Abstract
Type 2 diabetes is one of the fastest-growing health emergencies of the twenty-first century, in part due to its association with cardiovascular and renal disease. Successful implementation of evidence-based guidelines for the management of patients with diabetes and pre-diabetes has been shown to improve patient outcomes by controlling risk factors for cardiovascular and renal disease. Recommendations include the early introduction of lifestyle adjustments, supported by pharmacological tools. Despite the availability of regularly updated, evidence-based guidelines, guideline implementation in clinical practice is low. As a result, people living with type 2 diabetes are not consistently receiving ideal clinical care. Improving guideline adherence has the potential to improve quality of life and longevity in patients with type 2 diabetes. This article introduces Guardians For Health, a global initiative that aims to improve guideline adherence by simplifying patient management and encouraging patient participation in the implementation of guidelines for type 2 diabetes. Guardians For Health is supported by a global community of implementers, with tools to support decision-making and quality assurance. Through achieving better guideline adherence, Guardians For Health hopes to achieve its vision to “stop early mortality by reducing cardiovascular and kidney complications in people with type 2 diabetes”.
Keywords: Type 2 diabetes, Guardians For Health, Guideline implementation, Cardiorenal risk reduction, Mortality, Quality of life
Key Summary Points
| Type 2 diabetes (T2D) increases the risk of cardiovascular and renal disease and of premature death. |
| Adherence to evidence-based guidelines can reduce cardiorenal complications in T2D. |
| Guardians For Health (GFH) aims to facilitate guideline implementation in T2D. |
| GFH is based on shared decision-making between physicians and patients. |
| Through better guideline adherence, GFH hopes to stop premature mortality in T2D. |
Introduction
Type 2 diabetes (T2D) is a major and increasing threat to global health, mainly due to the high risk of microvascular and macrovascular complications [1]. Between 2000 and 2019, diabetes rose from 12th to 9th place among the leading causes of death worldwide, and from 14th to 8th place among the leading reasons for loss of disability-adjusted life-years [2]. Diabetes is also a major risk factor for the two highest global causes of mortality: ischaemic heart disease and stroke [2, 3]. Even impaired glucose tolerance, with an estimated prevalence of 10.6% of adults (541 million) globally, increases the risk of cardiovascular disease [4]. It has been estimated that, on average, the presence of T2D in middle-aged adults (aged 40–60 years) shortens their expected lifespan by 6 years [5]. However, this increases to 15 years if diabetes is accompanied by one cardiovascular complication and to 13 years in the presence of early diabetic kidney disease [6, 7].
Currently, the International Diabetes Federation estimates the global prevalence of diabetes to be 10.5% (537 million) among adults [4]. This is projected to increase to 12.2% (783 million) by 2045, making diabetes “one of the fastest growing global health emergencies of the twenty-first century” [4]. Given the large threat that diabetes poses to global health, it is concerning that almost every second person living with diabetes goes undetected (45.0%) [4]. Diabetes also has a significant financial impact, with the total healthcare expenditure for adults estimated at US$966 billion in 2021 [4]. This is expected to reach US$1.1 trillion by 2045, with costs relating to cardiovascular complications dominating [4, 8].
Evidence-based guidelines for the management of patients with diabetes, pre-diabetes, and cardiovascular disease in North America and Europe are regularly updated [3, 9–13]. The 2019 European Society of Cardiology guidelines were endorsed by 32 cardiac societies, and the pocket version was translated into eight languages (L.R., personal communication) [3, 14]. Early interventions based on lifestyle adjustments and supported by pharmacological tools have been shown to control various risk factors for cardiovascular disease [15–18]. These interventions aim to achieve modifiable risk factor targets and should be individualised for each patient. In patients with coronary artery disease (CAD) and T2D, this approach has produced morbidity and mortality rates similar to those seen in individuals without T2D [15]. Despite this success, surveys on guideline adherence reveal that patient management of T2D is far from satisfactory, suggesting that the implementation of guidelines is low [19, 20]. In support of this, US-based data from ~ 277,000 patients (2016–2018) show that fewer than one in ten individuals with T2D and atherosclerotic cardiovascular disease receive all guideline-recommended therapies [21–23].
In a 2016–2017 survey in 27 European countries (EUROASPIRE V), a large proportion of patients with T2D and CAD had not reached the clinical targets needed to manage/mitigate their cardiovascular risk [19]. Specifically, only 55.0% achieved blood pressure < 140/90 mmHg, 37.0% low-density lipoprotein cholesterol (LDLC) < 1.8 mmol/l (69.6 mg/dl) and 55% haemoglobin A1c (HbA1c) < 7.0% (53 mmol/mol) [19]. Guideline adherence related to lifestyle adjustments, such as smoking cessation, physical activity and weight management, was also low, showing no improvement compared with the results of the previous EUROASPIRE IV survey, conducted in 2012–2013 [20]. Similar observations of insufficient risk factor management have been reported from many parts of the world [21, 22, 24].
Ferrannini et al. recently addressed the question: “is coronary artery disease inevitable in type 2 diabetes?” [25]. In their review, two important obstacles were underlined: inadequate screening and inadequate management. The authors highlighted the need for improved guideline adherence, increased attention to those at risk of cardiovascular complications and simplified screening tools. They concluded that there are “reasons to believe that if screening and guideline adherence are improved, cardiovascular complications of dysglycaemia would be considerably reduced and possibly not inevitable”.
Guideline adherence needs to be increased to improve quality of life and longevity in patients with T2D. This article introduces and describes Guardians For Health (GFH), a global initiative that aims to improve guideline adherence by simplifying patient management and by encouraging patient participation in the implementation process. This article contains references to previously conducted studies, and does not contain any new studies with human participants or animals performed by any of the authors.
What Needs to be Done?
