Abstract
Background
During the last few years, increasing focus has been placed on heart failure with mildly reduced ejection fraction (HFmrEF), an intermediate phenotype from preserved to reduced ejection fraction (EF). However, clinical features and outcome of HFmrEF in elderly patients aged ≥ 70 yrs have been poorly investigated.
Methods
The present study retrospectively included all consecutive patients aged ≥ 70 yrs discharged from our Institution with a first diagnosis of HFmrEF, between January 2020 and November 2020. All patients underwent transthoracic echocardiography. The primary outcome was all-cause mortality, while the secondary one was the composite of all-cause mortality + rehospitalization for all causes over a mid-term follow-up.
Results
The study included 107 HFmrEF patients (84.3 ± 7.4 yrs, 61.7% females). Patients were classified as “old” (70–84 yrs, n = 55) and “oldest-old” (≥ 85 yrs, n = 52) and separately analyzed. As compared to the “oldest-old” patients, the “old” ones were more commonly males (58.2% vs 17.3%, p < 0.001), with history of coronary artery disease (CAD) (54.5% vs 15.4%, p < 0.001) and significantly lower EF (43.5 ± 2.7% vs 47.3 ± 3.6%, p < 0.001) at hospital admission. Mean follow-up was 1.8 ± 1.1 yrs. During follow-up, 29 patients died and 45 were re-hospitalized. Male sex (HR 6.71, 95% CI 1.59–28.4), history of CAD (HR 5.37, 95% CI 2.04–14.1) and EF (HR 0.48, 95% CI 0.34–0.68) were independently associated with all-cause mortality in the whole study population. EF also predicted the composite of all-cause mortality + rehospitalization for all causes. EF < 45% was the best cut-off value to predict both outcomes.
Conclusions
EF at hospital admission is independently associated with all-cause mortality and rehospitalization for all causes in elderly HFmrEF patients over a mid-term follow-up.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-023-02454-3.
Keywords: Elderly, Ejection fraction, Heart failure, HFmrEF, Outcome
Introduction
Heart failure with mildly reduced ejection fraction (HFmrEF), defined as symptoms and signs of heart failure (HF) with an ejection fraction (EF) between 41 and 49%, has been formally classified as a new phenotype of HF in 2016 European Society of Cardiology (ESC) guidelines [1]. According to the 2021 ESC guidelines, increased serum levels of natriuretic peptides and other evidence of structural heart disease make HFmrEF diagnosis more likely but are not mandatory if there is certainty regarding EF measurement [2]. Based on recent clinical trials and registries, HFmrEF accounts for ~ 13–24% of HF cases [3, 4]. The primary recognized cause of HFmrEF is coronary artery disease (CAD); accordingly, from an etiological point of view, patients with HFmrEF are more similar to those with heart failure with reduced ejection fraction (HFrEF) rather than those with preserved ejection fraction (HFpEF) [5]. According to literature data [6], HFmrEF patients are likely to be heterogeneous and may not have a single pathophysiological substrate. Given that EF is a dynamic index and may increase or decrease during the course of HF, HFmrEF may occur either as a recovery from HFrEF or a deterioration from HFpEF [7]. To date, several studies [5, 8–13] have evaluated epidemiology, pathophysiology and clinical outcomes of HFmrEF patients. However, the majority of individuals included in those studies were 70 years old or younger and only few studies [10, 14] were specifically focused on the assessment of HFmrEF in elderly patients. Because of the growing ageing of the population worldwide, HFmrEF patients aged ≥ 70 yrs will be more frequently encountered in contemporary clinical practice [15]. Accordingly, the present study was designed to investigate the main clinical, laboratory and echocardiographic features of HFmrEF patients aged ≥ 70 yrs, categorized in the two age subgroups of “old” (70–84 yrs) and “oldest-old” (≥ 85 yrs), and to evaluate the independent prognostic indicators of “all-cause mortality”, over a medium-term follow-up.
Methods
Study population
This retrospective observational study included all consecutive patients aged ≥ 70 yrs discharged from Internal Medicine Division of San Giuseppe MultiMedica Hospital (Milan), a tertiary university institution, with a main diagnosis of HFmrEF, between January 1st, 2020, and November 30th, 2020. The present study group was selected from a larger population of HF patients, object of another clinical investigation focused on the prevalence and clinical outcome of main echocardiographic and hemodynamic HF phenotypes [16].
HFmrEF diagnosis was established according to the 2021 ESC guidelines [2] and based on: (1) symptoms (dyspnea, fatigue, or decreased exercise capacity); (2) signs (edema or rales on chest auscultation); (3) a mildly reduced EF (41–49%) on transthoracic echocardiography (TTE) examination performed at admission to the Internal Medicine Division.
Exclusion criteria were: HFpEF (EF ≥ 50%), HFrEF (EF ≤ 40%), age < 70 yrs, hemodynamic instability requiring spoke-to-hub transfer, lacking of two-dimensional (2D) TTE performed during hospital stay, poor echocardiographic windows, lacking of a complete laboratory panel. Although this study was performed during the COVID-19 pandemic, COVID-19 patients were excluded from this retrospective analysis, to avoid the risk of bias related to concomitant COVID-19 disease.
HFmrEF patients were stratified in two major groups, according to their age: (1) HFmrEF patients aged 70–84 years (the “old” group); (2) HFmrEF patients aged ≥ 85 years (the “oldest-old” group). This cut-off was derived from previous studies conducted on elderly HF patients [17–19].
On the basis of the underlying etiology, following predominant clinical subtypes of HFmrEF were identified: (1) HF due to acute/chronic CAD; (2) HF due to acute/chronic valvular heart disease (VHD); (3) HF due to hypertensive cardiomyopathy; (4) HF due to acute/chronic pulmonary hypertension [2].
Main etiology of HF and both echocardiographic and clinical categories of HF were assessed according to the above-mentioned standardized criteria by two expert clinicians (C.L. and A.S.) within the first 24 h of admission to the Internal Medicine Division.
All following data were collected from patients’ hospital medical charts: age; gender; prevalence of relevant cardiovascular risk factors (hypertension, smoking, type 2 diabetes and dyslipidemia); main comorbidities, such as chronic kidney disease (CKD) defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min/m2 [20], history of CAD (previous acute coronary syndrome, previous percutaneous and/or surgical coronary revascularization), peripheral arteriopathy, previous stroke and/or transient ischemic attack, cognitive impairment, chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, hypothyroidism, anemia defined as hemoglobin < 12 g/dl for females or 13 g/dl for males, gastroesophageal reflux disease; blood tests comprehensive of complete blood count, serum creatinine and eGFR, serum levels of glucose, sodium, potassium, uric acid, low-density lipoprotein (LDL) cholesterol, thyroid-stimulating hormone, C-reactive protein (CRP), N-terminal pro-B-type natriuretic peptide (NTproBNP), high-sensitivity (HS) troponine; blood pressure measurements; electrocardiographic data (cardiac rhythm and pattern of intraventricular conduction); chest X-ray results; current medical treatment.
All procedures were in accordance with the ethical standards of our Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the local Ethics Committee (Committee’s reference number 464.2021).
Clinical prognostic scores
For each HFmrEF patient, following prognostic scores were retrospectively calculated: (1) the CHA2DS2-VASc [Congestive heart failure or left ventricular dysfunction (1 point), Hypertension (1 point), Age ≥ 75 years (2 points), Diabetes (1 point), Stroke/TIA (2 points), Vascular disease (1 point), Age 65–74 years (1 point), and Sex category (female; 1 point)] score [21]; (2) the HAS-BLED [Hypertension (1 point), Abnormal renal/liver function (1 or 2 points), Stroke (1 point), Bleeding history or predisposition (1 point), Labile international normalized ratio (1 point), Elderly (> 65 years) (1 point), Drugs/alcohol concomitantly (1 or 2 points)] score [22]; (3) the Charlson comorbidity index (CCI), which assigned 1 point for each of the following comorbidities: myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic lung disease, connective tissue disease, ulcer, chronic liver disease, diabetes; 2 points for each of hemiplegia, moderate or severe kidney disease, diabetes with end-organ damage, tumor, leukemia, lymphoma; 3 points for moderate or severe liver disease; 6 points for tumor metastasis or AIDS [23].
Conventional echocardiographic examination
All echocardiograms were performed by the same expert cardiologist (A.S.) within 24 h after hospital admission, using commercially available Philips Sparq ultrasound machine (Philips, Andover, Massachusetts, USA) with a 2.5 MHz transducer. All parameters were measured according to the Recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [24, 25].
