Abstract
Background
The Academic Research Consortium - High Bleeding Risk (ARC-HBR) initiative defined conditions associated with percutaneous coronary intervention (PCI)-related bleeding.
Aims
We sought to further explore these HBR conditions in the setting of transcatheter aortic valve replacement (TAVR).
Methods
Patients from the SCOPE 2 trial were stratified by their bleeding risk status based on the ARC-HBR definitions. Baseline and procedural characteristics, as well as key clinical outcomes including Bleeding Academic Research Consortium (BARC) 3-5 bleeding, were compared in ARC-HBR positive (HBR+) and ARC-HBR negative (HBR−) patients.
Results
Of 787 patients randomised in SCOPE 2 and included in this study, 633 were HBR+ (80.4%). Compared with HBR− patients, those HBR+ were older and more frequently presented with diabetes, a history of coronary artery disease, atrial fibrillation, prior cerebrovascular accident, and a Society of Thoracic Surgeons predicted risk of 30-day mortality (STS-PROM) (4.9±2.9% vs 3.3%±2.1%; p<0.0001). In addition, HBR+ patients were more frequently on oral anticoagulation therapy. At 1 year, HBR+ patients had higher rates of all-cause death (12.4% vs 4.3%, respectively, risk difference 8.09%; 95% confidence interval [CI]: 3.76-12.41; p=0.0002); the rates of BARC 3-5 type bleeding were relatively high but not statistically different compared with HBR− patients (7.7% vs 6.1%, risk difference 1.67%; 95% CI: -2.72 to 6.06; p=0.46). Subgroup analyses for bleeding events showed no significant interaction in terms of STS-PROM score, age, or medications.
Conclusions
The ARC-HBR criteria failed to isolate a subgroup of patients at higher bleeding risk in TAVR patients from a randomised trial. These findings have potential implications, especially for the selection of post-TAVR antithrombotic regimens based on individual bleeding-risk profiles. Specific HBR criteria should be defined for TAVR patients.
Introduction
Transcatheter aortic valve replacement (TAVR) has proven to be an effective and minimally invasive procedure in patients suffering from severe aortic stenosis1,2. TAVR is an alternative to surgical aortic valve replacement (SAVR) for a large proportion of patients with severe aortic stenosis, especially for those who are older or present with intermediate or high risk for surgery3,4. Since the late 2000’s, TAVR has led to constant improvement in clinical outcomes with the development of techniques and technological ameliorations along with increased operator experience. Although less prevalent compared with SAVR, the risk of major bleeding after TAVR has been estimated to be as high as approximately 6% and is associated with a three-fold increase in one-year mortality5,6,7.
Unlike percutaneous coronary intervention (PCI)-related bleeding risk conditions that have been recently defined by an Academic Research Consortium - High Bleeding Risk (ARC-HBR) consensus document8, those associated with major bleeding remain insufficiently explored after TAVR. The Valve Academic Research Consortium (VARC)-3 consensus provided an overview of risk assessment after TAVR that included the definitions of bleeding, but the conditions that increased this risk were not the subject of this initiative9. Some investigations based on local series or national registries aimed to evaluate the predictors and outcomes of major bleeding in TAVR patients7,10. Peripheral vascular disease, end-stage renal disease, and coagulopathy have been identified to increase the rates of major bleeding.
Against this background, we aimed to stratify patients from the SCOPE 2 trial11, a randomised comparison of the ACURATE neo (Boston Scientific) and the CoreValve Evolut (Medtronic) in 796 high-risk TAVR patients, according to their bleeding risk status based on the ARC-HBR definitions and to compare an array of key clinical outcomes including Bleeding Academic Research Consortium (BARC) 3-5 type bleeding complications in ARC-HBR positive (HBR+) and ARC-HBR negative patients (HBR-).
Methods
Study design
The SCOPE 2 trial design and rationale along with the principal 1-year results have been reported previously11. In brief, SCOPE 2 was a multicentre, randomised, parallel-design, non-inferiority, open-label trial carried out in 23 tertiary heart valve centres in 6 countries. It compared the safety and effectiveness of transfemoral TAVR using the ACURATE neo versus the CoreValve Evolut in patients with symptomatic severe aortic stenosis deemed to be at increased risk for mortality with SAVR, as assessed by the local Heart Teams. Inclusion criteria were the presence of symptomatic aortic stenosis with aortic annulus diameters covered by the sizes of the ACURATE neo and CoreValve Evolut valves. Left ventricular ejection fraction (<20%), pre-existing prosthetic valves in the aortic and/or mitral positions, bicuspid or unicuspid valves, severe mitral regurgitation, and/or peripheral anatomy inappropriate for transfemoral implant due to size, disease and degree of calcification or tortuosity of the aorta or ilio-femoral arteries represented the main criteria for exclusion. Complete details of the inclusion and exclusion criteria have been reported11. The trial was conducted according to the Declaration of Helsinki and Good Clinical Practice and was approved by the investigational review board or research ethics committee at each participating centre. All participants gave their informed consent. Procedural recommendations were per standard of care. The mode of anaesthesia was selected according to local standard practice. Pre- and post-dilatation were performed at the operator’s discretion, although predilatation is recommended by the manufacturer of the ACURATE neo valve. Access site closure was performed according to local practice. Minimally required laboratory analyses included haemoglobin, creatinine, and high-sensitivity troponin values. Dual antiplatelet therapy (preferably with aspirin and clopidogrel) was recommended for at least 3 months, followed by single antiplatelet therapy. In patients with an indication for oral anticoagulation or who had undergone recent coronary stent implantation, combination regimens and their duration were given at the discretion of the operator. Clinical follow-up was performed at 30 days and 1 year.
ARC-HBR criteria
Some of the ARC-HBR criteria needed to be modified or were not available because they were either not captured in the electronic data capture or represented criteria for exclusion in the trial, as summarised in Supplementary Table 1. This approach has been followed in other validation studies12,13,14,15. Major and minor ARC-HBR criteria applied in the current study are as follows: age ≥75 years (minor); oral anticoagulant or novel oral anticoagulant at discharge (major); estimated glomerular filtration rate (eGFR) <30 ml/min (major) and eGFR ≥30, <60 ml/min (minor); baseline haemoglobin <11 g/dL (major), and 11-12.9 g/dL for men and 11-11.9 g/dL for women (minor); thrombocytes at index procedure <100×109/L (major); non-steroidal anti-inflammatory drugs (NSAID) at discharge (minor); active cancer in the past 12 months (major); previous intracranial bleeding (major); any ischaemic stroke at any time not meeting the major criterion (minor). Patients were at HBR if at least one major criterion or two minor criteria were met10. An overall ARC-HBR score was calculated by adding 1 point for any major criterion and 0.5 for any minor criterion.
Outcomes
The primary endpoint was major or life-threatening bleeding (BARC type 3 or 5) at 12 months12. Other key clinical outcomes were the occurrence of death, stroke, hospitalisation for valve-related symptoms or worsened chronic heart failure, myocardial infarction, new permanent pacemaker implantation, and any arrhythmia responsible for haemodynamic disorders at 30 days and at 1 year. All patients were followed up to 12 months. The definitions of all endpoints have been reported previously10. An independent committee adjudicated events after a review of original source documents.
