Abstract
Background
Coronary microvascular dysfunction (CMD) is an important contributor to angina syndromes. Recently, two distinct endotypes were identified using combined assessment of coronary flow reserve (CFR) and minimal microvascular resistance (MR), termed structural and functional CMD.
Aims
We aimed to assess the relevance of the combined assessment of CFR and MR in patients with angina and no obstructive coronary arteries.
Methods
Patients with chronic coronary syndromes (CCS) and non-obstructive coronary artery disease (fractional flow reserve [FFR] ≥0.80) were selected (N=1,102). Functional CMD was defined as abnormal CFR in combination with normal MR and structural CMD as abnormal CFR with abnormal MR. Clinical endpoints were the incidence of major adverse cardiac events (MACE) and target vessel failure (TVF) at 5-year follow-up.
Results
Abnormal CFR was associated with an increased risk of MACE and TVF at 5-year follow-up. Microvascular resistance parameters were not associated with MACE or TVF at 5-year follow-up. The risk of MACE and TVF at 5-year follow-up was similarly increased for patients with structural or functional CMD compared with patients with normal microvascular function. There were no differences between both endotypes (p=0.88 for MACE, and p=0.55 for TVF).
Conclusions
Coronary microvascular dysfunction, identified by an impaired CFR, was unequivocally associated with increased MACE and TVF rates over a 5-year follow-up period. In contrast, impaired MR was not associated with 5-year adverse clinical events. Moreover, there was no significant difference in the risk of MACE and TVF between a low CFR accompanied by pathologically increased MR (structural CMD) or not (functional CMD). ClinicalTrials.gov: NCT04485234
Introduction
Coronary microvascular dysfunction (CMD) is increasingly recognised as an important contributor or even a sole cause of angina syndromes, and portends an increased risk for major adverse cardiac events1,2,3. Among patients with angina and no obstructive coronary artery disease (ANOCA) with documented CMD, two distinct endotypes have been previously identified using additional assessment of minimal microvascular resistance (MR), termed structural and functional CMD4,5. Structural CMD describes the more traditional CMD endotype and is characterised by a reduced coronary flow reserve (CFR) in the presence of an increase in minimal MR. This endotype is considered to represent architectural changes in the microvasculature such as capillary rarefaction and arteriolar obliteration, which impair microcirculation under maximally vasodilated conditions6. Functional CMD describes an endotype characterised by a reduced CFR in the presence of normal or decreased minimal MR. This is considered to be related to an increased demand for myocardial oxygen or disordered coronary autoregulation, both of which lead to increased coronary flow in resting conditions despite unaltered myocardial workload and normal microcirculation during maximally vasodilated conditions. Notwithstanding the distinctly different pathophysiology, both endotypes have previously been related to a similar prevalence of inducible myocardial ischaemia on cardiac magnetic resonance imaging, as well as to similarly reduced coronary perfusion efficiency during physical exercise5,7. The prognostic relevance of functional versus structural CMD in ANOCA patients has not been evaluated. We therefore aimed to evaluate the prognostic implications of structural versus functional CMD in patients with ANOCA.
Methods
Study population
The ILIAS (Inclusive Invasive Physiological Assessment in Angina Syndromes) registry is a global, multicentre initiative retrospectively pooling lesion-level coronary pressure and flow data, as well as vessel-level clinical outcome data. The registry is composed of 20 coronary physiology expert medical institutes from the Netherlands, Republic of Korea, Japan, Spain, Denmark, Italy and the United States of America. All data were gathered in local study protocols and ethical approval was obtained from local independent ethical commissions. Patients who underwent clinically indicated invasive coronary angiography and a comprehensive invasive physiological assessment of at least one native coronary artery were enrolled in the registry. Patients with haemodynamic instability, significant valvular pathology and prior coronary artery bypass graft surgery, as well as patients with acute coronary syndromes were excluded. Individual patient data for pooled analysis were collected using standardised spreadsheets and a fully compliant cloud-based clinical data platform (Castor EDC). Standardised definitions were used for all variables. The ILIAS Registry was registered at Clinicaltrials.gov: NCT04485234.
For this analysis, only those patients with ANOCA were selected. ANOCA was defined according to current consensus as the presence of stable anginal symptoms, a clinical indication for invasive coronary angiography, and no haemodynamically significant epicardial coronary artery disease, defined as fractional flow reserve (FFR)>0.80.
Coronary angiography and physiological assessment
Coronary angiography and intracoronary testing were performed in all institutions using similar, standard techniques. After diagnostic coronary angiography, invasive physiological indices were measured using either separate pressure- (PressureWire; Abbott) and Doppler velocity sensor-equipped coronary guidewires (FloWire; Philips-Volcano), dual pressure- and Doppler flow velocity-equipped guidewire (ComboWire; Philips-Volcano), or a temperature-sensitive pressure sensor-equipped guidewire (PressureWire; Abbott) using routine techniques. Intracoronary nitrate (100 or 200 μg) was administered before physiologic measurements. Using the Doppler velocity technique, baseline (bAPV) and hyperaemic (hAPV) average peak flow velocities were labelled as baseline and hyperaemic flow, respectively. Using the coronary thermodilution technique, resting and hyperaemic thermodilution curves were obtained in triplicate using three injections (4 mL each) of room‐temperature saline, and the inverse of the average basal (bTmn) and hyperaemic (hTmn) mean transit times were labelled as baseline and hyperaemic flow, respectively. Hyperaemia was induced by intravenous infusion of adenosine (140 μg/kg per min) or adenosine triphosphate (ATP; 150 μg/kg per min) through a peripheral or central vein, an intracoronary bolus injection of adenosine (20-200 mcg), or an intracoronary bolus injection of nicorandil (3 mg), according to local standards.
Follow-up and clinical assessment
Clinical follow-up was obtained at outpatient clinic visits or by telephone contact to ascertain the occurrence of major adverse cardiac events (MACE) and target vessel failure (TVF). MACE was defined as the composite of all-cause death, acute myocardial infarction and clinically driven (urgent) revascularisation by means of coronary artery bypass or percutaneous coronary intervention (PCI). TVF was defined as the composite of cardiac death, acute myocardial infarction not clearly attributable to a non-target vessel, and clinically driven (urgent) revascularisation of the target vessel by means of coronary artery bypass graft surgery or PCI. All patient-reported events were verified by evaluating hospital records or contacting the treating cardiologist or general practitioner.
Data analysis
CFR was calculated as the ratio of hyperaemic to basal coronary flow, and CFR <2.5 was considered abnormal. Minimal MR was calculated as the ratio of hyperaemic distal coronary pressure to hyperaemic distal coronary flow and expressed by the hyperaemic microvascular resistance index (HMR) for Doppler flow velocity measurements, and the index of microvascular resistance (IMR) for coronary thermodilution measurements. An HMR value of ≥2.5 and an IMR value of ≥25 were considered abnormal8.
