Abstract
Purpose
Rhesus macaques (Macaca mulatta) are the premier nonhuman primate model for studying human health and disease. We investigated if age was associated with clinically relevant ocular features in a large cohort of free-ranging rhesus macaques from Cayo Santiago, Puerto Rico.
Methods
We evaluated 120 rhesus macaques (73 males, 47 females) from 0 to 29 years old (mean ± SD: 12.6 ± 6.4) from September to December 2021. The ophthalmic evaluation included intraocular pressure (IOP) assessment, corneal pachymetry, biomicroscopy, A-scan biometry, automated refraction, and fundus photography after pupil dilation. The associations of age with the outcomes were investigated through multilevel mixed-effects models adjusted for sex and weight.
Results
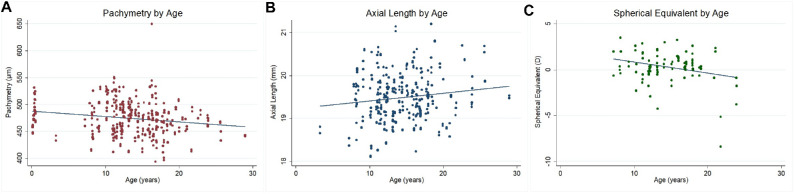
On average, IOP, pachymetry, axial length, and automated refraction spherical equivalent were 18.37 ± 4.68 mmHg, 474.43 ± 32.21 µm, 19.49 ± 1.24 mm, and 0.30 ± 1.70 diopters (D), respectively. Age was significantly associated with pachymetry (β coefficient = −1.20; 95% confidence interval [CI], −2.27 to −0.14; P = 0.026), axial length (β coefficient = 0.03; 95% CI, 0.01 to 0.05; P = 0.002), and spherical equivalent (β coefficient = −0.12; 95% CI, −0.22 to −0.02; P = 0.015). No association was detected between age and IOP. The prevalence of cataracts in either eye was 10.83% (95% CI, 6.34–17.89) and was significantly associated with age (odds ratio [OR] = 1.20; 95% CI, 1.06–1.36; P = 0.004). Retinal drusen in either eye was observed in 15.00% (95% CI, 9.60–22.68) of animals, which was also significantly associated with age (OR = 1.14; 95% CI, 1.02–1.27; P = 0.020).
Conclusions
Rhesus macaques exhibit age-related ocular associations similar to those observed in human aging, including decreased corneal thickness, increased axial length, myopic shift, and higher prevalence of cataract and retinal drusen.
Keywords: aging, animal models, nonhuman primate, rhesus macaque
Over 4% of the worldwide population is estimated to live with moderate to severe visual impairment or blindness.1 Among the four main causes, cataract, glaucoma, and age-related macular degeneration are conditions strongly associated with aging.2 Blindness due to cataract is a reversible condition, and the underlying mechanisms associated with the disease development and progression are mostly known.3 Conversely, the environmental and genetic contributions to diseases such as glaucoma and age-related macular degeneration are not fully understood, in large part because human data are confounded by medical interventions as well as social and environmental variation among subjects.4–6 Given these limitations, the evaluation of animal models in consistent environments is a valuable alternative to overcome the gaps and promote better understanding of these diseases. Studies of primates are especially valuable for their translational potential to humans due to shared general biology and physiology, including many shared ocular features.7
Rhesus macaques (Macaca mulatta) are the premier nonhuman primate (NHP) model for studying human health and disease.8 Members of the genus Macaca have over 90% DNA sequence similarity and highly conserved protein sequences with humans.8 The rhesus macaque lifespan is approximately three to four times shorter than that of humans, which provides an advantage for longitudinal research, as age-related changes occur over about a fourfold shorter time span.9 Importantly, the aging process of rhesus monkeys is highly similar to that of humans, including declines in physical health, physiological integrity, brain function, and peripheral immune regulation.9–11
Ocular studies of rhesus macaques have shown a close anatomical association between NHP and human visual systems, including features of the optic nerve head, retina, and lens.8,12,13 Although those similarities are well established, detailed data on the age association with ocular parameters such as increasing intraocular pressure (IOP) and axial length in rhesus macaques remain limited to a few studies based on small sample sizes of captive animals.14–16 Data from a free-ranging, naturalistic population, free from medical interventions, hold considerable promise to inform our understanding of the similarities and differences between the NHP and human visual systems and enhance translational outcomes. The population of rhesus macaques residing on the island of Cayo Santiago, a 15.2-hectare island located 1 km from the eastern coast of Puerto Rico, have provided a unique resource for medical and social research for over 80 years.17 The macaques live under naturalistic circumstances and are free to socialize with others in the population.9
The purpose of the current study was to quantify age-related differences in ocular features in a large cohort of free-ranging rhesus macaques from Cayo Santiago. We specifically aimed to understand the association of age with ocular structure, as well as with other age-related features commonly seen in humans as cataracts and retinal drusen.