Statistical analysis
Discrete variables are expressed as percentages with frequencies and were compared by the χ2 or Fisher’s exact tests. Continuous variables are reported as mean±standard deviation and were compared by t-test if normally distributed or the Wilcoxon rank sum test for a non-parametric distribution. Event rates were based on Kaplan-Meier estimates in time-to-first-event analyses if mortality was part of the endpoint, or on cumulative incidence functions with the delta method for the estimation of the standard error, taking mortality as competing risk into account otherwise. Hazard ratios (HR) with 95% confidence intervals (CI) were determined by Cox regression analysis, and event rates were compared with the log-rank or Gray’s test, respectively. The day of the procedure was taken as day 0. For patients without a procedure, the day of randomisation was taken as day 0. Interaction testing was performed to determine whether the relative risk of BARC 3 or 5 type bleeding and mortality measures at 12 months varied by age, number of anti-thrombotic medications, Society of Thoracic Surgeons (STS) score, and presence of oral anticoagulants at the time of the procedure. A landmark analysis was performed for BARC 3 or 5 type bleeding from 30 days to 12 months of follow-up according to presence or absence of oral anticoagulants (OAC) at day 30. Wolbers’s adaptation of Harrell’s C-statistic for survival data was used to describe the prediction accuracy of HBR for major bleeds16. The C-index was 0.52. All analyses were performed in the intention-to-treat population. A two-sided p-value <0.05 was considered significant. All statistical analyses were performed with SAS software (Version 9.4, SAS/STAT version 15.1; SAS Institute Inc.).
Results
Between April 2017 and April 2019, 796 patients with symptomatic severe aortic stenosis were randomised to ACURATE neo (n=398) versus CoreValve Evolut (n=398). Due to missing values, nine patients could not be classified as high or low bleeding risk patients. Of the remaining 787 patients, 633 patients (80.4%) were stratified as high bleeding risk (HBR+) according to the ARC-HBR criteria.
Baseline characteristics
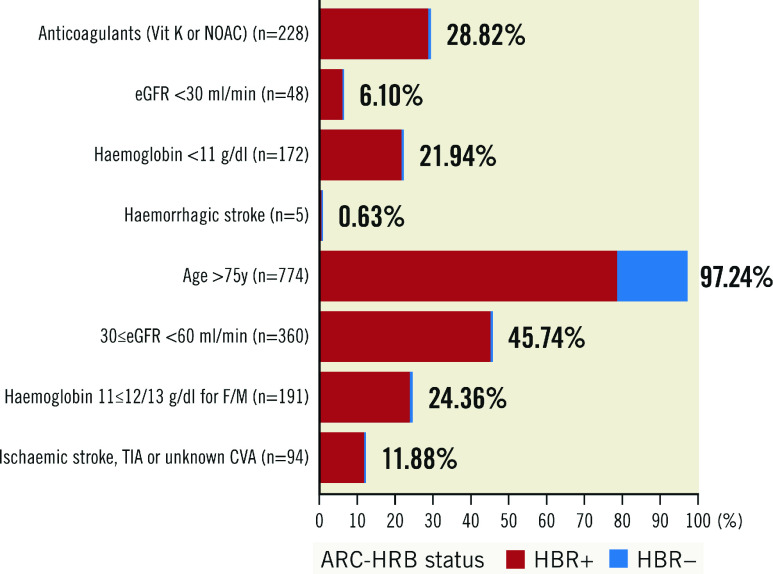
The main baseline characteristics of the study population are shown in Table 1. In brief, HBR+ patients were older and carried less favourable medical conditions. Compared to HBR- patients, they were more frequently diabetic and more frequently had a history of permanent pacemaker implantation, atrial fibrillation and/or a prior cerebrovascular accident. Their estimated STS predicted risk of mortality (STS-PROM) at 30 days was higher (4.9%±2.9% vs 3.3%±2.1%; p<0.0001). The distribution of medical conditions leading to HBR+ status is depicted in Figure 1. HBR+ patients were more frequently on OAC therapy (vitamin K antagonists [VKA]) or novel oral anticoagulants (NOAC), but the total number of antithrombotic medications was similar between groups (Table 2).
Table 1. Baseline characteristics of the patients stratified by ARC-HBR status.
|
HBR+ N=663 |
HBR− N=154 |
p-value | |
|---|---|---|---|
| Gender, female | 420 (66.3%) | 113 (73.4%) | 0.09 |
| Age (yrs) | 83.5±4.3 | 82.1±4.0 | <0.0001 |
| BMI (kg/m²) | 26.4±5.0 | 27.5±5.0 | 0.37 |
| Symptoms | |||
| NYHA Class III or IV | 424 (67.0%) | 87 (56.5%) | 0.02 |
| CCS Class III or IV | 30 (4.7%) | 10 (6.5%) | 0.41 |
| Syncope | 72 (11.4%) | 19 (12.3%) | 0.78 |
| STS predicted risk of mortality (%) | 4.9±2.9 | 3.3±2.1 | <0.0001 |
| Medical conditions and medical history | |||
| Diabetes | 189 (29.9%) | 32 (20.8%) | 0.028 |
| Dyslipidaemia | 326 (51.5%) | 78 (50.7%) | 0.86 |
| Hypertension | 547 (86.4%) | 129 (83.8%) | 0.44 |
| Current smoker | 22 (3.5%) | 5 (3.3%) | >0.99 |
| Coronary artery disease | 267 (42.2%) | 52 (33.7%) | 0.07 |
| COPD | 77 (12.2%) | 15 (9.7%) | 0.49 |
| Extracranial cerebral artery disease | 33 (5.2%) | 7 (4.6%) | 0.84 |
| Peripheral artery disease | 61 (9.6%) | 10 (6.5%) | 0.27 |
| Dialysis | 5 (0.8%) | 0 (0.0%) | 0.59 |
| History of atrial fibrillation | 252 (39.8%) | 12 (7.8%) | <0.001 |
| Previous pacemaker implantation | 66 (10.4%) | 8 (5.2%) | 0.046 |
| History of myocardial infarction | 54 (8.5%) | 12 (7.8%) | 0.87 |
| History of PCI | 164 (25.9%) | 38 (24.7%) | 0.84 |
| History of cardiac surgery | 40 (6.3%) | 4 (2.6%) | 0.08 |
| Previous aortic valvuloplasty | 11 (1.7%) | 1 (0.6%) | 0.48 |
| Prior cerebrovascular accident | 99 (15.6%) | 0 (0.0%) | <0.001 |
| CT findings | |||
| Aortic annulus perimeter (mm) | 74±5 | 74±5 | 0.18 |
| Aortic annulus area (mm²) | 423±56 | 420±55 | 0.50 |
| Area-derived diameter (mm) | 23 (22-24) | 23 (22-25) | 0.98 |
| Number of events (percentages). Mean±standard deviation or median (Q1-Q3). BMI: body mass index; CCS: Canadian Cardiovascular Society; COPD: chronic obstructive pulmonary disease; CT: computed tomography; HBR: high bleeding risk; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons | |||
Figure 1. Distribution of medical conditions leading to HBR+ status.
CVA: cardiovascular accident; eGFR: estimated glomerular filtration rate; F/M: female/male; HBR: high bleeding risk; NOAC: novel oral anticoagulants; TIA: transient ischaemic attack
Table 2. Anti-thrombotic medications at baseline, 30-day and 1-year follow-up, by HBR status.
