Abstract
Pancreatic cancer (PC) is one of the most dangerous diseases that threaten human life, and investigating the details affecting its progression or regression is particularly important. Exosomes are one of the derivatives produced from different cells, including tumor cells and other cells such as Tregs, M2 macrophages, and MDSCs, and can help tumor growth. These exosomes perform their actions by affecting the cells in the tumor microenvironment, such as pancreatic stellate cells (PSCs) that produce extracellular matrix (ECM) components and immune cells that are responsible for killing tumor cells. It has also been shown that pancreatic cancer cell (PCC)-derived exosomes at different stages carry molecules. Checking the presence of these molecules in the blood and other body fluids can help us in the early stage diagnosis and monitoring of PC. However, immune system cell-derived exosomes (IEXs) and mesenchymal stem cell (MSC)-derived exosomes can contribute to PC treatment. Immune cells produce exosomes as part of the mechanisms involved in the immune surveillance and tumor cell-killing phenomenon. Exosomes can be modified in such a way that their antitumor properties are enhanced. One of these methods is drug loading in exosomes, which can significantly increase the effectiveness of chemotherapy drugs. In general, exosomes form a complex intercellular communication network that plays a role in developing, progressing, diagnosing, monitoring, and treating pancreatic cancer.
Keywords: pancreatic cancer, exosome, diagnosis, prognosis, tumor, treatment, miRNA
1. Introduction
Pancreatic cancer (PC) is one of the most dangerous cancers related to the digestive tract, which ranks fourth and sixth in America and China, respectively, as the leading cause of cancer-related death (1). This disease symptoms usually do not appear until a large part of the pancreas is damaged, and the symptoms stage is too late to start treatment (2). The usual treatments for pancreatic cancer are surgery and chemotherapy (3). However, it seems that in most cases, after surgery, cancer recurs and can be dangerous to the patient’s life (4). In the meantime, tumor cells can suppress the related responses needed for tumor regression by affecting the immune system (5). Tumor cells usually perform this action through several mechanisms, which include the production of suppressive soluble cytokines, the expression of surface molecules, and the production of extracellular vesicles (EVs) (6, 7). EVs are expressed in two categories. The first category of classification is based on cell origin, how to separate from the cell, size, and contents; they are divided into three subclasses, namely, apoptotic bodies, microvesicles, and exosomes. In the new classification, EVs with a maximum diameter of 200 nm are classified as small EVs (sEVs) and EVs larger than 200 nm as medium/large EVs (m/lEVs). However, in articles that investigate the therapeutic properties of vesicles, authors usually use the word exosomes as one of the subfamilies of sEV. Due to the characteristics of exosomes, such as biocompatibility, low immunogenicity, small size, ability to pass through small vessels, and ease of isolation and storage, they have received much attention in the field of tumors.
Exosomes are double-layered, nano-sized vesicles that are produced by almost all cells, and their role is to maintain homeostasis and intercellular communication (8). These vesicles carry various components (proteins, lipids, and nucleic acids), and after reaching the target cells, they transfer these cargos to them by different methods, including fusion, and change the characteristics of the target cells (9). Like other cells, tumor cells communicate with the cells in the tumor microenvironment through the production of exosomes and change their features in a useful way for tumor growth (10). However, exosomes produced from tumor cells at different stages have different components (including different microRNAs) that can be used as potential markers for diagnosing and monitoring the pancreatic cancer treatment process, progress, and regression (11, 12). Therefore, the importance of investigating and isolating exosomes derived from tumor cells is increasing daily because they are suitable biological tools for understanding the tumor stage.
Meanwhile, exosomes derived from other cells, including immune cells, can be used as therapeutic agents in all tumors (13, 14). Moreover, investigation of tumor behavior in the therapeutic use of mesenchymal stem cell (MSC)-derived exosomes is very important due to the presence of these cells in most tumor tissues and their effect on the treatment outcome (15). Today, different methods are used in which the therapeutic potential of exosomes has increased, such as drug- and various miR-loaded exosomes and engineered exosomes for targeted delivery (16), which lead to better tumor regression compared with intact exosome applications (17). Therefore, examining studies that reveal the relationship between exosomes and PC is of particular importance and can lead to a better orientation of researchers toward new treatments based on exosomes and the early diagnosis of this cancer. In the following, we will discuss important aspects of the relationship between pancreatic cancer and exosomes, including the role of exosomes in progression and metastasis, their diagnostic applications, and their therapeutic uses ( Figure 1 ).
Figure 1.
Mechanisms in which exosomes can lead to tumor progression. These vesicles can increase proliferation, invasion, metastasis, drug resistance, and immune deviation by affecting different cells.
2. Tumor-derived exosomes in pancreatic cancer progression
Tumor cells, like other cells, produce EVs for intercellular communication and cell homeostasis maintenance. After being produced from a tumor cell, these vesicles can have an autocrine effect on the cell itself or a paracrine and endocrine effect on the cells in the tumor microenvironment or other tissues and lead to the formation of a tumor nano environment (TNE) (18). EVs are divided into different subtypes based on their size and formation model (19). Exosomes are more prevalent in laboratory and clinical applications due to their advantages, such as small size, biocompatibility, and manipulation capabilities (20). Today, it is said that exosomes should be called sEV because the exosome population produced from a cell or a set of cells is heterogeneous and does not all reflect a specific characteristic (21). Usually, the characterization of sEVs is complex, and according to MISEV2018, the EV source and preparation should be described quantitatively (22). Different characteristics of sEVs should be investigated to confirm their separation. For example, the size of the vesicles should be checked using dynamic light scanning (DLS) and their shape by electron microscopy (SEM and TEM). Also, these vesicles should be evaluated to examine the expression of surface markers such as CD83, CD63, CD9, and integrin, as well as intracytosolic markers such as ALIX, TSG101, syntenin, and HSP70 (22). The components of sEVs can be different based on the origin and biological functions of the producing cell. Various components of exosomes can change under cell conditions. For example, the upregulation of oncogenes in cancer cells can increase their exosome oncoprotein levels, such as HRS and EGFR type III (23). sEVs are captured by the target cell through several biological mechanisms, including fusion, pinocytosis, phagocytosis (macrophage and myeloid cells), receptor-mediated endocytosis, caveolae-mediated endocytosis, and lipid raft-mediated endocytosis (24). One of the main routes by which tumor-derived sEVs are removed by the target cell is receptor-mediated endocytosis, because these vesicles have ligands such as FASL, PD-L1, and TRAIL on their surface, which increases endocytosis (25). Also, the expression of growth factors and their binding to EGFR can stimulate the harvesting of sEVs through pinocytosis (26). In congruence with the latest suggestion, we refer to “exosomes” as “sEVs” (22) throughout this review.
Exosomes produced from tumor cells by affecting the target cells can facilitate the conditions for tumor cell proliferation, metastasis, drug resistance, tumor-specific immune suppression, and angiogenesis, and can actually lead to tumor progression (27). Exosomes usually affect their target cells in three ways. 1) After the release of exosomes and their movement toward the target cells, their surface molecules bind to the surface receptors of the target cell and lead to signal transduction through these receptors (juxtacrine signaling). 2) In some cases, the exosome surface molecules are cut adjacent to the target cell and then bind to their receptor and exert their effects (soluble signaling). 3) In the third method, which is more common than the previous two methods, exosomes are integrated with the target cell using various mechanisms such as fusion and receptor-mediated endocytosis or phagocytosis, and transfer their internal content (protein, lipid, nucleic acid) to it, which also leads to changing the responses and conditions of the target cell (28, 29). In new studies, it is said that exosomes, in addition to the usual cargo, can also transfer mitochondria to the target cell (30). Since the mitochondria play an important role in metabolism and maintaining cell proliferation, transferring mitochondria via exosomes to tumor cells can increase their proliferation ability (31, 32). It has also been shown that the transfer of mitochondria from MSCs by exosomes can increase the chemoresistance ability of tumor cells (33). Depending on the origin of the exosome, different responses can be initiated in the target cell. For example, exosomes derived from immune system cells can lead to the expansion or suppression of tumor growth based on their origin (34, 35). Due to their immunomodulatory properties, the presence of MSC-derived exosomes can lead to tumor progression (36). Also, exosomes produced from tumor cells and released into the tumor environment can suppress the responses of the immune cells and, by affecting the endothelial cells, increase the angiogenesis and mobility, migration, and metastasis of tumor cells (37, 38).
