Abstract
Phosphoinositide 3-kinases (PI3Ks) play a central role in tumourigenesis with recurrent activating mutations of its p110α subunit (PIK3CA) identified in several tumours. Although several PI3K inhibitors are approved for haematological malignancies, only alpelisib was approved in solid tumours and for the treatment of PIK3CA-related overgrowth spectrum (PROS) syndrome. Traditional PI3K inhibitors inhibit both wild-type and mutant PI3K with almost equal potency, thus limiting their efficacy due to on-target toxicity. Since the initiation of phase I clinical trials investigating next generation allosteric mutant and isoform selective PIK3CA inhibitors, there has been a surge in interest in PIK3CA targeting in solid tumours. Preclinical characterisation of these compounds showed that maximal mutant protein inhibition fails to elicit metabolic and glucose homoeostasis dysregulation, one of the dose limiting toxicities of both selective and pan PI3K inhibitors. While extreme selectivity can be hypothesised to grant activity and safety advantage to these novel agents, on the other hand reduced benefit can be speculated for patients harbouring multiple or rare PIK3CA mutations. This review summarises the current understanding of PI3K alterations and the state-of-the-art treatment strategies in PI3K driven solid tumours, while also exploring the potential intrinsic and acquired resistance mechanisms to these agents, and the emerging role of mutant selective PIK3CA inhibitors.
Subject terms: Oncology, Drug development
Introduction
The understanding of the molecular drivers of tumourigenesis, leading to the development of targeted therapies that block the signalling functions of oncogenes, has radically changed cancer care.
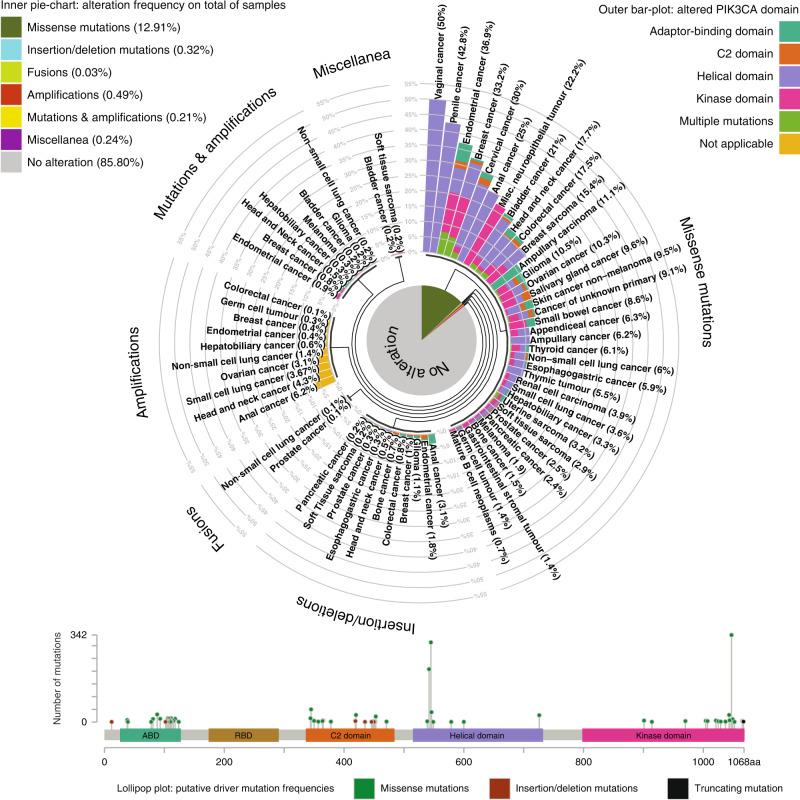
It has been more than 30 years since phosphatidylinositol 3-kinases, also called phosphoinositide 3-kinases (PI3K), were first discovered, while only in 2004 its somatic mutations, especially those occurring in its subunit p110α, were identified in different malignancies [1]. The widespread use of high-throughput sequencing allowed to recognise genetic dysregulations of the four members of the PI3K kinase family (α, β, δ, and γ), as one of the most frequent “driver” mechanisms in cancers, enabling their choice as prospective therapeutic targets. Various types of genomic alterations can dysregulate activity of all PI3K proteins, with PIK3CA/p110α being the most common gene altered detected in 14% of all solid tumours and rarely in haematological malignancies [2]. According to MSK-IMPACT Clinical Sequencing Cohort, endometrial cancer (40%) had the highest prevalence of PIK3CA mutations followed by breast cancer (34.59%), cervical cancer (30%), anal cancer (28%), head and neck cancer (24%), bladder cancer (23.4%) and colorectal cancer (19.17%) (Fig. 1) [3]. Once activated, PI3K mediates signals to a multitude of downstream effectors such as AKT1 and MTOR, as well as promoting additional cancer-benefitting factors such as recruitment of inflammatory cells and angiogenesis [4, 5] (Fig. 2).
Fig. 1. (Top Panel)Inner pie-chart: frequency of PIK3CA gene alterations according to type (Missense mutations, Insertion/deletion mutations, Fusions, Amplifications, Mutations & amplifications and Miscellanea) in 10945 samples of the MSK-IMPACT Clinical Sequencing Cohort [3].
Outer bar-plot: cumulative percentage of any given type of PIK3CA alteration according to cancer type, coloured according to the domain on which alteration is identified (Adaptor binding domain, C2 domain, Helical domain, Kinase domain). When multiple alterations are detected in the same sample “Multiple mutations” is reported, whereas when alterations affect the gene in its entirety “Not applicable” is reported. (Bottom Panel) Lollipop plot: putative driver mutation frequencies relative to domain and codon are reported.
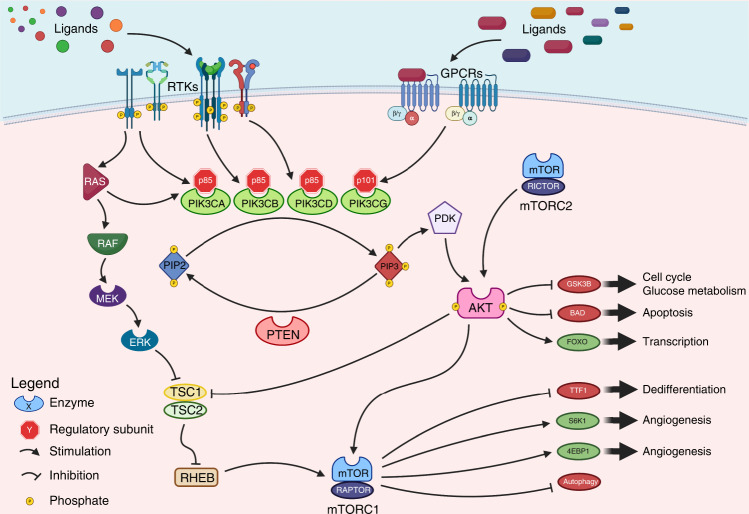
Fig. 2. A schematic representation of major class I PI3K signalling pathway.
PI3Ks are lipid kinases. The various isoforms of PI3K proteins are represented together with their preferred regulatory subunit. Upstream (receptor tyrosine kinases, G-protein coupled receptors and RAS/RAF intracellular signalling molecules) as well as downstream effectors (AKT and mTOR) are reported together with the most important transcriptional consequences of these signalling pathways [63]. Class IA members are heterodimers composed of a p110 catalytic subunit (p110α, p110β, or p110δ; encoded by PIK3CA, PIK3CB, or PIK3CD gene, respectively) and one p85 type regulatory subunit (p85α or its splicing variants p55α or p50α; p85β, or p55γ, encoded by PIK3R1-2-3, respectively). The most frequent alterations in solid tumours are observed in PIK3CA, located on chromosome 3q26.32. Class IB members are heterodimers composed of a p110γ (encoded by PIK3CG) catalytic subunit and a regulatory isoform p101 (PIK3R5) or p87 (PIK3R6) [8]. Either physiological or pathological stimulation by RTKs, GPCRs, or small GTPase such as RAS or RAF family member or activating mutations in p110α, facilitating kinase activation, or inactivating mutations in p85α, disrupting p85 binding thus relieving its inhibitor function, can activate PI3K signalling. After its activation PI3K converts PIP2 into PIP3, which interacts with AKT at the cellular membrane where it is phosphorylated and activated. PTEN is a negative regulator of PI3K, which dephosphorylates PIP3. AKT serves a range of cellular functions with effects on metabolism and cell survival, and through indirect activation of mTOR controls cell proliferation, survival, angiogenesis, immune-evasion, protein translation, autophagy and epithelial to mesenchymal transition (EMT) favouring tumour invasion and metastasis. mTOR exists as two multi-protein complexes, mTORC1 and mTORC2. mTORC2 lies upstream of AKT and activates AKT, whereas mTORC1 lies downstream of AKT and is activated via AKT-mediated inhibition of tuberous sclerosis complex 1 and 2 (TSC1 and TSC2). The PI3K/AKT/mTOR signalling pathway exhibits cross-talk with the RAS/MAPK pathway. PI3K phosphoinositide 3-kinase, RTK receptor tyrosine kinase, GPCR G protein-coupled receptor, PIP2 phosphatidylinositol 4,5 bisphosphate, PIP3 phosphatidylinositol 3, 4, 5 trisphosphate. Created with Biorender.com (accessed on 9 December 2022).
