Abstract
Background:
Scrophularia striata Boiss. (S. striata) is a flowering plant with several therapeutic properties including antiinflammatory, antioxidant, antimicrobial, and wound-healing activity. Regarding the side effects of drugs conventionally used for inflammatory bowel disease (IBD) treatment, we investigated the anticolitis properties of aqueous (SSAE) and hydroalcoholic (SSHE) extracts of S. striata on experimental colitis.
Materials and Methods:
The colitis was induced using acetic acid (3%) and 2 h before ulcer induction, each group of rats received orally three doses (150, 300, and 600 mg/kg, p.o.) of SSAE or SSHE for the next 5 days. Dexamethasone (1 mg/kg, i.p.) and mesalazine (100 mg/kg, p.o.) were used as reference drugs. Different parameters including weight of colon/height, ulcer index, total colitis index, levels of myeloperoxidase (MPO) and malondialdehyde (MDA) were investigated.
Results:
Total phenolic contents were 4.3 ± 0.2 and 7.1 ± 0.4 mg/g equivalent to gallic acid for SSAE and SSHE respectively. Three applied doses of SSHE and the highest dose of SSAE (600 mg/kg) could reduce all the macroscopic and pathologic indices of colitis and the levels of MPO and MDA. Two lesser doses of SSAE (150, 300 mg/kg) however, couldn’t diminish the histopathologic features of colitis and the values of MPO and MDA.
Conclusions:
S. striata, especially SSHE, which also contained more phenolic compounds, had an ameliorating effect on ulcerative colitis and possibly exerts this effect through its antioxidant, antiinflammatory and wound healing properties. Further investigations are required to introduce this plant as a novel alternative herbal drug for colitis treatment.
Keywords: Antiinflammatory agents, colitis, plant extracts, rats, Scrophularia, striata, ulcer
INTRODUCTION
Inflammatory bowel disease (IBD) is related to a widely gastrointestinal disorder characterized by idiopathic, chronic, and recurrent inflammation. Ulcerative colitis (UC) and Crohn's disease are two prime categories of IBD.[1] Although the precise etiology of IBD is not clearly illuminated, several factors including; environmental elements, genetics, immune system, and bacterial flora are considered to be contributed to the complex pathogenesis and increased inflammation of the disease.[2] UC confines colon and rectum with significant morbidity and is clinically characterized by disrupted integrity of the intestinal epithelial and diffuse inflammation of the colon mucosa.[3] Common symptoms of UC are colon ulceration, abdominal cramps and pain, fever, bloody diarrhea, anemia, and weight loss.[1,3] The main complications of UC include colon cancer, toxic megacolon, as well as extra-intestinal complications like skin involvement and osteoarthritis.[4] Oxidative stress is known as one of the major factors participated in the development and complication of the disease. Oxidative stress could result from increased inflammatory mediators, cytokines, and reactive oxygen species (ROS) or decreased activity of antioxidant capacity comprising myeloperoxidase (MPO) and malondialdehyde (MDA).[5] Conventional treatments for UC are mainly based on immunosuppressive agents, corticosteroids, and amino-salicylates which the latter are administered as first-line therapy.[1,3] However, adverse effects of these agents, temporary relief, and insufficient efficiency of treatment highlight the necessity of providing definitive treatment and safe novel remedies.[6,7] Increasing interest in alternative therapy and homeopathy, as well as traditional therapies like herbal medication, has suggested novel complementary therapeutics for UC and IBD treatment.[8] In this regard, natural compounds derived from traditional Iranian medicine have been proposed as alternative candidates to study as novel therapeutic agents for IBD treatment.[9] Scrophularia striata Boiss. (S. striata, Figwort, Tashnedari) is a member of the flowering plant of the Scrophulariaceae family with antioxidant, antiinflammatory, antinociceptive, anticancer, ulcer healing and immune-modulatory effects.[10] Members of this family could be found in temperate to cold climate areas including mountains of central Asia particularly in the Mediterranean area, central Europe, and North America.[11] Several species of Scrophulariaceae grow in Western and Northwestern regions of Iran that have been gathered for traditional medicine uses such as burns, wounds, scabies, ulcers, hemorrhoids, eczema, and infectious diseases.[12] A plethora of Scrophularia species have been isolated and examined as different classes of secondary metabolites with antioxidant activity including phenolic acids (cinnamic acid), iridoids (scropolioside A and scrovalentinoside), terpenoids, flavonoids (quercetin, isorhamnetin3-O-rutinoside and nepitrin), phenylpropanoids, and acetoside1 (a glycoside).[10,13,14] Azadmehr et al.[15] have identified the inhibitory effect of S. striata ethanolic and ethyl acetate extracts on the induction of proinflammatory mediators including IL-1β, TNF-α, and Prostaglandin E2 (PGE2) as well as nitric oxide (NO). In another study performed by same authors on peritonitis induced ex vivo, ethanolic extract of S. striata was able to reduce macrophage activation and peritoneal inflammation due to NO release inhibition.[16] On the other hand, total and methanolic extracts of S. striata improved full-thickness incisional and burn-induced and infected wounds respectively in animal models.[17,18] Given this information, herein, we investigated the in vivo antiulcerative effects of S. striata hydroalcoholic extract (SSHE) and S. striata aqueous extract (SSAE) on a rat model of colitis induced by acetic acid.
