Abstract
Objective:
To estimate the proportion of cases of Crohn’s disease (CD) and ulcerative colitis (UC) that could be prevented by modifiable lifestyle factors.
Design:
In a prospective cohort study of US adults from the Nurses’ Health Study (NHS; n=72,290), NHSII (n=93,909), and Health Professionals Follow-up Study (HPFS; n=41,871), we created modifiable risk scores (MRS; 0–6) for CD and UC based on established lifestyle risk factors, and healthy lifestyle scores (HLS; 0–9) derived from American healthy lifestyle recommendations. We calculated the population attributable risk by comparing the incidence of CD and UC between low (CD-MRS≤1, UC-MRS≤2, HLS≥7) and high-risk groups. We externally validated our findings in three European cohorts: the Swedish Mammography Cohort (n=37,275), Cohort of Swedish Men (n=40,810), and European Prospective Investigation into Cancer and Nutrition (n=404,144).
Results:
Over 5,117,021 person-years of follow-up (NHS, HPFS: 1986–2016; NHSII: 1991–2017), we documented 346 CD and 456 UC cases. Adherence to a low MRS could have prevented 42.9% (95% CI 12.2 to 66.1%) of CD and 44.4% (95% CI 9.0 to 69.8%) of UC cases. Similarly, adherence to a healthy lifestyle could have prevented 61.1% (95% CI 16.8 to 84.9%) of CD and 42.2% (95% CI 1.7 to 70.9%) of UC cases. In our validation cohorts, adherence to a low MRS and healthy lifestyle could have respectively prevented 43.9–51.2% and 48.8–60.4% of CD cases, and 20.6–27.8% and 46.8–56.3% of UC cases.
Conclusions:
Across six US and European cohorts, a substantial burden of IBD risk may be preventable through lifestyle modification.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) that affect an estimated 3.1 million adults in the US1 and another 1.3 million in Europe2. Globally, the incidence of IBD is increasing, particularly in newly industrialized countries3. IBD is associated with significant societal cost, with an estimated annual healthcare cost of $23,000 per-person in the US4. Thus, strategies to prevent IBD could substantially decrease morbidity associated with disease and healthcare costs. However, to date, no strategies exist to prevent the development of IBD.
One approach to prevent chronic diseases is via modification of lifestyle risk factors. Indeed, previous observational studies have identified several lifestyle factors to be associated with IBD5, but whether modification of these lifestyle factors could be an attractive prevention strategy is unknown. Thus, in this study, we created modifiable risk scores (MRS) based on established risk factors for IBD and estimated the proportion of IBD cases that could have been prevented using population attributable risk (PAR). As some of the established risk factors such as smoking and body mass index (BMI) have opposite associations with CD and UC6,7, we also estimated the proportion of cases that could be prevented by adhering to an overall healthy lifestyle, as recommended by the US Department of Health and Human Services (HHS), the US Department of Agriculture (USDA), and the American Heart Association (AHA).
METHODS
Study population
Our primary cohort included participants from three prospective cohorts: the Nurses’ Health Study (NHS), NHSII, and Health Professionals Follow-up Study (HPFS). Briefly, the NHS enrolled 121,700 female nurses (30–55 years) across 11 US states in 1976, while the NHSII cohort, established in 1989, followed a younger cohort of 116,429 female nurses (25–42 years) from 15 US states8. The HPFS cohort enrolled 51,529 male physicians (40–75 years) across all 50 states in 19869. Participants completed baseline and biennial questionnaires that assessed lifestyle factors, anthropomorphic data, and medical history. Dietary information was collected every four years via semi-quantitative food frequency questionnaires (SFFQ) beginning in 1986 for NHS and HPFS and 1991 for NHSII (defined as baseline). Follow-up rates for these cohorts have consistently exceeded 85%8,9.
We excluded participants who had missing baseline SFFQ or implausible daily caloric intake (<600 or >3500 kcal/day for women, <800 or >4200 kcal/day for men; n=67,671 (23%)), those who only completed baseline questionnaire (n=8,177 (2.8%)), those with a diagnosis of IBD at baseline (n=144 (0.05%)), and missing or implausible BMI (BMI <10 kg/m2; n=1,468 (0.5%)). The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health (#2001P001128).
We also used three large European cohorts to externally replicate our results: the Swedish Mammography Cohort (SMC; n=37,275), the Cohort of Swedish Men (CoSM; n=40,810), and the European Prospective Investigation into Cancer and Nutrition (EPIC; n=404,144; Supplementary Appendix). Briefly, the SMC and CoSM are parallel cohorts of females (40–74 years) and males (45–79 years)10, respectively, in Sweden, while the EPIC cohort is composed of both males and females (35–70 years) across 10 European countries11. Detailed medical, lifestyle, and dietary information were collected at baseline (1997 for SMC and CoSM, 1992–1999 for EPIC) via self-administered questionnaires in all cohorts (Supplementary Appendix).
Patient and Public Involvement
Patient or the public were not involved in the design or interpretation of this study.
Ascertainment of IBD diagnosis
Ascertainment of IBD diagnoses in NHS, NHSII and HPFS has been previously described in detail12. Briefly, participants first self-reported diagnoses of either CD or UC in biennial questionnaires. Supplementary questionnaires were then mailed requesting detailed information on IBD diagnoses and permission to review medical records. Records were then reviewed by two gastroenterologists blinded to exposure information. IBD cases were confirmed by endoscopic and histopathology findings and date of diagnosis defined as date of index endoscopy or surgery and pathology results. IBD cases in the validation cohorts were ascertained either through medical record review or validated definitions used in patient registers (Supplementary Appendix).
Assessment of lifestyle risk factors and other covariates
Briefly, non-dietary factors including BMI, family history of IBD, history of appendectomy (self-reported), physical activity, smoking status, and non-steroidal anti-inflammatory drug (NSAID) use were assessed from baseline and follow-up questionnaires. Dietary factors, including daily servings of fruit and vegetables and red meat, fiber intake in grams (g), and ratio of n3:n6 polyunsaturated fatty acid (PUFA) intake were ascertained using frequency of intake reported on every 4-year SFFQ and the Harvard Food Composition Database to calculate nutrient-level data13. BMI, smoking status, and NSAID use were updated every 2 years while physical activity and dietary variables were cumulative-averaged over the follow up time to better represent long-term patterns14. In external cohorts, covariates were ascertained at baseline only. Further details for the variables assessed in the primary and external cohorts are described in the Supplementary Appendix.
Statistical Analysis
We constructed MRS for each of CD and UC (CD-MRS and UC-MRS) based on established modifiable risk factors, including BMI6,15, smoking status7, NSAID use16, physical activity17, and daily consumption of fruit and vegetables18, fiber19,20, n3:n6 PUFAs21, and red meat 22,23. The directed acyclic graph for the proposed relationship between risk factors and outcomes is shown in Figure S1 (created using DAGitty v3.0)24. We defined low-risk criteria for each factor based on observed associations from prior literature, some of which had opposite relationships with CD and UC (Table 1)5. For example, never-smoking and non-obese BMI were considered low-risk for CD, while current-smoking and obese BMI were considered low-risk for UC6,7,15. For each participant, we assigned 1 point to each factor not meeting its low-risk criterion (0 otherwise) and summed each category for a total MRS of 0 to 6 points, so that higher scores reflected a greater number of disease-specific risk factors. The low-risk group (reference) was defined as a score 0–1, or 0–2 when there were too few cases of CD and UC (defined by non-convergence of the models) in the 0–1 group.
