study of 437 patients with CLD and 144 healthy volunteers who had received two doses of inactivated whole-virion SARS-CoV-2 vaccines showed a lower rate of neutralising antibody (nAb) positivity in patients with CLD than in controls (90%), without significant differences between non-cirrhotic CLD (77%), compensated cirrhosis (79%) and decompensated cirrhosis (77%).116 By contrast, both a multicentre study of 381 patients with non-alcoholic fatty liver disease (very few with advanced CLD) receiving two doses of inactivated vaccine, and a recent prospective study from Greece of patients receiving two doses of mRNA-based vaccines, showed adequate seroconversion rates in patients with non-cirrhotic CLD (88-96%) and in those with cirrhosis (97%).117,118 It remains unclear if disease severity or other biological factors such as inflammation determine vaccine response in cirrhosis. Further data are available from China, where Sinovac inactivated vaccines (CoronaVac, BBIBP-CorV, WIBP-CorV) led to nAb positivity in 72% and 66% of patients with compensated and decompensated cirrhosis, respectively; Child-Pugh grade B/C was an independent predictor of negative serological response.119 However, these vaccines are not available in Europe or North America, and larger studies in cirrhosis are urgently required to identify factors predicting poor vaccine response.
In patients with HCC, data on the immunogenicity of COVID-19 vaccines are limited. In a Chinese multicentre prospective study in patients with HCC who received two doses of inactivated whole-virion COVID-19 vaccines (CoronaVac, BBIBP-CorV, and WIBP-CorV), only 61% had positive nAbs at 45 days following the second dose.120 On the balance of current data, it seems that severity of liver disease, rather than presence of HCC, determines vaccine response, but as in other vulnerable disease groups better correlates of protection are required to guide booster dosing. EASL and AASLD recommend prioritising vaccination against SARS-CoV-2 in patients with HCC, considering the phase of the malignant disease, therapy, age and comorbidity. In addition, it is not recommended to interrupt locoregional or systemic therapy for HCC for the purpose of vaccination.121,122
The picture for LT recipients is clearer, with reduced immunogenicity demonstrated in several studies, with different vaccine types.[123], [124], [125], [126] In a study of 492 LT recipients and 307 controls matched by age and sex, detectable antibodies were observed in only 75% of patients after 3 months following the second dose of BNT162b2. Older age (>40 years, p = 0.016), shorter time from LT (<5 years, p = 0.004), and immunosuppression with antimetabolites (p = 0.029) were associated with nonresponse. LT recipients showed lower antibody titres than the control group (103 vs. 261 AU/ml, p <0.0001).127 In addition, immunosuppression with mycophenolate seemed to be associated with lower long-term antibody responses in LT recipients.128 The first studies evaluating immune responses after three doses have shown promising results with no serious adverse events or acute rejection episodes in LT recipients.129
Clinical effectiveness of COVID-19 vaccination in CLD and post-LT
There remains a need for data concerning the effectiveness of COVID-19 vaccination in vulnerable populations, including those with CLD and LT recipients, to inform public health policy (shielding, booster regimens etc.) in case of future COVID-19 waves and also to guide the use of antivirals (e.g. ritonavir-boosted nirmatrelvir) and monoclonal nAbs (e.g. tixagevimab/cilgavimab). Existing data primarily comes from large database studies; a US Veteran’s Affairs database study on 20,037 patients with cirrhosis who received at least one dose of mRNA vaccine demonstrated reduced protection from COVID-19 infection compared with healthy individuals (65% reduction after one dose, 79% reduction after two doses), which was further reduced in patients with decompensated cirrhosis compared with compensated cirrhosis (50% vs. 67%).130 A similar large dataset of nearly 3,500 patients with cirrhosis who developed COVID-19 demonstrated that vaccination was associated with lower risk of death (aHR 0.21, 95% CI 0.10-0.42).131 In LT recipients, a further registry study of 2,151 solid organ transplant recipients, including 603 (28%) LT recipients, demonstrated an almost 80% reduction in the incidence of symptomatic COVID-19 vs. unvaccinated solid organ transplant recipients.132 An early case series of 19 LT recipients with COVID-19 infection also supports favourable outcomes in fully vaccinated patients.133
Currently, most liver societies recommend that patients with CLD and LT recipients receive initial COVID-19 vaccination and booster dosing. As of September 2022, the only authorized booster is one dose of mRNA bivalent vaccine (Moderna or Pfizer) ≥2 months after the primary series or most recent booster.121,122 It is not recommended to withhold immunosuppression prior to or after COVID-19 vaccine administration for the purposes of increasing the likelihood of vaccine efficacy. LT candidates should receive a COVID-19 vaccine prior to transplantation whenever possible. If a COVID-19 vaccine is not administered prior to transplantation, the optimal time to administer the vaccine is at least 3 months post-LT. However, unanswered questions remain for patients with CLD and LT recipients, such as clarifying durability of protection (to inform future booster regimens) and identifying factors that predict breakthrough infection in these populations.
Safety of COVID-19 vaccination
The safety of COVID-19 vaccines remains a focus of great public and media interest. Vaccines are generally well tolerated, with pain at the injection site as the most frequent adverse event.120 However, documented serious adverse events are rare. The most well-described is myocarditis/pericarditis with mRNA and recombinant protein vaccines, although the incidence is extremely low at 10.6 per million. Other serious complications reported with adenoviral vaccines are Guillain-Barré syndrome and thrombosis or thrombocytopenia with an incidence of 7.8 and 3.0 per million, respectively.122 Additionally, a rare autoimmune hepatitis-like syndrome following COVID-19 vaccination has been described, with 32 cases documented in the literature until November 2021.134 This syndrome appears amendable to corticosteroid therapy with favourable outcomes.134 In patients with pre-existing AILD, there has consequently been concern that vaccination may induce a disease flare. Data are limited; Shroff et al. reported on a series of 16 patients with COVID-19 vaccine-related liver injury, of whom six had pre-existing AILD.135 Further data are needed, but there is no suggestion that COVID-19 vaccination should be withheld in these patients.