Methods
Study Population
The Cayo Santiago population of rhesus macaques was established in the late 1930s and started with 409 founders imported from India. Since then, the macaques have flourished, with the population now approaching 2000 individuals. The population is managed by the Caribbean Primate Research Center (CPRC) at the University of Puerto Rico. The CPRC maintains a detailed census of the population, recording the date of birth of each monkey, and provisions them daily with monkey chow and water. Aside from tetanus inoculation administered when animals are approximately 1 year old, no medical interventions are administered to the individuals. The animals at Cayo Santiago are free to aggregate into social units and to supplement their diets with insects and leaves found on the island; they are exposed to natural weather conditions and interact in affiliative, agonistic, and reproductive behaviors with other members of the population.9 Each individual is recognized and tracked by tattoos, ear notches, and facial features. Interventions are minimal except during the annual trapping period, when animals that are currently part of specific research programs can be trapped, sedated, and physically examined.
We performed comprehensive eye exams on 120 rhesus macaques (73 male, 47 female) from September to December 2021. We measured all individuals from two social groups that were trapped as part of an ongoing longitudinal study of aging on Cayo Santiago. To consider biological variables that may impact ocular features, we collected data on the age and weight of all study individuals (Table 1). Males and females in our dataset did not differ significantly in age (z = 0.680; P = 0.4964), but males were significantly heavier than females (z = −7.371; P < 0.0001).
Table 1.
Rhesus Macaque Age and Weight by Sex
| Mean ± SD | |||
|---|---|---|---|
| Variable | Males | Females | All |
| Age (y) | 12.40 ± 5.60 | 12.80 ± 7.62 | 12.56 ± 6.44 |
| Weight (kg) | 9.84 ± 3.20 | 5.97 ± 2.72 | 8.33 ± 3.56 |
Eye Examination Procedures
All of the procedures complied with the guidelines of ARVO. Animals were anesthetized using ketamine HCL (100 mg/mL) and xylazine (100 mg/mL) following the protocol approved by the animal welfare committee of the University of Puerto Rico (IACUC #A400117) and of the University of Calgary (AC19-0091). We examined both eyes from each study animal. The ophthalmic evaluation included IOP, corneal pachymetry, anterior segment biomicroscopy, and A-scan biometry. Automated refraction and fundus photography were performed after pupil dilation. All in vivo evaluation and testing were conducted by one experienced ophthalmic technologist (AF) with experience in NHP handling. We measured IOP using a hand-held tonometer. Central corneal thickness was measured using a Pachmate 2 ultrasonic pachymeter (DGH Technologies, Exton, PA, USA). Anterior segment biomicroscopy was performed using a handheld slit lamp (MicroClear, Jiangsu, China) with a focus on cataract diagnosis, considering any type of age-related lens opacification not associated with trauma. The A-scan was performed using VuPad (Sonomed Escalon, Lake Success, NY, USA) and provided measurements of axial length, anterior chamber depth, lens, and vitreous chamber. Pupils were dilated by using a drop of tropicamide 1% and one drop of cyclopentolate 1%. Static refraction was performed with the automated refractor KR 7000S (Topcon, Tokyo, Japan) and provided both refraction and keratometry information. Finally, fundus photography was performed using the posterior segment module from the TRC-NW400 camera (Topcon). Both pachymetry and A-scan were performed after instillation of one anesthetic drop of tetracaine 0.5% in each eye to guarantee the animal’s comfort. Automated refraction was performed at least 20 minutes from the administration of pupil dilation drops. The spherical equivalent was calculated as the refraction spherical component summed to half of the cylinder component. A-scan and automated refraction were not performed in individuals younger than 7 years old due to the limitations of the equipment associated with the short eye sizes. A rigid gas permeable contact lens (Boston EO; Boston Lens Materials, Boston, MA, USA) was fitted to each eye to improve the fundus photography image quality. Each image was analyzed by two independent retina experts (MG and IL) to determine retinal drusen presence. After the exams, the animals were kept monitored until the next day and were evaluated by the veterinarian before being released.
