Abstract
Purpose:
The authors examined the longitudinal stability of genetic and environmental influences on children’s productive language sample measures during the early school-age years.
Method:
Twin study methodology with structural equation modeling was used to derive univariate estimates of additive genetic (A), shared environmental (C), and nonshared environmental (E) effects on language measures at each of 2 time points, based on 487 twins at the 1st-grade time point and 387 twins at the 2nd-grade time point. To address questions of stability over time, the authors used longitudinal latent factor analysis.
Results:
Stability in the Conversational Language factor was accounted for almost entirely by shared genetic effects between 1st and 2nd grade, meaning no new genetic effects were observed at the 2nd time point. In contrast, nonshared environmental effects were entirely time point specific, meaning whatever nonshared environmental influences were operating at the first time point were not influencing individual variation in the language factor at the second time point.
Conclusion:
The discussion in this article centers on possible candidates for both genetic and nonshared environmental effects as well as implications for clinical practice and future research.
Keywords: language, elementary pupils, expressive language assessment
The present behavioral genetic study focused on examining the etiology of variance in children’s language production within discourse as it unfolded across the early school-age years. Despite the tendency of investigators to focus on the striking development of language within the first few years of life, spoken language skills continue to develop during the early school-age years, including diversification of vocabulary (cf. G.A. Miller & Gildea, 1987; J. F. Miller, Freiberg, Rolland, & Reeves, 1992; Nagy, Herman, & Anderson, 1985), derivational morphology (e.g., Nippold & Sun, 2008), and complex syntax (Eisenberg et al., 2008; Rice, Redmond, & Hoffman, 2006; Scott, 1984; Scott&Stokes, 1995).The transition into early elementary school is critical given the increased reliance on formalized instruction; the emphasis on explicit language-based skills, such as reading and writing; and the heightened role of peer relationships.
Differences in language skills have real-world implications for school-age children, in regard to both academic (e.g., Catts, Adolf, Hogan, & Weismer, 2005; DeThorne et al., 2006; Nathan, Stackhouse, Goulandris, & Snowling, 2004) and social success (Fujiki, Brinton, Isaacson, & Summers, 2001; Fujiki, Brinton, Robinson, & Watson, 1997; Redmond & Rice, 1998; Windsor, 1995); however, children’s developmental status is not static, and their trajectory is not always linear. For example, there is evidence of late-talking toddlers “catching up” in their spoken language skills by school age (e.g., Dale, Price, Bishop, & Plomin, 2003; Paul, 1996; Scarborough & Dobrich, 1990). Such phenotypic changes overtime lead to questions regarding the stability of genetic and environmental effects, as well as issues of gene–environment interaction. Genetic effects are not all set in motion at conception and constant across one’s lifetime. There is increasing evidence of change in genetic effects over time, with genes being “turned on and off” over time and their influences being mediated and moderated by environmental effects (Rutter, 2006). As a simplified illustration, Hewitt, Emde, and Plomin (2001) highlighted three possible models for change and stability in genetic effects over time, which we apply here to the issue of language development in particular: (a) complete genetic stability over time, meaning no new genetic effects impinge on language development over the period of time under study; (b) complete genetic specificity, meaning heritability in language use at each time point is due to different genes; and (c) a combination of genetic overlap and specificity, meaning some of the same genes influence language development across time points, whereas others contribute only at specific periods of development. Such stability and specificity can be examined for each etiological effect, including shared and nonshared environmental effects.
Methodologically, twin studies can tease apart genetic and environmental effects on language by comparing the phenotypic similarities of monozygotic (MZ) twins, who share 100% of their segregating genes, with dizygotic (DZ) twins, who share on average only 50% of such genetic material. As a consequence, the extent to which MZ twins are more similar than DZ twins in their language skills provides an estimate of heritability for that phenotype—that is, the proportion of the total phenotypic variation that can be attributed to genetic effects: . The similarity in MZ twins that can not be accounted for by genetic effects, is attributed to shared environmental effects that influence both twins within a pair . Shared environmental effects encompass any nongenetic factors that would be shared between twins. Such effects could include exposure to environmental toxins, birth trauma, and parenting practices. Remaining differences between MZ twins are attributed to nonshared environmental effects and measurement error . Nonshared environmental influences include nongenetic factors that differ between twins, such as injuries, illnesses, and aspects of interactions that are unique to each child.
Building on this same conceptual foundation, one can use multivariate behavioral genetic analysis to disentangle the genetic versus environmental sources of covariance of traits overtime to estimate stability of causal effects (cf. Plomin, 1986); specifically, twin correlations are estimated across time points, so that measurement at the first time point is correlated with the same measure at the later time point. The difference in cross-time correlations between MZ and DZ twins provides an indication of genetic influence on the stability between the two traits. Bivariate heritability refers to the extent to which genetic factors account for the phenotypic correlation between the traits (bivariate ). For example, a bivariate heritability of. 30 would suggest that 30% of the phenotypic correlation between timepoints is genetically mediated. A related concept, genetic correlation, is derived by dividing the genetic covariance of two variables by the square root of the product of the genetic variances. Genetic correlations estimate the extent to which genetic influences on one trait correlate with genetic influences on a second trait, independent of the heritabilities of the traits (Plomin, DeFries, McClearn, & McGuffin, 2008). In the present context, the genetic correlation indicates the extent to which genetic influences on language skills at the second time point are the same as those that influence language skills at the first time point.
Using the twin method, genetic effects on various language measures have been well documented, although the strength of estimates varies widely (cf. Plomin & Kovas, 2005, and Stromswold, 2001, for reviews).Such variability has been attributed to differences in form of measurement (DeThorne et al., 2008), environmental circumstances (Rowe, Jacobson, & Van den Oord, 1999), and child age (Plomin, Fulker, Corley, & DeFries, 1997). Despite the potential role age might play, few studies have the size and longevity to study the longitudinal stability in causal effects over time in the same sample. Spinath, Price, Dale, and Plomin (2004) examined the influence of age, sex, and severity on the heritability of child language skill using 1,943 twin pairs from The Twins Early Development Study (Trouton, Spinath, & Plomin, 2002).1 Using data from a parent-report instrument, the authors found that the heritability of individual differences in child language use ranged from .18 to .23 for girls and .27 to .34 for boys across the annual timepoints of 2, 3, and 4 years of age. Systematic differences related to age were not observed, and multivariate estimates of genetic or environmental stability were not provided.
Despite the paucity of analyses related to stability of genetic and environmental effects on child language, analyses focused on IQ, verbal IQ included, have revealed a trend toward increasing heritability with age (e.g., Haworth et al., 2010; Hoekstra, Bartels, & Boomsma, 2007; Plomin et al., 1997), as well as evidence of genetic stability (e.g., Hoekstra et al., 2007; Price, Dale, & Plomin, 2004). The finding of increasing heritability over time has been attributed to gene–environment correlations—or, more specifically, the notion that as children get older they gain increasing influence over their environments (Haworth et al., 2010; Rutter & Plomin, 1997; Scarr & McCartney, 1983). Environmental variance yoked to genetic variance in this way could increase heritability estimates over time (cf. Hopper, 2000; Plomin, DeFries, & Loehlin, 1977). Another possible explanation for increasing heritability over time would be the influence of “new” genes that come into play as children develop, which would imply findings of at least some genetic specificity at later time points. In contrast, however, early findings across domains have suggested high genetic stability, which has been attributed to the concept of “generalist genes” that operate throughout development and have an impact on multiple domains (Hoekstra et al., 2007; Plomin & Kovas, 2005).
In the present study, we examined the stability of genetic and environmental influences on children’s language measures taken from conversational interactions. In particular, we focused on conversational interactions, given their common use in phenotypic research on child language and their inherent social validity; it is through conversation that children express their thoughts, shape the impressions of others, and solidify their relationships. The current analyses build directly on DeThorne et al.’s (2008) study from the Western Reserve Reading Project (WRRP; Petrill, Deater-Deckard, Thompson, DeThorne, & Schatschneider, 2006), which used a sample of 380 twins to estimate the extent of genetic and environmental influences on children’s language measures at a mean age of 7 years (see also DeThorne & Hart, 2009). Measures taken from conversational language samples (i.e., mean length of utterance, number of total words, number of different words, measure D, total number of conjunctions, and development sentence scores) loaded strongly on a single factor and were therefore combined into one factor, labeled Conversational Language. Approximately 70% of variability in the children’s Conversational Language factor was attributed to genetic effects, with the remaining variance accounted for by nonshared environmental effects. Comparable language data are now available for a time point 1 year later, thereby providing a means of directly examining the stability of these causal influences over time.