Solid organ rejection post-COVID-19 vaccination is extremely rare but has also been described. A systematic review and meta-analysis including studies until May 2022 found 56 cases of acute rejection following COVID-19 vaccination. Allograft rejections occurred for cornea (n = 38, 67.8%), liver (n = 11, 19.6%), kidney (n = 6, 10.7%) and pancreas (n = 1, 1.8%) transplant recipients.15 Most patients were easily treated and recovered without any serious complications or requirement for long-term allograft rejection therapy. Therefore, there is again no suggestion that COVID-19 vaccination should be discouraged post-LT, since the benefits of vaccination far outweigh the risks.
Opportunities in the post-COVID era
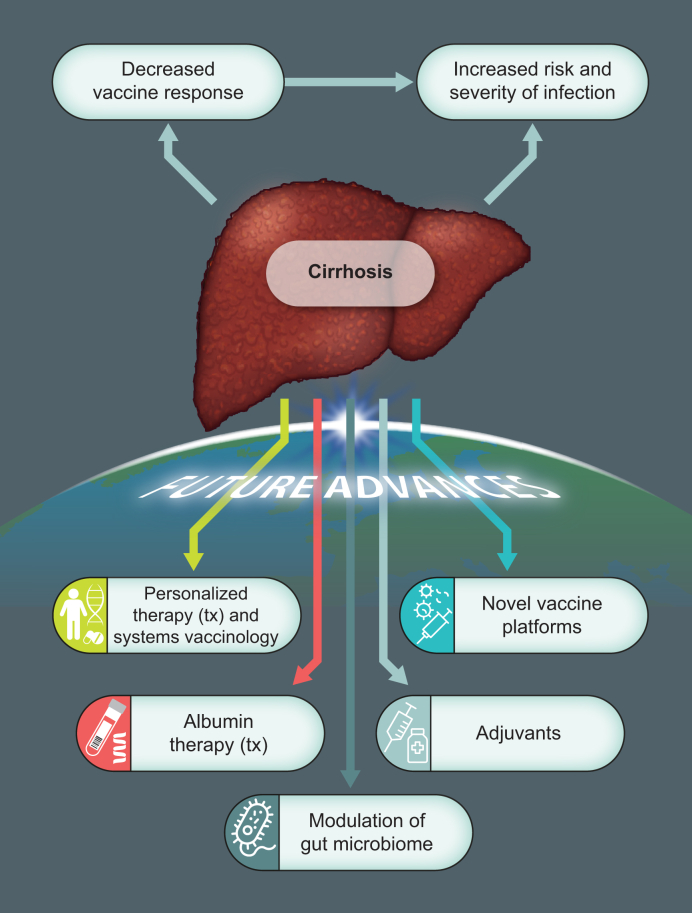
The COVID-19 pandemic has ushered in an era of unprecedented research and development into vaccination technology and platforms, which will have spillover benefits for vaccination against other communicable diseases. Most of the rapidly approved vaccines against SARS-CoV-2 were developed using novel platforms, such as viral vectors or mRNA, due to the flexibility and greater speed of development afforded by such technology. These platforms are here to stay and will be rapidly adopted into other disease areas, including liver disease (Fig. 1).
Fig. 1.
Overview of vaccination in cirrhosis focused on future developments: Vaccination technology and platforms.
Vaccine responses in cirrhosis are reduced as a consequence of cirrhosis-associated immune dysfunction; moreover, the consequences of infection are greater in these patients with higher rates of organ failure and mortality. Several advances in vaccine therapeutics may positively influence outcomes in patients with cirrhosis, such as: personalised vaccine therapy using systems biology approaches, novel vaccine platforms (e.g. mRNA technology), greater understanding of vaccine adjuvants, and modulation of inflammatory response (e.g. albumin or gut microbiome-directed therapeutics).
The immunobiology of adjuvants has also advanced rapidly in recent years. As noted, adjuvants are common additions to inactivated vaccines, and HBV vaccines with novel adjuvants are currently being trialled. Moreover, mRNA vaccines have further expanded insights into mechanisms of action of vaccine adjuvants, since the lipid nanoparticle-mRNA complex appears to independently stimulate innate immune signalling through MDA-5 receptors and thus requires no additional adjuvant.136 Advances in vaccine platform and adjuvant technologies will have clear benefits for patients with poor vaccine responses, including those with CLD and LT recipients.
Furthermore, systems biology, or systems vaccinology in this case, encompasses a multi-omics approach to comprehensively analyse immune responses to vaccination, and thus provides insight into mechanisms of poor response. These approaches have moved the field on from single, typically serological, measures of vaccine immunogenicity, and have already provided insights into the nature of COVID-19 vaccine responses.137 Future studies using systems vaccinology in liver disease may provide a surrogate transcriptional correlate of protection, to guide personalised approaches to vaccination and public health policy.
Finally, vaccine immunogenicity may be context dependent. Increasingly, we are aware of the impact of systemic inflammation, largely gut-derived, in cirrhosis. The gut microbiome is emerging as a central regulator of immunity, and recent human data demonstrates that manipulating the microbiome with broad-spectrum antibiotics results in decreased influenza vaccination responses.138 Consequently, addressing dysbiosis may be a novel therapeutic approach to augmenting vaccine responses in cirrhosis. Similarly, albumin is known to have immunomodulatory effects in cirrhosis,139 and co-administration with vaccines may be an option worth testing in patients with cirrhosis. Of note, albumin levels have been shown to independently predict response to HBV vaccination in populations without liver disease.140
To conclude, the COVID-19 pandemic has markedly accelerated vaccine research and development, which will benefit vulnerable patients, such as those with CLD or LT recipients, in the future. As the science-fiction writer William Gibson wrote, “The future is already here - it’s just not evenly distributed”.