Idelalisib was the first PI3K inhibitor to achieve FDA approval in 2014 in patients with relapsed follicular B-cell non-Hodgkin lymphoma or relapsed small lymphocytic lymphoma (SLL), and subsequently many other PI3K inhibitors reached approval in these diseases. In solid tumours, alpelisib was the first PI3K inhibitor to be granted FDA approval in 2019 in combination with fulvestrant in PIK3CA-altered hormone receptor (HR) positive advanced breast cancer [6]. Despite these successes, further development of PI3K inhibitors is hampered by poor drug tolerance and lack of consistent clinical benefit across different tumour types. However, the development of new isoform-selective PI3K inhibitors has opened a new era of treatment for PI3K-alter solid tumours.
Besides oncology, germline occurring PIK3CA mutations have also been characterised as causative of PIK3CA-related overgrowth spectrum (PROS), a rare syndrome characterised by overgrowth of regional tissue carrying this alteration [7].
This review summarises the current understanding of PI3K alterations and the state-of-the-art treatment strategies in PI3K-driven solid tumours, while also exploring the emerging class of mutant selective P13K inhibitors.
PI3K alterations in cancer
The most common oncogenic PI3K alterations are PIK3CA mutations and generally these are somatic and heterozygous dominant [8].
Mutations can occur in all PIK3CA domains. However, more than 80% mutations occur in exon 9 (helical domain: p.E542K and p.E545K) and in exon 20 (kinase domain: p.H1047R) (Fig. 1). Mutations in other catalytic isoforms and in regulatory subunits are rare, among these p85 regulatory subunit is the most frequently altered in cancer. According to TCGA analysis, somatic mutations of PIK3R1 (p85α) are commonly detected in endometrial cancer, metastatic prostate adenocarcinoma, glioblastoma, breast cancer, and colon adenocarcinoma [3]. Inactivating p85α mutations in the p110α interaction segment or premature truncations at Q572 can induce loss of activity and mediate indirect oncogenic transformation via p110α activation suggesting that this regulatory subunit acts as a tumour suppressor [9].
In breast and colon cancer, PIK3CA mutations tend to be early events and thus typically are clonal [10]. In breast cancer, PIK3CA mutations have been reported in ~40% of HR+, human epidermal growth factor receptor 2 negative (HER2−) [11], in ~30% of HER2 amplified and in ~10% of triple-negative breast cancer (TNBC) [12]. The prognostic role of these mutations is controversial with some studies reporting a good outcome in patients with HR+HER2− early breast cancer and poor prognosis in advanced setting [13]. Patients harbouring PIK3CA mutations are less sensitive to and endocrine therapies [14], but show more sensitivity towards endocrine therapy when added to a PI3K or mTOR inhibitor [15].
In colorectal cancer, PIK3CA mutations can co-occur with RAS mutations (in about 50%) and microsatellite instability (MSI), while no significant association is found with BRAF mutations [16]. A large retrospective analysis [17] and two additional meta-analyses [18, 19] showed that exon 20 PIK3CA mutations occurred in cetuximab-resistant patients and were associated with benefit loss. These findings suggest that targeting PI3K might be useful in PIK3CA mutant metastatic colorectal patients (mCRC).
In other solid tumours, the role of PIK3CA mutation is not clearly defined and does not correlate with any clinic-pathological characteristics.
Amplification of wild-type PIK3CA gene have been reported in various malignancies [20]; however, these alterations alone do not seem to display transforming capacity probably due to lower stimulation of the PI3K pathway. Gene fusions and insertion–deletions while rare, induce PIK3CA hyperactivation and can be found in in a large array of solid malignancies [21, 22].
PI3K inhibitors
PI3K inhibitors can be classified according to their isoform selectivity: pan-PI3K inhibitors and dual-PI3K inhibitors target either all or two PI3K isoforms at the same time, while isoform selective inhibitors target one specific isoform of PI3K. Mutant and isoform specific inhibitors target only mutant proteins of a specific isoform.
In what follows, we shall focus only on the results of clinical trials where these PI3K inhibitors were tested in solid tumours with mentions on other interesting and promising drugs.
Pan-PI3K inhibitors
Many pan-PI3K inhibitors have completed early phase clinical trials in solid tumours, but further development of most of them has been halted mainly due to their on-target and off-target toxicities [23–27]. Currently only two pan-PI3K inhibitors, buparlisib and copanlisib, are under investigation in clinical trials in solid tumours (Table 1; chemical structures and preclinical data of inhibitors are present in the Supplementary Material file named “chemical_structure_preclinical_info”).
Table 1.
PI3K inhibitors are reported together with isoform selectivity, inhibitory constant 50 (IC50) expressed in nM and FDA approval label, whenever applicable in both solid tumours and haematological malignancies.
| Drug | Type | PIK3CA (IC50) | PIK3CB (IC50) | PIK3CD (IC50) | PIK3CG (IC50) | Specific mutations | FDA label | Indication in ongoing trials | NCT number | Experimental combinations |
|---|---|---|---|---|---|---|---|---|---|---|
| Buparlisib (NVP-BKM120) | Pan PI3K | 52 | 166 | 116 | 262 | No | No | HNSCC (III) | NCT04338399 | |
| Pictilisib GDC-0941) | Pan PI3K | 3 | 33 | 3 | 75 | No | No | |||
| Pilaralisib (XL147) | Pan PI3K | 39 | 36 | 36 | 23 | No | No | |||
| Sonolisib (PX-866) | Pan PI3K | 0.1 | >300 | 2.9 | 1 | No | No | |||
| Copanlisib (BAY 80-6946) | Pan PI3K | 0.5 | 3.7 | 0.7 | 6.6 | No | FL after 2 lines | HR + BC(I/II); HER2 + BC(I/II); TNBC(I/II); NSCLC (I); solid tumours (I); MSS colorectal cancer (I/II); castration-resistant prostate cancer (I/II); gynaecological–peritoneal (I/II); solid tumours (I/II); thyroid cancer radioiodine refractory (I) | NCT04895579; NCT03842228; NCT03711058; NCT04253262; NCT05010096; NCT04345913; NCT05082025; NCT03803761; NCT03586661; NCT04317105; NCT04462471; NCT03939897; NCT04108858; NCT02465060; NCT03878524 | Durvalumab; Olaparib + Durvalumab; Nivolumab; Nivolumab + Ipilimumab; Rucaparib; Elimusertib; Eribulin; Fulvestrant; Niraparib; Vemurafenib; Fulvestrant + Abemaciclib; Trastuzumab + Pertuzumab |