Goals and Vision of Guardians For Health
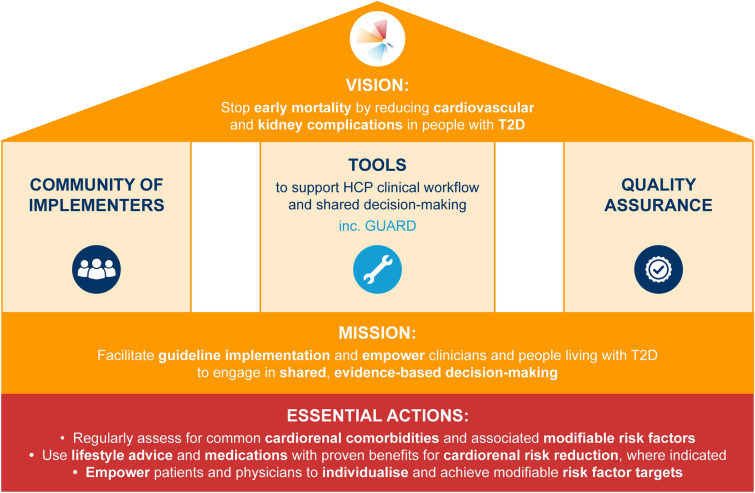
The United Nations has set out multiple sustainable development goals to be achieved by 2030, one of which is to reduce premature mortality from non-communicable diseases by a third [26]. Noting that T2D is a non-communicable disease with a tremendous impact on health and mortality, GFH was created by Boehringer Ingelheim as a global programme with a vision to “stop early mortality by reducing cardiovascular and kidney complications in people with T2D”. An overview of the structure of the GFH programme is provided in Fig. 1.
Fig. 1.
Structure of the Guardians For Health programme. Three pillars underpin the GFH approach and are essential for achieving the vision of the programme. In support of the mission statement, three essential actions are defined. GFH Guardians For Health, GUARD Guideline Application in Real Life Digital, HCP healthcare professional, T2D type 2 diabetes
Mission and Essential Actions
The mission of GFH is to “facilitate guideline implementation and empower clinicians and people living with T2D to engage in shared, evidence-based decision-making” (Fig. 1). Additionally, GFH aims to empower people with T2D with the knowledge needed to take control of their disease. The mission statement of GFH is supported by the following three essential actions:
Regularly assess for common cardiorenal comorbidities and associated modifiable risk factors;
Use lifestyle advice and medications with proven benefits for cardiorenal risk reduction, where indicated;
Empower patients and physicians to individualise and achieve modifiable risk factor targets.
The first essential action requires practitioners to recognise that cardiorenal comorbidities are common in T2D and are associated with a high risk of mortality [27]. For example, cardiovascular disease is reported to be the leading cause of mortality in persons with diabetes [28–31]. Additionally, heart failure (HF) and chronic kidney disease have been identified as not only the most common first cardiovascular or renal disease manifestations (6 times more common than peripheral artery disease and 4 times more common than stroke or myocardial infarction) but also two of the most serious complications in T2D. Specifically, HF and chronic kidney disease are associated with a high risk of cardiovascular and all-cause mortality (hazard ratio = 2.0 [95% CI 1.8–2.3] and hazard ratio = 2.1 [95% CI 1.8–2.3], respectively) [27]. Although GFH recognises that other non-cardiorenal comorbidities also contribute significantly to mortality in T2D (e.g. cancer and liver disease) [28, 29], these conditions and their risk factors do not currently fall under the scope of the initiative.
Given the burden of cardiorenal comorbidities in T2D, appropriate testing for these comorbidities, along with other modifiable risk factors, is essential. Guideline-directed testing includes a full history, a physical examination and laboratory testing (HbA1c, creatinine with estimated glomerular filtration rate [eGFR] and urinary albumin-creatinine ratio [UACR]) [3]. The choice of therapy should factor in any comorbidities or risk factors that are detected, including the use of organ-protecting therapies where appropriate, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, glucagon-like peptide-1 receptor agonists, sodium-glucose co-transporter-2 inhibitors, statins and acetylsalicylic acid [3, 13, 32, 33]. Of note, these therapies are recommended in guidelines for their organ-protecting benefits, regardless of blood pressure, cholesterol or glucose levels. Beyond the use of these organ-protecting therapies, achievement of individualised multifactorial targets is also essential and recommended by guidelines worldwide [3, 32–34].
Three Pillars
GFH is supported by three pillars, which are essential for achieving the vision of the programme (Fig. 1). These include the community of implementers, tools to support decision-making and quality assurance.
Pillar 1: Community of Implementers
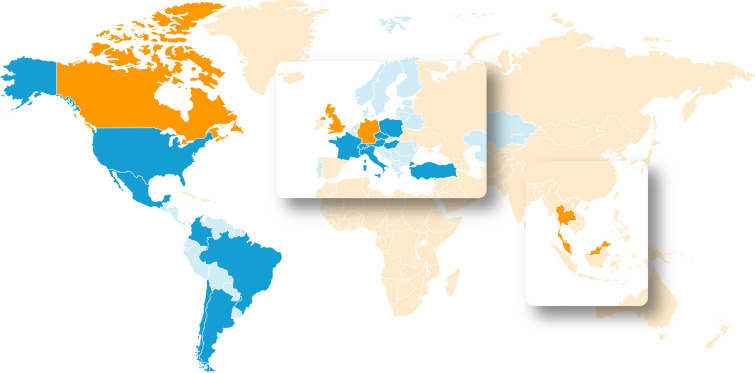
The process of achieving the GFH vision starts with the “community of implementers”—experts who are responsible for disseminating the message of GFH. Countries currently involved with the GFH programme are shown in Fig. 2. Although GFH is currently only operating in these countries, the success of the programme could lead to future engagement and uptake in other regions of the world.
Fig. 2.
Countries currently engaged with the Guardians For Health programme. Orange: GFH countries 2021; dark blue: GFH countries 2022; light blue: countries reporting to a regional office but not independently operating as a GFH country. GFH Guardians For Health
In participating countries, the GFH programme receives financial support and resources from Boehringer Ingelheim. Several local organisations (e.g. societies and patient organisations) also participate in the implementation of the programme in different ways. Examples of collaborating organisations include Kidney Disease Improving Global Outcomes (KDIGO) and International Diabetes Federation (IDF) Europe.
National steering committees are responsible for setting goals that align with the vision of GFH. Attainable goals are identified by learning from “bright spots”—examples of best practice at the local, national or international level. Once a plan of action is ready, the national steering committees drive change by implementing strategies on a national level. A key aspect of successful implementation is the mentorship of local healthcare provider (HCP) champions. GFH recruits HCPs through the action of local project managers and through partnerships with organisations (e.g. societies and patient organisations) that feature GFH activities.
