Highlights
-
•
Higher physical activity was associated with chance of prediabetes regression to normoglycemia.
-
•
Each 500 MET-minutes/week physical activity increased chance of prediabetes regression by 5%.
-
•
Physical activity level was not associated with the risk of prediabetes progression.
Keywords: Physical activity, Pre-diabetes, Type 2 diabetes, Normoglycemia, Metabolic equivalent
Abstract
The possible association of habitual physical activity (PA) and the risk of pre-diabetes (Pre-DM) progression to type 2 diabetes (T2D) or the chance of returning to normoglycemia was investigated. This cohort study included 1167 Pre-DM individuals (mean age of 53.5 years, and 45.3% men) who participated in the third phase of the Tehran Lipid and Glucose Study (2006–2008) and followed up to a median of 9 years. PA, including leisure time and job activities, was measured using a reliable and validated Iranian version of the Modifiable Activity Questionnaire and reported as metabolic equivalent (MET)-minutes per week. The odds ratios (ORs) and 95% confidence intervals (CIs) of incident T2D and returning to normoglycemia were estimated in relation to PA levels (i.e., per every 500 MET-minutes/week, or across categories of PA levels < 600 as a reference, 600–1500 and > 1500 MET-minutes/week). During the study follow-up, 39.0 % progressed to T2D, and 37.8% returned to normoglycemia. Compared to subjects with a PA < 600 MET-minutes/week, the chance of regression to normoglycemia increased by 58% [OR = 1.58, 95% CI = 1.03–2.40 ∼ relative risk (RR) = 1.32, 95% CI = 1.02–1.63] among the participants who had a PA > 1500 MET-minutes/week. We further noted that each 500 MET-min/week activity corresponded to an elevated chance of returning to normoglycemia by 5% (OR = 1.05, 95% CI = 1.01–1.11). The study’s findings provided evidence that higher daily PA levels may facilitate Pre-DM regression to normoglycemia. The beneficial effect of PA in Pre-DM subjects needs to exceed the recommended levels (i.e., 600 MET-minutes/week).
1. Introduction
Pre-diabetes (Pre-DM) is an intermediate state between normal glucose homeostasis and type 2 diabetes (T2D), characterized by elevated levels of fasting plasma glucose (100–125 mg/dL), 2-h plasma glucose (140–199 mg/dL), or glycated hemoglobin (HbA1c, 5.7–6.4%) (Association, 2020). The Pre-DM will progress into T2D within 10 years in 70% of patients (DeJesus et al., 2017). Less is known about the potential factors that affect the regression or progression of Pre-DM; however, lower fasting plasma glucose, greater insulin secretion, younger age, weight loss, and intensive lifestyle modifications may facilitate Pre-DM regression to normal glycemia (Alizadeh et al., 2022, Perreault et al., 2009).
Physical activity (PA) refers to “any bodily movement produced by skeletal muscles that requires energy expenditure” and includes “all movement during leisure time, for transport to get to and from places, or as part of a person’s work” (World Health Organization, 2018). As recommended by the American Diabetes Association (ADA), Pre-DM subjects should have at least 150 min/week PA (Colberg et al., 2016). Pre-DM subjects who followed a training program had better oral glucose tolerance, fasting blood glucose, and decreased glycated hemoglobin (HbA1C) (Jadhav et al., 2017). A meta-analysis of 16 randomized controlled trials reported that Pre-DM subjects who received lifestyle intervention, including increased PA levels, had a lower rate of progression to T2D after one (RR = 0.46, 95% CI = 0.32–0.66) and three years of follow-up (RR = 0.64, 95% CI = 0.53, 0.77) (Glechner et al., 2018).
Considering a high prevalence of insufficient PA among the Iranian population (Momenan et al., 2011), which is estimated to be responsible for 7.6% of developing T2D (Lee et al., 2012), here, we aimed to assess the possible association of PA levels (estimated as metabolic equivalent, MET-minutes per week) with the chance of regression to normoglycemia or progression to T2D within 9 years, in a cohort of middle-aged Pre-DM men and women.
