Abstract
We investigated the plasma levels of pesticides components namely polychlorinated biphenyls (PCBs), dieldrin, dichlorodiphenyldichloroethylene (DDE), ethion, malathion, and chlorpyrifos in recurrent pregnancy loss (RPL) cases, and tested their associations with placental oxidative stress (OS) biomarkers [nitric oxide (NO.), thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), and superoxide dismutase (SOD)] and with placental apoptotic/antiapoptotic indices (Bcl-2 and caspase-3), and evaluated their possible cut-off points to distinguish RPL cases. The study recruited 101 pregnant women divided into; G1 [n = 49, control, normal 1st-trimester pregnancy, normal obstetric history with at least one previous normal live birth], G2 [n = 26, cases with missed abortion (< 3 abortions) before 24 weeks of gestation], and G3 [n = 26, cases with missed abortion (≥ 3 abortions) before 24 weeks of gestation]. The plasma pesticide levels were analyzed by gas chromatography-mass spectrometry. Plasma human chorionic gonadotrophin (HCG), placental OS, Bcl-2, and caspase-3, were analyzed by their corresponding methods and kits. Plasma PCBs, DDE, dieldrin, and ethion levels were significantly higher in RPL cases than in normal pregnancies (p ≤ 0.001). These levels correlated positively with placental OS and apoptosis and negatively with plasma HCG levels. Also, these levels were reliable markers of risk to RPL. Malathion and chlorpyrifos were not detected in any of the study’s participants. Pesticides may be risk factors in cases of spontaneous RPL cases. They are associated with an increasing placental OS and placental apoptosis. Specific measures should be taken to decrease maternal exposure to these pollutants’ sources, especially in underdeveloped and developing countries.
Subject terms: Biochemistry, Biomarkers, Medical research, Molecular medicine, Pathogenesis, Risk factors
Introduction
Recurrent pregnancy loss (RPL) is the loss of three or more successive pregnancies before 24 weeks of gestation. It affects 2–3% of couples trying to get pregnant globally. About 50–70% of these cases are of unknown etiology1. Indeed, pregnancy is a sensitive phase for women, during which they are susceptible to harmful environmental contaminants that can negatively impact fetal health2–6. Exposure to these contaminants is a risk factor in a considerable percentage of RPL cases7–9.
Many of these contaminants act to disrupt the endocrine system functions i.e., endocrine disruptors (EDs). They can modify various biologic processes including immunometabolism and reproduction10. Exposure to EDs during pregnancy has detrimental effects including, but not limited to, preeclampsia, fetal growth restriction (FGR), pregnancy loss (PL), and preterm birth2–6. Organo-chlorine pesticides (OCPs) and organophosphate pesticides (OPPs) are examples of EDs6,8,10. The OCPs include many chemicals such as polychlorinated biphenyls (PCBs), dieldrin, aldrin, and dichlorodiphenyltrichloroethane (DDT) and its derivative the dichlorodiphenyldichloroethylene (DDE). The OPPs include a diversity of chemicals such as ethion, malathion, and chlorpyrifos11.
The current work investigated the plasma levels of PCBs, DDE, dieldrin, ethion, malathion, and chlorpyrifos in RPL cases and tested their association with the placental oxidative stress (OS) biomarkers [nitric oxide (NO.), thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), and superoxide dismutase (SOD)] and with placental apoptotic/antiapoptotic indices (BcL-2 and caspase-3). Also, the study evaluated the possible cut-off points of the plasma levels of these chemicals that could distinguish RPL cases.
Subjects and methods
The current case–control study was carried out in the Women’s Health Hospital, Assiut University. The study recruited 101 pregnant women between November 2022 and March 2023. Complete personal, medical, and obstetrics histories were taken, and physical and obstetric assessments, routine investigations, and sonography were done for all participants. The eligible women were grouped into three groups (G1, G2, and G3).
Inclusion criteria: G1: a healthy control group with a normal 1st-trimester pregnancy, normal obstetric histories, and at least one prior normal live birth (n = 49), G2: cases diagnosed with missed abortion and gave a history of < 3 abortions before 24 weeks of gestation (n = 26), and G3 cases diagnosed with missed abortion and gave a history ≥ 3 abortions before 24 weeks of gestation (G3; n = 26)12.
Exclusion criteria: women were excluded once they showed any systemic diseases or other possible causes of RPL such as endocrine disorders, uterine problems, immune-inflammatory diseases, possible chromosomal aberrations (family history), and infections such as TORCH infections13.
Plasma pesticides and human chorionic gonadotropin (HCG) analysis
Ten ml of blood was collected from the antecubital vein in an EDTA-containing tube, centrifuged at 4000 rpm for 10 min and the plasma was kept at − 20 °C. At the time of analysis, samples were allowed to warm up to room temperature. The plasma HCG levels were measured by HCG ELISA Kit (MyBioSource, Inc. San Diego, CA 92195-3308, USA, cat# MBS704531.
For pesticides analysis, n-hexane (1:1, v/v) was added to the samples, mixed well, and centrifuged at 6600 rpm (for 5 min at 25 °C) then the n-hexane layer was poured into an autosampler vial (1 µL was injected into the GC) and analyzed by gas chromatography-mass spectrometry (GC–MS).
Chemicals
Different types of PCBs, DDE, dieldrin, ethion, malathion, and chlorpyrifos with high purity (≥ 96.0%), methanol, and hexane were Aldrich pure grade.
Solution preparation
Pure standard pesticide components (1000 µg/mL) were used to prepare the standard solutions. 1 mg of them was dissolved in 1 mL of absolute methanol. The standard solutions were frozen and kept away from light, constantly checked for signs of degradation or evaporation till being used. Secondary dilutions were prepared using absolute methanol.
