Abstract
Maintenance therapies in multiple myeloma improve survival after induction treatment. This study characterizes the strategies for maintenance therapy being employed in currently enrolling clinical trials for patients with multiple myeloma and highlights how high-risk myeloma patients may be assigned to maintenance strategies incongruent with current US guidelines.
Keywords: Myeloma, Landscape, Lenalidomide, Maintenance
Despite dramatic advances in therapeutic options for multiple myeloma (MM), the disease is associated with considerable morbidity and mortality [1]. Current standard first-line therapy for fit and eligible patients with MM involves triplet or quadruplet induction, followed by autologous stem cell transplant (ASCT) and maintenance therapy [2].
Even in the setting of optimal therapy during the first line of treatment, disease relapse is expected [3]. Maintenance therapy post-ASCT delays disease progression and prolongs survival. The current standard for it is lenalidomide, an immunomodulatory drug FDA-approved in 2017 after several large, randomized phase 3 trials demonstrated significant improvement in progression free survival (PFS), and a large meta-analysis confirmed overall survival (OS) benefit [4–6] of its use in the maintenance setting. Although other agents such as daratumumab and ixazomib have been studied as maintenance strategies, no OS benefit has yet been seen [7, 8].
Patients with high-risk cytogenetic features historically have poorer outcomes than patients with standard risk MM. There is mounting interest in the use of combination therapy for maintenance, and doublet maintenance is currently recommended in national USA guidelines for patients with high-risk MM [9, 10]. The recent FORTE trial demonstrated greater PFS with the carfilzomib and lenalidomide combination versus lenalidomide alone, with an improvement in PFS noted in high-risk subset of patients [10]. Furthermore, the role of minimal residual disease (MRD) in maintenance therapy continues to evolve [11] and the use of MRD allows for a platform to evaluate discontinuation of continuous therapy for deep responders.
We sought to assess the current landscape of maintenance therapy in clinical trials for newly diagnosed MM. The objective of this study was to characterize ongoing clinical trials with regards to agent(s) being used, the proportion of randomized studies, and characterization of primary endpoints. We assessed the proportions of currently enrolling randomized studies that were evaluating: OS for a primary endpoint; PFS in direct comparison to lenalidomide, and. MRD in the decision to discontinue treatment. Finally, we assessed whether high-risk MM patients were being enrolled in these studies and the maintenance therapy utilized in these patients.
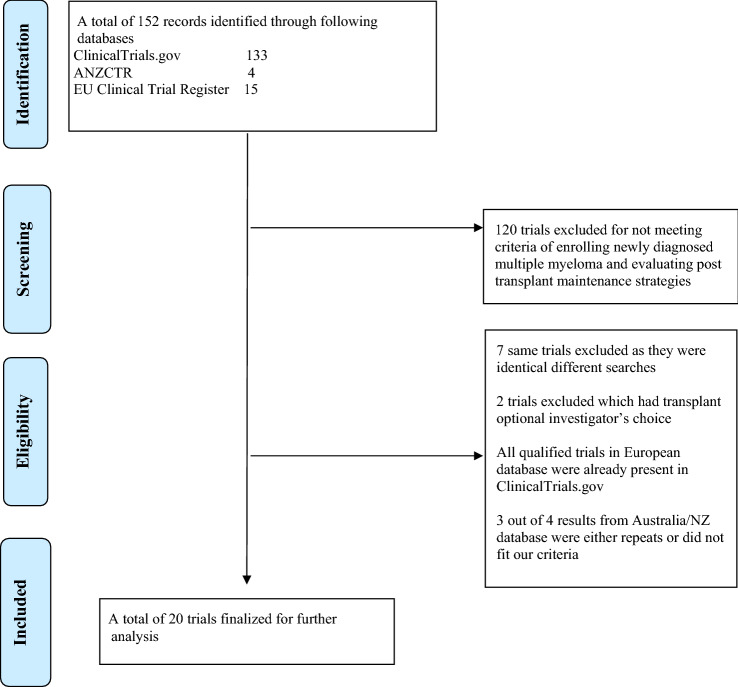
A comprehensive search was performed on the clinicaltrials.gov, clinicaltrialsregister.eu, and anzctr.org.au databases on March 2, 2022, using the keywords “newly diagnosed multiple myeloma”, “transplant-eligible multiple myeloma”, and “maintenance in multiple myeloma” as detailed in Fig. 1. Studies which were currently recruiting patients, or those which were active but not yet recruiting were included, but not those which had completed enrollment or were terminated. Studies were included if patients were planned to undergo ASCT after induction therapy, followed by maintenance strategy, and if at least one arm received maintenance therapy, with the latter specified in the trial description or interventions. Trials evaluating maintenance therapy after a salvage transplant or maintenance therapy in non-transplant settings, those without a defined maintenance therapy agent(s) and those investigating non-pharmacological interventions were excluded.