2. Methods
2.1. Study population
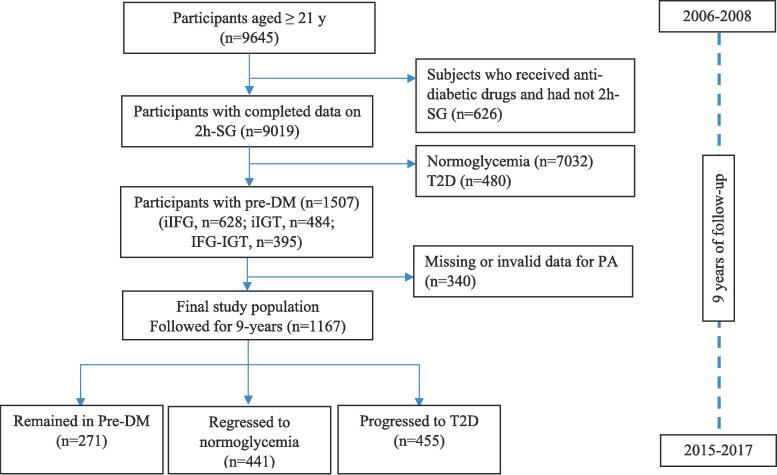
This study was conducted within the framework of the Tehran Lipid and Glucose Study (TLGS), an ongoing community-based prospective study started in 1999 on>15,000 participants aged 3–75 years to investigate and prevent non-communicable diseases in a representative sample in district 13 of Tehran, the capital city of Iran (Azizi et al., 2018). During the third phase of the TLGS (2006–2008), 9645 adult men and women (aged ≥ 21 y) completed the examinations. For the current study, a subset of the third phase participants diagnosed with Pre-DM (n = 1167) with complete data (i.e., demographics, lifestyle, anthropometric and biochemical measurements) was recruited. The study participants were followed up to the sixth phase of the TLGS examination (2015–2017) for a median of 9 years. The measurements (demographics, anthropometrics, and biochemical) were repeated at three-year intervals [in the fourth (2009–2011), fifth (2012–2014), and sixth (2015–2017) phases of the TLGS]. The flowchart of study participants is presented in Fig. 1.
Fig. 1.
The study flowchart.
Written informed consent was obtained from all participants. The ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved the study protocol (Ethics code: IR.SBMU.ENDOCRINE.REC.1401.121).
2.2. Demographic, lifestyle, anthropometric, and biochemical measurements
Details of data collection and measurements (i.e., determining the status of occupation, marriage, education level, smoking habits, medical history, and medications, as well as anthropometric measurements) have been reported elsewhere (Azizi et al., 2000). Education level was categorized based on the study years [as primary/illiterate, 0–6 years, secondary/diploma, 6–12 years, and higher, > 12 years]. Marital status was reported in three groups single, married, and divorced/widowed. Smoking habits were considered into two statuses of smokers (current smoking) and non-smokers (never or pastsmoking).
Systolic (SBP) and diastolic (DBP) blood pressures were measured using a standard mercury sphygmomanometer calibrated by the Institute of Standards and Industrial Research of Iran (Askari et al., 2014). Blood pressure was measured twice on the participants' right arm after a 15-minute rest in a sitting position, with at least a 30-second interval between the two measurements. The two measurements' mean was considered the participant’s blood pressure.
Details of biochemical measurements in the TLGS samples have been described in detail (Tohidi et al., 2014). Measurements were done after a 12-to 14-h overnight fasting at baseline and all subsequent examinations. In brief, serum glucose concentration was measured using an enzymatic colorimetric method using glucose oxidase (Pars Azmoon, Tehran, Iran). The standard 2 h-SG test followed oral glucose administration of 82.5 g glucose monohydrate solution (equivalent to 75 g anhydrous glucose; Cerestar EP, Spain). Serum TG was measured using the enzymatic colorimetric method (Pars Azmoon, Tehran, Iran) (Tohidi et al., 2014). Intra- and inter-assay coefficients of variation (CV) were<5.0% for all measurements.
