Background:
NAFLD is increasingly common among young people. Whether NAFLD carries a more benign course in younger adults is not known. We aimed to characterize genetic and metabolic risk factors for NAFLD and their effects on disease progression across age groups.
Methods:
We conducted a retrospective study of adults with NAFLD seen within Michigan Medicine, a tertiary care center, between 2010 and 2021. NAFLD was defined by hepatic steatosis on imaging, biopsy, or transient elastography in the absence of other chronic liver diseases. Cirrhosis was determined by validated International Classification of Diseases-9/10 codes or imaging. Fine-Gray competing risk models were generated, with incident cirrhosis and liver-related events (LREs) as the primary outcomes and death without cirrhosis or LREs as a competing risk. The primary predictor was the age category.
Results:
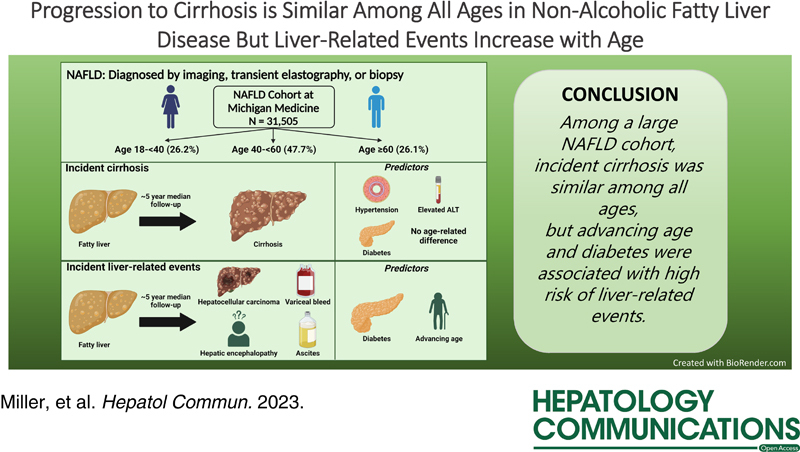
We included 31,505 patients with NAFLD, with 8,252 aged 18 to younger than 40, 15,035 aged 40 to younger than 60, and 8,218 aged 60 years or older years at diagnosis. Compared with older patients, young adults more often had obesity, higher ALT, and high-risk PNPLA3 alleles, and fewer had prevalent cirrhosis, hypertension, hyperlipidemia, and diabetes. The 10-year risk of incident cirrhosis was similar between ages (3.4% in age 18 to <40 vs 3.7% in age 40 to <60 vs 4.7% in age ≥60; p = 0.058). Predictors of LREs were advancing age and diabetes, with a significantly higher 10-year risk of LREs in the oldest age group (0.2% in age 18 to <40 vs 0.7% in age 40 to <60 vs 1.1% in age ≥60; p = 0.008).
Conclusions:
While the baseline prevalence of cirrhosis was higher among older adults, the rate of NAFLD progression to cirrhosis was similar in young and older adults. Older patients were more likely to have LREs.
INTRODUCTION
The prevalence of obesity has risen drastically since the mid-1970s.1,2 Obesity increasingly affects children and young adults,1 40% of adults aged 20–39 years in the US have obesity, on par with older adults,3 and young adults are increasingly affected by metabolic diseases, including diabetes mellitus and NAFLD.4–7
NAFLD is strongly associated with obesity and metabolic dysfunction and now affects 20%–30% of adults worldwide.7–9 While NAFLD can progress to decompensated cirrhosis and is projected to become the leading indication for liver transplantation in the US,10,11 only a minority of patients with NAFLD ultimately develop cirrhosis.12,13 Identifying the subset of NAFLD patients who will progress to advanced fibrosis or cirrhosis has been an ongoing challenge. Age is a risk factor for disease-specific mortality in NAFLD.14 However, this association may simply reflect longer exposure to hepatic steatosis and, therefore, a higher prevalence of cirrhosis in older patients. In contrast, among patients without cirrhosis, whether young adults with NAFLD have a more benign disease trajectory than older persons with NAFLD is unknown. Young adults with NAFLD may have a lower burden of metabolic diseases, notably diabetes.15 However, young adults with NAFLD may be more likely to have genetic risk factors for NAFLD, such as PNPLA3-rs738409-G, which is associated with an increased risk of cirrhosis.16 Notably, young patients with NAFLD potentially have decades of future “disease exposure” during which cirrhosis may develop. As the number of young adults with NAFLD grows, the global burden of cirrhosis and its complications is projected to be immense.17 However, precise data on the rate of progression to cirrhosis across age groups are limited. This limitation in the literature is especially important given the increasing prevalence of NAFLD in young adults [>30% of US adults aged 18–40 y18].
We aimed to compare young and older adults with NAFLD with respect to prevalent cirrhosis, risk of progression to cirrhosis, and liver-related events (LREs; including hepatic decompensation and HCC) and identify whether metabolic and laboratory-based risk factors demonstrate age-specific effects.
METHODS
Study design and cohort
This is a retrospective study of adults (≥18 y) with NAFLD seen at Michigan Medicine. We identified patients with hepatic steatosis based on liver biopsy, vibration-controlled transient elastography–controlled attenuation parameter >250 db/m,19 or imaging between January 1, 2010 and December 31, 2021. Identification of steatosis through imaging used a validated natural language processing algorithm,20,21 which searches imaging reports for the term “steato” or “fat” in the same sentence as “liver” or “hepat” in the absence of a term denoting negation (eg, “no”) or evaluation (eg, “rule out”). We excluded patients with a history of cancer other than nonmelanoma skin cancer,22 excess alcohol use (≥14/21 standard drinks/week in women/men, respectively), or other chronic liver diseases23 (Supplemental Table S1, http://links.lww.com/HC9/A311). The NAFLD index date was the date of the first study documenting the presence of hepatic steatosis. Patients were divided into 3 age groups based on age at the NAFLD index date: 18 to <40 years (young), 40 to <60 years (middle-aged), and ≥60 years (older).12
A subset of these patients was also participant in the Michigan Genomics Initiative (MGI), a prospective cohort of Michigan Medicine patients who underwent genotyping for research purposes. At the time of analysis, >80,000 MGI participants had undergone genotyping using an Illumina HumanCoreExome v.12.1 array.24
The Institutional Review Board of the University of Michigan approved this study. All MGI participants provided written informed consent for the use of their genetic data. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul.