Financial support
GM was part-funded by the Foundation for Liver Research, who had no input into the writing or submission of this manuscript.
Authors’ contributions
The manuscript was written by MPB and GM. The manuscript was critically reviewed by RJ.
Conflicts of interest
RJ is the inventor of ornithine phenylacetate, Yaq-001, DIALIVE, and Yaq-005. He is also the founder of Yaqrit Discovery, a spin out company from UCL, Hepyx Limited and Cyberliver. He has research collaborations with Yaqrit Discovery. GM is an inventor of 'Treatment of Pyroptosis in Liver Disease', and is a shareholder of Hepyx Limited.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We acknowledge the support of the COBALT consortium in the conceptualization of this manuscript.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100776.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Lin C., Tu P., Beitsch L.M. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel) 2020 Dec 30;9(1):16. doi: 10.3390/vaccines9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costantino C., Rizzo C., Rosselli R., Battista T., Conforto A., Cimino L., et al. Ten actions to counteract vaccine hesitancy suggested by the Italian society of hygiene, preventive medicine, and public health. Vaccines (Basel) 2022 Jun 27;10(7):1030. doi: 10.3390/vaccines10071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://ourworldindata.org/covid-vaccinations. Accessed 9th January 2023.
- 4.Chu C.M., Liaw Y.F. Increased incidence of fulminant hepatic failure in previously unrecognized HBsAg carriers with acute hepatitis independent of etiology. Infection. 2005 Jun;33(3):136–139. doi: 10.1007/s15010-005-4094-4. [DOI] [PubMed] [Google Scholar]
- 5.Saab S., Lee C., Shpaner A., Ibrahim A.B. Seroepidemiology of hepatitis A in patients with chronic liver disease. J Viral Hepat. 2005 Jan;12(1):101–105. doi: 10.1111/j.1365-2893.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 6.Marjot T., Moon A.M., Cook J.A., Abd-Elsalam S., Aloman C., Armstrong M.J., et al. Outcomes following SARSCoV- 2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCashland T.M., Preheim L.C., Gentry M.J. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis. 2000;181(2):757–760. doi: 10.1086/315245. [DOI] [PubMed] [Google Scholar]
- 8.Albillos A., Martin-Mateos R., Van der Merwe S., Wiest R., Jalan R., Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022 Feb;19(2):112–134. doi: 10.1038/s41575-021-00520-7. [DOI] [PubMed] [Google Scholar]
- 9.Arvaniti V., D'Amico G., Fede G., Manousou P., Tsochatzis E., Pleguezuelo M., et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010 Oct;139(4):1246–1256. doi: 10.1053/j.gastro.2010.06.019. 1256.e1-e1256. [DOI] [PubMed] [Google Scholar]
- 10.Gustot T., Felleiter P., Pickkers P., Sakr Y., Rello J., Velissaris D., et al. EPIC II Group of Investigators. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int. 2014 Nov;34(10):1496–1503. doi: 10.1111/liv.12520. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 Jun;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakaya H.I., Hagan T., Duraisingham S.S., Lee E.K., Kwissa M., Rouphael N., et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015 Dec 15;43(6):1186–1198. doi: 10.1016/j.immuni.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourati S., Cristescu R., Loboda A., Talla A., Filali A., Railkar R., et al. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun. 2016 Jan 8;7 doi: 10.1038/ncomms10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wörns M.A., Teufel A., Kanzler S., Shrestha A., Victor A., Otto G., et al. Incidence of HAV and HBV infections and vaccination rates in patients with autoimmune liver diseases. Am J Gastroenterol. 2008 Jan;103(1):138–146. doi: 10.1111/j.1572-0241.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- 15.Alhumaid S., Rabaan A.A., Dhama K., Yong S.J., Nainu F., Hajissa K., et al. Solid organ rejection following SARS-CoV-2 vaccination or COVID-19 infection: a systematic review and meta-analysis. Vaccines (Basel) 2022 Aug 10;10(8):1289. doi: 10.3390/vaccines10081289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verolet C.M., Posfay-Barbe K.M. Live virus vaccines in transplantation: friend or foe? Curr Infect Dis Rep. 2015 Apr;17(4):472. doi: 10.1007/s11908-015-0472-y. [DOI] [PubMed] [Google Scholar]
- 17.Willicombe M., Scanlon M., Loud F., Lightstone L. Should we be clinically assessing antibody responses to covid vaccines in immunocompromised people? BMJ. 2022 Apr 12;377:o966. doi: 10.1136/bmj.o966. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R., Mehta G., Jalan R. Acute-on-chronic liver failure. Clin Med (Lond) 2020 Sep;20(5):501–504. doi: 10.7861/clinmed.2020-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liaw Y.F., Yeh C.T., Tsai S.L. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95:2978–2980. doi: 10.1111/j.1572-0241.2000.02337.x. [DOI] [PubMed] [Google Scholar]
- 20.Sagnelli E., Coppola N., Messina V., Di Caprio D., Marrocco C., Marotta A., et al. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology. 2002 Nov;36(5):1285–1291. doi: 10.1053/jhep.2002.36509. [DOI] [PubMed] [Google Scholar]