| HBR+ | HBR− | ||
|---|---|---|---|
| At baseline | |||
| Aspirin | 305/633 (48.2%) | 99/154 (64.3%) | |
| Clopidogrel | 124/633 (19.6%) | 29/154 (18.8%) | |
| Prasugrel | 2/633 (0.3%) | 0/154 (0.0%) | |
| Ticagrelor | 6/633 (0.9%) | 0/154 (0.0%) | |
| Vitamin K antagonist | 91/633 (14.4%) | 0/154 (0.0%) | |
| NOAC | 142/633 (22.4%) | 0/154 (0.0%) | |
| No of antithrombotic medications | 0 | 111/633 (17.5%) | 52/154 (33.8%) |
| 1 | 389/633 (61.5%) | 76/154 (49.4%) | |
| 2 | 118/633 (18.6%) | 26/154 (16.9%) | |
| 3 | 15/633 (2.4%) | 0/154 (0.0%) | |
| At day 30 | |||
| Aspirin | 334/573 (58.3%) | 126/146 (86.3%) | |
| Clopidogrel | 252/573 (44.0%) | 73/146 (50.0%) | |
| Prasugrel | 1/573 (0.2%) | 0/146 (0.0%) | |
| Ticagrelor | 4/573 (0.7%) | 0/146 (0.0%) | |
| Vitamin K antagonist | 69/573 (12.0%) | 4/146 (2.7%) | |
| NOAC | 178/573 (31.1%) | 10/146 (6.9%) | |
| No of antithrombotic medications | 0 | 15/573 (2.6%) | 4/146 (2.7%) |
| 1 | 291/573 (50.8%) | 71/146 (48.6%) | |
| 2 | 254/573 (44.3%) | 71/146 (48.6%) | |
| 3 | 13/573 (2.3%) | 0/146 (0.0%) | |
| At 1 year | |||
| Aspirin | 255/504 (50.6%) | 109/137 (79.6%) | |
| Clopidogrel | 78/504 (15.5%) | 20/137 (14.6%) | |
| Prasugrel | 0/504 (0.0%) | 0/137 (0.0%) | |
| Ticagrelor | 0/504 (0.0%) | 0/137 (0.0%) | |
| Vitamin K antagonist | 55/504 (10.9%) | 4/137 (2.9%) | |
| NOAC | 165/504 (32.7%) | 17/137 (12.4%) | |
| No of antithrombotic medications | 0 | 26/504 (5.2%) | 4/137 (2.9%) |
| 1 | 404/504 (80.2%) | 116/137 (84.7%) | |
| 2 | 73/504 (14.5%) | 17/137 (12.4%) | |
| 3 | 1/504 (0.2%) | 0/137 (0.0%) | |
| Number of events (percentages). NOAC: novel oral anticoagulants | |||
Procedural characteristics and immediate outcome
The procedural characteristics did not vary significantly between HBR+ and HBR- patients (Table 3). A suture-mediated closure device was used in >80% of the patients. The immediate complication rates were similar between the two groups (13.4% vs 13.3%;p>0.99).
Table 3. Procedure characteristics and outcomes by ARC-HBR status.
| HBR+ N=633 | HBR– N=154 | p-value | ||
|---|---|---|---|---|
| Procedure performed | 620/633 (97.9%) | 151/154 (98.1%) | >0.99 | |
| Total procedure time (min) | 72±36 | 77±37 | 0.18 | |
| Total contrast volume administered (mL) | 132±53 | 135±70 | 0.51 | |
| General anaesthesia | 88/620 (14.2%) | 28/151 (18.5%) | 0.20 | |
| Type of access | Percutaneous | 616/619 (99.5%) | 151/151 (100.0%) | 0.39 |
| Surgical cut-down | 3/619 (0.5%) | 0/151 (0.0%) | ||
| Sheathless | 235/620 (37.9%) | 52/151 (34.4%) | 0.45 | |
| Suture device closure for main access site | No | 5/619 (0.8%) | 1/151 (0.7%) | 0.92 |
| ProStar | 131/619 (21.2%) | 29/151 (19.2%) | ||
| 1 ProGlide | 34/619 (5.5%) | 7/151 (4.6%) | ||
| 2 ProGlide | 335/619 (54.1%) | 88/151 (58.3%) | ||
| Other | 114/619 (18.4%) | 26/151 (17.2%) | ||
| Predilatation balloon valvuloplasty | 370/620 (59.7%) | 93/151 (61.6%) | 0.71 | |
| Size device (mm) | 27±2 | 26±2 | 0.08 | |
| Post-dilatation | 248/620 (40.0%) | 68/151 (45.0%) | 0.27 | |
| Procedural complications | 84/620 (13.5%) | 28/151 (13.2%) | >0.99 | |
| Valve malpositioning | 5/620 (0.8%) | 6/151 (4.0%) | 0.01 | |
| Coronary artery obstruction | 2/620 (0.3%) | 0/151 (0.0%) | >0.99 | |
| Haemodynamic instability | 9/620 (1.5%) | 0/151 (0.0%) | 0.21 | |
| Cardiac tamponade | 7/620 (1.1%) | 1/151 (0.7%) | >0.99 | |
| Annular rupture | 1/620 (0.2%) | 1/151 (0.7%) | 0.35 | |
| Conversion to open heart surgery | 2/620 (0.3%) | 0/151 (0.0%) | >0.99 | |
| Access vessel complication | 48/620 (7.7%) | 9/151 (6.0%) | 0.60 | |
| Bleeding | 16/620 (2.6%) | 1/151 (0.7%) | 0.22 | |
| Intraprocedural death | 3/620 (0.5%) | 0/151 (0.0%) | >0.99 | |
| Number of events (percentages). ARC-HBR: Academic Research Consortium - High Bleeding Risk; min: minutes; mL: millilitres; mm: millimetres | ||||
Outcomes at 30 days and 12 months
Estimates of clinical outcomes at 30-day follow-up are shown in Table 4. BARC 3 or 5 type bleeding did not differ significantly between HBR+ and HBR- patients. HBR+ patients had higher rates of composite all-cause death and disabling stroke (4.8% vs 1.3%, risk difference 3.50%; 95% CI: 1.00-5.99; p=0.0061).
Table 4. Endpoints at 30 days by ARC-HBR status.
| HBR+ N=633 | HBR– N=154 |
Risk difference [95% CI] |
p-value | |
|---|---|---|---|---|
| All-cause death | 19 (3.1%) [2.0-4.8] | 0 (0.0%) [NC-NC] | 3.08 [NC-NC] | NC |
| Cardiac death | 14 (2.3%) [1.4-3.8] | 0 (0.0%) [NC-NC] | 2.29 [NC-NC] | NC |
| Non-cardiac death | 5 (0.8%) [0.3-1.9] | 0 (0.0%) [NC-NC] | 0.81 [NC-NC] | NC |
| All strokes* | 27 (4.3%) [2.9-6.1] | 3 (2.0%) [0.5-5.3] | 2.35 [–0.40-5.09] | 0.09 |
| Disabling strokes* | 14 (2.2%) [1.3-3.6] | 2 (1.3%) [0.3-4.3] | 0.90 [–1.28-3.08] | 0.42 |
| Non-disabling strokes* | 13 (2.1%) [1.2-3.5] | 1 (0.6%) [0.1-3.3] | 1.45 [-0.25-3.14] | 0.09 |
| All-cause death and disabling strokes | 30 (4.8%) [3.4-6.8] | 2 (1.3%) [0.3-5.2] | 3.50 [1.00-5.99] | 0.0061 |