MATERIALS AND METHODS
Drugs and chemicals
Mesalazine and dexamethasone powders were purchased from Iran Hormone Company (Tehran, Iran). O-dianisidine dihydrochloride (ODD) and hexadecyl trimethyl ammonium bromide (HTAB) were purchased from Sigma Company (St. Louis, USA). Folin–Ciocalteu reagent was purchased from Solarbio Corporation (Beijing, China). Ethanol, methanol, glacial acetic acid, and formaldehyde were procured from Merck Company (Darmstadt, Germany). Ketamine (10%) and xylazine (2%) were both purchased from Alfasan Company (Woerden, Netherland).
Preparation of plant
S. striata was collected from Ilam province, Iran and Dr. M. Sadeghi-Dinani a Pharmacognosist from Isfahan School of Pharmacy and pharmaceutical Sciences authenticated its genus and variety.
Preparation of SSAH
Initially, the aerial parts of S. striata were collected and set to dry. Then the dried material was pulverized using an electronic mill. Approximately 500 g of the plant powder was mixed with distilled water (6 liters) and kept for 24 h followed by stirring for 2 h. The extract was then filtered and concentrated through the rotary evaporation process at 50°C.
Preparation of SSHE
The powdered plant (500 g) was mixed with ethanol 70% (4 L) and set aside to soak well for 24 h. Then, the extract was stirred for 2 h and filtered using the Büchner funnel.[19]
Yield value and total phenol assessment of extracts
Ten milliliters of extracts were weighted and placed on a Ben Mary to remove the solvent. Then it was placed for 3 h in an oven at a temperature of 100 to 105°C to obtain the dry weight of the extracts. This was repeated for three times.
Folin–Ciocalteu method was used to determine the amount of polyphenolic compounds in extracts. Briefly, SSAE and SSHE (0.5 g) were added separately to 10 mL ethanol 96° and brought to the desired concentration. Then different concentrations of gallic acid were prepared and in the presence of Folin–Ciocalteu reagent and sodium bicarbonate solution, its standard curve was drawn. Then the absorption of extract solutions was read at 765 nm wave length and their phenolic contents as gallic acid equivalent (GALeq, mg/g), of extract was calculated using standard curve. The experiment was repeated for three times.[20]
Animals
Herein, 60 male Wistar rats (180–220 g) were used. Rats were obtained from the animal house of Isfahan School of Pharmacy, Isfahan, Iran. Animals were grown up under the same controlled conditions of temperature, humidity, 12/12 h light/dark photoperiods while tap water and chow pellets were ad libitum. The national code number of IR.MUI.RESEARCH.REC.1399.749 was assigned to this research grant which indicates the acceptance of this research by the Iranian National Ethics Committee in Biological Research.
Acute colitis induction
After 24 h of fasting, rats were lightly anesthetized with isoflurane and a thin and flexible catheter with 2 mm inner diameter and 8 cm length was placed into the anus and 2 mL acetic acid 3% was injected. The rats were maintained in head-down position for 60 sec. to avoid bringing out the injected acetic acid before taking the catheter out.[21]
The groups of experimental animals
The rats were randomly divided into ten groups (n = 6) and interventions were done 2 h prior to colitis induction and continued daily for 5 days thereafter. The suspensions of extracts and reference drugs were made by 0.1% v/v Tween 80 in normal saline (vehicle) to obtain a final volume of 1 ml and administrated orally for 5 days as follow.
Group 1 (Normal): received vehicle (5 mL/kg) orally (p.o.) without colitis induction
Group 2 (Control colitis): received vehicle (5 mL/kg, p.o.) before colitis induction
Groups 3-5 (SSAE, 150, 300, 600 mg/kg, p.o.): received SSAE with three mentioned doses orally before colitis induction
Groups 6-8 (SSHE 150, 300, 600 mg/kg, p.o.): received SSAE with three mentioned doses orally before colitis induction.[22,23]
Group 9 (Dexa. 1): received dexamethasone (1 mg/kg) intraperitoneally (i.p.) before colitis induction
Group 10 (Mes. 100): received mesalazine (100 mg/kg, p.o.), before colitis induction
Evaluation of macroscopic features of colon
Sixth days after starting treatment, the rats were sacrificed by CO2 overdose. Colon sections were exposed, and distal colon (8 cm in length) were removed and incised longitudinally. The tissues were irrigated (by normal saline) and weighted. Images were then taken using Sony® camera followed by tissue fixing on a working sheet. Ulcer score (US) was determined according to the following scales[24]: No ulcer (0), Mucosal erythema, mild edema and inflammation (1) Moderate inflammation, bleeding, edema, thickness and superficial ulcer (2), Severe and deep ulcer and bleeding (3), and necrosis and/or perforation (4). Ulcer area was measured by Fiji-win 32 software. For comparing the extracts, ulcer index (UI) was calculated for each group by UI = US (ulcer score) + UA (ulcer aria).