Table 1.
Associations between modifiable risk factors and Crohn’s disease or ulcerative colitis, and definitions for ‘low-risk’ criteria used in calculation of modifiable risk scores (MRS).
| Crohn’s disease | Ulcerative Colitis | ||
|---|---|---|---|
| Risk Factor | “Low-Risk” Criterion for MRS | Risk Factor | “Low-Risk” Criterion for MRS |
| BMI6,15 | < 30 kg/m2 | BMI6 | ≥ 30 kg/m2 |
| Smoking7 | Never smokers | Smoking7 | Current smokers |
| NSAIDs16 | < 2x/week | NSAIDs16 | < 2x/week |
| Physical activity17 | Highest quintile (MET-hrs/wk) | Fruit & vegetables18 | Highest quintile (servings/d) |
| Fruit & vegetables18 | Highest quintile (servings/d) | Red meat22,23 | Lowest quintile (servings/d) |
| Fiber19,20 | Highest quintile (grams/d) | n3:n6 PUFA21 | Highest quintile (servings/d) |
BMI Body mass index. MET Metabolic equivalent of task. NSAIDs Non-steroidal anti-inflammatory drugs. PUFA Polyunsaturated fatty acid.
Additionally, we note that adherence to low-risk factors did not necessarily represent healthy habits, particularly for UC, where current smoking and obese BMI are protective. Thus, we additionally constructed healthy lifestyle scores (HLS), to assess adherence to healthy lifestyle recommendations by the US HHS and USDA Dietary Guidelines for Americans, and the AHA Guidelines for Healthy Living25–27 (Supplementary Appendix). Healthy criteria were defined as BMI ≥18.5 to <25 kg/m2; never smoking; physical activity ≥7.5 metabolic equivalent of task (MET)-hours/week; intakes of fruit and vegetables ≥8 servings/day, red meat <0.5 servings/day, fiber ≥25 g/day, fish ≥2 servings/week, nuts/seeds ≥0.5 servings/day; and alcohol consumption ≤1 drink (14g)/day (females) or ≤2 drinks (28g)/day (males; Table S1). We assigned 1 point for each healthy criterion met, and calculated HLS by summing across all categories (range 0–9), such that higher scores reflected healthier lifestyle. The healthy group (reference) was defined as a score of 7–9, as insufficient number of participants met 8 or 9 criteria, and the unhealthy group was defined as a score < 7.
We calculated person-time from date of return of baseline questionnaire to first of: date of IBD diagnosis, death, date of last returned biennial questionnaire, or end of follow-up (2016 for NHS, HPFS; 2017 for NHSII). We used Cox proportional hazards models to estimate multivariable-adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for CD and UC according to CD- and UC-MRS, respectively, as well as HLS. Models were stratified by age, time-period (2-year intervals), and cohort (NHS, NHSII, or HPFS), and were additionally adjusted for appendectomy and family history of IBD5.
In analyses that used NHS, NHSII and HPFS data only, all covariates except family history of IBD were modeled as time-varying. However, because EPIC only collected dietary and lifestyle data at baseline, analyses that compared NHS, NHSIII and HPFS data with the external cohorts were done using baseline data only.
We calculated the population attributable risk (PAR) for CD and UC to estimate the proportion of cases that could have been prevented through lifestyle modification, assuming a causal relationship. We used a binary term to compare (1) high- versus low-risk groups and (2) unhealthy versus healthy groups, as has been previously described28,29. For PAR calculations, exposure prevalence and aHRs were derived separately for each of the pooled NHS, NHSII and HPFS cohorts, pooled SMC and CoSM cohorts, and EPIC cohort. In this way, PAR could be interpreted as the proportion of cases in each cohort that could have been prevented if all individuals had been in the (1) low-risk group or (2) healthy group, assuming a causal relationship exists.
We conducted several exploratory and sensitivity analyses. First, we examined whether the relationship between MRS and IBD differed according to sex. Second, because the binary variables in MRS calculation could not account for incremental changes in IBD risk, we derived weighted-MRS scores (range 6–30) using refined categories of lifestyle factors, and used individuals in the lowest 15% of scores as the reference (Supplementary Appendix). Third, we estimated partial PAR for individual modifiable risk factors28. Fourth, because of the previously reported association between processed meat intake and risk of IBD30, we repeated our primary analysis, replacing red meat intake with processed meat intake for calculation of UC-MRS and including a term for processed meat in derivation of CD-MRS. Finally, to demonstrate that our scores are relatively specific to IBD, we performed a falsification analysis to determine the relationship between our MRS and rheumatoid arthritis (RA), a similar immune-mediated disease (Supplementary Appendix). We chose RA because it shares multiple risk factors with CD31,32, but has an inverse relationship with several factors used in the UC-MRS, such as BMI and smoking. In this way, we expect a priori that the PAR for RA according to CD-MRS might yield comparable results as in our primary analysis for CD, but that the PAR for RA according to UC-MRS should be substantially lower or zero. To further test the specificity of our scores for IBD, we performed additional falsification analyses for two non-immune mediated diseases, colorectal cancer (CRC) and cardiovascular disease (CVD; Supplementary Appendix).
Statistical calculations were performed in SAS version 9.4 (SAS Institute Inc, Cary, NC) and STATA 16.1/MP (StataCorp LLC), and statistical significance was defined as p < 0.05 using two-tailed tests. The proportional hazards assumption was tested by testing for significance of interaction terms between follow-up time and CD- and UC-MRS (CD: P=0.83, UC: P=0.08; Supplementary Appendix). Residual confounding for the primary analysis was assessed using the E-value method (Supplementary Appendix)33.
RESULTS
In our primary cohort, a total of 208,070 participants were included after exclusions (NHS: n=72,290, NHSII: n=93,909, and HPFS: n=41,871). During 5,117,021 person-years of follow-up, we ascertained 346 CD and 456 UC cases, with an incidence rate of 7 cases of CD and 9 cases of UC per 100,000 person-years. Baseline characteristics for the pooled primary cohort are shown in Table S2.