Statistical analysis
Data were analysed on a per-patient basis for clinical characteristics and on a per-vessel basis for all other calculations. Normality and homogeneity of the variances were tested using Shapiro-Wilk and Levene tests. Continuous variables are presented as mean±SD or median (first, third quartile [Q1, Q3]) and were compared with the Student’s t-test or the Mann-Whitney U test. Categorical variables are presented as counts and percentages and were compared using the Fisher's exact test. For vessel-to-patient analyses, robust regressions with Huber-White robust standard errors were used to adjust for clustering of vessels within patients, where appropriate. The association of continuous CFR, HMR, and IMR values, as well as the presence of abnormal MR with 5-year MACE and TVF was evaluated using (marginal) Cox proportional hazards models, adjusted for the effect of relevant clinical and angiographic characteristics (p<0.1 for inclusion). All clinical and angiographic characteristics (Table 1) were considered as covariates. Event rates over time across groups defined by normal/abnormal CFR and normal/abnormal MR were visualised using the unadjusted Kaplan-Meier method. The statistical significance of differences in event rates between groups was assessed with unadjusted (marginal) Cox proportional hazards models and is presented using hazard ratios (HR) with 95% confidence intervals (95% CI). For the MACE endpoint, Cox proportional hazard analysis was adjusted for the clustering of vessels within patients. All Cox proportional hazards models were preceded by verification of the proportional hazard assumption using Schoenfeld’s residuals. A p-value <0.05 (2-sided) was considered statistically significant. The Stata version 14.0 (StataCorp) software package was used for calculations.
Results
Patient population
A total of 2,322 patients underwent invasive physiological assessment in a total of 3,046 vessels. Among these, 320 patients had a non-stable indication for coronary angiography and obstructive coronary artery disease was present in 855 vessels. These were excluded for the current analysis. From this population, follow-up data were missing for 41 patients. The final study population consisted of 1,102 patients with 1,562 vessels who underwent a coronary angiography procedure between 1998 and 2018. The key baseline characteristics of the final study population are shown in Table 1. The mean age was 63±10.3 years and 69% of the patients were men.
Table 1. Baseline characteristics of the total study population and across the different groups defined by normal/abnormal CFR and MR.
| Total | CFR ≥2.5 | CFR <2.5 | p-value across CFR groups | |||||
|---|---|---|---|---|---|---|---|---|
| MR low | MR high | p-value | MR low | MR high | p-value | |||
| Patients | N=1,102 | N=420 | N=112 | N=344 | N=226 | |||
| Demographics | ||||||||
| Age, yrs | 63 (62-64) | 61 (60-62) | 62 (60-64) | 0.336 | 64 (63-65) | 66 (64-67) | 0.322 | <0.005 |
| Male sex, % | 69 (62-73) | 69 (65-74) | 72 (62-79) | 0.650 | 64 (59-69) | 72 (65-77) | 0.427 | 0.105 |
| BMI, kg/m2 | 26 (25–26) | 25 (25–25) | 26 (26–27) | 0.007 | 26 (25–26) | 26 (25–26) | 0.523 | 0.263 |
| LVEF, % | 61 (59–62) | 62 (61–63) | 62 (60–63) | 0.966 | 60 (59–61) | 60 (60–62) | 0.976 | 0.005 |
| Coronary risk factors | ||||||||
| Hypertension, % | 58 (52-63) | 52 (47-57) | 62 (52-71) | 0.147 | 58 (53-63) | 66 (59-72) | 0.044 | 0.008 |
| Diabetes, % | 24 (19-29) | 22 (18-26) | 22 (15-32) | 0.712 | 26 (21-31) | 24 (19-30) | 0.645 | 0.158 |
| Hyperlipidaemia, % | 62 (57-68) | 60 (55-65) | 56 (46-65) | 0.463 | 65 (59-69) | 65 (59-71) | 0.735 | 0.171 |
| Positive family history, % | 37 (32-47) | 35 (31-40) | 35 (26-46) | 0.575 | 37 (32-43) | 41 (34-48) | 0.425 | 0.211 |
| Current smoking, % | 22 (17-27) | 22 (18-27) | 24 (16-34) | 0.902 | 21 (17-26) | 22 (17-28) | 0.620 | 0.577 |
| Prior myocardial infarction, % | 14 (11-19) | 12 (9-16) | 14 (8-22) | 0.643 | 16 (13-21) | 17 (13-23) | 0.749 | 0.020 |
| Prior coronary intervention, % | 21 (17-27) | 20 (16-25) | 20 (13-29) | 0.702 | 24 (20-29) | 18 (13-24) | 0.390 | 0.560 |
| Medication | ||||||||
| Aspirin, % | 78 (73-83) | 80 (76-84) | 78 (68-85) | 0.231 | 77 (72-82) | 78 (71-83) | 0.986 | 0.597 |
| ACE inhibitor, % | 38 (33-44) | 35 (30-39) | 46 (36-56) | 0.124 | 38 (33-44) | 40 (34-47) | 0.533 | 0.287 |
| Beta blocker, % | 46 (40-52) | 43 (39-48) | 41 (32-52) | 0.455 | 47 (41-53) | 52 (45-59) | 0.541 | 0.058 |
| Calcium antagonist, % | 39 (33-45) | 36 (32-41) | 41 (32-52) | 0.388 | 38 (32-43) | 44 (37-51) | 0.171 | 0.345 |
| Nitrates, % | 36 (30-42) | 36 (32-42) | 31 (22-41) | 0.501 | 35 (30-41) | 39 (32-46) | 0.453 | 0.648 |
| Data presented as n (%) or mean±standard deviation. BMI: body mass index; CFR: coronary flow reserve; LVEF: left ventricular ejection fraction; MR: minimal micorvascular resistance | ||||||||
Clinical and physiological characteristics across CFR and MR groups
Patients were classified into 4 groups based on CFR (cut-off value <2.5) and the presence of high MR (cut-off value ≥25 for IMR and ≥2.5 mmHg/cm/s for HMR). A total of 793 vessels (50.7%) had normal CFR, of which 618 (77.9%) had normal MR and 175 (22.1%) had abnormal MR. Of the 769 vessels (49.3%) with abnormal CFR, 487 (63.3%) had normal MR consistent with functional microvascular dysfunction, and 282 (36.7%) had abnormal MR consistent with structural microvascular dysfunction.
Patients with abnormal CFR were older, more likely to have hypertension, and had higher rates of previous myocardial infarction. In the normal CFR group, there were no differences in clinical characteristics between the high and low MR subgroup. In the abnormal CFR group, patients with high MR were more likely to have hypertension than patients with low MR (58.6%; 95% CI: 53-63% vs 66.8%; 95% CI: 59-72%; p=0.044).