- 21.Zarski J.P., Bohn B., Bastie A., Pawlotsky J.M., Baud M., Bost-Bezeaux F., et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998 Jan;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 22.Benvegnù L., Fattovich G., Noventa F., Tremolada F., Chemello L., Cecchetto A., et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer. 1994;74:2442–2448. doi: 10.1002/1097-0142(19941101)74:9<2442::aid-cncr2820740909>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Tsai J.F., Jeng J.E., Ho M.S., Chang W.Y., Hsieh M.Y., Lin Z.Y., et al. Effect of hepatitis C and B virus infection on risk of hepatocellular carcinoma: a prospective study. Br J Cancer. 1997;76(7):968–974. doi: 10.1038/bjc.1997.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crespo J., Fábrega E., Casafont F., Rivero M., Heras G., de la Peña J., et al. Severe clinical course of de novo hepatitis B infection after liver transplantation. Liver Transpl Surg. 1999;5:175–183. doi: 10.1002/lt.500050301. [DOI] [PubMed] [Google Scholar]
- 25.Poland G.A., Jacobson R.M. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med. 2004 Dec 30;351(27):2832–2838. doi: 10.1056/NEJMcp041507. Erratum in: N Engl J Med. 2005 Feb 17;352(7):740. Erratum in: N Engl J Med. 2005 Jun 2;352(22):2362. [DOI] [PubMed] [Google Scholar]
- 26.Horlander J.C., Boyle N., Manam R., Schenk M., Herring S., Kwo P.Y., et al. Vaccination against hepatitis B in patients with chronic liver disease awaiting liver transplantation. Am J Med Sci. 1999 Nov;318(5):304–307. doi: 10.1097/00000441-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Aggeletopoulou I., Davoulou P., Konstantakis C., Thomopoulos K., Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017 Nov;27(6) doi: 10.1002/rmv.1942. [DOI] [PubMed] [Google Scholar]
- 28.Roni D.A., Pathapati R.M., Kumar A.S., Nihal L., Sridhar K., Tumkur Rajashekar S. Safety and efficacy of hepatitis B vaccination in cirrhosis of liver. Adv Virol. 2013;2013 doi: 10.1155/2013/196704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engler S.H., Sauer P.W., Golling M., Klar E.A., Benz C., Stremmel W., et al. Immunogenicity of two accelerated hepatitis B vaccination protocols in liver transplant candidates. Eur J Gastroenterol Hepatol. 2001 Apr;13(4):363–367. doi: 10.1097/00042737-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Tajes S., Pocurull A., Lens S., Mariño Z., Olivas I., Soy G., et al. Efficacy of an accelerated double-dose hepatitis B vaccine regimen in patients with cirrhosis. J Viral Hepat. 2021 Jul;28(7):1019–1024. doi: 10.1111/jvh.13509. [DOI] [PubMed] [Google Scholar]
- 31.Rosman A.S., Basu P., Galvin K., Lieber C.S. Efficacy of a high and accelerated dose of hepatitis B vaccine in alcoholic patients: a randomized clinical trial. Am J Med. 1997 Sep;103(3):217–222. doi: 10.1016/s0002-9343(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 32.Bronowicki J.P., Weber-Larivaille F., Gut J.P., Doffoël M., Vetter D. Comparaison de l'immunogénicité de la vaccination et de la sérovaccination contre le virus de l'hépatite B chez les malades atteints de cirrhose alcoolique [Comparison of immunogenicity of vaccination and serovaccination against hepatitis B virus in patients with alcoholic cirrhosis] Gastroenterol Clin Biol. 1997;21(11):848–853. [PubMed] [Google Scholar]
- 33.Keeffe E.B., Krause D.S. Hepatitis B vaccination of patients with chronic liver disease. Liver Transpl Surg. 1998;4:437. doi: 10.1002/lt.500040515. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.D., Chan C.Y., Yu M.I., Lu R.H., Chang F.Y., Lo K.J. Hepatitis B vaccination in patients with chronic hepatitis C. J Med Virol. 1999;59:463. [PubMed] [Google Scholar]
- 35.Wiedmann M., Liebert U.G., Oesen U., Porst H., Wiese M., Schroeder S., et al. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology. 2000;31:230. doi: 10.1002/hep.510310134. [DOI] [PubMed] [Google Scholar]
- 36.Koslinska-Berkan E., Kuydowicz J. The comparison of the humoral response among the patients with liver cirrhosis and steatosis of the liver after HBV vaccination. Przegl Epidemiol. 2006;60:199. [PubMed] [Google Scholar]
- 37.Pulendran B., S Arunachalam P., O'Hagan D.T. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021 Jun;20(6):454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villeneuve E., Vincelette J., Villeneuve J.P. Ineffectiveness of hepatitis B vaccination in cirrhotic patients waiting for liver transplantation. Can J Gastroenterol. 2000 Jul-Aug;14(Suppl B):59B–62B. doi: 10.1155/2000/548206. [DOI] [PubMed] [Google Scholar]
- 39.Arslan M., Wiesner R.H., Sievers C., Egan K., Zein N.N. Double-dose accelerated hepatitis B vaccine in patients with end-stage liver disease. Liver Transpl. 2001 Apr;7(4):314–320. doi: 10.1053/jlts.2001.23069. [DOI] [PubMed] [Google Scholar]
- 40.Domínguez M., Bárcena R., García M., López-Sanroman A., Nuño J. Vaccination against hepatitis B virus in cirrhotic patients on liver transplant waiting list. Liver Transpl. 2000 Jul;6(4):440–442. doi: 10.1053/jlts.2000.8313. [DOI] [PubMed] [Google Scholar]
- 41.Younossi Z.M., Stepanova M. Changes in hepatitis A and B vaccination rates in adult patients with chronic liver diseases and diabetes in the U.S. population. Hepatology. 2011;54:1167–1178. doi: 10.1002/hep.24510. [DOI] [PubMed] [Google Scholar]
- 42.Yue X., Black C.L., O'Halloran A., Lu P.J., Williams W.W., Nelson N.P. Hepatitis A and hepatitis B vaccination coverage among adults with chronic liver disease. Vaccine. 2018 Feb 21;36(9):1183–1189. doi: 10.1016/j.vaccine.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herta T., Petroff D., Engelmann C., Herber A., Aehling N., Scheuermann U., et al. Hepatitis B vaccination in patients with liver cirrhosis evaluated for liver transplantation - a simple intervention ensures high adherence. Ann Transpl. 2019 Sep 13;24:527–531. doi: 10.12659/AOT.917198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenner C.T., Herzog K., Chaudhari S., Bini E.J., Weinshel E.H. Knowledge, attitudes and barriers regarding vaccination against hepatitis A and B in patients with chronic hepatitis C virus infection: a survey of family medicine and internal medicine physicians in the United States. Int J Clin Pract. 2012;66:1009–1013. doi: 10.1111/ijcp.12013. [DOI] [PubMed] [Google Scholar]