Assessment of colonic histopathology
Colon tissues were cut in length into two equal sections. One section was transferred to −70°C to measure the MDA and MPO activity. Another section was fixed in 10% formalin for histological evaluation after staining with hematoxylin and eosin (H&E). Histological injuries were described based on Heidari et al. study.[25] Digital images related to the macroscopic and histological injuries were captured using a standard Zeiss® microscope equipped with a Sony® color video camera.[24]
Measurement of the MPO activity
Frozen colon tissues were weighed (0.1 g) and crushed. Then phosphate buffer (5 mL) supplemented with HTAB (0.5% w/v) was added and homogenized through three cycles for 45 sec with 1 min of interval. The homogenate solution was sonicated on ice for 10 sec and centrifuged at 4,000 rpm for 15 min. Next, 0.1 mL of supernatant was mixed with 2.9 mL phosphate buffer containing hydrogen peroxide (0.005%) and ODZ (0.167 mg/mL). The absorption was measured at a wavelength of 450 nm to estimate MPO activity at 0 and 3 min. MPO activity was stated as units (U) per gram (g) weight of wet colon tissue.[26]
Measurement of the MDA
One ml of potassium chloride (1.15% v/100 mg tissue) was added to each colonic tissue (0.1 g) and the sample was homogenized. Samples were centrifuged at 1200 rpm for 10 min and the supernatant was subjected to measure MDA content according to the MDA assay kit (Navand-Salamat, Urmia). Finally, the samples were centrifuged at 3000 rpm for 15 min and the absorbance was measured at 550 nm wave length. MDA levels were calculated by the following formula: MDA level (nmol/mg) = (MDA (optical density) OD – 0.0451)/0.1784.[27]
Statistical analysis
Data was reported as mean ± SEM for parametric and median (range) for nonparametric ones. The significant level was P < 0.05. The one-way analysis of variance (ANOVA) with Tukey as post hoc test and Mann–Whitney U test were used for the determination of differences between groups at parametric and nonparametric data, respectively using SPSS version 16 software.
RESULTS
Yield value of S. striata extracts
Yield values were 17.3 ± 1.1% w/w and 12.7 ± 0.8% w/w for SSAE and SSHE, respectively. The total phenolic content of SSAE and SSHE were 4.3 ± 0.2 and 7.1 ± 0.4 mg/g GALeq of dry extract, respectively.
Macroscopic results
In the normal group no erosion, ulcer or edema was found indicating no interference of handling and surgical procedure with experimental findings [Figure 1]. In contrast, the highest degree of tissue damage, ulceration and edema occurred in the colitis control group. Table 1 presented the SSAE and SSHE effects (150, 300, and 600 mg/kg) on macroscopic parameters in the acetic acid-induced colitis rats. Oral administration of SSAE and SSHE reduced ulcer area and score, ulcer index and weight of colons in comparison with the control group (at least P < 0.05). SSHE at the dose 600 mg/kg was significantly more effective than SSHE with similar dose (600 mg/kg) (P < 0.05) in reduction of ulcer index as well as area and score. Treatment with dexamethasone and mesalazine as reference drugs decreased the investigated parameters in comparison to control colitis group significantly (at least P < 0.01) [Table 1].
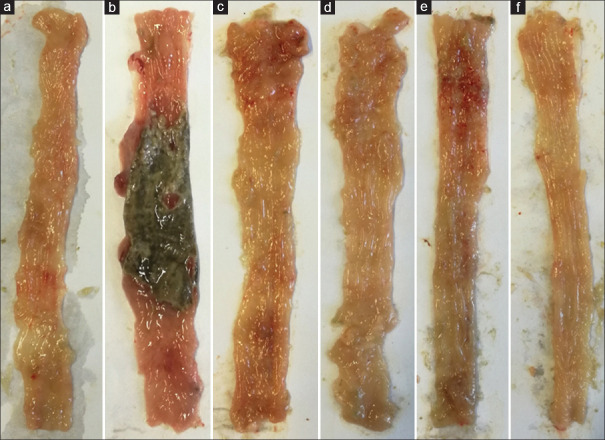
Figure 1.
Macroscopic image of colon tissue in rats. (a) Normal colon tissue with no sign of ulcer, necrosis, thickness and edema. (b) Control colitis group represent clear ulceration, necrosis, thickness and edema. (c) Colitis treated with S. striata aqueous extract (SSAE, 600 mg/kg), (d) Colitis treated with S. striata hydroalcoholic extract (SSHE, 600 mg/kg), (e) Colitis treated with mesalazine (100 mg/kg) and f) Colitis treated with dexamethasone (1 mg/kg) represent different significant degree of improvement in ulceration, edema and thickness of tissue with no sign of necrosis
Table 1.
