Abstract
Malignant melanoma is a highly aggressive cancer with metastatic potential to various locations such as the lymph nodes, lungs, liver, brain, and bone. After the lymph nodes, the lungs are the most common site of malignant melanoma metastases. Pulmonary metastases from malignant melanoma commonly presents as solitary or multiple solid nodules, sub-solid nodules or miliary opacities on CT chest. We present a case of pulmonary metastases from malignant melanoma in a 74-year-old man which presented unusually on CT chest as a combination of patterns like “crazy paving,” upper lobe predominance with subpleural sparing, and centrilobular micronodules. Video-assisted thoracoscopic surgery, wedge resection and tissue analysis were performed, which confirmed the diagnosis of malignant melanoma metastases, and the patient further underwent PET-CT for staging and surveillance. Patients with pulmonary metastases from malignant melanoma can have atypical imaging findings, therefore radiologists should be aware of these unconventional presentations to avoid any misdiagnoses.
Keywords: Malignant melanoma, Pulmonary metastases, Atypical presentation, Crazy paving, Oncology
Introduction
Melanoma comprises of only 1% of skin cancers but remains the leading cause of death amongst the skin cancers [1]. Malignant melanoma is renowned for its aggressive nature and high metastatic potential to a myriad of locations such as the lymph nodes, lungs, liver, brain, and bone via lymphatic and hematogenous routes [2]. The risk of metastasis is strongly dependent on the site of the primary tumor [3]. Melanomas arising on the head, neck and trunk carry a higher risk of metastasis than melanomas originating from the limbs [3].
The most frequent location of melanoma metastases is the lymphatic system, especially the local and regional lymph nodes surrounding the primary tumor [2]. After the lymph nodes, the most common site of distant metastases are the lungs and pleura, with an incidence rate of 80% [4]. Chest radiography (CXR) is the initial imaging modality utilized to detect suspected pulmonary metastases followed by chest computed tomography (CT) scan [5]. Positron emission tomography–computed tomography (PET-CT) serves as the most sensitive imaging modality to visualize the extent of pulmonary melanoma metastases [5].
Melanoma metastases to the lungs typically presents as well-defined, sharply delineated single or multiple solid nodules and rarely as semi-solid nodules or miliary invasions [6]. This article describes a case of melanoma metastases to the lungs that unusually presented as a spectrum of patterns such as bilateral diffuse consolidation and ground glass opacities with septal thickening, giving a “crazy-paving” pattern. The distribution was upper lobe predominant with subpleural sparing and multiple centrilobular micronodules were also noted on CT images. We provide additional histopathological analysis and PET-CT findings for correlation.
Case report
A 74-year-old man presented to our hospital with fever, nonproductive cough, malaise, and dyspnea. Patient endorses that he was diagnosed with melanoma of the scalp and upper back without evidence of metastatic disease one and a half years ago. He states that the lesions were surgically excised 3 months after initial diagnosis. He has no family history of melanomas but is a known diabetic and hypertensive. Patient was COVID negative and has received both doses of the Pfizer COVID vaccine. On physical examination, patient was tachypneic (respiratory rate ∼ 20), hypertensive (142/89 mm Hg), afebrile (temp – 98.2°F) with O2 saturation of 91% on room air. Bilateral crackles were heard upon auscultation. Patient was then referred for a CT chest without contrast for further evaluation. CT chest (Fig. 1) demonstrated bilateral diffuse consolidation and ground glass opacities with superimposed septal thickening; giving a “crazy paving” appearance. Airspace opacities predominantly involved the upper lobes with relative subpleural sparing. In addition, centrilobular micronodules were also noted. Thus, a broad imaging differential ranging from pulmonary hemorrhage, multifocal infection, hypersensitivity pneumonitis, pulmonary alveolar proteinosis and other interstitial lung diseases were considered. He was treated with prednisone and antibiotics and was recommended to follow up in 4 weeks for a repeat CT chest.