Diagnosis of cirrhosis and liver-related events
We defined cirrhosis based on imaging demonstrating cirrhosis, as described,20 or validated International Classification of Diseases (ICD) codes (Supplemental Table S1, http://links.lww.com/HC9/A311) for cirrhosis or portal hypertensive complications with positive predictive values of 94%–96%.25,26 We defined LREs based on ICD codes for ascites, variceal bleeding, hepatic encephalopathy, or HCC (Supplemental Table S1, http://links.lww.com/HC9/A311).27 The earliest date of ICD diagnosis or imaging study demonstrating cirrhosis was used to define the date of cirrhosis diagnosis. Patients with cirrhosis diagnosed before or ≤1 year after the NAFLD index date were considered to have prevalent cirrhosis and those diagnosed >1 year after the NAFLD index date to have incident cirrhosis. For analyses of incident cirrhosis and LREs, we excluded patients with both prevalent cirrhosis or LREs (before or ≤1 y after the NAFLD index date). We chose to exclude patients with baseline cirrhosis from our analysis of incident LRE because the interest of our study was identifying progression from noncirrhotic NAFLD to LREs, rather than from compensated cirrhosis to decompensation. We evaluated the specificity of ICD codes for cirrhosis among those with presumed prevalent cirrhosis (vs imaging as the gold standard) and found that specificity was similar across age groups (99.2%, 98.8%, and 98.7% in young, middle-aged, and older).
Genetic analyses
Among patients with available genetic data through MGI, we compared the distribution of genotypes of NAFLD risk alleles reported in a published study.16 The risk alleles evaluated were PNPLA3-rs738409-G, TM6SF2-rs58542926-T, GCKR-rs1260326-C, MBOAT7-rs626283-C, and HSD17B13-rs6834314-G (proxy for HSD17B13-rs72613567, which is not available in MGI; r 2 = 1.0). For all alleles except the TM6SF2 allele, we compared the proportion of patients with zero, 1, or 2 copies of the allele; for the TM6SF2-rs58542926 variant, we combined the CT and TT genotypes because the T allele has low allele frequency. We compared genotype distributions using a chi-squared test.
Risk factors for prevalent and incident cirrhosis
We identified factors associated with prevalent cirrhosis in the overall cohort and within each age group. First, we used univariable logistic regression to identify prespecified risk factors. Age and variables with p <0.1 on univariable analysis were included in the multivariate model, except that we did not include aspartate aminotransferase (AST) [alanine transaminase (ALT) was included instead] to avoid multicollinearity.
To identify risk factors associated with incident cirrhosis, we used Fine-Gray competing risk analyses with the development of cirrhosis as the primary event and death before the development of cirrhosis as the competing risk. Patients with prevalent cirrhosis or with follow-up <365 days were excluded from these analyses. The primary predictor was the age group. We conducted subgroup analyses based on baseline AST-to-platelet ratio index (APRI)28 as a noninvasive marker for baseline fibrosis status, and diabetes, obesity, and baseline ALT because they were associated with prevalent cirrhosis. Of the commonly used fibrosis markers, APRI is the only one that can be applied consistently across age groups as it does not include age.
Statistics
Quantitative variables were reported as median [interquartile range (IQR)] and categorical variables as numbers and percentages (%). Three-way comparisons were performed with Kruskal-Wallis statistics for continuous variables and the Fisher test for categorical variables.
A 2-sided p value < 0.05 was used to determine statistical significance throughout.
RESULTS
Cohort and baseline characteristics
We included 31,505 patients with NAFLD (Figure 1), of whom 8252 (26.2%) were aged 18 to <40 (young), 15,035 (47.7%) aged 40 to <60 (middle-aged), and 8218 (26.1%) aged ≥60 years (older) at NAFLD index date. The first modality used to identify hepatic steatosis was imaging in 29,930 (95.0%), transient elastography in 916 (2.9%), and biopsy in 659 (2.1%). Young people were slightly more likely to have NAFLD diagnosed through imaging than middle-aged and older patients (95.6% in young and 94.8% in middle-aged and older, p < 0.05).
FIGURE 1.
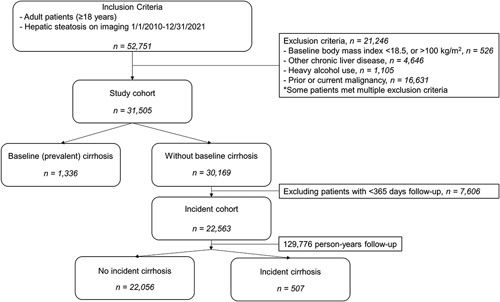
Study flowchart.
The baseline characteristics of the study cohort are reported in Table 1. Obesity was more common in those <60 years old (65.4%, 65.5%, and 57.0% in young, middle-aged, and older, respectively; p < 0.001). There was a stepwise increase in the prevalence of other metabolic diseases with advancing age, including hypertension, hyperlipidemia, and diabetes mellitus (16.1%, 29.6%, and 36.9% in young, middle-aged, and older; p < 0.001). Prevalent cirrhosis was also more frequent with advancing age (2.0, 4.0, and 6.2% in young, middle-aged, and older; p < 0.001). The older group had the lowest triglycerides, lowest LDL, and highest HDL levels. There was a stepwise decrease in baseline ALT with age (median 46.0, 38.5, and 32.0 U/L in young, middle-aged, and older; p < 0.001).
TABLE 1.