| Alpelisib (NVP-BYL719) | Selective (PIK3CA) | 4.6 | 1200 | 290 | 250 | No | PIK3Ca mutant HR+ and HER2– breast cancer | HR + BC (I/II/III); HER2 + BC (II/III); TNBC (I/II/III); colorectal cancer (I/II); HNSCC (I/II); gastric cancer (I/II); serous ovarian cancer (III); HPV-positive oropharingeal cancer (II); endometrial (I); meningioma (I) | NCT05143229; NCT04524000; NCT04208178; NCT04753203; NCT04216472; NCT03207529; NCT05038735; NCT04997902; NCT04729387; NCT04762979; NCT04526470; NCT04544189; NCT03631953; NCT03601507; NCT04251533; NCT05154487; NCT04862143; NCT05063786; NCT04943497; NCT03284957; NCT04188548 | Sacituzumab Govitecan; Fulvestrant; Trastuzumab + Pertuzumab; Capecitabine; Nab-paclitaxel; Enzalutamide; Tipifarnib; Olaparib; Aromatase inhibitor; Paclitaxel; Trametinib; Trastuzumab + Fulvestrant + /− Vinorelbine + /− Capecitabine + /− Eribulin; Ribociclib + Chemotherapy; Amcenestrant; LY3484356 |
| Taselisib (GDC-0032) | Dual (PI3K A + G) | 0.29 | 9.1 | 0.97 | 0.12 | No | No | Solid tumours (II) | NCT02465060 | |
| Inavolisib (GDC-0077/RG-6114) | Selective (PIK3CA) | 0.034 | 100 | 12 | 18 | No | No | HR + BC (I/II/III); HER2 + BC (I); colorectal cancer (I) | NCT04191499; NCT03006172; NCT04802759; NCT04589845 | Palbociclib + Fulvestrant; Letrozole; Letrozole + Palbociclib; Fulvestrant; Fulvestrant + Palbociclib; Fulvestrant + Palbociclib + Metformin; Trastuzumab + Pertuzumab; Bevacizumab; Giredestrant |
| Serabelisib (TAK-117/MLN1117/Petra 06) | Selective (PIK3CA) | 15 | 4500 | 1900 | 14,000 | No | No | |||
| MEN 1611 (CH5132799) | Selective (PIK3CA) | 14 | 120 | 500 | 36 | No | No | HR + HER2 + BC (I); colorectal (I/II) | NCT03767335; NCT04495621 | Trastuzumab + Fulvestrant; Cetuximab |
| CYH-33 | Selective (PIK3CA) | 5.9 | 600 | 79 | 225 | No | No | Solid tumours (I); ovarian cancer (II); HR + BC (I) | NCT04586335; NCT05043922; NCT04856371 | Olaparib, Fulvestrant; Fulvestrant + Palbociclib; Letrozole + Palbociclib |
| GSK2636771 | Selective (PIK3CB) | >5800 | 5.2 | 58 | >126,000 | No | No | Solid tumours (II) | NCT02465060 | |
| SAR260301 | Selective (PIK3CB) | 1500 | 23 | 470 | >10,000 | No | No | |||
| Eganelisib (IPI-549) | Selective (PIK3CG) | 3200 | 3500 | >8400 | 16 | No | No | TNBC(II); renal cell carcinoma (II) | NCT03961698 | Atezolizumab + Nab-paclitaxel; Atezolizumab + Bevacizumab |
| Duvelisib IPI-145 | Dual (PI3K GD) | 1600 | 85 | 2.5 | 27 | No | CLL after 2 lines; FL after 2 lines; small lymphocyte lymphoma after 2 lines | Melanoma (I/II); HNSCC(II) | NCT04688658; NCT05057247 | Nivolumab; Docetaxel |
| Tenalisib (RP6530) | Dual (PI3K GD) | >300 | >100 | 25 | 33 | No | No | ER + BC (II) | NCT05021900 | |
| AZD8154 | Dual (PI3K GD) | 60 | 1250 | 6 | 8 | No | No | |||
| AMG319 (ACP319) | Selective (PIK3CD) | 33,000 | 2700 | 18 | 850 | No | No | |||
| Idelalisib (CAL-101/GS-1001) | Selective (PIK3CD) | 820 | 570 | 2.5 | 89 | No | CLL relapsing; FL after 2 lines; small lymphocyte lymphoma after 2 lines | |||
| Umbralisib (TGR-1202) | Selective (PIK3CD) | >10,000 | >10,000 | 6.2 | 1400 | No | CLL relapsing; FL; MZL | |||
| Nemiralisib (GSK2269557) | Selective (PIK3CD) | 5000 | 1600 | 0.13 | 6300 | No | No | |||
| Leniolisib (CDZ173) | Selective (PIK3CD) | 240 | 420 | 11 | 2200 | No | No | |||
| Parsaclisib (INCB-50465) | Selective (PIK3CD) | >20,000 | >20,000 | 1.1 | >10,000 | No | No | |||
| Seletalisib (UCB-5857) | Selective (PIK3CD) | 3600 | 2100 | 12 | 280 | No | No | |||
| Zandelisib (PWT-143/ME-401) | Selective (PIK3CD) | 5000 | 210 | 5 | 2100 | No | No | |||
| IOA-244 | Selective (PIK3CD) | 19,000 | 2900 | 150 | >20,000 | No | No | Solid tumours (I) | NCT04328844 | Cisplatin + Pemetrexed |
| Linperlisib (YY-20394) | Selective (PIK3CD) | 1200 | 140 | 4.6 | 5200 | No | No | Thymic cancer (II) | NCT04975061 | |
| SHC014748 | Selective (PIK3CD) | 240 | 96 | 0.77 | 101 | No | No | |||
| LOXO-783 | Allosteric PIK3CA mutant selective | No | No | No | No | PIK3CA H1047R | No |
Breast cancer (I) solid tumours (I) |
NCT05307705 | Fulvestrant; Imlunestrant; Abemaciclib; Anastrozole; Exemestane; Letrozole; Paclitaxel |
| RLY-2608 | Allosteric PIK3CA mutant selective | No | No | No | No | PIK3CA E542K or E545K or H1047R | No |
Breast cancer (I) solid tumours (I) |
NCT05216432 | Fulvestrant |
| KA2237 | Undisclosed | No | No | No | No | Undisclosed | No | |||
| TQ-B3525 | Undisclosed | No | No | No | No | Undisclosed | No | Gynaecologic cancers (II); HR + BC(II) | NCT04836663; NCT04355520 | Fulvestrant |
| HMPL-689 | Undisclosed | No | No | No | No | Undisclosed | No | n |
Ongoing clinical trials are reported together with experimental indications and agents in experimental combination only in solid tumours that is the topic of this review. The Supplementary Material in the file “chemical_structure_preclinical_info” provides publicly available chemical structure and preclinical data of these inhibitors. We provided the PubChem URL and chemical structure of the PI3K inhibitors in a Supplementary document.
PI3K phosphatidylinositol 3-kinase, IC50 50% inhibitory concentration, NCT National Clinical Trial, FL follicular lymphoma, HNSCC head and neck squamous cell carcinoma, HR hormone receptor, BC breast cancer, TNBC triple-negative breast cancer, NSCLC non-small cell lung cancer, MSS microsatellite stable, HPV human papillomavirus, CLL chronic lymphocytic leukaemia, MZL marginal zone lymphoma.
Buparlisib (BKM120)
Buparlisib was tested in combination with fulvestrant vs fulvestrant plus placebo in two phase III trials in post-menopausal women with HR + HER2- breast cancer, progressing to an aromatase inhibitor with (BELLE-3) [28], or without (BELLE-2) [29] mTOR inhibitors. Both studies [28, 29] met the primary endpoint of improvement of median progression free survival (mPFS) in the group receiving buparlisib plus fulvestrant, albeit by a modest amount (Table 2). However, up to 25% of patients in both trials discontinued the treatment due to grade ≥3 adverse events (AEs) consisting of transaminitis, hyperglycaemia, and rash. In addition, in BELLE-3 trial depression, anxiety, and rare suicidal ideation were reported as drug-related toxicities, probably explained by buparlisib’s ability to cross the blood brain barrier. Nevertheless, BELLE-2 and BELLE-3 trials provided information supporting the combination of fulvestrant plus PI3K inhibitors in PIK3CA mutated HR + HER2- breast cancer. Indeed, a subgroup analysis conducted on both studies confirmed that the presence of PIK3CA mutations was associated with mPFS improvement (BELLE-2: mPFS 4.2 vs 1.6 months; HR 0.46; p = 0.0034; BELLE-3: mPFS 7.0 vs 3.2 months; HR 0.56; p = 0.0005) [28, 29].
Table 2.