Although two annual assessments span a limited period of development, 1 year is a fairly common window for examining behavioral change(Dale et al., 2003;Price et al., 2004; Spinath et al., 2004), and with this research, we are providing one of the first empirical tests of the causal stability in language during the early school-age years. Findings of stable versus new effects will inform predictions of whether findings of specific genetic or nonshared environmental effects on child language use would be consistent across development. From a clinical standpoint, understanding the timing and mechanism of both genetic and environmental influences on development could inform decisions regarding when and how to intervene. This study’s three specific research questions were as follows:
What is the extent of genetic and environmental effects on individuals’ productive language measures at each of two annual home visits?
What is the stability of genetic and environmental influences across the time points as indexed by the phenotypic correlation?
Is there evidence for “new” genetic or environmental influences on the productive language measures across time?
Method
Participants
All participants were from the WRRP (Petrill et al., 2006), a longitudinal study of reading and related cognitive skills that included annual home visits beginning at kindergarten/first grade and extending to fifth grade. The project received institutional review board approval through all participating institutions. The present study focused on the first two annual home visits at which conversational language samples were collected: These are referred to here and in previous publications as the second and third annual home visits (HV2 and HV3, respectively; also referred to in other publications as Wave 2 and Wave 3). The first home visit is not included in the present analyses because it did not include a systematic language sample.
Twins were selected from the WRRP on the basis of thepresenceoflanguagesampledatafromeitherHV2or HV3, as well as complete data on age, biological sex, and zygosity; this process resulted in 534 twins. Thirty-six cases were subsequently excluded because of a history of ambiguous or persistent hearing difficulties, resulting in an effective sample of 498twins with relevant data for atleast one of the two home visits. Specifically, 487 twins with a mean age of 7.13 years () had HV2 data, and 387 twins with a mean age of 8.31 years () had HV3 data. Longitudinal analyses were contingent on the 376 twins who had data from both home visits. The percentages of male twins were equally distributed across zygosity types and home visits, ranging from 39% to 41%. Approximately 92% of the total sample was Caucasian, and 94% of primary caregivers had completed high school. Based on a parent-report measure detailed in DeThorne et al. (2006), 20% of the included twins had seen a speech-language pathologist at some point in their development, with 78% never having seen a speech-language pathologist, and 2% having had ambiguous or missing data for this item.
Procedure
Each home visit was conducted by a pair of examiners who each simultaneously evaluated one child within a twin pair using a battery of standardized assessments and parent questionnaires. In addition, HV2 and HV3 included the recording of conversational language samples between the child and examiner during 15 min of free play with modeling clay. The rationale for conversational instead of narrative samples was based on the flexibility that the former provides to follow the child’s lead, the presence of established guidelines for eliciting samples (e.g., Leadholm & Miller, 1992), and the ability to control the length of the sample. All examiners in the present study were specifically trained in language sample collection guidelines from Leadholm and Miller (1992) that included specifications such as (a) offer information of interest to the child, (b) limit direct requests and closed-ended questions, and (c) try to introduce three to four different conversational topics (see DeThorne & Hart, 2009, for complete guidelines).
Samples were recorded on cassette tape or compact flash card. Transcription into Systematic Analysis of Language Transcripts (SALT, Version 8; see http://www.saltsoftware.com/) was completed by undergraduate and graduate students in speech and hearing science who were trained through review of the SALT tutorial and laboratory manual (Child Language & Literacy Lab, 2010; J. F. Miller, 2004), followed by transcription of practice samples until 85% agreement on both utterance boundaries and individual morphemes was achieved with an experienced transcriber. Transcription reliability was constantly monitored through independent retranscription of atleast every15thsample, paired with calculation of point-by-point agreement. Across 43HV2 samples, this process resulted in an average agreement of .90 (range: .70–1.00) for conversational unit (C-unit) boundaries and .91 (range: .80–.98) for individual morphemes. Analogous values across 45 HV3 samples resulted in a mean agreement of .93 (range: .84–.99) for C-unit boundaries and .92 (range: .81–.99) for morphemes.
Given that the frequent use of conjoining conjunctions during the school-age years may serve to inflate utterance length, segmentation was determined by conventions for C-units (Loban, 1976; Nippold, 1998), meaning that independent clauses joined by the conjunctions and, but, and or were segmented into separate utterances (i.e., C-units). Twins within each pair were transcribed by different research assistants who were naBve to twin zygosity. All transcribed samples were independently reviewed by a second research assistant for correction of explicit errors. Questions or disagreements related to transcription conventions were resolved through discussion at weekly laboratory meetings with the first author. After being transcribed and checked, dependent variables were derived through SALT. In the following sections, we describe the specific measures we selected given their ubiquitous nature in the child language literature, ease of automated analyses, and the existence of reasonable evidence of developmental change during the school-age years and/or ability to differentiate groups (cf. Leadholm & Miller, 1992; J. F. Miller et al., 1992; Rice, 2004; Rice et al., 2006; Scott & Windsor, 2000).
Total complete and intelligible C-units.
This variable (here after, TCICU) represented the total number of complete and intelligible child C-units within a 15-min sample, thereby serving as a general measure of productivity or volubility (Leadholm & Miller, 1992). This measure has revealed group differences between school-age children with language disabilities and their same-age peers (e.g., Scott & Windsor, 2000).
Number of total words (NTW).
NTW, a count of all root words from the first 100 complete and intelligible child C-units in each sample, provided an additional measure of volubility. Values were not derived for samples with fewer than 100 complete and intelligible C-units. NTW was derived on the basis of 100 C-units rather than the entire sample in order to be comparable to the measure used by DeThorne et al. (2008) and because the resulting measure appears less confounded by aspects of child temperament (DeThorne, Deater-Deckard, Mahurin-Smith, Coletto, & Petrill, 2011). Though often considered a measure of volubility or fluency (Leadholm & Miller, 1992; Scott & Windsor, 2000), NTW tends to correlate significantly with measures of linguistic complexity, such as mean length of utterance (MLU) and number of different words (NDW; e.g., DeThorne et al., 2008).
MLU.
A measure of average utterance length, MLU was derived from all complete and intelligible child C-units within the sample. Consistent with conventions from Leadholm and Miller (1992), we counted individual morphemes, including all root words and the following bound morphemes: plural /–s/, possessive /–s/, present progressive /–ing/, regular past tense/–ed/, third person singular /–s/, and contracted forms of DO and BE. Morphemes, rather than words, were the focus of the measure because of our interest in capturing variation in morphological complexity. Although often referred to as a measure of grammatical complexity, we present MLU more generally as a global measure of language productivity given its confound with other linguistic constructs, vocabulary in particular (DeThorne, Johnson, & Loeb, 2005; Hutchins, Brannick, Bryant, & Silliman, 2005).
NDW.
As a measure of lexical diversity, NDW provided a count of the total number of different root words used within the first 100 complete and intelligible child C-units in the sample. Based on transcription conventions for inflectional and derivational morphemes (Leadholm & Miller, 1992), the words friend and friendly would be counted as different words, but the words jump and jumped would be counted as the same root word. Validity evidence for NDW includes correlation with standardized vocabulary measures (e.g., Ukrainetz & Blomquist, 2002) and differentiation of child language ability (e.g., Watkins, Kelly, Harbers, & Hollis, 1995).
Total number of conjunctions (TNC).
Intended as a general measure of syntactic complexity (Leadholm & Miller, 1992; Nippold, 1998), TNC provided a frequency count of each child’s use of 12 earlier developing conjunction types within their first 100 complete and intelligible utterances: after, and, as, because, but, if, or, since, so, then, until, and while. This analysis relied on the automated conjunction count available via SALT based on the availability of reference data indicating developmental change in this measure during the target age range (e.g., Leadholm & Miller, 1992; J. F. Miller et al., 2005).