Baseline cohort characteristics by age group
| Trait | Age 18 to <40 (n = 8252); % | Age 40 to <60 (n = 15035); % | Age; ≥60 (n = 8218); % | p |
|---|---|---|---|---|
| Age (y) | 32.2 (27.0–36.4) | 50.6 (45.7–55.2) | 66.9 (63.2–72.1) | — |
| Male | 49.0 | 49.4 | 46.5 | <0.001 |
| Race | ||||
| White | 74.1 | 78.9 | 83.9 | <0.001 |
| Hispanic | 7.0 | 4.1 | 2.1 | — |
| Non-Hispanic Black | 8.4 | 8.7 | 6.4 | — |
| Asian | 6.1 | 4.7 | 4.2 | — |
| Other | 4.4 | 3.7 | 3.4 | — |
| Hypertension | 24.0 | 48.0 | 63.3 | <0.001 |
| Hyperlipidemia | 19.2 | 41.4 | 53.7 | <0.001 |
| Diabetes | 16.1 | 29.6 | 36.9 | <0.001 |
| BMI (kg/m2) | 32.9 (27.8–39.0) | 32.5 (28.3–37.9) | 30.8 (27.1–35.5) | <0.001 |
| Normal | 13.5 | 10.0 | 13.1 | <0.001 |
| Overweight | 21.1 | 24.5 | 29.9 | — |
| Class 1 obesity | 24.8 | 28.2 | 29.7 | — |
| Class 2 obesity | 19.1 | 18.8 | 16.1 | — |
| Class 3 obesity | 21.5 | 18.5 | 11.2 | — |
| Cirrhosis | 2.3 | 4.3 | 6.4 | <0.001 |
| Aspirin use | 16.6 | 38.4 | 61.5 | <0.001 |
| Statin use | 15.6 | 45.4 | 65.3 | <0.001 |
| Metformin use | 20.0 | 29.6 | 32.0 | <0.001 |
| Laboratory values | ||||
| Creatinine (mg/dL) (n = 26267) | 0.8 (0.7–0.9) | 0.8 (0.7–1.0) | 0.9 (0.8–1.1) | <0.001 |
| Hemoglobin A1c (%) (n = 9984) | 5.6 (5.3–6.2) | 5.9 (5.5–6.9) | 6.2 (5.8–7.2) | <0.001 |
| AST (U/L) (n = 25393) | 32.0 (23.0–48.0) | 31.0 (23.0–44.0) | 30.0 (23.0–42.0) | <0.001 |
| ALT (U/L) (n = 25460) | 46.0 (26.0–76.5) | 38.5 (26.0–60.0) | 32.0 (22.0–49.0) | <0.001 |
| <ULN | 17.5 | 19.0 | 27.7 | <0.001 |
| 1 to <2x ULN | 37.0 | 45.0 | 44.5 | — |
| 2 to <5x ULN | 34.9 | 29.6 | 22.1 | — |
| ≥5x ULN | 10.6 | 6.5 | 5.7 | — |
| Total bilirubin (mg/dL) (n = 25286) | 0.5 (0.4–0.8) | 0.5 (0.4–0.8) | 0.6 (0.4–0.8) | <0.001 |
| Alkaline phosphatase (U/L) (n = 25297) | 80.5 (66.0–101.0) | 84.0 (69.0–108.0) | 87.0 (69.0–114.0) | <0.001 |
| Albumin (g/dL) (n = 25495) | 4.4 (4.1–4.6) | 4.3 (4.1–4.5) | 4.2 (3.8–4.5) | <0.001 |
| Platelets (K/uL) (n = 24641) | 255.0 (211.5–303.5) | 241.0 (199.0–290.0) | 221.0 (176.5–271.0) | <0.001 |
| LDL (mg/dL) (n = 10834) | 103.0 (82.0–127.0) | 105.0 (81.0–129.0) | 89.0 (66.0–116.0) | <0.001 |
| HDL (mg/dL) (n = 11476) | 41.0 (35.0–49.0) | 44.0 (37.0–53.0) | 45.0 (37.0–55.0) | <0.001 |
| Triglycerides (mg/dL) (n = 12218) | 167.0 (112.0–254.2) | 162.0 (112.5–242.0) | 147.5 (105.6–209.0) | <0.001 |
Note: Continuous variables are reported as median (interquartile range). The upper limit of normal for alanine transaminase was defined as 19 U/L for women or 30 U/L for men.
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; ULN, upper limit of normal.
Genotypic data were available in 4,359 patients with similar availability in all age groups (9.4%, 10.6%, and 10.0% in young, middle-aged, and older, respectively). The frequency of the PNPLA3-rs738409-G allele was higher in the young (GG genotype 11.8%, 9.6%, and 8.8% in young, middle-aged, and older; p = 0.016; Figure 2). The frequency of NAFLD risk alleles in GCKR, HSD17B13, MBOAT7, and TM6SF2 was not different across age groups (Supplemental Figure S1, http://links.lww.com/HC9/A311).
FIGURE 2.
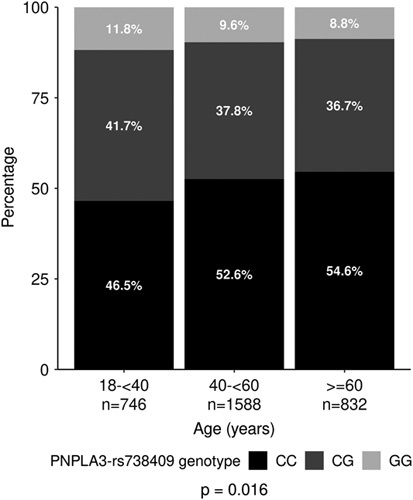
Distribution of PNPLA3-rs738409 genotype across age groups.
Factors associated with prevalent cirrhosis
Among patients presenting with cirrhosis, the percentage of decompensated cases was similar among ages (17.7% overall, with 19.8% in young, 17.5% in middle-aged, and 17.1% in older, p = 0.693). Older patients were also more likely to have had decompensation at the time of NAFLD diagnosis (0.7% overall, with 0.4% in young, 0.7% in middle-aged, and 1.1% in older, p = < 0.001).