Fig. 1.
Flow diagram depicting our search strategy and study inclusion
A total of 20 studies were identified and analyzed (Table 1). Of these, 11 (55.0%%) were randomized and nine (45.0%) were non-randomized.
Table 1.
Study Characteristics
| Trial characteristics | N (%) (N = 20) |
|---|---|
| Randomized | 11 (55.0) |
| Non-randomized | 9 (45.0) |
| Phase II study | 12 (60.0) |
| Phase III study | 8 (40.0) |
| US based enrollment | 9 (45.0) |
| Non-US-based enrollment | 11 (55.0) |
| Studies including high-risk patients | 19 (95.0) |
| Primary end point(s) | |
| Response based | 3 (25.0) |
| PFS based | 6 (30.0) |
| OS based | 2 (10.0) |
| MRD status | 11 (55.0) |
| Incidence of death based | 1 (5.0) |
| Proportion able to complete 1 year therapy | 1 (5.0) |
| FACT-MM TOI score | 1 (5.0) |
PFS= progression free survival; OS= overall survival; MRD= Minimal residual disease; FACT-MM TOI= Functional assessment of cancer therapy - multiple myeloma trial outcome index
A time to event outcome (either PFS or OS) was included as a primary endpoint in 8 of the 20 studies (40.0%). Four of the 20 (20.0%) included multiple primary endpoints in addition to PFS or OS. Six studies (30.0%) evaluated PFS and two (10.0%) included OS as primary outcomes. Of the 11 randomized studies, time to event-based was a primary endpoint in 7 (63.6%), with 2 evaluating OS (18.1%) and 5 PFS (45.5%).
MRD negativity was included as a primary endpoint in 11 studies (55.0%) and a secondary endpoint in 7 (35%). Two trials assessed discontinuation of maintenance therapy for those achieving MRD negativity (NCT04071457, NCT05091372). The former trial utilized MRD negativity at 24 months, and the latter utilized sustained MRD criteria defined as two separate MRD-negative evaluations at least 1 year apart.
Of the 9 non-randomized studies, 4 had a primary endpoint of MRD negativity rate (44.4%), two (22.2%) had response-based primary outcomes, and one (11.1%) had a time to event primary outcome.
Table 2 describes maintenance regimens utilized in the included studies. Among the non-randomized studies (n = 9), a single-drug maintenance strategy was utilized in 5 (55.6%), and combination regimen was utilized in the other 4 (44.4%). Among the randomized trials (n = 11), the intervention arm comprised of combination therapy in 8 studies (72.7%), and single arm therapy in 3. In the randomized studies (n = 11), the control arm was lenalidomide in 8, ixazomib in 1, isatuximab in 1 and combination maintenance therapy in 1 (NCT05091372). A total of three randomized studies assessed comparison of lenalidomide and another agent to lenalidomide alone with a primary endpoint powered for PFS (ACTRN12620000291987, NCT05243797, NCT05317416), and 2 randomized studies comparing these had OS as the primary outcome (NCT 04071457, NCT 03941860).
Table 2.
