Abstract
Agrichemicals such as organophosphorus pesticides’ metabolites (OPPMs) are more hazardous and pervasive than their parent pesticides. Parental germline exposure to such xenobiotics leads to an elevated susceptibility towards reproductive failures e.g. sub- or in-fertility. This study sought to examine the effects of low-dose, acute OPPM exposure on mammalian sperm function using buffalo as the model organism. The buffalo spermatozoa were briefly (2 h) exposed to metabolites of the three most prevalent organophosphorus pesticides (OPPs) viz. Omethoate (from Dimethoate), paraoxon-methyl (from methyl/ethyl parathion) and 3, 5, 6-trichloro-2-pyridinol (from chlorpyrifos). Exposure to OPPMs resulted in compromised structural and functional integrity (dose-dependent) of the buffalo spermatozoa typified by elevated membrane damage, increased lipid peroxidation, precocious capacitation and tyrosine phosphorylation, perturbed mitochondrial activity and function and (P < 0.05). This led to a decline in the in vitro fertilizing ability (P < 0.01) of the exposed spermatozoa, as indicated by reduced cleavage and blastocyst formation rates. Preliminary data indicate that acute exposure to OPPMs, akin to their parent pesticides, induces biomolecular and physiological changes in spermatozoa that compromise their health and function ultimately affecting their fertility. This is the first study demonstrating the in vitro spermatotoxic effects of multiple OPPMs on male gamete functional integrity.
Subject terms: Cellular imaging, Mechanism of action
Introduction
OPPs are amongst the most widely used synthetic pesticides around the globe. Despite being banned or restricted in many developed nations, their exposure is still prevalent, particularly in developing economies across the globe1–3. Consequently, a large fraction of the human and animal population is exposed to such pesticides rendering these living organisms vulnerable to various reproductive hazards4–7. Most pesticides including the OPPs contain at least one agent/metabolite that affects any of the reproductive or developmental endpoints in multiple mammalian species including humans8–10. The OPPs and their metabolites (OPPMs) are known mammalian male reproductive toxicants that damage the DNA of the spermatozoa, alter testicular-somatic cells’ function and adversely affect the semen quality (genotoxic and teratogenic effects)11–13.
The metabolites produced after the biotransformation of pesticides are considered more hazardous than their parent pesticides and they often persist, pervade the environment, and interact with various organisms for decades after exposure leading to widespread body burdens14–17. For example, the OPP, Dimethoate, O, O-Dimethyl S-[2-(methylamino)-2-oxoethyl] phosphorodithioate, a systemic and contact OPP insecticide and its metabolite, Omethoate, O, O-Dimethyl S-[2-(methylamino)-2-oxoethyl] phosphorothioate reportedly persist in the reproductive organs of mice and rats for weeks after exposure18–20. Omethoate is the toxic metabolite of dimethoate that has been implicated in pesticide toxicity in insects and mammals21,22. Epidemiological data and experimental studies indicate a correlation between exposure to OPPs and perturbed sperm functional parameters (SFPs) and a decline in fertility23–25. Spermatozoa are particularly vulnerable to the adverse effects of various agrichemical exposures and insults. The near lack of apoptotic mechanisms; repair, proofreading and protective enzymes; exceptionally high surface area/volume ratio (> 50:1) and a considerable proportion of unsaturated fatty acids (PUFAs) in membrane render them comparatively more susceptible to environmental insults vis-à-vis the female gamete and other somatic cells26–29. Besides, the male-specific traits e.g., the higher abundance of androgen receptors which can interact with several pesticides, altered testicular pathology, a decline in sperm count and reduction in sperm motility are also implicated in the elevated severity observed in male reproductive function30–34.
Notably, under the Indian grazing systems the livestock animals are reared as per the semi-intensive farming paradigm wherein the animals (e.g. buffaloes) are let out for grazing in open fields rather than being stall-fed35–39. This greatly increases their chances of exposure to agrichemicals such as OPPs. We chose buffalo as a model for this study because it is a primary dairy animal in South and Middle Eastern Asian countries40. A crucial milch and meat species, more people depend on buffalo for their livelihood than on any other livestock animal40. Nevertheless, it suffers from numerous reproductive constraints e.g., low conception rates (sub-fecundity) despite producing morphologically normal spermatozoa (idiopathic causes).
The anti-androgenic effects and the alterations in the reproductive enzyme pathways upon acute or chronic OPP exposure are known to negatively influence the mammalian spermatozoa quality and function apart from causing pernicious effects in the offspring41–44. Consequently, interest in defined, low-dose, acute or chronic pesticide exposure on the male reproductive system has recently increased apparently because of the lack of a consented definition of the chronic or low-level exposures in many nations, which makes it difficult to decide the class of the exposure23,44–46. Nonetheless, only a limited number of studies have investigated the effect of acute, in vitro OPPM exposure on livestock sperm health, function and fertility. Against this background, this work aimed to investigate the effects of low-dose, acute exposure to three OPP metabolites viz. omethoate (from dimethoate), paraoxon-methyl (from methyl parathion) and 3, 5, 6-trichloro-2-pyridinol or TCPy (from chlorpyrifos) on mammalian sperm function following exposure for 2 h at 38 °C. We used the buffalo sperm as the model and tested a continuum of doses ranging from 0.5 to 20 µM, based on previous in vitro OPP reproductive toxicology studies since the mammalian spermatozoa from larger animals reportedly serve as the best potential alternative to the use of live mammalian model organisms47–49.
Results
The mean representative motility of the control, vehicle (DMSO) control and the OPPM exposed spermatozoa are shown in Supplementary Fig. 1. No changes in the motility of the buffalo spermatozoa upon exposure to DMSO or at low doses (< 5 μM) were observed (Supplementary Fig. 1).
Sperm function parameters (SFPs)
Membrane integrity assessment
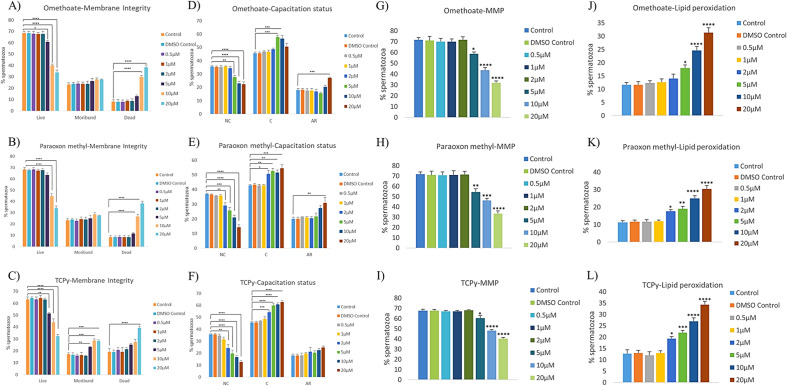
Membrane integrity is a crucial morphological/structural criterion for a spermatozoon to traverse the FRT and fertilize the oocyte. The CFDA-PI dual staining discerned three spermatozoa populations viz. live, dead and moribund (dying sperm, ROS producers) spermatozoa based on their fluorescence patterns which are indicative of the functional or compromised membrane integrity of mammalian sperm (Supplementary Fig. 2A). The structural integrity of the buffalo spermatozoa plasma membrane appeared to be compromised in a dose-dependent manner upon incubation with omethoate (Fig. 1A), paraoxon methyl (Fig. 1B), and TCPy (Fig. 1C).
Figure 1.
Sperm Functional Parameters. The buffalo spermatozoa were exposed to 0.5, 1, 2, 5, 10 and 20 μM of Omethoate, Paraoxon methyl, TCPy for the assessment of membrane integrity (A–C), capacitation status (D–F), mitochondrial membrane potential (G–I) and lipid peroxidation (J–L) using fluorescent staining. CFDA with PI (see text) was used to assess the membrane integrity of the spermatozoa that were categorized as live, dead or moribund. CTC (see text) was used to categorize the spermatozoa as non-capacitated (NC), capacitated (C), or acrosome-reacted (AR). JC-1 (see text) was used to calculate the percentage of spermatozoa with high and low mitochondrial membrane potential while BODIPY (see text) was used to distinguish the spermatozoa with high and low lipid peroxidation (LPO).
Capacitation status
Capacitation is an imperative process that a spermatozoon must go through before attaining the ability to fertilize an egg. Since the membrane dynamics undergo dramatic changes during capacitation, the spermatozoa have a limited shelf life thereafter and as expected, premature capacitation would negatively affect fertility. Chlortetracycline (CTC), which is used to detect Ca2+-related changes in the intracellular calcium re-distribution in the sperm head, particularly during capacitation, discerned three distinct fluorescent patterns. These were classified as non-capacitated (NC), capacitated (C) and acrosome-reacted (AR) in the control group and the OPPM exposed spermatozoa (Fig. 1D–F and Supplementary Fig. 2B). The exposure to OPPMs induced precocious capacitation in buffalo spermatozoa which increased linearly with concentration upon incubation with omethoate (Fig. 1D), paraoxon methyl (Fig. 1E), and TCPy (Fig. 1F).