Analyses
To examine the extent of genetic and environmental effects on individuals’ productive language measures at each of two annual home visits, we first compared intraclass correlations for MZ and DZ pairs on each measure. We extended these analyses using standard univariate twin models to obtain univariate estimates of genetic , shared environmental , and nonshared environmental effects for the observed measures, where refers to the proportion of variance due to additive genetic influences, refers to the proportion of variance due to shared environmental effects (i.e., those shared by individual pairs), and refers to the proportion of variance due to nonshared environmental effects (i.e., those unique to individuals within the pair), plus measurement error.
To examine the stability of genetic and environmental influences across the time points and to estimate the extent to which “new” genetic or environmental influences come into play, we used multivariate genetic models. In these analyses, the conversational language measures at each home visit were used as indicators of latent HV2 and HV3 Conversational Language factors. These latent factors subsume the common variance among the measures within each home visit. The use of latent factors in a genetically sensitive design is highly informative, because it enables one to focus on genetic and environmental influences that contribute to variance in the target ability (as indexed by the common variance among measures), independent of measure-specific variance and measure-specific error. In addition, estimates of relationships involving latent factors are more reliable (Loehlin, 2004).
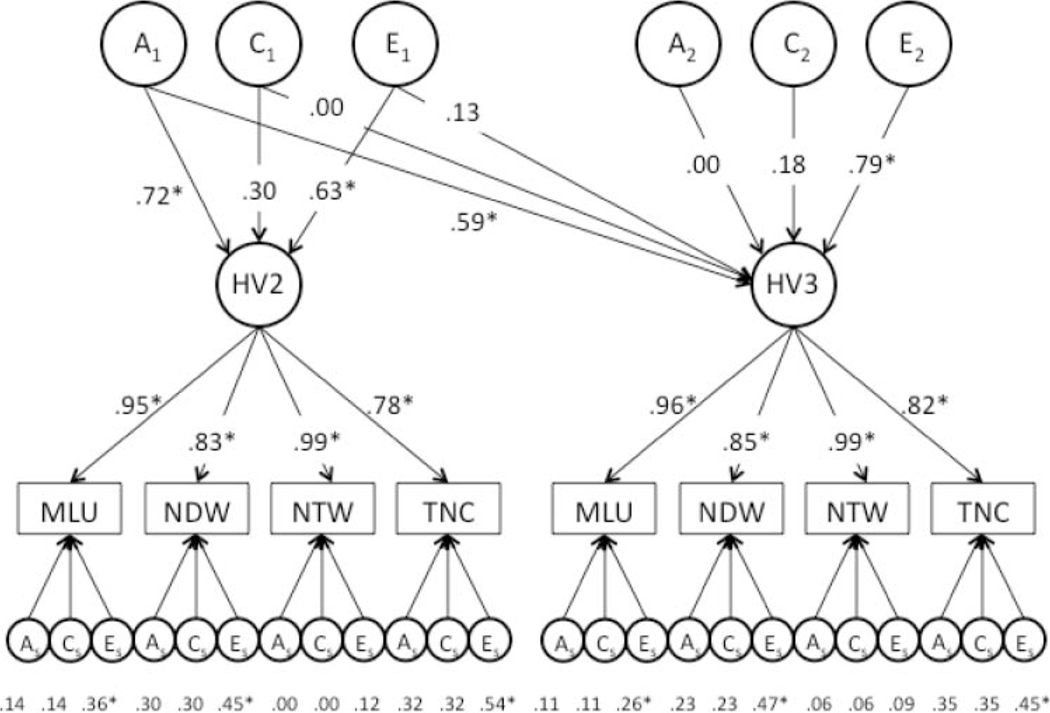
Our baseline model (shown in Figure 1) takes the form of a Cholesky decomposition model (Neale & Cardon, 1992). The variance and covariance between the HV2 and HV3 Conversational Language factors are decomposed into A, C, and E effects. The first set of factors, , , and , represent A, C, and E effects that contribute to the total phenotypic variance in HV2 Conversational Language scores, as well as the covariance between HV2 and HV3 Conversational Language, that is, the stability of Conversational Language from HV2 to HV3. The second set of latent factors, , , and , represent A, C, and E effects on HV3 Conversational Language that are independent of A, C, and E effects on HV2 Conversational Language, that is, wave-specific or “new” A, C, E effects that emerge at HV3. When the path coefficients in the Cholesky decomposition model are standardized, the proportion of the total phenotypic variance in each latent Conversational Language factor that is due to genetic influences can be estimated from the squared path coefficients from the genetic factors. For example, the heritability of HV2 Conversational Language is estimated by squaring the standardized factor loading for the first path coefficient for (that loads on HV2), and the heritability of HV3 Conversational Language is estimated by squaring and summing the standardized factor loadings of the second path coefficient for (that loads on HV3) and the path coefficient for . Similar calculations can be used to estimate the proportion of phenotypic variance in each measure due to shared environmental influences and nonshared environmental influences. Because A, C, and E are not measured directly but are inferred from patterns of twin similarity, they do not have a natural scale. In the current study, we fixed the total variance (i.e., the sum of A, C, and E) within each measure to equal 1. In addition to examining the variance and covariance between the latent HV2 and HV3 Conversational Language factors, it is possible to decompose the remaining measure-specific variance into , , and effects.
Figure 1.
Longitudinal latent factor analyses across language measures within a home visit (HV) using an ACE model. The top layer of arrows represents genetic and nonshared environmental effects on latent factors at each home visit. The middle layer of arrows represents the loadings of individual measures on the latent factor, and the bottom layer of arrows offers the genetic and environment effects on measure-specific variance, not accounted for by the factor. *p < .05.
Models were estimated from the raw data using full-information maximum likelihood, which yields maximum-likelihood estimates for the effects of interest while taking missing data into account. In maximum likelihood estimation, the values of the unknown parameters (i.e., , , and ) are iteratively adjusted until estimates are obtained that yield the smallest possible discrepancy between the model (expected twin variances and covariances) and the data (observed variances and covariances). All analyses were undertaken in Mx (Neale, Boker, Xie, & Maes, 2006). We used two statistics to ascertain model fit: (a) the Akaike information criterion (Akaike, 1987) and (b) the Bayesian information criterion (BIC; Raftery, 1995). These are indices of relative fit, in which smaller values indicate better model fit (i.e., the model that reproduces the observed variances and covariances with as few unknown estimated parameters as possible). The is estimated as likelihood , where likelihood is the maximized value of the likelihood function for the estimated model and is the number of parameters in the statistical model. The is estimated as likelihood , where represents the number of parameters and the number of observations in the fitted model. We designated model parameters as significant if their 95% confidence intervals (CIs) did not include 0. For the purpose of our analyses, scores were standardized on the whole sample to a mean of 0 and an SD of 1. In addition, for the genetic analyses, scores were adjusted for age and sex (McGue & Bouchard, 1984).
Results
Descriptives for all dependent variables are summarized in Table 1 by home visit and zygosity. Note that the number of participants fluctuated across variables on the basis of differing requirements in sample length. For example, TCICU was derived regardless of sample length, whereas NTW, NDW, and TNC required 100 complete and intelligible utterances. A couple of trends worth highlighting from Table 1 are the similar means across MZ twins and DZ twins, with a tendency toward greater variability in DZ twins across all measures except TCICU. Variance differences reached significance in two cases: MLU at HV2 (, ) and TCICU at HV3 (, ). There was also a consistent trend for measures to increase from HV2 to HV3, as one might expect developmentally. With MZ and DZ groups combined, skewness for individual dependent variables ranged from –.02 for TCICU at HV3 to .96 for TNC at HV2. Kurtosis ranged from –.31 for TCICU at HV3 to .98 for TNC at HV3.
Table 1.
Descriptive data on child language sample measures divided by zygosity and home visit (HV).
| Child language measure | MZ |
DZ |
||||
|---|---|---|---|---|---|---|
| n | M (SD) | Range | n | M (SD) | Range | |
| HV2 | ||||||
| TCICU | 205 | 139.78 (48.20) | 10–272 | 282 | 135.59 (45.61) | 10–236 |
| MLU | 197 | 5.65 (1.05) | 3–10 | 272 | 5.707 (1.26) | 3–10 |
| NTW | 163 | 524.73 (103.63) | 263–887 | 224 | 530.55 (112.37) | 314–887 |
| NDW | 163 | 189.96 (28.35) | 104–275 | 224 | 191.10 (29.29) | 126–274 |
| TNC | 163 | 36.83 (18.81) | 5–109 | 224 | 37.70 (20.34) | 5–109 |
| HV3 | ||||||
| TCICU | 178 | 141.02 (48.92) | 31–272 | 209 | 137.45 (47.38) | 21–279 |
| MLU | 174 | 5.98 (1.32) | 2–12 | 199 | 5.978 (1.41) | 2–10 |
| NTW | 136 | 555.99 (114.81) | 222–1,025 | 164 | 548.82 (128.89) | 281–904 |
| NDW | 136 | 199.99 (27.51) | 99–279 | 164 | 196.93 (32.15) | 125–267 |
| TNC | 136 | 42.13 (20.21) | 6–121 | 164 | 41.23 (20.86) | 6–95 |
Note. MZ = monozygotic; DZ = dizygotic; TCICU = total complete and intelligible conversational units (C-units); MLU = mean length of utterance/C-unit; NTW = number of total words; NDW = number of different words; TNC = total number of conjunctions.