Instrumentation
The GC–MS analytical system is equipped with temperature programming capability, splitless injector, capillary column, and Mass Quadrupole Spectrometry detector (GC/MS) (7890A/5975B), USA. A computer data system (MSD ChemStation E.0201.1177) was used for measuring the peak areas and heights from Agilent Technologies. The analytical column used was DB-1701P (30 m × 0.25 mm × 0.25 µm), Agilent Part No. 122-7732.
The oven temperature was set at 60 °C for 0.50 min, increased to 140 °C at 120 °C/min, 228 °C at 11 °C/min then to 234.22 °C for 1 min at 6.2 °C/min, 234.47 °C for 1 min at 0.25 °C/min, then increased to 260 °C for 5 min at 11 °C/min. The volume of the injected sample was 1 µL in splitless mode. The injector temperature was set at 250 °C. Helium (99.999%, purity) was used as carrier ramped flow, 0.5 mL/min for 10.9 min then 1 mL/min per min to 1 mL/min for 30 min.
The mass spectrometer was operated in electron impact (70 eV of ion energy), with 4.0 min solvent delay, SIM acquisition mode, mass quadrupole, and mass source kept at 150 °C and 230 °C.
Determinations of placental total protein and Bcl-2 and caspase-3
Ten gm placental specimens were washed three times with normal saline and twice with a phosphate buffer solution (PBS). After the aspiration of the PBS, a lysis solution was added (2 mL) then specimens were homogenized, and gently shaken for 1 h. The homogenates were then centrifuged for 10 min at 1500 rpm and the lysates were kept preserved in aliquots at – 80 °C still being used.
The total placental protein concentration was measured after the procedure of Lowry et al.14 using the Folin phenol reagent. Placental Bcl-2 and caspase-3 levels were measured by an Invitrogen™ Bcl-2 human sandwich ELISA Kit, and caspase-3 Human Instant ELISA Kit (Thermo Fisher Scientific Inc, A-1030 Vienna, Austria cat# BMS244-3, and BMS2012INST, respectively). The levels were expressed as ng/mg protein in placental tissue extract.
Determination of placental NO. levels
About 5 g placental samples were processed as previously discussed but here we used Krebs–Henseleit buffer solution for washing instead of the PBS and kept frozen at – 80 °C. To measure NO., 100 mg frozen placental tissue was homogenized with 2 mL of Krebs–Henseleit buffer. The NO. levels in the supernata were measured by a chemoluminescence technique15.
Determination of placental SOD activity and GSH levels
Twinty-grams specimens from the placentae were washed three times with normal saline. They were homogenized while ice-cold in Tris–HCl buffer (50 mM, pH 7.0)/1 mM EDTA to make a 10% (w/v) homogenate and centrifuged at 120,000 rpm for 30 min. One mM of phenyl-methane sulphonyl fluoride (PMSF) was added to the supenata and analyzed immediately for the SOD activity spectrophotometrically by the method described by Paoletti and Mocali16 where a unit of SOD activity referred to the amount of enzyme inhibiting the oxidation of NAD(P)H by 50% and measured as (U/mg protein in placental tissue extract).
The placental GSH concentrations were measured spectrophotometrically at 412 nm according to the method described by Beutler et al.17 by dithiobis-2-nitrobenzoate in 0.3 M phosphate solution. The concentration was expressed as µM/mg protein in placental tissue extract with the GSH M extinction coefficient 13,600.
Determination of placental TBARS at the syncytiotrophoblast brush border membrane (BBM)
At 4 °C, the maternal decidua was stripped off and 10 gm of the core portion (villous) of the placentas were collected, cut into minute fragments, and washed several times with Hepes/Tris buffer (10 mM, pH 7/mannitol (300 mM) to eliminate blood. the samples were homogenized, stirred vigorously, and filtered via cheesecloth. Then, PMSF was added and mixed with them to a final concentration of 0.2 mM before being centrifuged at 50.000 rpm for 30 min. The collected pellets were then treated with a 12 mM MgCl2 and centrifuged at 3000 rpm for 15 min to separate the BBM from non-BBM portions. The supernata were centrifuged again at 6000 rpm for 3 min and the pellets were then redissolved in 4 mL of the buffer and bypassed via a 26-gauge needle18. The pureness of BBM was evaluated by observing the enhancement of its marker alkaline phosphatase and the negative tests corresponding to other membranes namely Na+–K+ ATPase, succinate dehydrogenase, and cytochrome-C-reductase18,19.
To measure the lipid peroxidation, inhibition of any further reactions catalyzed by the presence of transition metals was achieved by treating the purified BBMs with a mixture of 5 mM EDTA/1 mM ascorbic/PMSF. The TBARS was measured according to the procedure of Cyanomon et al.20. Briefly, the samples were heated to 100 °C for 10 min with trichloroacetic acid (20%)/thiobarbituric acid reagents then cooled to room temperature, and the TBARS were measured spectrophotometrically at 532 nm.
Statistical analysis
SPSS version 26 was used to process and examine the data. The data distributions were reviewed using the Shapiro–Wilk test. The student’s t-test and one-way analysis of variance (ANOVA) compared continuous variables21. The chi-square test was used to compare categorical variables. We investigated the correlations between continuous variables by Pearson's correlation (r). The variable's ability to distinguish RPL cases was examined using the receiver operating characteristic curve (ROC)22. p-Values ≤ 0.05 were considered significant.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board at the Faculty of Medicine, Assiut University [IRB: 17300933]. Informed consent was obtained from all women involved in the study.
