Highlights
-
•
Alpha-fetoprotein secreting tumors in gynecologic cancers have a broad differential.
-
•
Surgical pathology may provide a more comprehensive picture of tumor heterogeneity than initial biopsy.
-
•
Yolk Sac Tumor components intermixed with carcinoma points towards a somatic derivative rather than a collision tumor.
-
•
Alpha-fetoprotein can be trended over time to gauge response to treatment of cancers that produce the protein.
Keywords: Alpha-fetoprotein, Yolk sac tumor, Endometrial carcinoma, Hepatoid, Endometroid, Uterine cancer
1. Introduction
Alpha-fetoprotein (AFP) producing tumors in the biologic female include yolk sac tumors (YST) of the ovary and the rare variants hepatoid adenocarcinoma of the endometrium and YST of the endometrium. Treatment of these rare variants is complicated by a lack of consensus on treatment. In this case, we describe the treatment course of a high-grade carcinoma with hepatoid, endometrioid, serous features and YST component in a 50-year-old female with significantly elevated AFP.
2. Case presentation
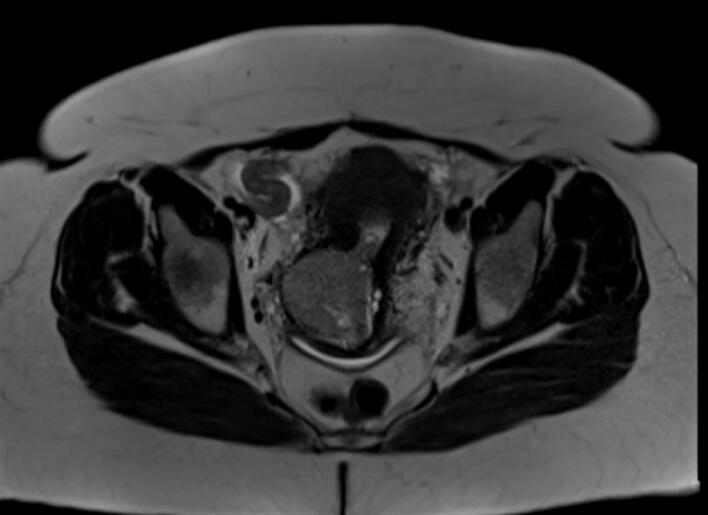
The patient was a 50-year-old G3P3 woman with a history of ductal carcinoma in situ treated by lumpectomy 5 years prior to presentation. She had never taken tamoxifen. At the time of presentation, the patient was still experiencing monthly menses. The patient had a routine pap smear that showed atypical glandular cells, at which time the patient was referred for transvaginal ultrasound. Transvaginal ultrasound revealed a 5.3 × 5.3 cm mass in the anterior lower uterine segment with increased vascularity, heterogeneous myometrium, and multiple leiomyomata. The patient was sent for cervical and endometrial biopsy and both specimens revealed poorly differentiated cells consistent with carcinoma. CT of the chest/abdomen/pelvis revealed 2–3 mm micronodules in the left upper and lower lobes of the lung as well as the heterogeneous uterine/cervical mass, however, additional imaging was recommended due to difficulty evaluating the mass in this modality. The patient was sent for MRI of the abdomen/pelvis which showed omental thickening and a 5.2 × 4.3 × 5.1 cm circumscribed mass expanding the cervical canal and umbilicated at the endometrium of the lower uterine segment (Fig. 1). No suspicious adnexal masses were noted with only a benign (functional) appearing 2.3 cm left ovarian cyst.
Fig. 1.
Pelvic MRI demonstrating umbilicated lower uterine segment mass at presentation (Axial T2 Haste).