- 45.Thudi K., Yadav D., Sweeney K., Behari J. Physicians infrequently adhere to hepatitis vaccination guidelines for chronic liver disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vento S., Garofano T., Renzini C., Cainelli F., Casali F., Ghironzi G., et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 47.Pramoolsinsap C., Poovorawan Y., Hirsch P., Busagorn N., Attamasirikul K. Acute, hepatitis-A super-infection in HBV carriers, or chronic liver disease related to HBV or HCV. Ann Trop Med Parasitol. 1999;93:745. doi: 10.1080/00034989958005. [DOI] [PubMed] [Google Scholar]
- 48.Keeffe E.B., Iwarson S., McMahon B.J., Lindsay K.L., Koff R.S., Manns M., et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- 49.Tsang S.W., Sung J.J. Inactivated hepatitis A vaccine in Chinese patients with chronic hepatitis B infection. Aliment Pharmacol Ther. 1999;13:1445. doi: 10.1046/j.1365-2036.1999.00628.x. [DOI] [PubMed] [Google Scholar]
- 50.Arguedas M.R., Johnson A., Eloubeidi M.A., Fallon M.B. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34:28. doi: 10.1053/jhep.2001.25883. [DOI] [PubMed] [Google Scholar]
- 51.Arslan M., Wiesner R.H., Poterucha J.J., Zein N.N. Safety and efficacy of hepatitis A vaccination in liver transplantation recipients. Transplantation. 2001;72:272. doi: 10.1097/00007890-200107270-00019. [DOI] [PubMed] [Google Scholar]
- 52.Stark K., Günther M., Neuhaus R., Reinke P., Schröder K., Linnig S., et al. Immunogenicity and safety of hepatitis A vaccine in liver and renal transplant recipients. J Infect Dis. 1999 Dec;180(6):2014–2017. doi: 10.1086/315125. [DOI] [PubMed] [Google Scholar]
- 53.Kramer J.R., Hachem C.Y., Kanwal F., Mei M., El-Serag H.B. Meeting vaccination quality measures for hepatitis A and B virus in patients with chronic hepatitis C infection. Hepatology. 2011 Jan;53:42–52. doi: 10.1002/hep.24024. [DOI] [PubMed] [Google Scholar]
- 54.Burman L.A., Norrby R., Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev Infect Dis. 1985;7:133. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- 55.Lipsky B.A., Boyko E.J., Inui T.S., Koepsell T.D. Risk factors for acquiring pneumococcal infections. Arch Intern Med. 1986;146:2179. [PubMed] [Google Scholar]
- 56.Baxter R., Yee A., Aukes L., Snow V., Fireman B., Atkinson B., et al. Risk of underlying chronic medical conditions for invasive pneumococcal disease in adults. Vaccine. 2016 Aug 5;34(36):4293–4297. doi: 10.1016/j.vaccine.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Imai K., Petigara T., Kohn M.A., Nakashima K., Aoshima M., Shito A., et al. Risk of pneumococcal diseases in adults with underlying medical conditions: a retrospective, cohort study using two Japanese healthcare databases. BMJ Open. 2018 Mar 2;8(3) doi: 10.1136/bmjopen-2017-018553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viasus D., Garcia-Vidal C., Castellote J., Adamuz J., Verdaguer R., Dorca J., et al. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 2011 Mar;90(2):110–118. doi: 10.1097/MD.0b013e318210504c. [DOI] [PubMed] [Google Scholar]
- 59.Kim T., Hong S.I., Park S.Y., Jung J., Chong Y.P., Kim S.H., et al. 2016 May. Clinical features and outcomes of spontaneous bacterial peritonitis caused by Streptococcus pneumoniae: a matched case-control study; p. 95e3796. Medicine (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirovino M., Lydick E., Grob P.J., Arrenbrecht S., Altorfer J., Schmid M. Pneumococcal vaccination: the response of patients with alcoholic liver cirrhosis. Hepatology. 1984 Sep-Oct;4(5):946–949. doi: 10.1002/hep.1840040527. [DOI] [PubMed] [Google Scholar]
- Härmälä S., Parisinos C., Shallcross L., O'Brien A., Hayward A. Effectiveness of pneumococcal and influenza vaccines to prevent serious health complications in adults with chronic liver disease: a protocol for a systematic review. BMJ Open. 2018 Mar 16;8(3) doi: 10.1136/bmjopen-2017-018223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duchini A., Viernes M.E., Nyberg L.M., Hendry R.M., Pockros P.J. Hepatic decompensation in patients with cirrhosis during infection with influenza A. Arch Intern Med. 2000 Jan 10;160(1):113–115. doi: 10.1001/archinte.160.1.113. [DOI] [PubMed] [Google Scholar]
- Schütte A., Ciesek S., Wedemeyer H., Lange C.M. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019 Apr;70(4):797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Marzano A., Marengo A., Ruggiero T., Allice T., Sanna C., Alessandria C., et al. Clinical impact of A/H1/N1/09 influenza in patients with cirrhosis: experience from a nosocomial cluster of infection. J Med Virol. 2013 Jan;85(1):1–7. doi: 10.1002/jmv.23454. [DOI] [PubMed] [Google Scholar]
- 65.Liu W.D., Yeh C.Y., Shih M.C., Sheng W.H. Clinical manifestations and risk factors for mortality of patients with severe influenza during the 2016-2018 season. Int J Infect Dis. 2020 Jun;95:347–351. doi: 10.1016/j.ijid.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Premkumar M., Devurgowda D., Dudha S., Maiwall R., Bihari C., Grover S., et al. A/H1N1/09 influenza is associated with high mortality in liver cirrhosis. J Clin Exp Hepatol. 2019 Mar-Apr;9(2):162–170. doi: 10.1016/j.jceh.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vilchez R.A., Fung J.J., Kusne S. Influenza A myocarditis developing in an adult liver transplant recipient despite vaccination: a case report and review of the literature. Transplantation. 2000 Aug 15;70(3):543–545. doi: 10.1097/00007890-200008150-00026. [DOI] [PubMed] [Google Scholar]