Angiographic and physiological characteristics are summarised in Table 2. In most cases, the LAD (n=779; 49%) was the interrogated vessel. A clinically irrelevant but statistically significant difference in FFR was present between normal and abnormal MR groups, both in patients with (0.89±0.05 vs 0.90±0.05; p<0.005) as well as in patients without CMD (0.90±0.06 vs 0.92±0.06; p<0.005). The same finding applied to CFR, where a clinically irrelevant but statistically significant difference was documented between normal and abnormal MR groups both in patients with (1.96±0.40 vs 1.87±0.42; p<0.005) as well as in patients without CMD (3.65±0.98 vs 3.32±0.73; p<0.005).
Table 2. Coronary angiography characteristics of the total study population and across the different groups defined by abnormal and normal CFR and MR.
| Total | CFR ≥2.5 | CFR <2.5 | p-value across CFR groups | |||||
|---|---|---|---|---|---|---|---|---|
| MR low | MR high | p-value | MR low | MR high | p-value | |||
| Vessels | N=1,562 | N=618 | N=175 | N=487 | N=282 | |||
| Interrogated vessel | ||||||||
| LAD, (%) | 779 (49) | 332 (53) | 72 (39) | 0.019 | 234 (46) | 141 (46) | 0.910 | 0.123 |
| RCX, (%) | 388 (25) | 129 (21) | 37 (24) | 0.932 | 151 (33) | 71 (25) | 0.136 | 0.090 |
| RCA, (%) | 386 (25) | 155 (26) | 65 (37) | 0.010 | 99 (20) | 67 (28) | 0.126 | 0.084 |
| Unknown, (%) | 9 (1) | 2 (0) | 1 (0) | n/a | 3 (1) | 3 (1) | n/a | |
| Quantitative analysis | ||||||||
| Diameter stenosis, % | 45.5±17.0 | 42.9±16.6 | 42.9±17.1 | 0.983 | 46.7±17.0 | 51.0±16.6 | 0.001 | <0.005 |
| Haemodynamic | ||||||||
| bHR | 68.9±11.6 | 67.8±10.9 | 68.5±11.3 | 0.650 | 70.4±12.1 | 69.9±12.4 | 0.756 | 0.019 |
| bPd | 94.9±15.2 | 94.4±14.2 | 99.0±14.6 | <0.005 | 93.4±15.5 | 96.0±16.5 | 0.019 | 0.184 |
| hPd | 81.5±14.9 | 80.5±13.9 | 86.6±12.5 | <0.005 | 79.3±15.5 | 84.1±15.9 | <0.005 | 0.3311 |
| bPa | 98.3±15.0 | 97.6±14.1 | 101.2±13.9 | <0.005 | 97.3±15.5 | 99.7±16.2 | 0.027 | 0.818 |
| hPa | 90.1±15.5 | 89.0±14.9 | 94.4±12.2 | <0.005 | 88.3±16.2 | 93.0±16.5 | <0.005 | 0.889 |
| bAPV | 17.2±7.4 | 15.7±4.9 | 9.86±2.1 | <0.005 | 23.4±8.1 | 14.1±3.7 | <0.005 | <0.005 |
| hAPV | 41.1±15.8 | 48.6±13.8 | 29.6±5.7 | <0.005 | 47.2±15.7 | 27.4±7.4 | <0.005 | <0.005 |
| bTmn | 0.77±0.44 | 0.80±0.29 | 1.43±0.47 | <0.005 | 0.41±0.23 | 0.88±0.39 | <0.005 | <0.005 |
| hTmn | 0.28±0.18 | 0.21±0.06 | 0.41±0.13 | <0.005 | 0.22±0.13 | 0.54±0.26 | <0.005 | <0.005 |
| Physiological metrics | ||||||||
| FFR | 0.90±0.06 | 0.90±0.06 | 0.92±0.06 | <0.005 | 0.89±0.05 | 0.90±0.05 | <0.005 | <0.005 |
| CFR | 2.76±1.10 | 3.65±0.98 | 3.32±0.73 | <0.005 | 1.96±0.40 | 1.87±0.42 | <0.005 | <0.005 |
| HMR | 2.29±0.86 | 1.79±0.38 | 3.00±0.56 | <0.005 | 1.84±0.42 | 3.31±0.79 | <0.005 | <0.005 |
| IMR | 21.8±14.0 | 15.8±4.52 | 34.9±9.68 | <0.005 | 15.76±4.67 | 42.7±21.1 | <0.005 | <0.005 |
| Data presented as n (%) or mean±standard deviation. APV: average peak velocity; b: baseline; CFR: coronary flow reserve; FFR: fractional flow reserve; h: hyperaemic; HMR: hyperaemic microvascular resistance; HR: heart rate; IMR: index of microvascular resistance; LAD: left anterior descending artery; MR: minimal resistance; RCA: right coronary artery; RCX: ramus circumflexus coronary artery; Pa: aortic pressure; Pd: distal pressure; Tmn: mean transit time | ||||||||
Clinical outcomes determined by CFR and MR
The median follow-up duration was 4.92 years (Q1, Q3: 1.99, 5.07). A total of 96 patients (9.1%) experienced at least one MACE, and a total of 94 vessels (6.0%) experienced at least one TVF during follow-up<strong (Table 3). Of the clinical and angiographic characteristics, age, gender, diabetes, familial predisposition for coronary artery disease, previous myocardial infarction, nitrate use and reduced left ventricular function were associated with 5-year MACE and TVF. After correction for these confounders, CFR as a continuous variable was independently associated with MACE (HR 0.67, 95% CI: 0.59-0.75; p<0.005) and TVF (HR 0.70, 95% CI: 0.59-0.83; p<0.005) at 5-year follow-up. Also, when restricting the analysis to patients with normal LV function, CFR as a continuous variable was independently associated with 5-year MACE (HR 0.66, 95% CI: 0.53-0.83; p<0.005) and TVF (HR 0.68, 95% CI: 0.51-0.91; p=0.009). In contrast, MR parameters were not associated with MACE or TVF at 5-year follow-up, whether HMR, IMR, or IMR corrected for wedge pressure was assessed (Supplementary Table 1-Supplementary Table 3).