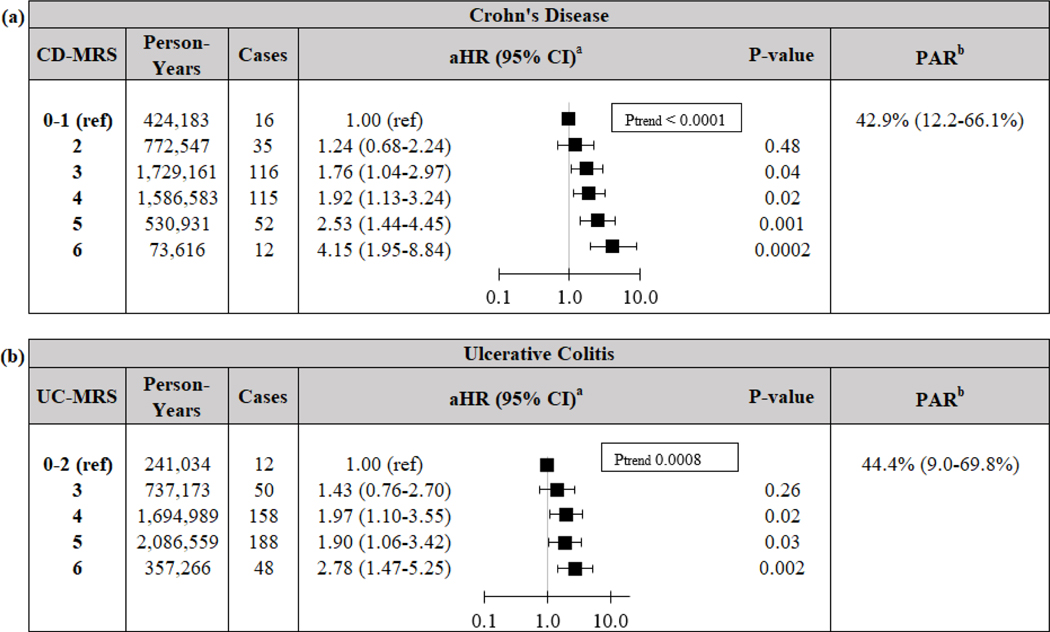
Compared to participants with a CD-MRS of 0–1, the aHR (95%CI) of those with a CD-MRS of 6 was 4.15 (1.95–8.84; Figure 1). Similarly, compared to those with a UC-MRS of 0–2, the aHR (95%CI) of those with a UC-MRS of 6 was 2.78 (1.47–5.25). Risk of CD and UC increased with each one-point increase in CD-MRS (Ptrend<0.0001) and UC-MRS (Ptrend=0.008), respectively. Our findings were similar for both females and males (all Pinteraction>0.19; Table S3). When using binary scores, those with a CD-MRS ≥ 2 had an aHR (95% CI) of 1.85 (1.12–3.06; p=0.02) for CD when compared to those with a score of 0–1. Similarly, those with a UC-MRS ≥ 3 had an aHR (95% CI) of 1.92 (1.08–3.40; p=0.03 for UC when compared to those with a score of 0–2.
Figure 1.
Risk and PAR of (a) Crohn’s disease and (b) ulcerative colitis according to modifiable risk scores. Reference level for UC set to 0–2 given low number of scores 0–1. a Cox models stratified by age (months), time-period (2-year intervals), and cohort (NHS, NHSII, or HPFS), and adjusted for appendectomy (yes/no), and family history of IBD (yes/no). b PAR for binary comparison between high-MRS (2+ risk factors for CD or 3+ risk factors for UC) and low-MRS (reference), adjusted for age (<40, 40 ≤ age <60, ≥ 60 years), cohort (NHS, NHSII, HPFS), appendectomy (yes/no) and family history of IBD (yes/no). CD Crohn’s Disease. CI Confidence Interval. aHR Multivariable-adjusted Hazard Ratio. MRS Modifiable Risk Score. PAR Population Attributable Risk. UC Ulcerative Colitis.
We estimated that adherence to low CD-MRS (0–1) and UC-MRS (0–2) could have prevented 42.9% (12.2–66.1%) of CD and 44.4% (9.0–69.8%) of UC, respectively (PAR; Figure 1). These findings were similar when incorporating processed meat intake in derivation of CD-MRS and UC-MRS (Supplementary Appendix). In a sensitivity analysis using weighted-criteria to define MRS, adherence to low CD-MRS and UC-MRS (lowest 15% of scores) could have prevented 41.0% (17.5–60.0%) of CD and 27.7% (7.5–45.7%) of UC (Table S4).
Falsification analysis yielded anticipated results (Supplementary Appendix). Adherence to low CD-MRS (0–1) could have prevented 32.3% (0.4–58.3%) of RA, 13.3% (2.3–23.9%) of CRC, and 14.0% (9.6–18.5%) of CVD. Conversely, adherence to low UC-MRS (0–2) was associated with higher risk of RA, CRC, and CVD compared to the UC-MRS > 2 group, and therefore PAR for adherence to a low UC-MRS could not be calculated. In other words, adherence to low UC-MRS could not prevent RA, CRC, or CVD in our cohorts (Supplementary Appendix).
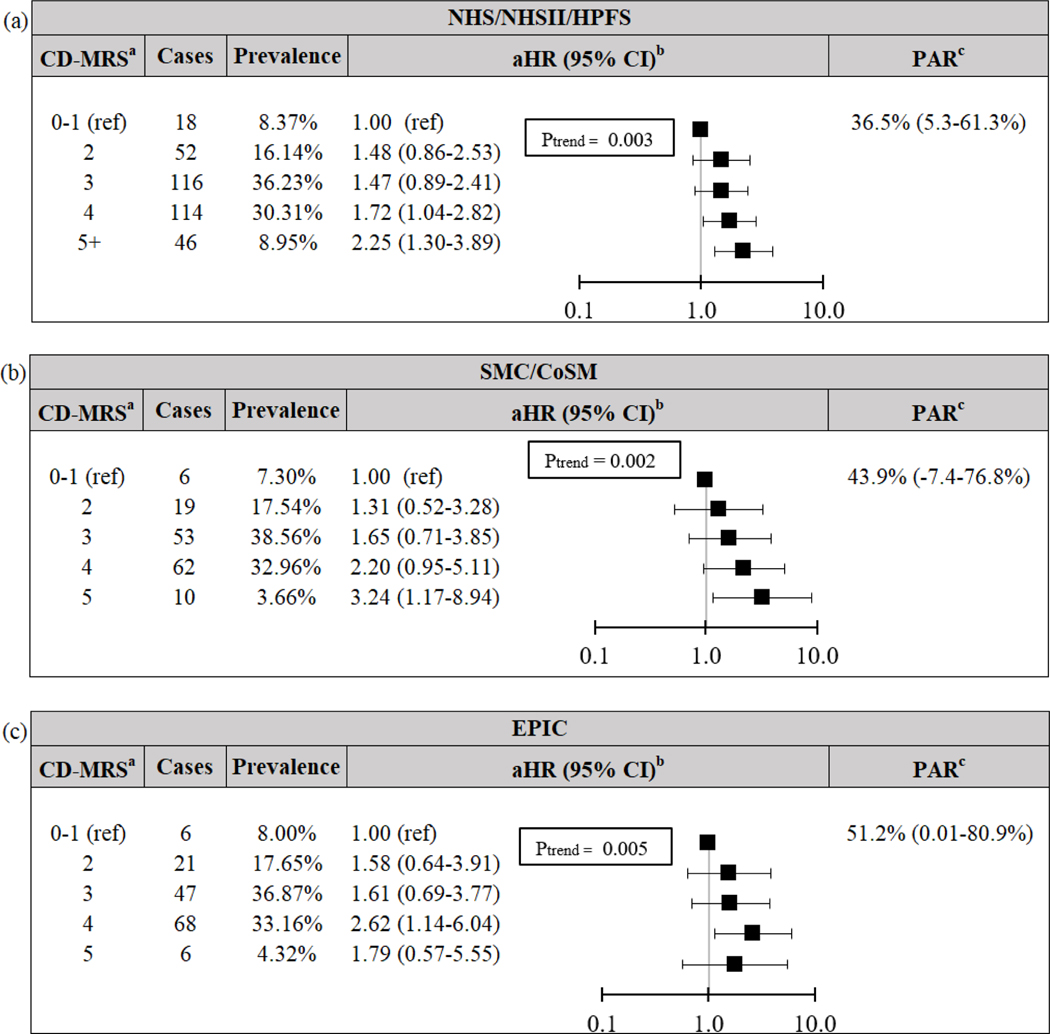
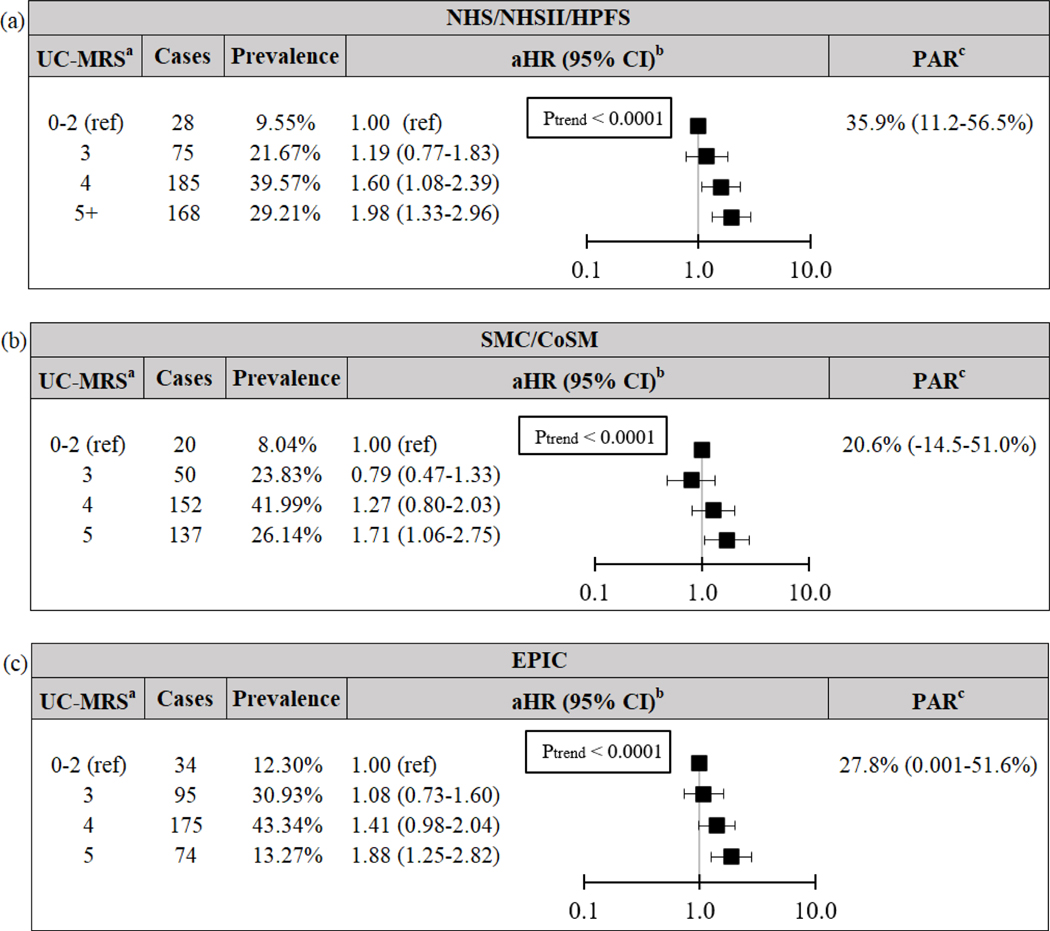
We also confirmed our primary findings using baseline data. In the pooled NHS, NHSII and HPFS cohort, baseline CD-MRS and UC-MRS remained significantly associated with increased risk of CD and UC, respectively (both Ptrend≤0.003; Figures 2–3). For CD, adherence to low baseline CD-MRS (0–1) could have prevented 36.5% (5.3–61.3%) of CD, while adherence to low baseline UC-MRS (0–2) could have prevented 35.9% (11.2–56.5%) of UC.