Mitochondria membrane potential
The mitochondria membrane potential (MMP) is an indicator of health since the mitochondrion is an important source of ATP. The JC-1 dye was used for the assessment of buffalo spermatozoal MMP post-exposure to OPPMs. It renders the mitochondria with high MMP as fluorescing bright green whereas the mitochondria with low membrane potential produced a dull fluorescence after labelling (Supplementary Fig. 2C). The MMP of buffalo spermatozoa diminished significantly upon incubation with OPPMs. Consequently, a dose-dependent decline in the percentage of spermatozoa with a high mitochondrial membrane potential upon incubation with omethoate (Fig. 1G), paraoxon methyl (Fig. 1H), and TCPy (Fig. 1I) was observed.
Lipid peroxidation
Lipid peroxidation has been implicated in the aetiology of defective spermatozoa (with high ROS) function in many mammalian species. The fluorophore, BODIPY ((4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene)) can incorporate into the spermatozoa and undergo a spectral emission shift upon interacting with reactive oxygen metabolites, indicating their oxidative stress. The incubation of buffalo spermatozoa with omethoate (Fig. 1J), paraoxon methyl (Fig. 1K), and TCPy (Fig. 1L) induced lipid peroxidation especially in the mid-piece as observed by BODIPY staining (Supplementary Fig. 2D). However, it was less evident in the sperm head or the rest of the sperm tail (Supplementary Fig. 2D).
Protein tyrosine phosphorylation
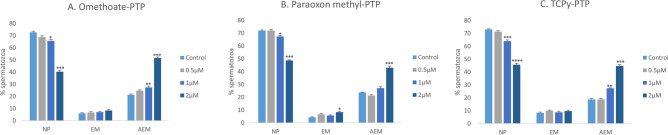
Exposure to OPPs/OPPMs may cause multiple asymptomatic effects at comparatively lower exposures rather than overt signs and symptoms50. We report similar subclinical toxic effects, particularly at doses ≤ 5 µM. Although these doses did not affect the sperm morphology, mass motility or any functional parameters (except capacitation), nonetheless, low doses (1 µM) appeared to negatively affect the phosphorylation status of the protein tyrosine residues present on the cell surface (Fig. 2 and Supplementary Fig. 3). Therefore, only the OPPM concentrations ≤ 5 µM were used in the subsequent experiments. A higher abundance of non-phosphorylated (NP) spermatozoa vis-à-vis the phosphorylated spermatozoa (EM and AEM patterns) was observed in the control and the OPPM treated samples (Fig. 2A–C and Supplementary Fig. 3). Notably, the localization of phosphorylated proteins on the sperm mid-piece always coincided with that of the equatorial region. The incubation with OPPMs appeared to induce the phosphorylation of tyrosine residues (a conserved feature of mammalian sperm capacitation) in the sperm proteins51 thus causing a reduction in the fraction of NP spermatozoa (Fig. 2).
Figure 2.
Protein tyrosine phosphorylation. Three major fluorescent patterns viz. non-fluorescent spermatozoa i.e. non-phosphorylated (NP), sperm bearing signal at equatorial region and mid-piece (EM) and spermatozoa with a signal at the acrosomal region, equatorial region and mid-piece (AEM).were observed during immunocytochemistry using a monoclonal anti-phosphotyrosine antibody (P1869; Sigma). The buffalo spermatozoa were exposed to 0.5, 1, and 2 μM of OPPMs viz. Omethoate (A), Paraoxon methyl (B), and TCPy (C) for 2 h.
Expression dynamics of metabolic genes and oxidative stress-related genes
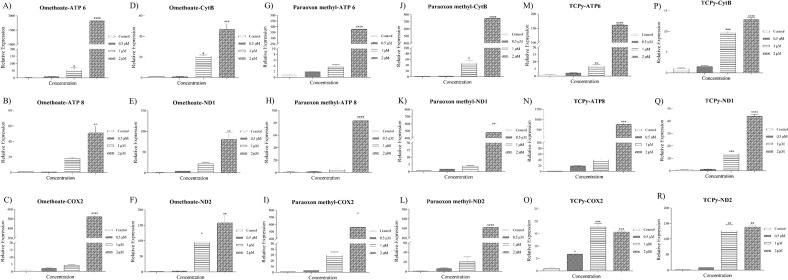
The relative expression profiles of the selected panel of metabolic genes (ATP 6, ATP 8, COX 2, CYT B, ND1 and ND2) from the mitochondrial genome were generated using RT-qPCR in the spermatozoa treated with OPPMs (Fig. 3). The exposure to OPPMs viz. Omethoate (Fig. 3A–F), paraoxon methyl (Fig. 3G–L), and TCPy (Fig. 3M–R) induced the expression of mitochondrial metabolic genes.
Figure 3.
Pattern of expression of metabolic and oxidative stress-related genes. Relative expression profiles of the mitochondrial metabolic genes viz. ATP6, ATP8, COX-2, CytB, ND-1 and ND-2 for the control group and the buffalo spermatozoa exposed to 0.5, 1, and 2 μM of Omethoate (A–F), Paraoxon methyl (G–L), and TCPy (M–R) were generated using RT-qPCR. The expression values are normalized to GAPDH and β-actin, and the error bars represent the standard error of the mean (SEM).
Computer-assisted sperm analysis (CASA)
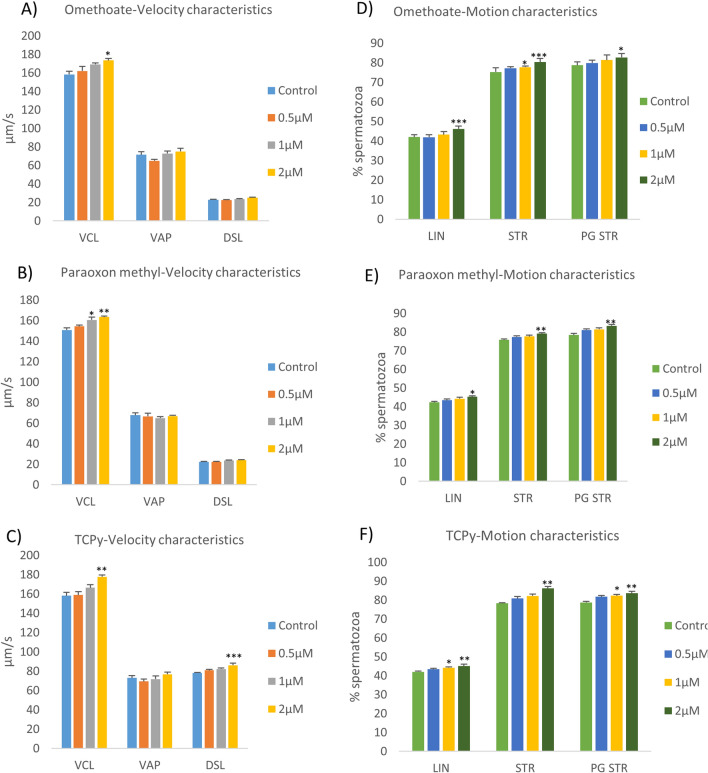
The buffalo sperm kinematic parameters were found to be significantly perturbed upon exposure to OPPMs (Fig. 4 and Supplementary Fig. 4). It is worth mentioning that although the lower doses of OPPMs didn’t affect the motility of the buffalo spermatozoa per se, their motion and velocity characteristics were however, altered upon exposure to Omethoate (Fig. 4A,D),Pparaoxon methyl (Fig. 4B,E), and TCPy (Fig. 4C,F). None of the OPPMs affected the lateral head displacement (Supplementary Fig. 4A–C) while only the Omethoate exposure significantly affected (P < 0.01) the beat cross frequency of the buffalo spermatozoa at 2 μM concentration (Supplementary Fig. 4D–F).
Figure 4.
Kinematics of buffalo spermatozoa. The velocity characteristics viz. curvilinear velocity (VCL), average path velocity (VAP) and (DSL) (A–C) and the motion characteristics viz. the percentage of linearity (LIN), the straightness coefficient (STR) and progressive STR (PGSTR) (D–F) were evaluated in the buffalo spermatozoa exposed to 0.5, 1, and 2 μM of Omethoate (A, D), Paraoxon methyl (B, E), TCPy (C, F) by Computer-assisted sperm analyzer (IVOS12.1, Hamilton-Thorne Biosciences, Beverly, MA, USA).
In vitro fertilization (IVF)
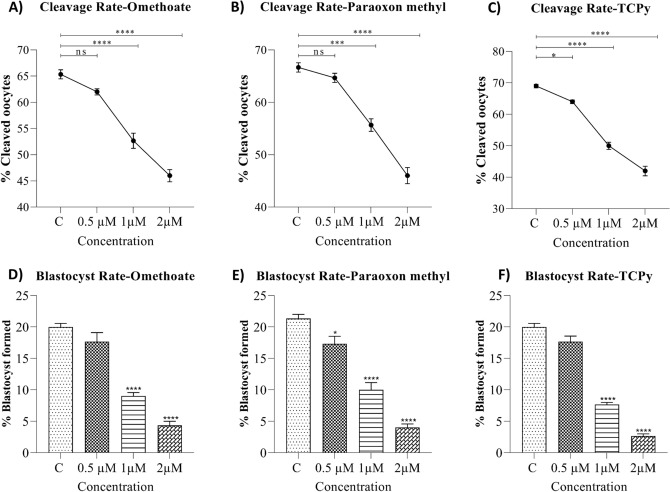
The incubation of the semen sample with pesticide metabolites hindered the fertilization in a dose-dependent manner (Fig. 5). The rate of oocyte cleavage (Fig. 5A–C) and formation of the blastocyst (Fig. 5D,E) declined significantly upon exposure to Omethoate (Fig. 5A,D), Paraoxon methyl (Fig. 5B,E), and TCPy (Fig. 5C,F) indicating detrimental effects of OPPM exposure on the fertilizing ability of the buffalo spermatozoa.