Univariate logistic regression analysis identified older age, diabetes, hypertension, class 3 obesity, elevated ALT, elevated AST, and AST-to-ALT ratio > 1 to be associated with prevalent cirrhosis in the overall cohort (Supplemental Table S2, http://links.lww.com/HC9/A311, Supplemental Figure S2, http://links.lww.com/HC9/A311). Black and Asian patients were less likely to have prevalent cirrhosis overall (ORs: 0.39 and 0.36, respectively, p < 0.001 in both). In multivariate analysis (Table 2, Supplemental Figure S3, http://links.lww.com/HC9/A311), older age, diabetes, and higher ALT were significantly associated with a higher prevalence of cirrhosis. Diabetes was strongly associated with prevalent cirrhosis in all age groups (ORs: 1.74, 2.75, and 2.17 in young, middle-aged, and older; p < 0.05 in all). Similarly, higher ALT was associated with prevalent cirrhosis, but this effect was seen primarily in the older age group. Surprisingly, higher body mass index (BMI) was only strongly associated with a higher prevalence of cirrhosis in the older age group and was paradoxically associated with lower odds of prevalent cirrhosis in the young. Hyperlipidemia was not associated with cirrhosis in the young but was associated with lower odds of prevalent cirrhosis in the middle-aged and older groups (ORs: 0.65 and 0.56, respectively).
TABLE 2.
Multivariate model for prediction of prevalent (baseline) cirrhosis
| Overall | Age 18 to <40 | Age 40 to <60 | Age ≥60 | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Age (y) | 1.02 (1.02–1.03) | <0.001 | 1.00 (0.98–1.03) | 0.734 | 1.04 (1.02–1.06) | 0.000 | 0.98 (0.97–1.00) | 0.084 |
| Male (vs female) | 1.03 (0.90–1.17) | 0.679 | 1.19 (0.86–1.64) | 0.294 | 0.95 (0.79–1.15) | 0.611 | 1.06 (0.85–1.31) | 0.620 |
| Race | ||||||||
| White | Referent | — | Referent | — | Referent | — | Referent | — |
| Black | 0.35 (0.25–0.49) | <0.001 | 0.58 (0.30–1.12) | 0.103 | 0.32 (0.20–0.51) | <0.001 | 0.27 (0.14–0.53) | <0.001 |
| Asian | 0.42 (0.28–0.64) | <0.001 | 0.45 (0.18–1.12) | 0.085 | 0.53 (0.30–0.93) | 0.027 | 0.36 (0.17–0.77) | 0.009 |
| Hispanic | 0.89 (0.66–1.21) | 0.467 | 0.68 (0.35–1.30) | 0.243 | 1.05 (0.70–1.58) | 0.807 | 1.02 (0.54–1.94) | 0.941 |
| Other | 0.69 (0.48–1.00) | 0.052 | 0.60 (0.24–1.48) | 0.268 | 0.73 (0.43–1.24) | 0.247 | 0.68 (0.36–1.27) | 0.224 |
| Diabetes | 2.44 (2.11–2.82) | <0.001 | 1.74 (1.14–2.66) | 0.011 | 2.75 (2.23–3.39) | <0.001 | 2.17 (1.72–2.74) | <0.001 |
| Hypertension | 0.92 (0.79–1.06) | 0.241 | 1.10 (0.74–1.62) | 0.637 | 0.92 (0.75–1.13) | 0.403 | 0.84 (0.65–1.08) | 0.170 |
| Hyperlipidemia | 0.64 (0.55–0.74) | 0.000 | 0.85 (0.54–1.34) | 0.489 | 0.65 (0.53–0.80) | <0.001 | 0.56 (0.44–0.71) | <0.001 |
| BMI | ||||||||
| Normal | Referent | — | Referent | — | Referent | — | Referent | — |
| Overweight | 0.92 (0.73–1.16) | 0.501 | 0.56 (0.35–0.90) | 0.016 | 1.01 (0.69–1.46) | 0.978 | 1.21 (0.81–1.81) | 0.363 |
| Class 1 obesity | 0.93 (0.74–1.17) | 0.522 | 0.32 (0.19–0.53) | <0.001 | 0.93 (0.64–1.34) | 0.689 | 1.56 (1.05–2.31) | 0.029 |
| Class 2 obesity | 1.08 (0.85–1.37) | 0.542 | 0.36 (0.21–0.61) | <0.001 | 1.23 (0.85–1.79) | 0.271 | 1.61 (1.05–2.46) | 0.029 |
| Class 3 obesity | 1.24 (0.97–1.57) | 0.082 | 0.53 (0.33–0.86) | 0.010 | 1.41 (0.97–2.04) | 0.071 | 1.69 (1.08–2.66) | 0.023 |
| ALT | ||||||||
| <ULN | Referent | — | Referent | — | Referent | — | Referent | — |
| 1 to <2× ULN | 1.25 (1.05–1.50) | 0.013 | 1.32 (0.81–2.16) | 0.269 | 0.99 (0.77–1.29) | 0.968 | 1.45 (1.09–1.93) | 0.010 |
| 2 to <5× ULN | 1.47 (1.21–1.78) | <0.001 | 1.68 (1.03–2.74) | 0.039 | 1.13 (0.86–1.50) | 0.373 | 1.75 (1.27–2.40) | 0.001 |
| ≥5× ULN | 1.91 (1.48–2.45) | <0.001 | 1.75 (0.97–3.18) | 0.064 | 1.83 (1.28–2.62) | 0.001 | 1.92 (1.22–3.02) | 0.005 |
Note: ORs and 95% CI are reported. Normal body mass index was defined as body mass index <23 in Asians and body mass index <25 in non-Asian patients. The upper limit of normal for alanine transaminase was defined as 19 U/L for women or 30 U/L for men.
Abbreviations: ALT, alanine transaminase; BMI, body mass index; ULN, upper limit of normal.