2.3. Physical activity measurements
Physical activity, including leisure time and job activities, was measured using a reliable and validated Iranian version (Momenan et al., 2012) of the Modifiable Activity Questionnaire (MAQ) (Kriska et al., 1990) at baseline (2006–2008). Details of the measurement have been reported by our TLGS research group elsewhere (Naseri et al., 2020, Sheikholeslami et al., 2018). In brief, participants were asked for their physical activities, as well as the frequency and duration were multiplied for each activity over the past 12 months to get the total number of minutes/year for each leisure time physical activity (LTPA) and then divided by 52 to estimate total time in minutes/week. The metabolic equivalent (MET) of total LTPA for each person was then calculated by multiplying the number of minutes/week of each LTPA by its Metabolic Task Equivalent (MET) value to get MET-min/week and adding the values across all reported LTPA to get a total LTPA value. Job physical activity (JPA) was calculated by multiplying the number of minutes per week of each of three categories of occupational activity (light, e.g., standing, moderate, e.g., housework, and heavy e.g., lifting loads) by assigned MET values for each category to get total MET-minutes/week. One MET is defined as the amount of consumed oxygen at resting, equal to 3.5 ml O2 per kg BW × min. The MET is a simple, practical, and easily understood procedure for expressing the energy cost of PA as a multiple of the resting metabolic rate (RMR). MET represents the ratio of energy expended during an activity to the rate of energy expended at rest; e.g., a 4 MET activity expends 4 times the energy used by the body at rest. If a person does a 4 MET activity for 30 min, he/she has done 4 × 30 = 120 MET-minutes (or 2.0 MET-hours) of PA (U.S. Department of Health and Human Services, 2008)(). The moderate-intensity activities are defined as 3.0 to 5.9 METs (e.g., walking 3 miles/hour), while vigorous-intensity activities are defined as ≥ 6.0 METs (e.g., running 6 miles/hour or tennis) (U.S. Department of Health and Human Services, 2008).
The subject’s total PA level was estimated by summing up LTPA and JPA and then was classified as low (MET < 600 min/wk), moderate (MET 600–1499 min/wk), and high (MET ≥ 1500 min/wk) levels (Kriska et al., 1990).
2.4. Definition of outcomes and co-variates
All Pre-DM subjects were assessed for the occurrence of the outcomes at three-year intervals (2009–2011, 2012–2014, or 2015–2017) during the study follow-up. Pre-DM was defined as having at least one of the IFG (100 ≤ FSG < 126 mg/dL) or IGT (140 ≤ 2 h-SG < 200 mg/dL) (Association, 2020). Returning to normal glycemia was defined as the first occurrence of both normal fasting glucose and normal glucose tolerance (NFG, i.e., FSG < 100 mg/dL and NGT, i.e., 2 h-SG < 140 mg/dL); T2D was defined as the first occurrence of FSG ≥ 126 mg/dL or 2 h-SG ≥ 200 mg/dL, or self-reported use of glucose-lowering medications. A self-reported positive family history of T2D (FHD) was defined as having at least one parent or sibling with T2D.
Hypertriglyceridemia was defined according to the NCEP ATP III diagnostic criteria as serum TG ≥ 150 mg/dL (1.69 mmol/L) or using lipid-lowering drugs (Grundy et al., 2005). Abdominal obesity was defined according to the report of the Iranian population by the Iranian National Committee of Obesity for the appropriate definition of metabolic syndrome as WC ≥ 95 cm for both genders (Azizi et al., 2010). The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) (James et al., 2014) was used for the definition of hypertension (HTN), i.e., SBP ≥ 140 mmHg, DBP ≥ 90 mmHg or medications for HTN.
2.5. Statistical methods
Statistical analyses were conducted using the SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA). Baseline characteristics of the participants were compared across the groups according to the final glycemic state, using analysis of variance (ANOVA) for the continuous variables with normal distribution or chi-square test for the categorical variables. Data are reported as mean (SD), median (inter-quartile range, IQR), or percent. The extreme outlier values for PA level were defined as values above or below three standard deviations; then they replaced outliers and extreme values with the nearest value that would not be considered extreme (Modeler, 2021).