| All-cause death and all strokes | 43 (6.9%) [5.2-9.2] | 3 (2.0%) [0.6-6.0] | 4.94 [1.95-7.94] | 0.0012 |
| Hospitalisation for valve-related symptoms or worsened CHF* | 13 (2.1%) [1.2-3.5] | 1 (0.7%) [0.1-3.4] | 1.46 [–0.27-3.19] | 0.09 |
| Life-threatening major bleeding (BARC type 3 or 5)* | 39 (6.2%) [4.5-8.3] | 5 (3.3%) [1.2-7.0] | 2.94 [–0.47-6.34] | 0.09 |
| Life-threatening major bleeding (BARC type 3b or 5)* | 15 (2.4%) [1.4-3.8] | 2 (1.3%) [0.3-4.3] | 1.09 [–1.10-3.27] | 0.33 |
| Myocardial infarction* | 2 (0.3%) [0.1-1.1] | 0 (0.0%) [NC-NC] | 0.32 [NC-NC] | NC |
| Valve-related dysfunction requiring repeat procedure* | 2 (0.3%) [0.1-1.1] | 0 (0.0%) [NC-NC] | 0.32 [NC-NC] | NC |
| Implantation of multiple valves* | 1 (0.2%) [0.0-0.9] | 4 (2.6%) [0.9-6.1] | –2.44 [–4.97-0.09] | 0.06 |
| New LBBB* | 92 (14.6%) [12.0-17.5] | 30 (19.7%) [13.8-26.4] | –5.06 [–12.0-1.84] | 0.15 |
| New permanent pacemaker implantation* | 95 (15.2%) [12.5-18.2] | 16 (10.6%) [6.3-16.1] | 4.64 [–1.02-10.31] | 0.11 |
| Any arrhythmia resulting in haemodynamic instability or requiring therapy | 30 (4.8%) [3.3-6.7] | 3 (2.0%) [0.5-5.3] | 2.79 [–0.01-5.60] | 0.051 |
| Number of events (percentages) [95% CI: confidence interval]. Percentages are Kaplan-Meier estimates or cumulative incidence estimates (indicated by *) taking mortality as a competing risk into account. BARC: Bleeding Academic Research Consortium; CHF: congestive heart failure; HBR: high bleeding risk; LBBB: left bundle branch block; NC: not calculated | ||||
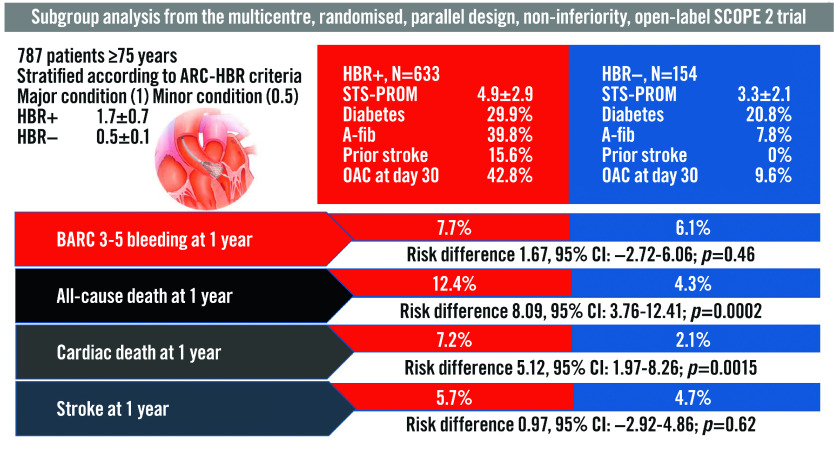
At 12 months, the rates of all-cause death were higher in HBR+ compared to HBR- patients (Table 5). However, the rates of BARC 3 or 5 type bleeding were not statistically different between groups (7.7% vs 6.1%, risk difference 1.67%; 95% CI: -2.72 to 6.06; p=0.46) (Central illustration, Figure 2). Compared to HBR- patients, HBR+ had more frequent access site-related BARC 3 or 5 type bleeding (3.0% vs. 0.7%, risk difference 2.36%; 95% CI: 0.49-4.23; p=0.0133). However, the rates of non-access site related BARC 3 or 5 type bleeding were not statistically different between groups (4.6% vs 4.8% for HBR+ and HBR- patients, respectively, risk difference -0.17%; 95% CI: -3.99 to -3.65; p=0.93).
Table 5. Endpoints at 1 year by ARC-HBR status.
| HBR+ N=633 | HBR– N=154 | Risk difference [95% CI] | p-value | |
|---|---|---|---|---|
| All-cause death | 73 (12.4%) [10.0-15.4] | 6 (4.3%) [2.0-9.4] | 8.09 [3.76-12.41] | 0.0002 |
| Cardiac death | 42 (7.2%) [5.4-9.6] | 3 (2.1%) [0.7-6.3] | 5.12 [1.97-8.26] | 0.0015 |
| Non-cardiac death | 31 (5.6%) [4.0-7.9] | 3 (2.3%) [0.7-7.0] | 3.33 [0.12-6.53] | 0.0422 |
| All strokes* | 35 (5.7%) [4.1-7.7] | 7 (4.7%) [2.1-9.0] | 0.97 [–2.92-4.86] | 0.62 |
| Disabling strokes* | 17 (2.8%) [1.7-4.3] | 4 (2.5%) [0.9-6.3] | 0.04 [–2.88-2.97] | 0.98 |
| Non-disabling strokes* | 18 (3.0%) [1.8-4.5] | 3 (2.0%) [0.6-5.4] | 0.93 [–1.70-3.57] | 0.49 |
| All-cause death and disabling strokes | 84 (14.1%) [11.6-17.2] | 9 (6.3%) [3.3-11.9] | 7.80 [2.89-12.71] | 0.0019 |
| All-cause death and all strokes | 100 (16.8%) [14.0-20.0] | 11 (7.5%) [4.2-13.2] | 9.25 [4.02-14.47] | 0.0005 |
| Hospitalisation for valve-related symptoms or worsened CHF* | 37 (6.3%) [4.5-8.4] | 4 (2.8%) [0.9-6.5] | 3.51 [0.20-6.83] | 0.0380 |
| Life-threatening major bleeding (BARC type 3 or 5)* | 48 (7.7%) [5.8-10.0] | 9 (6.1%) [3.0-10.7] | 1.67 [–2.72-6.06] | 0.46 |
| Life-threatening major bleeding (BARC type 3b or 5)* | 21 (3.4%) [2.2-5.1] | 3 (2.0%) [0.6-5.3] | 1.43 [–1.25-4.11] | 0.30 |
| Myocardial infarction* | 9 (1.5%) [0.7-2.8] | 0 (0.0%) [NC-NC] | 1.51 [NC-NC] | NC |
| Valve-related dysfunction requiring repeat procedure* | 4 (0.7%) [0.2-1.6] | 0 (0.0%) [NC-NC] | 0.66 [NC-NC] | NC |
| Endocarditis* | 4 (0.7%) [0.2-1.7] | 0 (0.0%) [NC-NC] | 0.69 [NC-NC] | NC |
| New LBBB* | 96 (15.4%) [12.7-18.3] | 30 (19.7%) [13.8-26.4] | –4.32 [–11.3-2.61] | 0.22 |
| New permanent pacemaker implantation* | 98 (15.8%) [13.0-18.7] | 16 (10.6%) [6.3-16.1] | 5.16 [–0.52-10.84] | 0.07 |
| Any arrhythmia resulting in haemodynamic instability or requiring therapy | 37 (6.1%) [4.4-8.2] | 4 (2.7%) [0.9-6.3] | 3.40 [0.18-6.62] | 0.0387 |
| Number of events (percentages) [95% CI: confidence interval]. Percentages are Kaplan-Meier estimates or cumulative incidence estimates (indicated by *) taking mortality as a competing risk into account. ARC-HBR: Academic Research Consortium - High Bleeding Risk; BARC: Bleeding Academic Research Consortium; CHF: congestive heart failure; LBBB: left bundle branch block; NC: not calculated | ||||
Central illustration. Subgroup analysis of the SCOPE 2 trial population according to ARC-HBR criteria.
A-fib: atrial filbrillation; ARC: Academic Research Consortium; CI: confidence interval; HBR: high bleeding risk; OAC: oral anticoagulants; STS-PROM: Society of Thoracic Surgeons predicted risk of mortality
Figure 2. Cumulative incidence curves.