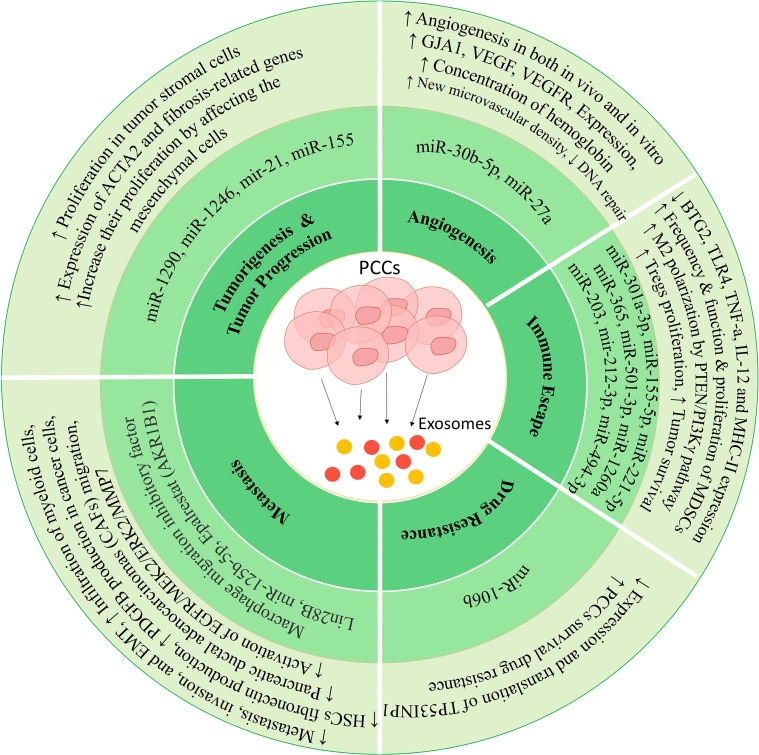
2.1. Tumorigenesis and tumor progression
Exosomes produced from pancreatic cancer cells (PCCs) differ from exosomes produced from normal pancreatic cells in terms of content and heterogeneity (39). Exosomes derived from tumor cells can transfer the oncopeptide of oncoproteins to the target cells, leading to phenotypic changes and tumorigenesis of the target cell (40). It has also been shown that people with tumors have more exosomes in their blood than healthy people, indicating that more exosomes are produced from tumor cells than from healthy cells (41). The results of the studies show that these exosomes can act as an initiator for the random mutations that occur in the target cell and lead to the transformation of a healthy cell into a tumor cell. Of course, it is worth mentioning that transforming a cell into a cancerous state requires an initiator and a promoter.
In a study conducted by Stefanius and colleagues, it was shown that using exosomes derived from PCC lines can lead to mutations in the KRAS gene (a proto-oncogene) and to the transformation of NIH/3T3 cells that have a mesenchymal origin. This study was conducted in two phases, in vivo and in vitro, and showed that if tumor cell exosomes were used as initiators and cadmium chloride (CdCl) as a promoter, the rate of tumor formation and cell transformation increased significantly compared with the control (42). It also leads to fundamental changes in the proteomics of these cells. Therefore, it was found that transferring various cargoes from tumor cells to healthy cells can lead to tumorigenesis. Among the different cargoes, non-coding RNAs play an important role in exosome applications. For example, exosomes derived from PCCs stimulate pancreatic stellate cells (PSCs) (43). These stimulated PSCs produce exosomes that contain miRNA-21, which can be transferred to other cells in tumor tissue, such as the pancreas, and alter their actions, leading to increased proliferation in tumor stromal cells (44). Cancer cells seem to activate PSCs by producing exosomes containing miRNA-1290 and miRNA-1246 and releasing them into the tumor environment (45). Also, exosomes derived from tumor cells have miRNA-155 by themselves, which can increase their proliferation by affecting the mesenchymal cells in the tumor environment (46). These mesenchymal cells and mesenchymal stem cells derived from bone marrow can differentiate into fibroblastic cells via cancer-derived exosomes, which play an important role in supporting the proliferation of pancreatic cancer cells (47).
2.2. Metastasis
Metastasis is a complex process that occurs via a set of cells and different factors in the tumor environment and is usually accompanied by neoangiogenesis (48, 49). This angiogenesis leads to the migration of tumor cells to distant places and their implantation and development in a new location (50). In a study, it has been shown that the expression level of Aldo-keto reductase family 1 member B1 (AKR1B1) in pancreatic cancer cells is associated with an increased level and release of exosomes from tumor cells (51). Moreover, previous studies show that epalrestat, which is an inhibitor of AKR1B1, can be used as an inhibitor of metastasis in different types of tumors (52). Considering that AKR1B1 leads to negative regulation of lysosome trafficking to endosomes and increases the formation of multivesicular bodies (MVBs) in cancer cells (53), the application of drugs such as epalrestat leads to a decrease in metastasis and reduces the amount of exosome production from cancer cells. Therefore, it can be concluded that the production of exosomes by PCCs can be a potential pathway to stimulate metastasis-related mechanisms.
Proteomic analysis of PCCs and normal pancreatic cell- derived exosomes shows that PCC exosomes carry various key factors regulating metastasis and molecules related to signaling required for metastasis and can lead to increased metastasis. Identifying the pathways leading to metastasis is crucial because cancer progression can be prevented by inhibiting these mechanisms. In the case of pancreatic cancer, one of the cells that play a role in the progression of metastasis is pancreatic ductal adenocarcinomas (PDACs) (54). These cells help tumor metastasis by producing exosomes that contain a large amount of macrophage migration inhibitory factor (MIF) (55, 56). These exosomes migrate to the liver, and after uptake by Kupffer cells, lead to the production of TGF-β from them (57). This cytokine affects hepatic stellate cells (HSCs) and leads to their activation, which is associated with the production of various components of the extracellular matrix and fibrosis of the liver tissue (58). The important point is that inhibiting MIF transfer by exosomes or using anti-TGF-β inhibits pancreatic cancer metastasis to the liver (57, 59). All these results show that exosomes derived from PDACs can play a role as a potential pathway in the metastasis of this cancer. One of the important and decisive components of exosomes as metastasis-regulating agents is the expression of various integrins through these vesicles. Integrin alpha V beta 5 (ITGavb5)-expressing exosomes bind specifically to Kupffer cells and induce tropism of these vesicles to the liver tissue. Other integrins, including ITGa6b4 and ITGa6b1 on sEVs, can bind to epithelial cells and resident fibroblasts in the lung and increase their tropism to the lung tissue (60–62).
Another study showed that exosomes derived from PCCs induce the ability to form premetastatic niches and tumor growth in their livers after being injected into normal mice (63). Flow cytometric studies show that these exosomes can lead to increased expression and activity of the transcription factor STAT3, as well as increased infiltration of myeloid cells, leading to the activity of HSCs in the liver and increased production of fibronectin from them (63). The injection of PCC-derived exosomes leads to an increase in the frequency of myeloid-derived suppressor cells (MDSCs) in the peripheral blood of mice (63). As immune response suppressors, these cells can help tumor growth and spread (64). It was also found that these exosomes can lead to a decrease in the adhesion of Panc02 cells and an increase in their migration, which increases their metastatic and invasive potential (63). Therefore, in addition to PDACs, pancreatic tumor cells can help tumor spread by increasing their metastatic properties by producing exosomes.
In addition to the mentioned cases, some studies have confirmed the presence of PSCs in metastatic tumors derived from pancreatic cancer. One of the studies investigated metastatic cells’ ability to induce the migration of pancreatic ductal adenocarcinomas (CAFs) and PSCs (as the main factor in tumor expansion) (65). The results of this study show that exosomes produced by PCCs stimulate the migration of these cells. Specific pathways have been proposed for this phenomenon. Still, one of the most important pathways is mediated by the exosomal protein Lin28B, and it was proven by Yue-Feng Zhang et al. that fusion of the PCC-derived exosomes by PSCs leads to the activation of the Lin28B/let-7/HMGA2/PDGFB-related pathway, which is normally associated with increased production of PDGFB in cancer cells and inhibits itself through miR let-7 expression (65, 66). Since the expression of miR let-7 is inversely correlated with the expression of HMGA2/PDGFB (67), the suppression of miR let-7 expression leads to increases in the expression of the HMGA2/PDGFB (65). Since PDGFB is a well-known chemokine, it seems that this molecule can help with PSCs and their migration to the secondary site of tumor formation during metastasis (68, 69). The important point is that Western blot analysis indicates the absence of PDGFB inside exosomes, and an increase in its expression was shown after the fusion of PCC-derived exosomes by PSCs (65).