Results
A significantly high percentage of working mothers and those who gave a history of smoking (active/passive), and chronic exposure to pesticides, insecticides, and herbicides were found in the G3 compared to G2 and G1 (Table 1). The maternal ages and the residence showed non-significant differences among the studied groups (Table 1).
Table 1.
Comparison of maternal ages and some risk factors among the studied groups.
| G1 (n = 49) No previous abortions |
G2 (n = 26) Abortions less than 3 times |
G3 (n = 26) Abortions more than 3 times |
p-Value | ||
|---|---|---|---|---|---|
| Maternal age (years) | 26.1 ± 5.1 | 27.7 ± 6.7 | 29.5 ± 6.4 | 0.077* | |
| Residence | Rural | 33 (67.3%) | 19 (73%) | 17 (65.3%) | 0.820 |
| Urban | 16 (32.7%) | 7 (27%) | 9 (34.6%) | ||
| Working mother | Non-working | 43 (87.8%) | 19 (73%) | 16 (61.5%) | 0.03 |
| Working | 6 (12.2%) | 7 (27%) | 10 (38.5%) | ||
| Smoking | No | 41 (83.6%) | 11 (42.3%) | 4 (15.4%) | < 0.001 |
| Passive | 4 (8.2%) | 11 (42.3%) | 15 (57.7%) | ||
| Active | 4 (8.2%) | 4 (15.4%) | 7 (27%) | ||
| History of chronic exposure to pesticides, insecticides, and herbicides | No | 43 (87.8%) | 17 (65.4%) | 5 (19.2%) | < 0.001 |
| Yes | 6 (12.2%) | 9 (34.6%) | 21 (80.8%) | ||
*One-way analysis of variance (ANOVA) was used for comparison while Chi-Square (Χ2) was used for the comparison of other variables.
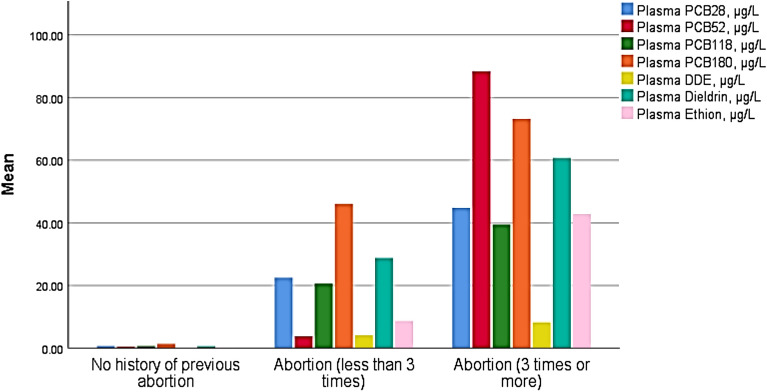
The current study detected PCB28, PCB52, PCB118, and PCB180 in 49.5%, 79.2%, 58.4%, and 60.4% of the study participants, respectively. The DDE and dieldrin were detected in 30.7% and ethion was detected in 45.5% of the study participants. The levels of all of them were significantly higher in G3 than in G2 and G1 and in G2 than in G1 (Table 2 and Fig. 1). The reverse was noticed regarding the HCG levels (Table 2). Malathion and chlorpyrifos were not detected in any of the study participants (data not shown in the table).
Table 2.
Comparison of plasma HCG, PCBs, DDE and dieldrin, and ethion levels in the three studied groups.
| G1 (n = 49) No previous abortion |
G2 (n = 26) Abortions less than 3 times |
G3 (n = 26) Abortions more than 3 times |
p-Value | |
|---|---|---|---|---|
| Plasma HCG (IU/L) | 88.6 ± 13.6 | 10.1 ± 2.7 | 8.9 ± 2.7 | < 0.001 |
| PCB28 (µg/L) | 0.7 ± 1.9 | 22.5 ± 34.4 | 44.8 ± 56.8 | < 0.001 |
| PCB52 (µg/L) | 0.4 ± 0.3 | 3.7 ± 1.6 | 88.4 ± 135.2 | < 0.001 |
| PCB118 (µg/L) | 0.6 ± 1.5 | 20.6 ± 28.4 | 39.4 ± 35.9 | < 0.001 |
| PCB180 (µg/L) | 1.4 ± 3.3 | 46.0 ± 75.3 | 73.2 ± 86.4 | < 0.001 |
| DDE (µg/L) | 0.02 ± 0.07 | 4.08 ± 10.2 | 8.2 ± 13.3 | 0.001 |
| Dieldrin (µg/L) | 0.6 ± 1.5 | 28.7 ± 75.7 | 60.7 ± 86.5 | < 0.001 |
| Ethion (µg/L) | 0.2 ± 0.5 | 8.7 ± 13.0 | 42.8 ± 139.8 | 0.048 |
One-way analysis of variance (ANOVA) was used for comparison.
PCBs, plasma polychlorinated biphenyls; DDE, dichlorodiphenyltrichloroethane.
Figure 1.
Clustered bar for the mean of plasma polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane (DDE), dieldrin, and ethion (µg/L) in the 3 studied groups.
