INTRODUCTION
The use of pacemakers and implantable cardioverter-defibrillators (ICDs) has become increasingly prevalent in the management of cardiac arrhythmias.1,2 However, with the increasing use of these devices, complications arising from lead placement have become more common. One such complication is Twiddler Syndrome, a rare but potentially serious condition characterized by lead dislodgement and cessation of ventricular pacing. The condition is caused by the patient’s repetitive manipulation of the pacemaker generator, leading to a change in the position of the leads.3 Other complications associated with pacemaker leads include lead dislodgement, phlebitis, myocardial perforation, and complete fragmentation of the pacemaker lead.4–6 These complications can cause significant morbidity and mortality, and it is crucial for clinicians to be aware of these potential complications to diagnose and treat them promptly.
The incidence of Twiddler Syndrome is estimated to be between 0.3–3% of all pacemaker implanted patients.7 Although the prevalence of this condition is relatively low, it can have significant consequences if not promptly diagnosed and treated. The diagnosis of Twiddler Syndrome can be challenging as it is often asymptomatic and can present with a wide range of symptoms such as device failure, sensing disturbances, and atrial or ventricular arrhythmias.8 Therefore, it is important for clinicians to have a high index of suspicion in patients with pacemakers, especially those who have a history of manipulating their device.
Early diagnosis and prompt treatment, such as lead repositioning or replacement, are crucial to prevent further complications and to ensure that the device continues to function properly. We present the case of a patient with an unusual presentation who was diagnosed with Twiddler syndrome.
CASE REPORT
A 72-year-old female patient with a history of coronary artery disease with stent placement, hypertension, hyperlipidemia, anemia, diabetes, atrial fibrillation, and paroxysmal atrial tachycardia presented to the clinic six weeks following the implantation of an automatic implantable cardioverter-defibrillator (AICD) with complaints of muscle spasms on her back and right side of the chest. The patient reported experiencing a thumping sensation from her back that radiated to the frontal side of her chest. On physical examination, it was noted that the muscle spasms occurred simultaneously with heartbeats and would cease upon laying in a supine position. The muscle spasms also were found to be fainter upon sitting upright and twisting the torso to the right.
The patient denied dyspnea, palpitations, or syncope. She denied previous manual manipulation of her AICD. Vital signs were stable upon presentation. Electrocardiogram revealed a ventricular paced rhythm at a rate of 65 beats per minute (bpm).
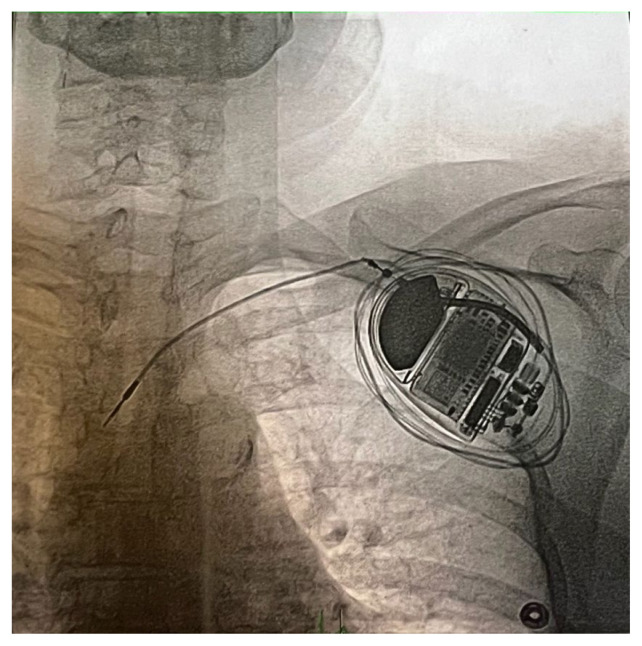
Interrogation of the AICD revealed that the right ventricular (RV) lead was not sensing. The percentage of ventricular pacing was 0.2%, in comparison to 10% atrial pacing. P-wave sensing was detected at 0.9 millivolts (mV) while an R-wave was not detected. Fluoroscopy confirmed that the RV lead was coiled around the AICD device and not placed in the right ventricle (Figure 1). Based on these findings, a diagnosis of Twiddler Syndrome was made. The patient underwent AICD system extraction, and an endocardial leadless pacing system was implanted. The post-operative course was uncomplicated.