Figure 5.
In vitro fertilization. The mean ± SEM for cleavage rate (A–C), and blastocyst formation rates (D–F) in the control group and buffalo spermatozoa exposed to 0.5, 1, and 2 μM of OPPM viz. Omethoate (A, D), Paraoxon methyl (B, E) and TCPy (C, F).
Discussion
This study was undertaken to assess the effects of acute exposure to three OPPMs viz. Omethoate Paraoxon-methyl, and TCPy on the structural and functional integrity of the bubaline spermatozoa. The OPPs and their metabolites (e.g. oxons) are known to persist in the environment, interact with various organisms and pervade long after the withdrawal of exposure. This not only affects the metabolic, physiologic and reproductive health of the exposed parent but also causes pernicious effects in the forthcoming generations. Surprisingly, most of the reproductive toxicological studies on OPPs/OPPMs have focused either on their roles as endocrine-disrupting chemicals (EDCs) or the epigenetic changes induced upon their exposure. The effects of low-dose, acute exposure to OPPMs on mammalian male gamete have not been studied in detail. Our results indicated that acute exposure to OPP metabolites viz. Omethoate, Paraoxon-methyl, and TCPy compromised bubaline spermatozoa function in a dose-dependent manner. The OPPM exposure resulted in impairment of sperm structural and functional integrity, perturbation in kinematic parameters, altered gene expression and decreased fertilizing ability indicating the toxicological implications of acute, low-dose pesticide metabolite exposure (Fig. 6).
Figure 6.
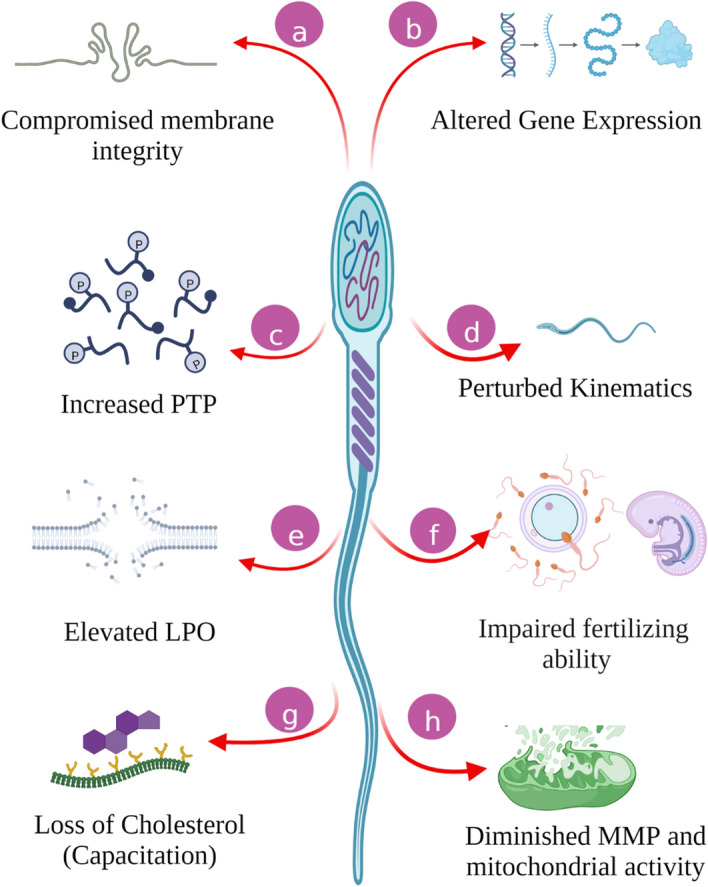
The overall effect of low-dose acute exposure of OPPMs on buffalo spermatozoa. The buffalo sperm were incubated with 0.5, 1, and 2 μM of Omethoate, Paraoxon methyl, TCPy for 2 h which compromised the structural and functional integrity of the exposed sperm as discussed in the text. PTP protein tyrosine phosphorylation, LPO lipid peroxidation, MMP mitochondria membrane potential.
Exposure to pesticides and their metabolites (OPPMs) are known to induce deleterious (cytotoxic) effects on the reproductive system of animals through distinct physiological mechanisms, components and pathways and are thus associated with deteriorated sperm quality38,52–55. For example, acute exposure (1–3 h) to Roundup (and its major component Glyphosate) has recently been reported to negatively impact the male gametes causing a reduction in sperm viability, mitochondrial activity, motility and acrosome integrity in a dose-dependent manner55. Notably, most of the studies on pesticide exposure have reported adverse impacts on one or more SFPs e.g., membrane integrity, MMP, capacitation and motility54,57,58. Many of these parameters are correlated with fertility and have been proposed as fertility biomarkers in addition to their reported associations with the life expectancy of the offspring59–62. We also observed a rise in the percentage of spermatozoa with impaired SFPs upon exposure to OPPMs (Figs. 1 and 6), particularly at higher doses (≥ 5 μM). The presence of pesticide residues or their metabolites (OPPMs) in the fluids surrounding spermatozoa exerts negative influences on the spermatozoa function (impairing survival and fertility) and is also implicated in developmental malformations or defects in the fetus/offspring39,54,63,64. Several studies across multiple mammalian species have focused on the effects and putative mechanisms of action of (organophosphorus) pesticides on the male reproductive system7,41,57,65. For instance, both in vivo (via oral gavage) and in vitro exposure to Chlorpyrifos have been shown to interfere with male reproductive functions leading to reduced fertility in mammals3,66. Our results agree with the aforementioned studies that exposure to these xenobiotics potentiates the perturbation of functional parameters of mammalian spermatozoa e.g., mitochondrial function and activity (Fig. 1G–I). The oxidative stress associated with pesticide exposure is known to trigger mitochondrial deficiency among other cytotoxic and genotoxic effects67,68. The alteration in the mitochondrial activity and function results in the manifestation of oxidative stress thus creating a vicious cycle and is itself entwined in it (discussed below). The buffalo spermatozoa have been reported to contain an elevated amount of unsaturated fatty acids (PUFA) vis-à-vis other bovids, which renders them vulnerable to oxidative stress-induced damage26–29,69. We observed a reduced MMP (Fig. 1G–I) along with a concomitant rise in lipid peroxidation (LPO) of bubaline spermatozoa (Fig. 1J–L) which has been demonstrated to be highly associated with Murrah buffalo bull fertility70. The exposure of mammalian spermatozoa to OPPs/OPPMs e.g., Methyl parathion or its metabolite, Methyl paraoxon (one of the most potent insecticides) reportedly caused oxidative stress through elevated LPO, as assessed by malondialdehyde production at 7 and 28 days post-treatment (dpt) in male mice31. A different OPP, Dimethoate (along with Chlorpyrifos) is amongst the most frequently used OPPs, especially in developing economies, as mentioned previously3,71. As expected, the exposure to Dimethoate (through oral gavage) has been reported to affect the reproductive performance of male mice leading to a decline in sperm motility, the fraction of live spermatozoa, hormone levels and alterations (degenerative changes) in testicular histology19,20. The Chlorpyrifos metabolite, TCPy and the Dimethoate metabolite, Omethoate are reportedly more toxic than their parent pesticides13,18,21,22. Overall, acute exposure to OPPMs negatively impacted the SFPs which are considered biomarkers of mammalian sperm fertility.
It is worth mentioning that capacitation was the only functional parameter that was affected at low OPPM exposure (2 µM, P < 0.01) to buffalo sperm (Fig. 1D–F). Neither the sperm mass motility nor any other SFP was significantly altered at 2 µM OPPM exposure (Supplementary Fig. 1). It has often been observed that such subclinical effects may appear to be subtle but can nonetheless, cause chronic illness through functional alterations in diverse biological processes50. The considerable rise in precociously capacitated sperm at low OPPM (Paraoxon Methyl and TCPy) exposure (2 µM) intrigued us to assess similar, capacitation-associated functional alterations e.g., PTP status and buffalo sperm kinematics. The acute exposure of OPPMs induced protein tyrosine phosphorylation (PTP) in the buffalo spermatozoa even at low doses (1 µM). PTP is an important intracellular mechanism that is implicated in many cellular processes e.g., regulating sperm functions. A rise in the tyrosine protein phosphorylation is considered a robust indicator of capacitation, in several species including humans72, rodents73, pigs74, and bovids including buffalo75,76. Most OPPs are well-known phosphorylating agents and our results indicate that the same is true for their metabolites (OPPMs) that induced dose-dependent tyrosine phosphorylation in buffalo spermatozoa (Fig. 2). Our observations are in agreement with these studies and are underpinned by the observed rise in the number of precociously capacitated spermatozoa51,72–76. Nevertheless, whether this rise in phosphorylation was genotoxic was not assessed in this study.