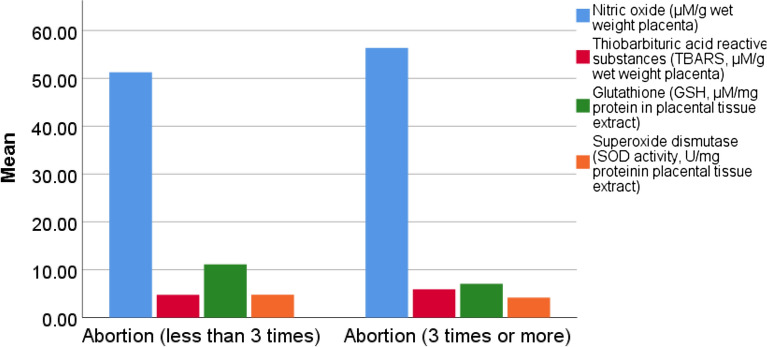
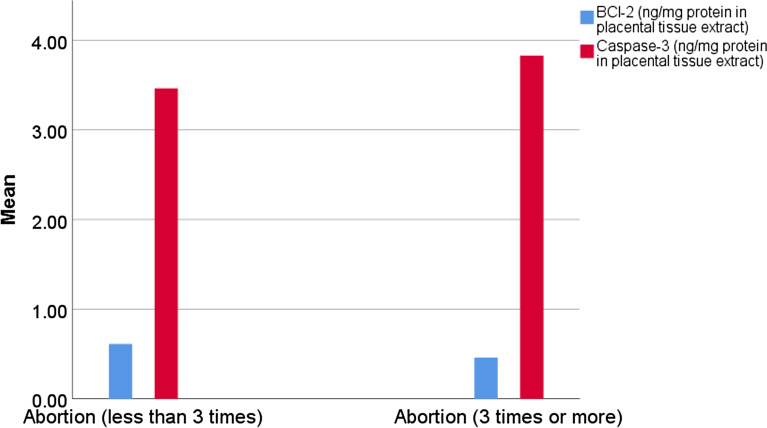
Placental GSH, SOD, and Bcl-2 levels were significantly lower in G3 than G2 and G1 and in G2 than G1 (Table 3, Figs. 2 and 3). The reverse was noticed regarding the placental TBARS and caspase-3 levels (Table 3, Figs. 2 and 3).
Table 3.
Placental oxidative stress biomarkers and apoptotic indices in G2 and G3.
| G2 (n = 26) Abortions less than 3 times |
G3 (n = 26) Abortions more than 3 times |
p-Value | |
|---|---|---|---|
| NO. (µM/g wet weight placental tissues) | 51.3 ± 18.9 | 56.3 ± 40.4 | 0.355 |
| TBARS (µM/g wet weight placental tissues) | 4.7 ± 0.7 | 5.8 ± 1.3 | < 0.001 |
| GSH (µM/mg protein in placental tissue extract) | 11.1 ± 2.8 | 7.1 ± 2.3 | < 0.001 |
| SOD (U/mg protein in placental tissue extract) | 4.8 ± 0.8 | 4.2 ± 0.8 | 0.007 |
| Bcl-2 (ng/mg protein in placental tissue extract) | 0.6 ± 0.2 | 0.4 ± 0.2 | 0.023 |
| Caspase-3 (ng/ protein in placental tissue extract) | 3.4 ± 0.5 | 3.8 ± 0.5 | 0.016 |
One-way analysis of variance (ANOVA) was used for comparison.
HCG, human chorionic gonadotropin; NO, placental nitric oxide; TBARS, thiobarbituric acid reactive substances; GSH, placental reduced glutathione; SOD, placental superoxide dismutase activity; Bcl-2, placental B-cell lymphoma 2; Caspase-3, placental cysteine-dependent aspartate specific protease-3.
Figure 2.
Clustered bar for the mean of placental nitric oxide (NO.), thiobarbituric acid reactive substances (TBARS), glutathione (GSH), superoxide dismutase activity (SOD) in the G2 and G3.
Figure 3.
Clustered bar for the mean of placental Bcl-2 and Caspase-3 levels in G2 and G3.
The plasma levels of PCBs, DDT, dieldrin, and ethion correlated positively with placental NO., TBARS, and caspase-3 levels while correlated negatively with plasma HCG and placental GSH, SOD, and Bcl-2 levels (Table 4).
Table 4.
Correlations of the plasma PCBs, DDE and dieldrin, and ethion levels with plasma HCG, the placental oxidative stress biomarkers, and placental Bcl-2 and caspase-3 concentrations in G2 and G3.
| Plasma HCG (IU/L) | NO (µM/g wet weight placental tissues) | TBARS (µM/g wet weight placental tissues) | GSH (µM/mg protein in placental tissue extract) | SOD (U/mg protein in placental tissue extract) | Bcl-2 (ng/ protein in placental tissue extract) | Caspase-3 (ng/ protein in placental tissue extract) | ||
|---|---|---|---|---|---|---|---|---|
| PCB28 (µg/L) | r | − 0.707 | 0.696 | 0.889 | − 0.775 | − 0.682 | − 0.636 | 0.743 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| PCB52 (µg/L) | r | − 0.519 | 0.494 | 0.743 | − 0.631 | − 0.481 | − 0.420 | 0.534 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.002 | < 0.001 | |
| PCB118 (µg/L) | r | − 0.821 | 0.847 | 0.942 | − 0.845 | − 0.820 | − 0.799 | 0.888 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| PCB180 (µg/L) | r | − 0.745 | 0.759 | 0.890 | − 0.765 | − 0.723 | − 0.699 | 0.802 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| DDE (µg/L) | r | − 0.616 | 0.602 | 0.881 | − 0.739 | − 0.576 | − 0.520 | 0.650 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Dieldrin (µg/L) | r | − 0.635 | 0.628 | 0.837 | − 0.706 | − 0.598 | − 0.548 | 0.666 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Ethion (µg/L) | r | − 0.341 | 0.309 | 0.513 | − 0.434 | − 0.318 | − 0.264 | 0.346 |
| P | 0.013 | 0.026 | < 0.001 | 0.001 | 0.022 | 0.059 | 0.012 | |
PCB, polychlorinated biphenyl; DDT, dichlorodiphenyltrichloroethane; HCG, human chorionic gonadotropin; NO., nitric oxide; TBARS, thiobarbituric acid reactive substances; GSH, glutathione; SOD, superoxide dismutase activity; BcI-2, lymphoma 2 protein; Caspase-3, cysteine-dependent aspartate specific protease; r, Pearson’s correlation coefficient; p, p-Value.