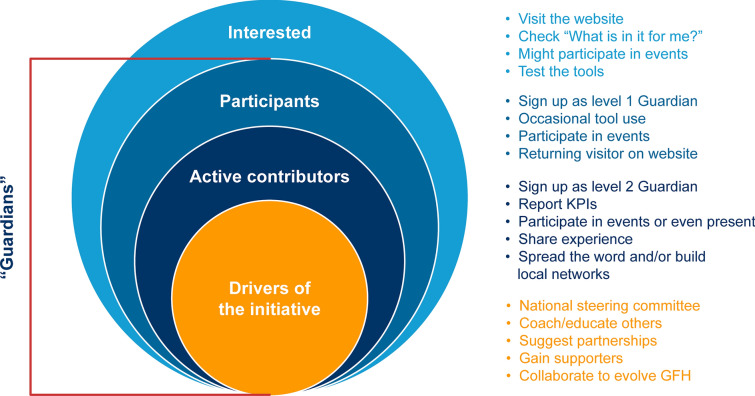
GFH allows local HCPs to choose their level of engagement with the programme (Fig. 3). Interested providers may visit the GFH website (guardiansforhealth.com) to learn more, participate in events and test the tools to support HCP clinical workflow. On the website, HCPs are given the option of signing up as “level 1” or “level 2” Guardians. Level 1 Guardians can access GFH tools to support decision-making and participate in events, and level 2 Guardians report key performance indicators to GFH and work towards spreading the word and/or building local networks. Examples of GFH events and activities that HCPs can engage with are provided in Table 1.
Fig. 3.
The Guardians For Health community will allow different levels of engagement with the programme. GFH Guardians For Health, KPI key performance indicator
Table 1.
Examples of Guardians For Health events, activities and HCP/patient support tools
| GFH events/activities | GFH tools |
|---|---|
| GFH/regional summits (e.g. GFH tool launching) | Newsletters |
| Congress symposia | Medical education videos |
| HCP workshops (e.g. Body Interact—interactive case simulation) | “Bright spots” podcasts and videos (examples of best practice at a local, national and international level) |
| National steering committee meetings | Regional GFH websites |
| Localisation of GFH materials | E-learning courses for healthcare assistants |
| Media campaigns (to drive awareness of GFH) | MSL slides and training |
| Collaboration with medical societies | Pathway development toolkit |
| Partnership with other CRM initiatives | Patient surveys |
| Regional round-table discussions with HCPs | Patient record books |
| GFH masterclasses | Performance trackers |
| Local Guardian network formation | HCP actions checklists |
| HCP Guidelines-at-a-Glance/pocket cards | |
| HCP digital decision support tools (e.g. GUARD pilot in Canada) |
The events, activities and support tools listed here have been piloted in selected countries and provide examples of opportunities for engagement with the programme
CRM cardiorenal-metabolic, GUARD Guideline Application in Real Life Digital, GFH Guardians For Health, HCP healthcare provider, MSL medical science liaison
The level of HCP engagement and key performance indicators are then carefully assessed by the national steering committee, and strategies are fine-tuned to further improve the adoption of guideline-directed practice for patients with T2D.
Together, the national steering committee and level 1 and 2 Guardians form a community of implementers who help drive change to achieve the vision of GFH.
Pillar 2: Tools to Support Decision-Making
The complexities of diabetes management in the modern era are well recognised by practising clinicians. Previously, glucose-lowering therapies for patients with T2D were limited to mainly insulin, sulphonylureas and metformin. However, over the past 3 decades, up to ten additional glucose-lowering medication categories have been introduced, making decision-making (e.g. which drugs to use, when and in which combination) difficult at times [13, 35]. Providers must simultaneously consider glucose-lowering ability, costs, side-effect profiles, contraindications and cardiorenal risk factors, as well as any non-glycaemic benefits of specific agents [13, 36]. Indeed, over the past 5 years, non-glycaemic benefits have become a dominant part of the discussion around the optimisation of T2D care.
Complex treatment decisions may be simplified through the use of decision support tools that summarise patient clinical characteristics, treatment preferences and ancillary data at the point of care. With the wide adoption of electronic health records, implementation of these tools has become easier, and their success has been demonstrated in diabetes care over the past decade [37, 38].
The main focus of GFH is on routine visits for diabetes care. The portfolio of tools will help both clinicians and patients to make evidence-based decisions and to overcome therapeutic inertia, identified as a major impediment to successful, proactive diabetes care [39]. To facilitate GFH’s mission of ensuring guideline implementation and empowering clinicians and people living with T2D to engage in shared, evidence-based decision-making, a toolkit has been developed to support clinical workflow. This includes action checklists, pocket cards to provide “Guidelines-at-a-Glance”, pragmatic tips delivered through brief educational videos and digital decision-support tools (Table 1). GFH tools are also available for patients themselves, since their perspectives and involvement are key for successful management of their chronic disease [13, 39]. These tools do not constitute a generalised patient support programme (e.g. medication handling guidance) but instead provide guidance and raise awareness about the importance of maintaining heart and kidney health and the benefits of guideline-directed therapies. Specifically, these tools will help people with T2D to take ownership of their care, be informed about treatment targets, and understand the importance of the various treatments and their anticipated results. In turn, this will facilitate successful interactions with their clinicians.
For example, the Guideline Application in Real Life Digital (GUARD) decision-support tool is being piloted in Canada. GUARD provides quick treatment advice for HCPs, based on Diabetes Canada guidelines [9]. It integrates digitised medical knowledge into clinical practice through an easy-to-use web-based application. GUARD stresses the individualisation of treatment targets, based on overall medical status and a cardiorenal risk assessment. It includes recommendations regarding management of glycaemia, lipids and blood pressure, and use of antiplatelet therapies. This tool supports HCP behavioural change and helps to overcome therapeutic inertia by targeting physical opportunities (relating to time, resources, location, etc.), social opportunities (opportunity afforded by interpersonal influences) and reflective motivation (involving plans and evaluations) [40].
Other resources are also being developed to inform the GFH community and to support guideline implementation. These include an interactive website (guardiansforhealth.com); educational videos; guideline synopses and comparisons, including print or digital “pocket cards” providing summaries in an accessible format; healthcare checklists/care pathways to assess a patient’s progress according to the GFH essential actions; and a patient record book (Table 1). The latter includes information about appointments with multidisciplinary healthcare teams, longitudinal cardiorenal risk factor status, individualised risk factor targets, and treatment and action plans agreed with their physician to reduce cardiorenal risk.
The cost of medicines is also an important variable to consider when making treatment decisions. In some countries (e.g. Thailand and Malaysia), GFH has carried out advocacy work with policymakers to raise awareness of the benefits of medicines for cardiorenal protection. This work intends to change the mindsets of policymakers by explaining the potential cost-saving benefits and medical rationale of such treatments.