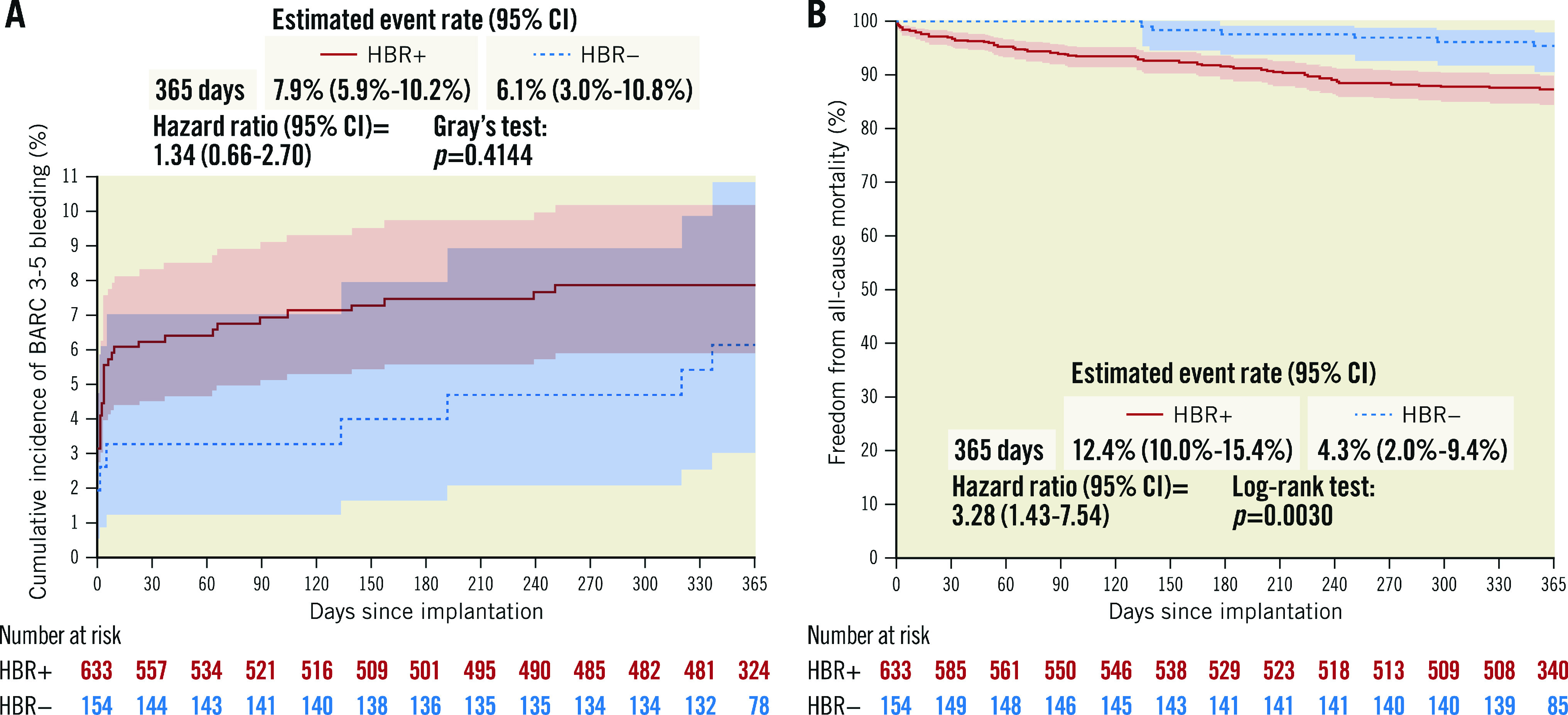
A) BARC 3-5 bleeding and B) freedom from all-cause mortality in HBR+ and in HBR– patients. BARC: Bleeding Academic Research Consortium; CI: confidence interval; HBR: high bleeding risk
Subgroup analyses for bleeding events showed no significant interaction with patients’ age (Figure 3). In addition, a landmark analysis conducted according to the presence or absence of OAC at day 30 to determine the risk of BARC 3-5 bleeding up to one year showed no difference between HBR+ and HBR- patients (Table 6). In the subgroup analysis for mortality at 12 months (Figure 4) a significant difference for intermediate-risk (STS 5-8) and older patients (81-85 and >85 years) was observed.
Figure 3. BARC 3-5 bleeding at one year by subgroups.
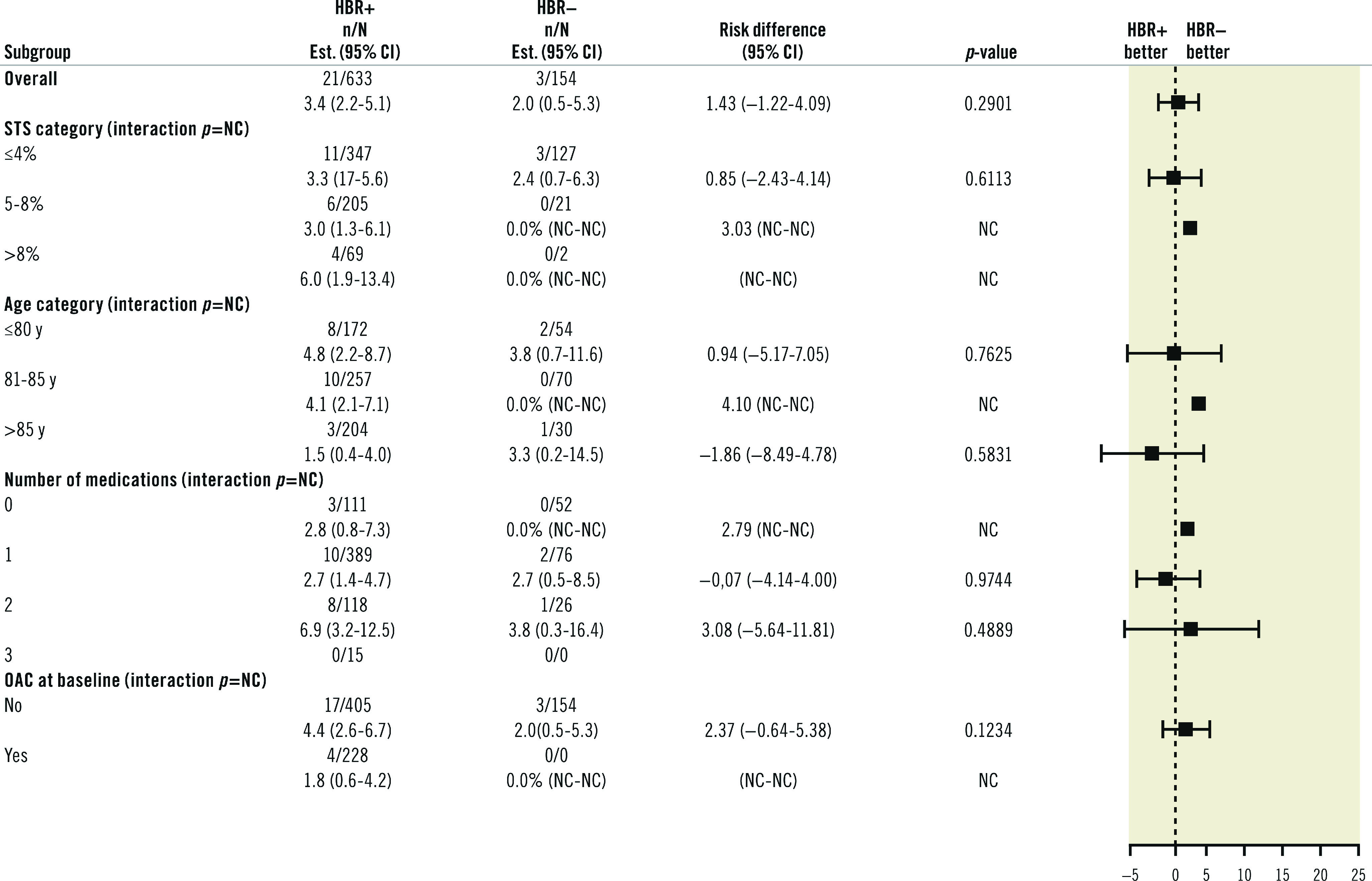
CI: confidence interval; HBR: high bleeding risk; OAC: oral anticoagulants; NC: not calculated; STS: Society of Thoracic Surgeons
Table 6. Landmark analysis according to OAC status at day 30 to assess the one-year BARC 3-5 bleeding risk.
|
OAC at day 30 (interaction p=0.1461) |
HBR+ | HBR– |
Risk difference [95% CI] |
p-value |
|---|---|---|---|---|
| No | 6/322 (1.9%) [0.8-3.9] | 1/132 (0.8%) [0.1-3.9] | 1.11 [–1.04-3.26] | 0.31 |
| Yes | 4/242 (1.7%) [0.6-4.0] | 2/14 (14.9%) [2.4-37.8] | –13.2 [–32.3-5.97] | 0.18 |
| Number of events (percentages) [95% CI: confidence interval]. Percentages are cumulative incidence estimates taking mortality as a competing risk into account; CI: confidence interval. HBR: high bleeding risk; OAC: oral anticoagulants | ||||
Figure 4. Mortality at one year by subgroups.