Not shown in the table, the placental Bcl-2 levels correlated positively with the placental levels of GSH and SOD levels (r = 0.830 and = 0.983, respectively, p < 0.001 for both) while negatively correlated with the placental levels of NO. and TBARS levels (r = − 0.977 and − 0.763, respectively, p < 0.001 for both). Opposite findings were noticed regarding the correlations of placental caspase-3 levels with placental GSH, SOD, NO., and TBARS levels (r = − 0.837, − 0.964, 0.971, and 0.831, respectively, and p < 0.001 for all).
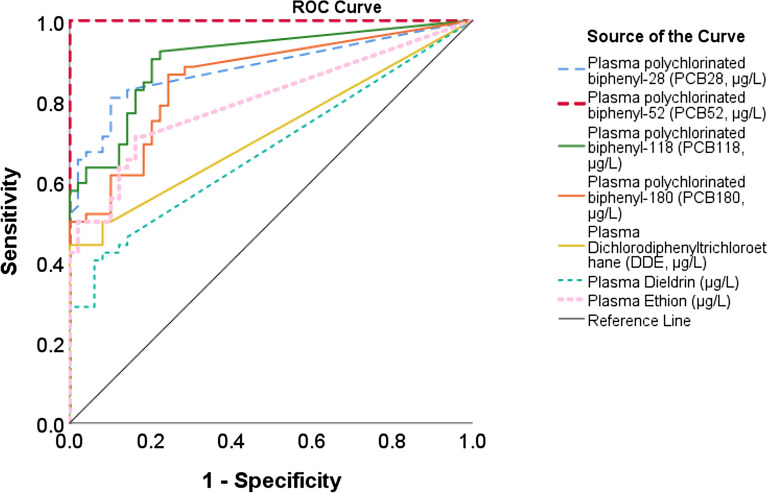
The cut-off points, AUC, sensitivity, and specificity for the ability of plasma PCBs, DDE, dieldrin, and ethion levels to detect cases of recurrent pregnancy loss are illustrated in Table 5 and Fig. 4.
Table 5.
Cut-off points, AUC, sensitivity, and specificity for the ability of plasma PCBs, DDE, dieldrin, and ethion, levels to detect RPL cases.
| Variable | Cut-off point | AUC | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| PCB28 (µg/L) | 14.2 | 0.88 | 48 | 100 |
| PCB52 (µg/L) | 4.3 | 1.00 | 69 | 100 |
| PCB118 (µg/L) | 4.5 | 0.90 | 60 | 96 |
| PCB180 (µg/L) | 5.9 | 0.86 | 52 | 90 |
| DDE (µg/L) | 6.1 | 0.72 | 29 | 100 |
| Dieldrin (µg/L) | 5.2 | 0.68 | 29 | 94 |
| Ethion (µg/L) | 3.6 | 0.80 | 42 | 100 |
AUC, area under the curve; PCB, plasma polychlorinated biphenyl; DDE, dichlorodiphenyltrichloroethane.
Figure 4.
Receiver operating characteristic (ROC) curve for the ability of plasma polychlorinated biphenyl (PCBs), dichlorodiphenyltrichloroethane (DDE), dieldrin, and ethion detect recurrent pregnancy loss (RPL) cases.
Discussion
Exposure to numerous banned pesticide components is inevitable due to their continued widespread use worldwide23–25. The sources of these components include industry (e.g., plasticizers), agriculture (e.g., pesticides and herbicides), and even house-related exposure (e.g., smoking, insecticides, and deteriorated paints)6,26. Exposure to these contaminants occurs through polluted food, water, or inhalation6. E-waste recycling is a major source of PCBs exposure27. Water samples from the River Nile in Egypt showed significantly higher than acceptable PCBs levels, especially PCB-138, indicating a continuous source of poisoning25.
The current study found higher levels of PCBs, DDE, dieldrin, and ethion in RPL cases than in normal pregnancies and these levels correlated with levels of placental OS and apoptosis and inversely with the plasma HCG levels. Also, the maternal plasma levels of these chemicals showed good to excellent abilities to distinguish RPL cases (reliable markers of risk to RPL).
These results were in accord with many previous studies3,8,26–28. These chemicals are EDs that might disrupt normal body homeostasis resulting in major health problems such as malignant transformation, abnormal reproduction, fetal maldevelopment, and diabetes mellitus26,28. Intrauterine exposure to these EDs especially during the early developmental phases has several negative impacts including the increasing rates of RPL2,3,8. Most of the OCPs’ bad effects come from the disruption of the signaling cascades for many hormones such as thyroid, HCG, and sex steroids6,7,10,24,29,30. Also, they impair the body’s antioxidant capacity and generate OS and thus are genotoxic31. Many of the pesticides’ components (e.g., PCBs) are xenoestrogens that disrupt the action of the endogenous estrogens and negatively impact human health. These impacts are more dangerous when exposure occurs early in intrauterine life29.
Being extremely lipophilic and non-degradable allows the EDs to accumulate and be concentrated in tissues (e.g., placenta), body fluids (e.g., blood and amniotic fluid), and the biological secretions (e.g., breast milk and semen)5,10,32. For the OCPs and OPPs the transport across the placenta involves both simple and active transport processes33,34.