Through these and other practical initiatives, GFH aims to simplify, standardise and systematise the delivery of high-quality and guideline-based diabetes care, with the goal of reducing morbidity and mortality from T2D.
Pillar 3: Performance Improvement/Quality Assurance
Variation in the quality of care has long been a problem when managing the health of an individual or a population. Care must be equitable, of a high standard and based on evidence. Management of diabetes or any other long-term condition requires a measure of performance improvement [41].
Quality assurance can be provided in many ways (e.g. via a simple audit of current practice) [41–45]. The audit cycle needs to be SMART (i.e, Specific, Measurable, Agreed/Achievable, Realistic and with a Target date) [41]. This will enable the setting of standards, the measurement of performance against peers, and evaluation of how to improve both patient experience and outcomes.
Gathering data and feedback is also essential for improvement and quality assurance [41]. There is an abundance of data, but using data in an appropriate manner to facilitate improvement is vital. For instance, looking at the outcomes of a target population (e.g. a minority ethnic group) in relation to the general population will allow the development of protocols and pathways to achieve the best outcomes in that population [46].
Performance improvement is dependent on a variety of factors, not least of which is the skill and knowledge of the HCP. Being up to date with the latest guidance/evidence and using such guidance in a manner that promotes best practice is imperative [39, 40]. Developing pathways and protocols to suit local population needs could ensure equity of care and reduce variation [13, 41].
Inertia has long been a detrimental factor in the management of long-term conditions; this can be both HCP- and patient-related [39, 40]. To achieve the best outcomes for patients, HCPs need to be aware of the latest developments/guidelines and be able to implement them effectively. Although HCPs have limited time available to spend with patients, these interactions are important and full advantage must be taken of them. Equally, patients need to be given as much information as possible, taking into consideration their health and digital literacy, to allow them to understand and take control of their condition. A well-motivated patient is likely to be engaged in shared decision-making, have improved adherence, and therefore see improvements in their own health and outcomes [39, 40].
Quality assurance is the bedrock of good practice, enabling the identification of gaps in both knowledge and the service that is provided. Quality assurance also sets the standard by which we measure ourselves and the requirements needed to achieve the best outcomes for patients.
Quality Monitoring
Quality assurance is achieved via the implementation of processes that define, ensure, maintain and improve quality, and is carried out by entities that are separate from those responsible for delivering the care [47, 48]. The only way to ensure best practice and outcomes whenever new guidelines or pathways are implemented is by way of quality assurance. This must be the bedrock of any healthcare improvement programme, and must show the positives and negatives of any intervention. Without measuring quality—whether in the form of patient-related outcomes, patient satisfaction or appropriate and timely use of therapies—it is not possible to justify a new programme to either patients or payers.
Quality assurance needs to be standardised, but also localised [47, 48] (i.e. there should be the same level of quality assurance across the board, and that assurance should be localised to the care provider and area). This is to ensure that the programme meets the criteria for cost effectiveness, safety and outcome measures. Monitoring should occur at regular intervals to ensure that the programme, pathway or guidance is being implemented and followed. Assurance can also be linked closely to key performance indicators, which can provide the necessary checks and balances to ensure sustainability and show proof of concept where needed. Quality assurance coupled with key performance indicators can provide the necessary information to indicate whether or not a programme is transferrable and reproducible in other areas. This can help to reduce variation in care and improve outcomes across the board.
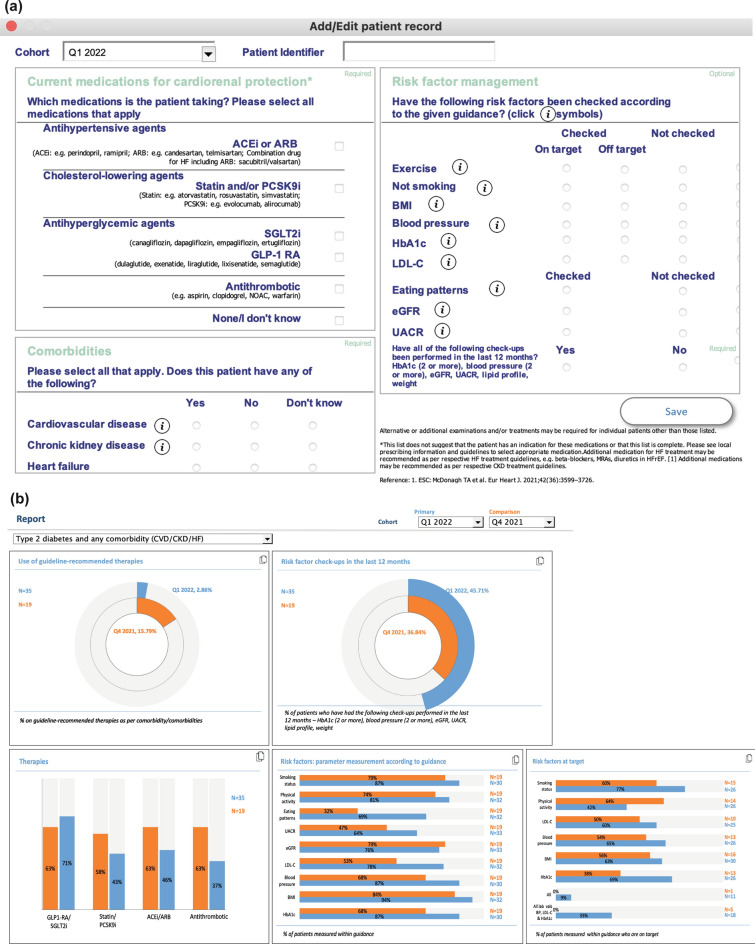
The GFH programme facilitates quality monitoring by encouraging participating HCPs to utilise an XLS-based dashboard performance tracking tool. HCPs can create and update patient records via the dashboard; these capture information relating to a patient’s cardiorenal comorbidities, the management of risk factors and the prescription of medications for cardiorenal protection (Fig. 4a). To monitor the quality of cardiorenal risk management, HCPs can perform an audit to assess whether patients with T2D who are at cardiorenal risk are receiving guideline-recommended therapies, have had their risk factors/laboratory parameters checked at guideline-recommended intervals and have met guideline-recommended targets for these parameters (e.g. exercise, body mass index and blood pressure). A key performance indicator for GFH is the proportion of individuals with T2D and atherosclerotic cardiovascular disease who receive treatments with three or four of the guideline-recommended medication classes. These medication classes include angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers, glucagon-like peptide-1 receptor agonists or sodium-glucose co-transporter-2 inhibitors, statins or proprotein convertase subtilisin/kexin type 9 and an anti-platelet such as acetylsalicylic acid. During the audit, it is recommended that several relevant medical records are randomly selected (e.g. n = 20) for analysis using the electronic medical record system. Reports can then be generated which display aggregated cardiorenal risk management data across multiple charts. These data can be compared between quarterly cohorts and used to track progress over time (Fig. 4b). Based on audit and feedback, HCPs can determine a course of action for quality improvement, setting an ambitious performance target supported by tools to drive behaviour change (e.g. reminders and checklists). Overall, GFH quality monitoring facilitates progress towards high-quality cardiorenal risk management.