PI3K inhibitor with publicly available clinical data arising from at least a phase II study in solid tumours are reported.
| Drug | Study ID | Phase | Indications | Experimental treatment | Control | ORR | mPFS (months) | Discontinuation rate (due to AE) |
|---|---|---|---|---|---|---|---|---|
| Pictilisib (GDC-0941) | FERG1 [23] | II | HR + HER2-BC (Part 1: previous aromatase inhibitor; regardless of PIK3CA mutation) | Pictilisib + Fulvestrant | Placebo + Fulvestrant | 7.9% vs 6.3% | 6.6 vs 5.1; HR 0.74 (0.51–1.06, p = 0.096) | 18% vs 3% |
| HR + HER2− BC (Part 2: previous aromatase inhibitor; PTs with PIK3CA mutation) | Pictilisib + Fulvestrant | Placebo + Fulvestrant | 7.3% vs 5% | 5.4 vs 10; HR 1.07 (0.53–2.18, p = 0.84) | 24% vs 5% | |||
| PEGGY [24] | II | HR + HER2− BC (chemo-naive with exception of previous Capecitabine; regardless of PIK3CA mutation) | Pictilisib + Paclitaxel | Placebo + Paclitaxel | 22% vs 19.6% | 8.2 vs 7.8; HR 0.95 (0.62–1.46, p = 0.83) | 25% vs 15.2% | |
| Pilaralisib (XL147) | NCT01013324 [25] | II | Advanced endometrial cancer (progressing to 1/2 previous CT lines; regardless of PIK3CA mutation) | Pilaralisib | NA | 6% | NA | 20.9% |
| Sonolisib (PX-866) | NCT01204099 [26, 27] | II | Advanced NSCLC (progressing to 1/2 previous CT lines; regardless of PIK3CA mutation) | PX-866 + Docetaxel | Placebo + Docetaxel | 6% vs 0% | 2 vs 2.9; HR NA | 0% vs 5% |
| Advanced HNSCC (progressing to 1/2 previous CT lines; regardless of PIK3CA mutation) | PX-866 + Docetaxel | Placebo + Docetaxel | 14% vs 5% | 3 vs 2.7; HR NA | NA | |||
| Buparlisib (BKM120) | BELLE-2 [29] | III | HR + HER2− BC (previous aromatase inhibitor) | Buparlisib + Fulvestrant | Placebo + Fulvestrant | 11.8% vs 7.7% | 6.9 vs 5.0; HR 0.78 (0.67–0.89, p < 0.001) | 39% vs 5% |
| BELLE-3 [28] | III | HR + HER2− BC (previous aromatase inhibitor and mTORi) | Buparlisib + Fulvestrant | Placebo + Fulvestrant | 8% vs 2% | 3.9 vs 1.8; HR 0.67 (0.53–0.84, p < 0.001) | 21% vs 5% | |
| BELLE-4 [30] | II/III | HER2-BC (chemo-naive) | Buparlisib + Paclitaxel | Placebo + Paclitaxel | 22.6% vs 27% | 8.0 vs 9.2; HR 1.18 (0.81–1.68) | 20.8% vs 7.2% | |
| BERIL-1 [31] | II | HNSCC | Buparlisib + Paclitaxel | Placebo + paclitaxel | 39% vs 14% | 4.6 vs 3.5; HR 0.65 (0.45–0.95, p = 0.011) | 10% vs 14% | |
| Copanlisib (BAY 80-6946) | NCI-MATCH [32] | II | Solid tumours (PIK3CA mutant and PTEN loss) | Copanlisib | NA | 16% | NA | 10% |
| Alpelisib (NVP-BYL719) | Solar-1 [6, 33] | III | PIK3CA mutant HR + HER2− BC (previous aromatase inhibitor, CDK4/6 inhibitor permitted) | Alpelisib + Fulvestrant | Placebo + Fulvestrant | 26.6% vs 12.8% | 11 vs 5.7; HR 0.65 (0.50–0.85, p < 0.001) | 25% vs 4.2% |
| BYLieve [37] | II | PIK3CA mutant HR + HER2− BC (previous CDK4/6 inhibitors + aromatase inhibitor or previous CDK4/6 inhibitor + Fulvestrant or previous chemotherapy) | Alpelisib + Fulvestrant | NA | 17% | 7.3 | 20% | |
| Eganelisib (IPI-549) | Mario-275a (NCT03980041) | II | Urothelial cancer (immunotherapy naive, platinum refractory) | Eganelisib + Nivolumab | Placebo + Nivolumab | 30% vs 25% | NA | NA |
| Mario-3 [43] | II | TNBC | Eganelisib + Atezolizumab + Nab-Paclitaxel | NA | NA | NA | NA | |
| Taselisib (GDC-0032) | Sandpiper [46] | III | PIK3CA mutant HR + HER2− BC (previous aromatase inhibitor) | Taselisib + Fulvestrant | Placebo + Fulvestrant | 28% vs 11.9% | 7.4 vs 5.4; HR 0.7 (p = 0.0037) | 17% vs 2% |
ORR overall response rate, mPFS median progression-free survival, AE adverse event, BC breast cancer, HR hormone receptor, HER2 Human Epidermal Growth Factor Receptor 2, mTORi: mammalian target of rapamycin inhibitor, PIK3CA phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha, NSCLC non-small cell lung cancer, CT chemotherapy, HNSCC head and neck squamous cell carcinoma, CDK cyclin-dependent kinase, TNBC triple-negative breast cancer, NA not available.
aFor Mario-275 trial, the only available data arise from press releases, we chose to report them here since, if confirmed on peer reviewed sources, they might be relevant in the future.
Buparlisib was also tested in combination with chemotherapy in randomised phase II BELLE-4 study [30] in chemo-naive HER2− advanced breast cancer, but it did not meet the mPFS primary endpoint. A lack of predictive biomarkers and the high burden of adverse responsible for treatment discontinuation impacted the results of the study.
Buparlisib (vs placebo) + paclitaxel was shown to be active in a phase II trial conducted in patients with platinum-pretreated recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) (Table 2) [31]. The median PFS was significantly longer in the buparlisib arm (4.6 vs 3.5 months, p = 0.01), as well as OS (10.4 vs 6.5 months, p = 0.041). Based on this result, the phase III trial BURAN (NCT04338399) is evaluating the efficacy of paclitaxel + buparlisib vs paclitaxel + placebo in patients with advanced HNSCC progressing after immunotherapy either as monotherapy or with a platinum-based regimen.
Copanlisib (BAY 80-6946)
Copanlisib was tested in the subprotocol Z1F of the phase II tumour-agnostic NCI-MATCH ECOG-ACRIN (EAY131) platform trial in advanced pretreated solid tumours, positive for PIK3CA mutation confirmed by NGS [32]. This subprotocol enrolled patients with 20 different tumour types, with 68% of them receiving 3 or more previous lines of systemic therapy. The study met its primary endpoint with an ORR of 16% (4/25 patients, 90% CI 6–33, p = 0.0341) showing promising clinical activity in tumours harbouring PIK3CA mutation. In line with other PI3K inhibitors, hyperglycaemia and gastrointestinal toxicities were the most observed grade 3/4 toxicities. Approximately 10% of patients discontinued treatment due to AEs, a rate relatively lower than other PI3K inhibitors (>20%).
Isoform-specific PI3K inhibitors
Isoform-specific PI3K inhibitors display a high potency and specificity towards one or more (usually two) specific isoform(s) of PI3K (Table 1), thus providing a better therapeutic window and tolerability profile. Among them, those targeting PI3Kα are being evaluated in advanced phase trials in solid tumours, whereas PI3Kβ, PI3Kγ and PI3Kδ inhibitors are under evaluation in early phase trials (Table 1).
PI3Kα inhibitors
Many PI3Kα inhibitors are under evaluation in clinical trials (Table 1), but to date, alpelisib is the only selective PI3Kα inhibitor approved by FDA in combination with fulvestrant in men and postmenopausal women with HR + /HER2–, PIK3CA-mutated advanced breast cancer [6].
Alpelisib (BYL719)
The activity of alpelisib in breast cancer was clinically validated by SOLAR-1 phase 3 study, which was the basis of FDA approval of this drug in HR + HER2− PI3K mutated advanced breast cancer pretreated with a prior line of endocrine therapy [6, 33]. The rationale for using this combination stems from preclinical and clinical studies where PIK3CA signalling was shown to mediate anti-oestrogen therapy resistance, resulting in an increase in oestrogen receptor (ER) translation in ER+ breast cancer cell lines, xenograft models, and patient samples [34–36]. In SOLAR-1 study, 572 patients, 341 of whom harboured PIK3CA mutations, were randomised to receive fulvestrant with alpelisib/placebo. The mutant group treated with alpelisib plus fulvestrant showed an improvement in mPFS compared to fulvestrant plus placebo not observed in PIK3CA wild type (WT) group (Table 2). Since the SOLAR-1 study lacked an alpelisib monotherapy arm, it is not completely clear if the anticancer effect of the combination therapy is due solely to PI3K inhibition or restoration of cellular sensitivity to anti-oestrogen therapy. The tolerability profile of alpelisib was in line with that of other PI3K inhibitors: AEs leading to discontinuation of treatment were reported in 25% of patients, compared to 4.2% in the placebo group. The most frequent high-grade AEs reported in the SOLAR-1 study were G3/G4 hyperglycaemia (36.6% in alpelisib plus fulvestrant group vs 0.7% in placebo + fulvestrant group), G3 rash (9.9% vs 0.3%) and G3 diarrhoea (6.7% vs 0.3%).