As mentioned before, exosomes produced by cells have different contents in different conditions (70). miRNA sequencing of exosomes derived from PC-1.0 cells (prometastatic cells associated with pancreatic cancer) (71) and PC-1 (as weakly invasive cells) showed that these exosomes differ in the amount of 62 miRNAs, and miR-125b-5p is highly upregulated in the exosomes of cells with high metastasis ability (72). Functional investigations show that these miRNAs play an important role in increased metastasis, invasion, and epithelial-to-mesenchymal transition (EMT) (73). Moreover, investigating the miR-125b-5p mechanism of action showed that this miRNA binds to STARD13, which plays an essential role in the good prognosis of pancreatic cancer (72). Adding PC-1.0-derived exosomes to weakly invasive pancreatic cancer cells increases their ability in metastatic processes. Therefore, these exosomes seem to increase the metastatic capacity of PC-1 cells by carrying miR-125b-5p and its binding to STARD13. The subsequent suppression of STARD13 expression in weakly metastatic PC-1 cells leads to the activation of EGFR/MEK2/ERK2/MMP7 signaling pathways related to metastasis in different cancer cells (72).
2.3. Angiogenesis
Since tumor cells grow more than the other cells in the tumor site, most of the oxygen is consumed by these cells (74). Usually, a hypoxia condition is established in the tumor microenvironment, which can ultimately lead to neoangiogenesis (75). A study published by Kai Chen et al. in 2022 showed that the amount of miR-30b-5p in exosomes derived from hypoxic PCCs is increased (76). After being transferred to endothelial cells by exosomes, this exosomal miRNA binds to the 3′UTR region and inhibits the expression of a gap junction-related protein called GJA1, which can lead to tube formation, increasing the migration of endothelial cells and ultimately increasing angiogenesis (76). The results of the in vivo phase in animal studies also confirm the results of the in vitro phase, in which the group receiving the exosomes derived from hypoxic PCCs had a higher hemoglobin concentration than the group treated with PBS. Moreover, the results of immunohistochemistry analysis showed that the injection of these exosomes increased the new microvascular density by increasing the population of CD31+ cells (76). In another study, microarray analysis showed an increase in another miRNA called miR-27a (77). In this study, exosomes derived from the PANC-1 cell line (pancreatic cancer cell line) were used to treat human microvascular endothelial cells (HMVECs), and their effects on invasion, proliferation, and angiogenesis were investigated. Moreover, the in vivo phase was performed with xenograft injection in nude mice. The results of this study show that exosomal miR-27a leads to the negative regulation of B-cell translocation gene 2 (BTG2) and is associated with increased angiogenesis in both in vivo and in vitro conditions (77). BTG2 is a molecule involved in DNA repair and has an antitumor role (78). Western blot analysis after the co-culture of exosomes derived from PANC-1 with HMVEC shows an increase in the production of proteins, such as VEGF, VEGFR, MMP-2, and MMP-9 in HMVEC, related to angiogenesis and metastasis (77).
2.4. Tumor immune escape
After the formation of tumor cells, the immune system can eliminate them by identifying new antigens (neoantigens) produced by tumor cells (immune surveillance) and preventing their spread (79). However, immune responses are usually suppressed in these areas due to the specific conditions of the tumor microenvironment (80). Most immune cells in this area are Tregs (81), M2 macrophages (82), and MDSCs (83). The inhibiting of the immune system occurs by various mechanisms, including direct contact between cells and inhibition by surface molecules such as CTLA-4 and PD-1 (84), as well as the production of cytokines that inhibit immune system responses, such as IL-10 and TGF-β (85). However, it has been shown that the transfer of exosomes from tumor cells to immune cells located at the tumor site can inhibit their responses (86). Macrophages around the tumor are known as tumor-associated macrophages (TAMs) and are derived from monocytes migrating to the tumor site (87), which have immune-suppressive effects (88). In this place, macrophages capture PCC-derived exosomes, leading to their polarization to the M2 phototype, which produces pro-tumorigenic factors (89). A study showed that exosomes derived from hypoxic PCCs by transferring miR-301a-3p to macrophages stimulate their polarization to M2 phenotype through the PTEN/PI3Kγ pathway (90). It has also been shown that exosomes derived from pancreatic tumor cells enriched with ezrin after inducing M2 polarization can increase tumor metastasis to the liver (91). On the other hand, when M2 macrophages are found in the tumor site, they can help tumor expansion by producing exosomes (92). Exosomes derived from M2 macrophages induced in the pancreatic tumor site containing various cargoes such as miR-155-5p, miR-221-5p (93), miR-365 (94), and miR-501-3p (95) contribute to tumor survival by influencing different pathways. For example, M2 exosomes containing miR-365 can suppress BTG2 expression. Since BTG2 plays a role in inhibiting the FAK/AKT pathway, therefore, inhibiting its expression leads to the activation of the FAK/AKT signaling pathway and increases the development of pancreatic cancer (94). Thus, PCC-derived exosomes lead to the suppression of immune responses in macrophages, and in this way, they can also increase their survival and progression.
In addition to macrophages, MDSCs also play a role in tumor-induced immune escape (96). Continuous stimulation signals and hypoxia at the tumor site lead to the differentiation of these cells into TAM-like cells (97), which have high pro-tumorigenic activity. Various studies have shown that PCC-derived exosomes containing miR-1260a and miR-494-3p can affect different populations of MDSCs and lead to increased proliferation and their immunosuppressive ability (98). In addition to the effect of PCC exosomes on the innate immune system cells, these exosomes can also affect the responses of T cells in two ways, direct and indirect (impact on antigen-presenting cells) (99). A study has shown that dendritic cells (DCs), after uptake of PCC-derived exosomes through the transfer of miR-203, lead to a decrease in the expression of TLR4, TNF-a, and IL-12, and by transferring mir-212-3p to this cell the MHC-II expression in DCs is reduced (100, 101). Therefore, exosomes derived from PCCs can reduce the ability of DCs to induce and initiate strong T-cell responses (102). In one of the new studies, it has been shown that exosomes derived from PCCs can increase the proliferation ability of Tregs (103). This study showed that SIRT1, SIRT2, ATM, AMPK, and SIRT6 were sequentially activated in T lymphocytes treated with exosomes (103). As the most important suppressive immune cells, these cells help inhibit immune responses by producing various cytokines, absorbing IL-2 in the environment, cell-to-cell contact, etc. (104). In general, it can be said that PCCs communicate with immune cells by exosomes in a two-way manner and can lead to tumor expansion and failure of immune surveillance.
2.5. Tumor drug resistance
One of our main problems in chemotherapy is the drug resistance of cancer cells (105). Gemcitabine (GEM) is one of the primary drugs involved in the chemotherapy of pancreatic cancer (106), whose application for a long time has led to the acquisition and expansion of drug resistance in tumor cells (107). Some other cells, including CAFs, can play a role in developing this drug resistance (108). A study by Yuan Fang et al. investigated the role of these fibroblasts and their exosomes in the drug resistance of PCC (109). The results of this study show that fibroblasts producing exosomes containing miR-106b can induce this phenomenon in PCCs (109). Moreover, the pretreatment of fibroblasts with miR-106b production-inhibiting factors leads to a decrease in the amount of miR-106b in exosomes and a decrease in the drug resistance of PCCs to gemcitabine (109). To understand the potential mechanisms where miR-106b leads to an increase in the drug resistance of PCCs, different targets of this miR were investigated, and it was found that this microRNA plays an essential role in the drug resistance of breast cancer by targeting the tumor protein p53-inducible nuclear protein gene 1 (TP53INP1) by binding to the messenger RNA (mRNA) 3′ end of this gene. The expression of this gene is regulated by factors such as p53, p73, and E2F1, and its product has two isoforms that play an important role in the function of p53 (110). Moreover, to confirm this, it was shown that overexpression of TP53INP1 leads to the reversal of the miR-106b function (109). Therefore, it can be said that exosomes derived from CAFs, by transferring miR-106b to PCCs, lead to the inhibition of the expression and translation of TP53INP1-related mRNA, which leads to an increase in their survival and gemcitabine drug resistance in PCCs.
Another study showed that the amount of five microRNAs was higher than others in CAF-derived exosomes, namely, miR-222, miR-221, miR-181a, miR-92a, and miR-21 (111). After being transferred to PCCs, investigations show that these microRNAs can increase chemoresistance to gemcitabine in the PCCs by inhibiting the expression and production of phosphatase and tensin homolog (PTEN)-related protein in vitro (111). PTEN plays an essential role in suppressing tumor formation (112). The reduction of its expression and the mutation in its gene are observed in many tumors, leading to uncontrolled tumor proliferation and expansion (113). Therefore, by producing these exosomes, CAFs contribute to the drug resistance of tumor cells and lead to cancer progression. To further confirm the role of CAF-derived exosomes in this study, it was shown that GW4869 treatment in vivo led to the inhibition of exosome production and decreased drug resistance (111). The tumor size in GW4869-treated mice was not associated with a significant increase compared to control mice, which indicates the importance of exosomes in the drug resistance of tumor cells and their cross-talk with other cells (111).