Phenotypic Correlations
In Tables 2 and 3, we present the phenotypic correlations across variables within HV2 and HV3, respectively. Cross-variable correlations within both visits demonstrated significant correlations across MLU, NDW, NTW, and TNC, all of which were large in effect size. In contrast, TCICU at both home visits correlated significantly with MLU and NTW but not with ND Wand TNC. It is worth noting here that NTW, NDW, and TNC were all derived from samples that were truncated to 100 utterances, there by potentially reducings hared variance with TCICU. Intravariable correlations revealed relative stability of measures across home visits, with the following coefficients and CIs: .29 [.20, .37] for TCICU, .52 [.45, .58] for MLU, .42 [.33, .51] for NDW, .46 [.36, .54] for NTW, and .47 [.37, .55] for TNC.
Table 2.
HV2 cross-variable correlations (with 95% confidence intervals [CIs] in brackets).
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. TCICU | — | ||||
| 2. MLU | .35 [.27, .42] | — | |||
| 3. NDW | .05 [.00, .17] | .77 [.73, .81] | — | ||
| 4. NTW | .24 [.15, .32] | .92 [.91, .94] | .84 [.81, .86] | — | |
| 5. TNC | .08 [.00, .19] | .72 [.68, .76] | .65 [.60, .70] | .76 [.73, .80] | — |
Table 3.
HV3 cross-variable correlations (with 95% CIs in brackets).
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. TCICU | — | ||||
| 2. MLU | .35 [.24, .44] | — | |||
| 3. NDW | .06 [.00, .22] | .80 [.75, .84] | — | ||
| 4. NTW | .06 [.00, .22] | .80 [.75, .84] | .84 [.80, .87] | — | |
| 5. TNC | .06 [.00, .22] | .80 [.75, .84] | .84 [.80, .87] | .81 [.76, .84] | — |
Univariate Analyses
In regard to the extent of genetic and environmental effects on individual measures, intraclass correlations and modeling results for HV2 and HV3 are presented in Tables 4 and 5, respectively. For HV2 measures, MZ twin correlations were consistently greater than the DZ twin correlations, which is indicative of genetic influences. The DZ twin correlations were greater than half the MZ twin correlations, which is indicative of shared environmental influences. An exception to this pattern was NTW, which yielded a DZ twin correlation less than half the MZ twin correlation. It should be noted, however, that for all measures, including NTW, the CIs for MZ and DZ twin correlations overlapped considerably, suggesting that shared environmental influences can be estimated only very imprecisely. The ACE estimates bear this out: All measures showed significant genetic influences, whereas shared environmental effects were significant only for TCICU. We probed this finding further by comparing fit statistics for the ACE model with an AE model, as shown in the lower half of Table 4. All measures except TCICU showed lower AIC and BIC values for the AE model than for the ACE model. For TCICU, the results were ambiguous: The AIC was lower for the ACE model than for the AE model, whereas the opposite pattern was seen for the BIC. It should be noted that TCICU at HV2 showed significant variance differences, which may partly account for these results. In sum, the overall pattern of results for HV2 indicated that shared environmental effects could be dropped from our models without a significant deterioration in fit.
Table 4.
Univariate MZ and DZ twin correlations and estimates of , , and for HV2, with nested AE model comparison.
| Variable/statistic | TCICU | MLU | NDW | NTW | TNC |
|---|---|---|---|---|---|
| MZ | |||||
| r | .74 | .55 | .52 | .50 | .50 |
| 95% CI | [.65, .82] | [.40, .68] | [.34, .67] | [.30, .65] | [.31, .38] |
| n pairs | 107 | 102 | 75 | 75 | 75 |
| DZ | |||||
| r | .42 | .37 | .27 | .32 | .20 |
| 95% CI | [.28, .54] | [.22, .50] | [.09, .49] | [.13, .48] | [.00, .38] |
| n pairs | 143 | 141 | 95 | 95 | 95 |
| ACE model parameter estimates | |||||
| .35 | .50 | .55 | .59 | .53 | |
| 95% CI | [.07, .66] | [.14, .71] | [.09, .67] | [.14, .71] | [.22, .66] |
| .33 | .11 | .00 | .00 | .00 | |
| 95% CI | [.03, .56] | [.00, .39] | [.00, .35] | [.00, .34] | [.00, .20] |
| .32 | .39 | .45 | .40 | .47 | |
| 95% CI | [.25, .42] | [.28, .53] | [.33, .63] | [.29, .59] | [.34, .66] |
| AE model vs. ACE model | |||||
| Fit statistics | |||||
| AIC (ACE) | 328.582 | 368.39 | 319.38 | 268.87 | 326.51 |
| BIC (ACE) | −784.54 | −697.08 | −542.60 | −385.97 | −539.03 |
| AIC (AE) | 331.21 | 366.95 | 317.38 | 267.66 | 324.51 |
| BIC (AE) | −785.02 | −699.58 | −545.32 | −388.21 | −541.76 |
| AE model parameter estimates | |||||
| .69 | .63 | .55 | .56 | .53 | |
| 95% CI | [.60, .75] | [.50, .72] | [.39, .67] | [.44, .71] | [.34, .66] |
| .31 | .37 | .45 | .40 | .47 | |
| 95% CI | [.25, .40] | [.28, .50] | [.33, .61] | [.29, .56] | [.34, .66] |
Note. Boldface type indicates lower Akaike information criterion (AIC) and Bayesian information criterion (BIC) values (indicating a better fitting model) for each model-fitting comparison. AIC (ACE/AE): AIC for the ACE/AE model. BIC (ACE/AE): BIC for the ACE/AE model.
Table 5.
Univariate MZ and DZ twin correlations and estimates of , , and for HV3, with nested AE and CE model comparisons.
| Variable/statistic | TCICU | MLU | NDW | NTW | TNC |
|---|---|---|---|---|---|
| MZ | |||||
| r | .67 | .37 | .25 | .25 | .41 |
| 95% CI | [.54, .76] | [.17, .54] | [.00, .47] | [.00, .48] | [.17, .61] |
| n pairs | 98 | 87 | 56 | 56 | 56 |
| DZ | |||||
| r | .54 | .22 | .20 | .25 | .35 |
| 95% CI | [.40, .64] | [.02, .41] | [.00, .41] | [.02, .47] | [.12, .54] |
| n pairs | 130 | 96 | 63 | 63 | 63 |
| ACE model parameter estimates | |||||
| .53 | .28 | .24 | .05 | .08 | |
| 95% CI | [.28, .79] | [.00, .52] | [.00, .50] | [.00, .47] | [.00, .53] |
| .23 | .09 | .06 | .21 | .30 | |
| 95% CI | [.00, .44] | [.00, .40] | [.00, .36] | [.00, .39] | [.00, .48] |
| .24 | .64 | .71 | .74 | .63 | |
| 95% CI | [.18, .33] | [.48, .82] | [.50, .95] | [.53, .92] | [.46, .79] |
| AE model vs. ACE model | |||||
| Fit statistics | |||||
| AIC (ACE) | 297.23 | 324.74 | 270.94 | 268.87 | 257.84 |
| BIC (ACE) | −695.95 | −506.07 | −384.94 | −385.97 | −391.49 |
| AIC (AE) | 298.74 | 322.90 | 268.99 | 267.66 | 257.50 |
| BIC (AE) | −696.94 | −508.68 | −387.55 | −388.21 | −393.30 |
| AE model parameter estimates | |||||
| .77 | .37 | .31 | .31 | .42 | |
| 95% CI | [.69, .83] | [.21, .52] | [.07, .50] | [.10, .49] | [.24, .57] |
| .23 | .63 | .69 | .69 | .58 | |
| 95% CI | [.17, .31] | [.48, .79] | [.50, .93] | [.51, .90] | [.44, .76] |
| CE model vs. ACE model | |||||
| Fit statistics | |||||
| AIC (ACE) | 297.23 | 324.74 | 270.94 | 268.87 | 257.84 |
| BIC (ACE) | −695.95 | −506.07 | −384.94 | −385.97 | −391.49 |
| AIC (CE) | 310.39 | 323.85 | 269.41 | 266.89 | 255.92 |
| BIC (CE) | −691.12 | −508.20 | −387.34 | −388.60 | −394.09 |
| CE model parameter estimates | |||||
| .59 | .29 | .21 | .25 | .35 | |
| 95% CI | [.50, .66] | [.15, .42] | [.04, .37] | [.08, .39] | [.20, .48] |
| .41 | .71 | .79 | .75 | .65 | |
| 95% CI | [.34, .50] | [.58, .85] | [.63, .96] | [.61, .92] | [.52, .80] |
Note. Boldface type indicates lower AIC and BIC values (indicating a better fitting model) for each model-fitting comparison. AIC (ACE/AE/CE) = AIC for the ACE/AE/CE model. BIC (ACE/AE/CE) = BIC for the ACE/AE/CE model.