When SOLAR-1 study was designed, CDK4/6 inhibitors, which have now become the standard treatment for HR + /HER2– advanced breast cancer, were not yet approved. The ongoing phase 2 trial BYLieve [37] and the phase 3 trial EPIK-B5 (NCT05038735) are evaluating the activity of alpelisib plus fulvestrant in chemonaïve patient, progressing on or after previous therapy with CDK4/6 inhibitor and endocrine therapy in PI3K mutated HR + HER2− advanced breast cancer.
There are currently ongoing phase II and III clinical trials that are evaluating alpelisib in different solid tumour in combination with targeted agents or chemotherapy as shown in Table 1.
CYH-33
Preliminary results of safety and activity of this drug were reported at the 2021 ESMO congress [38], where it was tested in pretreated solid tumours with or without PIK3CA mutations (NCT03544905). In 19 evaluable patients, 5 had PR (26%) and they belong to PIK3CA mutated group. The toxicity was comparable to other selective α-inhibitors with hyperglycaemia, transaminitis and gastrointestinal toxicity as the most common. These encouraging results have opened the way to test this drug in combination with other hormone therapies and targeted therapies in solid tumours in phase I and II studies (Table 1).
Inavolisib (GDC-0077)
This drug was tested as single agent, in combination with or with letrozole/fulvestrant or letrozole/fulvestrant and palbociclib in advanced PIK3CA mutant breast cancer (NCT03006172). This study also included an arm with inavolisib + palbociclib + fulvestrant + metformin to prevent PIK3CA induced hyperglycaemia. The rationale for combining PI3Kinhibitor with cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors stems from preclinical studies showing that eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), one of most characterised mTORC1 target, is involved both in protein synthesis and also in translation of key cell cycle regulators such as MYC or cyclin D1 (CCND1) [39]. When bound to CCND1, CDK4/6 phosphorylates retinoblastoma (RB1) thus causing its uncoupling from E2F transcription factors, with subsequent translocation into the nucleus and induction of transcription of genes promoting G1/S phase transition and cell cycle progression. The safety profile of inavolisib monotherapy or combinations was comparable to that reported for other inhibitors with hyperglycaemia as the most common AE, followed by stomatitis, neutropenia, diarrhoea, nausea, alopecia, and rash. No toxicity data has been disclosed for the metformin combination arm.
PI3Kβ inhibitors
Results of phase I trials are available for these two PI3Kβ inhibitors: SAR260301 [40], whose further testing was halted because pharmacokinetics and pharmacodynamics studies showed that this drug has a fast clearance responsible for inadequate target inhibition [40], and GSK2636771 (Table 2).
GSK2636771
This drug was tested with paclitaxel in PTEN-altered advanced gastric cancer progressing to frontline chemotherapy [41]. Out of 37 patients treated with recommended phase 2 dose (R2PD), mPFS was 12.1 weeks and OS was 33.4 weeks. PTEN-null tumours showed favourable mPFS (18.9 vs 11.6 weeks, p = 0.026) compared to PTEN partial loss tumours. The initial results of this trial are encouraging and open a new therapeutic strategy in this subgroup of gastric cancer patients having poor prognosis with very few therapeutic options available.
PI3Kγ inhibitors
PI3Kγ inhibitors are the latest addition to the growing repertoire of selective PI3K inhibitors in solid tumours. Only preliminary data is available for them, nevertheless, they could be important in future in combination with immunotherapy regimens [5]. PI3Kγ inhibitors can stimulate anticancer immunity by reducing the number of T regulatory (Treg) cells, activating intra-tumoural infiltration of CD4+ and CD8+ T cells, by inhibition of macrophages polarisation responsible for suppression of T cell activation, and by regulation of production of immunostimulatory cytokines in tumour microenvironment [5, 42].
Eganelisib (IPI-549)
It is the only PI3Kγ inhibitor that received Fast Track designation by FDA in solid tumours in platinum-refractory, immunotherapy-naive, advanced urothelial cancer (NCT03980041) and in combination with an immune checkpoint inhibitor and chemotherapy for the first-line treatment of patients with advanced/metastatic TNBC [43] (Tables 1 and 2).
PI3Kδ inhibitors
Many PI3Kδ inhibitors are under evaluation in early clinical trials in solid tumours (Table 1); however, the principal role of these inhibitors is in the treatment of haematological malignancies where multiple have reached regulatory approval. Their role in this setting is well established considering that idelalisib received FDA approval as early as 2014 for B cell malignancies [44]. PI3Kδ inhibitors, like PI3Kγ inhibitors, have a role in regulation of immune system by inhibition of Treg lymphocytes and activation of cytotoxic lymphocytes [5].
Dual PI3K inhibitors
The majority of dual PI3K inhibitors are under evaluation in early clinical trials in solid tumours (Table 2), with clinical data available only for taselisib.
Taselisib (GDC-0032)
Taselisib (GDC-0032) is a strong inhibitor of α and γ isoforms exhibiting less potency against β and δ isoform (Table 1). This drug showed to be active in a phase I trial in locally advanced or metastatic solid tumours in patients carrying PIK3CA mutation with an ORR of 36% vs 0% in WT patients [45]. The phase III SANDPIPER trial evaluated the efficacy of fulvestrant in combination with taselisib or placebo in HR + HER2− advanced breast cancer patients pretreated with a prior endocrine therapy [46]. The study was prematurely closed due to modest clinical benefit with only 2-month PFS advantage in taselisib arm and increased serious AEs (32.0% vs 8.9%) responsible for a high rate of discontinuations (16.8% vs 2.3%) and dose reductions (36.5% vs 2.3%). However, a retrospective analysis of data from this trial showed that 19% (n = 66) of patients with PIK3CA mutant tumours harboured multiple PIK3CA mutations. These patients had increased tumour shrinkage on taselisib compared to single mutant group (ORR 30.2% vs 18.1% p = 0.0493), thus suggesting increased sensitivity to PI3K inhibition in this subset of patients [47]. This finding is interesting considering that double PIK3CA-mutant tumours occur in 4% of all population and 12% in the PIK3CA mutant group according to MSK-IMPACT cohorts (Fig. 1)
Isoform and mutant selective PIK3CA inhibitors
The observation that most recurrent oncogenic mutations of PIK3CA occurred far from the ATP binding site sprouted rational drug design of allosteric inhibitors to engage pockets with altered tridimensional conformation in presence of specific oncogenic mutations. The absence of a favourable allosteric binding pocket on WT target or other isoforms dramatically reduces affinity of these pharmaceuticals to WT PIK3CA that is responsible for dysregulations of glucose metabolism, one of the most frequent dose limiting toxicity of ATP competitive PI3K inhibitors. Currently two investigational agents of this class have entered early phase 1 clinical trials: RLY-2608 and LOXO-783 and several in pre-clinical development, e.g. STX-478.
RLY-2608
Based on data presented at the AACR-NCI-EORTC conference on October 2021, this allosteric isoform-mutant selective PIK3CA inhibitor shows comparable inhibitory activity towards helical domain (E542K and E545K) and kinase domain (H1047R) mutations with an almost 10-fold affinity compared with WT PIK3CA and more than 200-fold selectivity vs other PI3K isoforms. This drug is currently under an early phase I clinical trial (NCT05216432).
LOXO-783
It is an allosteric mutant and isoform selective PIK3CA inhibitor with high brain penetrance. It displays selective inhibition of kinase domain PIK3CA H1047R mutation with a 90-fold selectivity compared to WT PIK3CA and no activity on other PI3K isoforms. This drug did not display preclinical activity on cell lines driven by other PIK3CA mutation or PTEN mutation. This investigational drug is currently undergoing early phase I clinical trial (NCT05307705).
ST-814 (Mut-Sel H1047 PI3Kα)
ST-814 is designed to be a wild-type-sparing, oral inhibitor of kinase-domain-mutant PI3Kα capable of penetrating the blood-brain barrier. It has shown in vitro selectivity in multiple head-to-head preclinical studies and dose-dependent anti-tumour activity in animal models at well-tolerated doses without causing metabolic dysregulation that leads to hyperinsulinaemia and hyperglycaemia, key challenges associated with targeting this mutation [48].
PI3K/AKT/mTOR pathway inhibitors
Inhibition of PI3K downstream effectors AKT/mTOR was also tested as an alternative strategy for targeting PI3K-aberrant tumours (Fig. 2). While no AKT inhibitor is approved by FDA till date, two allosteric mTOR inhibitors, temsirolimus and everolimus, have already been approved in different solid tumours.