Fig. 4.
Guardians For Health dashboard tracker. a Blank patient record. Patient data relating to prescribed classes of medication, cardiorenal comorbidities, risk factors and laboratory parameters are inputted here. Information ‘(i)’ buttons can be selected to provide definitions and to direct users to guideline recommendations about risk factor/parameter targets and assessment intervals. b Example reporting screen displaying aggregated cardiorenal risk management data. Information about the use of guideline-recommended therapies and the monitoring and management of risk factors is displayed. Drop-down menus allow cohorts to be filtered by the presence of different comorbidities and to compare between different quarters to assess progress in the provision of guideline-based care. ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, BMI body mass index, BP blood pressure, CKD chronic kidney disease, CVD cardiovascular disease, eGFR estimated glomerular filtration rate, GLP-1 RA glucagon-like peptide-1 receptor agonist, HbA1c haemoglobin A1c, HF heart failure, HFrEF heart failure with reduced ejection fraction, LDL-C low-density lipoprotein cholesterol, MRA mineralocorticoid receptor antagonist, NOAC new oral anticoagulant, PCSK9i proprotein convertase subtilisin/kexin type 9 inhibitor, SGLT2i sodium-glucose co-transporter-2 inhibitor, UACR urinary albumin-creatinine ratio
In France, a pilot study has recently been registered to evaluate the extent to which GFH-based tools improve the management of cardiorenal risk in patients with T2D compared with patients receiving their usual diabetes care. Specifically, this study will evaluate DiabetoWise, a platform for HCPs to help optimise the management of patients with T2D, in accordance with the Société Francophone de Diabetologie (SFD) guidelines [49]. This tool allows healthcare teams to create individual patient profiles and develop tailored treatment recommendations, which are accessed by HCPs through a specialised app. The tool provides remote monitoring, support for medical decision-making and contact via remote expertise with a referring diabetologist where needed [50]. Success will be measured by assessing the extent to which patients are managed according to SFD guideline recommendations, including the proportion of patients treated with therapies for cardiorenal protection and the proportion of patients meeting targets for specific laboratory parameters (e.g. HbA1c, LDLC and blood pressure).
Medical Education
It is imperative that HCPs responsible for patients with diabetes—especially primary care physicians and general practitioners—are cognizant of the essential features of this complex disease that affect many aspects of physical and mental health.
HCPs need to be aware of (and acquire) the skills needed to predict risk, screen, diagnose, follow-up and treat diabetes. The ultimate goal of diabetes management is to prevent complications and ensure a good quality of life for patients [13]. HCPs need to be able to differentiate between the various types of the disease. They must also be aware of the different characteristics and heterogeneity of the more common types of diabetes (type 1 diabetes, T2D and gestational diabetes) to better customise treatment options for patients [51].
Knowledge of risk factors for the development of the disease, such as family history, history of gestational diabetes, polycystic ovary syndrome and being overweight/obese, will help to improve screening [52–54]. Cooperation with local community stakeholders (local municipalities, local patient associations, etc.) can help to identify possible candidates for screening. Screening and the delivery of prevention programmes in community-based settings aligned to social support needs (e.g. food bank sites) offers a promising approach that may both reduce barriers to care and streamline the provision of care.
HCPs and patients must also be educated on the importance of, and the techniques for, maintaining glycaemic control. Specifically, self-monitoring of blood glucose at home using finger-stick tests or continuous glucose monitoring techniques may be conducted as appropriate and where available [13]. This is particularly important for those with more complex treatment regimens, including those associated with an increased risk of hypoglycaemia and those requiring periodic HbA1c measurements (at least every 3–6 months) [55]. Screening for risk factors (smoking, physical inactivity, unhealthy eating, disturbed sleeping patterns, hypertension, dyslipidaemia or obesity) and applying preventive/corrective measures are essential to ensure patient well-being and to monitor for the development of microvascular and macrovascular complications of diabetes [13, 16, 18, 53].
Cardiovascular disease (including CAD, HF, stroke, peripheral vascular disease) is the most important contributor to mortality in diabetes and has a significant impact on quality of life [34, 56, 57]. Renal disease is also recognised as a significant cause of mortality and reduced quality of life [56–58]. These risks must be addressed with periodic screening (either annually, or more frequently if needed), eGFR and UACR measurement and monitoring of ankle-brachial index [59, 60]. Where needed, specific treatments should be given for cardiorenal risk reduction, with the goal of reaching evidence-based HbA1c, blood pressure and lipid targets [10, 13]. Foot care and retinopathy screening and treatment are also important [55, 61].
HCPs need to be able to communicate effectively with their patients. To achieve this, there must be an understanding of the high heterogeneity of the patient population regarding health literacy and attitudes toward disease goals and treatment expectations [13]. Additionally, HCPs must be aware that specific patient populations (i.e. elderly people with frailty and comorbidities such as heart and kidney failure), as well as people treated with insulin, will need a tailored approach to care [13].
HCPs must also acquire and practice the art of empowering and motivating patients towards a healthier lifestyle [13, 39]. Devoting time to the patient and demonstrating empathy is essential to be able to build a productive patient/physician relationship that will provide benefit in the long term, such as regarding medication adherence [39, 40]. In short, engaging patients with shared decision-making is important to improve patient outcomes [13, 40].
Given the complexity of diabetes care, the provision of high-quality medical education with tools that are delivered in multiple modalities is vital. GFH provides this globally (Fig. 2) through e-learning courses and other developed materials (Table 1). Through this programme, the proper implementation of diabetes guidelines will lead to improved healthcare and outcomes for patients.