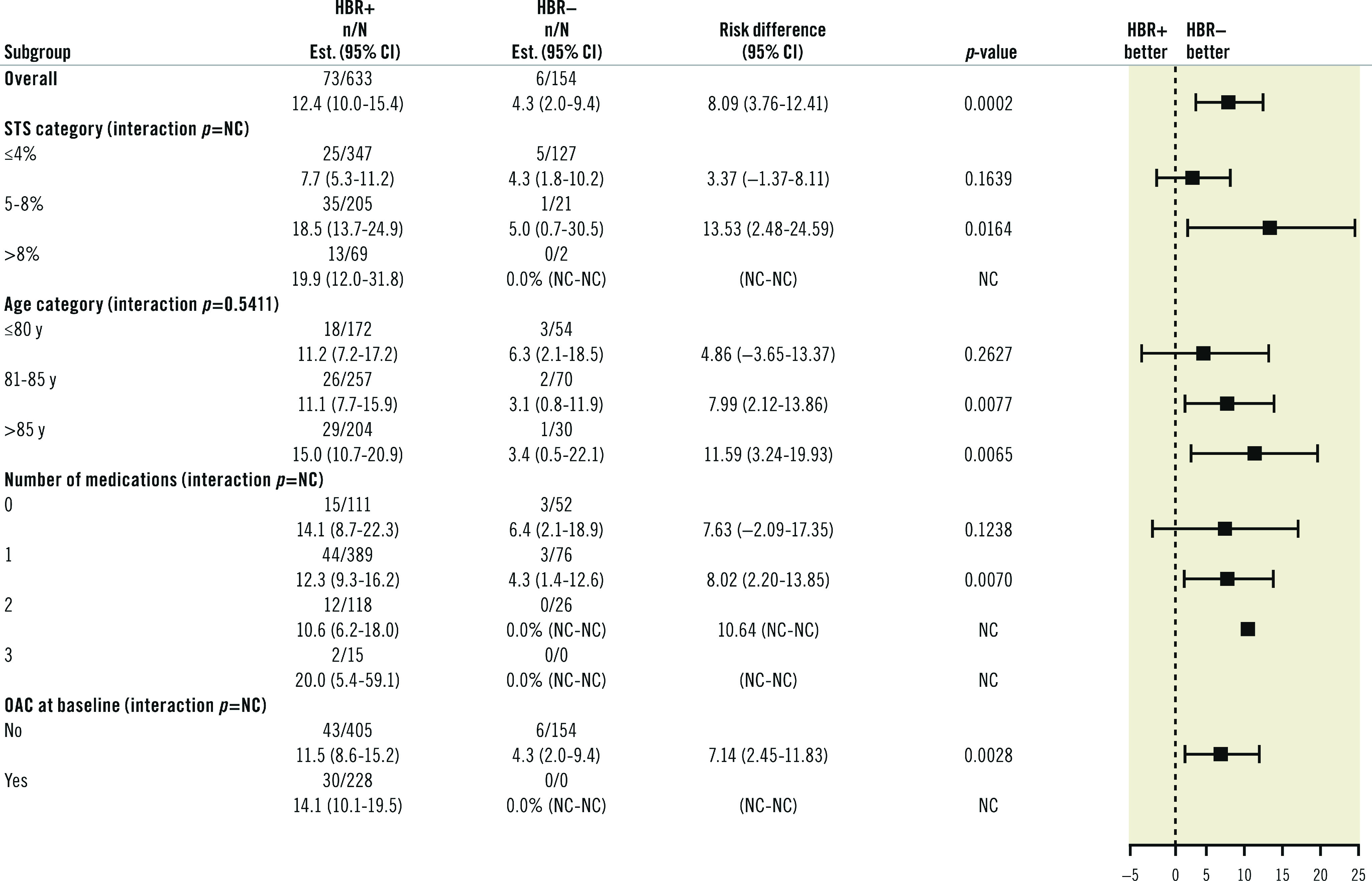
CI: confidence interval; HBR: high bleeding risk; OAC: oral anticoagulants; NC: not calculated; STS: Society of Thoracic Surgeons
Discussion
The present analysis of the SCOPE 2 trial indicates that HBR+ patients, identified based on ARC-HBR standards for PCI, had higher rates of death and stroke at 12 months, but similar rates of BARC 3 or 5 type bleeding compared to HBR- patients. These findings suggest that ARC-HBR criteria defined in a PCI population are not relevant to discriminate an increased bleeding risk in TAVR patients. A contributing role of age is likely, as TAVR patients are on average 15 to 20 years older than patients undergoing PCI. An age cut-off of 75 years, as defined by the ARC-HBR definitions for PCI to indicate a minor criterion, is probably not adequate for TAVR patients. Indeed, the rate of major or life-threatening bleeding was 3.6% in PARTNER 3 (mean age 73.3±5.8 years) and 10.4% in PARTNER II, where patients were older (81.5±6.7 years)3,4. Factors that increase the risk of bleeding after TAVR include a high prevalence of chronic kidney disease, liver disease, peripheral vasculopathy, acquired thrombocytopenia, colonic malignancy, and acquired reversible von Willebrand factor deficiency17,18,19,20,21. Since PCI is most frequently performed via the radial approach with smaller sheaths and catheters, the presence of peripheral vasculopathy is notably absent from ARC-HBR criteria while the size, presence of calcifications, and tortuosity of the iliac and femoral arteries are strongly associated with TAVR-related vascular complications and bleeding. More recently, Navarese et al have developed a 6-item algorithm comprising blood haemoglobin and serum iron concentrations, oral anticoagulation and dual antiplatelet therapy, common femoral artery diameter, and creatinine clearance that was able to identify patients at high risk of bleeding within 30 days after TAVR19. The role of anti-thrombotic medications is crucial in determining the rate of bleeding in these patients. However, we found that despite having more frequent use of OAC and being stratified according to bleeding-associated conditions, HBR+ patients had similar rates of severe bleeding compared to HBR-, which highlights the fact that important conditions reflecting the specificity of a TAVR population are missing in the ARC-HBR definition.
In the present study, approximately 7% of patients who underwent TAVR had BARC 3 or 5 type bleeding at one year, which is consistent with recent reports5,7. A previous study reported that life-threatening bleeding after TAVR, as defined by the VARC criteria, occurred in approximately 15 to 20% of TAVR procedures22. Our estimate of major bleeding complications in TAVR was 7.2%, which we believe to be a more contemporary estimate and could be further ameliorated using a single antithrombotic agent (e.g., a VKA or an NOAC in patients with atrial fibrillation, or aspirin in patients without) if PCI is not concurrently performed4.
Major bleeding or vascular complications have decreased as TAVR technology evolves into smaller device and sheath sizes22,23, and bleeding complications are inconsistent in the early literature24. However, major bleeding is still associated with a three-fold increase in one-year mortality following TAVR5,6,7. Patients referred to TAVR are elderly, frail and at risk for both bleeding and ischaemic complications7,17,25. Careful evaluation of risk and benefit is warranted to identify the optimal antithrombotic regimen, as major late bleeding complications are associated with an increased risk of mortality5. Our data support and strengthen a previous report26 suggesting that ARC-HBR criteria are not suitable for TAVR patients. Further studies and initiatives are warranted to determine the conditions qualifying HBR for the specific subset of patients requiring percutaneous valvular interventions.