Effect of S. striata aqueous (SSAE) and hydroalcoholic (SSHE) extracts on macroscopic parameters of colitis in rats
| Groups/dose (mg/kg) | Ulcer aera (cm2) (0-8) | Ulcer score (0-4) | Ulcer index (0-12) | Colon/height (g/cm) |
|---|---|---|---|---|
| Normal | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.10±0.02 |
| Control colitis | 7.46±1.02### | 3.66±0.40 ### | 11.12±1.81### | 0.22±0.04### |
| SSAE 150 | 3.82±0.68** | 3.00±0.38 | 6.82±1.51* | 0.14±0.02* |
| SSAE 300 | 4.29±1.14* | 2.33±0.22* | 6.62±1.36* | 0.13±0.03* |
| SSAE 600 | 2.71±1.03** | 2.16±0.23* | 4.87±1.23** | 0.10±0.02** |
| SSHE 150 | 1.48±0.50*** | 1.66±0.16** | 3.14±0.39*** | 0.09±0.02** |
| SSHE 300 | 1.29±0.65*** | 1.33±0.18 *** | 2.62±1.19*** | 0.09±0.02** |
| SSHE 600 | 0.45±0.16***,+ | 0.66±0.20 ***,+ | 1.11±0.68***,+ | 0.08±0.01*** |
| Dex. 1 | 0.42±0.12*** | 0.5±0.28*** | 0.92±0.46*** | 0.07±0.01*** |
| Mes. 100 | 3.04±0.71** | 1.33±0.30** | 4.37±1.2** | 0.11±0.01** |
Data are presented as Mean±standard error of mean (n=6). Dex. (Dexamethasone), Mes. (Mesalazine). The differences between groups were calculated based on ANOVA and Mann-Whitney tests. *P<0.05, **P<0.01, and ***P<0.001 represent significant difference compared to control group, +P<0.05 represent significant difference compared to SSAE 600 mg/kg, ###P<0.001: represent significant difference compared to normal group
Microscopic evaluation
According to the pathological evaluation, no pathological/histological damages were found in the normal group. Also, the mucosal layer, submucosa, and crypts were intact, and no leukocyte infiltration was observed [Figure 2]. In contrast, crypt damage, leucocyte infiltration, and inflammation at sub-mucosal and mucus layers were clearly found in control colitis rats [Table 2]. Administration of SSAE (600 mg/kg) reduced the crypt damage and total colitis index significantly in comparison to control group (P < 0.05), while two lower doses of SSAE were not effective in this regard [Table 2]. On the other hand, all histological parameters and total colitis index were meaningfully decreased after SSHE administration (150, 300, and 600 mg/kg) compared with the control group (at least P < 0.05). SSHE at the dose 600 mg/kg was significantly more effective than SSAE with similar dose (600 mg/kg) (P < 0.05) in reduction of total colitis index as well as inflammatory extent and crypt damage. In addition, dexamethasone and mesalazine both reduced the inflammation extent and severity, leucocytes infiltration, crypt damage, and total colitis index in comparison to the control group (at least P < 0.05) [Table 2].
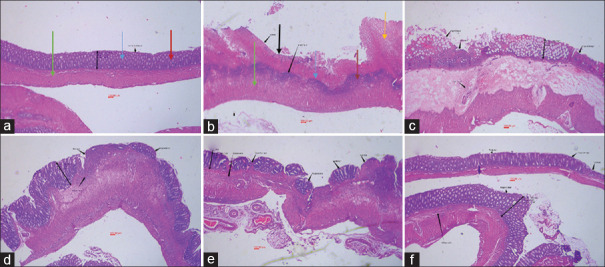
Figure 2.
Microscopic image of colon tissue after H and E staining (40 × magnification) in rats. (a) Normal tissue had normal mucosal (blue arrow) and sub-mucosal layer (green arrow), intact crypts (red arrow) with no leukocyte infiltration. (b) Control colitis group had crypt damage, mucosal layer destruction and necrosis (black arrow), thickness of sub-layer and leukocyte infiltration (yellow arrow). (c) Colitis treated with S. striata aqueous extract (SSAE, 600 mg/kg), (d) Colitis treated with S. striata hydroalcoholic extract (SSHE, 600 mg/kg), (e) Colitis treated with mesalazine (100 mg/kg) and (f) Colitis treated with dexamethasone (1 mg/kg) represent different significant degree of epithelial regeneration, diminished inflammation, leukocyte infiltration and crypt damage with no sign of necrosis
Table 2.