The following M-mode and 2D echocardiographic parameters were recorded: relative wall thickness (RWT), calculated with the formula RWT = 2 posterior wall thickness/left ventricular (LV) internal diameter at end-diastole; LV end-diastolic and end-systolic volumes; EF estimated with the biplane modified Simpson’s method [24] and calculated as the average value of two and five different biplane measurements in non-atrial fibrillation (AF) and AF patients, respectively; left atrial antero-posterior diameter and left atrial volume; right ventricular inflow tract and the tricuspid annular plane systolic excursion (TAPSE) using an apical four-chamber view; finally, the inferior vena cava (IVC) diameter by a subcostal view.
Doppler measurements included E/A ratio and average E/e′ ratio, the latter as an index of left ventricular filling pressure (LVFP) [25]. Systolic pulmonary artery pressure (SPAP) was derived by the modified Bernoulli equation, where SPAP = 4 × [tricuspid regurgitation velocity (TRV)]2 + right atrial pressure [26]. The latter was estimated from IVC diameter and collapsibility.
Degree of valvulopathy was assessed according to the AHA/ACC recommendations for the management of patients with VHD [27].
Outcome definition
The primary aim of the study was to identify the independent predictors of “all-cause mortality” in the whole population of HFmrEF patients, over a medium-term follow-up. The secondary purpose was to evaluate the independent predictors of the composite of “all-cause mortality + re-hospitalization for all causes” in the same study group.
Causes of death and rehospitalization for each HFmrEF patient were determined by accessing medical records available in the hospital archive and/or from telephone interviews.
Statistical analysis
HFmrEF patients enrolled in the study were stratified in two major groups: (1) HF patients aged 70–84 years (the “old” group); (2) HF patients aged ≥ 85 years (the “oldest-old” group). For the whole study population and for each group of elderly patients, continuous data were summarized as mean ± standard deviation, while categorical data were presented as number (percentage). Each continuous variable was checked through the Shapiro–Wilk test and all data were determined to be normally distributed. Continuous variables were compared using a two-sample independent t test, whereas categorical parameters were compared using the Chi-squared test or the Fisher’s exact test.
Univariate Cox regression analysis was performed to evaluate the effect of the following variables: (1) age and male sex (as demographics); (2) smoking, hypertension, type 2 diabetes and dyslipidemia (as cardiovascular risk factors); (3) previous history of CAD (as index of the atherosclerotic burden); (4) CHA2DS2-VASc score, HAS-BLED score and CCI (as clinical prognostic scores, expressed as continuous parameters); (5) serum hemoglobin, serum sodium, eGFR, serum CRP, serum NT-proBNP and serum HS troponine (as biochemical markers); (6) heart rate, AF and left bundle branch block (LBBB) pattern (as ECG parameters); (7) EF, average E/e′ ratio and TRV (as echoDoppler variables); (8) loop diuretics, beta blockers and statin therapy (as concerns discharge medical treatment), on the occurrence of both primary and secondary endpoints during follow-up period, in the whole study population. For each variable investigated, correspondent hazard ratios with 95% confidence intervals (CIs) were calculated. Only the variables with statistically significant association on univariate analysis were thereafter included in the multivariate Cox regression model.
The receiver operating characteristics (ROC) curve analysis was performed to establish the sensitivity and the specificity of EF for predicting the above-mentioned outcomes. Area under curve (AUC) was estimated. The optimal cutoff of EF was calculated using the maximum value of the Youden Index (determined as sensitivity + [1 − specificity]).
Kaplan–Meier survival curves were designed to measure differences between age groups and EF categories in the rates of “all-cause mortality” and “all-cause mortality + rehospitalization for all causes” respectively, over a medium-term follow-up, for the whole study population. The comparison between survival curves was assessed using the log-rank test.
Intra-observer and inter-observer variability analysis for EF assessment was conducted in a subgroup of 15 randomly selected HFmrEF patients. EF was blindly re-measured by the same cardiologist who performed all echocardiographic examinations (A.S.) and by a second one (M.L.). The intraclass correlation coefficient (ICC) with its 95% CI was used as a statistical method for assessing intra-observer and inter-observer measurement variability. An ICC of 0.70 or more was considered to indicate acceptable reliability.
Statistical analysis was performed with SPSS version 26 (SPSS Inc., Chicago, Illinois, USA), with two-tailed p values below 0.05 deemed statistically significant.
Results
Baseline characteristics
During the study period, 122 HFmrEF patients aged ≥ 70 yrs were selected from the original study population [16]; among them, 10 were excluded due to poor echocardiographic window and 5 due to lack of collaboration. Accordingly, this study retrospectively included a total of 107 consecutive HFmrEF patients (mean age 84.3 ± 7.4 yrs). The “old” group (n = 55) and the “oldest-old” group (n = 52) were separately analyzed.
Table 1 summarizes main demographics and clinical parameters recorded in the whole study population and in the two age groups at hospital admission.
Table 1.
Baseline clinical characteristics of the whole HFmrEF study population and of the two age groups
| Baseline clinical parameters | All patients (n = 107) |
“Old” group (70–84 yrs) (n = 55) |
“Oldest-old” group (≥ 85 yrs) (n = 52) |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (yrs) | 84.3 ± 7.4 | 78.4 ± 4.2 | 90.5 ± 4.1 | < 0.001 |
| Female sex (n, %) | 66 (61.7) | 23 (41.8) | 43 (82.7) | < 0.001 |
| Male sex (n, %) | 41 (38.3) | 32 (58.2) | 9 (17.3) | < 0.001 |
| Cardiovascular risk factors and comorbidities | ||||
| Hypertension (n, %) | 78 (72.9) | 31 (56.4) | 47 (90.4) | < 0.001 |
| Smoking (n, %) | 34 (31.8) | 25 (45.5) | 9 (17.3) | 0.002 |
| Type 2 diabetes mellitus (n, %) | 35 (32.7) | 24 (43.6) | 11 (21.1) | 0.01 |
| Dyslipidemia (n, %) | 28 (26.2) | 22 (40.0) | 6 (11.5) | < 0.001 |
| Anaemia (Hb < 12 F or 13 g/dl M) (n, %) | 39 (36.4) | 10 (18.2) | 29 (55.7) | < 0.001 |
| CKD (eGFR < 60 ml/min/m2) (n, %) | 64 (59.8) | 21 (38.2) | 43 (82.7) | < 0.001 |
| COPD (n, %) | 31 (29.0) | 22 (40.0) | 9 (17.3) | 0.009 |
| OSAS (n, %) | 12 (11.2) | 9 (16.4) | 3 (5.8) | 0.08 |
| Hypothyroidism (n, %) | 20 (18.7) | 6 (10.9) | 14 (26.9) | 0.03 |
| History of CAD (n, %) | 38 (35.5) | 30 (54.5) | 8 (15.4) | < 0.001 |
| Previous stroke (n, %) | 19 (17.7) | 14 (25.5) | 5 (9.6) | 0.03 |
| Peripheral arteriopathy (n, %) | 30 (28.0) | 21 (38.2) | 9 (17.3) | 0.02 |
| GERD (n, %) | 28 (26.2) | 13 (23.6) | 15 (28.8) | 0.54 |
| Cognitive impairment (n, %) | 36 (33.6) | 6 (10.9) | 30 (57.7) | < 0.001 |
| Physical examination | ||||
| Dyspnea (n, %) | 66 (61.7) | 46 (83.6) | 20 (38.5) | < 0.001 |
| Leg swelling (n, %) | 58 (54.2) | 30 (54.5) | 28 (53.8) | 0.94 |
| Body temperature ≥ 37.5° (n, %) | 32 (29.9) | 22 (40.0) | 10 (19.2) | 0.02 |
| Blood pressure values | ||||
| SBP (mmHg) | 135.8 ± 27.6 | 128.3 ± 30.7 | 143.7 ± 21.4 | 0.003 |
| DBP (mmHg) | 74.2 ± 13.6 | 73.8 ± 13.5 | 74.7 ± 13.8 | 0.73 |
| Chest X-ray | ||||
| Normal pattern (n, %) | 24 (22.4) | 6 (10.9) | 18 (34.6) | 0.003 |
| Congestion (n, %) | 81 (75.7) | 49 (89.1) | 32 (61.5) | < 0.001 |
| Pneumonia (n, %) | 36 (33.6) | 24 (43.6) | 12 (23.1) | 0.02 |
| ECG parameters | ||||
| AF (n, %) | 40 (37.4) | 15 (27.3) | 25 (48.1) | 0.03 |
| HR (bpm) | 83.3 ± 20.1 | 85.9 ± 24.5 | 80.6 ± 13.7 | 0.17 |
| LBBB (n, %) | 29 (27.1) | 20 (36.4) | 9 (17.3) | 0.03 |
| Clinical prognostic scores | ||||
| Charlson comorbidity index | 9.1 ± 2.9 | 8.3 ± 2.9 | 10.0 ± 2.7 | 0.002 |
| CHA2DS2-VASc score | 5.2 ± 1.6 | 5.2 ± 1.9 | 5.1 ± 1.1 | 0.74 |
| HAS-BLED score | 2.9 ± 1.2 | 2.4 ± 1.1 | 3.5 ± 1.1 | < 0.001 |
AF atrial fibrillation, CAD coronary artery disease, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, GERD gastrosophageal reflux disease, Hb hemoglobin, HFmrEF heart failure with mildly reduced ejection fraction, HR heart rate, LBBB left bundle branch block, OSAS obstructive sleep apnea syndrome, SBP systolic blood pressure
Significant P values are in bold
Overall, 75.7% of HFmrEF patients were ≥ 80 yrs old, with a higher prevalence of female sex (61.7%). Females represented 82.7% of HFmrEF patients in the “oldest-old” group; conversely, the majority of HFmrEF patients in the “old” group (58.2%) were males. In the whole study population arterial hypertension and CKD were observed in approximately two-third of patients, whereas one-third of them were affected by type 2 diabetes, chronic CAD, peripheral arteriopathy, anemia and cognitive impairment. Compared to the “old” group, the “oldest-old” one had significantly higher prevalence of hypertension, CKD, anemia and cognitive impairment. On the other hand, type 2 diabetes, dyslipidemia and CAD were significantly more prevalent among patients aged 70–84 yrs.