At HV3, the MZ and DZ twin correlations for NTW were identical, suggesting that resemblance between twins is entirely due to shared environmental influences. For all other measures, MZ twin correlations were consistently higher than DZ correlations, and DZ correlations were greater than half the MZ correlations, which is indicative of genetic and shared environmental influences. Again, however, the CIs for the MZ and DZ twin correlations overlapped substantially for each measure. The ACE estimates indicated that genetic and shared environmental influences were not significantly different from 0 for any measure, with the exception of TCICU, which showed significant genetic (but not shared environmental) influences. Model fit statistics for HV3 are shown in Table 5. We compared the ACE model with an AE model and, because the twin correlations for NTW suggested no genetic influences, a CE model. For all measures except TCICU, the AIC and BIC values for the reduced models (AE and CE) were lower than for the full ACE model. Comparisons between the fit statistics for the AE and CE models were more difficult to make, but in general, the AE model showed lower AIC and BIC values than the CE model. The best fitting model for TCICU was the full ACE model.
Multivariate Analyses
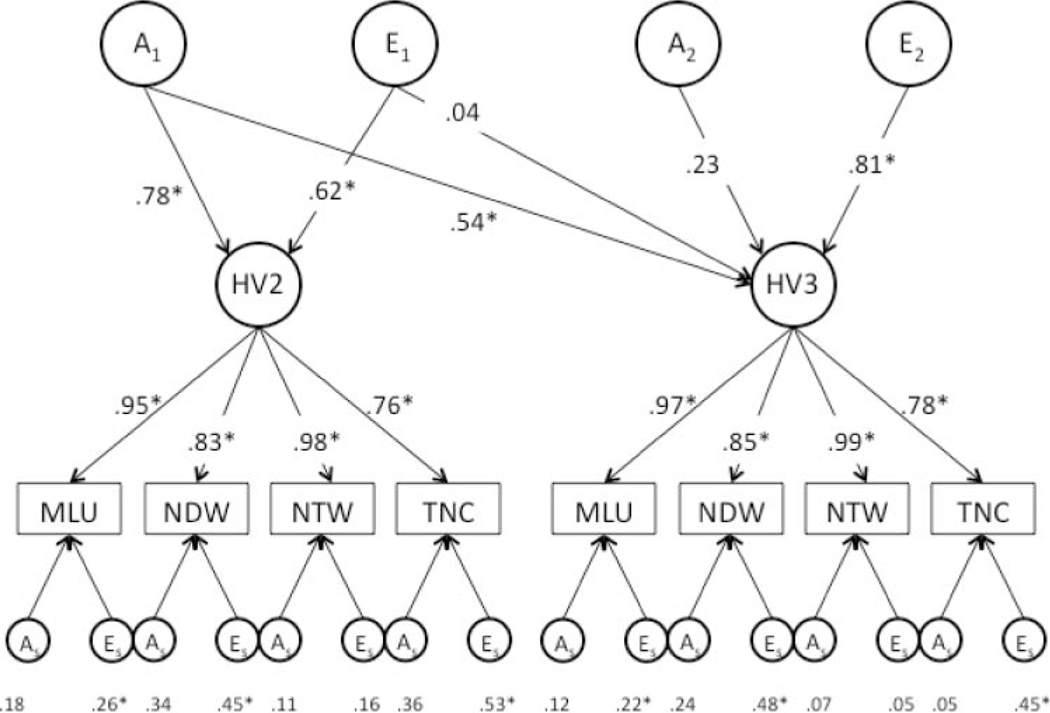
To address questions of stability over time, we relied on longitudinal latent factor analysis; specifically, MLU, NDW, NTW, and TNC were included within a latent Conversational Language factor for each home visit. TCICU was not included because of its lower phenotypic correlation with the other variables (see also DeThorne et al., 2008). Because an AE model emerged as the best fit for MLU, NDW, NTW, and TNC within the univariate analyses at both time points, we used an AE rather than an ACE model for the multivariate analyses (see Figure 2); however, the full ACE model is provided in Figure 1 for general comparison purposes. Factor loadings were large, ranging from .76 to .98 at HV2 and from .78 to .99 at HV3 (see the middle layer of arrows in Figure 2).
Figure 2.
Longitudinal latent factor analyses across language measures within a home visit (HV) using an AE model. The top layer of arrows represents genetic and nonshared environmental effects on latent factors at each home visit. The middle layer of arrows represents the loadings of individual measures on the latent factor, and the bottom layer of arrows offers the genetic and environment effects on measure-specific variance, not accounted for by the factor. *p < .05.
The standardized path estimates specified along-side the top layer of arrows in Figure 2 offer key information regarding the genetic and nonshared effects on the latent factors at each home visit; specifically, the standardized path estimate of .78 from A1 to HV2 specifies the genetic variance in the HV2 latent factor. When squared, this value provides the heritability of the HV2 factor, specifying that 62% (.782) of variance in HV2 conversational language skills is due to genetic effects. The value of .62 associated with indicates that 38% (.622) of the variance in HV2 conversational language skills is due to nonshared environmental effects. The path estimates of .54 and .04 running from and to HV3 indicate the amount of genetic and nonshared environmental variance at HV3 that overlaps with HV2. Finally, the paths from A2 and to HV3 represent the unique genetic and nonshared environmental variance associated with the HV3 Conversational Language factor. The bottom row of coefficients in Figure 2 represents the genetic and environmental effects on measure-specific variance ( and )—in other words, variance not accounted for by the conversational factors. In sum, the findings indicate significant genetic overlap across the two time points (.542 = .29); specifically, 62% of the variance in the conversational language factor at HV2 was heritable; 34% of the variance in the conversational language factor at HV3 was heritable (.542 + .232 = .34), and all of the genetic effects overlapped with HV2. In contrast, nonshared environmental effects were entirely specific to home visit: Thirty-eight percent (.622) of the variance in the Conversational Language factor at HV2 was due to nonshared environmental effects, and 66% (.812) of the variance in the Conversational Language factor at HV3 was due to nonshared environmental effects, all of which were unique to that home visit. A summary of the squared, standardized path estimates and their associated CIs at each home visit is provided in Table 6. The remaining variance attributed to individual measures was minimal and primarily due to nonshared environmental effects.
Table 6.
Squared, standardized path estimates from longitudinal analysis of latent language at HV2 and HV3.
| Home visit | Contribution of A1 | Contribution of A2 | |
|---|---|---|---|
| HV2 | .62 [.49, .71] | .62 [.49, .71] | |
| HV3 | .29 [.15, .42] | .05 [.00, .22] | .34 [.18, .49] |
| Contribution of E1 | Contribution of E2 | ||
|
| |||
| HV2 | .38 [.29, .51] | .38 [.29, .51] | |
| HV3 | .00 [.00, .04] | .66 [.51, .81] | .66 [.51, .82] |
Note. Numbers in brackets are 95% CIs. A1 and E1 represent A and E influences, respectively, accounting for all the variance in HV2 and the covariance of HV2 with HV3; A2 and E2 represent A and E influences, respectively, accounting for the remaining variance in HV3, independent of HV2.