Maintenance regimens utilized
| Nonrandomized studies | Total studies |
|---|---|
| Single agent maintenance | |
| Belantamab | 1 |
| Daratumumab | 1 |
| Iberdomide | 2 |
| Lenalidomide | 1 |
| Combination maintenance | |
| Belantamab + lenalidomide | 2 |
| Isatuximab + lenalidomide | 2 |
| Randomized Studies | Total studies | Control arm maintenance |
|---|---|---|
| Single agent maintenance: treatment arm | ||
| Elranatamab | 1 | Lenalidomide |
| Lenalidomide | 2 | Lenalidomide |
| Combination maintenance: treatment arm | ||
| Belantamab + lenalidomide | 1 | Belantamab + lenalidomide |
| Cellprotecta + isatuximab | 1 | Isatuximab |
| Daratumumab + lenalidomide | 2 | Lenalidomide |
| Daratumumab + ixazomib | 1 | Ixazomib |
| Ixazomib + lenalidomide | 1 | Lenalidomide |
| Selinexor + lenalidomide | 1 | Lenalidomide |
| Teclistamab + lenalidomide | 1 | Lenalidomide |
aCellprotect is a manufactured product consisting of invitro expanded and activated autologous NK cells
One study evaluating a regimen of ixazomib, lenalidomide, daratumumab and dexamethasone limited enrollment to standard risk disease (NCT03669445). No qualifying studies enrolled only “high-risk” disease. Among the 19 trials enrolling patients with high-risk disease, 8 (42.1%) permitted the use of single agent lenalidomide (Table 3).
Table 3.
Clinical trials included in current analysis
| Trial name | Trial identifier | Expected sample size | Randomized or non-randomized | Primary endpoint(s) |
|---|---|---|---|---|
| Belantamab mafodotin newly diagnosed transplant eligible multiple myeloma patients | NCT04802356 | 50 | Non-randomized | Incidence of death, adverse events, analytical alterations, and ocular events |
| Study of belantamab mafodotin as pre- and post-autologous stem cell transplant and maintenance for multiple myeloma | NCT04680468 | 47 | Non-randomized | MRD negativity rate |
| Iberdomide (Cc220) maintenance after asct in newly diagnosed MM patients | NCT04564703 | 160 | Non-randomized | Efficacy, rate of dose reductions/discontinuations |
| Study association of lenalidomide, ixazomib, dexamethasone and daratumumab in newly diagnosed standard risk multiple myeloma | NCT03669445 | 45 | Non-randomized | MRD negativity rate |
| Daratumumab after stem cell transplant in treating patients with multiple myeloma | NCT03346135 | 40 | Non-randomized | PFS |
| Post autologous transplant maintenance with isatuximab and lenalidomide in minimal residual disease positive multiple myeloma (HEME-18) | NCT05344833 | 50 | Non-randomized | MRD negativity rate |
| Belantamab mafodotin and lenalidomide for the treatment of multiple myeloma in patients with minimal residual disease positive after stem cell transplant | NCT04876248 | 20 | Non-randomized | MRD negativity rate |
| A study of isatuximab added to standard cybord induction and lenalidomide maintenance treatments in ND-TEMM | NCT04786028 | 65 | Non-randomized | Response rate (VGPR or better) |
| Iberdomide maintenance therapy in patients with multiple myeloma | NCT05177536 | 38 | Non-randomized | Proportion of subjects who complete 1 year of therapy |
| Clinical trial for autologus nk cells alone or in combination with isatuximab as maintenance for multiple myeloma | NCT04558931 | 60 | Randomized | Response rate (VGPR or better), change in MRD negativity rate |
| S1803, lenalidomide ± daratumumab/rHuPh20 as Post-ASCT maintenance for MM w/MRD to direct therapy duration (DRAMMATIC) | NCT04071457 | 1100 | Randomized | OS |
| A study of daratumumab plus lenalidomide versus lenalidomide alone as maintenance treatment in participants with newly diagnosed multiple myeloma who are minimal residual disease positive after frontline autologous stem cell transplant (AURIGA) | NCT03901963 | 214 | Randomized | MRD negativity rate |
| Daratumumab-bortezomib-dexamethasone (Dara-VCd) vs bortezomib-thalidomide-dexamethasone (VTd), then maintenance with ixazomib (IXA) or IXA-Dara | NCT03896737 | 400 | Randomized | PFS, MRD negativity rate |
| An ALLG phase 3 randomized trial of selinexor and lenalidomide—versus lenalidomide maintenance post autologous stem cell transplant for patients with newly diagnosed multiple myeloma | ACTRN12620000291987 | 232 | Randomized | PFS |
| A study of teclistamab in combination with lenalidomide versus lenalidomide alone in participants with newly diagnosed multiple myeloma as maintenance therapy following autologous stem cell transplantation (MajesTEC-4) | NCT05243797 | 1000 | Randomized | PFS |