The odds ratios (95% confidence intervals, CIs) of Pre-DM regression to normoglycemia or progression to T2D were estimated using multinomial logistic regression analysis; PA levels were included in the models both as continuous (per every 500 MET-minutes/week ∼ 90 min walking for exercise with a rate of 3.5 miles/hours) and categorical (<600 as reference, 600–1500 and > 1500 MET-minutes/week) variable. Potential covariates were selected based on scientific and statistical evidence (Alizadeh et al., 2022). A univariate analysis was performed for potential confounding variables, and those with PE < 0.2 were selected for the final multivariable model; PE (P-value for entry) determines which variables should be included in the multivariable model (). Finally, three models were conducted: Model 1) adjusted for age, sex, and FSG; Model 2) additionally adjusted for BMI, FHD, HTN, and high-TG; Model 3) additionally adjusted for smoking and occupation. Because our outcomes are common in the study population (>10%), the adjusted ORs derived from the logistic regression can no longer approximate the relative risks (RRs) (Zhang and Yu, 1998). The ORs (95% Cis) of the final models were, therefore, converted by the following formula to approximate the RR to adjust for outcomes incidence: RR = OR ÷ [(1-P0) + (P0 × OR)], in which P0 indicates the incidence of the outcome of interest in nonexposed group (i.e., the incidence of the outcome in the low-PA group in this study) (Zhang and Yu, 1998).
3. Results
The mean age of the study participants was 53.5 ± 13.5 y, and 45.3% were men. During a median follow-up of 9.3 years (inter-quartile range = 8.0–10.2 years), 39.0% of the participants progressed to T2D, and 37.8% regressed to normoglycemia. Table 1 represents the study participants’ baseline characteristics. Participants who regressed to normoglycemia were significantly younger and had lower FSG, 2 h-SG, and lower rates of HTN and abdominal obesity than those who progressed to T2D. They also had higher PA levels than those who remained Pre-DM (median = 1854, IQR = 1100–3223 MET-minutes/week vs. median = 1582, IQR = 704–2801). As indicated in Table2, full-adjusted OR for regression to normoglycemia among the participants who had a PA > 1500 MET-minutes/week was 1.58 (95% CI = 1.03–2.40), compared with people who had a PA < 600 MET-minutes/week. The estimated RR from adjusted OR was 1.32 (95% CI = 1.02–1.63).
Table 1.
Baseline (2006–2008) characteristics of the study participants (n = 1167).
| Remained Pre-DM (n = 271) |
Regressed to normoglycemia (n = 441) |
Progressed to T2D (n = 455) |
|
|---|---|---|---|
| Age (y) | 55.5 ± 13.2 | 51.1 ± 14.3 ab | 54.6 ± 12.5 |
| Men (%) | 49.8 | 43.1 | 44.8 |
| FHD (%) | 18.4 | 22.0 | 29.4 |
| Medications | |||
| Lipid-lowering (%) | 6.3 | 5.4 | 7.7 |
| BP-lowering (%) | 8.9 | 7.0 | 9.0 |
| Current smoker (%) | 10.0 | 10.0 | 8.6 |
| Education | |||
| Illiterate/primary | 11.5 | 14.5 | 11.9 |
| Secondary/diploma | 44.4 | 45.2 | 42.9 |
| Higher | 44.1 | 40.3 | 45.3 |
| Job status | |||
| Employed | 31.9 | 30.8 | 33.2 |
| Unemployed | 27.8 | 20.6 | 22.8 |
| House wife | 39.6 | 48.2 | 44.0 |
| Marital status | |||
| Single | 1.9 | 5.2 | 3.1 |
| Married | 88.5 | 85.7 | 85.9 |
| Divorced/widowed | 9.6 | 9.0 | 11.0 |
| PA (MET-min/week)† | 1582 (704–2801) | 1854 (1100–3223) b | 1764 (931–3222) |
| Low | 53.3 | 62.5 | 60.4 |
| Moderate | 23.0 | 23.1 | 23.1 |
| High | 23.7 | 14.0 | 16.5 |
| BMI (kg/m2) | 28.8 ± 3.9 | 28.5 ± 4.4 | 30.1 ± 5.1 |
| Abdominal obesity (%) | 57.5 | 55.1 a | 66.6 |
| HTN (%) | 27.3 | 24.0 a | 33.4 |
| High-TG (%) | 58.8 | 51.7 | 64.6 |
| FSG (mg/dL) | 101 ± 7.9 a | 97.3 ± 9.1ab | 105 ± 9.2 |
| 2 h-SG (mg/dL) | 135 ± 29.7 a | 131 ± 29.9 ab | 148 ± 30.4 |
Data are mean ± SD or percent.
T2D, type 2 diabetes; FHD, Family history of T2D; BMI, body mass index; Abdominal obesity (WC ≥ 95 cm); HTN, hypertension (systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg, or using medications for HTN); FSG, fasting serum glucose; 2 h-SG, 2-hours serum glucose; high-TG (serum triglyceride ≥ 150 mg/dL or using medications for hyperlipidemia);
Physical activity levels (Low < 600, moderate 600–1500 and high ≥ 1500 MET-min/week).