Discussion
The primary findings from the present study included the relative stability of conversational language measures. Excluding TCICU, measures at HV2 accounted for 18% to 27% of the variance in that same measure 1 year later. Such stability is consistent with other measures of language (e.g., Spinath et al., 2004). Particularly germane to the present study was the finding that stability in the Conversational Language factor was accounted for almost entirely by shared genetic effects. Longitudinal latent factor modeling indicated that 62% of the variance in children’s conversational language skills at HV2 was due to genetic effects, whereas heritability at HV3 was around 34%. The genetic effects at HV3 overlapped entirely with the genetic effects at HV2, thereby indicating no new genetic influences on children’s conversational language measures between the approximate ages of 7 and 8 years. In contrast to the stability in genetic effects, nonshared environmental effects were entirely time point specific, meaning whatever nonshared environmental influences were operating at HV2 were not the same influences on conversational language measures at HV3. In the discussion that follows, we focus on three areas: (a) comparisons with previous studies, particularly in terms of reconciling our finding of limited shared environmental effects; (b) speculation regarding candidate genes and nonshared environmental effects; and (c) implications of findings for clinical practice and future research.
Comparisons With Past Studies
Heritability estimates from the present study fall comfortably with in the wide ranges presented else where for individual differences in child language use (i.e., Plomin & Kovas, 2005; Stromswold, 2001), although most prior estimates come from parent report and more formalized tests. Two additional findings from the current study are worth noting in comparison to past studies. First, the present study demonstrated a trend toward lower heritability estimates at HV3 relative to HV2, although the overlapping CIs prevent a definitive interpretation. If the trend were clear, it would contrast with claims that the heritability of language tends to increase with age (Haworth et al., 2010; Hoekstra et al., 2007; Plomin et al., 1997; Stromswold, 2001). It is possible that our study was not sensitive to potential shifts in h2 because of the specific age of the participants, the limited 1-year trajectory, or the general nature of the measures used. Specific to child age, one might expect larger shifts in causal effects during key developmental transitions, such as entry into formal schooling or puberty. Of interest is that the one study that empirically assessed the stability of causal influences on child language (Hoekstra et al., 2007), actually reported a downward dip in heritability between the ages of 5 and 7 years despite the general increasing trend across other ages studied. On a related note, studies extended across longer time periods would likely be more prone to yield lower stability, a prediction we intend to assess in future analyses on the WRRP sample. It remains notable, however, that a 1-year time span, a common window for longitudinal study, yielded genetic stability paired with shifting nonshared effects on children’s conversational language measures, especially given the paucity of behavioral genetic work in this area. Future studies should consider longer time periods, key developmental transitions, and more finely tuned semantic and morphosyntactic measures.
Another note worthy finding compared with some past studies is the limited evidence of shared environmental influences on language. Shared environmental effects were statistically significant only for TCICU at HV2, with an estimate of .33 that barely reached statistical significance. For our specific measures of linguistic complexity, estimates of ranged from. 00 to .11 at HV2 and from.06 to .30 at HV3. Although other twin studies have found statistically significant shared environmental effects on a variety of traits, including child language (e.g.,Colledge et al., 2002; Samuelsson et al., 2005; Spinath et al., 2004), twin methodology has been critiqued for its overall inability to identify shared environmental effects, especially when the sample size is small (Bulik, Sullivan, Wade, & Kendler, 2000; Hewitt et al., 2001; Hopper, 2000). In fact, power to detect shared environmental effects was low in the present study. Achieving statistical significance for shared environmental estimates of 20% to 30% requires sample sizes ranging from 300 to over 1,000 twins, depending on the ratio of MZ to DZ twins and the distribution of remaining variance between A and E. Because of power limitations in the present study, it is impossible to claim that shared environmental effects do not influence individual differences in children’s language use.
In addition to low power, other factors may be contributing to the limited evidence of shared environmental effects in the present study. As previously mentioned, shared environmental factors are often thought to be more influential at younger ages; consequently, testing at younger ages may reveal stronger shared environmental effects. In addition, it is possible that the underrepresentation of families with lower parent education (i.e., no high school diploma) may limit the full variability of shared environmental experiences and thereby limiting estimates of such effects (cf. Rowe et al., 1999).
Candidate Genetic and Nonshared Environmental Effects
In terms of specific genes that may contribute to the genetic stability observed in the present study, candidates have been slow to emerge in the literature and difficult to replicate. Complex behavioral traits, such as language, are likely governed by numerous quantitative trait loci (QTLs), which individually are responsible for relatively small effects that are difficult to detect. Whereas most typical variation in language skill may be influenced by unique combinations of QTLs, it remains possible that language disorders are caused by a “heterogeneous collection of individually rare, highly penetrant mutations” (Plomin, 2005, pp., 1030–1032). As a case in point, FOXP2, on chromosomal region 7q31, has been implicated as a key DNA sequence in the human evolution of speech (Enard et al., 2002), and relatively rare mutations in this region have been linked with speech-language disorders (Lai, Fisher, Hurst, Vargha-Khadem, & Monaco, 2001; O’Brien, Zhang, Nishimura, Tomblin, & Murray, 2003). Other chromosomal regions affiliated with language disorders include 6p (Rice, Smith, & Gayan, 2009), 16q, 19q (SLI Consortium, 2002, 2004), and 13q (Bartlett et al., 2002). Regardless of which QTLs are identified, the genetic stability observed in the present study suggests that candidate genes found to influence conversational language measures at one time point during early school-age development are likely to be relevant at least 1 year later.
In regard to nonshared (or person-specific) environmental effects, the extent to which these effects were unique across time points was surprising, though not unprecedented (e.g., Hoekstra et al., 2007). Many of the anticipated nonshared environmental effects on child language, such as one twin’s exposure to injury or illness, would not be expected to shift within 1 year’s time, unless of course the event had occurred within that particular year. In the standard ACE model, nonshared environmental effects are difficult to interpret because the term encompasses measure-specific error. One advantage of the latent factor analyses is its ability to eliminate measure-specific error from estimates of nonshared effects (Loehlin, 2004). In theory, then, e2 estimates on the Conversational Language factor represent real nonshared effects, or at least effects that influence all measures within the factor.
The question then becomes, “What are these unique nonshared environmental effects?” Given that our study was not designed to answer this question, we are left to speculate on the basis of the nature of our findings. Although there is a range of intriguing possibilities, including examiner effects, child friendships, and classroom placement, the high correlation across our measures suggests that the nonshared effects could be attributed to the unique qualities of a particular corpus. As such, the nonshared effects would encompass not only the child’s intrinsic linguistic ability but also a complex amalgam represented by corpus-level factors such as topic, mood, and attention, both of the child and the examiner. Given that all the language measures were derived from one conversational sample and correlated highly, it is quite possible that factors influencing verbal engagement and shared communicative competence would have similar influences on all the derived measures and thus not be eliminated in the latent factor analyses.
Certainly reports related to the reliability of language sample measures suggest the likelihood of such effects (Bornstein, Haynes, Painter, & Genevro, 2000; Gavin&Giles, 1996).Despite this limitationin interpreting our results, two additional points are worth mentioning. First, such context effects are relevant for all forms of assessment, not just conversational samples (DeThorne et al., 2005; DeThorne & Watkins, 2006), and they might in fact explain the changes in nonshared effects within studies that use standardized assessments as well (e.g., Hoekstra et al., 2007). Second, we would argue that, rather than being “nonreal” effects, all factors that influence measures of child language should be understood as critical to providing meaningful assessment and intervention. If the goal is to minimize the impact of factors other than intrinsic linguistic ability, however, then one might consider using multiple measures and various assessment points (cf. DeThorne & Watkins, 2001).
Implications and Future Directions
To the extent that our findings reflect meaningful nonshared environmental effects on language, this study suggests the need to study environmental variables at the individual level. It has been noted that even family-level variables can have notably different effects on individuals within the same family (e.g., Moffitt, 2005; Rutter & Plomin, 1997); that does not mean that familywide variables are not important, only that children’s response to familywide variables may be highly individualized (cf. Rutter & Plomin, 1997). Environmental factors, ranging from diet and toxin exposure to parent interaction style, could have markedly different effects on individuals within the same family depending on that individual’s specific genetic makeup and environmental history. Clinical and educational practices could certainly benefit from identifying influential person-specific effects and their potentially complex interactions with other variables, so that we could move toward supporting individuals rather than treating fictitiously homogeneous populations.