Like other OCPs, PCBs have no placental barrier30. Chronic exposure to PCBs diminishes fertility and inversely impacts reproduction27. Their placental levels are associated with declines in syncytiotrophoblast volumes, placental disruption, and FGR6,10,24,35. Women with high PCBs levels showed abnormal menstruation, high rates of uterine fibroids, spontaneous PL, polycystic ovaries, and endometriosis7.
The DDE is a metabolite derived from the OCPs DDT that is utilized in agriculture36. The DDE has weak estrogen-like and androgen effects that may disturb their related signaling cascades impacting reproduction. Chronic exposure to low doses of DDT and/or DDE has been reported to cause spontaneous PL and their serum levels were associated with the occurrence of RPL9,37–39.
Exposure to dieldrin during pregnancy disrupts normal reproduction, negatively impacts fetal weight, and might result in skeletal anomalies40.
Ethion is an organophosphate pesticide (OPs) that acts not only through the inhibition of acetylcholinesterase enzyme but also through noncholinergic effects as well. The OPs are cytotoxic and can impair cellular homeostasis41. They induce OS mainly by increasing lipid peroxidation and decreasing antioxidants competence42. Exposure to OPs during pregnancy leads to their accumulation in the placenta disrupting fetal growth and development. Their levels in the maternal urine were associated with FGR, short gestational age, and RPL9,43,44.
The OS occurs as a result of a disturbance in the free radical/antioxidant balance and affects the cellular macromolecules and homeostasis45. The free radicals (FR) generation may be triggered by both endogenous and exogenous factors. The exogenous triggers include exposure to irradiation, pollution, smoking, heavy metals, and pesticides46. It is to be mentioned that all phases of normal pregnancy are associated with a controlled OS. It occurs due to the increase in the leukocytes number and the excess production of free radicals that are associated with the increase in antioxidants47.
The OS has been noticed in RPL cases but its exact underlying mechanism in the pathogenesis of these cases is still uncertain47–49. The accompanied increase in the free radicals production damage the placental tissues’ macromolecules (e.g., DNA, polypeptides, and unsaturated fatty acids) initiating these tissues’ death47. Bogavac et al. reported significantly higher plasma levels of SOD in cases with RPL than in the control healthy group. The reverse was noticed regarding the total antioxidants’ capacity. They concluded that these findings could be used as a predictor for RPL48 which went with our findings.
Apoptosis has a pivotal role in all stages of embryo and fetal development. The tight regulation of trophoblast growth/apoptosis balance is a crucial event, especially during the implantation process. When apoptosis exceeds the growth of trophoblasts bad consequences happen including FGR, preeclampsia, and preterm birth4,50. In compliance with our findings, disturbance of apoptotic/anti-apoptotic proteins balance has been recognized as a key feature in cases with RPL51. Exposure to environmental toxins might result in this disturbance and consequently RPL52. Pesticides can initiate apoptosis through the OS and their actions on the intrinsic pathway (mitochondrial or DNA injury) and/or the extrinsic pathway (through death receptors)34.
Conclusions
Pesticides may be risk factors in cases of spontaneous RPL cases. They are associated with an increasing placental OS and placental apoptosis. Specific measures should be taken to decrease maternal exposure to these pollutants’ sources, especially in underdeveloped and developing countries.
Abbreviations
- RPL
Recurrent pregnancy loss
- EDs
Endocrine disruptors
- PL
Pregnancy loss
- FGR
Fetal growth restriction
- OCPs
Organo-chlorine pesticides
- OPPs
Organophosphate pesticide
- PCBs
Polychlorinated biphenyls
- DDT
Dichlorodiphenyltrichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- OS
Oxidative stress
- NO
Placental nitric oxide
- TBARS
Thiobarbituric acid reactive substances
- GSH
Placental reduced glutathione
- SOD
Placental superoxide dismutase activity
- BcL-2
B-cell lymphoma-2
- Caspase-3
Cysteine–aspartate protease
- TORCH
Toxoplasmosis, rubella cytomegalovirus, herpes simplex, and HIV
- EDTA
Ethylenediamine tetraacetic acid
- HCG
Human chorionic gonadotropin
- GC–MS
Gas chromatography-mass spectrometry
- PBS
Phosphate buffer solution
- PMSF
Phenyl-methane sulphonyl fluoride
- BBM
Brush border membrane
- DNA
Deoxyribonucleic acid
Author contributions
Conceptualization and study design: M.A.H.E., A.F.A., and K.M.M., data curation: M.A.H.E., A.F.A., and K.M.M., investigation: M.A.H.E. and K.M.M., methodology: M.A.H.E., A.F.A., and K.M.M., software: K.M.M., validation: M.A.H.E., A.F.A., and K.M.M., writing—initial draft: M.A.H.E., and K.M.M., writing—review & editing: K.M.M. All authors reviewed and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Available by the corresponding author on sensible wish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-023-46570-6"
Change history
11/7/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-023-46570-6
References
- 1.Eggo RPL, Bender AR, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE guideline: Recurrent pregnancy loss: An update in 2022. Hum. Reprod. Open. 2023;2023(1):hoad002. doi: 10.1093/hropen/hoad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat. Rev. Dis. Primers. 2020;6(1):98. doi: 10.1038/s41572-020-00228-z. [DOI] [PubMed] [Google Scholar]
- 3.Sakali A-K, Papagianni M, Bargiota A, Rasic-Markovic A, Macut D, Mastorakos G. Environmental factors affecting pregnancy outcomes. Endocrine. 2023;2023:1–11. doi: 10.1007/s12020-023-03307-9. [DOI] [PubMed] [Google Scholar]