Incidence and predictors of incident cirrhosis
During a median (IQR) follow-up of 4.6 (2.5–8.0) years, 507 (2.2%) of 22,563 patients without prevalent cirrhosis were diagnosed with incident cirrhosis. The diagnosis was made by both ICD and imaging in 32%, imaging alone in 30%, and ICD alone in 38%. Follow-up time was similar in each age group, with a median of 4.4 years in the young, 5.0 years in the middle-aged, and 4.1 years in the older group. Among patients who developed incident cirrhosis, median time-to-cirrhosis was (IQR) 5.2 (2.4–9.1) years overall, 5.5 (2.9–10.0) years in the young, 6.1 (2.7–10.2) years in the middle-aged, and 3.8 (2.0–6.7) years in the older group. The cumulative 5 and 10-year incidence rates for cirrhosis were 1.5% and 3.9% overall, 1.3% and 3.4% for the young, 1.4% and 3.7% for the middle-aged, and 2.0% and 4.7% for the older age groups, respectively (p > 0.05 for both comparisons; Figure 3).
FIGURE 3.
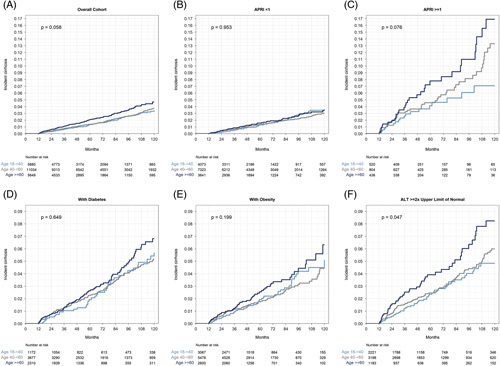
Cumulative incidence of cirrhosis, with death without cirrhosis modeled as a competing risk, stratified by age group. (A) Overall cohort. (B) Patients with APRI <1. (C) Patients with APRI ≥1. (D) Patients with diabetes. (E) Patients with obesity. (F) Patients with ALT ≥2 times the ULN, defined as 19 U/L for women or 30 U/L for men. Abbreviations: ALT, alanine transaminase; APRI, AST-to-platelet ratio index; ULN, upper limit of normal.
In univariable analysis, diabetes, hypertension, hyperlipidemia, higher BMI, and higher baseline ALT level were associated with an increased risk of cirrhosis. While the oldest age group was at modestly increased risk of incident cirrhosis in univariable analysis [HR: 1.33 (1.03–1.71) vs youngest], in multivariable analysis, the age group was not associated with risk of incident cirrhosis (Table 3). In addition, diabetes, hypertension, and higher ALT remained associated with an increased risk of cirrhosis, while hyperlipidemia and higher BMI (except class 3 obesity) were not. Black patients were significantly less likely to develop cirrhosis. The strongest predictors of incident cirrhosis were diabetes [HR: 2.05 (1.58–2.64) vs no diabetes] and ALT elevations [HR: 3.30 (2.21–4.94) for ALT 2 to <5 times upper limit of normal (ULN) and 5.05 (3.13–8.16) for ALT ≥ 5 times ULN vs < ULN; Table 3]. In several subgroup analyses, age group remained not associated with incident cirrhosis in patients with or without diabetes, obesity, or elevated baseline APRI (Figure 3, Supplemental Figure S4, http://links.lww.com/HC9/A311). Older patients were modestly more likely to develop incident cirrhosis in the subgroup with baseline ALT ≥2 times ULN.
TABLE 3.
Univariate and multivariate predictors of incident cirrhosis
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p | HR (95% Cl) | p |
| Age (y) | ||||
| 18 to <40 | Referent | — | Referent | — |
| 40 to <60 | 1.12 (0.90–1.40) | 0.304 | 0.86 (0.65–1.15) | 0.321 |
| ≥60 | 1.33 (1.03–1.71) | 0.026 | 1.03 (0.72–1.47) | 0.881 |
| Male (vs female) | 0.97 (0.82–1.16) | 0.776 | 1.21 (0.96–1.52) | 0.110 |
| Race | ||||
| White | Referent | — | Referent | — |
| Black | 0.50 (0.32–0.77) | 0.002 | 0.34 (0.18–0.64) | 0.001 |
| Asian | 0.56 (0.33–0.93) | 0.026 | 0.70 (0.38–1.30) | 0.262 |
| Hispanic | 1.21 (0.80–1.82) | 0.368 | 0.72 (0.40–1.29) | 0.269 |
| Other | 0.69 (0.38–1.25) | 0.216 | 0.49 (0.20–1.19) | 0.113 |
| Diabetes | 2.16 (1.81–2.59) | <0.001 | 2.05 (1.58–2.64) | <0.001 |
| Hypertension | 1.70 (1.39–2.08) | <0.001 | 1.51 (1.14–2.01) | 0.004 |
| Hyperlipidemia | 1.41 (1.17–1.70) | <0.001 | 1.07 (0.81–1.41) | 0.623 |
| BMI | ||||
| Normal | Referent | — | Referent | — |
| Overweight | 1.56 (0.94–2.58) | 0.084 | 1.33 (0.79–2.24) | 0.288 |
| Class 1 obesity | 1.75 (1.07–2.87) | 0.026 | 1.21 (0.72–2.04) | 0.467 |
| Class 2 obesity | 1.59 (0.94–2.67) | 0.082 | 1.01 (0.58–1.74) | 0.982 |
| Class 3 obesity | 2.69 (1.64–4.41) | <0.001 | 1.81 (1.07–3.07) | 0.028 |
| ALT | ||||
| <ULN | Referent | — | Referent | — |
| 1 to <2× ULN | 1.51 (1.08–2.12) | 0.017 | 1.70 (1.13–2.56) | 0.011 |
| 2 to <5× ULN | 2.58 (1.85–3.60) | <0.001 | 3.30 (2.21–4.94) | <0.001 |
| ≥5× ULN | 4.50 (3.04–6.66) | <0.001 | 5.05 (3.13–8.16) | <0.001 |
Note: HRs and 95% CI are reported. Normal body mass index was defined as body mass index <23 in Asians and body mass index <25 in non-Asian patients. The upper limit of normal for alanine transaminase was defined as 19 U/L for women or 30 U/L for men.
Abbreviations: ALT, alanine transaminase; BMI, body mass index; ULN, upper limit of normal.