In regard to genetic findings, it has been argued that even small genetic effects, if consistent, will lead to increases in heritability over time (Eaves, Long, & Heath, 1986), although such an increase was not observed across the two time points in the present study. As noted elsewhere, heritability does not eliminate environmental influence. In contrast, understanding the genetic mechanism often leads to more effective environmental arrangements and offers the promise of more effective interventions and preventions for cases of language disorder (Rutter & Plomin, 1997; see also the process proposed by Moffitt, 2005, in relation to psychopathology). Future behavioral genetic work could better inform clinical understanding by extending assessments of causal stability across longer time frames and expanding the types of measures employed. Similarly, clinical practices should serve to inform basic research. Behavioral observations of phenotypic variation and disassociations, as well as identification of useful treatment strategies, offer important insights into what key cognitive processes and deficits might be underlying language development and disability.
Acknowledgments
The Western Reserve Reading Project is supported by National Institute of Child Health and Human Development Grants HD38075, HD46167, and HD050307. In addition, transcription and analyses have been supported by the American Speech-Language-Hearing Foundation New Investigator Award; the University of Illinois at Urbana–Champaign Campus Research Board; and the Children, Youth and Families Consortium at The Pennsylvania State University. Interdisciplinary collaborations have been enhanced by the American Speech-Language-Hearing Association Advancing Academic–Research Careers (AARC) Award. In addition, we sincerely appreciate the time and effort of all participating families and affiliated research staff. Students responsible for language sample transcription of HV3 included, but were not limited to, Tanya Cooper, Lisa Mellman, Kelly O’Connor, Carly Sullivan, Elena Turner, Amy Woodrum, and Amy Van Nada.
Footnotes
See also www.teds.ac.uk.
References
- Akaike H. (1987). Factor analysis and the AIC. Psychometrika, 52, 317–332. [Google Scholar]
- Bartlett CW, Flax JF, Logue MW, Vieland VJ, Bassett AS, Tallal P, & Brzustowicz LM (2002). A major susceptibility locus for specific language impairment on 13q21. American Journal of Human Genetics, 71, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Haynes M, Painter KM, & Genevro JL (2000). Child language with mother and with stranger at home and in the laboratory: A methodological study. Journal of Child Language, 27, 407–420. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Wade TD, & Kendler KS (2000). Twin studies of eating disorders: A review. International Journal of Eating Disorders, 27, 1–20. [DOI] [PubMed] [Google Scholar]
- Catts HW, Adolf SM, Hogan TP, & Weismer SE (2005). Are specific language impairments and dyslexia distinct disorders? Journal of Speech, Language, and Hearing Research, 48, 1378–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Language Child & Lab Literacy. (2010). Specific guidelines for language sample transcription. Unpublished lab manual. [Google Scholar]
- Colledge E, Bishop DV, Koeppen-Schomerus G, Price TS, Happe FGE, Eley TC, ... Plomin R. (2002). The structure of language abilities at 4 years: A twin study. Developmental Psychology, 38, 749–757. [DOI] [PubMed] [Google Scholar]
- Dale PS, Price TS, Bishop DVM, & Plomin R. (2003). Outcomes of early language delay: I. Predicting persistent and transient language difficulties at 3 and 4 years. Journal of Speech, Language, and Hearing Research, 46, 544–560. [DOI] [PubMed] [Google Scholar]
- DeThorne LS, Deater-Deckard K, Mahurin-Smith J, Coletto M, & Petrill S. (2011). Volubility as a mediator in the associations between conversational language measures and child temperament. International Journal of Language and Communication Disorders, Advance online publication. doi: 10.1111/j.1460-6984.2011.00034.x [DOI] [PMC free article] [PubMed]
- DeThorne LS, & Hart SA (2009). Use of twin design to examine evocative gene–environment effects within a conversational context. European Journal of Developmental Science, 3, 175–194. [PMC free article] [PubMed] [Google Scholar]
- DeThorne LS,Hart SA,Petrill SA,Deater-Deckard K, Thompson LA, Schatschneider C, & Davison MD (2006). Children’s history of speech-language difficulties: Genetic influences and associations with reading. Journal of Speech, Language, and Hearing Research, 49, 1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeThorne LS, Johnson BW, & Loeb JW (2005). A closer look at MLU: What does it really measure? Clinical Linguistics & Phonetics, 19, 635–648. [DOI] [PubMed] [Google Scholar]
- DeThorne LS, Petrill SA, Hart SA, Channell RW, Campbell RJ, Deater-Deckard K, ... Vandenbergh DJ (2008). Genetic effects on children’s conversational language use. Journal of Speech, Language, and Hearing Research, 51, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeThorne LS, & Watkins RV (2001). Listeners’ perceptions of language skills in children. Language, Speech, and Hearing Services in Schools, 32, 142–148. [DOI] [PubMed] [Google Scholar]
- DeThorne LS, & Watkins RV (2006). Language abilities and nonverbal IQ in children with language impairment: Inconsistency across measures. Clinical Linguistics & Phonetics, 20, 641–658. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Long J, & Heath AC (1986). A theory of developmental change in quantitative phenotypes applied to cognitive development. Behavior Genetics, 16, 143–162. [DOI] [PubMed] [Google Scholar]
- Eisenberg SL, Ukrainetz TA, Hsu JR, Kaderavek JN, Justice LM, & Gillam RB (2008). Noun phrase elaboration in children’s spoken stories. Language, Speech, and Hearing Services in Schools, 39, 145–157. [DOI] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CS, Wieve V, Kitano T, ... Paabo, S. (2002, August 22). Molecular evolution of FOXP2, a gene involved in speech and language. Nature, 418, 869–871. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Brinton B, Isaacson T, & Summers C. (2001). Social behaviors of children with language impairment on the playgroup: A pilot study. Language, Speech, and Hearing Services in Schools, 32, 101–113. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Brinton B, Robinson LA, & Watson VJ (1997). The ability of children with specific language impairment to participate in a group decision task. Journal of Children’s Communication Development, 18, 1–10. [Google Scholar]
- Gavin WJ, & Giles L. (1996). Sample size effects on temporal reliability of language sample measures of preschool children. Journal of Speech and Hearing Research, 39, 1258–1262. [DOI] [PubMed] [Google Scholar]
- Haworth CMA, Wright MJ, Luciano M, Martin NG, de Geus EJC, van Beijsterveldt CEM, ... Plomin R. (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 15, 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt JK, Emde RN, & Plomin R. (2001). The twin method: What we can learn from a longitudinal study. In Emde RN & Hewitt JK (Eds.), Infancy to early childhood: Genetic and environmental influences on developmental change (pp. 12–22). New York, NY: Oxford University Press. [Google Scholar]
- Hoekstra RA, Bartels M, & Boomsma DI (2007). Longitudinal genetic study of verbal and nonverbal IQ from early childhood to young adulthood. Learning and Individual Differences, 17, 97–114. [Google Scholar]
- Hopper JL (2000). Why “common environmental effects” are so uncommon in the literature. In Spector TD, Snieder H, & MacGregor AJ (Eds.), Advances in twin and sib-pair analysis (pp. 151–165). London, UK: Oxford University Press. [Google Scholar]
- Hutchins TL, Brannick M, Bryant JB, & Silliman ER (2005). Methods for controlling amount of talk: Difficulties, considerations and recommendations. First Language, 25, 347–363. [Google Scholar]
- Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, & Monaco AP (2001, October 4). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature, 413, 519–523. [DOI] [PubMed] [Google Scholar]
- Leadholm BJ, & Miller JF (1992). Language sample analysis: The Wisconsin guide. Madison: Wisconsin Department of Public Instruction. [Google Scholar]
- Loban W. (1976). Language development: Kindergarten through grade twelve (Research Report No. 18). Urbana, IL: National Council of Teachers of English. [Google Scholar]