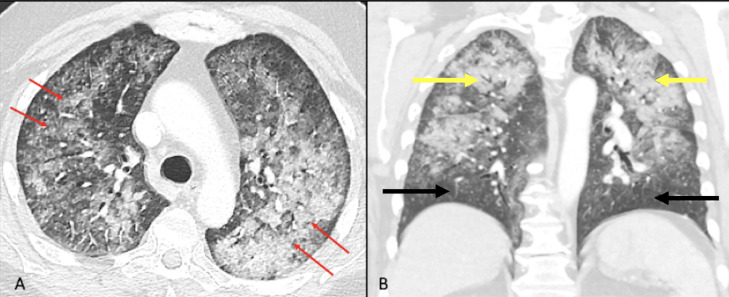
Fig. 1.
Axial CT chest image lung window (A) demonstrates bilateral diffuse consolidation and ground glass opacities with superimposed septal thickening, giving a “crazy-paving” pattern (red arrows). There is relative subpleural sparing. Coronal reformatted lung window (B) shows airspace disease predominantly involving upper lobes of lungs (yellow arrows) with relative sparing of bilateral lower lobes (black arrows).
During his 1-month follow up, a repeat CT chest without contrast (Fig. 2) demonstrated interval worsening of the previously visualized bilateral diffuse airspace disease. A bronchoalveolar lavage and a sputum culture were also performed during this visit and were found to be negative for tuberculosis, bacterial and fungal infections. A provisional diagnosis of interstitial lung disease was given and the patient was further referred to cardiothoracic surgery for wedge resection and tissue analysis.
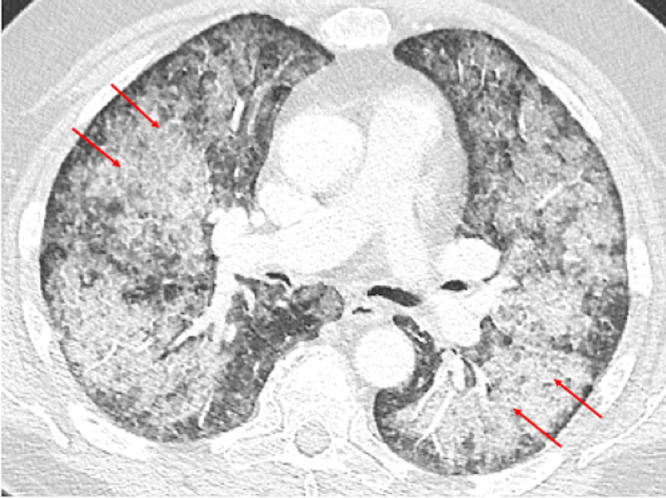
Fig. 2.
Axial CT chest image lung window performed 4 weeks after baseline CT demonstrates interval worsening of bilateral diffuse airspace disease with crazy-paving pattern (red arrows).
Two months after initial presentation to our hospital, the patient underwent video assisted thoracoscopic surgery (VATS) and wedge resection x3 of the right upper lobe (RUL), right middle lobe (RML) and the right lower lobe (RLL) with right pleural biopsy. Thoracostomy tube was placed. Follow up chest radiograph (Fig. 3) demonstrated bilateral diffuse airspace disease with expected postsurgical right subcutaneous emphysema and trace right pneumothorax. The presence of a right chest tube is also evident. The tissue samples were sent for histopathological analysis. The pathology revealed malignant cells with epithelioid morphology and cytoplasmic pigmentation; revealed to be melanin in the RUL, RML and RLL wedge resections (Fig. 4A and B). These histological features were consistent with malignant melanoma. In addition, the tumor cells were diffusely positive for pan-melanoma markers identified by immunohistochemistry confirming the histological interpretation of metastatic melanoma.