Figure 1 and Figure 2 show the unadjusted Kaplan-Meier curves for MACE and TVF according to normal versus abnormal CFR or MR, respectively. Abnormal CFR was associated with an increased risk for 5-year MACE (HR 2.05, 95% CI: 1.47-2.87; p<0.005), and 5-year TVF (HR 2.27, 95% CI: 1.47-3.49; p<0.005). Abnormal MR, in contrast, was not associated with an increased risk for 5-year MACE (HR 0.99, 95% CI: 0.69-1.42; p=0.979) or 5-year TVF (HR 1.00, 95% CI: 0.64-1.57; p=0.971). The Central illustration shows the unadjusted Kaplan-Meier estimates for MACE and TVF according to the groups defined by normal/abnormal CFR and MR. The risk for MACE (Table 4) and TVF (Table 5) at 5-year follow-up was increased for patients with abnormal CFR, regardless of the accompanying MR (MACE: HR 1.93, 95% CI: 1.37-2.72, for normal MR vs abnormal MR, HR 1.96, 95% CI: 1.33-3.04; p=0.877; TVF: HR 1.84, 95% CI: 1.23-2.75, for normal MR vs abnormal MR, HR 2.24, 95% CI: 1.39-3.52; p=0.553). Moreover, in patients with normal CFR, the risk for MACE and TVF at 5-year follow-up was low and did not differ between normal and abnormal MR (MACE: HR 0.65, 95% CI: 0.32-1.31; p=0.231 for abnormal MR compared to normal MR; TVF: HR 0.48, 95% CI: 0.19-1.22; p=0.124 for abnormal MR compared to normal MR).
Figure1. Unadjusted Kaplan-Meier time-to-event curves according to normal or abnormal coronary flow reserve (CFR).
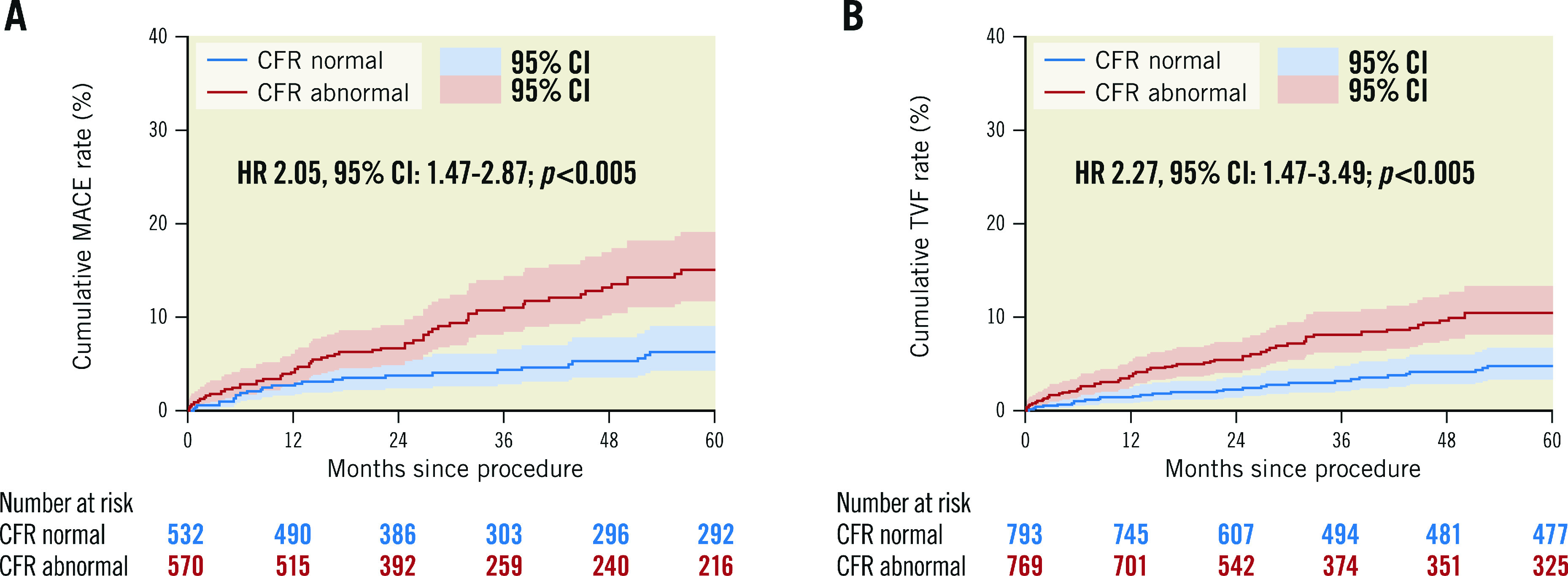
(A) MACE and (B) TVF up to 5-year follow-up. Hazard-ratio (HR) with 95% confidence intervals (CI) presented based on Cox-regression analysis corrected for confounders and adjusted for patient clustering in case of MACE. MACE: major adverse cardiac events; MR: microvascular resistance; TVF: target vessel failure
Figure 2. Unadjusted Kaplan-Meier time-to-event curves according to normal or abnormal microvascular resistance.
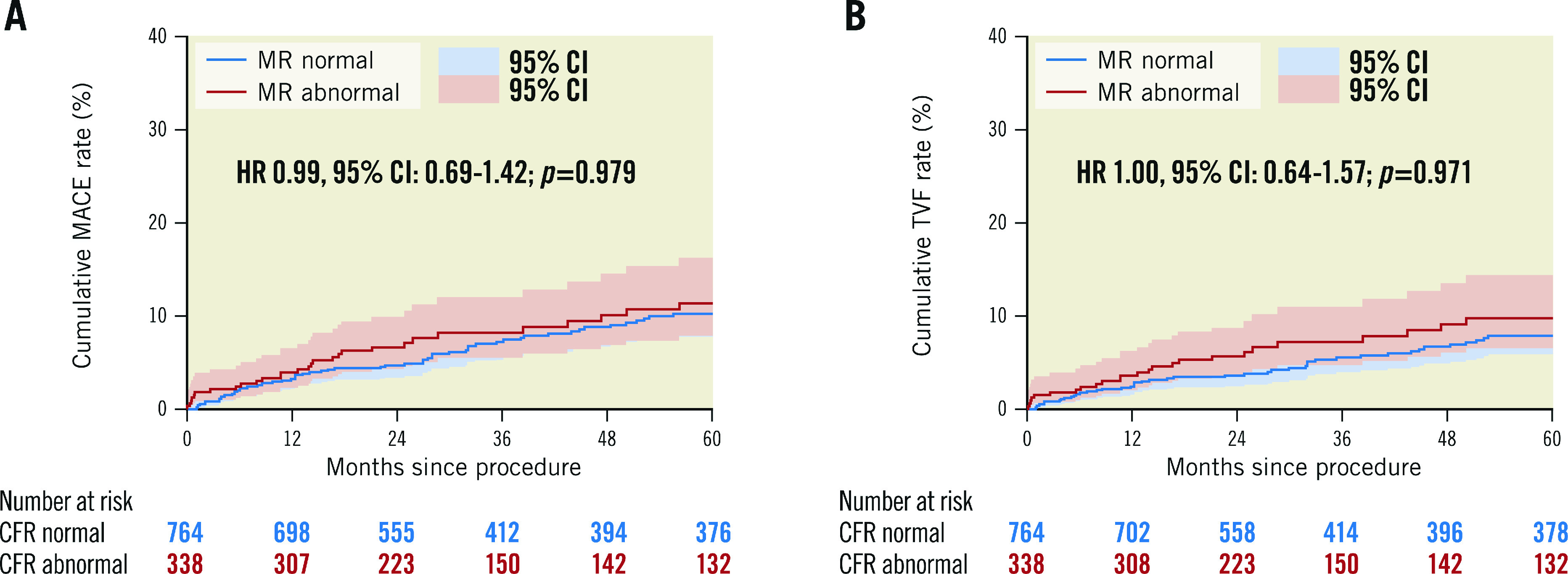
(A) MACE and (B) TVF up to 5-year follow-up. Hazard-ratio (HR) with 95% confidence intervals (CI) presented based on Cox-regression analysis corrected for confounders and adjusted for patient clustering in case of MACE. MACE: major adverse cardiac events; MR: microvascular resistance; TVF: target vessel failure
Central illustration. Unadjusted Kaplan Meier event estimates for MACE and TVF at 5-year follow-up.