Median (inter-quartile rage, IQR).
Significant difference with T2D and b Significant difference with Pre-DM (P < 0.05); analysis of variance was used with Bonferroni post hoc test (the logarithm of PA was used in the analysis due to its non-normal distribution).
Table 2.
The odds ratio (95% CI) of prediabetes (Pre-DM) regression to normoglycemia and progression to type 2 diabetes (T2D) in relation to physical activity levels.
|
Regressed to normoglycemia |
Progressed to T2D |
|||||
|---|---|---|---|---|---|---|
| PA (MET-min/week) | 600–1500 | ≥1500 | Per 500 | 600–1500 | ≥1500 | Per 500 |
| Crude | 1.40 (0.88–2.25) | 1.53 (1.02–2.29) | 1.04 (0.99–1.09) | 1.31 (0.82–2.08) | 1.41 (0.95–2.09) | 1.03 (0.98–1.08) |
| Model 1 | 1.37 (0.84–2.23) | 1.63 (1.08–2.46) | 1.06 (1.01–1.11) | 1.31 (0.82–2.10) | 1.40 (0.98–1.01) | 1.02 (0.97–1.07) |
| Model 2 | 1.32 (0.81–2.16) | 1.57 (1.04–2.40) | 1.05 (1.00–1.10) | 1.33 (0.82–2.16) | 1.43 (0.95–2.17) | 1.02 (0.97–1.07) |
| Model 3† | 1.32 (0.81–2.16) | 1.58 (1.03–2.40) | 1.05 (1.01–1.11) | 1.35 (0.83–2.19) | 1.45 (0.96–2.20) | 1.03 (0.98–1.08) |
Data are ORs and 95% CI.
Multinomial logistic regression was used.
Model 1, adjusted for age, sex, fasting serum glucose (FSG).
Model 2, additionally adjusted for BMI, FHD, HTN, High-TG.
Model 3, additionally adjusted for smoking and occupation.
The expected RRs from the obtained adjusted-ORs (25) were 1.19 (0.87–1.55) and 1.32 (1.02–1.63) for normoglycemia in the PA levels of 600–1500 and ≥ 1500, respectively. For T2D, the expected RRs from the obtained adjusted-ORs were 1.19 (0.89–1.52) and 1.30 (0.97–1.49) in the PA levels of 600–1500 and ≥ 1500, respectively.
Every 500 MET-minutes/week of PA was associated with an increased chance of returning to normoglycemia by 5% (OR = 1.05, 95% CI = 1.01–1.11). No significant association was observed between PA levels and the risk of developing T2D in Pre-DM subjects during the study follow-up.
4. Discussion
In a 9-year follow-up within a well-characterized cohort study, we observed that>1500 MET-min/week PA levels might facilitate regression to normoglycemia in Pre-DM subjects. Although, our findings indicated that the benefit of PA in Pre-DM subjects could be achieved even by relatively low levels (i.e., 5% increased chance of Pre-DM regression per each 500 MET-min/week ∼ 90 min walking for exercise with a rate of 3.5 miles/hours), considerable significant benefits are afforded for higher levels of PA (i.e., >1500 MET-min/week). Although previous studies reported the benefit of exercise programs on glucose homeostasis (Chang et al., 2020, Qu et al., 2022, Shah et al., 2021) and indicated the protective effect of PA against the development of T2D (Aune et al., 2015, Smith et al., 2016), it was not distinguished how much PA levels are needed to prevent developing T2D in Pre-DM subjects or facilitate Pre-DM regression to normoglycemia. To the best of our knowledge, this was the first cohort of Pre-DM subjects to assess the possible association of PA levels with the regression/ progression of Pre-DM simultaneously.
Several factors may affect the Pre-DM regression to normoglycemia; a previous report from our research group on a larger TLGS population indicated that regression to normoglycemia was associated with age [relative risk ratio (RRR) = 0.97, 95% CI = 0.95–0.99), female sex (RRR = 1.72, 95% CI = 1.18–2.50), high education level of ≥ 12 years (RRR = 2.10, 95% CI = 1.19–3.70), and combined IFG/IGT compared to IFG (RRR = 0.45, 95% CI = 0.29–0.70) (Alizadeh et al., 2022).