- 68.Härmälä S., Parisinos C.A., Shallcross L., O'Brien A., Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. 2019 Sep 6;9(9) doi: 10.1136/bmjopen-2019-031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaeta G.B., Stornaiuolo G., Precone D.F., Amendola A., Zanetti A.R. Immunogenicity and safety of an adjuvanted influenza vaccine in patients with decompensated cirrhosis. Vaccine. 2002 Dec 20;20(Suppl 5):B33–B35. doi: 10.1016/s0264-410x(02)00510-8. [DOI] [PubMed] [Google Scholar]
- 70.Burbach G., Bienzle U., Stark K., Rayes N., Neuhaus R., Serke S., et al. Influenza vaccination in liver transplant recipients. Transplantation. 1999 Mar 15;67(5):753–755. doi: 10.1097/00007890-199903150-00019. [DOI] [PubMed] [Google Scholar]
- 71.Soesman N.M., Rimmelzwaan G.F., Nieuwkoop N.J., Beyer W.E., Tilanus H.W., Kemmeren M.H., et al. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000 May;61(1):85–93. [PubMed] [Google Scholar]
- 72.Lawal A., Basler C., Branch A., Gutierrez J., Schwartz M., Schiano T.D. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am J Transpl. 2004 Nov;4(11):1805–1809. doi: 10.1111/j.1600-6143.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 73.Song J.Y., Cheong H.J., Ha S.H., Hwang I.S., Kee S.Y., Jeong H.W., et al. Clinical impact of influenza immunization in patients with liver cirrhosis. J Clin Virol. 2007 Jul;39(3):159–163. doi: 10.1016/j.jcv.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Lai S.W., Liao K.F., Lin C.L., Liu C.S., Hwang B.F. Association between cirrhosis and herpes zoster in a cohort study in Taiwan. Int J Clin Pract. 2021 Nov;75(11) doi: 10.1111/ijcp.14677. [DOI] [PubMed] [Google Scholar]
- 75.Wu P.H., Lin Y.T., Kuo C.N., Chang W.C., Chang W.P. No increased risk of herpes zoster found in cirrhotic patients: a nationwide population-based study in Taiwan. PLoS One. 2014 Apr 3;9(4) doi: 10.1371/journal.pone.0093443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim W., Kim S., Oh J., Jeong Y.J., Rhu J., Kim K.S., et al. Incidence and risk factors for herpes zoster after adult liver transplantation. Ann Surg Treat Res. 2019 Feb;96(2):95–99. doi: 10.4174/astr.2019.96.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrero J.I., Quiroga J., Sangro B., Pardo F., Rotellar F., Alvarez-Cienfuegos J., et al. Herpes zoster after liver transplantation: incidence, risk factors, and complications. Liver Transpl. 2004 Sep;10(9):1140–1143. doi: 10.1002/lt.20219. [DOI] [PubMed] [Google Scholar]
- 78.Hamaguchi Y., Mori A., Uemura T., Ogawa K., Fujimoto Y., Okajima H., et al. Incidence and risk factors for herpes zoster in patients undergoing liver transplantation. Transpl Infect Dis. 2015 Oct;17(5):671–678. doi: 10.1111/tid.12425. [DOI] [PubMed] [Google Scholar]
- 79.Dooling K.L., Guo A., Patel M., Lee G.M., Moore K., Belongia E.A., et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018 Jan 26;67(3):103–108. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mbinta J.F., Nguyen B.P., Awuni P.M.A., Paynter J., Simpson C.R. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longev. 2022 Apr;3(4):e263–e275. doi: 10.1016/S2666-7568(22)00039-3. [DOI] [PubMed] [Google Scholar]
- 81.Balloni A., Assael B.M., Ghio L., Pedrazzi C., Nebbia G., Gridelli B., et al. Immunity to poliomyelitis, diphtheria and tetanus in pediatric patients before and after renal or liver transplantation. Vaccine. 1999 Jun 4;17(20–21):2507–2511. doi: 10.1016/s0264-410x(99)00064-x. [DOI] [PubMed] [Google Scholar]
- 82.Yoeli J.K., Yoeli D., Miloh T.A., Rana A., Goss J.A., Munoz-Rivas F. Measles, mumps, rubella (vaccine) and varicella vaccines in pediatric liver transplant: an initial analysis of post-transplant immunity. Pediatr Transpl. 2019 Aug;23(5) doi: 10.1111/petr.13490. [DOI] [PubMed] [Google Scholar]
- 83.Liman A.Y.J., Wozniak L.J., de St Maurice A., Dunkel G.L., Wanlass E.M., Venick R.S., et al. Low post-transplant measles and varicella titers among pediatric liver transplant recipients: a 10-year single-center study. Pediatr Transpl. 2022 Sep;26(6) doi: 10.1111/petr.14322. [DOI] [PubMed] [Google Scholar]
- 84.Scinicariello F., Sato T., Lee C.S., Hsu H.C., Chan T.S., Tyring S.K. Detection of human papillomavirus in primary hepatocellular carcinoma. Anticancer Res. 1992 May-Jun;12(3):763–766. [PubMed] [Google Scholar]
- 85.Kao S.S., Li C.J., Wei J.C., Lin C.L., Chang R., Hung Y.M. Human papillomavirus infection is associated with decreased risk of hepatocellular carcinoma in chronic hepatitis C patients: taiwan nationwide matched cohort study. Cancers (Basel) 2022 Mar 2;14(5):1289. doi: 10.3390/cancers14051289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chin-Hong P.V., Reid G.E., AST Infectious Diseases Community of Practice Human papillomavirus infection in solid organ transplant recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transpl. 2019 Sep;33(9) doi: 10.1111/ctr.13590. [DOI] [PubMed] [Google Scholar]
- 87.Cabellos C., Viladrich P.F., Ariza J., Maiques J.M., Verdaguer R., Gudiol F. Community-acquired bacterial meningitis in cirrhotic patients. Clin Microbiol Infect. 2008 Jan;14(1):35–40. doi: 10.1111/j.1469-0691.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- 88.Pagliano P., Boccia G., De Caro F., Esposito S. Bacterial meningitis complicating the course of liver cirrhosis. Infection. 2017 Dec;45(6):795–800. doi: 10.1007/s15010-017-1039-7. [DOI] [PubMed] [Google Scholar]