- 4.El-Baz MA, El-Deeb TS, El-Noweihi AM, Mohany KM, Shaaban OM, Abbas AM. Environmental factors and apoptotic indices in patients with intrauterine growth retardation: A nested case-control study. Environ. Toxicol. Pharmacol. 2015;39(2):589–596. doi: 10.1016/j.etap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Kolan AS, Hall JM. Association of preterm birth and exposure to endocrine disrupting chemicals. Int. J. Mol. Sci. 2023;24(3):1952. doi: 10.3390/ijms24031952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Prakash A, et al. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health. 2020;8:553850. doi: 10.3389/fpubh.2020.553850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neblett MF, Curtis SW, Gerkowicz SA, Spencer JB, Terrell ML, Jiang VS, et al. Examining reproductive health outcomes in females exposed to polychlorinated biphenyl and polybrominated biphenyl. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-60234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fucic A, Duca RC, Galea KS, Maric T, Garcia K, Bloom MS, et al. Reproductive health risks associated with occupational and environmental exposure to pesticides. Int. J. Environ. Res. Public Health. 2021;18(12):6576. doi: 10.3390/ijerph18126576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey A, Jaiswar SP, Ansari NG, Deo S, Sankhwar P, Pant S, et al. Pesticide risk and recurrent pregnancy loss in females of subhumid region of India. Niger. Med. J. J. Niger. Med. Assoc. 2020;61(2):55. doi: 10.4103/nmj.NMJ_117_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y, Guo F-J, Liu K, Ding R, Wang Y. The effect of endocrine-disrupting chemicals (EDCs) on placental development. Front. Endocrinol. 2023;14:343. doi: 10.3389/fendo.2023.1059854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syafrudin M, Kristanti RA, Yuniarto A, Hadibarata T, Rhee J, Al-Onazi WA, et al. Pesticides in drinking water—a review. Int. J. Environ. Res. Public Health. 2021;18(2):468. doi: 10.3390/ijerph18020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, et al. Recurrent pregnancy loss: Diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum. Reprod. Update. 2020;26(3):356–367. doi: 10.1093/humupd/dmz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu N, Zhou X, Shi W, Ye M, Cao X, Chen S, et al. Integrative analysis of circulating microRNAs and the placental transcriptome in recurrent pregnancy loss. Front. Physiol. 2022;2022:1557. doi: 10.3389/fphys.2022.893744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ch L. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Michelakis ED, Archer SL. The measurement of NO in biological systems using chemiluminescence. Nitric Oxide Protocols. 1998;1998:111–127. doi: 10.1385/1-59259-749-1:111. [DOI] [PubMed] [Google Scholar]
- 16.Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD (P) H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- 17.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 18.Qanungo S, Mukherjea M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol. Cell. Biochem. 2000;215:11–19. doi: 10.1023/a:1026511420505. [DOI] [PubMed] [Google Scholar]
- 19.Shirazi S, Beechey R, Butterworth P. The use of potent inhibitors of alkaline phosphatase to investigate the role of the enzyme in intestinal transport of inorganic phosphate. Biochem. J. 1981;194(3):803–809. doi: 10.1042/bj1940803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cynamon HA, Isenberg JN, Nguyen CH. Erythrocyte malondialdehyde release in vitro: A functional measure of vitamin E status. Clin. Chim. Acta. 1985;151(2):169–176. doi: 10.1016/0009-8981(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW. Methods for testing statistical differences between groups in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2022:2. [Google Scholar]
- 22.Carrington AM, Manuel DG, Fieguth PW, Ramsay T, Osmani V, Wernly B, et al. Deep ROC analysis and AUC as balanced average accuracy, for improved classifier selection, audit and explanation. IEEE Trans. Pattern Anal. Mach. Intell. 2022;45(1):329–341. doi: 10.1109/TPAMI.2022.3145392. [DOI] [PubMed] [Google Scholar]
- 23.Lallas PL. The Stockholm convention on persistent organic pollutants. Am. J. Int. Law. 2001;95(3):692–708. [Google Scholar]
- 24.Zhu M, Yuan Y, Yin H, Guo Z, Wei X, Qi X, et al. Environmental contamination and human exposure of polychlorinated biphenyls (PCBs) in China: A review. Sci. Total Environ. 2022;805:150270. doi: 10.1016/j.scitotenv.2021.150270. [DOI] [PubMed] [Google Scholar]
- 25.Megahed AM, Dahshan H, Abd-El-Kader MA, Abd-Elall AMM, Elbana MH, Nabawy E, et al. Polychlorinated biphenyls water pollution along the River Nile, Egypt. Sci. World J. 2015;2015:1–7. doi: 10.1155/2015/389213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz B, Terekeci H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020;21:127–147. doi: 10.1007/s11154-019-09521-z. [DOI] [PubMed] [Google Scholar]
- 27.Montano L, Pironti C, Pinto G, Ricciardi M, Buono A, Brogna C, et al. Polychlorinated biphenyls (PCBs) in the environment: Occupational and exposure events, effects on human health and fertility. Toxics. 2022;10(7):365. doi: 10.3390/toxics10070365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020;8(8):703–718. doi: 10.1016/S2213-8587(20)30129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chmielewski J, Luszczki J, Czarny-Dzialak M, Dutkiewicz E, Król H, Gworek B, et al. Environmental exposition to xenoestrogens and related health effects. J. Elementol. 2021;26:3. [Google Scholar]