Temsirolimus
This mTORC1 inhibitor was approved for the treatment of advanced-stage RCC patients according to the results of the phase III Global ARCC trial, which showed to prolong mOS in treatment-naive metastatic patients compared to IFN-α (10.9 months vs 7.3 months; HR 0.73, 95% CI 0.58–0.92; p = 0.008) [49]
Everolimus
This mTORC1 inhibitor was approved for different indications including advanced RCC, HER2– HR+ breast cancer, pancreatic neuroendocrine tumours (NETs), and other selected NETs [15, 50–52]. The phase III RECORD-1 trial tested everolimus vs placebo in pretreated advanced RCC. A prolonged mPFS (4.0 vs 1.9 months, HR = 0.30, 95%CI 0.22–0.40, p < 0.0001) with everolimus was reported that did not translate neither in OS improvement, probably due to the possibility to make crossover in the placebo arm, nor in ORR that was <5%. The phase III RADIANT-3 trial tested everolimus vs placebo in advanced pancreatic NETs. Also here, the mPFS was improved in everolimus arm (mPFS: 11.0 vs 4.6 months, HR 0.35, 95% CI 0.07–0.45, p < 0.001), but the ORR was less than 5% [51]. The activity of everolimus was also confirmed in patients with non-functional gastro-intestinal or lung NETs with improved mPFS compared to placebo (mPFS: 11.0 vs 3.9 months, HR: 0.48, 95% CI: 0.35–0.67, p < 0.00001). In BOLERO-2 trial in HR + HER2− breast cancer patients pretreated with aromatase inhibitors, everolimus plus exemestane showed significantly increased ORR compared to exemestane plus placebo (9.5% vs 0.5% p < 0.001) and prolonged mPFS (6.9 vs 2.8 months HR: 0.43, 95% CI: 0.35–0.54, p < 0.001).
The incidences of adverse events in these studies were similar with G3/G4 stomatitis (4–8%) and hyperglycaemia (5–15%) as the most common ones.
Capivasertib
This AKT inhibitor showed to be active in combination with fulvestrant compared to fulvestrant + placebo in the phase II trial FAKTION in aromatase resistant HR+ breast cancer. At the ASCO 2022 meeting, an update of this study was reported that confirmed the better outcome in terms of mPFS and mOS in capivasertib + fulvestrant group compared to placebo + fulvestrant (mPFS 10.3 vs 4.8 months, HR 0.56, 95% CI: 0.38–0.81; p = 0.002; mOS 29.3 vs 23.4 months HR 0.66, 95% CI 0.45–0.97; p = 0.035). Response analysis showed greater OS benefit in patients harbouring PIK3CA, AKT1 and PTEN alterations compared to WT tumours [53], thus supporting the importance of tumour molecular analysis in treatment decision. Capivasertib is also tested in phase III CAPItello-292 trial (NCT04862663) in combination with fulvestrant + palbociclib vs fulvestrant + palbociclib in ER+ breast cancer.
Other than HR+ tumours, capivasertib also showed to be active in TNBC in combination with paclitaxel vs paclitaxel + placebo, and the improvement in outcome was particularly evident in PI3K mutant tumours (mPFS was 9.3 vs 3.7 months, HR 0.30 p = 0.01) [53]. These results have led to the phase III CAPItello-290 (NCT03997123) trial evaluating combination of capivasertib plus paclitaxel as first line in advanced TNBC.
Ipatasertib
This AKT inhibitor showed to be active in combination with abiraterone in a phase III trial in mCRPC. Patients with or without PTEN loss were randomised to receive placebo + abiraterone vs ipatasertib + abiraterone [54]. The addition of ipatasertib to abiraterone resulted in 23% risk reduction for radiological disease progression or death among patients carrying PTEN loss compared with placebo plus abiraterone as a first-line treatment. In addition, mPFS was improved in ipatasertib arm compared to placebo arm (18.5 vs 16.5 months, HR for progression or death 0.77, 95% CI 0.61–0.98, p = 0.034). In a subgroup of 250 patients who had an alteration predicted to be pathogenic in PIK3CA/AKT1/PTEN, the stratified HR for progression or death was 0.63 (95% CI 0.44–0.88) [54].
Resistance mechanisms to PI3K inhibition
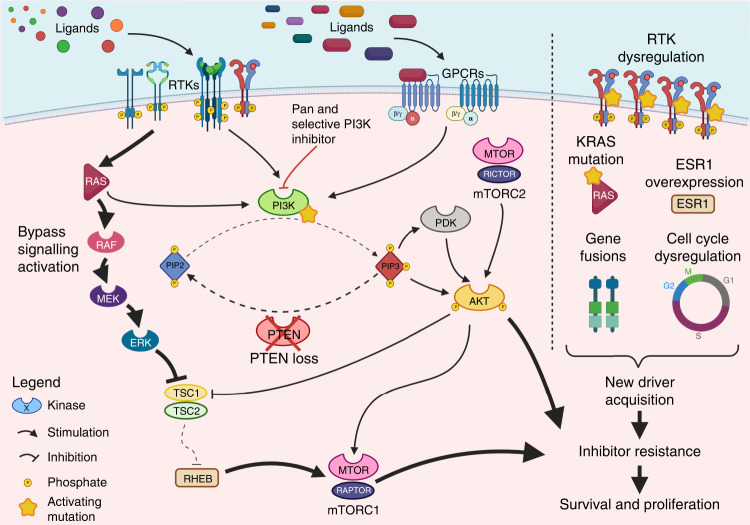
PI3K inhibitor resistance mechanisms can be classified as primary resistance, where no initial clinical response is observed either due to intrinsic refractoriness of the tumour or very rapid adaptation to PI3K inhibition, and secondary resistance arising during treatment when prolonged clinical response is followed by tumour escape. However, to the present date, clinical evidence highlighting potential resistance mechanisms are scarce, fragmentary, and non-conclusive both because the vast majority of PI3K inhibitors tested in solid tumours did not reach advanced phase clinical trials and also because of the retrospective nature of these research. A schematic representation of most frequently characterised resistance mechanisms to PI3K inhibition is available in Fig. 3.
Fig. 3. A schematic representation of most frequently observed PI3K resistance mechanisms.
Resistance can occur either by bypass signalling pathways activation, PTEN loss with subsequent build-up of PIP3 or acquisition/evolution of alternative driver mechanisms such as receptor tyrosine kinase (RTK) dysregulation, mutations activating pathway genes in MAPK pathway (such as KRAS), Cell Cycle dysregulation, upregulation of growth factor receptors or gene fusions. Regardless of the predominant mechanism the final effect is cancer cell survival and proliferation even in presence of effective PI3K inhibition. PI3K phosphoinositide 3-kinase, RTK receptor tyrosine kinase, GPCR: G protein-coupled receptor, PIP2 phosphatidylinositol 4, 5 bisphosphate, PIP3 phosphatidylinositol 3, 4, 5 trisphosphate, KRAS Kirsten rat sarcoma virus, ESR1 oestrogen receptor 1. Created with Biorender.com (accessed on 9th December 2022).
Primary resistance
Primary resistance is characterised by the lack of clinical benefit and progression of disease. Data from the retrospective analysis of patients enrolled in the NCT01870505 phase I trial assessing the aromatase inhibitor and alpelisib combination in PIK3CA mutant HR + HER2− BC highlighted loss of function mutations in the tumour suppressor phosphatase and tensin homologue (PTEN) as recurring alterations in 25% of patients that showed primary resistance to therapy, although concomitant ESR1 mutation conferring resistance to aromatase inhibitor might act as a confounding factor [55]. The identification of recurrent PTEN loss in patients lacking benefit from selective PI3K inhibition is consistent with preclinical evidence of switch in PI3K isoform dependency (from PI3Kα to PI3Kβ) in PTEN deficient cells with subsequent downstream signal propagation and inhibition escape (Fig. 3) [56].
Secondary resistance
Secondary resistance is characterised by an initial clinical benefit from selective PI3K targeting agent with subsequent evolution of adaptative resistance. In this setting, independent convergent evolution of one or more common resistance mechanisms can highlight their putative role as alterations conferring an evolutionary advantage to escape drug-induced selective pressure.
An analysis of different progressing disease sites donated by a deceased patient harbouring PIK3CA-mutant BC experiencing secondary resistance to alpelisib identified PTEN loss of function alteration as the most frequent shared acquired alteration across different progressing disease sites [56].