| Minimal residual disease guided maintenance therapy with belantamab mafodotin and lenalidomide after autologous hematopoietic cell transplantation in patients with newly diagnosed multiple myeloma | NCT05091372 | 94 | Randomized | MRD positive to MRD negative conversion rate |
| A study of daratumumab, bortezomib, lenalidomide and dexamethasone (dvrd) followed by ciltacabtagene autoleucel versus daratumumab, bortezomib, lenalidomide and dexamethasone (DVRd) followed by autologous stem cell transplant (ASCT) in participants with newly diagnosed multiple myeloma (CARTITUDE-6) | NCT05257083 | 750 | Randomized | PFS, sustained MRD negative CR |
| Isa-KRd vs KRd in newly diagnosed multiple myeloma patients eligible for autologous stem cell transplantation (IsKia TRIAL) (IsKia) | NCT04483739 | 300 | Randomized | MRD negativity rate |
| Testing the addition of ixazomib to lenalidomide in patients with evidence of residual multiple myeloma, OPTIMUM Trial | NCT03941860 | 510 | Randomized | OS, Change in FACT/GOG-Ntx TOI score, Change in FACT-MM TOI score |
| Study with elranatamab versus lenalidomide in patients with newly diagnosed multiple myeloma after transplant (MagnetisMM-7) | NCT05317416 | 366 | Randomized | MRD negativity rate, PFS |
In this review of currently enrolling MM maintenance trials, we demonstrate an encouraging variety of regimens being utilized, MRD-guided discontinuation approaches, as well as MRD-stratified enrollment being assessed. We note that OS is a primary endpoint in only a small minority of trials, and that many compare two drugs to lenalidomide alone, with an endpoint of PFS. Previous data from the FORTE trial have already shown that a two-drug combination (lenalidomide + carfilzomib) offers superior PFS to lenalidomide alone, raising questions as to whether there is true equipoise for the endpoint being studied in these randomized trials [10]. Furthermore, with the increasing number of active drugs available for patients with newly diagnosed MM, the question of optimal resource utilization arises. It may not be the best option to study all these drugs in a two versus one comparison in the maintenance setting against lenalidomide alone. A PFS endpoint is inherently biased towards combination therapy, which is currently being done for teclistamab (NCT05243797) and selinexor (ACTRN12620000291987).
It is taking increasingly longer to demonstrate an OS advantage in trials for patients with MM. Additionally, maintenance therapy is continuous, expensive, and may be toxic. Accordingly, the onus for maintenance therapy should be to change the natural course of the disease and not just delay disease progression [12]. In this setting, despite the logistical advantages, the use of PFS or MRD as an endpoint does not allow for ascertainment of whether a maintenance strategy is truly changing the course of the disease and helping patients live longer or better, or simply delaying time to biochemical progression. The use of PFS2, defined as time from initial randomization to disease progression on the "next-line" treatment or death from any cause, may serve as an acceptable alternative in this setting.
We note that quality of life (QOL) is seldom included in primary endpoint evaluations, although a study has suggested that a sizeable portion of the myeloma population prioritizes QOL metrics over PFS improvements [13, 14]. Implementation of QOL outcomes into these studies would allow for easier widespread adaptation of these therapies in a real-world population.
The current recommendation by USA guidelines for high-risk disease is doublet maintenance with lenalidomide and a proteasome inhibitors, although strong data beyond the FORTE trial for this recommendation are lacking [9]. Our study demonstrates that all currently enrolling randomized trials, except for one (NCT05091372), have control arms of single drug therapy, rendering high-risk patients potentially being subjected to treatment inferior to what is the prevailing standard of care. Future trials should address this, by designing specific risk-adapted strategies or specifically enrolling patients with high-risk disease. If delaying progression or deepening response is the goal of therapy, then the best potential standard of care should be offered to patients in the control arm, which may include doublet therapy.