3. Exosomes in pancreatic cancer diagnosis and monitoring
As mentioned earlier, exosomes (especially PCC-derived exosomes) are produced more in pancreatic cancer patients, and exosome serum levels are significantly increased compared with healthy individuals and non-pancreatic cancer patients (114). Therefore, according to the presence of these exosomes in the serum, their increase in pancreatic cancer, and their detection capability, they can be used in diagnosing and monitoring agents in PC (115). Apart from CA-199, which has FDA approval for the diagnosis of pancreatic cancer (116), PC lacks molecular biomarkers for early diagnosis, and the definition of a diagnosis system can significantly help reduce PC mortality worldwide (117). In the case of exosomes, RNA (especially micro RNAs) is usually used for diagnosis (115). Compared to free RNAs in peripheral blood, they have more diagnostic value in terms of quantity and quality (118–120). In addition to serum, plasma, and peripheral blood, other body fluids, such as pancreatic juice and saliva, can be used for exosome isolation and pancreatic cancer diagnosis (121). In addition, in some cases, exosomes derived from other cells (such as NK cells and macrophages) (95, 122) can help in the diagnosis of pancreatic cancer because they are involved in the responses related to the expansion or non-expansion of cancer. Usually, different exosomal miRNAs can help in tumor stage diagnosis, early diagnosis, prognosis assessment, drug resistance, tumor cell viability, and tumor recurrence after treatment. Table 1 summarizes the details of some exosomal miRNAs detected in recent studies ( Table 1 ) The important point about the diagnostic use of exosomal miRNAs is that they play a significant role in the maintenance, expansion, proliferation, and invasion of pancreatic cancer, as mentioned previously (130). In addition to miRNAs, other components of exosome internal contents such as proteins, mRNAs, long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) can also be used as diagnostic tools ( Table 2 ) (143).
Table 1.
Exosomal miRNA role in the diagnosis of prostatic cancer (PC).
| Exosomal cargo | Cargo type | Origin or cell source | Biomarker or other roles | Number of patients | Year | Ref. |
|---|---|---|---|---|---|---|
| Glypican-1 | Protein (heparan sulfate proteoglycans) | Serum | 1) Diagnostic biomarker for dividing PC 2) Benign pancreatic disease biomarker 3) Prediction of prognosis |
190 | 2015 | (123) |
| Exosomal integrin | Protein (adhesion) | N/A | Prognostic | 27 | 2010 | (124) |
| Migration inhibitory factor (MIF) | Protein (cytokine) | 1) Plasma 2) Serum |
Liver metastasis prognostic marker | 40 | 2015 | (61) |
| Sox2ot | lncRNA | Peripheral blood | Early diagnosis | 61 | 2018 | (125) |
| MALAT-1 | lncRNA | Peripheral blood | Early diagnosis | N/A | 2020 | (126) |
| HULC | lncRNA | Peripheral blood | Early diagnosis | N/A | 2020 | (126) |
| GPC1 | mRNA | Peripheral blood | 1) Early PC diagnosis 2) Tumor stage |
133 | 2017 | (127) |
| WASF2 | mRNA | Serum | 1) Early PC diagnosis 2) Tumor stage |
27 | 2019 | (128) |
| IARS | circRNA | 1) Culture medium 2) Peripheral blood |
1) Tumor stage 2) Survival evaluation |
92 | 2018 | (129) |
Table 2.
Role of other types of exosomal cargos in pancreatic cancer diagnosis.
| Exosomal miRNA | Origin or cell source | Biomarker or other roles | Number of patients | Year | Ref. |
|---|---|---|---|---|---|
| miR-17-5p | Serum | 1) Diagnostic for PC advanced stage 2) Metastasis |
49 | 2013 | (131) |
| Plasma | Diagnostic biomarker for dividing PC and non-PC | 20 | 2015 | (132) | |
| miRNA-21 | 1) Portal vein blood (serum) 2) Pancreatic juice |
1) Diagnostic chronic pancreatitis 2) Early diagnostic 3) Prognostic 4) Recurrence diagnostic 5) Tumor survival evaluation |
49 | 2013 | (131) |
| 55 | 2019 | (133) | |||
| 35 | 2019 | (121) | |||
| miRNA-210 | 1) Peripheral blood | 1) Early diagnosis 2) Tumor stage |
40 | 2020 | (134) |
| 2) PSC | Prognostic (gemcitabine resistance) | In vitro | 2020 | (135) | |
| miRNA-10b | Peripheral blood (serum) | Early diagnosis | 36 | 2020 | (136) |
| 40 | 2017 | (137) | |||
| miRNA-3976 | Serum | Early diagnosis | 190 | 2015 | (138) |
| miR-222 | 1) Peripheral blood 2) PCC |
1) Tumor stage diagnosis 2) Survival evaluation 3) Prognostic |
Cell line/in vitro | 2018 | (139) |
| miRNA-1246 | Peripheral blood (serum) | Early diagnosis | 15 | 2017 | (139) |
| miRNA-451a | 1) Portal vein blood | 1) Recurrence diagnostic 2) Tumor survival evaluation |
55 | 2019 | (133) |
| 2) Plasma | Prognostic and recurrence | 46 | 2018 | (140) | |
| miRNA-155 | 1) Pancreatic juice | 1) Early diagnosis 2) Prognostic |
35 | 2019 | (121) |
| 2) PCC | Prognostic for gemcitabine resistance | 1) In vitro | 2017 | (141) | |
| 2) In vivo = 45 | |||||
| miRNA-4644 | Saliva | Early diagnosis | 12 | 2016 | (142) |
| miR-3607-3p | NK cells | Prognostic | 40 | 2019 | (122) |
4. Therapeutic applications of exosomes in pancreatic cancer
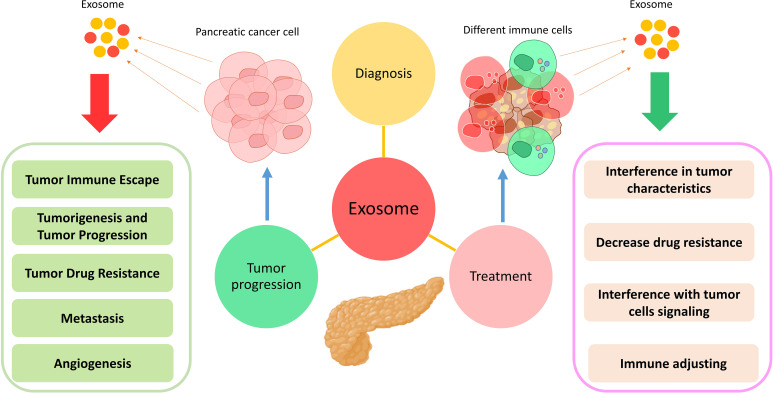
Pancreatic cancer is usually treated by chemotherapy, radiotherapy, and surgery (144). However, each treatment has different side effects that can affect and even threaten the patient’s life (145). Therefore, it is essential to use methods that reduce the dose and application times of these treatments. In vitro results show that using exosomes can reduce the effective dose of antibiotics used in infectious diseases (146). Moreover, the effectiveness of these vesicles in treating other conditions, such as autoimmune diseases, transplant rejection, neurodegenerative diseases, infectious diseases, and liver-related diseases, has been proven (147–149). In the meantime, the use of exosomes has been widely used in the treatment of various cancers (92). The results of studies have shown that successive injections of exosomes do not have any toxic effect on the body, their size is so small that they can pass through various vascular and capillary barriers, and they have immunocompatible and biocompatible properties suitable for use in therapeutic applications (20, 150). For these reasons, the application of exosomes has more priority than cell therapy. The antitumor role of exosomes derived from immune system inflammatory cells, including DCs, macrophages, NK cells, and T cells, has been well demonstrated (92). However, exosomes in the treatment of cancers are used in two ways: in the first case, exosomes in their naive and intact form are used (151), and in the second case, exosomes act as drug carriers (152). Moreover, the use of engineered exosomes, known as iExosomes, has increased in recent years. In fact, exosomes that contain loaded drugs, microRNAs, siRNAs, and CRISPR/Cas system components are considered part of this therapeutic approach (153). In some cases, exosomes are manipulated by surface molecules in order to increase therapeutic efficiency. For example, iExosomes can be engineered to express a chemokine receptor to a greater extent and migrate to the desired site in a targeted manner (154). Exosomes can perform their therapeutic function by affecting various mechanisms ( Figure 2 ).