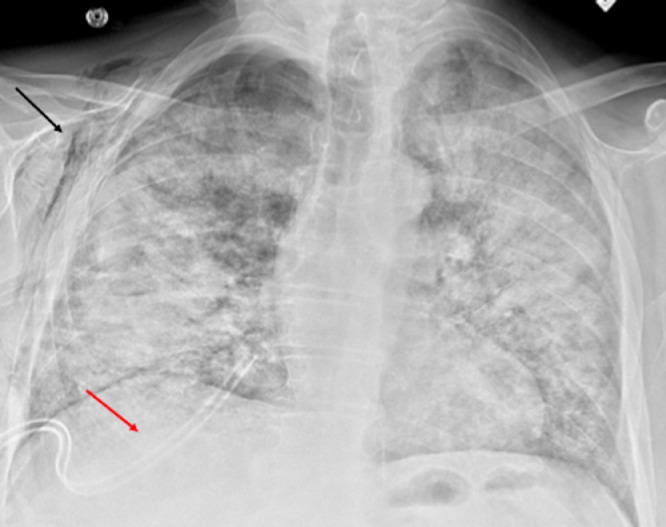
Fig. 3.
Postoperative chest radiograph after wedge resections of right lung demonstrates bilateral diffuse airspace disease with expected postsurgical right subcutaneous emphysema (black arrow) and trace right pneumothorax. Right chest drainage tube is noted in the lung base (red arrow).
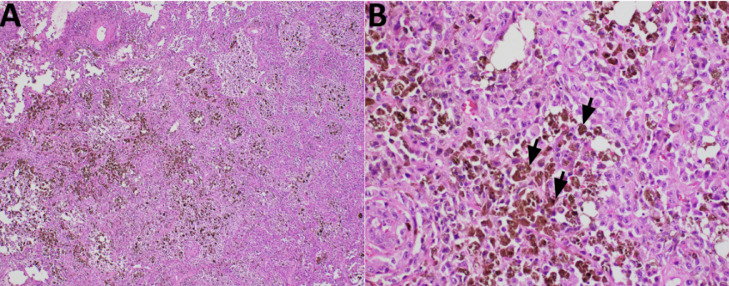
Fig. 4.
Representative H&E-stained slides showing the tumor histology. (A) The low-power picture shows diffuse growth of tumor containing dark-brown pigmentation. (B) At higher power magnification, the tumor cells show malignant nuclear features with melanin production (black arrows), consistent with metastatic melanoma.
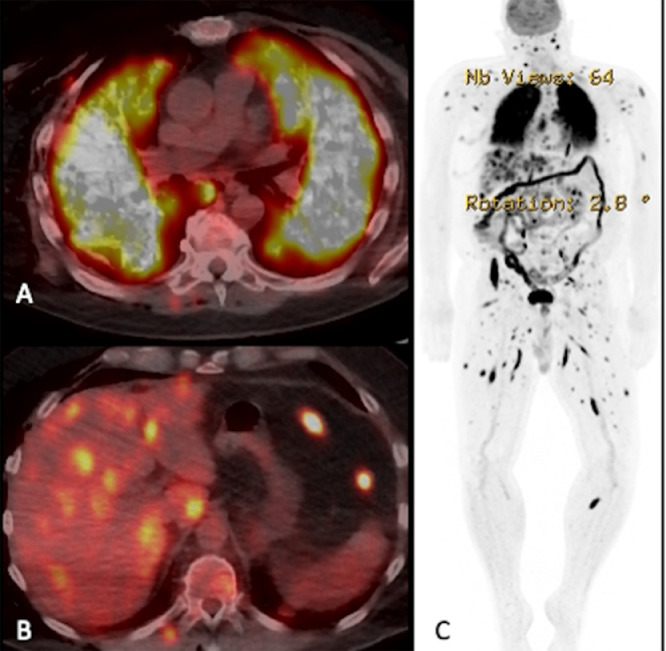
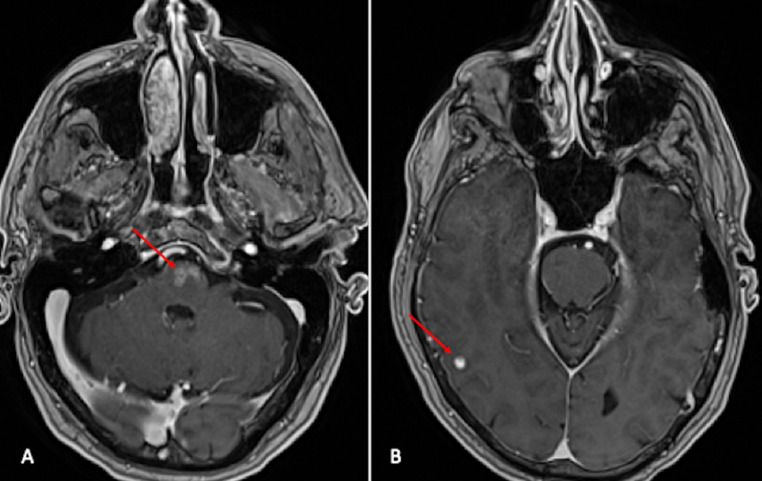
Fluorodeoxyglucose (FDG) PET-CT was also performed post VATS for restaging and surveillance of the malignant melanoma metastases. FDG PET-CT axial section (Fig. 5A) at the level of lungs demonstrates prominent uptake of FDG in bilateral diffuse airspace disease, consistent with pathology proven metastatic melanoma. FDG PET-CT axial section at the level of liver (Fig. 5B) shows multiple avid FDG uptake involving both lobes of the liver, posterior chest wall and peritoneum; favoring metastatic disease. FDG PET image (Fig. 5C) demonstrates extensive FDG avid metastatic disease involving the whole body. Additional MRI of brain (Fig. 6) was done for staging which showed enhancing multiple intraparenchymal metastases in the ventral pontomedullary junction of the brainstem and in the right cerebral cortex. The patient was referred for immunotherapy as radiotherapy confers risk due to extensive brain metastases. Patient refused treatment and was discharged to hospice care.
Fig. 5.
FDG PET-CT axial section at the level of lungs (A) demonstrates avid uptake of FDG in bilateral diffuse airspace disease, consistent with pathology proven metastatic melanoma. FDG PET-CT axial section at the level of liver (B) demonstrates multiple avid FDG uptake involving both lobes of the liver, posterior chest wall and peritoneum, favoring metastatic disease. FDG PET image (C) demonstrates extensive FDG avid metastatic disease involving whole body.