Behaviour Change
As the treatment landscape of chronic diseases such as T2D expands, the uptake of new therapies often requires change on the part of the patient and the treating clinician. Changing behaviour, however, is not as simple as informing the patient or provider of the changes that they should implement based on new evidence; in fact, education alone is a poor intervention for sustained behavioural change [62]. Ironically, understanding the reason as to why change is hard provides the context needed to promote change [63]. Furthermore, helping HCPs to understand how the patient-provider relationship can help people with T2D to maintain the motivation for self-management is an important aspect of the GFH programme.
It is generally accepted that behavioural habits form easily and, once formed, can be difficult to change [64]. This may be due to the limited attentional capacity of humans. Shifting from conscious, intentional behaviour to automatic behaviour increases the ability to function. Change disrupts this automatic behaviour and requires attentional focus. Furthermore, environmental factors can pose a barrier to change [63]. For clinicians, the need for time, training and confidence can represent obstacles to incorporating behavioural change into practice [63]. For patients, pleasure, convenience and immediate gratification can lead to unhealthy behaviours and can make change difficult [65].
Despite these difficulties, change can be achieved. Recent research suggests that a useful framework for behavioural change is to link behaviour to capability, opportunity and motivation [63, 66, 67]. Furthermore, in situations where any of these factors are problematic, change can be supported by establishing a change-based relationship [63]. The core of this relationship is the adoption of an attitude of non-judgmental curiosity towards behaviour. This promotes a collaborative and empowering relationship by strengthening a bond alliance (a respectful, empathic relationship towards change), a goal alliance (a shared outcome with personal value to the individual changing) and a task alliance (an agreement on what is required of whom to achieve change).
It is important to clearly define the current behaviour when supporting change using a change-based relationship [63]. Behavioural science has supported the conclusion that specificity assists with both appropriate goal setting and the problem-solving needed to develop confidence in a new behaviour. Once the behaviour has been identified, the relational aspect allows for identification of the individual’s readiness to change. One way to conceptualise readiness is by using a traffic light metaphor: red, in the circumstance where the person is not ready to change a specific behaviour; yellow, where the person is ambivalent; and green, where the person is ready (Fig. 5). This conceptualisation can be developed in a collaborative manner and has the advantage of allowing the approach to be tailored to each individual. In the case of situations with a green light, where motivation is present, goals can be successfully implemented that take into consideration capability and opportunity and utilise effective strategies (e.g. goal setting/action plans, behaviour shaping, stimulus control and reinforcement management). In situations of ambivalence (yellow light), behavioural intervention can be improved with an understanding of the benefits and risks of changing or not changing, as well as an exploration of personal, meaningful (i.e. values-based) reasons to change. Finally, in cases where the individual is not ready (red light), motivational communication can be used to maintain the relationship and explore underlying reasons for being unprepared to change. In turn, this can evoke ambivalence, and, using the concepts of bond, task and goal alliance, support the individual in moving toward change.
Fig. 5.
Readiness assessment as a framework for behavioural modification
Conclusion
T2D is a major threat to global health. Not only is diabetes one of the leading causes of death worldwide, but it is also a major risk factor for cardiovascular and renal disease. To improve patient outcomes, evidence-based guidelines recommend that risk factors for cardiovascular and renal disease be controlled through the early introduction of lifestyle adjustments, supported by pharmacological tools. Despite this, the implementation of guidelines in clinical practice is low, and patients with T2D are infrequently offered ideal guideline-directed clinical care. GFH is a global initiative with a vision to “stop early mortality by reducing cardiovascular and kidney complications in people with T2D”. To achieve this, the initiative aims to improve guideline adherence by simplifying patient management and empowering clinicians and patients living with T2D to engage in shared, evidence-based decision-making.
Acknowledgements
The authors thank Silvio Inzucchi of Yale School of Medicine, New Haven, CT, USA, for drafting section 2.3.2 Pillar 2: Tools to support decision-making.
Funding
This work was supported by Boehringer Ingelheim. Boehringer Ingelheim also paid the Rapid Service for this article.
Medical Writing and Editorial Assistance
Melissa Lawton, MSci, of Meditech Media provided writing support, which was contracted and funded by Boehringer Ingelheim.
Author Contributions
Naresh Kanumilli, Javed Butler, Konstantinos Makrilakis, Lars Ryden, Michael Vallis, Christoph Wanner, Shelley Zieroth and Alice Cheng are members of the Guardians For Health steering committee. All authors contributed equally to the writing of the manuscript, reviewed, and critically revised the manuscript and approved the final version for submission.
Disclosures
Naresh Kanumilli has received grants or contracts from AstraZeneca, Boehringer Ingelheim and Liva Health; has received consulting fees from AstraZeneca, Boehringer Ingelheim and Novo Nordisk; has received payment for lectures and presentations from Abbot, AstraZeneca, Boehringer Ingelheim, Lilly, Mylan, Novo Nordisk and Sanofi; has received support for conference attendance from AstraZeneca, Boehringer Ingelheim and Mylan; and has an unpaid leadership or fiduciary role in the Primary Care Diabetes society and the South Asian Health Foundation. Javed Butler has been a consultant to 3iveLabs, Abbott, American Regent, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardiac Dimensions, Cardior, CVRx, Edwards, Element Sciences, Faraday, FastBio, FIRE1, G3 Pharmaceuticals, Impulse Dynamics, Inventiva, Ionis, Janssen, Lexicon, LivaNova, Medtronics, Merck, Novo Nordisk, Otsuka, Pfizer, Roche, Sanofi, Sequana and Vifor. Konstantinos Makrilakis has received honoraria for congress speeches from AstraZeneca, Abbot Hellas, AEBE, Boehringer Ingelheim, Novo Nordisk Hellas, Pharmaserv-Lilly, Roche, Sanofi, Servier, Vianex/MSD and Winmedica; has received support for congress attendance from AstraZeneca, Boehringer Ingelheim, Novo Nordisk Hellas, Sanofi and Vianex/MSD; has participated on an advisory board affiliated with Abbot, AstraZeneca, Boehringer Ingelheim, Novo Nordisk Hellas and Sanofi; their institution has also received support for research activities from AstraZeneca, Abbot Hellas, AEBE, Boehringer Ingelheim, Novo Nordisk Hellas, Pharmaserv-Lilly, Roche, Sanofi, Servier, Vianex/MSD and Winmedica. Lars Rydén has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Bayer AG, Boehringer Ingelheim and Novo Nordisk. Michael Vallis has received investigator-driven research contracts from Abbot Diabetes Care, Bausch Health and Novo Nordisk; consulting fees from Abbot Diabetes Care, Bausch Health, Boehringer Ingelheim, Novo Nordisk, Roche Diabetes Care and Sanofi; has received payment for lectures, presentations or speakers bureaus from Abbot Diabetes Care, Bausch Health, Boehringer Ingelheim, Lifescan, Merck, Novo Nordisk, Roche Diabetes Care and Sanofi; has received support for congress attendance from Abbot Diabetes Care, Novo Nordisk and Roche Diabetes Care. Christoph Wanner has received honoraria for lectures from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim and Fresenius Medical Care; has received honoraria for their involvement with steering committees and for participation on an advisory board affiliated with AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GSK, MSD and Vifor. Shelley Zieroth has received research grant support, served on advisory boards for, or had speaker engagements with Abbott, Akcea, AstraZeneca, Amgen, Alnylam, Bayer, BMS, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Novo Nordisk, Otsuka, Pfizer, Roche, Servier and Vifor Pharma; serves on a clinical trial committee or as a national lead for studies sponsored by AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis and Pfizer. Ahmad Alhussein is a full-time employee of Boehringer Ingelheim. Alice Cheng has received consulting fees from Abbot, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Eisai, Eli Lilly, HLS Therapeutics, Insulet, Janssen, Novo Nordisk, Sanofi and Takeda; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Abbot, Amgen, AstraZeneca, Bausch, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, GSK, HLS Therapeutics, Insulet, Janssen, Medtronic, Merck, Novo Nordisk, Pfizer and Sanofi; has participated on a clinical trial advisory board affiliated with Applied Therapeutics and Sanofi; and is the Immediate Past Chair of Diabetes Canada.