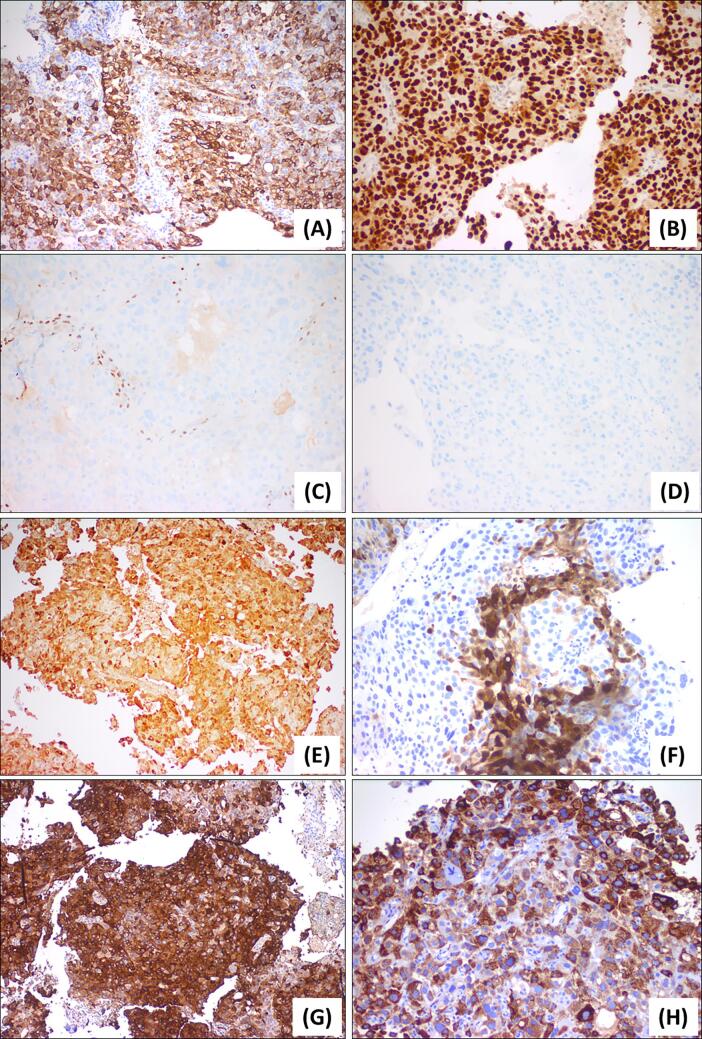
The patient was referred to our institution for gynecologic oncology evaluation and internal pathologist review of biopsy specimens of the uterus and cervix. Pathology review demonstrated sheets of polygonal cells with abundant eosinophilic cytoplasm and pleomorphic nuclei that were diffusely positive for glypican 3, HepPar1, arginase, and AFP, suggesting hepatoid features (see Fig. 2). There was also patchy staining for epithelial marker CK7 and diffuse positivity for pan-cytokeratin. There was scattered staining for SALL-4, a germ cell tumor marker. Additional immunohistochemistry showed positive staining for p16 and mutant P53, with a high Ki67 index and focal staining for CEA. These findings suggested a high-grade hepatoid carcinoma, possibly endometrioid carcinoma with hepatoid features. Serum cancer antigen 125 (CA125) at this time was found to be elevated at 70.6 U/mL. Serum AFP level was obtained based on discussion at tumor board and was found to be extremely elevated at 8,463.0 ng/mL (Normal Range: 0.0–8.3 ng/mL). Myriad Colaris genetic testing revealed no clinically significant mutations.
Fig. 2.
Initial biopsy results demonstrating staining of (A) CK7, (B) P53, (C) ER, (D) PR, (E) AFP, (F) Arginase, (G) Glypican 3, (H) HepPar1.
The patient underwent four cycles of chemotherapy every 21 days with carboplatin (Target AUC = 6 mg/mL/min) and paclitaxel (175 mg/m2). At initiation of the fourth cycle, the patient’s AFP level fell to 7,399 ng/mL. However, at her follow up appointment 13 days later, the patient was found to have markedly increased AFP to 11,432 ng/mL. Interval imaging showed a decrease in size of the uterine tumor from 5.3 × 5.3 cm to 3.4 × 3.4 cm and lack of new pulmonary nodules. Nonetheless, CT also revealed the emergence of bilateral adnexal complex cystic lesions originating from the patient's ovaries, with dimensions of 8.8 × 7.0 cm on the right side and 6.5 × 8.1 cm on the left side. The decision was made to proceed with definitive hysterectomy, salpingo-oophorectomy, and cytoreduction. Intraoperatively, there was extensive uterine and cervical disease, peritoneal carcinomatosis, and large bilateral pelvic masses extending to the level of the anterior abdominal wall, without hepatic or splenic involvement. At the completion of surgery, there was no evidence of residual disease. The patient had an excellent laboratory response to surgery, with an AFP level of 1,618 ng/mL 22 days post-op. The patient had an approximately 2-month chemo holiday following her surgery to allow for recovery.