Pre-DM subjects are recommended to have at least 150 min/week of moderate-intensity activities (i.e., defined as 3.0 to 5.9 MET) (Colberg et al., 2016). Having a PA level ≥ 600 MET-min/week meets the United States physical activity guidelines (USDHHS, 2008) (U.S. Department of Health and Human Services, 2008). In the current study, 59.7% of Pre-DM subjects had fewer PA levels than the recommendations. The prevalence of inactivity was previously reported by 69.8% (95% CI = 68.7–70.8) in the Iranian population, and only 30.2% (95% CI = 27.2–33.1%) of men and 30.3% (95% CI = 27.7–32.8%) of women were reported to meet the recommended PA levels (8). About 8.3% (95% CI = 6.4–10.2) of premature deaths are attributed to inadequate levels of PA (Momenan et al., 2011). Furthermore, insufficient PA levels are responsible for 7% (3.9–9.6%) of T2D worldwide (Carlson et al., 2018); in Iran, estimated population-attributable fractions (PAF) associated with physical inactivity were reported as 7.6 (3.8–11.8) (Lee et al., 2012).
Adhering to the PA recommendations (at least 150 min/week of moderate-intensity activities) significantly improved glycemic control and cardiometabolic health among adults with pre-DM (Qu et al., 2022). An 8-week moderate exercise program (i.e., 30 min PA with heart rate max 7% for 5 days/week) in pre-DM subjects significantly improved glycemic control and enhanced total antioxidant capacity (Shah et al., 2021). The aerobic-based activities spread as either 3 short (10–15 min) or frequent brief (1–5 min) bouts improved postprandial glucose and glycated hemoglobin in subjects with Pre-DM and T2D (Chang et al., 2020). A 5-year follow-up study of Pre-DM subjects reported that moderate-to-vigorous-intensity PA was associated with improved insulin sensitivity and β-cell function and a higher probability of Pre-DM regression to normoglycemia in middle-aged women (Færch et al., 20172017). A dose–response meta-analysis reported a 26% (95% CI = 20–31%) reduced risk of T2D among those who achieved 11.25 MET-h/week (equivalent to 150 min/week of moderate activity), compared to inactive individuals (Smith et al., 2016); higher levels of PA (22.5 and 60 MET-h/week) was associated with further risk reductions by 36 and 53% (Smith et al., 2016). Another meta-analysis showed that high vs. low levels of total PA and LTPA was associated with a reduced risk of developing T2D by 35% (RR = 0.65, 95 %=CI 0.59–0.71) and 26% (RR = 0.74, 95 % CI = 0.70–0.79), respectively; vigorous, moderate and low-intensity PA had a preventive effect about 39, 32 and 34% (RR = 0.61, 95 % CI 0.51–0.74, RR = 0.68, 95 % CI 0.52–0.90, RR = 0.66, 95 % CI 0.47–0.94), respectively (Aune et al., 2015).
The current study had some limitations and strengths that should be considered. The main limitation of the current study is its observational design, i.e., it cannot determine causation, rule out possible recall bias, and remain unknown or residual confounding. A lack of sedentary time data also limited the finding. Although using METs as estimated habitual PA levels, assuming the same average intensity for each person for a particular activity may be another limitation it is nonetheless a robust metric, especially for self-reported PA data. Furthermore, due to potential changes in individual's PA levels and changes in other T2D risk factors during the study follow-up, some degree of misclassification might have occurred, which could lead to biased estimated hazard ratios towards the null, as inherent in any prospective study. A major strength of this study was the use of a population-based cohort with detailed data on the most important potential confounders and multiple measurements of glycemic parameters enabled us to monitor the study participants’ glycemic changes more precisely over time and detect the occurrence of the outcomes at mid-interval periods. Using multinomial logistic regression enabled us to include the exposure and three-categorized outcome variables (i.e., normal glycemia, Pre-DM, and T2D), improved the study power, and provided us the chance of comparing the Odds of the outcomes simultaneously.
The findings of this longitudinal follow-up support the generally accepted notion of a graded association between PA and metabolic health and provide more evidence in support of the clinically meaningful role of PA in increasing chance of returning to normoglycemia in Pre-DM adults. We highlighted the necessity for achieving a higher daily PA levels than the current recommendations in subjects at risk of T2D.