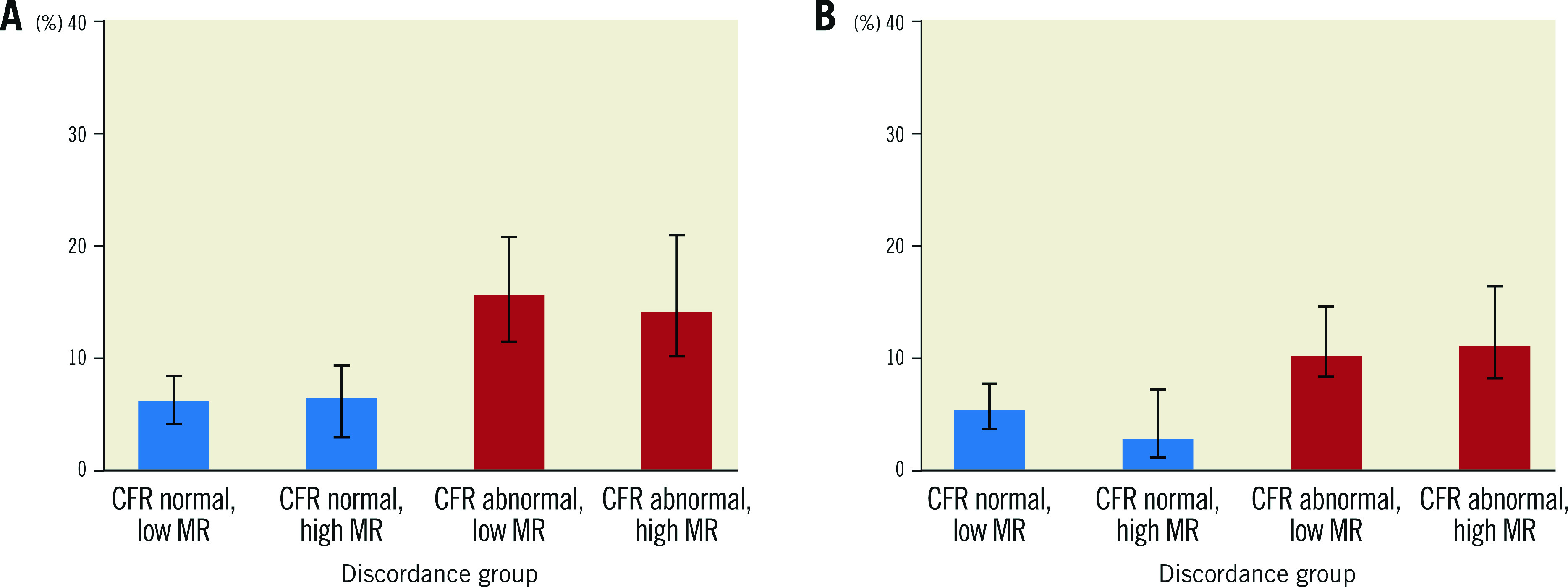
Event estimates according different coronary microvascular dysfunction endotypes according to normal or abnormal coronary flow reserve (CFR) and the presence or absence of abnormal microvascular resistance for (A) MACE and (B) TVF. MACE: major adverse cardiac events; MR: microvascular resistance; TVF: target vessel failure
Table 4. Cox-regression analysis after correction for confounders for MACE at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
| HR | Std. err. | 95% CI | p-value | |
|---|---|---|---|---|
| CFR norm/MR low | reference | – | – | – |
| CFR norm/MR high | 0.65 | 0.23 | 0.32-1.31 | 0.231 |
| CFR abn/MR low | 1.93 | 0.34 | 1.37-2.72 | <0.005 |
| CFR abn/MR high | 1.96 | 0.34 | 1.33-3.04 | <0.005 |
| Data presented as hazard ratio (HR) with standard error (std. err.), 95% confidence interval (CI) and corresponding p-value for multivariate Cox-regression analysis. | ||||
Table 5. Cox-regression analysis after correction for confounders for TVF at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
| HR | Std. err. | 95% CI | p-value | |
|---|---|---|---|---|
| CFR norm/MR low | reference | – | – | – |
| CFR norm/MR high | 0.48 | 0.23 | 0.19-1.22 | 0.124 |
| CFR abn/MR low | 1.84 | 0.37 | 1.23-2.75 | 0.003 |
| CFR abn/MR high | 2.21 | 0.53 | 1.39-3.52 | 0.001 |
| Data presented as hazard ratio (HR) with standard error (std. err.), 95% confidence interval (CI) and corresponding p-value for multivariate Cox-regression analysis. | ||||
The use of other cut-off values (CFR <2.0, IMR >40) did not alter the outcomes with respect to MACE and TVF at 5 years (data not shown). There was no interaction between the technique used to measure coronary flow or the technique by which hyperaemia was achieved and the clinical outcomes documented above.
Discussion
In this large registry of comprehensive coronary physiology measurements, we evaluated the prognostic impact of structural versus functional microvascular dysfunction in patients with ANOCA. We documented that CMD identified solely by impaired CFR (≤2.5) was unequivocally associated with increased MACE and TVF rates over a 5-year follow-up period. Moreover, we found no significant difference in the risk for MACE and TVF in patients with CMD, whether a low CFR was accompanied by pathologically increased MR or not. As such, our data document that both subtypes of CMD, whether structural or functional in origin, portend a similarly impaired prognosis during a 5-year follow-up period compared with normal microvascular function.