Exposure of mammalian spermatozoa to the OPPs has also been demonstrated to perturb many of the sperm kinematic parameters e.g., chlorpyrifos exposure to buffalo spermatozoa reportedly results in a reduction in straight line velocity (STR) and average path velocity (VAP)37. Most of these parameters are not perceptible by the naked eye, however, exhibit sensitivity to various reproductive toxicants such as OPPs77. Many of the velocity and motion characteristics of the buffalo sperm were found to be altered upon OPPM exposure, apparently in response to increased capacitation (Figs. 4 and 1D–F). Many of these characteristics are crucial to the fertilizing ability of the mammalian spermatozoa, for instance, the straight line velocity (STR) has been proposed to play crucial roles in sperm transport through the FRT and oocyte-penetration77. Largely, the results of the CASA experiments indicated perturbed motility and velocity parameters upon OPPM exposure that can potentially affect their reproductive function akin to the OPPs35,38. Interestingly, the observed alterations in motility-related spermatozoal kinematics parameters have been ascribed to the elevated mitochondrial gene activity affecting ATP production, which results in precocious capacitation77,78. The motility patterns, which are very crucial for the fertilizing ability of mammalian spermatozoa are dependent on energy sources79,80. Therefore, we selected a panel of mitochondrial metabolic genes to assess if exposure to OPPMs affects mitochondrial activity. We observed an elevated expression of genes involved in mitochondrial bioenergetics upon acute OPPM exposure (Fig. 3). Mitochondrial oxidative phosphorylation (OXPHOS) and glycolysis are the two main pathways to generate ATP79,80 and metabolic mitochondrial genes such as ATP6, ATP8 and the NADPH dehydrogenase subunits (ND1, ND2, and ND4) are involved in ATP formation81,82. The induction of the OXPHOS pathway is known to produce a large amount of ATP in sperm that in turn induces sperm capacitation and acrosome reaction, as observed in this study79–84. As mentioned earlier, the altered mitochondrial function and impaired structural, functional and motility parameters are expected to cause a considerable decline in the fertilizing ability of the spermatozoa e.g. in IVF83,84. In agreement with these studies, we observed a significant reduction in the in vitro fertilizing ability of spermatozoa upon low OPPM exposure (0.5 μM, TCPy) (Fig. 5). A rise in the precocious capacitation upon exposure to OPPMs could be implicated in their impaired fertilizing ability since it was the only SFP affected at dosage ≤ 5 μM82, Furthermore, it is worth mentioning that the paternal seminal fluid is capable of influencing the developmental programming effects in the progeny and dictating changes in the uterine luminal components85. Such exposures can lead to the impairment of various biochemical pathways and high ROS production resulting in low-quality embryos that consequently leads to poor clinical outcomes in IVF programs37,84,86,87. A growing body of evidence indicates that prenatal exposure to environmental xenobiotics e.g., OPPMs can adversely affect fertility, in utero and post-natal development and may have multigenerational effects as addressed under the novel, E-DOHaD (Environmentally-induced Developmental Origins of Health and Disease) model of trans- or inter-generational inheritance17,38,88,89. For instance, men exposed to pesticide residues had children with an elevated risk of male reproductive developmental disorders including birth defects such as cryptorchidism, hypospadias, reduced fertility, and stunted growth and development leading to clinical manifestations45,90–92. Hence, it would be interesting to assess the effects of OPPM exposure at different embryonic cell stages and post-natal stages in the offspring through adulthood.
There are certain caveats and limitations of this study that are worth discussing. For example, the epigenetic changes and genotoxicity of the OPPM exposure were not ascertained. Besides, a study is warranted to understand the effects of cumulative, minimal dosage, acute and chronic exposure on male reproductive physiology. This is because the mechanisms underlying chronic and acute exposure have been proposed to be distinct. For example, in rodents, the mechanisms behind the reduction of fertilizing ability of spermatozoa at 7- and 28-days post-treatment (dpt) of methyl parathion have been ascribed to the sperm surface remodelling events and acrosomal defects, respectively31,84. We had chosen OPPM dosage based on previous reproductive toxicology studies wherein a huge variation in the selected concentrations (0.005 μg/mL to 750 μM) was observed37,38,41. Importantly, the assessment of pesticide concentration in representative semen samples (as reported in food products and air samples) would additionally help employ the bio-available equivalent doses for similar studies92–99. Overall, the spermatotoxic effects of three OPPMs used in this study appear to share the mechanisms of toxicity as reported for various their parental OPPs100,101. The dose-dependent rise in PTP, LPO, and OXPHOS gene expression indicated the involvement of regulatory protein modifications (phosphorylation) and mitochondrial function in mediating the reproductive toxicity (decreased fertility) of OPPMs.
Conclusion
Exposure to environmental pollutants such as OPPs and their metabolites (OPPMs) appears to be one of the preponderant, previously unidentified pathological factors to be associated with idiopathic male infertility102,103. By using the mammalian (Bubaline) spermatozoa as a model, our results revealed that the transient exposure to OPPMs viz. Paraoxon-methyl, Pmethoate and TCPy at low doses (0.5–2 μM) detrimentally affects the functions of the male gamete. Acute exposure to OPPMs resulted in elevated membrane damage, perturbed mitochondrial function, and increased phosphorylation leading to precocious capacitation and impaired fertility. Nevertheless, caution should be exercised while extrapolating the results of these in vitro models to in vivo studies. Moreover, since most couples often share lifestyle habits and diet choices, occupy the same niche, and together transmit the molecular memory of their past environmental experience to their offspring (equal genetic contribution) both parents should be considered in future reproductive toxicity studies. Notably, a male exposed to pesticides (bodily fluids) may also render a female’s uterine environment susceptible to exposure during coitus104,105.
Methods
Reagents All chemicals, media and reagents including the commercial formulation of OPPMs used in this study were procured from Sigma-Aldrich Chemical Co. Ltd, (USA) unless stated otherwise. All plasticware was procured from Nunc Inc. (ThermoScientific, USA). The OPPM formulations were diluted with dimethyl sulfoxide (DMSO).
Sample collection and processing
Frozen semen straws of Murrah buffalo bulls (N = 9) were procured from the Artificial Breeding Research Centre (ABRC), ICAR-NDRI, India. The straws were thawed by immersing them in a water bath at 38 °C for 30 s. The contents were collected into 15 mL centrifugation tubes containing 2 mL working Sp-TALP medium (1 mM 60% Na-lactate and 0.98 mM Na-Pyruvate in 2X filtered stock mixed with an equal amount of Mili-Q water) (Supplementary sheet—Methods: Table 1). The spermatozoa were separated from the seminal plasma and extender components by centrifuging at 280×g for 6 min (thrice) in the working Sp-TALP medium. The supernatant was discarded each time and the sperm pellet obtained after the final wash was subject to the swim-up technique. The obtained fraction containing the motile spermatozoa was suspended in 250 μL working-NCM (non-capacitating, Sp-TALP medium). Since the semen straws were procured from a commercial government-funded farm that operates under standard conditions, specific authorization from the Ethics Committee was not required to conduct this study.
Experimental design
The motile spermatozoa (10 × 106) obtained from the previous step were incubated with different concentrations (0.5 µM, 1 µM, 2 µM, 5 µM, 10 µM, 20 µM) of three pesticide metabolites of the three most rampantly used OPPs viz. Omethoate, Paraoxon-methyl and 3,5,6-Trichloropyridinol or TCPy106 for 2 h at 37 °C in a CO2 incubator along with negative control (no pesticide metabolites) and vehicle control (DMSO only). The continuum of doses selected for this study was based on the previously reported concentrations employed in various in vitro and in vivo reproductive toxicological studies on mammalian spermatozoa survival and function37,39,41,107. Likewise, the time of incubation was decided based on previously published literature on acute toxicity, most of which have reported an incubation time between 1 and 3 h37,41,56.
Sperm function parameters (SFPs)
After incubation with OPPMs, the control and treated buffalo spermatozoa were evaluated for intactness of structural and functional integrity. The examination of sperm-membrane integrity, acrosome reaction and lipid peroxidation were done by using carboxyfluorescein diacetate-propidium iodide (CFDA-PI) and 4, 4-Difluoro-4-bora-3a, 4a-diaza-s-indacene (BODIPY) dyes, respectively, as per the method described by Singh and colleagues69. However, the assessment of mitochondrial membrane potential (MMP) by JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide) and capacitation status by Chlortetracycline (CTC), a fluorescent chelate probe of Ca2+ was done by the methods described by Saraf and co-workers108. Thereafter, the excess stains were removed by washing the stain-incubated spermatozoa with 200 µL of sperm-TALP by centrifugation at 800×g for 3 min. The pelleted spermatozoa were used to make a thin smear onto which a few drops of mounting medium, Dabco® 33-LV were placed and was observed at 1000 × magnification under an Olympus BX-51 fluorescence microscope using appropriate filters. A minimum of n = 200 spermatozoa (in triplicates) were evaluated, in a minimum of 10 fields for observing fluorescent patterns. The images of the two filters were merged to obtain the final image, wherever required.