We measured IOP with a TonoVet tonometer (iCare, Vantaa, Finland) using the “dog” setting. As this device does not have a specific configuration for macaques, we performed a cannulation experiment in five adult rhesus macaques (not included in our study) following established protocols.18 We then generated the following calibration formula for the TonoVet data IOP = −6.03 + 0.91 × TonoVet value.
Statistical Methods
We used Stata 14.0 (StataCorp, College Station, TX, USA) for statistical analyses. Frequency tables were used for descriptive analysis. We evaluated the associations of age with IOP, pachymetry, axial length, and autorefraction spherical equivalent values on a per-eye basis through multilevel mixed-effects models with a random intercept to account for intra-subject correlation.19 We report the results generated by including both eyes, but to be comprehensive we also ran the analyses including only the right eye or only the left eye. The outcomes were highly consistent, and we report those generated by including both eyes. We investigated the associations of age with cataract and retinal drusen prevalence on a per-individual basis using multiple logistic regressions. All of the models included age, sex, and weight. P ≤ 0.05 was considered statistically significant.
Results
The mean IOP, corneal thickness, axial length, and automated refraction spherical equivalent are presented in Table 2. We found significant associations between age and corneal thickness, axial length, and spherical equivalent (Fig. 1). For each year of age, the corneal thickness decreased by 1.20 µm, the axial length increased 0.03 mm, and the spherical equivalent decreased 0.12 diopters (D). There was also a main association of sex with IOP and axial length. Males had IOP that was 2.04 mmHg lower than females, and axial lengths that were 0.43 mm longer than females. Moreover, there was a significant association of weight and IOP, such that for each 1-kg increase in weight the intraocular pressure increased by 0.86 mmHg (Table 3). When including corneal thickness in the model for IOP, we noted a significant association between IOP and corneal thickness (β coefficient = 0.03; 95% CI, 0.01–0.04; P = 0.007); however, adding this variable did not change the main effects of sex, age, or weight on IOP. The association of age and corneal thickness also holds if we remove infants from the analysis (P = 0.041).
Table 2.
Features of Ocular Parameters Measured in a Free-Ranging Population of Rhesus Macaques
| Variable | Mean ± SD | Range |
|---|---|---|
| IOP (mmHg) | 18.37 ± 4.68 | 4–33 |
| Pachymetry (µm) | 473.06 ± 32.16 | 394–551 |
| A-scan (mm) | ||
| Anterior chamber depth | 3.45 ± 0.24 | 2.59–4.06 |
| Lens | 3.31 ± 0.25 | 2.81–4.05 |
| Vitreous chamber depth | 12.71 ± 0.54 | 11.47–14.11 |
| Axial length | 19.46 ± 0.56 | 18.11–20.81 |
| Refraction (D) | ||
| Spheric | 1.19 ± 1.77 | −6.75 to 6.00 |
| Cylinder | −1.79 ± 1.53 | −6.75 to −0.25 |
| Spherical equivalent | 0.30 ± 1.70 | −8.38 to 3.50 |
| K1 | 51.21 ± 2.76 | 42.50–55.87 |
| K2 | 53.21 ± 2.46 | 48.37–60.37 |
| KM | 52.41 ± 2.74 | 47.12–63.75 |
K1, flat keratometry; K2, steep keratometry; KM, mean curvature.
Figure 1.
Association of age with pachymetry (A), axial length (B), and spherical equivalent (C).
Table 3.
Multilevel Mixed-Effects Model Analysis for IOP, Pachymetry, Axial Length, and Spherical Equivalent
| IOP (mmHg) | Pachymetry (µm) | Axial Length (mm) | Spherical Equivalent (D) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | β Coefficient (95% CI) | P | β Coefficient (95% CI) | P | β Coefficient (95% CI) | P | β Coefficient (95% CI) | P |
| Sex | ||||||||
| Female | Ref. | — | Ref. | — | Ref. | — | Ref. | — |
| Male | −2.04 (−3.87 to −0.22) | 0.028 | 11.21 (−3.02 to 25.44) | 0.123 | 0.43 (0.11 to 0.76) | 0.008 | 0.46 (−1.03 to 1.95) | 0.549 |
| Age (y) | −0.10 (−0.23 to 0.04) | 0.162 | −1.20 (−2.27 to −0.14) | 0.026 | 0.03 (0.01 to 0.05) | 0.002 | −0.12 (−0.22 to −0.02) | 0.015 |
| Weight (kg) | 0.86 (0.57 to 1.15) | <0.001 | 0.53 (−1.73 to 2.79) | 0.647 | 0.05 (−0.03 to 0.12) | 0.219 | −0.05 (−0.40 to 0.30) | 0.785 |
Text in bold denotes statistically significant values.