Pathophysiology of coronary microvascular dysfunction
Structural CMD may represent those patients with architectural changes in their coronary circulation, such as arteriolar obliteration, microvascular obstruction and/or capillary rarefaction6. In line with previous studies, our study found that this group tends to have a more traditional cardiovascular risk profile and an increased prevalence of hypertension9. A study by Rahman et al on the pathophysiology of CMD indicated two potential mechanisms for inducible ischaemia due to structural CMD7. They found that patients with structural CMD had a significantly higher prevalence of exercise-related hypertension and impaired systemic vasodilatation in response to acetylcholine, which in healthy individuals induces endothelial-dependent vasodilation. Therefore, an adequate increase in coronary flow in reaction to shear stress during exercise may be inhibited in patients with structural CMD and reduce maximal coronary blood flow (CBF), leading to higher minimal resistance in the coronary microcirculation. Theoretically, these patients benefit from targeted life-style interventions (smoking cessation, weight loss) and treatment to improve afterload-reduction and vascular remodelling, such as beta blockers, ACE inhibitors or statins9,10.
In contrast, those patients with diminished CFR and preserved MR, referred to as functional CMD, are characterised by a preserved maximal CBF, but an increased resting CBF7. In our study, patients with functional CMD indeed show significantly higher resting CBF as compared with patients with structural CMD (bAPV: 23.4±8.1 vs 14.1±3.7; p<0.005; bTmn: 0.41±0.23 vs 0.54±0.26 p<0.005). Elevated resting CBF and concomitantly reduced CFR were previously found to be prognostically important in patients with chronic coronary syndromes3. The elevated resting CBF can be explained by either impaired coronary autoregulation, or by increased myocardial oxygen demand at rest. The study by Rahman et al suggested the latter, providing evidence for higher resting myocardial oxygen demand and an ineffective metabolic resting state in patients with functional CMD in comparison with structural CMD despite equivalent myocardial work load7. In theory, targeted therapy for these patients should aim to improve basic myocardial metabolism. However, scientific evidence for this CMD subgroup is lacking and future research is needed to assess potential therapeutic options.
Diagnostic value of microvascular resistance indices
Historically, the presence of pathologically increased minimal MR due to structural changes in the microvasculature has been coined as the mechanism of microvascular dysfunction, causing myocardial ischaemia by impairing the vasodilator response upon an increase in demand. Several authors have suggested that the identification of high MR is thereby a more reliable tool to identify the presence of CMD than CFR, mainly because of concerns regarding the impact of resting state variation on the assessment of resting coronary flow11. However, in our ANOCA population, 32% of patients had a normal MR despite the fact that CFR was abnormal, and these patients were at increased risk for adverse cardiac event rates during follow-up. The risk for adverse events in these patients with abnormal CFR but normal minimal MR was as large as that in patients with impaired CFR and high MR. These findings are in line with previous data from Rahman and colleagues, who documented that diminished CFR is associated with a higher prevalence of inducible myocardial ischaemia and reduced global perfusion reserve in addition to reduced coronary perfusion efficiency during physical exercise, regardless of whether it is accompanied by normal or abnormal MR7.
On the other hand, 9% of patients in our study had abnormally high MR despite the presence of a normal CFR. These patients, who are considered to have CMD in ANOCA protocols12, were at equivalent risk for adverse events compared with patients in whom both CFR and MR were normal. Likewise, a recent publication by Toya et al reported no additional risk stratification of HMR in combination with CFR in an ANOCA population13. All of this is in line with Rahman et al, who documented benign myocardial perfusion results and physiological exercise characteristics in patients with normal CFR but elevated MR7. Despite these initial data suggesting a lack of diagnostic and prognostic impact of solitarily increased MR, it remains unknown whether such increased MR in the presence of normal vasodilator reserve still identifies a specific type of ANOCA pathophysiology that may benefit from targeted medical treatment strategies to improve the wellbeing of these patients. Moreover, it needs to be noted that the correlation of the CMD endotypes in this study and abnormal vasomotor abnormalities assessed by increasing doses of acetylcholine, remains elusive but may allow for further stratification of ANOCA CMD endotypes14. Further studies are required to specifically elucidate the origin and diagnostic impact of increased minimal MR in the presence of a normal vasodilator reserve in ANOCA patients, and to evaluate its relevance for angina symptoms and the effect of targeted medical treatment.
Prognostic value of microvascular resistance indices
In contrast with the findings in this study, previous studies found significant associations between MR indices and clinical outcomes. In the case of IMR, however, the prognostic value has mainly been derived from study populations including patients with ST-segment elevation myocardial infarction (STEMI), where IMR ≥40 was documented to provide the highest discriminatory value for the occurrence of adverse events15,16. It has been documented that, in the setting of STEMI patients, post-PCI IMR ≥40 is significantly associated with microvascular obstruction, larger infarct size, as well as adverse left ventricular remodelling17. The pathophysiological substrate for high event rates in patients with abnormal IMR values in the setting of STEMI is therefore well established. In chronic coronary syndromes, which apply to the current study population, the prognostic value of IMR for adverse events has been less well established. In such a population, the prevalence of exceedingly high IMR values of ≥40 is very low. A sensitivity analysis in the current study population showed that an IMR ≥40 was not associated with MACE or TVF at 5-year follow-up in this study population (data not shown). A previous study from Lee et al, in a mixed patient population including stable angina and acute coronary syndrome patients and using different cut-off values of 2.0 for CFR and 23 for IMR, has suggested that both CFR and IMR independently improved the risk stratification of patients with high FFR18. The discrepancy with our findings may be explained by the inclusion of unstable patients and consequently a relatively higher rate of cardiac death and myocardial infarction, that drove the differences in MACE rates in their paper. Moreover, in a study by Nishi and colleagues, elevated post-PCI IMR values (IMR >25) were associated with a combined MACE endpoint that was driven by periprocedural myocardial infarctions19. These data enforce the association between elevated post-PCI IMR values and the extent of myocardial injury, which is, however, not relevant in the setting of ANOCA and was therefore not part of the clinical endpoints in the present study.
The prognostic value of the Doppler-derived HMR is less frequently studied. Recent studies documented superior diagnostic utility of HMR over IMR for the prediction of the extent of left ventricular infarction20 and the identification of microvascular dysfunction post-STEMI8. However, similar to the findings for IMR, HMR was not associated with MACE or TVF at 5-year follow-up in this study.
Despite the lack of prognostic value of both IMR and HMR in patients with ANOCA in this study population, their incorporation into diagnostic strategies may allow the identification of specific pathophysiological substrates in angina syndromes. As indicated by Rahman et al, there is a significant difference in the physiological explanation of abnormal CFR in patients with functional (normal MR) and structural (abnormal MR) CMD. It is possible that these differences translate into different treatment targets and future studies assessing the clinical implications of these pathophysiologic characteristics are therefore needed.