- 89.Thulstrup A.M., Mølle I., Svendsen N., Sørensen H.T. Incidence and prognosis of tuberculosis in patients with cirrhosis of the liver. A Danish nationwide population based study. Epidemiol Infect. 2000 Apr;124(2):221–225. doi: 10.1017/s0950268899003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baijal R., Praveenkumar H.R., Amarapurkar D.N., Nagaraj K., Jain M. Prevalence of tuberculosis in patients with cirrhosis of liver in western India. Trop Doct. 2010 Jul;40(3):163–164. doi: 10.1258/td.2010.090463. [DOI] [PubMed] [Google Scholar]
- 91.Dhiman R.K., Saraswat V.A., Rajekar H., Reddy C., Chawla Y.K. A guide to the management of tuberculosis in patients with chronic liver disease. J Clin Exp Hepatol. 2012 Sep;2(3):260–270. doi: 10.1016/j.jceh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2):768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ioannou G.N., Liang P.S., Locke E., Green P., Berry K., O'Hare A.M., et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology. 2021;74(1):322–335. doi: 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarin S.K., Choudhury A., Lau G.K., Zheng M.H., Ji D., Abd-Elsalam S., et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14(5):690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iavarone M., D'Ambrosio R., Soria A., Triolo M., Pugliese N., Del Poggio P., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73(5):1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mandour M.O., Rafique K.K., Koh J.M., Iliadou K., Forton D., Singanayagam A. 0415—Characteristics of sars-cov2 and liver cirrhosis: a single-centre experience in the United Kingdom. Hepatology. 2020;72(1 SUPPL):261A–262A. [Google Scholar]
- 97.Suresh S., Siddiqui M.B., Abu Ghanimeh M., Nimri F., Karrick M., Musleh M., et al. Clinical outcomes in hospitalized COVID-19 patients with chronic liver disease and cirrhosis. Hepatology. 2020;72:263A. 263A. [Google Scholar]
- 98.Shalimar, Elhence A., Vaishnav M., Kumar R., Pathak P., Soni K.D., et al. Poor outcomes in patients with cirrhosis and corona virus disease-19. Indian J Gastroenterol. 2020;39(3):285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qi X., Liu Y., Wang J., Fallowfield J.A., Wang J., Li X., et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70(2):433–436. doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moon A.M., Webb G.J., Aloman C., Armstrong M.J., Cargill T., Dhanasekaran R., et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu F., Long X., Ji G., Zhang B., Zhang W., Zhang Z., et al. Clinically significant portal hypertension in cirrhosis patients with COVID-19: clinical characteristics and outcomes. J Infect. 2020;81(2):e178–e180. doi: 10.1016/j.jinf.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ge J., Pletcher M.J., Lai J.C., Consortium N.3C. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national covid cohort collaborative study. Gastroenterology. 2021;161(5):1487–1501.e5. doi: 10.1053/j.gastro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bajaj J.S., Garcia-Tsao G., Biggins S.W., Kamath P.S., Wong F., McGeorge S., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Middleton P., Hsu C., Lythgoe M.P. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol. 2021;8(1) doi: 10.1136/bmjgast-2021-000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo M., Ballester M.P., Soffientini U., Jalan R., Mehta G. SARS-CoV-2 infection and liver involvement. Hepatol Int. 2022 Aug;16(4):755–774. doi: 10.1007/s12072-022-10364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vora S.M., Lieberman J., Wu H. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol. 2021;21(11):694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szabo G., Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 108.Soffientini U., Beaton N., Baweja S., Weiss E., Bihari C., Habtesion A., et al. The lipopolysaccharidesensing caspase(s)-4/11 are activated in cirrhosis and are causally associated with progression to multi-organ injury. Front Cel Dev Biol. 2021;9 doi: 10.3389/fcell.2021.668459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan P., Shen M., Yu Z., Ge W., Chen K., Tian M., et al. Author Correction: SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12(1):5306. doi: 10.1038/s41467-021-25629-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Junqueira C., Crespo Ranjbar S., Lewandrowski M., Ingber J., de Lacerda L.B., et al. vol. 3. Res Sq [Preprint]; 2021. (SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release). rs-153628. [Google Scholar]
- 111.Kim D., Adeniji N., Latt N., Kumar S., Bloom P.P., Aby E.S., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2021;19(7):1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. Jul, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Umemura T., Nishikawa K., Mutoh Y., Sasano H., Kozaki K., Yamada T., et al. Usage experience of remdesivir for SARS-CoV-2 infection in a patient with chronic cirrhosis of Child-Pugh class C. J Antimicrob Chemother. 2021 Jun 18;76(7):1947–1948. doi: 10.1093/jac/dkab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Antony S.J., Singh J., de Jesus M., Lance J. Early use of tocilizumab in respiratory failure associated with acute COVID -19 pneumonia in recipients with solid organ transplantation. IDCases. 2020 Jun 26;21 doi: 10.1016/j.idcr.2020.e00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iavarone M., Tosetti G., Facchetti F., Topa M., Lombardi A., D’Ambrosio R., et al. Delayed and suboptimal response to two doses of SARS-CoV-2 messenger RNA vaccine in European patients with compensated and decompensated cirrhosis of difference aetiologies. Hepatology. 2021;74(6):1391A. [Google Scholar]