In another retrospective analysis [55] of the patients of NCT01870505 trial experiencing secondary resistance to alpelisib and aromatase inhibitor, de novo emergence of PTEN alterations was a frequent occurrence (up to 22%). Apart from PTEN, the aforementioned analysis identified many other recurring genomic aberrations as putative cause of secondary resistance, all of which can be hypothesised to confer PI3K independency. Consistent with preclinical rationales, both AKT/mTOR pathway alterations, inducing PIK3CA downstream mediator activation, or RTK/RAS/RAF/MAPK pathway dysregulation, inducing PIK3CA bypass, or overexpression of upstream RTKs including epidermal growth factor receptor (EGFR), HER2 and HER3, platelet derived growth factor receptor (PDGFRA/B) or fibroblastic growth factor receptor 1 (FGFR1) were enriched after secondary resistance was established (Fig. 3) [55]. Despite these recurrent observations, traditionally strategies combining PI3K inhibitors with mTOR inhibitors or other upstream RTK were not clinically effective with only an increasing in toxicity [57, 58].
These insights arise only from PIK3CA inhibitor resistance in breast cancer, and as such it is unknown if these putative resistance mechanisms could be shared across other solid tumours or other inhibition escape strategies could also evolve.
An additional important resistance mechanism, the extent of which still needs to be fully explored, is secondary to the double effect of PI3K inhibitors on glucose metabolism and cancer cell growth. Non mutant selective PI3K inhibitors have comparable inhibitory function both on tumoural and healthy tissue PI3K proteins. Inhibition of WT protein in liver and other tissues induce a decrease in glucose uptake due to AKT-mediated down regulation of glucose transporters (GLUTs). This causes subsequent hyperglycaemia and compensatory hyperinsulinaemia and metabolic disturbances that may activate insulin receptor (INSR) or the insulin-like growth factor receptor 1 (IGF-1R) on cancer cells (Fig. 3). In this setting preclinical studies showed that treatment with sodium-glucose-co-transporter 2 (SGLT2) inhibitors, decreasing renal reabsorption of glucose and consequently reducing insulin production, lead to increasing anticancer effects and tolerability of PIK3CA inhibitors [59].
Based on these observations and evidence arising from preclinical studies a growing effort is being directed towards designing combination strategies to overcome either intrinsic or acquired resistance. A comprehensive summary of active trials involving PI3K inhibitor combinations with other drugs is available in Table 1.
Discussion and conclusions
Oncogenic changes in PI3K proteins are common in a variety of human cancers, with PIK3CA (p110α) mutations being the most common. The observation of independent convergent evolution of these alterations across different tumour types, as well as extensive preclinical and clinical evidence highlight their importance as major contributors of cancer progression.
The earliest PI3K inhibitors tested in solid tumours were pan-inhibitors without selectivity to a particular PI3K isoform. These drugs did not yield promising activities in solid tumours, and they showed high levels of toxicities due to their on-target and off-target activities.
The subsequent generation of PI3K inhibitors, designed to target specific isoforms of PI3K, showed greater efficacies and better toxicity profiles. In solid tumours, alpelisib was the first selective PI3Kα inhibitor to be granted FDA approval in combination with fulvestrant in PIK3CA mutant HR+ advanced breast cancer [6]. Despite this success, further development of PI3K inhibitors was hampered due to lack of activity across different tumour types. It should be noted that PI3K alterations were not a requirement for inclusion in many of the trials. The efficacy results were relatively better when trials enrolled only patients with PI3K mutations or when a subgroup analysis with just PI3K mutant group was performed, emphasising the need of proper patient selection. Furthermore, most trials only tested PI3K alterations in primary tumour lesions, making it difficult to determine whether tumours spreading on distant sites were responsive to PI3K inhibition.
The advent of allosteric mutant and isoform selective PIK3CA inhibitors, some of which are currently being evaluated in early phase I clinical trials, has revived hope in the search for improved PI3K inhibitors. This family of drugs are strong inhibitors of only mutant proteins, sparing the wild-type PI3K, thus avoiding glucose metabolic dysregulations, one of the most prevalent dose-limiting toxicities of ATP competitive PI3K inhibitors.
Primary and secondary resistances are a challenging issue in the development of PI3K inhibitors. The mechanistic data is scarce and incomplete in this regard; however, loss of PTEN function has been identified as a frequent mediator of these resistances. Loss of PTEN activity leads to PIP3 accumulation at the plasma membrane, which activates the AKT/mTOR pathway to drive cell growth, proliferation, and survival. As a result, targeting the other critical players in the AKT/mTOR pathway, which are typically involved in PI3K resistance, may assist to avoid drug resistance.
Future perspectives
Drug resistance is frequently caused by conformational changes in the targeted protein, which prevents the drug from binding to the target protein. Orthosteric drugs bind at the active site, whereas allosteric drugs bind elsewhere on the protein surface and allosterically change the conformation of the protein binding site. Allosteric drugs can resensitize an active site and restore the actions of orthosteric drugs. The strategy of combining allosteric and orthosteric drugs has been successfully employed to overcome drug resistances in BCR-ABL1 mutant chronic myeloid leukaemia (CML) and EGFR mutant lung cancer. Similarly, allosteric drugs may be explored for overcoming PI3K drug resistance, either by making it accessible to sterically blocked ATP-competitive orthosteric drugs, or by producing an altered, druggable PIP2 binding site [60].
Mutations that can rescue or resensitize a mutant protein to an allosteric drug by allosterically altering its conformation have been successfully explored for a few diseases such as Wiskott–Aldrich syndrome (WAS) and galactosemia [60]. Similar mutations may be explored in the kinase domain of PI3K. Other targeting strategies may include inhibiting p85, the non-catalytic subunit of PI3K, which has been found in breast cancer to dramatically reduce PI3K activity, induce cell death, and sensitise it to Trastuzumab treatment [61]. Finally, “molecular glues” that tighten and simplify the connection of an E3 ligase with a disease-causing protein for ubiquitination and subsequent degradation have emerged as a game-changing strategy for novel drug discovery and could be explored for mutant PI3K protein degradation too [62].
Supplementary information
chemical structures and preclinical data
Author contributions
CB conceptualised and supervised the study. All authors were involved in data curation, investigation, methodology, writing, reviewing, and editing. The final version was approved by all authors.
Data availability
Not applicable.
Competing interests
MR reports travelling funding for Sanofi. CP remunerated Consultant and/or Speaker for: Angelini Pharma, AstraZeneca, BMS, Eisai, General Electric, Ipsen, MSD; Protocol Steering Committee Member for: BMS, Eisai and MSD. VS reports research grants from Eli Lilly/Loxo Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, Boston Pharmaceuticals, and Helsinn Pharmaceuticals, and a grant and an advisory board/consultant positions with Eli Lilly/Loxo Oncology during the conduct of the study, as well as research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, IderaPharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-Cancer Therapy Evaluation Program, The University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, PharmaMar, Medimmune, advisory board/consultant positions with Helsinn, Incyte, QED Pharma, Daiichi Sankyo, Signant Health, Novartis, Relay Therapeutics, Pfizer, Roche, and Medimmune, travel funds from PharmaMar, Incyte, ASCO, and ESMO, and other support from Medscape outside the submitted work. GC received honoraria for speaker’s engagement: Roche, Seattle Genetics, Novartis, Lilly, Pfizer, Foundation Medicine, NanoString, Samsung, Celltrion, BMS, MSD; honoraria for providing consultancy: Roche, Seattle Genetics, NanoString; honoraria for participating in Advisory Board: Roche, Lilly, Pfizer, Foundation Medicine, Samsung, Celltrion, Mylan; honoraria for writing engagement: Novartis, BMS; honoraria for participation in Ellipsis Scientific Affairs Group; Institutional research funding for conducting phase I and II clinical trials: Pfizer, Roche, Novartis, Sanofi, Celgene, Servier, Orion, AstraZeneca, Seattle Genetics, AbbVie, Tesaro, BMS, Merck Serono, Merck Sharp Dome, Janssen-Cilag, Philogen, Bayer, Medivation, Medimmune. All remaining authors have declared no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02221-1.