When analyzing each component of the A-scan evaluation separately, age was associated with decreasing anterior chamber depth (β coefficient = −0.01; 95% CI, −0.02 to −0.01; P = 0.002) and increasing lens thickness (β coefficient = 0.04; 95% CI, 0.03–0.05; P < 0.001). In addition, we found a sex effect such that the vitreous chamber was deeper in males (β coefficient = 0.36; 95% CI, 0.04–0.68; P = 0.029). When analyzing each component of the automated refraction, age was associated with decreasing spherical component value (β coefficient = −0.16; 95% CI, −0.26 to −0.06; P = 0.001) and increasing cylindrical component value (β coefficient = 0.08; 95% CI, 0.01–0.15; P = 0.039). No association between keratometry parameters and sex, age or weight were observed (P > 0.05).
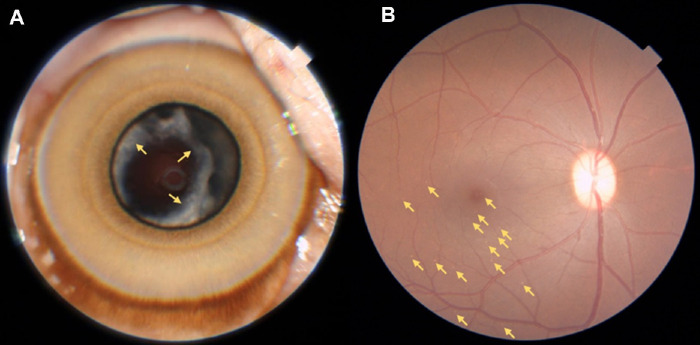
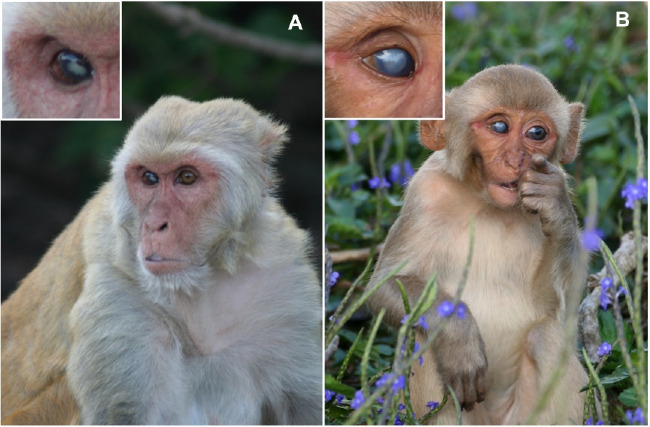
Cataract (Fig. 2A) in either eye was observed in 13 animals (seven unilateral and six bilateral cases) representing a frequency of 10.83% (95% CI, 6.34–17.89). Retinal drusen (Fig. 2B) in either eye was observed in 18 animals (10 unilateral and eight bilateral cases), representing a frequency of 15.00% (95% CI, 9.60–22.68). Older individuals were more likely to have cataract (odds ratio [OR] = 1.20; 95% CI, 1.06–1.36; P = 0.004) and retinal drusen (OR = 1.14; 95% CI, 1.02–1.27; P = 0.020) (Table 4). For each 1 increasing year of age, the odds of having cataract increased by 20% and the odds of having retinal drusen increased by 14%. The mean number of drusen on the 26 affected eyes was 8.9 ± 7.6. Although the presence or absence of drusen itself was age related (binary variable, P = 0.020), the actual number of drusen present was not age related (continuous variable, P = 0.393). Ocular abnormalities that were not age related were less frequent and included corneal scars/leucoma (2.50%) (see Fig. 3), eyelid laceration (0.83%), conjunctiva (0.83%), and iris nevus (0.83%); the first two likely due to trauma events and/or infections that occurred during typical social behaviors.
Figure 2.
(A) Cataract, right eye, female, 16.4 years old. Yellow arrow indicates cortical lens opacities. (B) Retinal drusen, right eye, male, 19.1 years old. Yellow arrows indicate drusen concentrated on macular and temporal inferior regions.
Table 4.