5. Availability of data and materials
Data will be presented upon forwarding the request to the corresponding author (mirmiran@endocrine.ac.ir) and confirmation of the director of RIES (azizi@endocrine.ac.ir).
Funding
This work was not supported by any funding agency.
CRediT authorship contribution statement
Zahra Bahadoran: Conceptualization, Methodology, Software, Writing – original draft. Parvin Mirmiran: Data curation, Visualization, Investigation, Supervision. Maryam Shabani: Conceptualization, Methodology, Software, Writing – original draft. Fereidoun Azizi: Data curation, Visualization, Investigation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management.
Contributor Information
Zahra Bahadoran, Email: z.bahadoran@endocrine.ac.ir.
Parvin Mirmiran, Email: mirmiran@endocrine.ac.ir.
Maryam Shabani, Email: Maryam.shabani@hiau.ac.ir.
Fereidoun Azizi, Email: azizi@endocrine.ac.ir.
Data availability
Data will be made available on request.
References
- Alizadeh Z., Baradaran H.R., Kohansal K., Hadaegh F., Azizi F., Khalili D. Are the determinants of the progression to type 2 diabetes and regression to normoglycemia in the populations with pre-diabetes the same? Front. Endocrinol. 2022;13:1041808. doi: 10.3389/fendo.2022.1041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari S., Asghari G., Ghanbarian A., Khazan M., Alamdari S., Azizi F. Seasonal variations of blood pressure in adults: Tehran lipid and glucose study. Arch. Iran. Med. 2014;17(6):441–443. [PubMed] [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S15-S33. [DOI] [PubMed]
- Aune D., Norat T., Leitzmann M., Tonstad S., Vatten L.J. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015;30(7):529–542. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- Azizi F., Madjid M., Rahmani M., Emami H., Mirmiran P., Hadjipour R. Tehran Lipid and Glucose Study (TLGS): rationale and design. Iranian Journal of Endocrinology and Metabolism. 2000;2(2):77–86. [Google Scholar]
- Azizi F., Hadaegh F., Khalili D., Esteghamati A., Hosseinpanah F., Delavari A., et al. Appropriate definition of metabolic syndrome among Iranian adults: report of the Iranian National Committee of Obesity. Arch. Iran. Med. 2010;13(5):426–428. [PubMed] [Google Scholar]
- Azizi F., Zadeh-Vakili A., Takyar M. Review of Rationale, Design, and Initial Findings: Tehran Lipid and Glucose Study. Int. J. Endocrinol. Metab. 2018;16(4 Suppl):e84777. doi: 10.5812/ijem.84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.A., Adams E.K., Yang Z., Fulton J.E. Percentage of deaths associated with inadequate physical activity in the united states. Prev. Chronic Dis. 2018;15 doi: 10.5888/pcd18.170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.R., Russell B.M., Dempsey P.C., Christie H.E., Campbell M.D., Francois M.E. Accumulating Physical Activity in Short or Brief Bouts for Glycemic Control in Adults With Prediabetes and Diabetes. Can. J. Diabetes. 2020;44(8):759–767. doi: 10.1016/j.jcjd.2020.10.013. [DOI] [PubMed] [Google Scholar]
- Colberg S.R., Sigal R.J., Yardley J.E., Riddell M.C., Dunstan D.W., Dempsey P.C., Horton E.S., Castorino K., Tate D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus R.S., Breitkopf C.R., Rutten L.J., Jacobson D.J., Wilson P.M., Sauver J.S. Incidence Rate of Prediabetes Progression to Diabetes: Modeling an Optimum Target Group for Intervention. Popul. Health Manag. 2017;20(3):216–223. doi: 10.1089/pop.2016.0067. [DOI] [PubMed] [Google Scholar]
- Færch K., Witte D.R., Brunner E.J., Kivimäki M., Tabák A., Jørgensen M.E., Ekelund U., Vistisen D. Physical Activity and Improvement of Glycemia in Prediabetes by Different Diagnostic Criteria. J. Clin. Endocrinol. Metab. 2017;102(10):3712–3721. doi: 10.1210/jc.2017-00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glechner A., Keuchel L., Affengruber L., Titscher V., Sommer I., Matyas N., Wagner G., Kien C., Klerings I., Gartlehner G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Primary care diabetes. 2018;12(5):393–408. doi: 10.1016/j.pcd.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Spertus J.A., Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Jadhav R.A., Hazari A., Monterio A., Kumar S., Maiya A.G. Effect of Physical Activity Intervention in Prediabetes: A Systematic Review With Meta-analysis. J. Phys. Act. Health. 2017;14(9):745–755. doi: 10.1123/jpah.2016-0632. [DOI] [PubMed] [Google Scholar]
- James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., Lackland D.T., LeFevre M.L., MacKenzie T.D., Ogedegbe O., Smith S.C., Svetkey L.P., Taler S.J., Townsend R.R., Wright J.T., Narva A.S., Ortiz E. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Kriska A.M., Knowler W.C., LaPorte R.E., Drash A.L., Wing R.R., Blair S.N., Bennett P.H., Kuller L.H. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;4:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- Lee I.-M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Modeler. Handling Outliers and Extreme Values 2021 [Dec 18, 2022]. Available from: https://www.ibm.com/docs/en/spss-modeler/18.2.0?topic=tab-handling-outliers-extreme-values.