- Loehlin JC (2004). Latent variable models: An introduction to factor, path, and structural analysis (4th ed). Mahwah, NJ: Erlbaum. [Google Scholar]
- McGue M, & Bouchard TJ Jr. (1984). Adjustment of twin data for the effects of age and sex. Behavior Genetics,14, 325–343. [DOI] [PubMed] [Google Scholar]
- Miller GA, & Gildea PM (1987). How children learn words. Scientific American, 257(3), 94–99. [DOI] [PubMed] [Google Scholar]
- Miller JF (2004). The Systematic Analysis of Language Transcripts user’s guide (Research version 8.0) [Computer software]. Madison, WI: University of Wisconsin. [Google Scholar]
- Miller JF, Freiberg C, Rolland M, & Reeves MA (1992). Implementing computerized language sample analysis in the public school. Topics in Language Disorders, 12, 69–82. [Google Scholar]
- Miller JF, Long S, McKinley N, Thormann S, Jones MA, & Nockerts A. (2005). Language sample analysis II: The Wisconsin guide. Madison: Wisconsin Department of Public Instruction. [Google Scholar]
- Moffitt TE (2005). The new look of behavioral genetics in developmental psychopathology: Gene–environment interplay in antisocial behaviors. Psychological Bulletin, 131, 533–554. [DOI] [PubMed] [Google Scholar]
- Nagy WE,Herman PA,&Anderson RC.(1985).Learning words from context. Reading Research Quarterly, 20, 233–253. [Google Scholar]
- Nathan L, Stackhouse J, Goulandris N, & Snowling MJ (2004). The development of early literacy skills among children with speech difficulties: A test of the “critical age hypothesis.” Journal of Speech, Language, and Hearing Research, 47, 377–391. [DOI] [PubMed] [Google Scholar]
- Neale M, Boker SM, Xie G, & Maes HH (2006). Mx statistical manual (6th ed.). Richmond: Virginia Commonwealth University. [Google Scholar]
- Neale MC, & Cardon LR (1992). Methodology for genetic studies of twins and families. Boston, MA: Kluwer Academic. [Google Scholar]
- Nippold MA (1998). Later language development: The school-age and adolescent years (2nd ed.). Austin, TX: Pro-Ed. [Google Scholar]
- Nippold MA, & Sun L. (2008). Knowledge of morphologically complex words: A developmental study of older children and young adolescents. Language, Speech, and Hearing Services in Schools, 39, 365–373. [DOI] [PubMed] [Google Scholar]
- O’Brien EK, Zhang X, Nishimura C, Tomblin B, & Murray JC (2003). Association of specific language impairment (SLI) to the region of 7q31. American Journal of Human Genetics, 72, 1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. (1996). Clinical implications of the natural history of slow expressive language development. American Journal of Speech-Language Pathology, 5, 5–21. [Google Scholar]
- Petrill SA, Deater-Deckard K, Thompson LA, DeThorne LS, & Schatschneider C. (2006). Reading skills in early readers: Genetic and shared environmental effects. Journal of Learning Disabilities, 39, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R. (1986). Multivariate analysis and developmental behavioral genetics: Developmental change as well as continuity. Behavior Genetics, 16, 25–43. [DOI] [PubMed] [Google Scholar]
- Plomin R. (2005). Finding genes in child psychology and psychiatry: When are we going to be there? Journal of Child Psychology and Psychiatry, 46, 1030–1038. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, & Loehlin JC (1977). Genotype–environment interaction and correlation in the analysis of human behavior. Psychological Bulletin, 84, 309–322. [PubMed] [Google Scholar]
- Plomin R, De Fries JC, McClearn GE, & McGuffin P. (2008). Behavioral genetics (5thed.). NewYork, NY: Freeman. [Google Scholar]
- Plomin R, Fulker DW, Corley R, & DeFries JC (1997). Nature, nurture, and cognitive development from 1 to 16 years: A parent–offspring adoption study. Psychological Science, 8, 442–447. [Google Scholar]
- Plomin R, & Kovas Y. (2005). Generalist genes and learning disabilities. Psychological Bulletin, 131, 592–617. [DOI] [PubMed] [Google Scholar]
- Price TS, Dale PS, & Plomin R. (2004). A longitudinal genetic analysis of low verbal and nonverbal cognitive abilities in early childhood. Twin Research, 7, 139–148. [DOI] [PubMed] [Google Scholar]
- Raftery AE (1995). Bayesian model selection in social research. In Marsden PV (Ed.), Sociological methodology (pp. 111–163). Cambridge, MA: Blackwell. [Google Scholar]
- Redmond SM, & Rice ML (1998). The socioemotional behaviors of children with SLI: Social adaption or social deviance? Journal of Speech, Language, and Hearing Research, 41, 688–700. [DOI] [PubMed] [Google Scholar]
- Rice M. (2004). Growth models of developmental language disorders. In Rice ML & Warren SF (Eds.), Developmental language disorders: From phenotypes to etiologies (pp. 207–240). Mahwah, NJ: Erlbaum. [Google Scholar]
- Rice ML, Redmond SM, & Hoffman L. (2006). Mean length of utterance in children with specific language impairment an in younger control children shows concurrent validity and stable and parallel growth trajectories. Journal of Speech, Language, and Hearing Research, 49, 793–808. [DOI] [PubMed] [Google Scholar]
- Rice ML, Smith SD, & Gayan J. (2009). Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language impairment. Journal of Neurodevelopmental Disorders, 1, 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, Jacobson KC, & Van den Oord EJCG(1999). Genetic and environmental influences on vocabulary IQ: Parental education level as moderator. Child Development, 70, 1151–1162. [DOI] [PubMed] [Google Scholar]
- Rutter M. (2006). Genes and behaviour: Nature–nurture interplay explained. London, UK: Blackwell. [Google Scholar]
- Rutter M, & Plomin R. (1997). Opportunities for psychiatry from genetic findings. British Journal of Psychiatry, 171, 209–219. [DOI] [PubMed] [Google Scholar]
- Samuelsson S, Byrne B, Quain P, Wadsworth S, Corley R, DeFries JC, ... Olson R. (2005). Environmental and genetic influences on prereading skills in Australia, Scandinavia, and the United States. Journal of Educational Psychology, 97, 705–722. [Google Scholar]
- Scarborough HS, & Dobrich W. (1990). Development of children with early language delay. Journal of Speech and Hearing Research, 33, 70–83. [DOI] [PubMed] [Google Scholar]
- Scarr S, & McCartney K. (1983). How people make their own environments: A theory of genotype Y environment effects. Child Development, 54, 424–435. [DOI] [PubMed] [Google Scholar]
- Scott CM (1984). Adverbial connectivity in conversations of children 6 to 12. Journal of Child Language, 11, 423–452. [DOI] [PubMed] [Google Scholar]
- Scott CM, & Stokes SL (1995). Measures of syntax in school-age children and adolescents. Language, Speech, and Hearing Services in Schools, 26, 309–319. [Google Scholar]
- Scott CM, & Windsor J. (2000). General language performance measures in spoken and written narrative and expository discourse of school-age children with language learning disabilities. Journal of Speech, Language, and Hearing Research, 43, 324–339. [DOI] [PubMed] [Google Scholar]
- Consortium SLI. (2002). A genomewide scan identifies two novel loci involved in specific language impairment. American Journal of Human Genetics, 70, 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium SLI. (2004). Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. American Journal of Human Genetics, 74, 1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinath FM, Price TS, Dale PS, & Plomin R. (2004). The genetic and environmental origins of language ability and disability. Child Development, 75, 445–454. [DOI] [PubMed] [Google Scholar]
- Stromswold K. (2001). The heritability of language: A review and meta-analysis of twin, adoption, and linkage studies. Language, 77, 647–723. [Google Scholar]
- Trouton A, Spinath FM, & Plomin R. (2002). Twins Early Development Study(TEDS):A multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Research, 5, 444–448. [DOI] [PubMed] [Google Scholar]
- Ukrainetz TA, & Blomquist C. (2002). The criterion validity of four vocabulary tests compared to a language sample. Child Language Teaching and Therapy, 18, 59–78. [Google Scholar]
- Watkins RV, Kelly DJ, Harbers HM, & Hollis W. (1995). Measuring children’s lexical diversity: Differentiating typical and impaired language learners. Journal of Speech and Hearing Research, 38, 1349–1355. [DOI] [PubMed] [Google Scholar]
- Windsor J. (1995). Language impairment and social competence. In Fey ME, Windsor J, & Warren SF (Eds.), Language intervention: Preschool through the elementary years (Vol. 5, pp. 213–238). Baltimore, MD: Brookes. [Google Scholar]