Figure 2.
Risk and PAR of Crohn’s disease according to baseline modifiable risk score for (a) pooled NHS/NHSII/HPFS cohort, (b) pooled SCM/CoSM cohort, and (c) EPIC cohort. aNSAID data missing from external cohorts, thus maximum MRS = 5. bCox models adjusted for baseline age (years) and cohort. cPAR for 2+ risk factors compared to reference (0–1), adjusted for age (<40, 40 ≤ age <60, ≥ 60 years) and cohort. CD Crohn’s Disease. CI Confidence Interval. CoSM Cohort of Swedish Men. EPIC European Prospective Investigation into Cancer and Nutrition. HPFS Health Professional’s Follow-up Study. aHR Multivariable-adjusted Hazard Ratio. MRS Modifiable Risk Score. NHS Nurses’ Health Study. PAR Population Attributable Risk. SMC Swedish Mammography Cohort.
Figure 3.
Risk and PAR of ulcerative colitis according to baseline modifiable risk score for (a) pooled NHS/NHSII/HPFS cohort, (b) pooled SCM/CoSM cohort, and (c) EPIC cohort. aNSAID data missing from external cohorts, thus maximum MRS = 5. UC-MRS adapted such that “low-risk” criterion for smoking defined as never-smoking only. Given the low incidence of UC-MRS 0–1, reference level set to 0–2. bCox models adjusted for baseline age (years) and cohort. cPAR for 3+ risk factors compared to reference, adjusted for age (<40, 40 ≤ age <60, ≥ 60 years) and cohort. CI Confidence Interval. CoSM Cohort of Swedish Men. EPIC European Prospective Investigation into Cancer and Nutrition. HPFS Health Professional’s Follow-up Study. aHR Multivariable-adjusted Hazard Ratio. MRS Modifiable Risk Score. NHS Nurses’ Health Study. PAR Population Attributable Risk. SMC Swedish Mammography Cohort. UC Ulcerative colitis.
Our findings were similar in the external cohorts. For CD, low baseline CD-MRS (0–1) could have prevented 43.9% (−7.4–76.8%) and 51.2% (0.01–80.9%) of CD in the pooled SMC and CoSM cohort and EPIC, respectively. Similarly, for UC, low baseline UC-MRS (0–2) could have prevented 20.6% (−14.5–51.0%) and 27.8% (0.001–51.6%) of UC in the pooled SMC and CoSM cohort and EPIC, respectively.
We also calculated the proportion of IBD cases that could have been prevented by adherence to American healthy lifestyle guidelines. In the pooled NHS, NHSII and HPFS cohort, baseline HLS was associated with decreased risk of CD and UC (Ptrend≤0.004 and 0.02, respectively; Table 2). Adherence to a healthy lifestyle (HLS 7–9) could have prevented 61.1% (16.8–84.9%) of CD and 42.2% (1.7–70.9%) of UC cases. These results were consistent in external cohorts (Table 2). Adherence to a healthy lifestyle could have prevented 48.8% (−37.4–89.8%) and 60.4% (4.1–87.6%) of CD in the pooled SMC and CoSM cohort and EPIC, respectively, and 56.3% (1.3–85.1%), and 46.8% (9.7–72.5%) of UC in the pooled SMC and CoSM cohort and EPIC, respectively.