Fig. 6.
Axial section post contrast T1 weighted images (A and B) demonstrates enhancing intraparenchymal lesions concerning for metastases in the ventral pontomedullary junction of the brainstem (red arrow in Fig. A) and in the right cerebral cortex (red arrow in Fig. B).
Discussion
Malignant melanoma has the potential to disseminate to various locations such as the lymph nodes, lungs, brain, bone, and liver [2]. The lungs and pleura are the most common site of distant metastases from malignant melanoma with 85% of end-stage melanoma patients showing evidence of lung metastases at autopsy [7]. Moreover, the prognosis of patients with metastatic malignant melanoma is very poor, with a 5-year survival rate of less than 10% [8].
There is a relative lack of information in literature pertaining the unusual radiological presentations of malignant melanoma metastases to the lungs. The most common presentation of pulmonary metastases from malignant melanoma reported in literature is in the form of solitary or multiple solid nodules [6]. They could also present as subsolid nodules [9], [10], [11], [12]. Some of the rare CT patterns of malignant melanoma metastases to the lungs that are documented in literature are “crazy-paving,” “halo-sign,” ground glass opacities and a synchronous presentation of solid and subsolid nodules [6,[13], [14], [15]].
However, our case is distinct and unusual in the sense that on CT chest the pulmonary metastases from malignant melanoma presented as a combination of findings such as bilateral diffuse consolidation and ground glass opacities with superimposed septal thickening, giving a “crazy paving” appearance. The air space opacities were predominantly seen involving the upper lobes with relative subpleural sparing. Multiple centrilobular micronodules were also present.