Limitations
The present analysis has several limitations. The present report describes a post hoc subgroup analysis according to bleeding risk in the SCOPE 2 trial. Outcomes were assessed among ARC-HBR+ and ARC-HBR- patients. As per trial protocol, the current subgroup analysis should be considered exploratory and hypothesis-generating as the trial did not meet its primary objective and was not powered for either comparative assessment of bleedings nor for this patient stratification. In addition, some of the ARC-HBR criteria were not captured in the trial. Most uncaptured medical conditions (cirrhosis and all severe coagulation conditions, active cancer with bad prognosis) were criteria for exclusion in the SCOPE 2 trial, and some of them (brain malformations) are very rare in this TAVR population. In addition, ischaemic strokes and transient ischaemic attacks were classified as minor criteria in the absence of timing of the events. We assumed that this would not significantly impact the determination of the patients’ groups.
Conclusion
ARC-HBR criteria defined for PCI patients did not identify a subset of TAVR patients at increased rates of BARC 3 or 5 bleeding in the SCOPE 2 trial. Specific HBR criteria should be defined for TAVR patients. These findings are clinically relevant and have potential important implications, especially for the selection of post-TAVR antithrombotic regimens based on individual bleeding risk profiles.
Impact on daily practice
Severe bleeding after TAVR is associated with increased morbidity and mortality. As opposed to PCI-related risks of bleeding that have been defined by an ARC initiative, the conditions leading to severe bleeding after TAVR remain insufficiently explored. The present report confirms and strengthens the fact that conditions stratifying high bleeding risk criteria in a PCI population are not relevant to qualify high bleeding risk in the subset of valvular patients requiring percutaneous intervention. High bleeding risk criteria should be defined in a way that is specific to TAVR patients. A dedicated initiative is warranted.
Acknowledgments
The authors are grateful to Mrs. Dupic for her skillful assistance in reviewing the present manuscript.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Supplementary data
Comparison of definitions between the ARC-HBR criteria and the present study.
Acknowledgments
Funding
The SCOPE 2 Trial is an investigator-initiated trial, funded by CERIC.
Conflict of interest statement
P. Garot is the medical Director and a shareholder of CERC. M-C. Morice is the CEO and a shareholder of CERC and a minor shareholder of Electroducer. C. Tamburino has received speaker fees from Medtronic. S. Bleiziffer has received speaker fees from Boston Scientific and Medtronic. M. Cunnington has received speaker and consultant fees from Medtronic. A. Wolf is a proctor for Boston Scientific, Edwards Lifesciences, and Medtronic. M. Barbanti has received consultant fees from Edwards Lifesciences. P. Pagnotta is a proctor for Boston Scientific, Cardia, and Gore Medical. F. Bedogni has received personal fees from Abbott Vascular, Boston Scientific, Medtronic, Meril Life Sciences, and Terumo. E. Van Belle has received personal fees from Philips/Volcano. M. Vasa-Nicotera is a proctor for Boston Scientific and Medtronic. A. Chieffo has received speaker fees from Abiomed, Abbott Vascular, Biosensors, Cardinal Health, and Magenta, and consultant fees from Abiomed, Abbott Vascular, Biosensors, Cardinal Health, and Magenta. K. Bogaerts has received consultant fees from the Cardiovascular European Research Center. C. Hengstenberg is a proctor for Boston Scientific; reports institutional grants from Boston Scientific; has received speaker fees from Boston Scientific; and has received consultant fees from Boston Scientific. D. Capodanno has received speaker fees from AstraZeneca, Biosensors, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Menarini, Pfizer, and Sanofi, and consultant fees from Abbott Vascular, Amgen, AstraZeneca, and Bayer. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Abbreviations
- ARC
Academic Research Consortium
- BARC
Bleeding Academic Research Consortium
- DAPT
dual antiplatelet therapy
- HBR
high bleeding risk
- OAC
oral anticoagulant
- PCI
percutaneous coronary intervention
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- STS-PROM
Society of Thoracic Surgeons - predicted risk of mortality
Contributor Information
Philippe Garot, Institut Cardiovasculaire Paris-Sud, Hôpital Privé Jacques Cartier, Ramsay-Santé, Massy, France.
Antoinette Neylon, Institut Cardiovasculaire Paris-Sud, Hôpital Privé Jacques Cartier, Ramsay-Santé, Massy, France.
Marie-Claude Morice, Institut Cardiovasculaire Paris-Sud, Hôpital Privé Jacques Cartier, Ramsay-Santé, Massy, France.
Corrado Tamburino, Division of Cardiology Azienda Ospedaliero Universitaria "Policlinico‐Vittorio Emanuele" University of Catania, Catania, Italy.
Sabine Bleiziffer, Department of Thoracic and Cardiovascular Surgery, Heart and Diabetes Center Northrhein-Westfalia, University Hospital, Ruhr-University Bochum, Bad Oeynhausen, Germany.
Holger Thiele, Department of Cardiology, Leipzig Heart Center, University of Leipzig, Leipzig, Germany.
Smita Scholtz, Department of Interventional Cardiology, Heart and Diabetes Center North Rhine Westfalia, Bad Oeynhausen, Germany.
Rene Schramm, Department of Thoracic and Cardiovascular Surgery, Heart and Diabetes Center Northrhein-Westfalia, University Hospital, Ruhr-University Bochum, Bad Oeynhausen, Germany.
James Cockburn, Department of Cardiology, Brighton & Sussex University Hospitals NHS Trust, Brighton, United Kingdom.
Michael Cunnington, Department of Cardiology, Leeds General Infirmary, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom.
Alexander Wolf, Department of Interventional Cardiology, Elisabeth Hospital Essen, Essen, Germany.
Marco Barbanti, Department of Cardio-Thoracic-Vascular diseases and transplantation, Azienda Ospedaliero-Universitaria Policlinico “G. Rodolico-San Marco”, Catania, Italy.
Didier Tchetche, Groupe CardioVasculaire Interventionnel, Clinique Pasteur, Toulouse, France.
Paolo Pagnotta, Department of Cardiovascular Medicine, Humanitas Clinical and Research Center, Milano, Italy.
Martine Gilard, Department of Cardiology, Brest University Hospital, Brest, France.
Francesco Bedogni, Cardiology Department, IRCCS Policlinico San Donato, Milano, Italy.
Eric Van Belle, Department of Cardiology, Lille University Hospital, Lille, France.
Mariuca Vasa-Nicotera, Department of Cardiology, Goethe University Hospital Frankfurt, Frankfurt am Main, Germany.
Alaide Chieffo, Interventional Cardiology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Kris Bogaerts, KU Leuven, Faculty of Medicine, I-BioStat, Leuven, Belgium and UHasselt, I-BioStat, Hasselt, Belgium.
Christian Hengstenberg, Department of Internal Medicine II, Medical University of Vienna, Vienna, Austria.
Davide Capodanno, Division of Cardiology Azienda Ospedaliero Universitaria "Policlinico‐Vittorio Emanuele" University of Catania, Catania, Italy.