Figure 2.
Application of exosomes in the diagnosis, treatment, and progression of pancreatic cancer. Exosomes exert their effects by changing various characteristics in different types of cells.
4.1. Adjusting immunity
DC-derived exosomes (DEXs), which have been previously primed with pancreatic cancer-derived antigens, contain specific MHC-I/II–peptide complexes that can trigger antitumor responses in CD4+ and CD8+ T cells (155, 156). In addition, these exosomes can lead to the maturation of immature DCs, which is associated with the production of various cytokines and the activation of tumor-specific T cells (157, 158). Moreover, other studies have shown that heat shock proteins (HSPs) carried by exosomes can reduce the activation threshold and lead to the activation of T cells (159). Another study used DCs loaded with PCC-derived exosomes for pancreatic cancer treatment (159). Considering that exosomes derived from PCCs are a suitable source for various tumor antigens, DCs, after harvesting these exosomes, provide peptides and other tumor antigens and can help T cells to perform antitumor activities. Moreover, the results of this study show that intratumoral injection of these DCs can lead to modulating the responses of MDSCs and Treg cells (159).
4.2. Interference with tumor cell signaling
Sixty-three different mutations have been identified in somatic genes (160); 50% of them are associated with tumor-suppressor genes such as KRAS, TP53, SMAD4, and CDKN2A (161). KRAS is a mutation in 90% of pancreatic cancer patients during which a substitution in the 12th amino acid (replacement of glycine with aspartate) has occurred (KRASG12D ), and this mutation plays an essential role in tumor proliferation and metastasis (162). In a study by Kamerkar et al., human foreskin fibroblast cells were used for exosome isolation and therapeutic application (163). Investigations show that these exosomes have shRNA or siRNA that inhibits the expression of oncogenic KRAS. The results of this study show that the expression level of KRASG12D in the pancreatic cancer cell line treated with these exosomes has decreased (163). Moreover, animal studies have demonstrated the reduction of metastasis in these models (163). In addition, exosomes can be used as carriers (164). Due to their characteristics, MSC-derived exosomes are usually suitable drug carriers for various therapeutic purposes (15). In a study by Yujin Lee et al., after exosomes were isolated from the supernatant of umbilical cord-derived MSCs, miR-145-5p was loaded into them, and these exosomes were intratumorally administered to mice (165). This microRNA is associated with decreased pancreatic cancer tumor cells (166, 167) and exerts its antitumor effects by affecting TGF-β/Smad3 pathways (168). The results of this study also show that these exosomes are attached to the Smad3 3′-UTR after being captured by tumor cells by endocytosis [investigated by Luciferase (Gluc) activity assays] and lead to the reduction of xenograft tumor growth according to the immunohistochemistry and TUNEL assay results in both in vivo (BALB/c nude mice) and in vitro conditions (165). Moreover, in another study, exosomes have been used as CRISPR/Cas9 system DNA plasmid carriers to target KRASG12D (169, 170). Exosomes loaded with CRISPR/Cas9 can target the mutated KrasG12D oncogenic allele in PCCs, thereby leading to the reduced proliferative ability of cancer cells and suppression of tumor growth in simultaneous subcutaneous and orthotopic models (169, 171). Therefore, exosomes can be used as promising carriers in pancreatic cancer gene therapy (169). Another gene whose overexpression is associated with pancreatic cancer is P21-activated kinase 4 (PAK4), which is associated with increased proliferation, survival, migration, and metastasis of these tumor cells (172, 173). Therefore, in a study, PCC-derived exosomes were isolated by ultracentrifugation, and siRNA was loaded into them by electroporation (172). In the next step, the colocalization of exosomes into PANC-1 cells was confirmed by fluorescent microscopy. The results of this study show that using these exosomes in vivo (intratumor injection) and in vitro leads to a decrease in PAK4 expression, a reduction in tumor growth, and an increase in the survival of mouse models (172). Moreover, the results of H&E staining show extensive apoptosis of tumor cells (172).
4.3. Decreasing drug resistance
Exosomes can be used as carriers for delivering chemotherapy drugs with high efficiency and biocompatibility characteristics ( Table 3 ) (179). Since gemcitabine is used as the first line of chemotherapy in pancreatic cancer (180), in a study conducted in 2020 by Yong-Jiang et al., this drug was loaded into autologous exosomes derived from PANC-1 cells (ExoGEM), and its therapeutic efficiency was evaluated in vivo and in vitro (181). The animal model was created through intraperitoneal injection of PANC-1 cells into BALB/c nude mice, and exosomes were injected intravenously (182). The results of this study show that drug loading in exosomes has led to the improvement of gemcitabine’s release profile, delivery, cell absorption, and therapeutic efficacy (181). Therefore, due to the increase in drug efficiency in combination with exosomes, the use of these exosomes led to a significant decrease in tumor growth and increased survival of mice in a dose-dependent manner (181). The interesting point is that the use of these exosomes led to the reduction of side effects associated with chemotherapy drugs and led to targeted tumor therapy in mice.
Table 3.
Encapsulated exosomes in efficient chemo drug delivery.
| Drug | Exosome source | Cell line/animal model | Loading method | Result | Year | Ref. |
|---|---|---|---|---|---|---|
| Gemcitabine monophosphate (GEMP) and paclitaxel | BM-MSCs | 1) MiaPaca-2 cells 2) 3D tumor spheroids 3) Nude mice |
Electroporation | 1) ↑ Endocellular dose of GEMP 2) ↑ Uptake of GEM, especially GEMP 3) ↑ Therapeutic efficacy 4) ↓ Cancer proliferative activity and chemoresistance |
2020 | (174) |
| Oxaliplatin | BM-MSCs | 1) PANC-02 cells 2) C57BL/6 mice |
Electroporation | 1) ↑ Cellular uptake efficiency 2) ↑ Cytotoxicity and immunogenic cell death response 3) ↑ In vivo antitumor efficacy and enhanced immunity |
2021 | (175) |
| Gemcitabine | BM-MSCs | CFPAC-1 cells | Priming of MSCs | 1) ↓ Cancer-related cell proliferation by 70% to 80% 2) Inhibit HUVEC proliferation |
2015 | (176) |
| Gemcitabine and deferasirox | M1 macrophage | 1) PANC-1 cells 2) 3D tumor spheroids |
Electroporation | 1) ↑ Iron removal efficacy 2) Reverse drug resistance 3) ↑ hENT1 expression for the transport of drug combinations into cells 4) ↑ Accumulation of chemotherapeutic agents in the cytosol 5 ) ↓ Invasiveness of GEM resistance |
2021 | (177) |
| Curcumin | 1) PANC-1 2) MIA PaCa-2 |
1) PANC-1 2 ) MIA PaCa-2 |
Methanol sonication | ↓ Pancreatic adenocarcinoma cell viability | 2015 | (178) |
4.4. Interference in tumor characteristics
The results of the studies show that the amount of miR-1231 in exosomes isolated from the peripheral blood of PC patients is associated with a significant decrease compared with healthy individuals (183). Moreover, the presence of miR-1231 in exosomes derived from bone marrow mesenchymal stem cells has been proven (184). It is also possible to increase the expression of this miRNA and its transport into exosomes in vitro by using pRNAT-U6 vector transfection with MSCs (185). In a study by Shang et al. in 2020 investigating the therapeutic effects of MSCs–exosomal miR-1231, two cell lines related to PC, namely, Panc-1, which had the highest expression of miR-1231, and BxPC-3, which had the lowest expression of miR-1231, were selected (185). After co-incubation with PCC-related cell lines, these exosomes fuse with them and transfer miR-1231 along with other exosome contents into them and change the actions of the cells. To check the efficiency of transferring this miR into cancer cells, the expression level of EGFR and cyclin E as its direct targets (186) was evaluated by immunoblots. The results showed that transferring this miR to PCCs leads to decreased proliferation ability (185). Moreover, the examination of wound-healing assays to investigate the migration ability of PCCs and the ability of invasion using Matrigel-coated Transwell chambers shows a significant decrease in migration and invasion in exosome-recipient cells (185). In addition, it has been demonstrated in this study that the adhesion of tumor cells to the matrix is also reduced in tumor cells receiving exosomes (185). In addition, the results of animal phase studies show that using exosomes with overexpressing miR-1231 in pancreatic tumor model mice leads to a decrease in the size, weight, and growth of tumors and an increase in survival in mice (185).