Effect of S. striata aqueous (SSAE) and hydroalcoholic (SSHE) extracts on microscopic parameters of colitis in rats
| Groups/dose (mg/kg) | Inflam. severity (0-3) | Inflam. extent (0-3) | Leucocyte infiltration (0-3) | Crypt Damage (0-4) | Total colitis index (0-13) |
|---|---|---|---|---|---|
| Normal | 0.0 (0-0) | 0.0 (0-0) | 0.0 (0-0) | 0.0 (0-0) | 0.0 (0-0) |
| Control colitis | 3.0 (3-3) ### | 3.0 (3-3) ### | 3.0 (2-3) ### | 4.0 (3-4) ### | 12.5 (12-13) ### |
| SSAE 150 | 3.0 (2-3) | 3.0 (2-3) | 3.0 (2-3) | 3.5 (2-4) | 11.6 (9-13) |
| SSAE 300 | 3.0 (2-3) | 3.0 (2-3) | 2.5 (1-3) | 3.0 (2-4) | 10.8 (7-13) |
| SSAE 600 | 2.5 (1-3) | 2.5 (2-3) | 2.0 (1-3) | 3.0 (2-3) * | 9.0 (6-12)* |
| SSHE 150 | 2.0 (1-2)*** | 2.0 (1-3)* | 2.0 (1-2)* | 2.5 (1-3)* | 8.6 (4-10)** |
| SSHE 300 | 1.5 (0-2)*** | 2.0 (1-2)* | 1.0 (1-2)*** | 2.0 (1-3)** | 6.3 (3-7) *** |
| SSHE 600 | 1.0 (0-2)*** | 1.0 (1-2)***,+ | 1.0 (1-1)*** | 1.5 (1-2)***,+ | 4.6 (3-7) ***+ |
| Dex.1 | 1.0 (0-1)*** | 1.0 (0-1)*** | 1.0 (0-1)*** | 1.0 (1-1)*** | 3.1 (1-5) *** |
| Mes.100 | 2.0 (1-3)* | 1.5 (1-3)** | 2.0 (1-2)* | 3 (2-3)* | 8.4 (5-11) ** |
Data are presented as Means±SEM (n=6). Dex. (Dexamethasone), Mes. (Mesalazine). The differences between groups were calculated based on Mann-Whitney test. *P<0.05, **P<0.01, ***P<0.001 represent significant difference compared to control group, +P<0.05 represent significant difference compared to SSAE 600 mg/kg, ###P<0.001 represent significant difference compared to normal group
MPO activity and MDA value in colonic tissue
Figures 3 and 4 represented the levels of MPO and MDA respectively in experimental groups that received SSAE, SSHE, dexamethasone, and mesalazine. According to the results, changes of MPO activity and MDA value were similar to pathologic findings as they were significant in SSAE (600 mg/kg), SSHE (150,300, and 600 mg/kg) groups as well as two reference groups (dexamethasone and mesalazine) compared to control group (at least P < 0.05).
Figure 3.
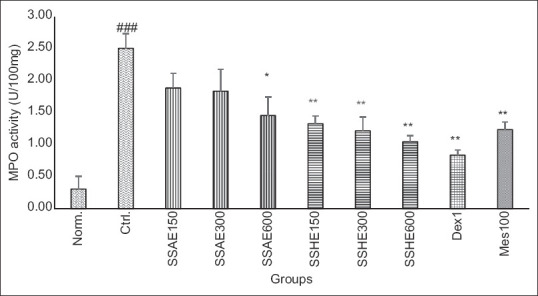
Effect of S. striata on colonic MPO activity (U/100 mg). Norm; Normal group, Ctrl; Control colitis group, SSAE; Colitis treated with S. striata aqueous extract, 150, 300, 600 mg/kg, SSHE; Colitis treated with S. striata hydroalcoholic extract, 150, 300, 600 mg/kg, Dex; Colitis treated with dexamethasone 1 mg/kg, Mes; Colitis treated with mesalazine 100 mg/kg. Data are stated as Mean ± standard error of mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to control group. ###P < 0.001 showed the significant level between normal and control group
Figure 4.