In the subset of patients with genetic data available and at least one year of follow-up (n = 3166), 71 patients developed incident cirrhosis. PNPLA3-rs738409-GG genotype was associated with an increased risk of incident cirrhosis (HR: 2.49 [1.29–4.79], p = 0.006) after adjustment for sex, age category, and genetic principal components 1–10. After additional adjustment for diabetes status, obesity, and ALT ≥2× ULN, the association between GG genotype and incident cirrhosis was no longer significant [HR: 2.08 (0.94–4.63), p = 0.073], partly due to missingness in BMI values. The rs738409-CG genotype was not associated with an increased risk of incident cirrhosis.
Incidence and predictors of liver-related events
During a median (IQR) follow-up of 4.6 (2.5–8.0) years, 74 (0.3%) of 22,121 patients had LREs [53 (71.6%) hepatic decompensation and 21 (28.4%) HCC] with cumulative 5 and 10-year incidences of 0.15% and 0.67% for the overall cohort. Among those who developed LREs, time-to-event was median (IQR) 7.2 (4.8–10.4) years, with 10.9 (4.9–16.5) years in the young, 8.1 (5.7–10.6) years in the middle-aged, and 5.7 (4.2–7.3) years in the older. The cumulative 5 and 10-year incidences of LREs were significantly higher in the older age groups (p = 0.008 in a 3-way comparison) and are given as follows: 0.14% and 0.20% for the young, 0.09% and 0.68% for the middle-aged, and 0.31% and 1.15% for the older age groups, respectively (Figure 4).
FIGURE 4.
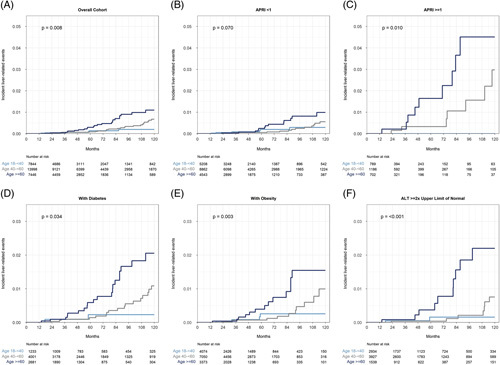
Cumulative incidence of LREs, with death without hepatic decompensation modeled as a competing risk, stratified by age group. (A) Overall cohort. (B) Patients with APRI <1. (C) Patients with APRI ≥1. (D) Patients with diabetes. (E) Patients with obesity. (F) Patients with ALT ≥2 times the ULN, defined as 19 U/L for women or 30 U/L for men. Abbreviations: ALT, alanine transaminase; APRI, AST-to-platelet ratio index; LRE, liver-related event; ULN, upper limit of normal.
In both univariable and multivariable analyses, only diabetes and advancing age were associated with an increased risk of LREs (Table 4). PNPLA3 genotype was not associated with incident LREs due to a small number of cases (n = 3). In multivariable analysis, diabetes was the strongest predictor [HR: 4.48 (2.18-9.18) vs no diabetes] followed by age 60 years or older [HR: 3.11 (1.17–8.31)] vs age 18- <40 y]. Sex, race, elevated ALT, hypertension, and hyperlipidemia were not significantly associated with LREs. In most subgroups, including those with diabetes, obesity, elevated baseline ALT, and elevated APRI, advancing age was significantly associated with the risk of LREs (Figure 4, Supplemental Figure S5, http://links.lww.com/HC9/A311). Among subgroups of patients using versus not using aspirin, statins, or metformin, only metformin use was significantly associated with LREs in the oldest age group though there was a trend toward increased LREs with advancing age in all subgroups (Supplemental Table S3, http://links.lww.com/HC9/A311).
TABLE 4.
Univariate and multivariate predictors of LREs
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p | HR (95% Cl) | p |
| Age (y) | ||||
| 18 to <40 | Referent | — | Referent | — |
| 40 to <60 | 1.17 (0.63–2.16) | 0.618 | 0.92 (0.36–2.35) | 0.857 |
| ≥60 | 2.35 (1.25–4.41) | 0.008 | 3.11 (1.17–8.31) | 0.024 |
| Male (vs female) | 1.33 (0.84–2.11) | 0.217 | 1.80 (0.94–3.46) | 0.078 |
| Race | ||||
| White | Referent | — | Referent | — |
| Black | 0.65 (0.24–1.77) | 0.395 | 0.70 (0.21–2.35) | 0.564 |
| Asian | 0.25 (0.03–1.78) | 0.165 | 0.74 (0.10–5.81) | 0.779 |
| Diabetes | 4.83 (2.79–8.34) | <0.001 | 4.48 (2.18–9.18) | <0.001 |
| Hypertension | 1.38 (0.83–2.31) | 0.214 | 0.82 (0.38–1.78) | 0.616 |
| Hyperlipidemia | 1.32 (0.81–2.17) | 0.266 | 0.67 (0.32–1.39) | 0.280 |
| BMI | ||||
| Normal | Referent | — | Referent | — |
| Overweight | 0.40 (0.10–1.60) | 0.195 | 0.46 (0.11–1.97) | 0.292 |
| Class 1 obesity | 0.79 (0.24–2.57) | 0.697 | 0.75 (0.21–2.70) | 0.655 |
| Class 2 obesity | 1.30 (0.41–4.15) | 0.657 | 1.11 (0.31–4.05) | 0.871 |
| Class 3 obesity | 2.12 (0.71–6.35) | 0.181 | 2.25 (0.64–7.85) | 0.204 |
| ALT | ||||
| <ULN | Referent | — | Referent | — |
| 1 to <2× ULN | 1.02 (0.49–2.11) | 0.964 | 1.04 (0.43–2.56) | 0.924 |
| 2 to <5× ULN | 1.29 (0.62–2.71) | 0.500 | 1.41 (0.52–3.83) | 0.505 |
| ≥5× ULN | 1.98 (0.72–5.41) | 0.185 | 1.59 (0.38–6.72) | 0.526 |
Note: HRs and 95% CI are reported. Normal body mass index was defined as body mass index <23 in Asians and body mass index <25 in non-Asian patients. The upper limit of normal for alanine transaminase was defined as 19 U/L for women or 30 U/L for men.