Figure 1.
Fluoroscopy shows the RV lead coiled around the AICD device.
Abbreviations: AICD (automatic implantable cardioverter-defibrillator)
DISCUSSION
This case described a diagnosis of Twiddler Syndrome in a patient denying a history of manipulating their pacemaker generator. She presented with muscle spasms of the back and chest and was found to have a dislodged lead on physical examination. As the diagnosis was caught early, the AICD system was extracted, and a leadless pacing system was implanted.
In Twiddler’s syndrome, patients typically present with lead dislodgement due to repetitive manipulation of the pacemaker generator.3 This leads to migration of the leads and can result in various device malfunctions, including changes in pacing threshold or output, loss of capture, changes in sensing or pacing parameters, increase in battery consumption, pocket hematoma formation, and pain or discomfort at the implant site.2,9 The repetitive manipulation of the device causes stimulation of the ipsilateral phrenic nerve, resulting in pacing of the diaphragm and abdominal pulsation. Continued rotation of the leads also may result in stimulation of the brachial plexus and manifest as rhythmic arm.10 Although the patient denied any history of intentional device manipulation, unintentional or subconscious manipulation could not be ruled out. As a result of the electric stimulation, the patient presented with muscular spasms in the back and chest.
Patients with Twiddler’s syndrome may experience symptoms related to the underlying heart condition that the device was implanted to treat, such as palpitations, shortness of breath, or chest pain.11 They also can develop severe symptoms such as syncope secondary to neurological etiology, as well as fatal cardiac arrhythmias due to lack of proper pacing. Therefore, it is important to counsel patients on how to manage their pacemaker properly and the potential risks of its malfunction.
The diagnosis of Twiddler Syndrome can be challenging as it often is asymptomatic and can present with a wide range of symptoms.12 It is important for clinicians to have a high index of suspicion for this condition in patients with pacemaker therapy, especially if they present with muscle spasms, chest or abdominal pain, or syncope. Furthermore, patients with a history of repetitive manipulation of their pacemaker generator should be monitored closely for the development of Twiddler Syndrome.3 The diagnostic workup for Twiddler’s syndrome typically includes a combination of physical examination, medical history, and diagnostic tests. The healthcare professional will use a programmer or handheld device to interrogate the pacemaker or ICD and check its function, including battery status, pacing and sensing thresholds, and lead impedance. An electrocardiogram can be used to check for changes in heart rate or rhythm that may be related to the device manipulation. A chest x-ray can be used to check for displacement of the device or lead dislodgement. Other imaging studies such as computed tomography or magnetic resonance imaging can be used to check for displacement of the device or lead dislodgement, and to evaluate the extent of device manipulation.13–15
Variations of the syndrome have been reported in association with other devices such as implantable cardioverter-defibrillators and chemotherapy infusion pumps.10,16 Risk factors for the development of Twiddler Syndrome include loose subcutaneous tissue, commonly found in elderly and obese patients, as this allows for the rotation of the pulse generator. To prevent manipulation of the device and lead migration, suturing the device to the fascia has been suggested as a preventive measure.10
In addition to suturing the device to the fascia, other management options for Twiddler Syndrome include lead repositioning or replacement, and in some cases, pacemaker extraction and replacement with a leadless pacing system.17 The patient in this case report underwent AICD system extraction and an endocardial leadless pacing system was implanted.
CONCLUSIONS
Twiddler Syndrome is a rare complication of pacemaker therapy that can be fatal if not promptly diagnosed and treated. The condition is characterized by lead dislodgement, which results in the cessation of ventricular pacing. The symptom presentation can be variable, making it challenging to diagnose. However, a high index of suspicion and further investigation are crucial to diagnose and treat this condition properly.