Protein tyrosine phosphorylation (PTP) status
The protein tyrosine phosphorylation (PTP) status of the buffalo spermatozoa was assessed using an indirect immunofluorescence assay as described by Saraf et al.109. The higher doses of ≥ 10 µM of the OPPMs were omitted from further experiments (explained later). The control and the treated spermatozoa were washed with PBS at 300×g for 5 min and a 20 µL sperm suspension was then smeared onto a clean glass slide and air dried. Thereafter, the spermatozoa were fixed in 4% paraformaldehyde for 1 h at 4 °C and washed with PBS (3 ×). The spermatozoa were permeabilized using methanol and the slides were blocked using 5% BSA in PBS for 2 h at room temperature. Subsequently, the smears were incubated with monoclonal anti-phosphotyrosine antibody (P1869; Sigma, 1:100) in 1% BSA for 3 h at 37 °C and then washed with PBS (3 ×). Afterwards, the smears were incubated with FITC-conjugated anti-mouse IgG antibody produced in goat (F4018; Sigma, 1:100) and then washed thoroughly with PBS. After the final washing step, the coverslip was mounted onto a dried glass slide. A drop of mounting medium, Dabco® 33-LVwas placed on the slide and the cells were then observed under a BX-51 Olympus fluorescence microscope at 1000 × magnification using a FITC filter. The spermatozoa were assessed for the percentage of different phosphorylation patterns and a minimum of 200 spermatozoa were counted (in three technical replicates) across the slide. The three patterns of tyrosine phosphorylation (pattern NP—no fluorescence; pattern EM—fluorescence over the equatorial region and mid-piece and pattern AEM—fluorescence over the acrosomal area, equatorial region and mid-piece) were counted and expressed as percentages.
Expression dynamics of the metabolic and oxidative stress-related genes
Total RNA was isolated from different experimental groups and the control group of spermatozoa as described previously by Batra and colleagues110 using the TRI Reagent, RNA isolation reagent (Sigma-Aldrich, USA). The isolated RNA was quantified using a Nanodrop ND-1000 UV–Vis spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA). The cDNA synthesis and RT-qPCR optimization were done as described by Batra et al.110 (Supplementary sheet-Methods). The primer designing tool at NCBI, the Primer-BLAST was used to design primers for the metabolic genes (ATP 6, ATP 8, COX 2, CYT B, ND1 and ND2) and two reference genes viz. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and β-actin in the buffalo spermatozoa. Intron-spanning primers were designed, wherever possible and the self-annealing sites, mismatches and secondary structures in the primers were checked using OligoCalc111. The specificity of each set of primers was again checked using the BLAST alignment tool and in silico PCR112 was run for each set of primers before commercial synthesis (Sigma-Aldrich, USA). The MIQE113 guidelines were followed at every step, wherever possible. The relative quantification of all the genes was done on a Bio-rad CFX-96 Touch Deep Well Real-Time PCR system platform using the iTaqUniversal SYBR Green Supermix (Bio-Rad, USA) in a 10 μL reaction mix. The thermal profile was 95 °C for 5 min, 40 cycles consisting of denaturation at 95 °C for 15 s, annealing at variable optimized temperatures for 20 s, extension at 72 °C for 20 s, followed by the melt curve protocol with 10 s at 95 °C and then 60 s each at 0.5 °C increments between 65 and 95 °C. The melt curve analysis ensures a specific, unique product formation and ascertains primer dimer formation. A no-template control (NTC) was included in each plate to confirm the absence of nucleic acid contamination. The mean sample Cq (Cycle of quantification) values for the various metabolic and oxidative stress-related genes were calculated for duplicate samples and their relative expression was calculated by ΔΔCt method, as described previously114.
Sperm kinematics using computer-assisted sperm analyzer
The spermatozoa incubated with pesticide metabolites (OPPMs) were subject to computer-assisted sperm analysis (CASA) for estimating the velocity and motion parameters of the buffalo spermatozoa. Computer-assisted sperm analyzer (IVOS12.1, Hamilton-Thorne Biosciences, Beverly, MA, USA) was used to evaluate the kinetic characteristics. The motility and movement parameters like the curvilinear velocity (VCL, µm/s), linear velocity (VSL, µm/s), average path velocity (VAP, µm/s), the mean amplitude of lateral head displacement (ALH, µm), the percentage of linearity i.e. the ratio between VSL and VCL (LIN, %), the straightness coefficient which is the ratio between VSL and VAP (STR, %) and the frequency with which the actual sperm trajectory crossed the average path trajectory (BCF, Hz) were recorded in duplicates for all the experimental groups. The CASA software settings were as follows: temperature = 38 °C, frame rate = 60 Hz, frames acquired = 30, minimum contrast = 35, minimum cell size = 5 pixels, cell size = 9 pixels, cell intensity = 110 pixels, progressive cells (VAP cut-off = 50 m/s, STR cut-off = 70%), slow cells (VAP cut-off = 30/s and VSL cut-off = 15/s). The spermatozoa (N = 500) were observed in a minimum of five optical fields around the central reticulum of the chamber for sperm motility analysis.
IVF (in vitro fertilization) Study
The IVF experiments were done as previously explained by Batra et al.105. Briefly, buffalo ovaries from the slaughtered animals were transported to the laboratory in physiological saline (0.9%, w/v NaCl) containing strepto-penicillin (50 mg/L) within 2–3 h of slaughter. After washing the ovaries with normal saline, the follicular fluid was aspirated using a vacuum pump (Cook) in HEPES-buffered hamster embryo culture (HH) medium. After extensive washing with HH medium, 15 cumulus-oocyte complexes (COCs) were placed in 100 µL droplets of maturation medium (HEPES buffered TCM199 modified with 10% (v/v) fetal bovine serum, (FBS), 0.005% (w/v) streptomycin, 0.01% (w/v) sodium pyruvate and 0.005% (w/v) glutamine supplemented with 5.0 μg/mL FSH, 10 μg/mL LH, 1 μg/mL estradiol 17-β, 50 ng/mL epidermal growth factor (EGF), 64 μg/mL cysteamine and 50 μL ITS). The dishes were cultured in duplicate for 24 h at 38.5 °C in a humidified atmosphere of 5% CO2 in an incubator. The IVF (in vitro fertilization) was carried out using 50 µL of spermatozoa suspension (1 × 106/mL) from OPPM-treated samples along with a control (vehicle). In vitro fertilization (IVF) also was carried out as per the procedure described in the abovementioned study. The IVC (in vitro culture) droplets of 100 µL were prepared following the progressive removal of the media from IVM droplets which was later replaced with IVC medium (mCR2aa—modified Charles Rosenkrans medium with amino acids containing 0.8% BSA, fatty acid-free, and 50 mg/mL gentamicin). After 12 h of IVF, oocytes were denuded mechanically by brief vortexing. The oocytes were subsequently washed five times in the IVC medium and transferred to IVC droplets in groups of 15. A total of 50 µL of IVC medium was replaced with fresh medium after 48 h. The replacement medium constituted of mCR2aa supplemented with 10% (v/v) FBS, 50 mg/mL of gentamicin and 0.8% BSA (fatty acid-free). The second replacement was done 48 h after the first replacement. The cleavage rates were assessed at the time of the first medium replacement after IVC while blastocyst rates were determined 7 days post-IVC.
Statistical analysis
The differences in the various SFPs, heterogeneity in phosphorylation, differential gene expression levels and kinematic parameters and the differences in cleavage and blastocyst rates within the different dosage groups were subjected to the Shapiro–Wilk normality test while the Brown–Forsythe test was employed to test the differences in their variance (standard deviation). One-way ANOVA and Tukey’s post-hoc test were used to measure the differences in the function parameters of the spermatozoa, phosphorylation status, mitochondrial gene expression, kinematic behaviour and the cleavage and blastocyst formation rates between the different dosage groups on GraphPad Prism 8.0 (for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com). A P-value < 0.05 was considered to be statistically significant.
Supplementary Information
Acknowledgements
SC is thankful to SERB-DST for the PDF grant. The assistance of Ankit Ror, Aakash Kumar, and Seema Karanwal in the IVF study and Dr Devender Kumar Suthar (ICAR-CIRB) in CASA experiments is gratefully acknowledged.
Author contributions
The study was conceptualized and designed by S.C., A.K., R.K., and T.K.D. Sample collection, microscopy and expression analysis was done by S.C. & V.B. The IVF was performed by S.C. & A.P. R.K. & T.K.D. performed statistical analyses. R.K. and A.K. implemented and conducted CASA. The manuscript was written by V.B. & S.C. Figures were designed by V.B., T.K.D. and S.C. All the authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Bill & Melinda Gates Foundation (Grant Number OPP1154401).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Supplementary Material & Information: Supplementary sheet-Methods and Supplementary Figs. 1, 2, and 3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shivani Chhillar and Vipul Batra.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35541-6.