Abbreviations: ALT, alanine transaminase; BMI, body mass index; LRE, liver-related event; ULN, upper limit of normal.
DISCUSSION
Among over 30,000 adults with hepatic steatosis, younger patients had a lower prevalence of diabetes but a higher prevalence of obesity, elevated ALT, and the PNPLA3-rs738409-G allele than their older counterparts. Progression to cirrhosis was similar in young, middle-aged, and older patients, but the risk of LREs was greater in older patients despite the exclusion of patients with baseline cirrhosis or a history of hepatic decompensation. Our finding that adult NAFLD patients of all ages demonstrate similar rates of progression to cirrhosis suggests that NAFLD is not necessarily benign in young patients. With >30% prevalence of NAFLD among Americans younger than 40 years,18 this study provides direct evidence that the prevalence of NAFLD-related cirrhosis is likely to grow dramatically in the near future. Prevalent cirrhosis was more than 3 times as common in older than young patients (6.3% vs 2.3%), which may reflect a longer average duration of NAFLD exposure.29,30
Few studies have focused on evaluating NAFLD disease trajectory in young adults compared with older adults. One recent territory-wide study of people with NAFLD and type 2 diabetes in Hong Kong found that age 50 years or older was associated with a higher incidence of LREs versus age younger than 40 years.31 While our study found the progression to cirrhosis overall to be similar across ages, we likewise found that the risk of LREs increased with advancing age and the presence of diabetes. Our study had important differences from the study in Hong Kong. First, we included both patients with and without diabetes to assess its impact on cirrhosis development and LRE, which is important as diabetes increases the risk of cirrhosis in NAFLD.12,32 Next, we excluded patients with baseline cirrhosis from our time-to-event analyses for hepatic decompensation, mitigating biases that prolonged cirrhosis may have on subsequent decompensation. Finally, our cohort had a higher proportion of young patients (26% vs 11%), increasing the sample of young patients who may decompensate.
The prevalence of metabolic and genetic risk factors for NAFLD differed considerably based on age group. Younger patients tended to have higher BMI and baseline ALT but less often had hypertension, hyperlipidemia, and diabetes. This is consistent with prior work in NAFLD demonstrating an inverse correlation between age and both obesity and ALT,33,34 and the general trend of increased metabolic comorbidities with age.4–6 These differences in metabolic profile have important prognostic significance, as both diabetes and obesity are linked to disease progression.31,35 Carriage of high-risk PNPLA3 alleles has been associated with earlier age of NAFLD diagnosis16 and with cirrhosis/advanced fibrosis36 and HCC.16,37 There is growing evidence that these variants are associated with disease progression in patients with established NAFLD.38 The higher prevalence of PNPLA3 risk alleles, obesity, and liver enzyme elevations in our young patients may “cancel out” their lower prevalence of diabetes and presumably shorter duration of steatosis resulting in a similar rate of incident cirrhosis as the older patients.
Strengths of our study include its large sample size of >30,000 patients, with a balanced representation of young and older adults, and a long-follow up period: a median of nearly 5 years and a total of >130,000 person-years. Furthermore, genotypic data were available in a large number of patients (n = 4359). There were several limitations to our study. First, this study was based on a single tertiary center with a predominantly White population, and the results may not be representative of the general population in the US or other countries. As a referral center, the incidence of cirrhosis and LREs in our patients is expected to be higher than in primary or secondary care settings. Whether our findings are generalizable to lower risk populations is unclear. Second, while we made every effort to identify the earliest date at which there was objective evidence of NAFLD, it was not possible to define the exact date at which NAFLD first developed. Third, while we made every effort to exclude patients with heavy alcohol use and other causes of chronic liver diseases, we relied on ICD codes and patient self-report in the medical record rather than standardized tools, such as AUDIT-C or biomarkers, which is a limitation inherent in a retrospective cohort of this size. Fourth, patients in our cohort had NAFLD determined primarily by imaging studies, and there is a possibility of referral bias, given that indications for abdominal imaging in younger patients may differ from older patients. Finally, due to the retrospective nature of this study, it can be challenging to identify when NAFLD first developed: routine screening for hepatic steatosis with imaging is not recommended, so we simply used the earliest date that there was evidence of steatosis in our medical system. Similarly, the exact date of cirrhosis development may be unclear if patients were not regularly monitored, and ICD codes and imaging may not be sensitive in identifying patients with compensated cirrhosis though this would apply across all age groups. We reported that the sensitivity of ICD codes for cirrhosis in our cohort was 46%,39 so there likely were cases of cirrhosis which were missed. We partially addressed this by evaluating LREs as an outcome, which are less likely to be underdiagnosed as they result in symptoms. We mitigated this by excluding patients with clear confounding indications for obtaining imaging, most notably cancers other than nonmelanoma skin cancer.
In conclusion, young adults with NAFLD demonstrated similar rates of progression to cirrhosis as older patients but experienced a significantly lower risk of LREs during a median follow-up of 4.6 years. Given the increasing prevalence of NAFLD in children and young adults, simple and reliable noninvasive strategies to assess baseline fibrosis have importance across all age groups. Our findings that older patients are at the highest risk of LREs in the short to medium term are consistent with current guidelines focusing on older patients. However, the increasing prevalence of NAFLD in children and young adults, and a similarly high rate of incident cirrhosis in younger patients compared with older ones forewarn a wave of NAFLD-related cirrhosis and related complications hitting younger persons in the future.
Supplementary Material
Acknowledgments
CONFLICTS OF INTEREST
This study was supported in part by a Clinical, Translational, and Outcomes Research Award from the AASLD to Vincent L. Chen. Vincent L. Chen and Anna S. Lok received research support from KOWA and Astra Zeneca (to University of Michigan). Anna S. Lok serves on the DSMB of Novo Nordisk. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: ALT, alanine transaminase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; ICD, International Classification of Diseases; IQR, interquartile range; LRE, liver-related event; MGI, Michigan Genomics Initiative; ULN, upper limit of normal.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Matthew J. Miller, Email: mmillers@med.umich.edu.