The “crazy paving” pattern can be described as ground glass opacities with superimposed septal thickening [16]. This “crazy paving” pattern used to be a characteristic finding of pulmonary alveolar proteinosis but is now considered as a nonspecific finding which can be seen be in many acute and chronic conditions [16]. In fact, upon literature review more than 40 diseases can show the “crazy paving” pattern [16]. In addition to pulmonary alveolar proteinosis, the most common conditions that can present this pattern include viral and bacterial pneumonia, acute respiratory distress syndrome (ARDS), acute interstitial pneumonia (AIP), pulmonary hemorrhage, drug induced pneumonia and has even been described in cases of COVID-19 pneumonia [16,17]. Moreover, some possible differentials for upper lobe predominant conditions are respiratory bronchiolitis, pneumocystis pneumonia, and hypersensitivity pneumonitis [18]. In addition, centrilobular micronodules can be seen in can be seen in infectious, inflammatory, vascular pathologies [19].
Since our patient presented with a combination of all of these unique patterns; various provisional differential diagnoses such as hypersensitivity pneumonitis, pulmonary hemorrhage, multifocal infection, pulmonary alveolar proteinosis and other interstitial lung diseases were considered initially. The patient was treated with steroids and antibiotics. Upon follow-up, the patient did not respond to treatment and further investigations showed a negative sputum culture and bronchoalveolar lavage. On repeat imaging, the patient also had significant worsening of the previously reported CT findings. Thus, VATS with wedge resection and tissue analysis was performed for a definitive diagnosis. The histopathological diagnosis was straightforward and showed pigmented cells consistent with malignant metastatic melanoma. FDG PET-CT was performed after the VATS procedure, which further confirmed our diagnosis as there was avid FDG uptake in the bilateral lungs and other sites such as the liver, posterior chest wall, peritoneum etc. This case highlights that pulmonary metastases from malignant melanoma can present as atypical patterns on CT chest and thus radiologists should be aware of this phenomenon in order to prevent any misdiagnosis.
Conclusion
This article describes a rare radiological presentation of pulmonary metastases from malignant melanoma. On CT chest, the presentation was a spectrum of multiple patterns like “crazy paving,” upper lobe predominance with subpleural sparing, and centrilobular micronodules. Therefore, radiologists should be aware of these unconventional presentations to avoid any misdiagnosis. In patients with a history of malignant melanoma, metastases should be considered as a differential diagnosis for any abnormal lung parenchymal patterns detected on imaging.
Patient consent
A written informed consent was obtained from our patient regarding the publication of their case. Our patient was fully informed about the details of their case report. They were made aware that their identity will be anonymized and that their case details may be published in an online medical journal, website or any other form of publication that is accessible to the general public.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Wang JY, Wang EB, Swetter SM. What is melanoma? JAMA. 2023;329(11):948. doi: 10.1001/jama.2022.24888. [DOI] [PubMed] [Google Scholar]
- 2.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV., Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pidhorecky I, Lee RJ, Proulx G, Kollmorgan DR, Jia C, Driscoll DL, et al. Risk factors for nodal recurrence after lymphadenectomy for melanoma. Ann Surg Oncol. 2001;8(2):109–115. doi: 10.1007/s10434-001-0109-2. [DOI] [PubMed] [Google Scholar]