References
- 1.Frazier LM. Reproductive disorders associated with pesticide exposure. J. Agromed. 2007;12(1):27–37. doi: 10.1300/J096v12n01_04. [DOI] [PubMed] [Google Scholar]
- 2.Sengupta P, Banerjee R. Environmental toxins: Alarming impacts of pesticides on male fertility. Hum. Exp. Toxicol. 2014;33(10):1017–1039. doi: 10.1177/0960327113515504. [DOI] [PubMed] [Google Scholar]
- 3.Alaa-Eldin EA, El-Shafei DA, Abouhashem NS. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. Environ. Sci. Pollut. Res. Int. 2017;24(2):1532–1543. doi: 10.1007/s11356-016-7912-6. [DOI] [PubMed] [Google Scholar]
- 4.Meeker JD, Ryan L, Barr DB, Hauser R. Exposure to nonpersistent insecticides and male reproductive hormones. Epidemiology. 2006;17(1):61–68. doi: 10.1097/01.ede.0000190602.14691.70. [DOI] [PubMed] [Google Scholar]
- 5.Ahouangninou C, Martin T, Edorh P, Bio-Bangana S, Samuel O, St-Laurent L, Dion S, Fayomi B. Characterization of health and environmental risks of pesticide use in market gardening in the rural city of Tori-Bossito in Benin, West Africa. J. Environ. Protect. 2012;3(3):241–248. doi: 10.4236/jep.2012.33030. [DOI] [Google Scholar]
- 6.Ambali SF, Shittu M, Ayo JO, Esievo KA, Ojo SA. Vitamin C alleviates chronic chlorpyrifos induced alterations in serum lipids and oxidative parameters in male wistar rats. Am. J. Pharmacol. Toxicol. 2011;6(4):109–118. doi: 10.3844/ajptsp.2011.109.118. [DOI] [Google Scholar]
- 7.Dziewirska E, Radwan M, Wielgomas B, Klimowska A, Radwan P, Kałużny P, Hanke W, Słodki M, Jurewicz J. Human semen quality, sperm DNA damage, and the level of urinary concentrations of 1N and TCPY, the biomarkers of nonpersistent insecticides. Am. J. Mens Health. 2019;13(1):1557988318816598. doi: 10.1177/1557988318816598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grewal KK, Sandhu GS, Kaur R, Brar RS, Sandhu HS. Toxic impacts of cypermethrin on behavior and histology of certain tissues of albino rats. Toxicol. Int. 2010;17(2):94–98. doi: 10.4103/0971-6580.72679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kara M, Öztaş E. Reproductive toxicity of insecticides. In: Aral F, Payan-Carreira R, Quaresma M, editors. Animal Reproduction in Veterinary Medicine. IntechOpen; 2020. [Google Scholar]
- 10.Elbetieha A, Da'as SI, Khamas W, Darmani H. Evaluation of the toxic potentials of cypermethrin pesticide on some reproductive and fertility parameters in the male rats. Arch. Environ. Contam. Toxicol. 2001;41(4):522–528. doi: 10.1007/s002440010280. [DOI] [PubMed] [Google Scholar]
- 11.Bustos-Obregon E, Niels S, Jose R, John L, Pflieger-Bruss S, Henrik L. Adverse effects of exposure to agropesticides on male reproduction. J. Pathol. Microbiol. Immunol. 2001;109(S103):S233–S242. doi: 10.1111/j.1600-0463.2001.tb05772.x. [DOI] [Google Scholar]
- 12.Hossain F, Ali O, D'Souza UJ, Naing DK. Effects of pesticide use on semen quality among farmers in rural areas of Sabah, Malaysia. J. Occup. Health. 2010;52(6):353–360. doi: 10.1539/joh.l10006. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Fourth National Report in Human Exposure to Environmental Chemicals, Updated Tables. http://www.cdc.gov/expsurereport/index.html. Accessed 24 May 2022.
- 14.Bolognesi C, Merlo FD. Pesticides: Human health effects. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Elsevier; 2011. pp. 438–453. [Google Scholar]
- 15.Soliman MM, Attia HF, El-Ella GA. Genetic and histopathological alterations induced by cypermethrin in rat kidney and liver: Protection by sesame oil. Int. J. Immunopathol. Pharmacol. 2015;28(4):508–520. doi: 10.1177/0394632015575950. [DOI] [PubMed] [Google Scholar]
- 16.Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018;17:1101–1136. doi: 10.17179/excli2018-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil. Steril. 2019;112(4):613–621. doi: 10.1016/j.fertnstert.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 18.International Programme on Chemical Safety (IPCS). Environmental Health Criteria 90. Dimethoate. http://www.inchem.org/documents/ehc/ehc/ehc90.htmexternalicon4/20/13. (1989). [DOI] [PubMed]
- 19.Afifi NA, Ramadan A, El-Aziz MI, Saki EE. Influence of dimethoate on testicular and epididymal organs, testosterone plasma level and their tissue residues in rats. Dtsch. Tierarztl. Wochenschr. 1991;98(11):419–423. [PubMed] [Google Scholar]
- 20.Farag AT, El-Aswad AF, Shaaban NA. Assessment of reproductive toxicity of orally administered technical dimethoate in male mice. Reprod. Toxicol. 2007;23(2):232–238. doi: 10.1016/j.reprotox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 21.IPCS. Global Assessment of the State-of-the-Science of Endocrine Disruptors. Damstra, T., Barlow, S., Bergman, A., Kavlock, R. & Van Der Kraak, G. (Eds), http://ehp.niehs.nih.gov/who/preface.pdf. (2002).
- 22.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2021. https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf. (2021).
- 23.Perry MJ. Effects of environmental and occupational pesticide exposure on human sperm: A systematic review. Hum. Reprod. Update. 2008;14(3):233–242. doi: 10.1093/humupd/dmm039. [DOI] [PubMed] [Google Scholar]
- 24.Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, Hauser R, Chavarro JE. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum. Reprod. 2015;30(6):1342–1351. doi: 10.1093/humrep/dev064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figueroa ZI, Young HA, Mumford SL, Meeker JD, Barr DB, Gray GM, Perry MJ. Pesticide interactions and risks of sperm chromosomal abnormalities. Int. J. Hyg. Environ. Health. 2019;222(7):1021–1029. doi: 10.1016/j.ijheh.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson L. Pesticide perturbation of sperm cell function. Bull. Environ. Contam. Toxicol. 1990;45(6):876–882. doi: 10.1007/BF01701087. [DOI] [PubMed] [Google Scholar]
- 27.Aitken RJ, Koopman P, Lewis SE. Seeds of concern. Nature. 2004;432(7013):48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 28.Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017;84(10):1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- 29.Sebastian R, Raghavan SC. Exposure to Endosulfan can result in male infertility due to testicular atrophy and reduced sperm count. Cell Death Discov. 2015;1:15020. doi: 10.1038/cddiscovery.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piña-Guzmán B, Solís-Heredia MJ, Quintanilla-Vega B. Diazinon alters sperm chromatin structure in mice by phosphorylating nuclear protamines. Toxicol. Appl. Pharmacol. 2005;202(2):189–198. doi: 10.1016/j.taap.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Piña-Guzmán B, Solís-Heredia MJ, Rojas-García AE, Urióstegui-Acosta M, Quintanilla-Vega B. Genetic damage caused by methyl-parathion in mouse spermatozoa is related to oxidative stress. Toxicol. Appl. Pharmacol. 2006;216(2):216–224. doi: 10.1016/j.taap.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Kalender Y, Kaya S, Durak D, Uzun FG, Demir F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012;33(2):141–148. doi: 10.1016/j.etap.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Shittu M, Ambali SF, Ayo JO, Fatihu MY, Sulaiman MM, Yaqub LS. Evaluation of chronic chlorpyrifos-induced reproductive toxicity in male wistar rat: protective effects of vitamin C. J. Exp. Integr. Med. 2013;3(1):23–30. doi: 10.5455/jeim.041012.or.047. [DOI] [Google Scholar]
- 34.Mosbah R, Yousef MI, Maranghi F, Mantovani A. Protective role of Nigella sativa oil against reproductive toxicity, hormonal alterations, and oxidative damage induced by chlorpyrifos in male rats. Toxicol. Ind. Health. 2016;32(7):1266–1277. doi: 10.1177/0748233714554675. [DOI] [PubMed] [Google Scholar]
- 35.Rhind SM. Endocrine disrupting compounds and farm animals: their properties, actions and routes of exposure. Domest. Anim. Endocrinol. 2002;23(1–2):179–187. doi: 10.1016/s0739-7240(02)00155-8. [DOI] [PubMed] [Google Scholar]
- 36.Tiemann U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: A review. Reprod. Toxicol. 2008;25(3):316–326. doi: 10.1016/j.reprotox.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Selvaraju S, Nandi S, Gupta PS, Ravindra JP. Effects of heavy metals and pesticides on buffalo (Bubalus bubalis) spermatozoa functions in vitro. Reprod. Domes. Anim. 2011;46(5):807–813. doi: 10.1111/j.1439-0531.2010.01745.x. [DOI] [PubMed] [Google Scholar]
- 38.Ghuman SS, Ratnakar U, Bedi JS, Gill JPS. Impact of pesticide residues on fertility of dairy animals: A review. Indian J. Anim. Sci. 2013;83(12):1243–1255. [Google Scholar]
- 39.Sharma A, Mollier J, Brocklesby R, Caves C, Jayasena CN, Minhas S. Endocrine-disrupting chemicals and male reproductive health. Reprod. Med. Biol. 2020;19(3):243–253. doi: 10.1002/rmb2.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherf BD. World Watch List for Domestic Animal Diversity. 3. Food and Agriculture Organization of the United Nations; 2000. [Google Scholar]
- 41.Salazar-Arredondo E, de JesúsSolís-Heredia M, Rojas-García E, Hernández-Ochoa I, Quintanilla-Vega B. Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reprod. Toxicol. 2008;25(4):455–460. doi: 10.1016/j.reprotox.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 42.Ngoula, F. et al. Reproductive and developmental toxicity of insecticides. In Insecticides, Advances in Integrated Pest Management (Perveen, F. Ed), 429–457. (IntechOpen, 2012). ISBN: 978-953-307-780-2.