References
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG PARTNER 2 Investigators. Transcatheter or Surgical Aortic Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022 Feb 14;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- Bendayan M, Messas N, Perrault LP, Asgar AW, Lauck S, Kim DH, Arora RC, Langlois Y, Piazza N, Martucci G, Lefèvre T, Noiseux N, Lamy A, Peterson MD, Labinaz M, Popma JJ, Webb JG, Afilalo J. Frailty and Bleeding in Older Adults Undergoing TAVR or SAVR: Insights From the FRAILTY-AVR Study. JACC Cardiovasc Interv. 2020;13:1058–68. doi: 10.1016/j.jcin.2020.01.238. [DOI] [PubMed] [Google Scholar]
- Ullah W, Jafar M, Zahid S, Ahmed F, Khan MZ, Sattar Y, Fischman DL, Virani SS, Alam M. Predictors of In-Hospital Mortality in Patients With End-Stage Renal Disease Undergoing Transcatheter Aortic Valve Replacement: A Nationwide Inpatient Sample Database Analysis. Cardiovasc Revasc Med. 2022;34:63–8. doi: 10.1016/j.carrev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- Khan H, Gilani A, Qayum I, Khattak T, Haq F, Zahid Anwar, Khan MA, Asjad SJ, Abbas S, Inayat A. An Analysis of the Predictors of Major Bleeding After Transcatheter Aortic Valve Transplantation Using the National Inpatient Sample (2015-2018). Cureus. 2021;13:e16022. doi: 10.7759/cureus.16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation. 2019;140:240–61. doi: 10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARC-3 WRITING COMMITTEE: Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717–46. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- Tchetche D, Van der, Dumonteil N, Chieffo A, Van Mieghem, Farah B, Buchanan GL, Saady R, Marcheix B, Serruys PW, Colombo A, Carrie D, De Jaegere, Fajadet J. Adverse impact of bleeding and transfusion on the outcome post-transcatheter aortic valve implantation: insights from the Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Am Heart J. 2012;164:402–9. doi: 10.1016/j.ahj.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Tamburino C, Bleiziffer S, Thiele H, Scholtz S, Hildick-Smith D, Cunnington M, Wolf A, Barbanti M, Tchetchè D, Garot P, Pagnotta P, Gilard M, Bedogni F, van Belle, Vasa-Nicotera M, Chieffo A, Deutsch O, Kempfert J, Søndergaard L, Butter C, Trillo-Nouche R, Lotfi S, Möllmann H, Joner M, Abdel-Wahab M, Bogaerts K, Hengstenberg C, Capodanno D. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients With Symptomatic Severe Aortic Stenosis: SCOPE 2 Randomized Clinical Trial. Circulation. 2020;142:2431–42. doi: 10.1161/CIRCULATIONAHA.120.051547. [DOI] [PubMed] [Google Scholar]
- Cao D, Mehran R, Dangas G, Baber U, Sartori S, Chandiramani R, Stefanini GG, Angiolillo DJ, Capodanno D, Urban P, Morice MC, Krucoff M, Goel R, Roumeliotis A, Sweeny J, Sharma SK, Kini A. Validation of the Academic Research Consortium High Bleeding Risk Definition in Contemporary PCI Patients. J Am Coll Cardiol. 2020;75:2711–22. doi: 10.1016/j.jacc.2020.03.070. [DOI] [PubMed] [Google Scholar]
- Corpataux N, Spirito A, Gragnano F, Vaisnora L, Galea R, Svab S, Gargiulo G, Zanchin T, Zanchin C, Siontis GCM, Praz F, Lanz J, Hunziker L, Stortecky S, Pilgrim T, Räber L, Capodanno D, Urban P, Pocock S, Heg D, Windecker S, Valgimigli M. Validation of high bleeding risk criteria and definition as proposed by the academic research consortium for high bleeding risk. Eur Heart J. 2020;41:3743–9. doi: 10.1093/eurheartj/ehaa671. [DOI] [PubMed] [Google Scholar]
- Gragnano F, Spirito A, Corpataux N, Vaisnora L, Galea R, Gargiulo G, Siontis GCM, Praz F, Lanz J, Billinger M, Hunziker L, Stortecky S, Pilgrim T, Bär S, Ueki Y, Capodanno D, Urban P, Pocock SJ, Mehran R, Heg D, Windecker S, Räber L, Valgimigli M. Impact of Clinical Presentation on Bleeding Risk after Percutaneous Coronary Intervention and Implications for the ARC-HBR Definition. EuroIntervention. 2021;17:e898–909. doi: 10.4244/EIJ-D-21-00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–61. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- Ferlini M, Mauri S, Rossini R. Dual antiplatelet therapy after TAVR: a drop in the bucket? Int J Cardiol. 2019;280:46–8. doi: 10.1016/j.ijcard.2019.01.069. [DOI] [PubMed] [Google Scholar]
- Ullah W, Zahid S, Hamzeh I, Birnbaum Y, Virani SS, Alam M. Trends and Predictors of Transcatheter Aortic Valve Implantation Related In-Hospital Mortality (From the National Inpatient Sample Database). Am J Cardiol. 2021;143:97–103. doi: 10.1016/j.amjcard.2020.12.031. [DOI] [PubMed] [Google Scholar]
- Navarese EP, Zhang Z, Kubica J, Andreotti F, Farinaccio A, Bartorelli AL, Bedogni F, Rupji M, Tomai F, Giordano A, Reimers B, Spaccarotella C, Wilczek K, Stepinska J, Witkowski A, Grygier M, Kukulski T, Wanha W, Wojakowski W, Lesiak M, Dudek D, Zembala MO, Berti S a Joint Effort of the Italian and Polish Cardiac Interventional Societies. Development and Validation of a Practical Model to Identify Patients at Risk of Bleeding After TAVR. JACC Cardiovasc Interv. 2021;14:1196–206. doi: 10.1016/j.jcin.2021.03.024. [DOI] [PubMed] [Google Scholar]
- Zahid S, Ullah W, Khan MU, Abbas S, Ud Din, Uddin MF, Inayat A, Ubaid A, Salman F, Thakkar S, Salama A, Khan MZ. Trends, predictors, and outcomes of major bleeding after transcatheter aortic valve implantation, from national inpatient sample (2011-2018). Expert Rev Cardiovasc Ther. 2021;19:557–63. doi: 10.1080/14779072.2021.1924678. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang T, Lan R, Dai Q, Kang L, Wang L, Wang Y, Xu W, Xu B. Meta-Analysis Comparing Results of Transcatheter Versus Surgical Aortic-Valve Replacement in Patients With Severe Aortic Stenosis. Am J Cardiol. 2020;125:449–58. doi: 10.1016/j.amjcard.2019.10.057. [DOI] [PubMed] [Google Scholar]
- Kolte D, Vlahakes GJ, Palacios IF, Sakhuja R, Passeri JJ, Inglessis I, Elmariah S. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J Am Coll Cardiol. 2019;74:1532–40. doi: 10.1016/j.jacc.2019.06.076. [DOI] [PubMed] [Google Scholar]
- Dangas GD, Tijssen JGP, Wohrle J, Sondergaard L, Gilard M, Mollmann H, Makkar RR, Herrmann HC, Giustino G, Baldus S, De Backer, Guimaraes AHC, Gullestad L, Kini A, von Lewinski, Mack M, Moreno R, Schafer U, Seeger J, Tchetche D, Thomitzek K, Valgimigli M, Vranckx P, Welsh RC, Wildgoose P, Volkl AA, Zazula A, van Amsterdam, Mehran R, Windecker S, GALILEO Investigators. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. 2020;382:120–9. doi: 10.1056/NEJMoa1911425. [DOI] [PubMed] [Google Scholar]
- Généreux P, Head SJ, Van Mieghem, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317–26. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Mangieri A, Montalto C, Poletti E, Sticchi A, Crimi G, Giannini F, Latib A, Capodanno D, Colombo A. Thrombotic Versus Bleeding Risk After Transcatheter Aortic Valve Replacement: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2088–101. doi: 10.1016/j.jacc.2019.08.1032. [DOI] [PubMed] [Google Scholar]
- Bor WL, Chan Pin, Brouwer J, Nijenhuis VJ, Peper J, Timmers L, Rensing BJWM, Swaans MJ, Ten Berg. Assessment of the Academic Research Consortium for High Bleeding Risk Criteria in Patients Undergoing TAVR. JACC Cardiovasc Interv. 2021;14:1265–7. doi: 10.1016/j.jcin.2021.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of definitions between the ARC-HBR criteria and the present study.