Future trials should emphasize distinct maintenance strategies adapted for risk as well as depth of response. For example, a standard risk patient who has achieved a deep response (measurable residual disease negativity) following transplant, may be over-treated with intensive maintenance strategies incorporating two or three agents given for years. Indeed, for such patients, whether maintenance of any sort is necessary is a question worth revisiting. Conversely, a high-risk patient with residual disease may not be suited well on a lenalidomide control arm in these studies, and may be best included in clinical trials assessing novel combination maintenance approaches. As such, current “one size fits all” maintenance trials may indeed simultaneously over-treat and under-treat some patients.
Our current study has numerous limitations. While several databases and search terms were included in our search, some studies may have been missed. As we evaluated currently ongoing trials, we do not have access to the results of these.
This current study analyzing the landscape of current maintenance trials demonstrates that there are many promising designs that adapt treatment based on the depth of responses and incorporate newer agents. However, there remains room for improvement, most notably to identify the ideal maintenance regimens for patients with high-risk disease and inclusion of patient-centered endpoints.
Acknowledgements
Syed Maaz Tariq: Data acquisition, wrote the paper. Al-Ola Abdallah: Edited the paper. Aaron Goodman: Edited the paper. Douglas Sborov: Edited the paper. Ghulam Rehman Mohyuddin: Designed the study, data acquisition, wrote the paper. Alec Britt: Wrote the paper, data acquisition. All the authors approved the final version of the manuscript.
Funding
No funding was acquired for this work.
Data availability
All data from which this study was generated is publicly available on clinical trials.gov.
Declarations
Conflict of Interest
The authors have no competing interests other than the following for DS: DS reports consulting for Janssen, SkylineDx, GlaxoSmithKline, Legend Biotech, Amgen and Celgene, AG reports consulting for EUSA Pharma and Seattle Genetics.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fonseca R, et al. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia. 2017;31(9):1915–1921. doi: 10.1038/leu.2016.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Hamed R, et al. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9(4):44. doi: 10.1038/s41408-019-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotta M, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113(14):3383–3391. doi: 10.1182/blood-2008-07-170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy PL, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279–3289. doi: 10.1200/JCO.2017.72.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attal M, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 6.Jackson GH, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57–73. doi: 10.1016/S1470-2045(18)30687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreau P, Sonneveld P, F.T.C.S. Investigators Daratumumab (DARA) maintenance or observation (OBS) after treatment with bortezomib, thalidomide and dexamethasone (VTd) with or without DARA and autologous stem cell transplant (ASCT) in patients (pts) with newly diagnosed multiple myeloma (NDMM): CASSIOPEIA Part 2. Blood. 2021;39(15_Suppl):8004–8004. [Google Scholar]
- 8.Kumar SK, et al. Ixazomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma: long-term follow-up including ixazomib maintenance. Leukemia. 2019;33(7):1736–1746. doi: 10.1038/s41375-019-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinic, M. MSMART Guidelines. 2022. https://www.msmart.org/mm-treatment-guidelines. Accessed 25 Jul 2022
- 10.Gay F, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: Results from the FORTE trial. J Clin Oncol. 2019;37(15):8002–8002. doi: 10.1200/JCO.2019.37.15_suppl.8002. [DOI] [Google Scholar]
- 11.Gambella M, et al. Minimal residual disease by flow cytometry and allelic-specific oligonucleotide real-time quantitative polymerase chain reaction in patients with myeloma receiving lenalidomide maintenance: a pooled analysis. Cancer. 2019;125(5):750–760. doi: 10.1002/cncr.31854. [DOI] [PubMed] [Google Scholar]
- 12.Cliff ERS, Rehman-Mohyuddin G. Overall survival as a primary end point in multiple myeloma trials. Nat Rev Clin Oncol. 2022 doi: 10.1038/s41571-022-00665-7. [DOI] [PubMed] [Google Scholar]
- 13.Mohyuddin GR, et al. Use of endpoints in multiple myeloma randomized controlled trials over the last 15 years: a systematic review. Am J Hematol. 2021;96(6):690–697. doi: 10.1002/ajh.26166. [DOI] [PubMed] [Google Scholar]
- 14.Fleischer A, et al. Is PFS the right endpoint to assess outcome of maintenance studies in multiple myeloma? Results of a patient survey highlight quality-of-life as an equally important outcome measure. Blood. 2021;138(Supplement 1):836–836. doi: 10.1182/blood-2021-152648. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data from which this study was generated is publicly available on clinical trials.gov.