2.1. Surgical pathology
The hysterectomy specimen revealed a 3.5 cm tumor arising from the lower uterine segment and involved the endocervix and uterine serosa, with additional tumor involving the bilateral ovaries, fallopian tubes, rectosigmoid serosa, omentum, and diaphragm. The tumor had papillary, cystic, and solid growth patterns. Tumor cells had high-grade features with marked cytologic atypia and eosinophilic globules. The tumor was also associated with abundant psammomatous-type calcifications. Lymphovascular invasion was present and one pelvic lymph node was positive for metastatic carcinoma. These findings staged the tumor as Stage IVB according to American Joint Committee on Cancer (AJCC) criteria.
Tumor cells were positive for hepatoid markers glypican 3, HepPar1, and AFP. Interestingly, germ cell marker SALL-4 was again patchy positive in the uterine tumor but diffusely positive in the ovarian tumor. Other positive markers were CK7, p16, and CDX2, and focally positive CK20, and epithelial membrane antigen (EMA). P53 had mutant overexpression. Tumor cells were negative for ER, Napsin-A, high-risk HPV, and WT-1. PAX8 was noncontributory. The tumor was microsatellite instability (MSI) stable with no loss of mismatch repair (MMR) protein expression. PD-L1 was negative in tumor cells.
Overall, this was a complex tumor ultimately diagnosed as high-grade carcinoma with hepatoid, endometrioid, and serous features and somatically-derived YST. SALL-4 suggests germ cell tumor differentiation, and positive AFP and glypican 3 and patchy positive EMA suggest YST. Positive AFP and glypican 3 staining are also seen in hepatoid component of tumors. The positivity of CK7 points to an epithelial carcinoma component, and CDX2 is often expressed in endometrioid adenocarcinoma. The psammomatous calcifications, marked atypia, positive p16 and mutant p53 point towards high-grade serous features. The overall morphology and immunohistochemical profile seen in this tumor is best characterized as a high-grade carcinoma with overlapping features of hepatoid, endometrioid, and serous carcinoma and somatically derived YST.
2.2. Adjusting the chemotherapy course for new findings
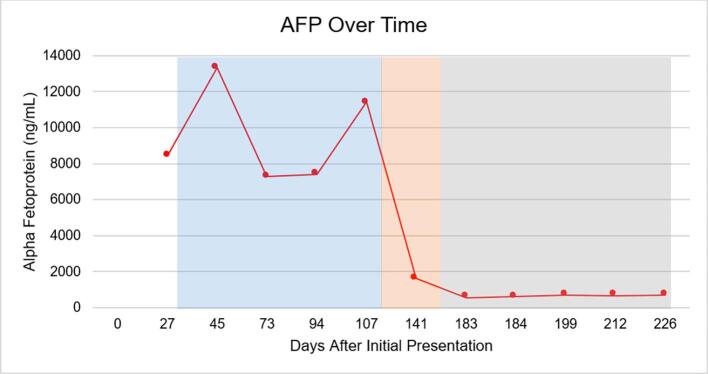
Multidisciplinary tumor board, including a YST expert from MD Anderson, convened to evaluate treatment moving forward. Subsequently, the patient underwent four cycles of bleomycin (30 units, CD1), etoposide (100 mg/m2/day, CD1-5), and cisplatin (20 mg/m2/day, CD1-5) (BEP) every 21 days to better target the large yolk sac components of the heterogeneous cancer. The patient had an excellent AFP response to BEP with a drop to 532 ng/mL after cycle one, shown in Fig. 3. However, during BEP treatment the patient experienced multiple adverse effects including recurrent supraventricular tachycardia, symptomatic anemia requiring transfusions, as well as neutropenic fever and pancytopenia resulting in 2 hospitalizations.
Fig. 3.
Trend of AFP level over time from presentation. Blue shading represents the time the patient was on four cycles of paclitaxel/carboplatin and recovery (Days 31–119). Orange shading represents the time the patient underwent surgical cytoreduction and recovery (Days 119–167). Surgical pathology revealed primary YST component. Subsequently, gray shading represents the time the patient underwent four cycles of BEP (Days 167–226). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
After recovery from hospitalization due to pancytopenia following cycle four of BEP, the patient underwent PET scan and was found to have enlarged left iliac chain and bilateral inguinal lymph nodes demonstrating mild FDG uptake. However,image guided inguinal lymph node and external iliac lymph node biopsies were negative for metastatic malignancy. At this time the patient’s AFP was stable with a plan for interval PET/CT and high-dose BEP and stem-cell rescue if disease recurred.