Table 2.
Preventable fraction of CD and UC cases due to American healthy lifestyle recommendations.
| NHS/NHSII/HPFS | ||||
|---|---|---|---|---|
| Crohn’s Disease | ||||
| Healthy lifestyle score a | Cases | Prevalence | aHR (95% CI) b | PAR (95% CI) c |
| 7–9 | 5 | 4.24% | 1.00 (ref) | 61.1% (16.8 – 84.9%) |
| 6 | 29 | 7.44% | 3.13 (1.21–8.09) | |
| 5 | 37 | 16.50% | 1.67 (0.65–4.26) | |
| 4 | 92 | 26.06% | 2.58 (1.05–6.37) | |
| 3 | 91 | 25.59% | 2.57 (1.04–6.34) | |
| 2 | 71 | 15.18% | 3.42 (1.38–8.51) | |
| 0–1 | 21 | 4.99% | 3.01 (1.13–8.02) | |
| Ptrend | 0.004 | |||
| Ulcerative Colitis | ||||
| Healthy lifestyle score a | Cases | Prevalence | aHR (95% CI) b | PAR (95% CI) c |
| 7–9 | 10 | 4.24% | 1.00 (ref) | 42.2% (1.7 – 70.9%) |
| 6 | 30 | 7.44% | 1.58 (0.77–3.23) | |
| 5 | 71 | 16.50% | 1.61 (0.83–3.13) | |
| 4 | 109 | 26.06% | 1.50 (0.78–2.87) | |
| 3 | 132 | 25.59% | 1.85 (0.97–3.53) | |
| 2 | 72 | 15.18% | 1.72 (0.88–3.34) | |
| 0–1 | 32 | 4.99% | 2.39 (1.17–4.87) | |
| Ptrend | 0.02 | |||
| SMC/CoSM | ||||
|---|---|---|---|---|
| Crohn’s Disease | ||||
| Healthy lifestyle score a | Cases | Prevalence | aHR (95% CI) b | PAR (95% CI) c |
| 7–9 | 2 | 2.62% | 1.00 (ref) | 48.8% (−37.4 – 89.8%) |
| 6 | 16 | 10.19% | 1.99 (0.46–8.66) | |
| 5 | 34 | 23.32% | 1.86 (0.45–7.74) | |
| 4 | 36 | 30.88% | 1.51 (0.36–6.27) | |
| 3 | 39 | 22.31% | 2.27 (0.55–9.41) | |
| 2 | 19 | 8.79% | 2.85 (0.66–12.23) | |
| 0–1 | 4 | 1.89% | 2.77 (0.51–15.14) | |
| Ptrend | 0.08 | |||
| Ulcerative Colitis | ||||
| Healthy lifestyle score a | Cases | Prevalence | aHR (95% CI) b | PAR (95% CI) c |
| 7–9 | 4 | 2.62% | 1.00 (ref) | 56.3% (1.3 – 85.1%) |
| 6 | 21 | 10.19% | 1.27 (0.44–3.70) | |
| 5 | 79 | 23.32% | 2.10 (0.77–5.72) | |
| 4 | 123 | 30.88% | 2.52 (0.93–6.83) | |
| 3 | 82 | 22.31% | 2.35 (0.86–6.41) | |
| 2 | 41 | 8.79% | 3.03 (1.09–8.46) | |
| 0–1 | 9 | 1.89% | 3.05 (0.94–9.89) | |
| Ptrend | 0.0004 | |||
| EPIC | ||||
|---|---|---|---|---|
| Crohn’s Disease | ||||
| Healthy lifestyle score a | Cases | Prevalence | aHR (95% CI) b | PAR (95% CI) c |
| 7–9 | 4 | 6.50% | 1.00 (ref) | 60.4% (4.1 – 87.6%) |
| 6 | 17 | 12.48% | 2.13 (0.71 – 6.34) | |
| 5 | 18 | 22.62% | 1.21 (0.41 – 3.57) | |
| 4 | 52 | 27.40% | 2.82 (1.02 – 7.81) | |
| 3 | 43 | 20.45% | 3.09 (1.11 – 8.63) | |
| 2 | 12 | 8.86% | 1.97 (0.64 – 6.12) | |
| 0–1 | 4 | 1.69% | 3.43 (0.86 – 13.76) | |
| Ptrend | 0.005 | |||
| Ulcerative Colitis | ||||
| Healthy lifestyle score a | Cases | Prevalence | aHR (95% CI) b | PAR (95% CI) c |
| 7–9 | 13 | 6.50% | 1.00 (ref) | 46.8% (9.7 – 72.5%) |
| 6 | 29 | 12.48% | 1.21 (0.62 – 2.38) | |
| 5 | 65 | 22.62% | 1.45 (0.78 – 2.68) | |
| 4 | 123 | 27.40% | 2.20 (1.22 – 3.98) | |
| 3 | 103 | 20.45% | 2.43 (1.34 – 4.42) | |
| 2 | 44 | 8.86% | 2.37 (1.25 – 4.48) | |
| 0–1 | 7 | 1.69% | 1.97 (0.78 – 4.99) | |
| Ptrend | <0.001 | |||
Maximum value set to 7+ given few participants with scores of 8–9 in primary cohort.
Cox models adjusted for baseline age (years) and cohort (NHS, NHSII, HPFS, SMC, CoSM).
PAR for HLS ≤ 6 compared to reference category (7–9), adjusted for age (<40, 40 ≤ age <60, ≥ 60 years) and cohort. CD Crohn’s Disease. CI Confidence Interval. aHR Multivariable-adjusted Hazard Ratio. PAR Population Attributable Risk. UC Ulcerative Colitis.
Additionally, we explored the contribution of individual lifestyle factors and risk of CD and UC in our primary cohorts (Tables S5–S6). Low fiber intake conferred the largest PAR for CD (27.9%) followed by past or current smoking (14.4%), and low physical activity (12.9%; Table S7). Low fruit and vegetable intake contributed the largest PAR for UC (20.1%), followed by past smoking (18.0%) and low n3:n6 PUFA (11.0%; Table S7). In comparison, family history of IBD conferred a PAR of 12.2% (8.0–16.2%) for CD and 8.8% (5.4–12.1%) for UC.
Finally, we used the E-value method to assess for residual confounding in the relationship between binary MRS scores and IBD used in our primary PAR analysis (Table S8). To explain an aHR of 1.85 for CD with a CD-MRS ≥ 2, an unmeasured confounder would need to have a risk ratio of 3.10 with each of the CD-MRS exposure and CD outcome, after controlling for the measured confounders, to fully explain away the observed relationship. Similarly, the observed aHR of 1.92 for UC with a UC-MRS ≥ 3 would need to be explained by an unmeasured confounder that was associated with a 3.25-fold risk with each of the UC-MRS exposure and UC outcome, after controlling for measured confounders. Weaker confounding could otherwise not explain away the observed relationships33.
DISCUSSION
In three large prospective US cohorts, we demonstrate that modifiable lifestyle factors could substantially decrease the burden of IBD. We found that 43% of CD and 44% of UC cases could have been prevented by adhering to low-risk modifiable lifestyle factors, assuming a causal relationship exists. Moreover, adherence to American healthy lifestyle recommendations could have prevented 61% of CD cases and 42% of UC cases. These findings were consistent across three European cohorts. In comparison, in our primary cohorts, family history of IBD had a modest PAR of 12% for CD and 9% for UC.