References
- 1.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Kwok-Shing Ng P, Kucherlapati M, Chen F, Liu Y, Tsang YH, et al. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell. 2017;31:820.e3–32.e3. doi: 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway. Nat Rev Clin Oncol. 2018;15:273–91. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 5.Petroni G, Buque A, Zitvogel L, Kroemer G, Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2021;39:310–45. doi: 10.1016/j.ccell.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–40. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 7.Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, Parker VE, Blumhorst C, Darling T, et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am J Med Genet A. 2014;164A:1713–33. doi: 10.1002/ajmg.a.36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–28. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16:463–74. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, et al. The evolutionary history of 2,658 cancers. Nature. 2020;578:122–8. doi: 10.1038/s41586-019-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32:208–17. doi: 10.1016/j.annonc.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Andre F, Hurvitz S, Fasolo A, Tseng LM, Jerusalem G, Wilks S, et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol. 2016;34:2115–24. doi: 10.1200/JCO.2015.63.9161. [DOI] [PubMed] [Google Scholar]
- 13.Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020;31:377–86. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogino S, Nosho K, Kirkner GJ, Shima K, Irahara N, Kure S, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477–84. doi: 10.1200/JCO.2008.18.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 18.Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2012;23:1518–25. doi: 10.1093/annonc/mdr464. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Liu Z, Deng D, Tan A, Liao M, Mo Z, et al. Anti-epidermal growth factor receptor monoclonal antibody-based therapy for metastatic colorectal cancer: a meta-analysis of the effect of PIK3CA mutations in KRAS wild-type patients. Arch Med Sci. 2014;10:1–9. doi: 10.5114/aoms.2014.40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croessmann S, Sheehan JH, Lee KM, Sliwoski G, He J, Nagy R, et al. PIK3CA C2 domain deletions hyperactivate phosphoinositide 3-kinase (PI3K), generate oncogene dependence, and are exquisitely sensitive to PI3Kalpha inhibitors. Clin Cancer Res. 2018;24:1426–35. doi: 10.1158/1078-0432.CCR-17-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:811–21. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuylsteke P, Huizing M, Petrakova K, Roylance R, Laing R, Chan S, et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann Oncol. 2016;27:2059–66. doi: 10.1093/annonc/mdw320. [DOI] [PubMed] [Google Scholar]
- 25.Matulonis U, Vergote I, Backes F, Martin LP, McMeekin S, Birrer M, et al. Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecol Oncol. 2015;136:246–53. doi: 10.1016/j.ygyno.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Levy B, Spira A, Becker D, Evans T, Schnadig I, Camidge DR, et al. A randomized, phase 2 trial of Docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic non-small-cell lung cancer. J Thorac Oncol. 2014;9:1031–5. doi: 10.1097/JTO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 27.Jimeno A, Bauman JE, Weissman C, Adkins D, Schnadig I, Beauregard P, et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Oral Oncol. 2015;51:383–8. doi: 10.1016/j.oraloncology.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Leo A, Johnston S, Lee KS, Ciruelos E, Lonning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–16. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin M, Chan A, Dirix L, O’Shaughnessy J, Hegg R, Manikhas A, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4) Ann Oncol. 2017;28:313–20. doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 31.Soulieres D, Faivre S, Mesia R, Remenar E, Li SH, Karpenko A, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18:323–35. doi: 10.1016/S1470-2045(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 32.Damodaran S, Zhao F, Deming DA, Mitchell EP, Wright JJ, Gray RJ, et al. Phase II study of copanlisib in patients with tumors with PIK3CA mutations: results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol Z1F. J Clin Oncol. 2022:40:1552–61. 10.1200/JCO.21.01648 [DOI] [PMC free article] [PubMed]
- 33.Narayan P, Prowell TM, Gao JJ, Fernandes LL, Li E, Jiang X, et al. FDA approval summary: alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin Cancer Res. 2021;27:1842–9. doi: 10.1158/1078-0432.CCR-20-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toska E, Castel P, Chhangawala S, Arruabarrena-Aristorena A, Chan C, Hristidis VC, et al. PI3K inhibition activates SGK1 via a feedback loop to promote chromatin-based regulation of ER-dependent gene expression. Cell Rep. 2019;27:294.e5–306.e5. doi: 10.1016/j.celrep.2019.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets M, et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science. 2017;355:1324–30. doi: 10.1126/science.aah6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rugo HS, Lerebours F, Ciruelos E, Drullinsky P, Ruiz-Borrego M, Neven P, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–98. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Liu J, Zhao H, Zhang Y, Liu Q, Zou B, et al. 33O A phase I study to evaluate safety, pharmacokinetics (PK), and preliminary efficacy of CYH33, a phosphatidylinositol 3-kinase α (PI3Kα) inhibitor, in patients (pts) with advanced solid tumours. Ann Oncol. 2021;32:S14. doi: 10.1016/j.annonc.2021.01.048. [DOI] [Google Scholar]
- 39.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–13. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedard PL, Davies MA, Kopetz S, Juric D, Shapiro GI, Luke JJ, et al. First-in-human trial of the PI3Kbeta-selective inhibitor SAR260301 in patients with advanced solid tumors. Cancer. 2018;124:315–24. doi: 10.1002/cncr.31044. [DOI] [PubMed] [Google Scholar]
- 41.Jung M, Kim C, Kim H, Lee C, Lee H, Bae W, et al. SO-10 An open-label, multi-centre, phase Ib/II study of PI3Kβ selective inhibitor GSK2636771 administered in combination with paclitaxel in patients with advanced gastric cancer having alterations in PI3K/Akt pathway. Ann Oncol. 2021;32:S206. doi: 10.1016/j.annonc.2021.05.034. [DOI] [Google Scholar]
- 42.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton E, Lee A, Swart R, Newton G, O’Connell B, Roberts J, et al. Abstract PS11-32: Mario-3 phase II study safety run-in evaluating a novel triplet combination of eganelisib (formerly IPI-549), atezolizumab (atezo), and nab-paclitaxel (nab-pac) as first-line (1L) therapy for locally advanced or metastatic triple-negative breast cancer (TNBC) Cancer Res. 2021;81:PS11-32–PS11-32. doi: 10.1158/1538-7445.Sabcs20-ps11-32. [DOI] [Google Scholar]
- 44.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juric D, Krop I, Ramanathan RK, Wilson TR, Ware JA, Sanabria Bohorquez SM, et al. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017;7:704–15. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dent S, Cortes J, Im YH, Dieras V, Harbeck N, Krop IE, et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Ann Oncol. 2021;32:197–207. doi: 10.1016/j.annonc.2020.10.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasan N, Razavi P, Johnson JL, Shao H, Reznik E, Smith ML, et al. Double PIK3CA mutations in cis enhance PI3Kα oncogene activation and sensitivity to PI3Kα inhibitors in breast cancer. Ann Oncol. 2019;30:iii1. doi: 10.1093/annonc/mdz095. [DOI] [Google Scholar]
- 48.Buckbinder L, St. Jean DJ, Tieu T, Wang W, Kryukov G, Jonsson P, et al. Abstract LB194: discovery and characterization of a mutant selective PI3Kα H1047X inhibitor with a best-in-class profile. Cancer Res. 2022;82:LB194. doi: 10.1158/1538-7445.AM2022-LB194. [DOI] [Google Scholar]
- 49.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 50.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 51.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–77. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423–33. doi: 10.1200/JCO.19.00368. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398:131–42. doi: 10.1016/S0140-6736(21)00580-8. [DOI] [PubMed] [Google Scholar]
- 55.Razavi P, Dickler MN, Shah PD, Toy W, Brown DN, Won HH, et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat Cancer. 2020;1:382–93. doi: 10.1038/s43018-020-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518:240–4. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curigliano G, Martin M, Jhaveri K, Beck JT, Tortora G, Fazio N, et al. Alpelisib in combination with everolimus +/− exemestane in solid tumours: phase Ib randomised, open-label, multicentre study. Eur J Cancer. 2021;151:49–62. doi: 10.1016/j.ejca.2021.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Hyman DM, Tran B, Paz-Ares L, Machiels JP, Schellens JH, Bedard PL, et al. Combined PIK3CA and FGFR inhibition with alpelisib and infigratinib in patients with PIK3CA-mutant solid tumors, with or without FGFR alterations. JCO Precis Oncol. 2019;3:1–13. doi: 10.1200/PO.19.00221. [DOI] [PubMed] [Google Scholar]
- 59.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, Jang H, Nussinov R. PI3K inhibitors: review and new strategies. Chem Sci. 2020;11:5855–65. doi: 10.1039/d0sc01676d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Folgiero V, Di Carlo SE, Bon G, Spugnini EP, Di Benedetto A, Germoni S, et al. Inhibition of p85, the non-catalytic subunit of phosphatidylinositol 3-kinase, exerts potent antitumor activity in human breast cancer cells. Cell Death Dis. 2012;3:e440. doi: 10.1038/cddis.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sasso JM, Tenchov R, Wang D, Johnson LS, Wang X, Zhou QA. Molecular glues: the adhesive connecting targeted protein degradation to the clinic. Biochemistry. 2022. 10.1021/acs.biochem.2c00245 [DOI] [PMC free article] [PubMed]
- 63.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
chemical structures and preclinical data
Data Availability Statement
Not applicable.