Emily Harding-Theobald, Email: emihar@umich.edu.
Jacob V. DiBattista, Email: jacob.dibattista@gmail.com.
Zhe Zhao, Email: zzhaozhe@umich.edu.
Karn Wijarnpreecha, Email: dr.karn.wi@gmail.com.
Anna S. Lok, Email: aslok@umich.edu.
Vincent L. Chen, Email: vichen@med.umich.edu.
REFERENCES
- 1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 4. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief. 2020;364:8. [PubMed] [Google Scholar]
- 5. Sullivan PW, Ghushchyan VH, Ben-Joseph R. The impact of obesity on diabetes, hyperlipidemia and hypertension in the United States. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2008;17:1063–71. [DOI] [PubMed] [Google Scholar]
- 6. Mendola ND, Eberhardt MS. Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United States. 2018;319:8. [PubMed] [Google Scholar]
- 7. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatol Baltim Md. 2018;67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol Baltim Md. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 9. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 10. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148;(3):547–555. [DOI] [PubMed] [Google Scholar]
- 11. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatol Baltim Md. 2014;59:2188–95. [DOI] [PubMed] [Google Scholar]
- 12. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng CH, Xiao J, Lim WH, Chin YH, Yong JN, Tan DJH, et al. Placebo effect on progression and regression in NASH: Evidence from a meta-analysis.. Hepatology. 2022;75:1647–61. [DOI] [PubMed] [Google Scholar]
- 14. Golabi P, Paik JM, Herring M, Younossi E, Kabbara K, Younossi ZM. Prevalence of high and moderate risk nonalcoholic fatty liver disease among adults in the United States, 1999-2016. Clin Gastroenterol Hepatol. 2021;S1542-3565:01339–2. [DOI] [PubMed] [Google Scholar]
- 15. Bardugo A, Bendor CD, Zucker I, Lutski M, Cukierman-Yaffe T, Derazne E, et al. Adolescent nonalcoholic fatty liver disease and type 2 diabetes in young adulthood. J Clin Endocrinol Metab. 2021;106:e34–44. [DOI] [PubMed] [Google Scholar]
- 16. Walker RW, Belbin GM, Sorokin EP, Van Vleck T, Wojcik GL, Moscati A, et al. A common variant in PNPLA3 is associated with age at diagnosis of NAFLD in patients from a multi-ethnic biobank. J Hepatol. 2020;72:1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–64. [DOI] [PubMed] [Google Scholar]
- 18. Kim D, Cholankeril G, Loomba R, Ahmed A. Prevalence of fatty liver disease and fibrosis detected by transient elastography in adults in the United States, 2017-2018. Clin Gastroenterol Hepatol. 2021;19:1499–501.e2. [DOI] [PubMed] [Google Scholar]
- 19. Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17:630–7.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen VL, Du X, Chen Y, Kuppa A, Handelman SK, Vohnoutka RB, et al. Nature Publishing Group. Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology. Nat Commun. 2021;12:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen VL, Chen Y, Du X, Handelman SK, Speliotes EK. Genetic variants that associate with cirrhosis have pleiotropic effects on human traits. Liver Int. 2020;40:405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagström H, Adams LA, Allen AM, Byrne CD, Chang Y, Grønbæk H, et al. Administrative coding in electronic health care record-based research of NAFLD: an expert panel consensus statement. Hepatol Baltim. 2021;74:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–37.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dey R, Schmidt EM, Abecasis GR, Lee S. A fast and accurate algorithm to test for binary phenotypes and its application to PheWAS. Am J Hum Genet. 2017;101:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burkholder DA, Moran IJ, DiBattista JV, Lok AS, Parikh ND, Chen VL. Accuracy of International Classification of Diseases-10 codes for cirrhosis and portal hypertensive complications. Dig Dis Sci. 2022;67:3623–3631; October 21, 2021. cited May 7, 2022. Accessed August 1, 2022. 10.1007/s10620-021-07282-x [DOI] [PubMed] [Google Scholar]
- 27. Chen VL, Oliveri A, Miller MJ, Wijarnpreecha K, Du X, Chen Y, et al. PNPLA3 genotype and diabetes identify patients with non-alcoholic fatty liver disease at high risk of incident cirrhosis. Gastroenterology. 2023;S0016-5085:00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- 29. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–12. [DOI] [PubMed] [Google Scholar]
- 30. Frith J, Day CP, Henderson E, Burt AD, Newton JL. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55:607–13. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Wong GLH, Yip TCF, Cheung JTK, Tse YK, Hui VWK, et al. Risk of liver-related events by age and diabetes duration in patients with diabetes and nonalcoholic fatty liver disease. Hepatol Baltim. 2022;76:1409–22. [DOI] [PubMed] [Google Scholar]
- 32. Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020;51:1149–59. [DOI] [PubMed] [Google Scholar]
- 33. Soresi M, Noto D, Cefalù AB, Martini S, Vigna GB, Fonda M, et al. Nonalcoholic fatty liver and metabolic syndrome in Italy: results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. 2013;50:241–9. [DOI] [PubMed] [Google Scholar]
- 34. Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, et al. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J Gastroenterol WJG. 2014;20:14185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- 36. Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trépo E, Nahon P, Bontempi G, Valenti L, Falleti E, Nischalke HD, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: evidence from a meta-analysis of individual participant data. Hepatol Baltim. 2014;59:2170–7. [DOI] [PubMed] [Google Scholar]
- 38. Koo BK, Lee H, Kwak SH, Lee DH, Park JH, Kim W. Long-term effect of PNPLA3 on the aggravation of nonalcoholic fatty liver disease in the biopsy-proven cohort. Clin Gastroenterol Hepatol. 2022;21:1105–1107. [DOI] [PubMed] [Google Scholar]
- 39. Wijarnpreecha K, Li F, Lundin SK, Suresh D, Song MW, Tao C, et al. Higher mortality among lean patients with non-alcoholic fatty liver disease despite fewer metabolic comorbidities. Aliment Pharmacol Ther. 2023;57:1014–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