Multiple Logistic Regression for Cataract and Retinal Drusen in Either Eye
| Cataract | Retinal Drusen | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P |
| Sex | ||||
| Female | Ref. | — | Ref. | — |
| Male | 0.45 (0.07–2.77) | 0.389 | 1.54 (0.30–7.94) | 0.605 |
| Age (y) | 1.20 (1.06–1.36) | 0.004 | 1.14 (1.02–1.27) | 0.020 |
| Weight (kg) | 0.99 (0.66–1.48) | 0.957 | 1.12 (0.80–1.55) | 0.505 |
Text in bold denotes statistically significant values.
Figure 3.
An adult (A) and a juvenile (B) rhesus macaque (Macaca mulatta) on Cayo Santiago with visible leucoma in the right eyes. The sources of the leucomas are unknown, but they were likely to be caused by trauma from their environment or conspecifics. Photo credits: Lauren J. N. Brent (A) and Amanda D. Melin (B).
Discussion
We investigated age-related ocular features in a large cohort of free-ranging rhesus macaques. We found that older individuals had thinner corneas, longer axial length, more myopic refractive error, and higher prevalence of cataract and retinal drusen. Below we discuss and contextualize these findings in more detail. Rhesus macaques age in a manner that closely resembles humans, including similar periods of development, maturation, reproduction, and senescence, albeit within a fourfold shorter time span.9 These monkeys display typical aging hallmarks, such as thinning and greying hair, atrophying skin, declining motor activity, decreasing stature, and redistribution of body fat, and they also exhibit pathological signs of age-related diseases such as cancer, cardiovascular disease, and metabolism disorders.9,10,20–22 With continual increases in human longevity, conditions such as glaucoma and age-related macular degeneration are expected to be major causes of vision impairment and blindness,23,24 highlighting the importance of identifying a good animal biomedical model for these and related ocular conditions.
Our study shows age-related differences in corneal thickness, axial length, and refraction in rhesus macaque eyes. Although these features are not necessarily associated with disease per se, they contribute to a growing understanding of changes that happen in the eye as we age. Our results indicate a corneal thickness decrease rate of 1.20 µm per year. Human studies also show declines in corneal thickness, but extensive variation in the estimated rates is present, due to differing methodologies and devices used for measurement, with rates ranging from 0.30 µm/y when using optical devices25–27 to 1.20 µm/y when using ultrasound-based devices.28,29 Corneal thinning has been attributed to loss of both epithelial and endothelial cells, which has been demonstrated to be associated with the aging process. However, other underlying causes such as changing in overall cell distribution and limbal epithelial stem cell dysfunction are under study.25,26,29,30 Importantly, corneal thinning rate has been found to be significantly greater in glaucoma cases,29 reinforcing the importance of a model with similar corneal characteristics when studying the disease. Regarding axial length, although the Sorbys's classical statement indicates that the axial eye growth in humans is completed at the age of 13 years,31 recent studies have demonstrated that the eye of those with persistent emmetropia and hyperopia continues to grow between 18 and 22 years, at a slow rate of approximately 0.03 to 0.04 mm/y.32,33 In our sample of rhesus macaques, we found an axial length difference of +0.03 mm/y, slightly lower than a previous publication in captive rhesus macaques that reported a difference of +0.05 mm/y,16 a difference possibly due to a broader range of ages in their analysis. Finally, as a consequence of the axial length increase, a myopic shift on the static spherical equivalent is expected to be observed.34 Our results, indeed, showed a change rate of −0.12 D/y. Longitudinal studies with human populations have demonstrated that a 0.1-mm change in axial length corresponds to a −0.24-D change in refraction, whereas the myopic shift in adults is mostly attributable to cataract formation.35
We found that IOP was not significantly associated with age. This is perhaps surprising given that a recent study on captive rhesus macaques reported higher IOP values in older individuals.16 However, a previous study on rhesus macaques from Cayo Santiago reports results similar to those we find here—that is, that age was not a significant predictor of IOP.36 Results from human studies are also variable, with some reports indicating higher IOP,37 lower IOP,38,39 or no IOP association40,41 in older individuals relative to younger individuals. It is important to add that many of the studies of humans and captive animals do not include weight in their analysis, so it is possible that the associations of higher IOP with older age and male sex could be confounded by the individual's weight. We included weight in our multivariate models and found a positive association between IOP and weight, which is in agreement with expectations and available research.37,42 Future studies focused on disentangling the effects of sex, age, and weight on IOP are recommended.