Limitations
The results from the present study should be interpreted in consideration of some limitations. First, this is an observational study and patients were treated according to clinical guidelines applicable at the time of the coronary angiography, but decisions in treatment were ultimately at the discretion of the treating physician. Second, no detailed information on the medication profiles of enrolled patients was available during the follow-up period, nor specifics regarding angina burden. The impact of CMD endotypes on angina burden and the impact of medical therapy could therefore not be evaluated. Moreover, there is no information on specific clinical endpoints susceptible in the ANOCA population, such as hospitalisation for arrythmias, anginal symptoms or heart failure. The impact of different CMD endotypes on these endpoints could not be evaluated. Furthermore, it is important to note that our study population largely consists of male patients. Hereby, there might be an underrepresentation of female patients, and the results of this study should be interpreted with this consideration. Finally, despite the length of follow-up and the large number of included patients and vessels, this study is subject to the basic limitations of a retrospective registry. For example, follow-up data were missing for 41 patients. Therefore, these conclusions should be confirmed in a prospective study.
Conclusions
Coronary microvascular dysfunction, identified by an impaired CFR, was unequivocally associated with increased MACE and TVF rates over a 5-year follow-up period. In contrast, MR was not associated with 5-year adverse clinical events. Moreover, in patients with CMD there was no difference in the risk for MACE and TVF between a low CFR accompanied by pathologically increased MR (structural CMD) or not (functional CMD).
Impact on daily practice
This study shows that CMD, identified only by an impaired CFR, was associated with increased rates of MACE and TVF over a 5-year follow-up. There was no difference in the risk for MACE and TVF whether a low CFR was accompanied by pathologically increased MR (structural CMD) or not (functional CMD).
Supplementary data
Cox-regression analysis for dichotomous and continuous physiological indices after correction for confounders for MACE at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
Cox-regression analysis for dichotomous and continuous physiological indices after correction for confounders for TVF at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
Cox-regression analysis for dichotomous and continuous physiological indices after correction for confounders for individual components of MACE at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
Table 3. Five-year absolute MACE and TVF rates across groups defined by abnormal/normal CFR and MR.
| Total | CFR ≥2.5 | CFR <2.5 | |||||
|---|---|---|---|---|---|---|---|
| MR low | MR high | Total | MR low | MR high | Total | ||
| MACE at 5 years, n (%) | 96 (9.1) | 23 (5.8) | 6 (6.3) | 29 (5.5) | 42 (12.6) | 25 (11.2) | 67 (11.7) |
| All-cause death | 37 (3.5) | 10 (2.5) | 1 (1.1) | 11 (2.1) | 16 (4.8) | 10 (4.5) | 26 (4.6) |
| Acute myocardial infarction | 13 (1.2) | 4 (1.0) | 1 (1.1) | 5 (1.0) | 2 (0.6) | 6 (2.7) | 8 (1.4) |
| Urgent revascularisation | 90 (4.3) | 25 (6.3) | 7 (7.4) | 32 (6.0) | 35 (10.5) | 23 (10.3) | 58 (10.2) |
| TVF at 5 years, n (%) | 94 (6.0) | 27 (4.3) | 4 (2.5) | 31 (3.9) | 40 (8.3) | 23 (7.6) | 63 (8.2) |
| Cardiac death | 32 (2.0) | 9 (1.4) | 0 (0) | 9 (1.1) | 14 (2.9) | 9 (3.0) | 23 (2.9) |
| Acute myocardial infarction (target vessel) | 18 (1.2) | 5 (0.8) | 1 (0.6) | 6 (0.7) | 5 (1.0) | 7 (2.3) | 12 (1.6) |
|
Urgent revascularisation (target vessel) |
81 (5.2) | 25 (4.1) | 4 (2.5) | 29 (3.7) | 31 (6.5) | 21 (7.1) | 52 (6.8) |
| Data presented as median [25th- 75th IQ] or n (%). CFR: coronary flow reserve; MACE: major adverse cardiac events; and MR: microvascular resistance; TVF: target vessel failure | |||||||
Acknowledgments
Conflict of interest statement
T. van de Hoef has received consulting fees, speaker fees and institutional research grants from Abbott and Philips. J.M. Lee received research grants from Abbott and Philips. M. Echavarria-Pinto has received speaker fees from Abbott and Philips. B-K. Koo has received institutional research grants from Abbott Vascular and Philips/Volcano. J.J. Piek has received support as a consultant for Philips/Volcano, and has received institutional research grants from Philips. H. Matsuo received lecture fees from Philips, Abbott Medical, Boston Scientific Japan and Zeon Medical. J. Escaned received speaker fees and was advisory board member for Abbott and Philips. The other authors have no relationship with industry related to this work.
Abbreviations
- ANOCA
angina and no obstructive coronary artery disease
- CFR
coronary flow reserve
- CMD
coronary microvascular dysfunction
- FFR
fractional flow reserve
- HMR
hyperaemic microvascular resistance
- ICA
invasive coronary angiography
- ICFT
intracoronary function testing
- IMR
index of microvascular resistance
- MACE
major adverse cardiac events
- MR
microvascular resistance
- TVF
target vessel failure
Contributor Information
Coen K.M. Boerhout, Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam, the Netherlands.
Guus A. de Waard, Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, the Netherlands.
Joo Myung Lee, Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Hernán Mejía-Rentería, Hospital Clínico San Carlos, IDISSC, and Universidad Complutense de Madrid, Madrid, Spain.
Seung Hun Lee, Division of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Republic of Korea.
Ji-Hyun Jung, Sejong General Hospital, Sejong Heart Institute, Bucheon, Republic of Korea.
Masahiro Hoshino, Gifu Heart Center, Department of Cardiovascular Medicine, Gifu, Japan.
Mauro Echavarria-Pinto, Hospital General ISSSTE Querétaro - Facultad de Medicina, Universidad Autónoma de Querétaro, Querétaro, Mexico.
Martijn Meuwissen, Department of Cardiology, Amphia Hospital, Breda, the Netherlands.
Hitoshi Matsuo, Gifu Heart Center, Department of Cardiovascular Medicine, Gifu, Japan.
Maribel Madera-Cambero, Tergooi Hospital, Department of Cardiology, Blaricum, the Netherlands.
Ashkan Eftekhari, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Mohamed A. Effat, Division of Cardiovascular Health and Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Tadashi Murai, Tsuchiura Kyodo General Hospital, Department of Cardiology, Tsuchiura City, Japan.
Koen Marques, Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, the Netherlands.
Yolande Appelman, Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, the Netherlands.
Joon-Hyung Doh, Department of Medicine, Keimyung University Dongsan Medical Center, Daegu, Republic of Korea.
Evald Høj Christiansen, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Rupak Banerjee, Mechanical and Materials Engineering Department, University of Cincinnati, Cincinnati, OH, USA; and Research Services, Veteran Affairs Medical Center, Cincinnati, OH, USA.
Chang-Wook Nam, Department of Medicine, Inje University Ilsan Paik Hospital, Goyang, Republic of Korea.