Compliance with Ethics Guidelines
This article describes the theoretical framework underlying the Guardians For Health program. It contains references to previously conducted studies, and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Leading causes of death and disability - A visual summary of global and regional trends 2000–2019. 2019. Available from: https://www.who.int/data/stories/leading-causes-of-death-and-disability-2000-2019-a-visual-summary. Cited 30 Jun 2022.
- 3.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas, 10th Edition. 2021. Available from: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf. Cited 24 Mar 2022.
- 5.Rao-Kondapally-Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen CP, Chang CH, Tsai MK, Lee JH, Lu PJ, Tsai SP, et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017;92(2):388–396. doi: 10.1016/j.kint.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya V, Gangan N, Sheehan J. Impact of cardiovascular complications among patients with type 2 diabetes mellitus: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2015;15(3):487–497. doi: 10.1586/14737167.2015.1024661. [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Canada Clinical Practice Guidelines Expert Committee Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42:S1–S325. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice Committee 10 Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 11.Diabetes Canada Clinical Practice Guidelines Expert Committee. Tobe SW, Gilbert RE, Jones C, Leiter LA, Prebtani APH, et al. Treatment of hypertension. Can J Diabetes. 2018;42:S186–S189. doi: 10.1016/j.jcjd.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Diabetes Canada Clinical Practice Guidelines Expert Committee. Mancini GBJ, Hegele RA, Leiter LA. Dyslipidemia. Can J Diabetes. 2018;42:S178–S185. doi: 10.1016/j.jcjd.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2022;45(11):2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Society of Cardiology. 2019 guidelines on diabetes, pre-diabetes and cardiovascular diseases developed in collaboration with the EASD. 2019. Available from: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Diabetes-Pre-Diabetes-and-Cardiovascular-Diseases-developed-with-the-EASD. Cited 30 Jun 2022.
- 15.Anselmino M, Malmberg K, Ohrvik J, Ryden L, Euro Heart Survey Investigators Evidence-based medication and revascularization: powerful tools in the management of patients with diabetes and coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur J Cardiovasc Prev Rehabil. 2008;15(2):216–223. doi: 10.1097/HJR.0b013e3282f335d0. [DOI] [PubMed] [Google Scholar]
- 16.Oellgaard J, Gæde P, Rossing P, Rørth R, Køber L, Parving HH, et al. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia. 2018;61(8):1724–1733. doi: 10.1007/s00125-018-4642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 18.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 19.Ferrannini G, De Bacquer D, De Backer G, Kotseva K, Mellbin L, Wood D, et al. Screening for glucose perturbations and risk factor management in dysglycemic patients with coronary artery disease-a persistent challenge in need of substantial improvement: a report from ESC EORP EUROASPIRE V. Diabetes Care. 2020;43(4):726–733. doi: 10.2337/dc19-2165. [DOI] [PubMed] [Google Scholar]
- 20.Gyberg V, De Bacquer D, De Backer G, Jennings C, Kotseva K, Mellbin L, et al. Patients with coronary artery disease and diabetes need improved management: a report from the EUROASPIRE IV survey: a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc Diabetol. 2015;14:133. doi: 10.1186/s12933-015-0296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson AJ, Ardissino M, Haynes K, Shambhu S, Eapen ZJ, McGuire DK, et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc. 2021;10(2):e016835. doi: 10.1161/JAHA.120.016835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold SV, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Mues KE, et al. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618–620. doi: 10.1161/CIRCULATIONAHA.119.041730. [DOI] [PubMed] [Google Scholar]
- 23.Ardissino M, Nelson AJ, Haynes K, Eapen Z, Shambhu S, Carnicelli A, et al. Abstract 14740: Achieving guideline-directed therapy in US patients with diabetes and cardiovascular disease: alarming gaps, compelling opportunities. Circulation. 2019;140:A14740. [Google Scholar]
- 24.Gitt AK, Drexel H, Feely J, Ferrieres J, Gonzalez-Juanatey JR, Thomsen KK, et al. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur J Prev Cardiol. 2012;19(2):221–230. doi: 10.1177/1741826711400545. [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini G, Norhammar A, Gyberg V, Mellbin L, Ryden L. Is coronary artery disease inevitable in type 2 diabetes? From a glucocentric to a holistic view on patient management. Diabetes Care. 2020;43(9):2001–2009. doi: 10.2337/dci20-0002. [DOI] [PubMed] [Google Scholar]
- 26.United Nations Department of Economic and Social Affairs. Transforming our world: The 2030 agenda for sustainable development. 2016, Available from: https://sdgs.un.org/2030agenda. Cited 27 Jul 2022.