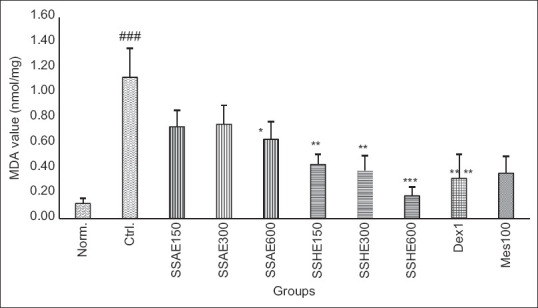
Effect of S. striata on colonic MDA level (nmol/mg). Norm; Normal group, Ctrl; Control colitis group, SSAE; Colitis treated with S. striata aqueous extract, 150, 300, 600 mg/kg, SSHE; Colitis treated with S. striata hydroalcoholic extract, 150, 300, 600 mg/kg, Dex; Colitis treated with dexamethasone 1 mg/kg, Mes; Colitis treated with mesalazine 100 mg/kg. Data are stated as Mean ± standard error of mean (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to control group. ###P < 0.001 showed the significant level between normal and control group
DISCUSSION
The present study indicated the ulcer healing, antiinflammatory and antioxidant effect of SSAE and SSHE by determining of macroscopic (ulcer index), microscopic (total colitis index) parameters as well as MPO and MDA activity on acetic acid-induced colitis rats. According to the results, all test doses of SSAE and SSHE reduced macroscopic parameters, while SSHE was just able to decrease microscopic parameters, MPO, and MDA activity. In addition, SSHE at the dose of 600 mg/kg exhibited the lowest ulcer index, total colitis score, and MPO and MDA levels compared to other interventional groups. Given that the extracts were administered orally, it is concluded that their active ingredients were available orally to the body and treatment for 5 days was able to achieve the desired result.[28] On the other hand, considering that the SSHE with all doses tested has been able to significantly reduce the indicators of ulcerative colitis, it can be concluded that it has more effective substances or these effective substances have been more available to the body.[29] The results showed that there was no significant difference between different doses of an extract but SSHE at the greatest used dose (600 mg/kg) was significantly more effective than SSAE at the same dose. Considering that the hydroalcoholic extract (SSHE) has a higher lipophilic content compared to the aqueous extract (SSAE), it is concluded that the better effectiveness of the SSHE is likely due to lipid-soluble ingredients such as steroids, saponins and iridoids.[10,13] In addition the results of total phenol content of extracts showed that the phenolic content of SSHE was higher than aqueous extract (7.1 vs. 4.3). This can be partly described by the fact that most of the active ingredients of this plant are in the category of phenols.[13] Phenolic acids (cinnamic acid), flavonoids (quercetin, isorhamnetin3-O-rutinoside and nepitrin) and flavonols, iridoids (scropolioside A and scrovalentinoside), saponins, tannins, and phenylpropanoids are among the active ingredients found in the extracts of this plant.[10,13,30] For each of these active ingredients, a variety of biological effects have been reported such as: antioxidants, antiinflammatory, analgesic, antitumor, wound healing, antibacterial and spasmolytic, some of which may have contributed to their anticolitis properties.[10] In a study conducted by Bas et al.[31] on the antiinflammatory properties of this plant, it was found that the iridoids (scrovalentinoside) had inhibitory effects on proinflammatory mediators and inflammation. Another study conducted by Ahmed et al.[32] on a similar species of the same genus (S. deserti) showed the antidiabetic and antiinflammatory effects of the plant and these effects were attributed to scropolioside-D2 and harpagoside-B contents in the plant. Moreover, the analgesic and antinociceptive effects of ethanolic (100, 200 mg/kg) and hydroalcoholic extracts (150–900 mg/kg) of S. striata on different models of experimentally induced pain such as hot plate and formalin tests have been studied and confirmed by researchers.[22,23] Due to the fact that oxidative stress and ROS are harmful per se and induce inflammatory and proinflammatory factors, in this study it was decided to measure the levels of MDA and MPO in rat colons as oxidative and proinflammatory markers.[33,34] Our findings showed the effectiveness of this plant, especially SSHE in reducing the levels of MDA and MPO, which confirmed the previous properties of this plant as an antioxidant and antiinflammatory agent.[35] In this regard, Shao et al.[36] investigated the effects of a traditional Chinese medicine named Suqingwan (SQW) in DSS-induced mouse model of UC. They evaluated levels of MPO which is associated with neutrophil infiltration and MDA as an oxidative stress biomarker. They reported that MDA and MPO levels were reduced in the treated groups which is in accordance with our findings. In the present study, we used acid acetic to induce colitis in rat model and investigated the antiinflammatory effects of S. striata extracts. This model has been used by many researchers for several years. This method has received a lot of attention due to its availability, cheapness, appropriate induction rate, good response to current therapies and pathological similarities with human colitis.[37] For example, Tanideh et al.[38] used the same acetic acid 3% method to induce colitis and investigated the effects of Melillotus Officinalis aqueous extract on colitis parameters. They reported that administration of 2,000 mg/kg of M. officinali showed a protective effect on colonic mucosa, while decreased the inflammatory cells and leukocyte infiltration. Moreover, acetic acid enhanced the levels of MDA and MPO in rat colonic mucosa compared to normal groups. Similarly, treatment with extract (1,000 and 2,000 mg/kg) for a period of seven days decreased the MDA and MPO levels and increased the glutathione (GSH) content at a significant level. Azadmehr et al.[15] evaluated the effects of S. striata ethyl acetate extract on the inhibition of proinflammatory cytokines including IL1β, TNF-α, and PGE2 that are produced by lipopolysaccharide (LPS) activated macrophages. They reported that S. striata extract (200 mg/ml) could inhibit the IL1β, TNF-α, and PGE2 production. In addition, neuroprotective and cognition enhancing properties of S. striata (200 mg/kg/d) has reported by Abedi et al.[39] They indicated that this effect was comparable with vitamin E (200 mg/kg/day) and attributed to antioxidant and radical scavenging properties of S. striata. Since this study is the first report on the beneficial anticolitis effects of S. striata, it is recommended that further studies be conducted on its safety profile and clinical efficacy as well as its active ingredients and more detailed mechanisms.