Giampaolo Niccoli, Department of Cardiovascular Medicine, Catholic University of the Sacred Heart, Institute of Cardiology, Rome, Italy.
Masafumi Nakayama, Gifu Heart Center, Department of Cardiovascular Medicine, Gifu, Japan; Cardiovascular Center, Toda Central General Hospital, Toda, Japan.
Nobuhiro Tanaka, Department of Cardiology, Tokyo Medical University, Hachioji Medical Center, Tokyo, Japan.
Eun-Seok Shin, Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea.
Marcel A.M. Beijk, Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam, the Netherlands.
Paul Knaapen, Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, the Netherlands.
Javier Escaned, Hospital Clínico San Carlos, IDISSC, and Universidad Complutense de Madrid, Madrid, Spain.
Tsunekazu Kakuta, Tsuchiura Kyodo General Hospital, Department of Cardiology, Tsuchiura City, Japan.
Bon-Kwon Koo, Department of Internal Medicine, Seoul National University Hospital, Cardiovascular Center, Seoul, Republic of Korea.
Jan J. Piek, Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam, the Netherlands.
Tim P. van de Hoef, Department of Cardiology, Amsterdam UMC – location AMC, Amsterdam, the Netherlands; Department of Cardiology, Amsterdam UMC – location VUmc, Amsterdam, the Netherlands; Department of Cardiology, NoordWest Ziekenhuisgroep, the Netherlands.
References
- Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de, Bax M, Damman P, Delewi R, Hassell ME, Piek MA, Chamuleau SA, Voskuil M, van Eck-Smit, Verberne HJ, Henriques JP, Koch KT, de Winter, Tijssen JG, Piek JJ, Meuwissen M. Impaired Coronary Autoregulation Is Associated With Long-term Fatal Events in Patients With Stable Coronary Artery Disease. Circ Cardiovasc Interv. 2013;6:329–35. doi: 10.1161/CIRCINTERVENTIONS.113.000378. [DOI] [PubMed] [Google Scholar]
- Ford TJ, Corcoran D, Oldroyd KG, McEntegart M, Rocchiccioli P, Watkins S, Brooksbank K, Padmanabhan S, Sattar N, Briggs A, McConnachie A, Touyz R, Berry C. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Angina (CorMicA) stratified medicine clinical trial. Am Heart J. 2018;201:86–94. doi: 10.1016/j.ahj.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, Chiribiri A, Perera D. Coronary Microvascular Dysfunction Is Associated With Myocardial Ischemia and Abnormal Coronary Perfusion During Exercise. Circulation. 2019;140:1805–16. doi: 10.1161/CIRCULATIONAHA.119.041595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía-Rentería H, van der, van de, Heemelaar J, Ryan N, Lerman A, van Royen, Escaned J. Targeting the dominant mechanism of coronary microvascular dysfunction with intracoronary physiology tests. Int J Cardiovasc Imaging. 2017;33:1041–59. doi: 10.1007/s10554-017-1136-9. [DOI] [PubMed] [Google Scholar]
- Rahman H, Demir OM, Khan F, Ryan M, Ellis H, Mills MT, Chiribiri A, Webb A, Perera D. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol. 2020;75(20):2538–49. doi: 10.1016/j.jacc.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RP, de Waard, De Silva, Lumley M, Asrress K, Arri S, Ellis H, Mir A, Clapp B, Chiribiri A, Plein S, Teunissen PF, Hollander MR, Marber M, Redwood S, van Royen, Perera D. Doppler Versus Thermodilution-Derived Coronary Microvascular Resistance to Predict Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction or Stable Angina Pectoris. Am J Cardiol. 2018;121:1–8. doi: 10.1016/j.amjcard.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozàkovà M, La Canna, Nardi M, Guelfi D, Salvetti M, Morizzo C, Vittone F, Rosei EA. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J Hypertens. 2003;21:625–31. doi: 10.1097/00004872-200303000-00030. [DOI] [PubMed] [Google Scholar]
- Lim TK, Choy AJ, Khan F, Belch JJ, Struthers AD, Lang CC. Therapeutic development in cardiac syndrome X: a need to target the underlying pathophysiology. Cardiovasc Ther. 2009;27:49–58. doi: 10.1111/j.1755-5922.2008.00070.x. [DOI] [PubMed] [Google Scholar]
- Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–61. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, Prescott E, Karam N, Appelman Y, Fraccaro C, Buchanan GL, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049–69. doi: 10.4244/EIJY20M07_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya T, Ahmad A, Corban MT, Ӧzcan I, Sara JD, Sebaali F, Escaned J, Lerman LO, Lerman A. Risk Stratification of Patients With NonObstructive Coronary Artery Disease Using Resistive Reserve Ratio. J Am Heart Assoc. 2021;10:e020464. doi: 10.1161/JAHA.120.020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwaidi J, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–41. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick D, Haig C, Ahmed N, Carberry J, Yue May, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Hood S, Watkins S, Davie A, Mahrous A, Mordi I, Ford I, Radjenovic A, Oldroyd KG, Berry C. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment–Elevation Myocardial Infarction. Circulation. 2016;134:1833–47. doi: 10.1161/CIRCULATIONAHA.116.022603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010;3:715–22. doi: 10.1016/j.jcin.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol. 2016;67:1158–69. doi: 10.1016/j.jacc.2015.12.053. [DOI] [PubMed] [Google Scholar]
- Nishi T, Murai T, Ciccarelli G, Shah S, Kobayashi Y, Derimay F, Waseda K, Moonen A, Hoshino M, Hirohata A, Yong ASC, Ng MKC, Amano T, Barbato E, Kakuta T, Fearon WF. Prognostic Value of Coronary Microvascular Function Measured Immediately After Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study. Circ Cardiovasc Interv. 2019;12:e007889. doi: 10.1161/CIRCINTERVENTIONS.119.007889. [DOI] [PubMed] [Google Scholar]
- Patel N, Petraco R, Dall’Armellina E, Kassimis G, De Maria, Dawkins S, Lee R, Prendergast BD, Choudhury RP, Forfar JC, Channon KM, Davies J, Banning AP, Kharbanda RK. Zero-Flow Pressure Measured Immediately After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Provides The Best Invasive Index for Predicting the Extent of Myocardial Infarction at 6 Months: an OxAMI study (Oxford Acute Myocardial Infarction). JACC Cardiovasc Interv. 2015;8:1410–21. doi: 10.1016/j.jcin.2015.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cox-regression analysis for dichotomous and continuous physiological indices after correction for confounders for MACE at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
Cox-regression analysis for dichotomous and continuous physiological indices after correction for confounders for TVF at 5-year follow-up across the different groups by normal/abnormal CFR and MR.
Cox-regression analysis for dichotomous and continuous physiological indices after correction for confounders for individual components of MACE at 5-year follow-up across the different groups by normal/abnormal CFR and MR.