- Momenan A.A., Delshad M., Mirmiran P., Ghanbarian A., Azizi F. Leisure Time Physical Activity and Its Determinants among Adults in Tehran: Tehran Lipid and Glucose Study. Int. J. Prev. Med. 2011;2(4):243–251. [PMC free article] [PubMed] [Google Scholar]
- Momenan A.A., Delshad M., Sarbazi N., Rezaei Ghaleh N., Ghanbarian A., Azizi F. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch. Iran. Med. 2012;15(5):279–282. [PubMed] [Google Scholar]
- Naseri P., Amiri P., Masihay-Akbar H., Jalali-Farahani S., Khalili D., Azizi F. Long-term incidence of cardiovascular outcomes in the middle-aged and elderly with different patterns of physical activity: Tehran lipid and glucose study. BMC Public Health. 2020;20(1):1–10. doi: 10.1186/s12889-020-09747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault L., Kahn S.E., Christophi C.A., Knowler W.C., Hamman R.F. Diabetes Prevention Program Research G. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care. 2009;32(9):1583–1588. doi: 10.2337/dc09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Chen K., Chen J., Zhang J. Trends in adherence to recommended physical activity and its effects on cardiometabolic markers in US adults with pre-diabetes. Trends in adherence to recommended physical activity and its effects on cardiometabolic markers in US adults with pre-diabetes. 2022;10(5):e002981. doi: 10.1136/bmjdrc-2022-002981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Z., Shah I., Malik M.O., Ullah I. Effect of short duration moderate intensity physical activity on glycemic control and antioxidant status of prediabetic population. Saudi Med. J. 2021;42(6):660–665. doi: 10.15537/smj.2021.42.6.20210019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikholeslami S., Ghanbarian A., Azizi F. The Impact of Physical Activity on Non-communicable Diseases: Findings from 20 Years of the Tehran Lipid and Glucose Study. Int. J. Endocrinol. Metab. 2018;16(4 Suppl):e84740. doi: 10.5812/ijem.84740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.D., Crippa A., Woodcock J., Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527–2545. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidi M., Ghasemi A., Hadaegh F., Derakhshan A., Chary A., Azizi F. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy Iranian adults: Tehran Lipid and Glucose Study. Clin. Biochem. 2014;47(6):432–438. doi: 10.1016/j.clinbiochem.2014.02.007. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Available at : https://health.gov/sites/default/files/2019-09/paguide.pdf.
- World Health Organization . World Health Organization; 2018. Global action plan on physical activity 2018–2030: more active people for a healthier world: at-a-glance. [Google Scholar]
- Zhang J., Yu K.F. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
Further reading
- CfD C. Prevention; Physical activity guidelines for Americans: 2008. Prevention, Control CfD. [Google Scholar]
- Committee IR. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms. http://www ipaq ki se/scoring pdf. 2005.
- Gjersvik P. This is a good title. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2013;133(2):129. doi: 10.4045/tidsskr.12.1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be presented upon forwarding the request to the corresponding author (mirmiran@endocrine.ac.ir) and confirmation of the director of RIES (azizi@endocrine.ac.ir).
Data will be made available on request.