CONCLUSION
In overall, hydroalcoholic and aqueous extracts of S. striata were effective in relieving the symptoms of colitis. In addition, both extracts (especially at greater doses) were able to decrease MPO, and MDA activity indicating their antioxidant and antiinflammatory capacity. Although the total phenolic content, including flavonoids and tannins, as well as steroids and iridoids are the most likely active ingredients in these results, more research is needed to determine exactly which active ingredients are involved.
Financial support and sponsorship
This study was financially supported by Vice Chancellor for Research and Technology in Isfahan University of Medical Sciences as grant number 399976.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This study was financially supported by Vice Chancellor for Research and Technology in Isfahan University of Medical Sciences as grant number 399976.
REFERENCES
- 1.Freidman S, Blumberg RS. Inflammatory bowel disease. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. New York: Mc Graw Hill Education; 2018. pp. 2259–62. [Google Scholar]
- 2.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;10:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seyedian SS, Nokhostin F, Dargahi MM. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113–22. doi: 10.25122/jml-2018-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yangyang RY, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349–55. doi: 10.1053/j.sempedsurg.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Dziąbowska-Grabias K, Sztanke M, Zając P, Celejewski M, Kurek K, Szkutnicki S, et al. Antioxidant therapy in inflammatory bowel diseases. Antioxidants. 2021;10:412. doi: 10.3390/antiox10030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Zh, Wang Sh, Li J. Treatment of inflammatory bowel disease: A comprehensive review. Front Med. 2021;10:765474. doi: 10.3389/fmed.2021.765474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan P, Chen H, Guo Y, Bai AP. Advances in treatment of ulcerative colitis with herbs: From bench to bedside. World J Gastroenterol. 2014;20:14099–104. doi: 10.3748/wjg.v20.i39.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amiot A, Peyrin-Biroulet L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Therap Adv Gastroenterol. 2015;8:66–82. doi: 10.1177/1756283X14558193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke F, Yadav PK, Ju LZ. Herbal medicine in the treatment of ulcerative colitis. Saudi J Gastroenterol. 2012;18:3–10. doi: 10.4103/1319-3767.91726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamri P. A mini-review on phytochemistry and pharmacological activities of Scrophularia striata. J Herbmed Pharmacol. 2019;8:85–9. [Google Scholar]
- 11.Zargoosh Z, Ghavam M, GBacchetta G, Tavili A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci Rep. 2019;9:16021. doi: 10.1038/s41598-019-52605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farahmandzad F, Riyahi N, Mahdavi E. The effects of Scrophularia striata extract on apoptosis in glioblastoma cells in cell culture. Int J Adv Chem Eng Biol. 2015;2:62–3. [Google Scholar]
- 13.Mahboubi M, Kazempour N, Boland Nazar AR. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of Scrophularia Striata Boiss extracts. Jundishapur J Nat Pharm Prod. 2013;8:15–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Pasdaran A, Hamedi A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm Biol. 2017;55:2211–33. doi: 10.1080/13880209.2017.1397178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azadmehr A, Maliji G, Hajiaghaee R, Shahnazi M, Afaghi A. Inhibition of pro-inflammatory cytokines by ethyl acetate extract of Scrophularia striata. Trop J Pharm Res. 2012;11:893–7. [Google Scholar]
- 16.Azadmehr A, Afshari A, Baradaran B, Hajiaghaee R, Rezazadeh S, Monsef-Esfahani H. Suppression of nitric oxide production in activated murine peritoneal macrophages in vitro and ex vivo by Scrophularia striata ethanolic extract. J Ethnopharmacol. 2009;124:166–9. doi: 10.1016/j.jep.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Ghashghaii A, Hashemnia M, Nikousefat Z, Zangeneh M, Zangeneh A. Wound healing potential of methanolic extract of Scrophularia striata in rats. Pharm Sci. 2017;23:256–63. [Google Scholar]
- 18.Tanideh N, Haddadi MH, Rokni-Hosseini MH, Hossienzadeh M, Mehrabani D, Sayehmiri K, et al. The healing effect of Scrophularia striata on experimental burn wounds infected to Pseudomonas aeruginosa in rat. World J Plast Surg. 2015;4:16–23. [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemi-Dehkordi N. Iranian Herbal Pharmacopoeia. 1st ed. Tehran: Ministry of Health Publications; 2002. pp. 200–5. [Google Scholar]