3. Discussion
3.1. Diagnosis
Serum AFP elevation is found in several malignancies including yolk sac tumors, hepatic cancers, and gastric cancers and indicates cellular de-differentiation. Physiologically, expression of AFP is increased in the first trimester of pregnancy and is secreted by the fetal liver and yolk sac to increase neovascularization and decrease apoptosis (Galle et al., 2019). In the gynecological oncology patient, increased serum AFP is most commonly associated with YST, also known as endodermal sinus tumors, and is a reliable marker for disease progression and response to treatment (Tanyi and Scholler, 2012 Jan 1).
However, in the case of our patient, endometrial biopsy results favored hepatoid carcinoma of the endometrium. The initial biopsy pointed toward a primary hepatoid carcinoma of the endometrium owing to the strong staining of glypican 3, HepPar1, arginase, and AFP. Additionally, biopsy showed only scattered SALL-4 staining, which is typically strongly positive in YST but is also known to be positive in hepatoid carcinoma of the endometrium (Liu et al., 2021). Further, the epithelial staining of the biopsy for CK7 and diffuse positivity for pan-cytokeratin sample initially favored hepatoid carcinoma of the endometrium over YST (Tochigi et al., 2003 Jul). In combination with the pink, polygonal cellular architecture of the sample, this led to the initial treatment of the cancer as hepatoid carcinoma versus YST of the endometrium. However, increasing AFP following cycle four of paclitaxel/carboplatin and new bilateral ovarian masses led to the decision for urgent debulking. Owing to the often heterogeneous nature of these tumors, surgical pathology is of utmost importance in planning a treatment path forward.
3.2. Surgical pathology
YST are malignant primitive germ cell tumors of the ovary. However, epithelial carcinomas have been shown to have YST differentiation, which is described as “somatically derived YST.” This case was a high-grade carcinoma with YST component in the uterus. There are several reports of ovarian carcinoma with somatically-derived YST in the ovary, but there is only one other report of uterine carcinoma with somatically-derived YST (Hodgson et al., 2021 May 1, McNamee et al., 2016 Nov).
Another differential diagnosis to consider is primary YST of the endometrium with associated carcinoma, which would be a collision tumor. There are few cases of primary YST of the uterus and fewer with associated carcinoma component (Wang et al., 2021 Oct, Fadare et al., 2019, Damato et al., 2016 Jul). These cases have occurred in women in their third decade and are speculated to arise from germ cell precursors that have been misplaced during embryological migration. However, our case had YST components intermixed with the carcinoma component, which points towards a carcinoma with somatically derived YST, rather than two different neoplastic processes as would be in a collision tumor. Additionally, SALL-4 was only focally positive in the uterine tumor, which points against a primary germ cell tumor of the endometrium (Fadare et al., 2019). Based on histology and immunohistochemistry alone, we cannot definitively exclude the possibility of a small ovarian lesion metastasizing to the uterus. However, the initial imaging showed benign adnexal structures and a large uterine mass, which is more supportive of a uterine primary. Our tumor was also negative for WT-1, which can be positive in both primary endometrial and adnexal tumors but tends to be positive more often in adnexal tumors. It is not unusual for SALL-4 to have variable expression in poorly or undifferentiated uterine carcinomas, and tumor heterogeneity and viability can also play a role in expression (Fadare et al., 2019). Given the clinical and morphological features, the tumor was best classified as a high-grade carcinoma with somatically derived YST.
3.3. Treatment
The treatment of heterogeneous tumors can be extremely challenging. In the case of our patient, treatment with paclitaxel/carboplatin initially led to a reduction in AFP and uterine tumor volume, suggesting partial platinum-responsiveness of the carcinoma. However, the sudden interval development of bilateral ovarian masses and increase in AFP after cycle four of paclitaxel/carboplatin suggested a fast-growing tumor component that was unresponsive to paclitaxel/carboplatin, warranting urgent surgical intervention. The variation of pathology between the initial biopsy of the endometrium with the new bilateral ovarian masses demonstrates the challenges in charting a treatment course with biopsy results alone.
Further, the heterogeneous nature of endometrial carcinoma with hepatoid, endometrioid, and somatically derived YST components muddies the treatment pathway. Because the YST component arises from the same primary carcinoma, it is postulated that this component will be less responsive to traditional YST treatment (Roth et al., 2011 Sep). However, this was not the case in our patient, who responded well to adjuvant treatment with BEP, which has produced similarly successful responses in those with pure primary YST of the endometrium (Ge and Bi, 2021). Nonetheless, despite stabilization of the AFP level and negative lymph node biopsies, the patient is likely to need additional cycles of chemotherapy in the future, with possible stem cell rescue due to her history of multiple hospitalizations from chemo-induced pancytopenia.