In accordance with previous studies in humans and captive rhesus macaques, we found cataracts to be strongly associated with aging.2,14 Several population-based studies in humans have reported increased risk of cataracts ranging from 6% to 24% for each increasing year in age, similar to our finding of 20%.2,41,43,44 Similarly, a higher frequency of retinal drusen is expected in older individuals.45,46 Our findings indicate a frequency of retinal drusen of 15% in the population and its significant association with age, with the odds of having drusen increasing by 14% for each year of age. Previous studies evaluating drusen in captive rhesus macaques have shown variable results with frequencies ranging from 5% to 61% of the population,47–51 variability potentially explained by several factors such as age and sex distribution, genetic differences among colonies, environment, methods of examination, and criteria used to define drusen occurrence. The lack of a significant association between the number of drusen may possibly be due to the low prevalence of drusen in the population when compared to the frequencies reported by other studies that had evaluated this parameter and found an association.48,50 Our presence/absence analysis was sufficiently powered, but the more sensitive analysis of the number of drusen may require a larger sample size. Retinal drusens may not cause visual impairment per se but are considered one of the main risk factors for age-related macular degeneration and therefore a target for investigations of the disease process.45,46,52
Biomedical research in ophthalmology is often reliant on rodents and rabbits, due to the advantages of short lifespan, well-known genetic features, low-cost maintenance, and ease of handling.53,54 Limitations of such models, however, include differential optic nerve head structure and lamina cribosa and dissimilarities of retinal ganglion cell subtypes when compared to primates,16 which restricts the results translation for human diseases as glaucoma. Moreover, non-primate mammals do not have a macula,55,56 again limiting the potential of this model when considering age-related macular degeneration research. We join other researchers in advocating for the rhesus macaques as a model for eye diseases. Here, we present an extensive and detailed study on IOP, corneal thickness, axial length, refraction, and cataract and retinal drusen prevalence in rhesus monkeys spanning a large age range. Importantly, this is the first study of ocular features in a naturalistic free-ranging population. Given that aging and disease development often differ between captive and free-ranging animals,9,21,57 our results might represent the most realistic scenario available, well suited for studying natural history of diseases. Limitations of this study include the fact that axial length and static refraction assessments were not performed in individuals younger than 3 and 7 years old, respectively, which could have underestimated the rates of eye growth and myopia shift per year in the general population; we were not able to perform examinations using optical coherence tomography due to logistic limitations of having the equipment located in situ in the field; and finally, our analysis is relied on structural rather than functional features.
In conclusion, rhesus macaques have age-related ocular changes similar to those observed in human aging, including higher prevalence of cataract and drusen, decreasing corneal thickness, increasing axial length, and myopic shift. These results are further evidence that rhesus macaques are a valuable model for study of age-related eye diseases.
Acknowledgments
The authors thank their colleagues for important support in data collection on the Cayo Santiago site, especially Allysa Arre, Nahiri Barreto, Josué Negrón, Daniel Phillips, and the Caribbean Primate Research Center staff. We appreciate constructive feedback from the editor and two anonymous reviewers on a previous version of this manuscript.
Supported by grants from the New Frontiers in Research Foundation (NFRFE-2018-02159), Natural Sciences and Engineering Research Council (RGPIN-2017-03782), Canada Research Chairs Program (950-231257), National Aging Institute (1R56AG071023, R01AG060931), BrightFocus Foundation (G2020047), National Institutes of Health (NIH R01-EY030770), and University of Calgary Eyes High Fellowship (AGF).