At physical examination, dyspnea and fever were more frequently reported in “old” patients, whereas systolic blood pressure at hospital admission was significantly higher in the “oldest-old” group. Congestive signs and pneumonia on chest X-ray were more frequently diagnosed in the “old” group. At ECG analysis, AF was present in 37.4% of HFmrEF patients, with higher prevalence in the “oldest-old” group, whereas LBBB pattern was much more common in the “old” group.
Assessment of clinical prognostic scores at hospital admission revealed that CCI (10.0 ± 2.7 vs 8.3 ± 2.9, p = 0.002) and HAS-BLED score (3.5 ± 1.1 vs 2.4 ± 1.1, p < 0.001) were significantly higher in the “oldest-old” group than in the “old” one, suggesting higher comorbidity burden and increased bleeding risk in HFmrEF patients aged ≥ 85 yrs in comparison to those aged 70–84 yrs, while CHA2DS2-VASc score was similar in the two groups (5.1 ± 1.1 vs 5.2 ± 1.9, p = 0.74).
Regarding blood parameters, HFmrEF patients were characterized by mild anemia, moderate decline in eGFR and increased serum levels of CRP, NT-proBNP and HS troponin. Compared to “old” patients, the “oldest-old” ones had greater impairment in eGFR and significantly lower serum levels of hemoglobin, glucose and LDL cholesterol. On the other hand, serum levels of CRP, NT-proBNP and HS troponin were significantly higher in the “old” group than in the “oldest-old” one (Table 2).
Table 2.
Biochemical parameters of the whole HFmrEF study population and of the two age groups at hospital admission
| Biochemical parameters | All patients (n = 107) |
“Old” group (70–84 yrs) (n = 55) |
“Oldest-old” group (≥ 85 yrs) (n = 52) |
P value |
|---|---|---|---|---|
| Serum hemoglobin (g/dl) | 11.7 ± 2.6 | 13.0 ± 2.5 | 10.5 ± 2.4 | < 0.001 |
| Serum platelets (× 103/µl) | 259 ± 127 | 264 ± 97 | 254 ± 154 | 0.68 |
| Serum glucose (mg/dl) | 129 ± 61 | 142 ± 80 | 115 ± 24 | 0.02 |
| Serum creatinine (mg/dl) | 1.55 ± 0.96 | 1.15 ± 0.62 | 1.98 ± 1.06 | < 0.001 |
| eGFR (ml/min/m2) | 49.5 ± 27.8 | 63.2 ± 26.2 | 35.0 ± 21.4 | < 0.001 |
| Serum sodium (mEq/l) | 137 ± 7 | 136 ± 6 | 138 ± 8 | 0.14 |
| Serum potassium (mEq/l) | 4.3 ± 0.9 | 4.1 ± 0.9 | 4.5 ± 0.7 | 0.01 |
| Serum uric acid (mg/dl) | 8.4 ± 3.0 | 7.9 ± 2.8 | 8.8 ± 3.2 | 0.12 |
| Serum LDL-cholesterol (mg/dl) | 76 ± 35 | 87 ± 42 | 64 ± 20 | < 0.001 |
| Serum TSH (uU/ml) | 2.7 ± 3.4 | 1.8 ± 1.3 | 3.7 ± 4.6 | 0.004 |
| Serum CRP (mg/dl) | 7.3 ± 7.4 | 9.7 ± 8.8 | 4.7 ± 4.3 | < 0.001 |
| Serum NT-proBNP (pg/ml) | 5759 ± 6509 | 7634 ± 6510 | 3775 ± 5948 | 0.002 |
| Serum HS troponin (ng/ml) | 193 ± 255 | 320 ± 298 | 60 ± 76 | < 0.001 |
CRP C-reactive protein, eGFR estimated glomerular filtration rate, HFmrEF heart failure with mildly reduced ejection fraction, HS high-sensitive, LDL low-density lipoprotein, NT-proBNP N-terminal pro-brain natriuretic peptide, TSH thyroid stimulating hormone
Significant P values are in bold
On TTE examination performed at the admission, HFmrEF patients showed normal biventricular cavity sizes, moderate LV hypertrophy, left atrial (LA) enlargement and mild biventricular systolic dysfunction, as assessed by EF and TAPSE respectively; a moderate-to-severe mitral and tricuspid regurgitation was diagnosed in approximately half of the whole population; accordingly, LVFP and TRV were moderately increased in the whole study group. In comparison to the “oldest-old” HFmrEF patients, the “old” ones were found with significantly greater LV and right ventricular diastolic dimensions and significantly reduced biventricular systolic function. Notably, an EF < 45% was significantly more prevalent among HFmrEF patients aged 70–84 yrs than in those aged ≥ 85 yrs (56.4 vs 17.3%, p < 0.001). Moreover, “old” patients showed a significantly increased prevalence of congestive echocardiographic signs. Indeed, LVFP (assessed by the average E/e′ ratio) and TRV values were significantly higher in HFmrEF patients aged 70–84 yrs than in those aged ≥ 85 yrs and a moderate-to-severe mitral regurgitation was more frequently observed in the “old” group than in the “oldest-old” one. On the other hand, HFmrEF patients aged ≥ 85 yrs were diagnosed with significantly smaller LV diastolic dimensions, greater RWT, larger LA size and higher EF. In addition, a moderate-to-severe aortic stenosis was much more commonly detected in HFmrEF patients aged ≥ 85 yrs than in those aged 70–84 yrs (48.1 vs 18.2%, p < 0.001) (Table 3).
Table 3.