- 30.Gingrich J, Ticiani E, Veiga-Lopez A. Placenta disrupted: Endocrine disrupting chemicals and pregnancy. Trends Endocrinol. Metab. 2020;31(7):508–524. doi: 10.1016/j.tem.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwivedi N, Mahdi AA, Deo S, Ahmad MK, Kumar D. Assessment of genotoxicity and oxidative stress in pregnant women contaminated to organochlorine pesticides and its correlation with pregnancy outcome. Environ. Res. 2022;204:112010. doi: 10.1016/j.envres.2021.112010. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Garg VK, Ramamurthy PC, Singh J, Pandey A. Impact And Prospects of Pesticides on Human and Environmental Health. Current Developments in Biotechnology and Bioengineering. Elsevier; 2023. pp. 1–32. [Google Scholar]
- 33.Yin S, Zhang J, Guo F, Poma G, Covaci A, Liu W. Transplacental transfer mechanism of organochlorine pesticides: An in vitro transcellular transport study. Environ. Int. 2020;135:105402. doi: 10.1016/j.envint.2019.105402. [DOI] [PubMed] [Google Scholar]
- 34.Samarghandian S, Farkhondeh T, Ashrafizadeh M, Talebi M, Aschner M, Darroudi M. The Effects of Organophosphate Pesticides on Mitochondria. Elsevier; 2023. pp. 587–600. [Google Scholar]
- 35.Laufer BI, Neier K, Valenzuela AE, Yasui DH, Schmidt RJ, Lein PJ, et al. Placenta and fetal brain share a neurodevelopmental disorder DNA methylation profile in a mouse model of prenatal PCB exposure. Cell Rep. 2022;38(9):110442. doi: 10.1016/j.celrep.2022.110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang G-R. Persistent organochlorine pesticides in aquatic environments and fishes in Taiwan and their risk assessment. Environ. Sci. Pollut. Res. 2018;25:7699–7708. doi: 10.1007/s11356-017-1110-z. [DOI] [PubMed] [Google Scholar]
- 37.Longnecker MP, Klebanoff MA, Dunson DB, Guo X, Chen Z, Zhou H, et al. Maternal serum level of the DDT metabolite DDE in relation to fetal loss in previous pregnancies. Environ. Res. 2005;97(2):127–133. doi: 10.1016/S0013-9351(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 38.Interdonato L, Siracusa R, Fusco R, Cuzzocrea S, Di Paola R. Endocrine disruptor compounds in environment: Focus on women’s reproductive health and endometriosis. Int. J. Mol. Sci. 2023;24(6):5682. doi: 10.3390/ijms24065682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venners SA, Korrick S, Xu X, Chen C, Guang W, Huang A, et al. Preconception serum DDT and pregnancy loss: A prospective study using a biomarker of pregnancy. Am. J. Epidemiol. 2005;162(8):709–716. doi: 10.1093/aje/kwi275. [DOI] [PubMed] [Google Scholar]
- 40.Sharma D, Kumari S, Rani P, Onteru SK, Roy P, Tyagi RK, et al. Organochlorine pesticide dieldrin upregulate proximal promoter (PII) driven CYP19A1 gene expression and increases estrogen production in granulosa cells. Reprod. Toxicol. 2021;106:103–108. doi: 10.1016/j.reprotox.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Voorhees JR, Rohlman DS, Lein PJ, Pieper AA. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Front. Neurosci. 2017;10:590. doi: 10.3389/fnins.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rambabu L, Megson IL, Eddleston M. Does oxidative stress contribute to toxicity in acute organophosphorus poisoning? A systematic review of the evidence. Clin. Toxicol. 2020;58(6):437–452. doi: 10.1080/15563650.2019.1693589. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson KK, van den Dries MA, Gaillard R, Pronk A, Spaan S, Tiemeier H, et al. Organophosphate pesticide exposure in pregnancy in association with ultrasound and delivery measures of fetal growth. Environ. Health Perspect. 2019;127(8):087005. doi: 10.1289/EHP4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suwannakul B, Sapbamrer R, Wiwattanadittakul N, Hongsibsong S. Organophosphate pesticide exposures in early and late pregnancy influence different aspects of infant developmental performance. Toxics. 2021;9(5):99. doi: 10.3390/toxics9050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel R, Rinker L, Peng J, Chilian WM. Reactive oxygen species: The good and the bad reactive oxygen species (ROS) in living cells. Toxics. 2018;7:8. [Google Scholar]
- 46.Cosa G. Singlet Oxygen: Applications in Biosciences and Nanosciences: Royal Society of Chemistry. InTech; 2016. [Google Scholar]
- 47.Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, Florek E. Association of oxidative stress on pregnancy. Oxid. Med. Cell. Longevity. 2020;2020:1–12. doi: 10.1155/2020/6398520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogavac M, Jakovljević A, Nikolić A, Milošević Tošić M, Perić T, Belopavlović Z. Biomarkers of oxidative stress in pregnant women with recurrent miscarriages. J. Lab. Med. 2019;43(2):101–114. [Google Scholar]
- 49.Zejnullahu VA, Zejnullahu VA, Kosumi E. The role of oxidative stress in patients with recurrent pregnancy loss: A review. Reprod. Health. 2021;18(1):1–12. doi: 10.1186/s12978-021-01257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod. Biol. Endocrinol. 2005;3(1):1–12. doi: 10.1186/1477-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michita RT, Zambra FMB, Fraga LR, Sanseverino MT, Schuler-Faccini L, Chies JAB, et al. The role of FAS, FAS-L, BAX, and BCL-2 gene polymorphisms in determining susceptibility to unexplained recurrent pregnancy loss. J. Assist. Reprod. Genet. 2019;36:995–1002. doi: 10.1007/s10815-019-01441-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye Y, Jiang S, Zhang C, Cheng Y, Zhong H, Du T, et al. Environmental pollutant benzo [a] pyrene induces recurrent pregnancy loss through promoting apoptosis and suppressing migration of extravillous trophoblast. BioMed Res. Int. 2020;2020:1–10. doi: 10.1155/2020/8983494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available by the corresponding author on sensible wish.