Disclosure: A.G. Fernandes, None; P. Alexopoulos, None; A. Burgos-Rodriguez, None; M.I. Martinez, None; M. Ghassibi, None; I. Leskov, None; L.J.N. Brent, None; N. Snyder-Mackler, None; J. Danias, None; G. Wollstein, None; J.P. Higham, None; A.D. Melin, None
References
- 1. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021; 9(2): e130–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021; 9(2): e144–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S.. Age-related cataract. Lancet. 2005; 365(9459): 599–609. [DOI] [PubMed] [Google Scholar]
- 4. Weinreb RN, Aung T, Medeiros FA.. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014; 311(18): 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang HW, Sun P, Chen Y, et al.. Research progress on human genes involved in the pathogenesis of glaucoma (Review). Mol Med Rep. 2018; 18(1): 656–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Hou XW, Liang G, Pan CW.. Metabolomics in glaucoma: a systematic review. Invest Ophthalmol Vis Sci. 2021; 62(6): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mustari MJ. Nonhuman primate studies to advance vision science and prevent blindness. ILAR J. 2017; 58(2): 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picaud S, Dalkara D, Marazova K, Goureau O, Roska B, Sahel JA.. The primate model for understanding and restoring vision. Proc Natl Acad Sci USA. 2019; 116(52): 26280–26287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiou KL, Montague MJ, Goldman EA, et al.. Rhesus macaques as a tractable physiological model of human ageing. Philos Trans R Soc Lond B Biol Sci. 2020; 375(1811): 20190612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK.. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004; 305(5689): 1423–1426. [DOI] [PubMed] [Google Scholar]
- 11. Watowich MM, Chiou KL, Montague MJ, et al.. Natural disaster and immunological aging in a nonhuman primate. Proc Natl Acad Sci USA. 2022; 119(8): e2121663119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burgoyne CF. The non-human primate experimental glaucoma model. Exp Eye Res. 2015; 141: 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moshiri A, Chen R, Kim S, et al.. A nonhuman primate model of inherited retinal disease. J Clin Invest. 2019; 129(2): 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age (Omaha). 1997; 20(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wendt M, Croft MA, McDonald J, Kaufman PL, Glasser A.. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2008; 86(5): 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin KH, Tran T, Kim S, et al.. Age-related changes in the rhesus macaque eye. Exp Eye Res. 2021; 212: 108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kessler MJ, Rawlins RG.. A 75-year pictorial history of the Cayo Santiago rhesus monkey colony. Am J Primatol. 2016; 78(1): 6–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He Z, Bui BV, Vingrys AJ.. The rate of functional recovery from acute IOP elevation. Invest Ophthalmol Vis Sci. 2006; 47(11): 4872–4880. [DOI] [PubMed] [Google Scholar]
- 19. Ying GS, Maguire MG, Glynn R, Rosner B.. Tutorial on biostatistics: linear regression analysis of continuous correlated eye data. Ophthalmic Epidemiol. 2017; 24(2): 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turnquist JE, Kessler MJ.. Free-ranging Cayo Santiago rhesus monkeys (Macaca mulatta): I. Body size, proportion, and allometry. Am J Primatol. 1989; 19(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 21. Mattison JA, Colman RJ, Beasley TM, et al.. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017; 8: 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colman RJ. Non-human primates as a model for aging. Biochim Biophys Acta Mol Basis Dis. 2018; 1864(9 pt A): 2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121(11): 2081–2090. [DOI] [PubMed] [Google Scholar]
- 24. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2(2): e106–e116. [DOI] [PubMed] [Google Scholar]
- 25. Galgauskas S, Norvydaitė D, Krasauskaitė D, Stech S, Ašoklis RS.. Age-related changes in corneal thickness and endothelial characteristics. Clin Interv Aging. 2013; 8: 1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y, Hong J, Deng SX, Xu J.. Age-related changes in human corneal epithelial thickness measured with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2014; 55(8): 5032–5038. [DOI] [PubMed] [Google Scholar]
- 27. Hashemi H, Asgari S, Emamian MH, Mehravaran S, Fotouhi A.. Five year changes in central and peripheral corneal thickness: the Shahroud Eye Cohort Study. Cont Lens Anterior Eye. 2016; 39(5): 331–335. [DOI] [PubMed] [Google Scholar]
- 28. Iyamu E, Osuobeni E.. Age, gender, corneal diameter, corneal curvature and central corneal thickness in Nigerians with normal intra ocular pressure. J Optom. 2012; 5(2): 87–97. [Google Scholar]
- 29. Mwanza JC, Tulenko SE, Budenz DL, et al.. Longitudinal change in central corneal thickness in the TEMA eye survey. Am J Ophthalmol. 2018; 186: 10–18. [DOI] [PubMed] [Google Scholar]
- 30. Roszkowska AM, Colosi P, D'Angelo P, Ferreri G. Age-related modifications of the corneal endothelium in adults. Int Ophthalmol. 2004; 25(3): 163–166. [DOI] [PubMed] [Google Scholar]