Main conventional echoDoppler parameters of the whole HFmrEF study population and of the two age groups
| EchoDoppler parameters | All patients (n = 107) |
“Old” group (70–84 yrs) (n = 55) |
“Oldest-old” group (≥ 85 yrs) (n = 52) |
P value |
|---|---|---|---|---|
| IVS (mm) | 13.5 ± 3.4 | 12.0 ± 2.5 | 15.1 ± 3.4 | < 0.001 |
| PW (mm) | 10.4 ± 1.5 | 10.2 ± 1.3 | 10.6 ± 1.7 | 0.17 |
| LVEDD (mm) | 46.8 ± 7.5 | 51.4 ± 7.3 | 41.8 ± 3.7 | < 0.001 |
| RWT | 0.45 ± 0.09 | 0.40 ± 0.06 | 0.51 ± 0.08 | < 0.001 |
| LVEDV (ml) | 77.0 ± 29.1 | 91.5 ± 32.0 | 61.7 ± 14.6 | < 0.001 |
| LVESV (ml) | 42.4 ± 17.6 | 51.7 ± 19.0 | 32.5 ± 8.1 | < 0.001 |
| EF (%) | 45.4 ± 3.6 | 43.5 ± 2.7 | 47.3 ± 3.6 | < 0.001 |
| EF < 45% (%) | 40 (37.4) | 31 (56.4) | 9 (17.3) | < 0.001 |
| E/A ratio | 1.05 ± 0.51 | 1.25 ± 0.57 | 0.87 ± 0.37 | < 0.001 |
| Average E/e′ ratio | 17.3 ± 5.1 | 19.5 ± 5.0 | 15.1 ± 4.1 | < 0.001 |
| LA A-P diameter (mm) | 47.4 ± 9.8 | 45.1 ± 9.0 | 49.6 ± 11.0 | 0.02 |
| LAV (ml) | 88.4.1 ± 35.6 | 78.4 ± 29.6 | 98.4 ± 41.1 | 0.004 |
| RVIT (mm) | 31.7 ± 8.4 | 35.0 ± 8.5 | 28.3 ± 7.0 | < 0.001 |
| TAPSE (mm) | 17.8 ± 4.4 | 17.0 ± 4.1 | 18.7 ± 4.5 | 0.04 |
| Moderate-to-severe MR (n, %) | 59 (55.1) | 41 (74.5) | 18 (34.6) | < 0.001 |
| Moderate-to-severe AR (n, %) | 25 (23.4) | 15 (27.3) | 10 (19.2) | 0.32 |
| Moderate-to-severe AS (n, %) | 35 (32.7) | 10 (18.2) | 25 (48.1) | < 0.001 |
| Moderate-to-severe TR (n, %) | 53 (49.5) | 38 (69.1) | 15 (28.8) | < 0.001 |
| TRV (m/s) | 2.94 ± 0.57 | 3.11 ± 0.62 | 2.76 ± 0.45 | 0.001 |
| IVC (mm) | 20.0 ± 6.1 | 22.3 ± 6.1 | 17.6 ± 5.2 | < 0.001 |
| SPAP (mmHg) | 43.9 ± 15.0 | 48.7 ± 16.2 | 38.7 ± 11.6 | < 0.001 |
| Aortic root (mm) | 31.5 ± 4.0 | 35.6 ± 3.5 | 27.5 ± 4.1 | < 0.001 |
| Ascending aorta (mm) | 33.5 ± 3.9 | 36.5 ± 4.2 | 30.6 ± 4.5 | < 0.001 |
A-P antero-posterior, AR aortic regurgitation, AS aortic stenosis, EF ejection fraction, HFmrEF heart failure with mildly reduced ejection fraction, IVC inferior vena cava, IVS interventricular septum, LA left atrial, LAV left atrial volume, LVEDD left ventricular end-diastolic diameter, LVEDV left ventricular end-diastolic volume, LVESV left ventricular end-systolic volume, MR mitral regurgitation, PW posterior wall, RVIT right ventricular inflow tract, RWT relative wall thickness, SPAP systolic pulmonary artery pressure, TAPSE tricuspid annular plane systolic excursion, TR tricuspid regurgitation, TRV tricuspid regurgitation velocity
Significant P values are in bold
A detailed analysis of HF characteristics and hospitalization parameters recorded in the whole population of HFmrEF patients and in the two age groups is reported in Table 4.
Table 4.
Main HFmrEF characteristics and hospitalization data in the whole study population and in the two age groups
| HF characteristics and hospitalization parameters | All patients (n = 107) |
“Old” group (70–84 yrs) (n = 55) |
“Oldest-old” group (≥ 85 yrs) (n = 52) |
P value |
|---|---|---|---|---|
| NYHA functional class | ||||
| Class III (n, %) | 42 (39.3) | 20 (36.4) | 22 (42.3) | 0.53 |
| Class IV (n, %) | 65 (60.7) | 35 (63.6) | 30 (57.7) | 0.53 |
| Etiology of HF | ||||
| Acute/chronic CAD (n, %) | 41 (38.3) | 32 (58.2) | 9 (17.3) | < 0.001 |
| Acute/chronic VHD (n, %) | 21 (19.6) | 9 (16.4) | 12 (23.1) | 0.38 |
| Hypertensive cardiomyopathy (n, %) | 37 (34.6) | 11 (20.0) | 26 (50.0) | 0.001 |
| Acute/chronic pulmonary hypertension (n, %) | 8 (7.5) | 3 (5.4) | 5 (9.6) | 0.41 |
| Reasons for hospitalizations | ||||
| Congestive heart failure (n, %) | 81 (75.7) | 49 (89.1) | 32 (61.5) | < 0.001 |
| Pneumonia/bronchitis/respiratory failure/PE (n, %) | 44 (41.1) | 29 (52.7) | 15 (28.8) | 0.01 |
| Infections (urinary tract, intestine, endocarditis) (n, %) | 32 (29.9) | 22 (40.0) | 10 (19.2) | 0.02 |
| Gastro-intestinal disorders (n, %) | 20 (18.7) | 6 (10.9) | 14 (26.9) | 0.03 |
| Severe anaemia (Hb < 8 g/dl) (n, %) | 13 (12.1) | 3 (5.5) | 10 (19.2) | 0.03 |
| Severe CKD (eGFR < 15 ml/min/m2) (n, %) | 16 (14.9) | 4 (7.3) | 12 (23.1) | 0.02 |
| Cancers (n, %) | 13 (12.1) | 3 (5.5) | 10 (19.2) | 0.03 |
| Hyponatriemia (n, %) | 13 (12.1) | 3 (5.5) | 10 (19.2) | 0.03 |
| Hypernatriemia (n, %) | 14 (13.1) | 3 (5.5) | 11 (21.1) | 0.02 |
| Neurological disorders (n, %) | 13 (12.1) | 6 (10.9) | 7 (13.5) | 0.68 |
| ≥ 2 reasons for hospitalizations (n %) | 55 (51.4) | 22 (40.0) | 33 (63.5) | 0.01 |
| Discharge therapy | ||||
| Antiplatelets (n, %) | 45 (42.0) | 30 (54.5) | 15 (28.8) | 0.007 |
| Anticoagulants (n, %) | 40 (37.4) | 15 (27.3) | 25 (48.1) | 0.03 |
| ACEIs/ARBs (n, %) | 50 (46.7) | 32 (58.2) | 18 (34.6) | 0.01 |
| CCB (n, %) | 52 (48.6) | 18 (32.7) | 34 (65.4) | < 0.001 |
| BB (n, %) | 59 (55.1) | 39 (70.9) | 20 (38.5) | < 0.001 |
| Digoxin (n, %) | 19 (17.7) | 14 (25.4) | 5 (9.6) | 0.03 |
| Loop diuretics (n, %) | 78 (72.9) | 46 (83.6) | 32 (61.5) | 0.01 |
| Aldosterone antagonists (n, %) | 39 (36.4) | 30 (54.5) | 9 (17.3) | < 0.001 |
| Statins (n, %) | 34 (31.8) | 28 (50.9) | 6 (11.5) | < 0.001 |
| Oral hypoglicemyc agents (n, %) | 32 (29.9) | 22 (40.0) | 10 (19.2) | 0.02 |
| Insulin (n, %) | 24 (22.4) | 18 (32.7) | 6 (11.5) | 0.008 |
| Length of hospital stay (days) | 10.0 ± 4.1 | 8.0 ± 3.3 | 12.0 ± 3.8 | < 0.001 |
ACEIs angiotensin-converting enzyme inhibitors, ARBs angiotensin II receptor blockers, BB beta blockers, CAD coronary artery disease, CCB calcium-channel blockers, CKD chronic kidney disease, eGFR estimated glomerular filtration rate, Hb hemoglobin, HFmrEF heart failure with mildly reduced ejection fraction, PE pulmonary embolism, VHD valvular heart disease
Significant P values are in bold
More than half of the study population (60.7%) was in New York Heart Association (NYHA) functional class IV, while the remaining 39.3% was in NYHA functional class III, with no statistically significant difference between the two age groups (p = 0.53). In the whole cohort of elderly HFmrEF patients, CAD and hypertensive cardiomyopathy were the two most common etiologies of HFmrEF. CAD was the leading cause of HFmrEF among the “old” group (58.2% of cases), whereas hypertensive cardiomyopathy was the most frequent HFmrEF cause among the “oldest-old” one (50% of cases). Congestive HF and respiratory diseases were the two main reasons for hospitalization both in the whole population and in the two study groups. However, when compared to “old” patients, the “oldest old” ones were more frequently hospitalized due to gastrointestinal diseases, severe anaemia (Hb < 8 g/dl), severe CKD (eGFR < 15 ml/min/m2), electrolyte disorders (hypo- and hypernatremia) and cancer. On the other hand, “old” patients were hospitalized for respiratory and non-respiratory infections more often than the “oldest-old” ones. A number ≥ 2 of reasons for hospitalization admission was observed in 51.4% of the whole study group, with significantly higher prevalence among the “oldest-old” patients in comparison to the “old” ones (63.5% vs 40.0%, p = 0.01).