Few studies have examined the total contribution of lifestyle factors on IBD development at a population level. In an Italian cohort, smoking, oral contraceptive use, and lack of breast feeding accounted for roughly 30% of the attributable risk for IBD34, while Brant et al estimated that current tobacco use conferred a 47% attributable risk for CD35. To our knowledge, our study represents the first to comprehensively assess the contribution of modifiable lifestyle and dietary factors to the risk of CD and UC. Nonetheless, our estimates are similar to those published for other immune-mediated diseases. For example, in two prior studies, modification of lifestyle risk factors could have prevented 41% of RA36 and 48% of psoriasis cases37. Further, similar to our study, family history had only a modest contribution to risk of RA and psoriasis (~20% each).
Importantly, our data suggest that adherence to an overall healthy lifestyle may prevent a modest proportion of cases of CD and UC. While unhealthy factors such as obesity and smoking have been inversely associated with risk of UC6,38, we saw that their contribution was outweighed by the total effect of healthy living. That is, more UC cases could have been prevented by adherence to a healthy lifestyle (42–56%) as compared to adherence to ‘traditional’ UC risk factors assessed by our UC-MRS scores (21–44%). Thus, current guidelines for healthy living, which are primarily recommended to reduce cardiovascular disease risk, may have additional benefits for prevention of other immune-mediated diseases such as IBD.
A key assumption of our findings is that the relationship between lifestyle factors and IBD development is causal. Though this has yet to be established, several lines of evidence support the critical role of environmental and lifestyle factors in development of IBD. First, in genome-wide association studies, genetic factors account for less than 15% of the total variance of IBD39. Similarly, in monozygotic twins, concordance for disease is estimated to be around only 15% for UC and 30% for CD40,41. Second, the high incidence of IBD in industrialized societies and sharp rise of IBD in developing countries also suggest that Westernization of diet and environment influences disease development3. Further, in immigrants who move from low- to high-incidence countries, risk for IBD is higher in second-versus first-generation immigrants42. Finally, the dietary and lifestyle factors considered here have also been linked with systemic inflammation, microbial dysbiosis, and gut permeability, providing mechanistic plausibility for a causal relationship43–46. Thus, although family history of IBD was the single strongest risk factor for IBD in our cohorts (aHR (95%CI) = 4.53 (3.38–6.07) for CD and 3.24 (2.45–4.29) for UC), the collective impact of environmental factors on IBD development is likely greater.
Though there are currently no known disease prevention strategies for CD and UC, dietary and lifestyle modifications may change the immunologic and microbiologic milieu necessary for disease development and therefore could serve as a strategy for IBD prevention. This may be of particular relevance to high-risk groups, such as first-degree relatives of IBD patients, who have an estimated 2–17% risk of developing the disease over their lifetime47. Similar strategies have been applied in other immune-mediated diseases, including type I diabetes48 and in unaffected first-degree relatives of those with RA49.
Our study has several strengths. Exposure data were collected prospectively, minimizing the risk of recall or selection bias. Diet and physical activity variables were cumulatively averaged to account for long-term patterns. We used validated methods to assess lifestyle factors across all cohorts50,51, and updated them over time to minimize exposure misclassification. Compared to prior studies, we considered a comprehensive list of modifiable lifestyle factors in the quantification of PAR, and avoided use of non-modifiable factors, pre-clinical markers of disease, and surrogates for proximal disease exposures in our MRS52. Further, falsification analysis demonstrated that our scores are relatively specific for IBD. For example, though associations and PAR estimates were similar for RA, a chronic immune-mediated disease with shared risk factors for CD, the corresponding PARs, and therefore preventable cases, were lower in CRC and CVD in spite of similar direction of association. This is largely due to differences in strength of associations and prevalence of risk factors, and presence of other modifiable risk factors such as alcohol and medications or supplements which are strongly associated with these other conditions29,53. We also note that the follow up period in our cohorts coincided with a significant rise in the incidence of IBD in the western countries, allowing us to examine relevant secular changes in lifestyle and dietary behaviors3. In our primary cohorts and EPIC, cases of IBD were confirmed through blinded, medical record review by two gastroenterologists, minimizing outcome misclassification bias. Additionally, while several PAR values had wide CIs, potentially due to a limited number of cases or a high standard error introduced by a broad exposure definition52, the large majority did not cross 0%, increasing confidence in potential importance of dietary and lifestyle modifications in preventing CD and UC. Finally, our findings were largely reproducible in three European prospective cohorts, confirming external validity.
We also acknowledge several limitations. Mean age of IBD diagnosis (~45 years) for our cohort was higher than the typical age of onset of IBD, thus younger onset disease may be under-represented. Given the stronger genetic association with early-onset disease54, our PAR figures may overestimate the potential for lifestyle modifications in preventing early-onset IBD. Nonetheless, this finding may remain relevant for older-onset disease, which may be driven more heavily by environmental and lifestyle factors. Early lifestyle factors such as antibiotic exposure and breastfeeding, which have not been associated with IBD risk in these cohorts55, environmental factors including pollution, and socioeconomic factors were also not considered as these may not be readily modifiable52. We also acknowledge that we did not have information on several other potentially modifiable factors such as stress in our cohorts. Thus, residual confounding may exist and affect the validity of PAR estimates if all confounders are not modeled28,52. However, as most observed relationships between environmental and lifestyle factors and risk of IBD rarely exceed relative risk ratios of 3.005, we feel the E-value analysis for residual confounding builds confidence in the validity of our results. We note that longitudinal data were not available for all cohorts thus time-varying exposures could not be used in our external cohorts. PAR is also affected by exposure prevalence, which may differ across non-Western countries, and therefore generalizability may be limited. Finally, because of our limited sample size, we could not independently explore the contribution of modifiable lifestyle factors to risk of IBD in high-risk individuals, defined as those with a first degree relative with IBD.
CONCLUSION:
Across six US and European cohorts, we confirmed that a substantial proportion of CD and UC risk may be preventable through modification of lifestyle risk factors, or adherence to a healthy lifestyle. Further prospective interventional studies are needed to determine whether lifestyle modification is effective for the primary prevention of IBD, particularly in high-risk population and younger-onset disease.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Several modifiable lifestyle and dietary risk factors have been identified for Crohn’s disease (CD) and ulcerative colitis (UC), and are widely thought to contribute to disease pathogenesis.
One approach to the prevention of chronic diseases is via modification of lifestyle and dietary factors.
However, the extent by which adherence to low-risk factors or a healthy lifestyle could decrease the burden of CD and UC is unknown.
WHAT THIS STUDY ADDS
In three prospective US cohorts, adherence to low-risk factors could have prevented 42.9% (95% CI 12.2 to 66.1%) of CD and 44.4% (95% CI 9.0 to 69.8%) of UC cases, while adherence to a healthy lifestyle could have prevented 61.1% (16.8 to 84.9%) of CD and 42.2% (1.7 to 70.9%) of UC cases. These findings were largely confirmed in three external European cohorts.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Assuming a causal relationship exists, a substantial proportion of the burden of IBD may be preventable through lifestyle modification. Lifestyle modification may be an attractive strategy for future prevention strategies in IBD.