Another antitumor miRNA loaded in exosomes is miR-34a, whose efficacy has been evaluated in treating pancreatic cancer (187). The results of this study show that miR-34a loaded inside exosomes derived from HEK293 cells can reduce tumor growth in both in vivo and in vitro conditions (187). Bcl-2, an anti-apoptotic molecule that leads to increased tumor cell survival (188), is one of the direct targets of miR-34a (189). In PCCs receiving exosomes (Panc28 and Miapaca-2 cells), the amount of miR-34a is associated with a significant increase. Meanwhile, qRT-PCR results show that Bcl-2 expression in these cells is significantly reduced compared with the control group. At the same time, the expression level of pro-apoptotic proteins such as Bax and P53 has significantly increased (187). Moreover, Annexin-V/PI results show increased apoptosis in the group receiving exosomes containing miR-34a. In addition, the investigation of the effect of these exosomes in vivo confirms the results of the in vitro phase. It shows that the use of these exosomes in pancreatic cancer model mice leads to a reduction in tumor growth (187).
In another study, instead of drug loading in MSCs–exosomes, these cells were primed with paclitaxel (PTX) as an antitumor drug (190, 191). The results of this study show that this action can lead to the production of exosomes from these cells (murine SR4987 line) that carry this drug inside them and, after reaching the tumor cell and merging with it, deliver the drug to them. Using these exosomes on the pancreatic cancer-related cell line (CFPAC-1) decreased tumor cell proliferation (190).
5. Conclusion and future perspective
Exosomes are very important in investigating the conditions and characteristics of cells. Due to the higher production of exosomes by tumor cells, the amount of these vesicles in the blood of people with tumors usually increases, and the analysis of these exosomes can help in the early diagnosis of various cancers. It is worth noting that the content of the different types of tumor cell-derived exosomes is similar in many cases. This issue can be challenging in accurately diagnosing tumors by exosomes. On the other hand, exosomes can be used in the treatment of pancreatic cancer, and in general, various mechanisms that can lead to tumor cell survival, proliferation, signaling, and tumor-related immune inhibition can be targeted and inverted by exosomes. Table 4 summarizes the studies in which exosomes have been used to diagnose and treat pancreatic cancer in ClinicalTrials.gov. Considering the problems related to the homogeneity in isolated exosomes, storage difficulty, and difficulty in large amount isolation of exosomes for treatment, this field of research requires further development and standardization of related methods. In addition, as shown in Table 4 , most of the studies related to the therapeutic application of exosomes in pancreatic cancer are limited to laboratory and animal studies, and their road to the clinical phases requires studies related to safety.
Table 4.
Exosome-related studies in the clinical trial phase.
| Study title | Study type | Intervention model | Estimated enrollment | Intervention | Status | Phase | Last update | NTC number |
|---|---|---|---|---|---|---|---|---|
| iExosomes in Treating Participants With Metastatic Pancreas Cancer With KrasG12D Mutation | Treatment | Single group assignment | 28 participants | Exosomes with KRAS G12D siRNA | Recruiting | Phase 1 | 2022 | NCT03608631 |
| ExoLuminate Study for Early Detection of Pancreatic Cancer | Diagnostic | Observational cohort |
1,000 participants | N/A | Not yet recruiting | N/A | 2022 | NCT05625529 |
| Diagnostic Accuracy of Circulating Tumor Cells (CTCs) and Onco-exosome Quantification in the Diagnosis of Pancreatic Cancer -PANC-CTC | Diagnostic | Observational cohort |
52 participants | N/A | Completed | N/A | 2018 | NCT03032913 |
| Circulating Extracellular Exosomal Small RNA as Potential Biomarker for Human Pancreatic Cancer | Diagnostic | Single group assignment | 102 participants | Venous sampling | Unknown | N/A | 2020 | NCT04636788 |
| New Biomarkers in Pancreatic Cancer Using EXPEL Concept (PANEXPEL) | Diagnostic | Observational cohort |
200 participants | N/A | Recruiting | N/A | 2022 | NCT03791073 |
Author contributions
KJ was responsible for the conception and design of the study and for the invitation of co-authors to participate in the study. XF and HL wrote the original manuscript draft. KJ and JQ reviewed and edited the manuscript critically for important intellectual content and provided comments and feedback for the scientific contents of the manuscript. All authors read, revised, and approved the final manuscript.
Funding Statement
This work was supported by the Zhejiang Provincial Science and Technology Project (grant no. LGF21H160004 to XF) and Jinhua Municipal Science and Technology Projects (grant no. 2021-3-040 to KJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Yeo TP, Lowenfels AB. Demographics and epidemiology of pancreatic cancer. Cancer J (2012) 18(6):477–84. doi: 10.1097/PPO.0b013e3182756803 [DOI] [PubMed] [Google Scholar]
- 2. Kleeff J, Korc M, Apte M, La Vecchia C, Johnson C, Biankin A, et al. Pancreatic cancer. Nat Rev Dis Primers (2016) 2(1):1–22. doi: 10.1038/nrdp.2016.22 [DOI] [PubMed] [Google Scholar]
- 3. Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J gastrointest Oncol (2011) 2(3):157. doi: 10.3978/j.issn.2078-6891.2011.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: challenge of the facts. World J Surg (2003) 27(10):1075–84. doi: 10.1007/s00268-003-7165-7 [DOI] [PubMed] [Google Scholar]
- 5. Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J gastroenterol: WJG (2014) 20(32):11160. doi: 10.3748/wjg.v20.i32.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez-Bosch N, Vinaixa J, Navarro P. Immune evasion in pancreatic cancer: from mechanisms to therapy. Cancers (2018) 10(1):6. doi: 10.3390/cancers10010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman A, Hao W. The role of exosomes in pancreatic cancer microenvironment. Bull Math Biol (2018) 80(5):1111–33. doi: 10.1007/s11538-017-0254-9 [DOI] [PubMed] [Google Scholar]
- 8. Farooqi AA, Desai NN, Qureshi MZ, Nogueira Librelotto DR, Gasparri ML, Bishayee A, et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv (2018) 36(1):328–34. doi: 10.1016/j.biotechadv.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 9. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Controlled Release (2015) 219:278–94. doi: 10.1016/j.jconrel.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 10. Boyiadzis M, Whiteside T. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia (2017) 31(6):1259–68. doi: 10.1038/leu.2017.91 [DOI] [PubMed] [Google Scholar]
- 11. Alipoor SD, Mortaz M, Varahram M, Movassaghi M, Kraneveld AD, Garssen J, et al. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front Immunol (2018) 9:819. doi: 10.3389/fimmu.2018.00819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan Y, Luo X, Lv W, Hu W, Zhao C, Xiong M, et al. Tumor-derived exosomal components: the multifaceted roles and mechanisms in breast cancer metastasis. Cell Death Dis (2021) 12(6):1–18. doi: 10.1038/s41419-021-03825-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan W, Jiang S. Immune cell-derived exosomes in the cancer-immunity cycle. Trends Cancer (2020) 6(6):506–17. doi: 10.1016/j.trecan.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 14. Tavasolian F, Hosseini A, Rashidi M, Soudi S, Abdollahi E, Momtazi B, et al. The impact of immune cell-derived exosomes on immune response initiation and immune system function. Curr Pharm Design (2021) 27(2):197–205. doi: 10.2174/1381612826666201207221819 [DOI] [PubMed] [Google Scholar]
- 15. Heris RM, Shirvaliloo M, Abbaspour-Aghdam S, Hazrati A, Shariati A, Youshanlouei HR, et al. The potential use of mesenchymal stem cells and their exosomes in parkinson’s disease treatment. Stem Cell Res Ther (2022) 13(1):1–14. doi: 10.1186/s13287-022-03050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hazrati A, Mirsanei Z, Heidari N, Malekpour K, Rahmani-Kukia N, Abbasi A, et al. The potential application of encapsulated exosomes: a new approach to increase exosomes therapeutic efficacy. Biomed Pharmacother (2023) 162:114615. doi: 10.1016/j.biopha.2023.114615 [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Controlled Release (2021) 329:894–906. doi: 10.1016/j.jconrel.2020.10.020 [DOI] [PubMed] [Google Scholar]
- 18. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest (2016) 126(4):1208–15. doi: 10.1172/JCI81135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van der Pol E, Böing N, Harrison A, Sturk P, Nieuwland A, Mattson MP. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev (2012) 64(3):676–705. doi: 10.1124/pr.112.005983 [DOI] [PubMed] [Google Scholar]
- 20. Rezaie J, Ajezi S, Biray Avci Ç, Karimipour M, Geranmayeh MH, Nourazarian A, et al. Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol (2018) 55(4):3372–93. doi: 10.1007/s12035-017-0582-7 [DOI] [PubMed] [Google Scholar]