At discharge, the majority of the “old” HFmrEF patients were prescribed with cardioprotective drugs, such as antiplatelets, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), beta blockers, loop diuretics, aldosterone antagonists and statins. On the other hand, anticoagulants were more frequently prescribed in the “oldest-old” HFmEF patients.
Finally, the length of hospital stay for the whole study population was 10.0 ± 4.1 days and it was significantly longer in “oldest-old” patients in comparison to the “old” ones (12.0 ± 3.8 vs 8.0 ± 3.3 days, p < 0.001).
Survival analysis
Mean follow-up time was 1.8 ± 1.1 yrs. During the follow-up period, 29 patients died and 45 were re-hospitalized. All-cause mortality was significantly higher among “old” patients than in “oldest-old” ones (Fig. 1, Panel A), whereas prevalence of rehospitalization for all causes did not statistically differ between the two groups (Fig. 1, Panel B). Compared to “oldest-old” patients, “old” ones showed significantly higher incidence of all-cause mortality and cardiovascular deaths. Rehospitalization rates were similar in the two groups, but “old” patients were more frequently readmitted for cardiovascular causes, while “oldest-old” ones were rehospitalized mainly due to other reasons, principally anemia and severe CKD (Table 5).
Fig. 1.

Kaplan Meier curves drawn to compare the rates of the primary outcome of “all-cause mortality” (A) and the secondary outcome of “all-cause mortality + rehospitalization for all causes” (B) in the two HFmrEF age groups. HFmrEF heart failure with mildly reduced ejection fraction
Table 5.
Primary and secondary outcomes evaluated at 1.8-year follow-up in the whole study population and in the two age groups
| Outcome at 1.8-year follow-up (HFmrEF age groups) | All patients (n = 107) |
“Old” group (70–84 yrs) (n = 55) |
“Oldest-old” group (≥ 85 yrs) (n = 52) |
P value |
|---|---|---|---|---|
| All-cause mortality + re-hospitalizations for all causes (n, %) | 74 (69.1) | 40 (72.7) | 34 (65.4) | 0.41 |
| All-cause mortality (n, %) | 29 (27.1) | 20 (36.4) | 9 (17.3) | 0.03 |
| Cardiovascular deaths (n, %) | 13 (12.1) | 12 (21.8) | 1 (1.9) | 0.001 |
| Non-cardiovascular deaths (n, %) | 16 (14.9) | 8 (14.5) | 8 (15.4) | 0.90 |
| In-hospital deaths (n, %) | 8 (7.5) | 7 (12.7) | 1 (1.9) | 0.03 |
| Time from hospital admission to death (months) | 13.6 ± 10.7 | 12.9 ± 11.6 | 17.9 ± 9.9 | 0.01 |
| Re-hospitalizations for all causes (n, %) | 45 (42.0) | 20 (36.4) | 25 (48.1) | 0.22 |
| Cardiovascular causes of rehospitalizations (n, %) | 29 (27.1) | 21 (38.2) | 8 (15.4) | 0.008 |
| Congestive heart failure (n, %) | 18 (16.8) | 14 (25.4) | 4 (7.7) | 0.01 |
| Acute coronary syndrome (n, %) | 4 (3.7) | 3 (5.5) | 1 (1.9) | 0.33 |
| Acute ischemic stroke (n, %) | 3 (2.8) | 2 (3.6) | 1 (1.9) | 0.59 |
| Deep venous thrombosis (n, %) | 5 (4.7) | 3 (5.5) | 2 (3.8) | 0.69 |
| Non-cardiovascular causes of rehospitalizations (n, %) | 16 (14.9) | 4 (7.3) | 12 (23.1) | 0.02 |
| Pneumonia (n, %) | 4 (3.7) | 1 (1.8) | 3 (5.8) | 0.28 |
| Severe anemia (Hb < 8 g/dl) (n, %) | 3 (2.8) | 1 (1.8) | 2 (3.8) | 0.52 |
| Severe CKD (eGFR < 15 ml/min/m2) (n, %) | 7 (6.5) | 1 (1.8) | 6 (11.5) | 0.04 |
| Gastro-intestinal disorders (n, %) | 2 (1.9) | 1 (1.8) | 1 (1.9) | 0.96 |
| Time from hospital admission to rehospitalizations (months) | 15.6 ± 11.5 | 12.8 ± 10.0 | 17.8 ± 12.3 | 0.02 |
CKD chronic kidney disease, eGFR estimated glomerular filtration rate, Hb hemoglobin, HFmrEF heart failure with mildly reduced ejection fraction
Significant P values are in bold
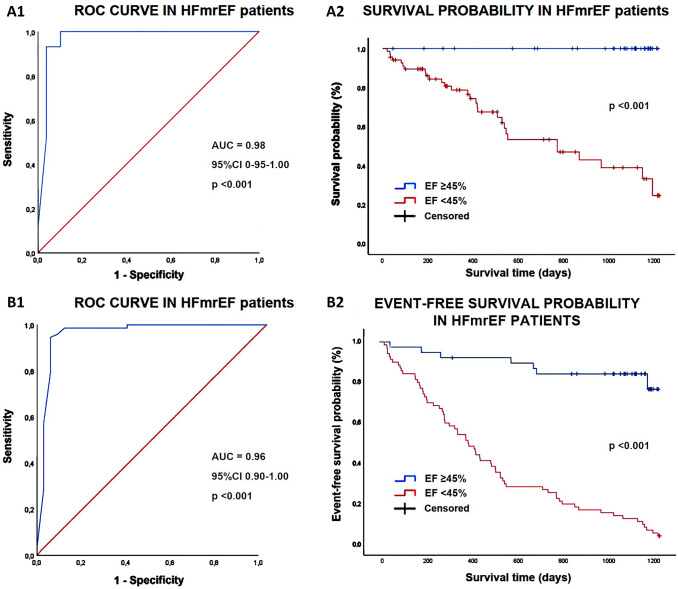
Multivariate Cox regression analysis performed for identifying independent predictors of “all-cause mortality” is reported in Table 6. Male sex (HR 6.71, 95% CI 1.59–28.4, p = 0.01), history of CAD (HR 5.37, 95% CI 2.04–14.1, p = 0.02) and EF (HR 0.48, 95% CI 0.34–0.68, p < 0.001) were independently associated with the primary outcome in the whole study population. An EF < 45% showed the greatest sensitivity and specificity for predicting the primary outcome in our cohort of HFmrEF patients (100% sensitivity, 90% specificity, AUC = 0.98). Prognostic ROC curves and Kaplan–meier survival curves drawn to compare “all-cause mortality” rates in HFmrEF patients categorized according to EF values (< 45% and ≥ 45%, respectively), are illustrated in Fig. 2, Panels A1 and A2.
Table 6.