- 43.Jawaid A, Jehle KL, Mansuy IM. Impact of parental exposure on offspring health in humans. Trends Genet. 2021;37(4):373–388. doi: 10.1016/j.tig.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Peiris-John RJ, Wickremasinghe R. Impact of low-level exposure to organophosphates on human reproduction and survival. Trans. R. Soc. Trop. Med. Hyg. 2008;102(3):239–245. doi: 10.1016/j.trstmh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014;230(2):146–156. doi: 10.1016/j.toxlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Moreira S, Pereira SC, Seco-Rovira V, Oliveira PF, Alves MG, Pereira ML. Pesticides and male fertility: A dangerous crosstalk. Metabolites. 2021;11(12):799. doi: 10.3390/metabo11120799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorenzetti S, Altieri I, Arabi S, Balduzzi D, Bechi N, Cordelli E, Galli C, Ietta F, Modina SC, Narciso L, Pacchierotti F, Villani P, Galli A, Lazzari G, Luciano AM, Paulesu L, Spanò M, Mantovani A. Innovative non-animal testing strategies for reproductive toxicology: The contribution of Italian partners within the EU project ReProTect. Ann. Istiut. Sanita. 2011;47(4):429–444. doi: 10.4415/ANN_11_04_16. [DOI] [PubMed] [Google Scholar]
- 48.Getting the measure of replacement, reduction and refinement—NC3Rs. https://www.nc3rs.org.uk. Aug 3, 2017. Accessed 16 Aug 2022.
- 49.Hubrecht RC, Carter E. The 3Rs and humane experimental technique: Implementing change. Animals. 2019;9(10):754. doi: 10.3390/ani9100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landrigan PJ. Pesticides and human reproduction. JAMA Intern. Med. 2018;178(1):26–27. doi: 10.1001/jamainternmed.2017.5092. [DOI] [PubMed] [Google Scholar]
- 51.Naresh S, Atreja SK. The protein tyrosine phosphorylation during in vitro capacitation and cryopreservation of mammalian spermatozoa. Cryobiology. 2015;70(3):211–216. doi: 10.1016/j.cryobiol.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Clair E, Mesnage R, Travert C, Séralini GÉ. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol. In Vitro. 2012;26(2):269–279. doi: 10.1016/j.tiv.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Martenies SE, Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology. 2013;307:66–73. doi: 10.1016/j.tox.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry MJ, Venners SA, Chen X, Liu X, Tang G, Xing H, Barr DB, Xu X. Organophosphorous pesticide exposures and sperm quality. Reprod. Toxicol. 2011;31(1):75–79. doi: 10.1016/j.reprotox.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sifakis S, Mparmpas M, Soldin OP, Tsatsakis A. Pesticide exposure and health related issues in male and female reproductive system. In: Stoytcheva M, editor. Pesticides: Formulations, Effects, Fate. InTech; 2011. pp. 495–526. [Google Scholar]
- 56.Nerozzi C, Recuero S, Galeati G, Bucci D, Spinaci M, Yeste M. Effects of Roundup and its main component, glyphosate, upon mammalian sperm function and survival. Sci. Rep. 2020;10(1):11026. doi: 10.1038/s41598-020-67538-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amir S, Tzatzarakis M, Mamoulakis C, Bello JH, Eqani S, Vakonaki E, Karavitakis M, Sultan S, Tahir F, Shah S, Tsatsakis A. Impact of organochlorine pollutants on semen parameters of infertile men in Pakistan. Environ. Res. 2021;195:110832. doi: 10.1016/j.envres.2021.110832. [DOI] [PubMed] [Google Scholar]
- 58.Knapke ET, Magalhaes DP, Dalvie MA, Mandrioli D, Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology. 2022;465:153017. doi: 10.1016/j.tox.2021.153017. [DOI] [PubMed] [Google Scholar]
- 59.Larsson B, Rodríguez-Martínez H. Can we use in vitro fertilization tests to predict semen fertility? Anim. Reprod. Sci. 2000;60–61:327–336. doi: 10.1016/s0378-4320(00)00089-0. [DOI] [PubMed] [Google Scholar]
- 60.Muller CH. Rationale, interpretation, validation, and uses of sperm function tests. J. Androl. 2000;21(1):10–30. [PubMed] [Google Scholar]
- 61.Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: A cohort study of 43,277 men. Am. J. Epidemiol. 2009;170(5):559–565. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]
- 62.Joffe M. What has happened to human fertility? Hum. Reprod. 2010;25(2):295–307. doi: 10.1093/humrep/dep390. [DOI] [PubMed] [Google Scholar]
- 63.Kamarianos A, Karamanlis X, Goulas P, Theodosiadou E, Smokovitis A. The presence of environmental pollutants in the follicular fluid of farm animals (cattle, sheep, goats, and pigs) Reprod. Toxicol. 2003;17(2):185–190. doi: 10.1016/s0890-6238(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Zhang P, Zhao Y, Zhang H. Low dose carbendazim disrupts mouse spermatogenesis might be through estrogen receptor related histone and DNA methylation. Ecotoxicol. Environ. Saf. 2019;176:242–249. doi: 10.1016/j.ecoenv.2019.03.103. [DOI] [PubMed] [Google Scholar]
- 65.Joshi SC, Mathur R, Gulati N. Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol. Ind. Health. 2007;23(7):439–444. doi: 10.1177/0748233707080908. [DOI] [PubMed] [Google Scholar]
- 66.Ikpeme EV, Okonko LE, Udensi OU. Detrimental effects of chlorpyrifos and cypermethrin on reproductive physiology ofmale albino rats. Res. J. Environ. Toxicol. 2016;10(1):68–74. doi: 10.3923/rjet.2016.68.74. [DOI] [Google Scholar]
- 67.Choudhary N, Joshi SC. Reproductive toxicity of endosulfan in male albino rats. Bull. Environ. Contam. Toxicol. 2003;70(2):285–289. doi: 10.1007/s00128-002-0189-0. [DOI] [PubMed] [Google Scholar]
- 68.Harchegani AB, Rahmani A, Tahmasbpour E, Kabootaraki HB, Rostami H, Shahriary A. Mechanisms of diazinon effects on impaired spermatogenesis and male infertility. Toxicol. Ind. Health. 2018;34(9):653–664. doi: 10.1177/0748233718778665. [DOI] [PubMed] [Google Scholar]
- 69.Lenzi A, Gandini L, Picardo M, Tramer F, Sandri G, Panfili E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front. Biosci. 2000;5:E1–E15. doi: 10.2741/lenzi. [DOI] [PubMed] [Google Scholar]
- 70.Singh RK, Kumaresan A, Chhillar S, Rajak SK, Tripathi UK, Nayak S, Datta TK, Mohanty TK, Malhotra R. Identification of suitable combinations of in vitro sperm-function test for the prediction of fertility in buffalo bull. Theriogenology. 2016;86(9):2263–2271.e1. doi: 10.1016/j.theriogenology.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 71.Miranda-Contreras L, Gómez-Pérez R, Rojas G, Cruz I, Berrueta L, Salmen S, Colmenares M, Barreto S, Balza A, Zavala L, Morales Y, Molina Y, Valeri L, Contreras CA, Osuna JA. Occupational exposure to organophosphate and carbamate pesticides affects sperm chromatin integrity and reproductive hormone levels among Venezuelan farm workers. J. Occup. Health. 2013;55(3):195–203. doi: 10.1539/joh.12-0144-fs. [DOI] [PubMed] [Google Scholar]
- 72.Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic. Biol. Med. 1997;22(4):643–656. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 73.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121(4):1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 74.Kalab P, Peknicova J, Geussova G, Moos J. Regulation of protein tyrosine phosphorylation in boar sperm through a cAMP dependent pathway. Mol. Reprod. Dev. 1998;51:304–314. doi: 10.1002/(SICI)1098-2795(199811)51:3<304::AID-MRD10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 75.Siddique RA, Atreja SK. Effect of Spermine-NONOate on acrosome reaction and associated protein tyrosine phosphorylation in Murrah buffalo (Bubalus bubalis) spermatozoa. Anim. Reprod. Sci. 2012;131(1–2):81–87. doi: 10.1016/j.anireprosci.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Gali JM, Atreja SK. Identification of capacitation associated tyrosine phosphoproteins in buffalo (Bubalus bubalis) and cattle spermatozoa. Anim. Reprod. Sci. 2011;123(1–2):40–47. doi: 10.1016/j.anireprosci.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 77.ESHRE Andrology Special Interest Group, European Society for Human Reproduction and Embryology Guidelines on the application of CASA technology in the analysis of spermatozoa. Hum. Reprod. 1998;13(1):142–145. doi: 10.1093/humrep/13.1.142. [DOI] [PubMed] [Google Scholar]
- 78.Gillan L, Kroetsch T, Maxwell WM, Evans G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci. 2008;103(3–4):201–214. doi: 10.1016/j.anireprosci.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Uzunhisarcikli M, Kalender Y, Dirican K, Kalender S, Ogutcu A, Buyukkomurcu F. Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of vitamins C and E. Pestic. Biochem. Physiol. 2007;87(2):115–122. doi: 10.1016/j.pestbp.2006.06.010. [DOI] [Google Scholar]
- 80.Larsen L, Scheike T, Jensen TK, Bonde JP, Ernst E, Hjollund NH, Zhou Y, Skakkebaek NE, Giwercman A. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population: The Danish First Pregnancy Planner Study Team. Hum. Reprod. 2000;15(7):1562–1567. doi: 10.1093/humrep/15.7.1562. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Z, Fan X, Lv Y, Lin Y, Wu D, Zeng W. Glutamine protects rabbit spermatozoa against oxidative stress via glutathione synthesis during cryopreservation. Reprod. Fertil. Dev. 2017;29(11):2183–2194. doi: 10.1071/RD17020. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Z, Umehara T, Okazaki T, Goto M, Fujita Y, Hoque S, Kawai T, Zeng W, Shimada M. Gene expression and protein synthesis in mitochondria enhance the duration of high-speed linear motility in boar sperm. Front. Physiol. 2019;10:252. doi: 10.3389/fphys.2019.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kedechi S, Zribi N, Louati N, Menif H, Sellami A, Lassoued S, Ben Mansour R, Keskes L, Rebai T, Chakroun N. Antioxidant effect of hydroxytyrosol on human sperm quality during in vitro incubation. Andrologia. 2017;49(1):12595. doi: 10.1111/and.12595. [DOI] [PubMed] [Google Scholar]
- 84.Piña-Guzmán B, Sánchez-Gutiérrez M, Marchetti F, Hernández-Ochoa I, Solís-Heredia MJ, Quintanilla-Vega B. Methyl-parathion decreases sperm function and fertilization capacity after targeting spermatocytes and maturing spermatozoa. Toxicol. Appl. Pharmacol. 2009;238(2):141–149. doi: 10.1016/j.taap.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 85.Batra V, Norman E, Morgan HL, Watkins AJ. Parental programming of offspring health: The intricate interplay between diet, environment. Reprod. Dev. Biomol. 2022;12(9):1289. doi: 10.3390/biom12091289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farr SL, Cooper GS, Cai J, Savitz DA, Sandler DP. Pesticide use and menstrual cycle characteristics among premenopausal women in the Agricultural Health Study. Am. J. Epidemiol. 2004;160(12):1194–1204. doi: 10.1093/aje/kwi006. [DOI] [PubMed] [Google Scholar]
- 87.Jia ZZ, Zhang JW, Zhou D, Xu DQ, Feng XZ. Deltamethrin exposure induces oxidative stress and affects meiotic maturation in mouse oocyte. Chemosphere. 2019;223:704–713. doi: 10.1016/j.chemosphere.2019.02.092. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Wang YL, Chen MH, Zhang Z, Xu BH, Liu R, Xu L, He SW, Li FP, Qi ZQ, Wang HL. Methoxychlor exposure induces oxidative stress and affects mouse oocyte meiotic maturation. Mol. Reprod. Dev. 2016;83(9):768–779. doi: 10.1002/mrd.22683. [DOI] [PubMed] [Google Scholar]
- 89.Final Report Summary—E-DOHAD (Environmentally-induced Developmental Origins of Health and Disease). https://cordis.europa.eu/project/id/311765/reporting. Accessed May 2022.