- 21. Mastoridis S, Minani Bertolino G, Whitehouse G, Dazzi F, Sanchez-Fueyo A, Martinez-Llordella M. Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front Immunol (2018) 9:1583. doi: 10.3389/fimmu.2018.01583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Théry C, Witwer WK, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina A, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J extracellular vesicles (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao Y, Qin Y, Wan C, Sun Y, Meng J, Huang J, et al. Small extracellular vesicles: a novel avenue for cancer management. Front Oncol (2021) 11:638357. doi: 10.3389/fonc.2021.638357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J extracellular vesicles (2014) 3(1):24641. doi: 10.3402/jev.v3.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qian K, Fu W, Li T, Zhao J, Lei C, Hu S, et al. The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy. J Exp Clin Cancer Res (2022) 41(1):286. doi: 10.1186/s13046-022-02492-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qi R, Zhao Y, Guo Q, Mi X, Cheng M, Hou W, et al. Exosomes in the lung cancer microenvironment: biological functions and potential use as clinical biomarkers. Cancer Cell Int (2021) 21(1):1–16. doi: 10.1186/s12935-021-01990-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic (2008) 9(6):871–81. doi: 10.1111/j.1600-0854.2008.00734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vyas N, Dhawan J. Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries. Cell Mol Life Sci (2017) 74(9):1567–76. doi: 10.1007/s00018-016-2413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol (2018) 16(1):1–13. doi: 10.1186/s12951-018-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hough KP, Trevor JL, Strenkowski JG, Wang Y, Chacko BK, Tousif S, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol (2018) 18:54–64. doi: 10.1016/j.redox.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berridge MV, Crasso C, Neuzil J. Mitochondrial genome transfer to tumor cells breaks the rules and establishes a new precedent in cancer biology. Mol Cell Oncol (2018) 5(5):e1023929. doi: 10.1080/23723556.2015.1023929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malekpour K, Hazrati A, Soudi S, Hashemi SM. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Controlled Release (2023) 354:755–69. doi: 10.1016/j.jconrel.2023.01.059 [DOI] [PubMed] [Google Scholar]
- 33. Li C, Cheung MK, Han S, Zhang Z, Chen L, Chen J, et al. Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Bioscience Rep (2019) 39(5). doi: 10.1042/BSR20182417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Y, Liu T, Zhou M. Immune-Cell-Derived exosomes for cancer therapy. Mol Pharmaceutics (2022) 19(9):3042–56. doi: 10.1021/acs.molpharmaceut.2c00407 [DOI] [PubMed] [Google Scholar]
- 35. Shen M, Ren X. New insights into the biological impacts of immune cell-derived exosomes within the tumor environment. Cancer Lett (2018) 431:115–22. doi: 10.1016/j.canlet.2018.05.040 [DOI] [PubMed] [Google Scholar]
- 36. Shojaei S, et al. Effect of mesenchymal stem cells-derived exosomes on tumor microenvironment: tumor progression versus tumor suppression. J Cell Physiol (2019) 234(4):3394–409. doi: 10.1002/jcp.27326 [DOI] [PubMed] [Google Scholar]
- 37. Zhou J, et al. Mesenchymal stem cell derived exosomes in cancer progression, metastasis and drug delivery: a comprehensive review. J Cancer (2018) 9(17):3129. doi: 10.7150/jca.25376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol (2012) 22:342–9. doi: 10.1016/j.semcancer.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 39. Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, Shimosegawa T. Exosomes derived from pancreatic stellate cells: MicroRNA signature and effects on pancreatic cancer cells. Pancreas (2017) 46(1):19–27. doi: 10.1097/MPA.0000000000000722 [DOI] [PubMed] [Google Scholar]
- 40. Xu R, et al. Extracellular vesicles in cancer–implications for future improvements in cancer care. Nat Rev Clin Oncol (2018) 15(10):617–38. doi: 10.1038/s41571-018-0036-9 [DOI] [PubMed] [Google Scholar]
- 41. Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, et al. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer (2018) 17(1):1–8. doi: 10.1186/s12943-018-0869-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stefanius K, et al. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. Elife (2019) 8:e40226. doi: 10.7554/eLife.40226.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jin G, et al. Molecular mechanism of pancreatic stellate cells activation in chronic pancreatitis and pancreatic cancer. J Cancer (2020) 11(6):1505. doi: 10.7150/jca.38616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J Cell communication Signaling (2014) 8(2):147–56. doi: 10.1007/s12079-014-0220-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masamune A, Yoshida N, Hamada S, Takikawa T, Nabeshima T, Shimosegawa T. Exosomes derived from pancreatic cancer cells induce activation and profibrogenic activities in pancreatic stellate cells. Biochem Biophys Res Commun (2018) 495(1):71–7. doi: 10.1016/j.bbrc.2017.10.141 [DOI] [PubMed] [Google Scholar]
- 46. Pang W, Su J, Wang Y, Feng H, Dai X, Yuan Y, et al. Pancreatic cancer-secreted miR-155 implicates in the conversion from normal fibroblasts to cancer-associated fibroblasts. Cancer Sci (2015) 106(10):1362–9. doi: 10.1111/cas.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richards KE, et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene (2017) 36(13):1770–8. doi: 10.1038/onc.2016.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walsh LA, Roy D.M, Reyngold M, Giri D, Snyder A, Turcan S, et al. RECK controls breast cancer metastasis by modulating a convergent, STAT3-dependent neoangiogenic switch. Oncogene (2015) 34(17):2189–203. doi: 10.1038/onc.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kerbel RS, Benezra R, Lyden DC, Hattori K, Heissig B, Nolan DJ, et al. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci (2008) 105(34):E54–4. doi: 10.1073/pnas.0804876105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol (2008) 215(3):211–3. doi: 10.1002/path.2350 [DOI] [PubMed] [Google Scholar]
- 51. Ji J, et al. AKR1B1 promotes pancreatic cancer metastasis by regulating lysosome-guided exosome secretion. Nano Res (2022) 15(6):5279–94. doi: 10.1007/s12274-022-4167-z [DOI] [Google Scholar]
- 52. Wu X, Li X, Fu Q, Cao Q, Chen X, Wang M, et al. AKR1B1 promotes basal-like breast cancer progression by a positive feedback loop that activates the EMT program. J Exp Med (2017) 214(4):1065–79. doi: 10.1084/jem.20160903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakamura K, Kusama K, Bai R, Sakurai T, Isuzugawa K, Godkin JD, et al. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PloS One (2016) 11(6):e0158278. doi: 10.1371/journal.pone.0158278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giovannetti E, Van Der Borden C, Frampton A, Ali A, Firuzi O, Peters G. Never let it go: stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol (2017) 44:43–59. doi: 10.1016/j.semcancer.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Wang X-F. A niche role for cancer exosomes in metastasis. Nat Cell Biol (2015) 17(6):709–11. doi: 10.1038/ncb3181 [DOI] [PubMed] [Google Scholar]
- 56. Ray K. Pancreatic cancer exosomes prime the liver for metastasis. Nat Rev Gastroenterol Hepatol (2015) 12(7):371–1. doi: 10.1038/nrgastro.2015.93 [DOI] [PubMed] [Google Scholar]
- 57. Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol (2015) 17(6):816–26. doi: 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hazrati A, Malekpour K, Soudi S, Hashemi SM. Mesenchymal stromal/stem cells and their extracellular vesicles application in acute and chronic inflammatory liver diseases: emphasizing on the anti-fibrotic and immunomodulatory mechanisms. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.865888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ellermeier J, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic CancerBifunctional siRNA against pancreatic cancer. Cancer Res (2013) 73(6):1709–20. doi: 10.1158/0008-5472.CAN-11-3850 [DOI] [PubMed] [Google Scholar]
- 60. Garofalo M, Villa A, Rizzi N, Kuryk L, Rinner B, Cerullo V, et al. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J Controlled Release (2019) 294:165–75. doi: 10.1016/j.jconrel.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 61. Hoshino A, Costa-Silva B, Shen T.-L, Rodrigues G, Hashimoto A, Tesic Mark M., et al. Tumour exosome integrins determine organotropic metastasis. Nature (2015) 527(7578):329–35. doi: 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Manzo G. Specific and aspecific molecular checkpoints as potential targets for dismantling tumor hierarchy and preventing relapse and metastasis through shielded cytolytic treatments. Front Cell Dev Biol (2021) 9:665321. doi: 10.3389/fcell.2021.665321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu Z, et al. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget (2017) 8(38):63461. doi: 10.18632/oncotarget.18831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: important contributors to tumor progression and metastasis. J Cell Physiol (2018) 233(4):3024–36. doi: 10.1002/jcp.26075 [DOI] [PubMed] [Google Scholar]