- 27.Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–1618. doi: 10.1111/dom.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenquist KJ, Fox CS, et al. Mortality trends in type 2 diabetes. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, et al., editors. Diabetes in America. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [PubMed] [Google Scholar]
- 29.Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8(4):e011295. doi: 10.1161/JAHA.118.011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(1):74. doi: 10.1186/s12933-022-01516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavallari I, Bhatt DL, Steg PG, Leiter LA, McGuire DK, Mosenzon O, et al. Causes and risk factors for death in diabetes: a competing-risk analysis from the SAVOR-TIMI 53 trial. J Am Coll Cardiol. 2021;77(14):1837–1840. doi: 10.1016/j.jacc.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 32.de Boer IH, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, et al. Executive summary of the 2020 KDIGO diabetes management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839–848. doi: 10.1016/j.kint.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63(2):221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 34.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 35.White JR., Jr A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82–86. doi: 10.2337/diaspect.27.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan DM, Lachin JM, Bebu I, Burch HB, Buse JB, Cherrington AL, et al. Glycemia reduction in type 2 diabetes—microvascular and cardiovascular outcomes. N Engl J Med. 2022;387(12):1075–1088. doi: 10.1056/NEJMoa2200436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson MJ, Nathan AG, Huang ES. Personalized decision support in type 2 diabetes mellitus: current evidence and future directions. Curr DiabRep. 2013;13(2):205–212. doi: 10.1007/s11892-012-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichardo-Lowden A, Umpierrez G, Lehman EB, Bolton MD, DeFlitch CJ, Chinchilli VM, et al. Clinical decision support to improve management of diabetes and dysglycemia in the hospital: a path to optimizing practice and outcomes. BMJ Open Diabetes Res Care. 2021;9(1):e001557. doi: 10.1136/bmjdrc-2020-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35(11):1735–1745. doi: 10.1007/s12325-018-0819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrzal PK, Mohseni AA, Fournier C, Goldenberg R, Hollahan D, Jin S, et al. Persons with diabetes and general/family practitioner perspectives related to therapeutic inertia in type 2 diabetes mellitus using qualitative focus groups and the theoretical domains framework: results from the MOTION study. Can J Diabetes. 2022;46(2):171–180. doi: 10.1016/j.jcjd.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 41.National Institute for Health and Care Excellence . Principles for best practice in clinical audit. London: National Institute for Health and Care Excellence; 2002. [Google Scholar]
- 42.Ackermann EW, Mitchell GK. An audit of structured diabetes care in a rural general practice. Med J Aust. 2006;185(2):69–72. doi: 10.5694/j.1326-5377.2006.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 43.Abou El-Enein NY, Abolfotouh MA. An audit of diabetes care at 3 centres in Alexandria. East Mediterr Health J = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2008;14(3):636–646. [PubMed] [Google Scholar]
- 44.Khattab MS, Swidan AM, Farghaly MN, Swidan HM, Ashtar MS, Darwish EA, et al. Quality improvement programme for diabetes care in family practice settings in Dubai. East Mediterr Health J = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2007;13(3):492–504. [PubMed] [Google Scholar]
- 45.Pruthu TK, Majella MG, Nair D, Ramaswamy G, Palanivel C, Subitha L, et al. Does audit improve diabetes care in a primary care setting? A management tool to address health system gaps. J Nat Sci Biol Med. 2015;6:S58–62. doi: 10.4103/0976-9668.166087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr DiabRep. 2013;13(6):814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Health Foundation . Quality improvement made simple. London: The Health Foundation; 2021. [Google Scholar]
- 48.Jabbal J, Lewis M. Approaches to better value in the NHS: improving quality and cost. London: The King's Fund; 2018.
- 49.Darmon P, Bauduceau B, Bordier L, Detournay B, Gourdy P, Guerci B, et al. Prise de position de la Société Francophone du Diabète (SFD) sur les stratégies d’utilisation des traitements anti-hyperglycémiants dans le diabète de type 2–2021. Médecine des Maladies Métaboliques. 2021;15(8):781–801. doi: 10.1016/j.mmm.2021.10.014. [DOI] [Google Scholar]
- 50.Boehringer Ingelheim. Boehringer Ingelheim partners with Sêmeia to launch a pilot phase of the DiabetoWise solution, a platform that aims to optimize the management of patients with T2D. 4 April 2023, Available from: https://www.boehringer-ingelheim.fr/communiqu%C3%A9s-de-presse/boehringer-ingelheim-est-partenaire-de-semeia-pour-lancer-une-phase-pilote-0.
- 51.World Health Organization . Classification of diabetes mellitus. Geneva: WHO; 2019. [Google Scholar]
- 52.Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes. 2015;6(2):234–244. doi: 10.4239/wjd.v6.i2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ismail L, Materwala H, Al KJ. Association of risk factors with type 2 diabetes: a systematic review. Comput Struct Biotechnol J. 2021;19:1759–1785. doi: 10.1016/j.csbj.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, et al. Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes. 2012;61(9):2369–2374. doi: 10.2337/db11-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. London: National Institute for Health and Care Excellence; 2022. [Google Scholar]
- 56.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H, WHO Multinational Study Group Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44:S14–21. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 57.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–2243. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 58.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(11):1465–1508. doi: 10.1016/j.jacc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 60.American Diabetes Association Professional Practice Committee Addendum. 11. Chronic kidney disease and risk management: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45(9):2182–2184. doi: 10.2337/dc22-ad08a. [DOI] [PubMed] [Google Scholar]
- 61.Internal Clinical Guidelines Team. National Institute for Health and Care Excellence: Clinical guidelines. Diabetic Foot Problems: Prevention and Management. London: National Institute for Health and Care Excellence (UK); 2015. [PubMed]
- 62.Arlinghaus KR, Johnston CA. Advocating for behavior change with education. Am J Lifestyle Med. 2018;12(2):113–116. doi: 10.1177/1559827617745479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vallis M, Lee-Baggley D, Sampalli T, Ryer A, Ryan-Carson S, Kumanan K, et al. Equipping providers with principles, knowledge and skills to successfully integrate behaviour change counselling into practice: a primary healthcare framework. Public Health. 2018;154:70–78. doi: 10.1016/j.puhe.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Gardner B. A review and analysis of the use of 'habit' in understanding, predicting and influencing health-related behaviour. Health Psychol Rev. 2015;9(3):277–295. doi: 10.1080/17437199.2013.876238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atkins L, Francis J, Islam R, O'Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12(1):77. doi: 10.1186/s13012-017-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whittal A, Störk S, Riegel B, Herber OR. Applying the COM-B behaviour model to overcome barriers to heart failure self-care: a practical application of a conceptual framework for the development of complex interventions (ACHIEVE study) Eur J Cardiovasc Nurs. 2021;20(3):261–267. doi: 10.1177/1474515120957292. [DOI] [PubMed] [Google Scholar]