REFERENCES
- 1.Silverman BG, Gross TP, Kaczmarek RG, Hamilton P, Hamburger S. The epidemiology of pacemaker implantation in the United States. Public Health Rep. 1995;110(1):42–46. [PMC free article] [PubMed] [Google Scholar]
- 2.Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States. J Am Coll Cardiol. 2011;58(10):1001–1006. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Jin C, Iwai S, Jacobson J, Ferrick A. A case of Twiddler’s syndrome with a subcutaneous implantable cardioverter-defibrillator. HeartRhythm Case Rep. 2022;8(8):596–597. doi: 10.1016/j.hrcr.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gul EE, Kayrak M. Common pacemaker problems: Lead and pocket complications. In: Das MR, editor. Modern Pacemakers - Present and Future. Rijeka, Croatia: InTech Europe; 2011. [Google Scholar]
- 5.Gupta AK, Burgos Claudio MI, Hus N. Unusual delayed complication of pacemaker leads. Cureus. 2020;12(7):e9479. doi: 10.7759/cureus.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khattak F, Khalid M, Gaddam S, Ramu V, Brahmbhatt V. A rare case of complete fragmentation of pacemaker lead after a high-velocity theme park ride. Case Rep Cardiol. 2018;2018:4192964. doi: 10.1155/2018/4192964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandal S, Pande A, Kahali D. A rare case of very early pacemaker Twiddler’s syndrome. Heart Views. 2012;13(3):114–115. doi: 10.4103/1995-705X.102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh V, Barsoum EA, Morcus R, Azab B, Lafferty J, Kohn J. Unique presentation of Twiddler’s syndrome. World J Cardiol. 2013;5(6):207–209. doi: 10.4330/wjc.v5.i6.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayliss CE, Beanlands DS, Baird RJ. The pacemaker-twiddler’s syndrome: A new complication of implantable transvenous pacemakers. Can Med Assoc J. 1968;99(8):371–373. [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson WJ, Tuohy KA, Tilkemeier P. Twiddler’s syndrome. N Engl J Med. 2003;348(17):1726–1727. doi: 10.1056/NEJM200304243481722. [DOI] [PubMed] [Google Scholar]
- 11.Liang JJ, Fenstad ER. Twiddler’s syndrome. Lancet. 2013;382(9909):e47. doi: 10.1016/S0140-6736(13)61419-1. [DOI] [PubMed] [Google Scholar]
- 12.Tahirovic E, Haxhibeqiri-Karabdic I. Twiddler’s Syndrome: Case report and literature review. Heart Views. 2018;19(1):27–31. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_89_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharawat R, Saadat M. Twiddler’s syndrome. Acta Med Acad. 2016;45(2):169–170. doi: 10.5644/ama2006-124.174. [DOI] [PubMed] [Google Scholar]
- 14.Salahuddin M, Cader FA, Nasrin S, Chowdhury MZ. The pacemaker-twiddler’s syndrome: An infrequent cause of pacemaker failure. BMC Res Notes. 2016;9:32. doi: 10.1186/s13104-015-1818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weir RA, Murphy CA, O’Rourke B, Petrie CJ. Twiddler’s syndrome: A rare cause of implantable cardioverter defibrillator malfunction. Eur Heart J. 2016;37(46):3439. doi: 10.1093/eurheartj/ehv383. [DOI] [PubMed] [Google Scholar]
- 16.Boyle NG, Anselme F, Monahan KM, et al. Twiddler’s syndrome variants in ICD patients. Pacing Clin Electrophysiol. 1998;21(12):2685–2687. doi: 10.1111/j.1540-8159.1998.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 17.Khalilullah M, Khanna SK, Gupta U, Padmavati S. Pacemaker twiddler’s syndrome: A note on its mechanism. J Cardiovasc Surg (Torino) 1979;20(1):95–100. [PubMed] [Google Scholar]