- 116.Ai J., Wang J., Liu D., Xiang H., Guo Y., Lv J., et al. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol. 2022;20:1516. doi: 10.1016/j.cgh.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J., Hou Z., Liu J., Gu Y., Wu Y., Chen Z., et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol. 2021;75:439. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bakasis A.D., Bitzogli K., Mouziouras D., Pouliakis A., Roumpoutsou M., Goules A.V., et al. Antibody Responses after SARS-CoV-2 Vaccination in Patients with Liver Diseases. Viruses. 2022;14(2):207. doi: 10.3390/v14020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang J., Zhang Q., Ai J., Liu D., Liu C., Xiang H., et al. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: a prospective multicenter study. Hepatol Int. 2022 Jun;16(3):691–701. doi: 10.1007/s12072-022-10332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qi X., Wang J., Zhang Q., Ai J., Liu C., Li Q., et al. Safety and immunogenicity of COVID-19 vaccination in patients with hepatocellular carcinoma (CHESS-NMCID 2101): a multicenter prospective study. J Med Virol. 2022 Nov;94(11):5553–5559. doi: 10.1002/jmv.27992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.https://www.aasld.org/sites/default/files/2022-10/AASLD%20COVID-19%20Guidance%20Document%2010.06.2022F.pdf.
- 123.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timmermann L., Globke B., Lurje G., Schmelzle M., Schöning W., Öllinger R., et al. Humoral immune response following SARS-CoV-2 vaccination in liver transplant recipients. Vaccines (Basel) 2021 Dec 1;9(12):1422. doi: 10.3390/vaccines9121422. PMID: 34960168; PMCID: PMC8703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rashidi-Alavijeh J., Frey A., Passenberg M., Korth J., Zmudzinski J., Anastasiou O.E., et al. Humoral response to SARS-cov-2 vaccination in liver transplant recipients-A single-center experience. Vaccines (Basel) 2021 Jul 4;9(7):738. doi: 10.3390/vaccines9070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Herrera S., Colmenero J., Pascal M., Escobedo M., Castel M.A., Sole-González E., et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transpl. 2021 Dec;21(12):3971–3979. doi: 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guarino M., Esposito I., Portella G., Cossiga V., Loperto I., Tortora R., et al. UniNa Collaborating Group Humoral response to 2-dose BNT162b2 mRNA COVID-19 vaccination in liver transplant recipients. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1534–1541.e4. doi: 10.1016/j.cgh.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Toniutto P., Falleti E., Cmet S., Cussigh A., Veneto L., Bitetto D., et al. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol. 2022 Jul;77(1):152–162. doi: 10.1016/j.jhep.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Bello A.D. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.John B.V., Deng Y., Scheinberg A., Mahmud N., Taddei T.H., Kaplan D., et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med. 2021;181(10):1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.John B.V., Deng Y., Schwartz K.B., Taddei T.H., Kaplan D.E., Martin P., et al. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022 Jul;76(1):126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aslam S., Adler E., Mekeel K., Little S.J. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021 Oct;23(5) doi: 10.1111/tid.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moon A.M., Webb G.J., García-Juárez I., Kulkarni A.V., Adali G., Wong D.K., et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun. 2022 Apr;6(4):889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chow K.W., Pham N.V., Ibrahim B.M., Hong K., Saab S. Autoimmune hepatitis-like syndrome following COVID-19 vaccination: a systematic review of the literature. Dig Dis Sci. 2022 Sep;67(9):4574–4580. doi: 10.1007/s10620-022-07504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shroff H., Satapathy S.K., Crawford J.M., Todd N.J., VanWagner L.B. Liver injury following SARS-CoV-2 vaccination: a multicenter case series. J Hepatol. 2022 Jan;76(1):211–214. doi: 10.1016/j.jhep.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C., Lee A., Grigoryan L., Arunachalam P.S., Scott M.K.D., Trisal M., et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022 Apr;23(4):543–555. doi: 10.1038/s41590-022-01163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arunachalam P.S., Scott M.K.D., Hagan T., Li C., Feng Y., Wimmers F., et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021 Aug;596(7872):410–416. doi: 10.1038/s41586-021-03791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hagan T., Cortese M., Rouphael N., Boudreau C., Linde C., Maddur M.S., et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019 Sep 5;178(6):1313–1328.e13. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fernández J., Clària J., Amorós A., Aguilar F., Castro M., Casulleras M., et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019 Jul;157(1):149–162. doi: 10.1053/j.gastro.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 140.Udomkarnjananun S., Takkavatakarn K., Praditpornsilpa K., Nader C., Eiam-Ong S., Jaber B.L., et al. Hepatitis B virus vaccine immune response and mortality in dialysis patients: a meta-analysis. J Nephrol. 2020 Apr;33(2):343–354. doi: 10.1007/s40620-019-00668-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.