Univariate and multivariate Cox regression analysis to identify the main variables independently associated with “all-cause mortality” at 1.8-year follow-up in the whole HFmrEF population
| Variables | Univariate cox regression analysis | Multivariate cox regression analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (yrs) | 0.96 | 0.92–1.00 | 0.04 | 1.02 | 0.96–1.08 | 0.54 |
| Male sex | 22.7 | 6.79–76.2 | < 0.001 | 6.71 | 1.59–28.4 | 0.01 |
| Smoking | 4.03 | 1.90–8.54 | < 0.001 | 1.44 | 0.63–3.32 | 0.38 |
| Hypertension | 1.10 | 0.52–2.33 | 0.80 | |||
| Type 2 diabetes mellitus | 1.82 | 0.86–3.85 | 0.12 | |||
| Dyslipidemia | 1.73 | 0.80–3.72 | 0.16 | |||
| Previous history of CAD | 8.97 | 3.80–21.1 | < 0.001 | 5.37 | 2.04–14.1 | 0.02 |
| CHA2DS2-VASc score | 1.05 | 0.82–1.35 | 0.67 | |||
| HAS-BLED score | 1.23 | 0.96–1.59 | 0.10 | |||
| CCI | 1.27 | 1.12–1.44 | < 0.001 | 1.14 | 0.95–1.38 | 0.15 |
| Serum hemoglobin (g/dl) | 0.93 | 0.81–1.08 | 0.35 | |||
| Serum sodium (mEq/l) | 0.99 | 0.94–1.05 | 0.85 | |||
| eGFR (ml/min/m2) | 0.99 | 0.98–1.01 | 0.59 | |||
| Serum CRP (mg/dl) | 1.01 | 0.96–1.06 | 0.70 | |||
| Serum NT-proBNP (pg/ml) | 1.00 | 0.99–1.01 | 0.18 | |||
| Serum HS troponin (ng/ml) | 1.00 | 0.99–1.00 | 0.74 | |||
| Heart rate (bpm) | 1.00 | 0.98–1.02 | 0.85 | |||
| Atrial fibrillation | 1.14 | 0.55–2.37 | 0.72 | |||
| LBBB | 1.27 | 0.59–2.71 | 0.53 | |||
| EF (%) | 0.52 | 0.42–0.66 | < 0.001 | 0.48 | 0.34–0.68 | < 0.001 |
| Average E/e′ ratio | 1.03 | 0.96–1.11 | 0.39 | |||
| TRV (m/s) | 1.24 | 0.68–2.26 | 0.48 | |||
| Loop diuretics | 0.85 | 0.37–1.92 | 0.69 | |||
| Beta blockers | 0.77 | 0.37–1.59 | 0.48 | |||
| Statins | 0.65 | 0.25–1.69 | 0.37 | |||
CAD coronary artery disease, CCI Charlson comorbidity index, CRP C-reactive protein, eGFR estimated glomerular filtration rate, HFmrEF heart failure with mildly reduced ejection fraction, HS high-sensitive, LBBB left bundle branch block, NT-proBNP N-terminal pro-brain natriuretic peptide, TRV tricuspid regurgitation velocity
Significant P values are in bold
Fig. 2.
Prognostic ROC curves and Kaplan–meier survival curves drawn to compare the rates of “all-cause mortality” (A1, A2) and the composite of “all-cause mortality + rehospitalization for all causes” (B1, B2) in HFmrEF patients, categorized according to EF < 45% and ≥ 45%
On multivariate Cox regression analysis, CCI (HR 1.55, 95% CI 1.08–1.27, p < 0.001) and EF (HR 0.75, 95% CI 0.66–0.85, p < 0.001) were independently associated with the composite of “all-cause mortality + rehospitalization for all causes” in the entire study population (Supplemental Table 7). A CCI ≥ 10 (99% sensitivity, 100% specificity, AUC = 0.99) and an EF < 45% (95% sensitivity, 99% specificity, AUC = 0.96) showed the greatest sensitivity and specificity for predicting the secondary outcome in our study group. The prognostic ROC curves and Kaplan–meier curves drawn for comparing the rates of “all-cause mortality + reospitalization for all causes” in HFmrEF patients categorized according to EF values (< 45% and ≥ 45%, respectively), are depicted in Fig. 2, panels B1 and B2.
Measurement variability
Intra-observer and inter-observer agreement in the assessment of EF, expressed as ICC (95% CI), was 0.91 (0.76–0.97) and 0.83 (0.56–0.94), respectively.
Discussion
Main findings of the study
In this monocentric study, carried out on a retrospective cohort of consecutive elderly patients aged ≥ 70 yrs and hospitalized due to symptoms and signs of HF and diagnosed with mildly reduced EF (41–49%) on TTE examination, demonstrated that, EF at hospital admission was the main independent predictor of both the primary outcome of “all-cause mortality” and the secondary one of “all-cause mortality and re-hospitalization for all causes” over a medium-term follow-up. ROC curve analysis indicated that an EF < 45% was the best cut-off value for predicting both outcomes. On multivariate Cox regression analysis, male sex and history of CAD were other independent prognostic indicators for all-cause mortality, whereas CCI independently predicted “all-cause mortality and re-hospitalization for all causes”.
Our results revealed that the elderly HFmrEF patients included in the present study showed completely different clinical features, when categorized in age groups. Notably, compared to the “oldest-old” patients (aged ≥ 85 yrs), the “old” ones (aged 70–84 yrs): (1) were more commonly males with previous history of CAD and increased atherosclerotic burden; (2) had a lower prevalence of AF, CKD and multicomorbities; (3) were found with lower EF associated with increased prevalence of clinical, radiological and echocardiographic signs of pulmonary congestion. Conversely, the “oldest-old” patients (aged ≥ 85 yrs) were mostly females, with long history of hypertension and CKD, generally affected by hypertensive cardiomyopathy, AF and multicomorbidities, with near-normal EF and lower prevalence of clinical and instrumental congestive signs. Survival analysis highlighted that the “old” group had a significantly higher overall mortality rate than the “oldest-old” group, over a medium-term follow-up, whereas the prevalence of rehospitalization for all causes was similar in the two groups of HFmrEF patients. Due to the increased severity of cardiac disease, patients aged 70–84 yrs had a significantly higher incidence of cardiovascular deaths, in-hospital deaths and rehospitalization for cardiovascular causes than those aged ≥ 85 yrs.
Comparison with previous studies and interpretation of results
To date, literature data regarding HFmrEF are mainly derived from observational single-centre or multicentre studies, sub-analyses of clinical trials, and large registries, such as the ESC HF Long-Term Registry [5, 9, 10, 28–31]. However, the majority of studies [5, 8–13] included HFmrEF patients aged < 70 yrs and literature data concerning HFmrEF patients aged ≥ 70 yrs are scanty.
A number of studies [5, 9, 10, 28–31] reported that clinical features of patients with HFmrEF were more similar to HFrEF than HFpEF. In particular, compared to patients with HFpEF patients, those with HFmrEF were more commonly men, younger, more frequently affected by chronic CAD (50–60% of cases) and less likely to have hypertension, AF and non-cardiac comorbidities.
In our study, clinical, instrumental and prognostic characteristics of elderly HFmrEF patients aged 70–84 yrs were similar to those of HFrEF patients, whereas the “oldest-old” ones had several analogies with HFpEF patients. Notably, in our retrospective cohort of HFmrEF patients, those aged 70–84 yrs had significantly higher prevalence of male sex, type 2 diabetes, dyslipidemia and history of CAD, in comparison to the “oldest-old” ones. These findings were in line with previous studies [29, 32]. It is likely that the HFmrEF elderly patients included in our study might be a transition phenotype of “old” patients with HFrEF who are recovering, or of “oldest-old” patients with HFpEF who are declining. Indeed, a substantial proportion of HF patients may show dynamic changes in EF over time, especially those with ischaemic disease and HFmrEF may be a transition from one category to another [8, 33–39].
Concerning HFmrEF prognosis at 1–3 years follow-up, literature data are still controversial. It has been reported that all-cause mortality in HFmrEF patients: (1) was less than HFrEF but similar to HFpEF [39]; (2) was similar to HFrEF and HFpEF [29]; (3) was higher than HFpEF patients [14, 30, 40, 41]. The follow-up data about the “old” group of HFmrEF patients included in our study would be consistent with an increased mortality rate in HFmrEF patients compared to HFpEF patients [14, 30, 40, 41].
Our findings highlighted that EF on TTE examination at hospital admission was the strongest independent predictor of both all-cause mortality and rehospitalization for all causes in elderly HFmrEF patients. These findings confirmed the incremental prognostic value of EF for mortality risk stratification in HF patients [16, 42]. EF is currently the most widely used index of LV systolic function. It is noninvasive, easy to obtain, well-known and understood by the majority of internists and cardiologists. In routine clinical care, EF is used to classify HF types and repeated EF assessments may help clinicians to guide and/or optimize cardioprotective treatment [43]. Despite these advantages, EF has several limitations, including the geometric assumptions made in its calculation, its high load-dependence and the significant intraobserver, interobserver and test–retest variability [44, 45]. Moreover, EF may be overestimated in the presence of severe aortic or mitral regurgitation [46] and, most of all, may not intercept subtle and/or subclinical myocardial dysfunction in the presence of ventricular hypertrophy, aortic stenosis, cardiac amyloidosis or diabetic cardiomyopathy [47].