4. Conclusion
We encountered a rare case of high-grade carcinoma with hepatoid, endometrioid, and serous features with somatically derived YST component, and elevated serum AFP in a pre-menopausal woman with past medical history of breast cancer. After treatment with 4 cycles of platinum-based chemotherapy, the patient underwent surgical debulking. Stabilization of AFP levels was achieved with 4 cycles of BEP with a plan for high dose chemotherapy and stem cell rescue therapy in the event of recurrence.
Funding Source for Publication
C.O. is a Burroughs Wellcome Fund Scholar supported by a Burroughs Wellcome Fund Physician Scientist Institutional Award to the Texas A&M University Academy of Physician Scientists (Grant ID: 1020069).
Patient Consent on File
Consent for the publication of patient history, imaging or other identifiable material was obtained by the authors and on file at the time of article submission to the journal stating that the patient gave consent with the understanding that this information may be publicly available.
CRediT authorship contribution statement
Cailin O'Connell: Investigation, Visualization, Writing – original draft. Sylvia Jang: Investigation, Writing – original draft. Paloma Monroig-Bosque: Investigation, Visualization, Writing – review & editing. Anne Alaniz: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We give special acknowledgement to Dr. Michael Deavers, the expert pathologist who handled the extensive work-up and analysis of this case
References
- Damato S., Haldar K., McCluggage W.G. Primary Endometrial Yolk Sac Tumor With Endodermal-Intestinal Differentiation Masquerading as Metastatic Colorectal Adenocarcinoma. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2016 Jul;35(4):316–320. doi: 10.1097/PGP.0000000000000236. [DOI] [PubMed] [Google Scholar]
- Fadare O., Shaker N., Alghamdi A., Ganesan R., Hanley K.Z., Hoang L.N., et al. 2019. Endometrial tumors with yolk sac tumor-like morphologic patterns or immunophenotypes: an expanded appraisal. Mod. Pathol. Off. J U S Can. Acad. Pathol. Inc. Dec;32(12):1847–60. [DOI] [PubMed]
- Galle P.R., Foerster F., Kudo M., Chan S.L., Llovet J.M., Qin S., et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver. Int. 2019;39(12):2214–2229. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
- Ge H., Bi R. Pure primary yolk sac tumor of the endometrium tends to occur at a younger age: A case report and literature analysis. SAGE. Open. Med. Case. Rep. 2021 doi: 10.1177/2050313X211027734. Jun 28;9:2050313X211027734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A., Ghorab Z., Parra-Herran C. Somatically Derived Yolk Sac Tumor of the Ovary in a Young Woman. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2021 May 1;40(3):296–300. doi: 10.1097/PGP.0000000000000673. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhou R., Wang S., Zhang G. Extra-Hepatic Hepatoid Carcinomas in Female Reproductive System: Three Case-Reports with a Literature Review. Cancer. Manag. Res. 2021;13:1625–1636. doi: 10.2147/CMAR.S288913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee T., Damato S., McCluggage W.G. Yolk sac tumours of the female genital tract in older adults derive commonly from somatic epithelial neoplasms: somatically derived yolk sac tumours. Histopathology. 2016 Nov;69(5):739–751. doi: 10.1111/his.13021. [DOI] [PubMed] [Google Scholar]
- Roth L.M., Talerman A., Levy T., Sukmanov O., Czernobilsky B. Ovarian yolk sac tumors in older women arising from epithelial ovarian tumors or with no detectable epithelial component. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2011 Sep;30(5):442–451. doi: 10.1097/PGP.0b013e3182164386. [DOI] [PubMed] [Google Scholar]
- Tanyi J.L., Scholler N. Oncology biomarkers for gynecologic malignancies. Front. Biosci-Elite. 2012 Jan 1;4(3):1097–1110. doi: 10.2741/e444. [DOI] [PubMed] [Google Scholar]
- Tochigi N., Kishimoto T., Supriatna Y., Nagai Y., Nikaido T., Ishikura H. Hepatoid carcinoma of the ovary: a report of three cases admixed with a common surface epithelial carcinoma. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2003 Jul;22(3):266–271. doi: 10.1097/01.PGP.0000055173.04957.66. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao S., Zhao M., Wang D., Chen H., Jiang L. Use of targeted therapy and immunotherapy for the treatment of yolk sac tumors in extragonadal pelvic sites: two case reports. Gland. Surg. 2021 Oct;10(10):3045–3052. doi: 10.21037/gs-21-663. [DOI] [PMC free article] [PubMed] [Google Scholar]