- 90.Gore AC. Neuroendocrine targets of endocrine disruptors. Hormones. 2010;9(1):16–27. doi: 10.14310/horm.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rezg R, Mornagui B, Benahmed M, Chouchane SG, Belhajhmida N, Abdeladhim M, Kamoun A, El-fazaa S, Gharbi N. Malathion exposure modulates hypothalamic gene expression and induces dyslipedemia in Wistar rats. Food Chem. Toxicol. 2010;48(6):1473–1477. doi: 10.1016/j.fct.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Chiu YH, Williams PL, Gillman MW, Gaskins AJ, Mínguez-Alarcón L, Souter I, Toth TL, Ford JB, Hauser R, Chavarro JE, EARTH Study Team Association between pesticide residue intake from consumption of fruits and vegetables and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technology. JAMA Intern. Med. 2018;178(1):17–26. doi: 10.1001/jamainternmed.2017.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassan A, Zayed SM, Bahig MR. Metabolism of organophosphorus insecticides. XI. Metabolic fate of dimethoate in the rat. Biochem. Pharmacol. 1969;18(10):2429–2438. doi: 10.1016/0006-2952(69)90359-1. [DOI] [PubMed] [Google Scholar]
- 94.Food and Agriculture Organization/World Health Organization (FAO/WHO). 4.10 Dimethoate, omethoate, and formothion (T**). In Pesticide Residues in Food-1996. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group. FAO Plant Production and Protection Paper, 140, (1997).
- 95.Marino, T. et al. Evaluation of the toxicity of 3,5,6-trichloro-2-pyridinol (TCP) to the early life stages of the rainbow trout, Oncorhynchus mykiss Walbaum. Lab Project Number: 991173. Unpublished study prepared by The Dow Chemical Co. (1999).
- 96.Petty DG, Getsinger KD, Woodburn KB. A review of the aquatic environmental fate of triclopyr and its major metabolites. J. Aquat. Plant Manag. 2003;41(2):69–75. [Google Scholar]
- 97.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Exp. Anal. Environ. Epidemiol. 2005;15(4):297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- 98.Cáceres T, He W, Naidu R, Megharaj M. Toxicity of chlorpyrifos and TCP alone and in combination to Daphnia carinata: The influence of microbial degradation in natural water. Water Res. 2007;41(19):4497–4503. doi: 10.1016/j.watres.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 99.Montesano MA, Olsson AO, Kuklenyik P, Needham LL, Bradman AS, Barr DB. Method for determination of acephate, methamidophos, omethoate, dimethoate, ethylenethiourea and propylenethiourea in human urine using high-performance liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J. Eposure Sci. Environ. Epidemiol. 2007;17(4):321–330. doi: 10.1038/sj.jes.7500550. [DOI] [PubMed] [Google Scholar]
- 100.Urióstegui-Acosta M, Tello-Mora P, Solís-Heredia MJ, Ortega-Olvera JM, Piña-Guzmán B, Martín-Tapia D, González-Mariscal L, Quintanilla-Vega B. Methyl parathion causes genetic damage in sperm and disrupts the permeability of the blood-testis barrier by an oxidant mechanism in mice. Toxicology. 2020;438:152463. doi: 10.1016/j.tox.2020.152463. [DOI] [PubMed] [Google Scholar]
- 101.Ortega-Olvera JM, Winkler R, Quintanilla-Vega B, Shibayama M, Chávez-Munguía B, Martín-Tapia D, Alarcón L, González-Mariscal L. The organophosphate pesticide methamidophos opens the blood-testis barrier and covalently binds to ZO-2 in mice. Toxicol. Appl. Pharmacol. 2018;360:257–272. doi: 10.1016/j.taap.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Jeng HA. Exposure to endocrine disrupting chemicals and male reproductive health. Front. Public Health. 2014;2:55. doi: 10.3389/fpubh.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Swan SH. Temporal trend in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update. 2017;23(6):646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Solhjou KA, Hosseini SE, Vahdati A, Edalatmanesh MA. Changes in the hypothalamic-pituitary-gonadal axis in adult male rats poisoned with proteus and biscaya insecticides. Iran. J. Med. Sci. 2019;44(2):155–162. [PMC free article] [PubMed] [Google Scholar]
- 105.Batra V, Bhushan V, Ali SA, Sarwalia P, Pal A, Karanwal S, Solanki S, Kumaresan A, Kumar R, Datta TK. Buffalo sperm surface proteome profiling reveals an intricate relationship between innate immunity and reproduction. BMC Genomics. 2021;22(1):480. doi: 10.1186/s12864-021-07640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ministry of Agriculture & Farmers Welfare. GoI—Pesticide-Wise Consumption of Indigenous Pesticides During 2016–2017 to 2020–2021. http://ppqs.gov.in/statistical-database.
- 107.Kaur S, Khera KS. Pesticide toxicity and avifauna of Punjab. Asian J. Anim. Sci. 2015;10(1):74–80. doi: 10.15740/HAS/TAJAS/10.1/74-80. [DOI] [Google Scholar]
- 108.Saraf KK, Singh RK, Kumaresan A, Nayak S, Chhillar S, Lathika S, Datta TK, Mohanty TK. Sperm functional attributes and oviduct explant binding capacity differs between bulls with different fertility ratings in the water buffalo (Bubalus bubalis) Reprod. Fertil. Dev. 2019;31(2):395–403. doi: 10.1071/RD17452. [DOI] [PubMed] [Google Scholar]
- 109.Saraf KK, Kumaresan A, Chhillar S, Nayak S, Lathika S, Datta TK, Gahlot SC, Karan P, Verma K, Mohanty TK. Spermatozoa with high mitochondrial membrane potential and low tyrosine phosphorylation preferentially bind to oviduct explants in the water buffalo (Bubalus bubalis) Anim. Reprod. Sci. 2017;180:30–36. doi: 10.1016/j.anireprosci.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 110.Batra V, Maheshwarappa A, Dagar K, Kumar S, Soni A, Kumaresan A, Kumar R, Datta TK. Unusual interplay of contrasting selective pressures on β-defensin genes implicated in male fertility of the Buffalo (Bubalus bubalis) BMC Evol. Biol. 2019;19(1):214. doi: 10.1186/s12862-019-1535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kibbe WA. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35:W43–W46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuhn RM, Haussler D, Kent WJ. The UCSC genome browser and associated tools. Brief. Bioinform. 2013;14(2):144–161. doi: 10.1093/bib/bbs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 114.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta deltaC(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. Supplementary Material & Information: Supplementary sheet-Methods and Supplementary Figs. 1, 2, and 3.