- 4.Younes R, Abrao FC, Gross J. Pulmonary metastasectomy for malignant melanoma: prognostic factors for long-term survival. Melanoma Res. 2013;23(4):307–311. doi: 10.1097/CMR.0b013e3283632cbe. [DOI] [PubMed] [Google Scholar]
- 5.Detterbeck FC, Grodzki T, Gleeson F, Robert JH. Imaging requirements in the practice of pulmonary metastasectomy. J Thorac Oncol. 2010;5(6):134–139. doi: 10.1097/JTO.0b013e3181dcf64d. [DOI] [PubMed] [Google Scholar]
- 6.Borghesi A, Tironi A, Michelini S, Scrimieri A, Benetti D, Maroldi R. Two synchronous lung metastases from malignant melanoma: the same patient but different morphological patterns. Eur J Radiol Open. 2019;6:287–290. doi: 10.1016/j.ejro.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damsky WE, Rosenbaum LE, Bosenberg M. Decoding melanoma metastasis. Cancers (Basel) 2010;3(1):126–163. doi: 10.3390/cancers3010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20(1):1–17. doi: 10.1016/j.soc.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalpiaz G, Asioli S, Fanti S, Rea G, Marchiori E. Rapidly growing pulmonary ground-glass nodule caused by metastatic melanoma lacking uptake on 18F-FDG PET-CT. J Bras Pneumol. 2018;44(2):171–172. doi: 10.1590/s1806-37562017000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okita R, Yamashita M, Nakata M, Teramoto N, Bessho A, Mogami H. Multiple ground-glass opacity in metastasis of malignant melanoma diagnosed by lung biopsy. Ann Thorac Surg. 2005;79(1):1–2. doi: 10.1016/j.athoracsur.2004.03.096. [DOI] [PubMed] [Google Scholar]
- 11.Mizuuchi H, Suda K, Kitahara H, Shimamatsu S, Kohno M, Okamoto T, et al. Solitary pulmonary metastasis from malignant melanoma of the bulbar conjunctiva presenting as a pulmonary ground glass nodule: report of a case. Thorac Cancer. 2015;6(1):97–100. doi: 10.1111/1759-7714.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang MJ, Kim MA, Park CM, Lee CH, Goo JM, Lee HJ. Ground-glass nodules found in two patients with malignant melanomas: different growth rate and different histology. Clin Imaging. 2010;34(5):396–399. doi: 10.1016/j.clinimag.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Kalchiem-Dekel O, Maimon N, Shack AR, Smoliakov A, Shaco-Levy R, Fruchter O, et al. An unusual presentation of malignant melanoma metastatic to the lungs and bronchi. Bilateral ground-glass opacities with a “crazy paving” component. Am J Respir Crit Care Med. 2015;191(8):954–955. doi: 10.1164/rccm.201411-1954IM. [DOI] [PubMed] [Google Scholar]
- 14.Alves Júnior SF, Zanetti G, Marchiori E. CT halo sign in pulmonary metastases from a melanoma. QJM. 2019;112(2):133–134. doi: 10.1093/qjmed/hcy190. [DOI] [PubMed] [Google Scholar]
- 15.Dalpiaz G, Kawamukai K, Parisi AM, La Torre L, Forcella D, Leuzzi G. Ground-glass opacity of the lung in a patient with melanoma: “the radiological seed of doubt”. Rev Esp Med Nucl Imagen Mol. 2015;34(6):390–392. doi: 10.1016/j.remn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Zhao J, Yang Q, Xiong W, Zhen G, Xu Y, et al. Pulmonary melanoma and “crazy paving” patterns in chest images: a case report and literature review. BMC Cancer. 2016;16:592. doi: 10.1186/s12885-016-2630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gbadamosi WA, Hanai B, Kim P, Anthony T, Rivera Z. Radiological finding of crazy-paving pattern in COVID-19 pneumonia. Cureus. 2022;14(6):e26107. doi: 10.7759/cureus.26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi YR, Chang YJ, Kim SW, Choe KH, Lee KM, An JY. Crazy paving radiography finding in asymptomatic pulmonary alveolar proteinosis. Asian Cardiovasc Thorac Ann. 2015;23(5):588–590. doi: 10.1177/0218492314548232. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Dabiri B, Hammer MM. Micronodular lung disease on high-resolution CT: patterns and differential diagnosis. Clin Radiol. 2021;76(6):399–406. doi: 10.1016/j.crad.2020.12.025. [DOI] [PubMed] [Google Scholar]