Acknowledgement
We would like to thank the participants and staff of the Nurses’ Health Study (NHS), NHSII, Health Professionals Follow-up Study, Swedish Mammography Cohort, Cohort of Swedish Men, and the European Prospective Investigation into Cancer and Nutrition (EPIC) for their valuable contributions.
Grant Support
Funded by UM1 CA186107 NHS cohort infrastructure grant, U01 CA176726 NHSII cohort infrastructure grant, and U01 CA167552 HPFS cohort infrastructure grant; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is also funded by the VR 2017–00644 SMC and CoSM cohorts Swedish research infrastructure (SIMPLER) grant. The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS) - Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology - ICO (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford) (United Kingdom). This study was also funded by a senior research award from the Crohn’s and Colitis Foundation to HK and a senior research award from the Crohn’s and Colitis Foundation to ATC. EL was funded from NIH T32 DK007191 during work on this manuscript and is currently funded by an American College of Gastroenterology junior faculty development award. Funding sources did not participate in study design, analysis, interpretation, drafting of manuscript, or submission process.
Disclosures
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: HK is supported by the American College of Gastroenterology Senior Research Award and the Beker Foundation; HK has received consulting fees from Abbvie and Takeda; HK has also received grant funding from Pfizer and Takeda; ATC is the Stuart and Suzanne MGH Research Scholar; JFL reports funding from Janssen corporation for work unrelated to this manuscript; OO has been PI on projects at Karolinska Institutet, partly financed by investigator-initiated grants from Janssen, Takeda, and Ferring, and Karolinska Institutet has received fees for lectures and participation on advisory boards from Janssen, Ferring, Takeda, and Pfizer; OO also reports a grant to Karolinska Institutet from Pfizer in the context of a national safety monitoring program; ATC has received consulting fees from Bayer Pharma AG, Pfizer Inc., and Boehringer Ingelheim for work unrelated to this manuscript. SSMC has received travel grants from Abbvie and Takeda. There are no other relationships or activities that could appear to have influenced the submitted work.
Abbreviations:
- BMI
Body mass index
- CD
Crohn’s disease
- HPFS
Health Professionals Follow-up Study
- IBD
Inflammatory bowel disease
- NHS
Nurses’ Health Study
- NHSII
Nurses’ Health Study II
- NSAIDs
non-steroidal anti-inflammatory drugs
- SFFQ
semi-quantitative food frequency questionnaire
- UC
ulcerative colitis
Footnotes
Collaborators: EPIC-IBD investigators: Pilar Amian, Aurelio Barricarte, Marie-Christine Boutron-Ruault, Franck Carbonnel, Olof Grip, Marc J Gunter, Rudolf Kaaks, Tim Key, María Dolores Chirlaque López, Robert Luben, Giovanna Masala, Jonas Manjer, Bas Oldenburg, Anja Olsen, Kim Overvad, Elio Riboli, Maria José Sánchez, Carlotta Sacerdote, Anne Tjønneland, Rosario Tumino, Roel Vermeulen, W. M. Monique Verschuren, Nick Wareham
Ethical Approval:
The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health (#2001P001128), and the IRB allowed participants’ completion of questionnaires to be considered as implied consent.
Transparency declaration:
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Data Sharing:
Further information including the procedures to obtain and access data from the Nurses’ Health Studies and Health Professionals Follow-up Study is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.
References:
- 1.Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166–1169. doi: 10.15585/mmwr.mm6542a3 [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Gönczi L, Lakatos PL, Burisch J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J Crohns Colitis. Published online February 14, 2021. doi: 10.1093/ecco-jcc/jjab029 [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 4.Park KT, Ehrlich OG, Allen JI, et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020;26(1):1. doi: 10.1093/IBD/IZZ104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. 2019;157(3):647–659.e4. doi: 10.1053/j.gastro.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 6.Mendall MA, Jensen CB, Sørensen TIA, Ängquist LH, Jess T. Body mass index in young men and risk of inflammatory bowel disease through adult life: A population-based Danish cohort study. Sci Rep. 2019;9(1). doi: 10.1038/s41598-019-42642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and Inflammatory Bowel Disease: A Meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. www.mayoclinicproceedings.com1462 [DOI] [PubMed] [Google Scholar]
- 8.History | Nurses’ Health Study. Accessed March 2, 2020. https://www.nurseshealthstudy.org/about-nhs/history
- 9.Health Professionals Follow-Up Study - About the Study. Accessed March 2, 2020. https://sites.sph.harvard.edu/hpfs/about-the-study/
- 10.Harris H, Håkansson N, Olofsson C, Stackelberg O, Julin B, Åkesson A. The Swedish mammography cohort and the cohort of Swedish men: study design and characteristics of two population-based longitudinal cohorts. OA Epidemiology. 2013;1(2):16. http://ki.se/imm/ [Google Scholar]
- 11.Riboli E, Hunt K, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6b):1113–1124. doi: 10.1079/PHN2002394 [DOI] [PubMed] [Google Scholar]
- 12.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61(12):1686–1692. doi: 10.1136/gutjnl-2011-301574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvard TH Chan School of Public Health Nutrition Department. Nutrient Tables. Accessed March 2, 2020. https://regepi.bwh.harvard.edu/health/ [Google Scholar]
- 14.Willett WC. Nutritional Epidemiology. Oxford University Press; 1998. doi: 10.1093/acprof:oso/9780195122978.001.0001 [DOI] [Google Scholar]
- 15.Chan SSM, Chen Y, Casey K, et al. Obesity is associated with increased risk of Crohn’s disease, but not ulcerative colitis: A pooled analysis of five prospective cohort studies. Clinical Gastroenterology and Hepatology. 2021;0(0). doi: 10.1016/j.cgh.2021.06.049 [DOI] [PubMed] [Google Scholar]
- 16.Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, Nonsteroidal Anti-inflammatory Drug Use, and Risk for Crohn Disease and Ulcerative Colitis A Cohort Study. Ann Intern Med. 2012;156:350–359. https://annals.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Xu KQ, Qin XR, Wen-Lu, Yan-Liu, Wang XY. Association between physical activity and inflammatory bowel disease risk: A meta-analysis. Digestive and Liver Disease. 2016;48(12):1425–1431. doi: 10.1016/j.dld.2016.08.129 [DOI] [PubMed] [Google Scholar]
- 18.Li F, Liu X, Wang W, Zhang D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(6):623–630. doi: 10.1097/MEG.0000000000000330 [DOI] [PubMed] [Google Scholar]
- 19.Zeng L, Hu S, Chen P, Wei W, Tan Y. Macronutrient intake and risk of Crohn’s disease: Systematic review and dose–response meta-analysis of epidemiological studies. Nutrients. 2017;9(5). doi: 10.3390/nu9050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–977. doi: 10.1053/j.gastro.2013.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananthakrishnan AN, Khalili H, Song M, et al. Genetic Polymorphisms in Fatty Acid Metabolism Modify the Association between Dietary n3:n6 Intake and Risk of Ulcerative Colitis: A Prospective Cohort Study. Inflamm Bowel Dis. 2017;23(11):1898–1904. doi: 10.1097/MIB.0000000000001236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. American Journal of Gastroenterology. 2010;105(10):2195–2201. doi: 10.1038/ajg.2010.192 [DOI] [PubMed] [Google Scholar]