Consistent with literature data [48, 49], our findings confirmed the increased mortality risk for males with a worse systolic function and an ischemic HF etiology.
Multivariate Cox analysis also revealed that CCI score, calculated at hospital admission, independently predicted the composite of “all-cause mortality + reospitalization for all causes” in the whole group of HFmrEF patients. The CCI, which is a summed score of 19 comorbidities weighted according to severity [23], can be easily obtained from the patients’ electronic medical records and/or from International Classification of Diseases (ICD) code at discharge [50]. This comorbidity index has been found to predict clinical outcome in different cardiovascular [51–53] and non-cardiovascular [54–56] conditions. Consistent with literature data [57], in our study “old” HFmrEF patients showed lower CCI scores than the “oldest-old” ones.
Concerning medical treatment at discharge, significant differences were observed between the two age groups of HFmrEF patients. Indeed, beta-blockers, loop diuretics, aldosterone antagonists and statins were more frequently prescribed in “old” patients than in “oldest old” ones. This finding could be attributed to the fact that “old” HFmrEF patients were more frequently diagnosed with EF < 45% and congestive clinical and instrumental signs, whereas “oldest-old” patients suffered from HFmrEF of hypertensive etiology with less degree of systolic dysfunction. Due to their frequent CAD history, antiplatelets were more commonly prescribed in “old” patients than in “oldest-old” ones. Notably, despite greater HAS-BLED scores and in front of similar CHA2DS2-VASc scores, “oldest-old” patients were more frequently discharged with anticoagulant therapy, probably due to higher prevalence of AF.
Implications for clinical practice
With improvements in acute coronary syndrome management, the prevalence of HFmrEF will probably increase over that of HFrEF within the next few years [58]. It is noteworthy that HFmrEF patients are an heterogenous and dynamic group of patients, rather than a unique subtype. This assumption is particularly evident in elderly HFmrEF patients, when they are categorized and evaluated according to age groups. In particular, HFmrEF in elderly patients aged ≥ 70 yrs may include “old” patients (aged 70–84 yrs) who have recovered from previous HFrEF and “oldest-old” patients (aged ≥ 85 yrs) who have deteriorated from previous HFpEF. As highlighted by our retrospective analysis, “old” and “oldest-old” HFmrEF patients have demographic, clinical and echocardiographic features which resemble those of HFrEF and HFpEF patients, respectively. A TTE-derived EF < 45%, obtained at hospital admission, might help the clinicians to identify, among HFmrEF patients, those with increased mortality and rehospitalization risk, over a medium-term follow-up. Our results would suggest that an EF value between 41 and 49% might not identify an univocal typology HF subtype, particularly in the elderly HF patients. In other terms, an EF range 41–49% could be too large in internal and geriatric practice, since it could include two completely different phenotypes of elderly HFmrEF patients, such as those described in the present study. We believe that an EF cut-off value of 45% might better distinguish HF patients with mild systolic dysfunction (EF between 50 and 45%) from those with moderate systolic dysfunction (EF between 44 and 40%). Moreover, EF should not be considered as a static value, but rather a dynamic parameter that may rapidly change over time, particularly in patients with chronic CAD who undergo cardioprotective treatment and/or multiple percutaneous coronary interventions or surgical coronary revascularization [48, 59, 60]. Accordingly, echocardiography follow-up should be implemented in clinical practice for measuring EF trajectory over time and determining the clinical course of HFmrEF. Finally, serial EF assessment might contribute to guide pharmacological treatment and improve prognosis. Indeed, being CAD the primary cause of HFmrEF, initiation and adequate up-titration of cardioprotective drugs for the management of coronary disease may represent the key to improve prognosis of these patients [61].
Limitations
The main limitations of the present study were its retrospective nature, its monocentric design and the small sample size of HFmrEF patients included. However, the great number of major adverse clinical outcomes over a mid-term follow-up, allowed us to perform an accurate survival analysis in both “old” and “oldest-old” HFmrEF patients.
Furthermore, given that the elderly HF patients we enrolled were admitted to a Division of Internal Medicine, and not of Geriatric Medicine, a comprehensive geriatric assessment, which is the cornerstone for a reliable estimate of prognosis in older patients, was not performed; however, even if we did not use the Multidimensional Prognostic Index (MPI) as prognostic indicator in our study population, a detailed description of the patients’ cognitive status and comorbidities was provided as accurately as possible. In addition, EF was obtained at hospital admission only, and echocardiographic data about previous hospitalizations or at the time of discharge were not collected. Therefore, diagnosis was only established on the basis of single time-point EF measurement. Finally, similarly to our previous studies performed in very old hospitalized patients [16, 62], body surface area (BSA) could not be precisely assessed in all the elderly patients enrolled, due to the poor global conditions of the majority of them, bedridden and frequently uncooperative. For this reason, echocardiographic measures were not indexed to BSA.
Conclusions
In the present study, for demographic, clinical and echocardiographic characteristics HFmrEF patients aged 70–84 yrs resembled those with HFrEF. Conversely, those aged ≥ 85 yrs were more similar to HFpEF ones. As a result, in our analysis HFmrHF seems to configure a transitional stage between HFrEF and HFpEF rather than a unique subtype.
EF is independently associated with all-cause mortality and re-hospitalization for all causes over a medium-term follow-up in HFmrEF patients aged 70 years and older.
Echocardiography follow-up should be implemented in clinical practice for measuring EF trajectory over time and determining the clinical course of HFmrEF.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1—Univariate and multivariate Cox regression analysis to identify the main variables independently associated with the composite of “all-cause mortality + rehospitalization for all causes” in the whole HFmrEF population. CAD, coronary artery disease; CCI, Charlson comorbidity index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mildly reduced ejection fraction; HS, high-sensitive; LBBB, left bundle branch block; NT-proBNP, N-terminal pro-brain natriuretic peptide; TRV, tricuspid regurgitation velocity. (DOCX 49 KB)
Author contributions
AS: conceptualization; data curation; investigation; methodology; software; visualization; writing—original draft. CL: conceptualization; data curation; investigation; methodology; writing—review and editing. MB: data curation; investigation; methodology; writing—review and editing. GLN, ML: supervision; validation; writing—review and editing. SH: conceptualization; supervision; validation; writing—review and editing.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Conflict of interest
We wish to confirm that there are no conflicts of interest associated with this publication. Andrea Sonaglioni declares that he has no conflict of interest. Chiara Lonati declares that she has no conflict of interest. Marta Behring declares that she has no conflict of interest. Gian Luigi Nicolosi declares that he has no conflict of interest. Michele Lombardo declares that he has no conflicts of interest. Sergio Harari reports grants and personal fees from Roche, AstraZeneca and Boehringer Ingelheim, outside the submitted work.
Ethical approval
All procedures performed in the present study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Need for informed consent was not required due to the retrospective nature of this study.