- 31. Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA.. Refraction and its components during the growth of the eye from the age of three. Memo Med Res Counc. 1961; 301(special): 1–67. [PubMed] [Google Scholar]
- 32. Fledelius HC, Christensen AS, Fledelius C.. Juvenile eye growth, when completed? An evaluation based on IOL-Master axial length data, cross-sectional and longitudinal. Acta Ophthalmol. 2014; 92(3): 259–264. [DOI] [PubMed] [Google Scholar]
- 33. McCullough S, Adamson G, Breslin KMM, McClelland JF, Doyle L, Saunders KJ.. Axial growth and refractive change in white European children and young adults: predictive factors for myopia. Sci Rep. 2020; 10(1): 15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong HB, Machin D, Tan SB, Wong TY, Saw SM.. Ocular component growth curves among Singaporean children with different refractive error status. Invest Ophthalmol Vis Sci. 2010; 51(3): 1341–1347. [DOI] [PubMed] [Google Scholar]
- 35. Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G.. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019; 96(8): 556–567. [DOI] [PubMed] [Google Scholar]
- 36. Dawson WW, Brooks DE, Hope GM, et al.. Primary open angle glaucomas in the rhesus monkey. Br J Ophthalmol. 1993; 77(5): 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han X, Yang T, Zhang J, et al.. Longitudinal changes in intraocular pressure and association with systemic factors and refractive error: Lingtou Eye Cohort Study. BMJ Open. 2018; 8(2): e019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baek SU, Kee C, Suh W.. Longitudinal analysis of age-related changes in intraocular pressure in South Korea. Eye (Lond). 2015; 29(5): 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Hu Q, Li X, et al.. Intraocular pressure of adults in a coastal province in southern China: the Fujian cross-sectional eye study. Ann Palliat Med. 2021; 10(12): 12390–12402. [DOI] [PubMed] [Google Scholar]
- 40. Han X, Niu Y, Guo X, Hu Y, Yan W, He M.. Age-related changes of intraocular pressure in elderly people in southern China: Lingtou Eye Cohort Study. PLoS One. 2016; 11(3): e0151766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernandes AG, Berezovsky A, Watanabe SES, et al.. Prevalence of ocular findings regardless of visual acuity status in older adults from the Brazilian Amazon Region. Sci Rep. 2021; 11(1): 23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Viljanen A, Hannukainen JC, Soinio M, et al.. The effect of bariatric surgery on intraocular pressure. Acta Ophthalmol. 2018; 96(8): 849–852. [DOI] [PubMed] [Google Scholar]
- 43. Chang JR, Koo E, Agrón E, et al.. Risk factors associated with incident cataracts and cataract surgery in the Age-Related Eye Disease Study (AREDS): AREDS Report Number 32. Ophthalmology. 2011; 118(11): 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hashemi H, Pakzad R, Yekta A, et al.. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye (Lond). 2020; 34(8): 1357–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan KN, Mahroo OA, Khan RS, et al.. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog Retin Eye Res. 2016; 53: 70–106. [DOI] [PubMed] [Google Scholar]
- 46. Heesterbeek TJ, Lorés-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI.. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020; 40(2): 140–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stafford TJ, Anness SH, Fine BS.. Spontaneous degenerative maculopathy in the monkey. Ophthalmology. 1984; 91(5): 513–521. [DOI] [PubMed] [Google Scholar]
- 48. Hope GM, Dawson WW, Engel HM, Ulshafer RJ, Kessler MJ, Sherwood MB.. A primate model for age related macular drusen. Br J Ophthalmol. 1992; 76(1): 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Monaco WA, Wormington CM.. The rhesus monkey as an animal model for age-related maculopathy. Optom Vis Sci. 1990; 67(7): 532–537. [DOI] [PubMed] [Google Scholar]
- 50. Gouras P, Ivert L, Landauer N, Mattison JA, Ingram DK, Neuringer M.. Drusenoid maculopathy in rhesus monkeys (Macaca mulatta): effects of age and gender. Graefes Arch Clin Exp Ophthalmol. 2008; 246(10): 1395–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yiu G, Tieu E, Munevar C, et al.. In vivo multimodal imaging of drusenoid lesions in rhesus macaques. Sci Rep. 2017; 7(1): 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang X, Sivaprasad S.. Drusen and pachydrusen: the definition, pathogenesis, and clinical significance. Eye (Lond). 2021; 35(1): 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Del Amo EM, Urtti A.. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp Eye Res. 2015; 137: 111–124. [DOI] [PubMed] [Google Scholar]
- 54. Pang IH, Clark AF.. Inducible rodent models of glaucoma. Prog Retin Eye Res. 2020; 75: 100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kostic C, Arsenijevic Y.. Animal modelling for inherited central vision loss. J Pathol. 2016; 238(2): 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wisely CE, Sayed JA, Tamez H, et al.. The chick eye in vision research: an excellent model for the study of ocular disease. Prog Retin Eye Res. 2017; 61: 72–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M.. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci USA. 2002; 99(14): 9591–9595. [DOI] [PMC free article] [PubMed] [Google Scholar]