- 23.Ge J, Han TJ, Liu J, et al. Meat intake and risk of inflammatory bowel disease: A meta-analysis. Turkish Journal of Gastroenterology. 2015;26(6):492–497. doi: 10.5152/tjg.2015.0106 [DOI] [PubMed] [Google Scholar]
- 24.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol. 2017;45(6):dyw341. doi: 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 25.American Heart Association. Guidelines for Healthy Living. Accessed April 21, 2021. https://www.heart.org/en/healthy-living
- 26.U.S. Department of Agriculture US Department of Health and Human Services. 2010 Dietary Guidelines for Americans. Accessed April 21, 2021. www.dietaryguidelines.gov
- 27.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA - Journal of the American Medical Association. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes and Control. 2007;18(5):571–579. doi: 10.1007/s10552-006-0090-y [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Wang K, Song M, Giovannucci EL. A comparison of methods in estimating population attributable risk for colorectal cancer in the United States. Int J Cancer. 2021;148(12):2947–2953. doi: 10.1002/ijc.33489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narula N, Wong ECL, Dehghan M, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ. 2021;374:n1554. doi: 10.1136/bmj.n1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaccardelli A, Friedlander HM, Ford JA, Sparks JA. Potential of Lifestyle Changes for Reducing the Risk of Developing Rheumatoid Arthritis: Is an Ounce of Prevention Worth a Pound of Cure? Clin Ther. 2019;41(7):1323–1345. doi: 10.1016/j.clinthera.2019.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, di Franco M. Dietary habits and nutrition in rheumatoid arthritis: Can diet influence disease development and clinical manifestations? Nutrients. 2020;12(5):1456. doi: 10.3390/nu12051456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 34.Corrao G, Tragnone A, Caprilli R, et al. Risk of inflammatory bowel disease attributable to smoking, oral contraception and breastfeeding in Italy: a nationwide case-control study. Cooperative Investigators of the Italian Group for the Study of the Colon and the Rectum (GISC). Int J Epidemiol. 1998;27(3):397–404. Accessed October 10, 2019. https://academic.oup.com/ije/article-abstract/27/3/397/625360 [DOI] [PubMed] [Google Scholar]
- 35.Brant SR, Wang MH, Rawsthorne P, et al. A population-based case-control study of CARD15 and other risk factors in Crohn’s disease and ulcerative colitis. American Journal of Gastroenterology. 2007;102(2):313–323. doi: 10.1111/j.1572-0241.2006.00926.x [DOI] [PubMed] [Google Scholar]
- 36.Sparks JA, Chen CY, Hiraki LT, Malspeis S, Costenbader KH, Karlson EW. Contributions of familial rheumatoid arthritis or lupus and environmental factors to risk of rheumatoid arthritis in women: A prospective cohort study. Arthritis Care Res (Hoboken). 2014;66(10):1438–1446. doi: 10.1002/acr.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naldi L, Chatenoud L, Linder D, et al. Cigarette Smoking, Body Mass Index, and Stressful Life Events as Risk Factors for Psoriasis: Results from an Italian Case-Control Study. J Invest Dermatol. 2005;125:61–67. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. American Journal of Gastroenterology. 2012;107(9):1399–1406. doi: 10.1038/ajg.2012.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brant SR. Update on the heritability of inflammatory bowel disease: The importance of twin studies. Inflamm Bowel Dis. 2011;17(1):1–5. doi: 10.1002/ibd.21385 [DOI] [PubMed] [Google Scholar]
- 41.Pack M. IBD: Fishing for missing heritability in IBD. Nat Rev Gastroenterol Hepatol. 2015;12(6):318–319. doi: 10.1038/nrgastro.2015.72 [DOI] [PubMed] [Google Scholar]
- 42.Agrawal M, Burisch J, Colombel JF, C. Shah S. Viewpoint: Inflammatory Bowel Diseases Among Immigrants From Low- to High-Incidence Countries: Opportunities and Considerations. J Crohns Colitis. 2020;14(2):267–273. doi: 10.1093/ecco-jcc/jjz139 [DOI] [PubMed] [Google Scholar]
- 43.Jenkins AP, Trew DR, Crump BJ, et al. Do non-steroidal anti-inflammatory drugs increase colonic permeability? Gut. 1991;32(1):66–69. doi: 10.1136/gut.32.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papoutsopoulou S, Satsangi J, Campbell BJ, Probert CS. Review article: impact of cigarette smoking on intestinal inflammation—direct and indirect mechanisms. Aliment Pharmacol Ther. 2020;51(12):1268–1285. doi: 10.1111/apt.15774 [DOI] [PubMed] [Google Scholar]
- 45.Amre DK, D’Souza S, Morgan K, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for crohn’s disease in children. American Journal of Gastroenterology. 2007;102(9):2016–2025. doi: 10.1111/j.1572-0241.2007.01411.x [DOI] [PubMed] [Google Scholar]
- 46.Wu G, Chen J, Hoffmann C, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science (1979). 2011;334(6052):105–108. doi: 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos MPC, Gomes C, Torres J. Familial and ethnic risk in inflammatory bowel disease. Ann Gastroenterol. 2018;31(1):14–23. doi: 10.20524/aog.2017.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knip M, Virtanen SM, Seppä K, et al. Dietary Intervention in Infancy and Later Signs of Beta-Cell Autoimmunity. N Engl J Med. 2010;363:1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparks JA, Iversen MD, Yu Z, et al. Disclosure of personalized rheumatoid arthritis risk using genetics, biomarkers, and lifestyle factors to motivate health behavior improvements: A randomized controlled trial HHS Public Access. Arthritis Care Res (Hoboken). 2018;70(6):823–833. doi: 10.1002/acr.23411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185(7):570–584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. Am J Epidemiol. 1992;135(10). https://academic.oup.com/aje/articleabstract/135/10/1114/67168 [DOI] [PubMed] [Google Scholar]
- 52.Rockhill B, Newman B, Weinberg C. Use and Misuse of Population Attributable Fractions. Am J Public Health. 1998;1:15–19. doi: 10.2105/ajph.88.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang MH, Hahn RA, Teutsch SM, Hutwagner LC. Multiple risk factors and population attributable risk for ischemic heart disease mortality in the United States, 1971–1992. J Clin Epidemiol. 2001;54(6):634–644. doi: 10.1016/S0895-4356(00)00343-7 [DOI] [PubMed] [Google Scholar]
- 54.Ananthakrishnan AN, Huang H, Nguyen DD, Sauk J, Yajnik V, Xavier RJ. Differential effect of genetic burden on disease phenotypes in crohn’s disease and ulcerative colitis: Analysis of a north american cohort. American Journal of Gastroenterology. 2014;109(3):395–400. doi: 10.1038/ajg.2013.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalili H, Ananthakrishnan AN, Higuchi LM, Richter JM, Fuchs CS, Chan AT. Early life factors and risk of inflammatory bowel disease in adulthood. Inflamm Bowel Dis. 2013;19(3):542–547. doi: 10.1097/MIB.0b013e31828132f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further information including the procedures to obtain and access data from the Nurses’ Health Studies and Health Professionals Follow-up Study is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.