- 20.Blainski A, Lopes GC, Palazzo de Mello JC. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852–65. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadraei H, Asghari G, Khanabadi M, Minaiyan M. Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res Pharm Sci. 2017;12:322–9. doi: 10.4103/1735-5362.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alimohammadi B, Azhdari-Zarmehri H, Dolatshahi M. The antinociceptive effect of hydroalcoholic extracts of Scrophularia striata Boiss.on mice using hot plate test. J Torbat Heydariyeh Univ Med Sci. 2015;3:1–7. [Google Scholar]
- 23.Sofiabadi M, Azadmehr A, Hajiaghaei R, Rezazadeh S, Ajdari Zarmehri H. The effect of ethanolic extract of Scrophularia striata on pain in male rats. J Med Plant. 2012;2:113–9. [Google Scholar]
- 24.Minaiyan M, Asghari G, Taheri D, Saeidi M, Nasr-Esfahani S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna J Phytomed. 2014;4:127–36. [PMC free article] [PubMed] [Google Scholar]
- 25.Heidari B, Sajjadi SE, Minaiyan M. Effect of Coriandrum sativum hydroalcoholic extract and its essential oil on acetic acid- induced acute colitis in rats. Avicenna J Phytomed. 2016;6:205–14. [PMC free article] [PubMed] [Google Scholar]
- 26.Motavallian A, Minaiyan M, Rabbani M, Mahzouni P, Andalib S. Anti-inflammatory effects of alosetron mediated through 5-HT 3 receptors on experimental colitis. Res Pharm Sci. 2019;14:228–36. doi: 10.4103/1735-5362.258489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niknami E, Sajjadi SE, Talebi A, Minaiyan M. Protective effect of Vitis vinifera (Black Grape) seed extract and oil on acetic acid-induced colitis in rats. Int J Prev Med. 2020;11:102. doi: 10.4103/ijpvm.IJPVM_362_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latifi G, Ghannadi A, Minaiyan M. Anti-inflammatory effect of volatile oil and hydroalcoholic extract of Rosa damascena Mill. on acetic acid-induced colitis in rats. Res Pharm Sci. 2015;10:514–22. [PMC free article] [PubMed] [Google Scholar]
- 29.Minaiyan M, Ghassemi-Dehkordi N, Mahzouni P, Ahmadi NS. Anti-inflammatory effect of Helichrysum oligocephalum DC extract on acetic acid-induced acute colitis in rats. Adv Biomed Res. 2014;3:87. doi: 10.4103/2277-9175.128000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharafati-Chaleshtori R, Sharafati-Chaleshtori F, Sharafati-Chaleshtori A, Ashrafi K. Antimicrobial effects and evaluation of total phenols, flavonoids and flavonols contents of ethanolic extracts of Scrophularia striata. J Shahrekord Univ Med Sci. 2010;11:32–7. [Google Scholar]
- 31.Bas E, Recio MC, Abdallah M, Máñez S, Giner RM, Cerdá-Nicolás M, et al. Inhibition of the pro-inflammatory mediators’ production and anti-inflammatory effect of the iridoid scrovalentinoside. J Ethnopharmacol. 2007;110:419–27. doi: 10.1016/j.jep.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed B, Al-Rehaily AJ, Al-Howiriny TA, El-Sayed KA, Ahmad MS. Scropolioside-D2 and harpagoside-B: Two new iridoid glycosides from Scrophularia deserti and their antidiabetic and antiinflammatory activity. Bio Pharm Bull. 2003;26:462–7. doi: 10.1248/bpb.26.462. [DOI] [PubMed] [Google Scholar]
- 33.Davies MJ, Hawkins CL. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid Redox Signal. 2020;32:957–81. doi: 10.1089/ars.2020.8030. [DOI] [PubMed] [Google Scholar]
- 34.Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017 doi: 10.1155/2017/4535194. 10.4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azadmehr A, Hajiaghaee R, Zohal MA, Maliji G. Protective effects of Scrophularia striata in ovalbumin-induced mice asthma model. DARU J Pharm Sci. 2013;21:1–7. doi: 10.1186/2008-2231-21-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao J, Liu Z, Wang L, Song Z, Chang H, Han N, et al. Screening of the optimized prescription from Suqingwan in terms of its therapeutic effect on DSS-induced ulcerative colitis by its regulation of inflammatory and oxidative mediators. J Ethnopharmacol. 2017;202:54–62. doi: 10.1016/j.jep.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Minaiyan M, Ghanadian SM, Hossaini M. Protective effect of Apium graveolens L. (Celery) seeds extracts and luteolin on acetic acid-induced colitis in rats. Int J Prev Med. 2021;12:100. doi: 10.4103/ijpvm.IJPVM_651_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanideh N, Bahrani M, Khoshnood-Mansoorkhani MJ, Mehrabani D, Firoozi D, Koohi-Hosseinabadi O, et al. Evaluating the effect of Melillotus officinalis L. Aqueous extracts on healing of acetic acid-induced ulcerative colitis in male rats. Ann Colorectal Res. 2016;4:e42856. [Google Scholar]
- 39.Abedi A, Bamdad K, Yaghoubi Shahir H, Dehghany R, Moradi A, Saadati H. Comparison of the protective effects of Scrophularia striata extract with vitamin E on cognitive function, anxiety and pain sensitivity in diazinon-exposed male rats. Physiol Pharmacol. 2021;25:47–56. [Google Scholar]