Human and animal rights
All the procedures performed in the present study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 2.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 3.Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%) Eur J Heart Fail. 2014;16:1049–1055. doi: 10.1002/ejhf.159. [DOI] [PubMed] [Google Scholar]
- 4.Farmakis D, Simitsis P, Bistola V, et al. Acute heart failure with mid-range left ventricular ejection fraction: clinical profile, in-hospital management, and short-term outcome. Clin Res Cardiol. 2017;106:359–368. doi: 10.1007/s00392-016-1063-0. [DOI] [PubMed] [Google Scholar]
- 5.Rickenbacher P, Kaufmann BA, Maeder MT, et al. Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) Eur J Heart Fail. 2017;19:1586–1596. doi: 10.1002/ejhf.798. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi A, Novak E, Platts AE, et al. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid-range ejection fraction. Eur J Heart Fail. 2017;19:1597–1605. doi: 10.1002/ejhf.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Li P, Zhao H, et al. Heart failure with mid-range ejection fraction: a distinctive subtype or a transitional stage? Front Cardiovasc Med. 2021;8:678121. doi: 10.3389/fcvm.2021.678121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuji K, Sakata Y, Nochioka K, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail. 2017;19:1258–1269. doi: 10.1002/ejhf.807. [DOI] [PubMed] [Google Scholar]
- 9.Koh AS, Tay WT, Teng THK, et al. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail. 2017;19:1624–1634. doi: 10.1002/ejhf.945. [DOI] [PubMed] [Google Scholar]
- 10.Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1574–1585. doi: 10.1002/ejhf.813. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 12.Sweitzer NK, Lopatin M, Yancy CW, et al. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–1156. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Gök G, Kılıç S, Sinan ÜY, et al. Epidemiology and clinical characteristics of hospitalized elderly patients for heart failure with reduced, mid-range and preserved ejection fraction. Heart Lung. 2020;49:495–500. doi: 10.1016/j.hrtlng.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Gavina C, Carvalho DS, Valente F, et al. 20 Years of real-world data to estimate the prevalence of heart failure and its subtypes in an unselected population of integrated care units. J Cardiovasc Dev Dis. 2022;9:149. doi: 10.3390/jcdd9050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonaglioni A, Lonati C, Tescaro L, et al. Prevalence and clinical outcome of main echocardiographic and hemodynamic heart failure phenotypes in a population of hospitalized patients 70 years old and older. Aging Clin Exp Res. 2022;34:1081–1094. doi: 10.1007/s40520-021-02025-4. [DOI] [PubMed] [Google Scholar]
- 17.Sung SH, Wang TJ, Cheng HM, et al. Clinical characteristics and outcomes in the very elderly patients hospitalized for acute heart failure: importance of pharmacologic guideline adherence. Sci Rep. 2018;8:14270. doi: 10.1038/s41598-018-32684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tromp J, Shen L, Jhund PS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74:601–612. doi: 10.1016/j.jacc.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 19.Obata H, Izumi T, Yamashita M, et al. Characteristics of elderly patients with heart failure and impact on activities of daily living: a registry report from super-aged society. J Card Fail. 2021;27:1203–1213. doi: 10.1016/j.cardfail.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 22.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 28.Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I, et al. Mid-range left ventricular ejection fraction: clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol. 2017;240:265–270. doi: 10.1016/j.ijcard.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Crespo-Leiro MG, Anker SD, Maggioni AP, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–625. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor JR, Kapoor R, Ju C, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. 2016;4:464–472. doi: 10.1016/j.jchf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Vedin O, Lam CSP, Koh AS, et al. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail. 2017;10:e003875. doi: 10.1161/CIRCHEARTFAILURE.117.003875. [DOI] [PubMed] [Google Scholar]
- 32.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart failure survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 33.Mann DL, Burkhoff D. Is myocardial recovery possible and how do you measure it? Curr Cardiol Rep. 2012;14:293–298. doi: 10.1007/s11886-012-0264-z. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013. doi: 10.1161/CIRCULATIONAHA.110.954388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. doi: 10.1001/jamacardio.2016.1325. [DOI] [PubMed] [Google Scholar]
- 37.Lupón J, Díez-López C, de Antonio M, et al. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail. 2017;19:1615–1623. doi: 10.1002/ejhf.824. [DOI] [PubMed] [Google Scholar]
- 38.Cho JH, Choe WS, Cho HJ, et al. Comparison of characteristics and 3-year outcomes in patients with acute heart failure with preserved, mid-range, and reduced ejection fraction. Circ J. 2019;83:347–356. doi: 10.1253/circj.CJ-18-0543. [DOI] [PubMed] [Google Scholar]
- 39.Raja DC, Samarawickrema I, Das S, et al. Long-term mortality in heart failure with mid-range ejection fraction: systematic review and meta-analysis. ESC Heart Fail. 2022;9:4088–4099. doi: 10.1002/ehf2.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobbs FD, Roalfe AK, Davis RC, et al. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES) Eur Heart J. 2007;28:1128–1134. doi: 10.1093/eurheartj/ehm102. [DOI] [PubMed] [Google Scholar]
- 41.Nadruz W, Jr, West E, Santos M, et al. Heart failure and midrange ejection fraction: implications of recovered ejection fraction for exercise tolerance and outcomes. Circ Heart Fail. 2016;9:e002826. doi: 10.1161/CIRCHEARTFAILURE.115.002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshihisa A, Ichijo Y, Sato Y, et al. Comprehensive clinical characteristics of hospitalized patients with mid-range left ventricular ejection fraction. Eur J Prev Cardiol. 2020;27:2084–2088. doi: 10.1177/2047487319859689. [DOI] [PubMed] [Google Scholar]
- 43.Bristow MR, Kao DP, Breathett KK, et al. Structural and functional phenotyping of the failing heart: is the left ventricular ejection fraction obsolete? JACC Heart Fail. 2017;5:772–781. doi: 10.1016/j.jchf.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otterstad JE, Froeland G, St John Sutton M, et al. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 45.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–274. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Kamperidis V, Marsan NA, Delgado V, et al. Left ventricular systolic function assessment in secondary mitral regurgitation: left ventricular ejection fraction vs. speckle tracking global longitudinal strain. Eur Heart J. 2016;37:811–816. doi: 10.1093/eurheartj/ehv680. [DOI] [PubMed] [Google Scholar]
- 47.Sonaglioni A, Nicolosi GL, Rigamonti E, et al. Molecular approaches and echocardiographic deformation imaging in detecting myocardial fibrosis. Int J Mol Sci. 2022;23:10944. doi: 10.3390/ijms231810944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke CL, Grunwald GK, Allen LA, et al. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:680–686. doi: 10.1161/CIRCOUTCOMES.111.000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez-Sellés M, Doughty RN, Poppe K, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur J Heart Fail. 2012;14:473–479. doi: 10.1093/eurjhf/hfs026. [DOI] [PubMed] [Google Scholar]
- 50.D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/S0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 51.Schelde AB, Schmidt M, Madsen M, et al. Impact of the Charlson Comorbidity Index score on risk prediction by single-photon emission computed tomography myocardial perfusion imaging following myocardial infarction. Clin Epidemiol. 2019;11:901–910. doi: 10.2147/CLEP.S211555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shuvy M, Zwas DR, Keren A, et al. The age-adjusted Charlson comorbidity index: a significant predictor of clinical outcome in patients with heart failure. Eur J Intern Med. 2020;73:103–104. doi: 10.1016/j.ejim.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 53.Zhang F, Wong C, Chiu Y, et al. Prognostic impact of comorbidity measures on outcomes following acute coronary syndrome: a systematic review. Int J Clin Pract. 2021;75:e14345. doi: 10.1111/ijcp.14345. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita K, Watanabe M, Mine S, et al. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today. 2018;48:632–639. doi: 10.1007/s00595-018-1630-2. [DOI] [PubMed] [Google Scholar]
- 55.Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53:235–240. doi: 10.1093/ejcts/ezx215. [DOI] [PubMed] [Google Scholar]
- 56.Sonaglioni A, Lombardo M, Albini A, et al. Charlson comorbidity index, neutrophil-to-lymphocyte ratio and undertreatment with renin-angiotensin-aldosterone system inhibitors predict in-hospital mortality of hospitalized COVID-19 patients during the omicron dominant period. Front Immunol. 2022;13:958418. doi: 10.3389/fimmu.2022.958418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dădârlat-Pop A, Sitar-Tăut A, Zdrenghea D, et al. Profile of obesity and comorbidities in elderly patients with heart failure. Clin Interv Aging. 2020;15:547–556. doi: 10.2147/CIA.S248158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marwick TH. Ejection fraction pros and cons: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2360–2379. doi: 10.1016/j.jacc.2018.08.2162. [DOI] [PubMed] [Google Scholar]
- 60.Lupón J, Gavidia-Bovadilla G, Ferrer E, et al. Dynamic trajectories of left ventricular ejection fraction in heart failure. J Am Coll Cardiol. 2018;72:591–601. doi: 10.1016/j.jacc.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 61.Ito M, Wada H, Sakakura K, et al. Clinical characteristics and long-term outcomes of patients with acute decompensated heart failure with mid-range ejection fraction. Int Heart J. 2019;60:862–869. doi: 10.1536/ihj.18-631. [DOI] [PubMed] [Google Scholar]
- 62.Sonaglioni A, Lombardo M, Baravelli M, et al. AnatoMy and physIopathoLogy of the heArt in a ceNtenarian cOhort (MILANO study) Am Heart J. 2018;205:12–20. doi: 10.1016/j.ahj.2018.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1—Univariate and multivariate Cox regression analysis to identify the main variables independently associated with the composite of “all-cause mortality + rehospitalization for all causes” in the whole HFmrEF population. CAD, coronary artery disease; CCI, Charlson comorbidity index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mildly reduced ejection fraction; HS, high-sensitive; LBBB, left bundle branch block; NT-proBNP, N-terminal pro-brain natriuretic peptide; TRV, tricuspid regurgitation velocity. (DOCX 49 KB)
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.