- 65. Zhang Y-F, Zhou Y-Z, Zhang B, Huang S-F, Li P-P, He X-M, et al. Pancreatic cancer-derived exosomes promoted pancreatic stellate cells recruitment by pancreatic cancer. J Cancer (2019) 10(18):4397. doi: 10.7150/jca.27590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Y, et al. Lin28B facilitates the progression and metastasis of pancreatic ductal adenocarcinoma. Oncotarget (2017) 8(36):60414. doi: 10.18632/oncotarget.19578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 pathway in cancer. Front Genet (2017) 8:31. doi: 10.3389/fgene.2017.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kugel S, Sebastián C, Fitamant J, Ross KN, Saha SK, Jain E, et al. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell (2016) 165(6):1401–15. doi: 10.1016/j.cell.2016.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Strell C, et al. Stroma-regulated HMGA2 is an independent prognostic marker in PDAC and AAC. Br J Cancer (2017) 117(1):65–77. doi: 10.1038/bjc.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma L. MicroRNA and metastasis. Adv Cancer Res (2016) 132:165–207. doi: 10.1016/bs.acr.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 71. Hirota M, et al. Production of scatter factor-like activity by a nitrosamine-induced pancreatic cancer cell line. Carcinogenesis (1993) 14(2):259–64. doi: 10.1093/carcin/14.2.259 [DOI] [PubMed] [Google Scholar]
- 72. Wu M, Tan X, Liu P, Yang Y, Huang Y, Liu X, et al. Role of exosomal microRNA-125b-5p in conferring the metastatic phenotype among pancreatic cancer cells with different potential of metastasis. Life Sci (2020) 255:117857. doi: 10.1016/j.lfs.2020.117857 [DOI] [PubMed] [Google Scholar]
- 73. Banzhaf-Strathmann J, Edbauer D. Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell communication Signaling (2014) 12(1):1–13. doi: 10.1186/1478-811X-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chang J, Erler J. Hypoxia-mediated metastasis. Tumor Microenviron Cell Stress (2014) p:55–81. doi: 10.1007/978-1-4614-5915-6_3 [DOI] [PubMed] [Google Scholar]
- 75. Acker T, Plate KH. Role of hypoxia in tumor angiogenesis–molecular and cellular angiogenic crosstalk. Cell Tissue Res (2003) 314(1):145–55. doi: 10.1007/s00441-003-0763-8 [DOI] [PubMed] [Google Scholar]
- 76. Chen K, et al. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int J Biol Sci (2022) 18(3):1220. doi: 10.7150/ijbs.67675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shang D, Xie C, Hu J, Tan J, Yuan Y, Liu Z, et al. Pancreatic cancer cell–derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J Cell Mol Med (2020) 24(1):588–604. doi: 10.1111/jcmm.14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mao B, Xiao H, Zhang Z, Wang D, Wang G. MicroRNA−21 regulates the expression of BTG2 in HepG2 liver cancer cells. Mol Med Rep (2015) 12(4):4917–24. doi: 10.3892/mmr.2015.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim R. Cancer immunoediting: from immune surveillance to immune escape. Cancer Immunother (2007) p:9–27. doi: 10.1016/B978-012372551-6/50066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Terry S, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol (2017) 11(7):824–46. doi: 10.1002/1878-0261.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Facciabene A, Motz GT, Coukos G. T-Regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res (2012) 72(9):2162–71. doi: 10.1158/0008-5472.CAN-11-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ma Y-S, Wu TM, Ling CC, Yu F, Zhang J, Cao PS, et al. M2 macrophage-derived exosomal microRNA-155-5p promotes the immune escape of colon cancer by downregulating ZC3H12B. Mol Therapy-Oncolytics (2021) 20:484–98. doi: 10.1016/j.omto.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med (2016) 94(5):509–22. doi: 10.1007/s00109-015-1376-x [DOI] [PubMed] [Google Scholar]
- 84. Jiang X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer (2019) 18(1):1–17. doi: 10.1186/s12943-018-0928-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang S, Ning Q, Yang L, Mo Z, Tang S. Mechanisms of immune escape in the cancer immune cycle. Int Immunopharmacol (2020) 86:106700. doi: 10.1016/j.intimp.2020.106700 [DOI] [PubMed] [Google Scholar]
- 86. Ichim TE, et al. Exosomes as a tumor immune escape mechanism: possible therapeutic implications. J Trans Med (2008) 6(1):1–7. doi: 10.1186/1479-5876-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mantovani A, et al. The origin and function of tumor-associated macrophages. Immunol Today (1992) 13(7):265–70. doi: 10.1016/0167-5699(92)90008-U [DOI] [PubMed] [Google Scholar]
- 88. Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev (2006) 25(3):315–22. doi: 10.1007/s10555-006-9001-7 [DOI] [PubMed] [Google Scholar]
- 89. Linton SS, et al. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PloS One (2018) 13(11):e0206759. doi: 10.1371/journal.pone.0206759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer MetastasisTumor-promoting effects of hypoxic exosomal miR-301a. Cancer Res (2018) 78(16):4586–98. doi: 10.1158/0008-5472.CAN-17-3841 [DOI] [PubMed] [Google Scholar]
- 91. Chang Y-T, Peng HY, Hu CM, Huang SC, Tien SC, Jeng YM. Pancreatic cancer-derived small extracellular vesical ezrin regulates macrophage polarization and promotes metastasis. Am J Cancer Res (2020) 10(1):12. [PMC free article] [PubMed] [Google Scholar]
- 92. Hazrati A, et al. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. biomark Res (2022) 10(1):1–25. doi: 10.1186/s40364-022-00374-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther (2021) 29(3):1226–38. doi: 10.1016/j.ymthe.2020.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li X, et al. miR-365 secreted from M2 macrophage-derived extracellular vesicles promotes pancreatic ductal adenocarcinoma progression through the BTG2/FAK/AKT axis. J Cell Mol Med (2021) 25(10):4671–83. doi: 10.1111/jcmm.16405 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95. Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res (2019) 38(1):1–20. doi: 10.1186/s13046-019-1313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pergamo M, Miller G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther (2017) 24(3):100–5. doi: 10.1038/cgt.2016.65 [DOI] [PubMed] [Google Scholar]
- 97. Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med (2010) 207(11):2439–53. doi: 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Basso D, et al. PDAC-derived exosomes enrich the microenvironment in MDSCs in a SMAD4-dependent manner through a new calcium related axis. Oncotarget (2017) 8(49):84928. doi: 10.18632/oncotarget.20863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Waldenmaier M, Seibold T, Seufferlein T, Eiseler T. Pancreatic cancer small extracellular vesicles (Exosomes): a tale of short-and long-distance communication. Cancers (2021) 13(19):4844. doi: 10.3390/cancers13194844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol (2014) 292(1-2):65–9. doi: 10.1016/j.cellimm.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 101. Ding G, et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget (2015) 6(30):29877. doi: 10.18632/oncotarget.4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Armstrong EA, Beal EW, Chakedis J, Paredes AZ, Moris D, Pawlik TM, et al. Exosomes in pancreatic cancer: from early detection to treatment. J Gastrointestinal Surg (2018) 22(4):737–50. doi: 10.1007/s11605-018-3693-1 [DOI] [PubMed] [Google Scholar]
- 103. Shen T, et al. BxPC-3-derived small extracellular vesicles induce FOXP3+ treg through ATM-AMPK-Sirtuins-mediated FOXOs nuclear translocations. Iscience (2020) 23(8):101431. doi: 10.1016/j.isci.2020.101431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol (2012) 3:51. doi: 10.3389/fimmu.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer (2013) 13(10):714–26. doi: 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- 106. Carmichael J, Fink U, Russell R, Spittle M, Harris A, Spiessi G, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer (1996) 73(1):101–5. doi: 10.1038/bjc.1996.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers (2017) 9(11):157. doi: 10.3390/cancers9110157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ham I-H, Lee D, Hur H. Role of cancer-associated fibroblast in gastric cancer progression and resistance to treatments. J Oncol (2019) 2019:46–51. doi: 10.1155/2019/6270784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu W, et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp Cell Res (2019) 383(1):111543. doi: 10.1016/j.yexcr.2019.111543 [DOI] [PubMed] [Google Scholar]
- 110. Shahbazi J, Lock R, Liu T. Tumor protein 53-induced nuclear protein 1 enhances p53 function and represses tumorigenesis. Front Genet (2013) 4:80. doi: 10.3389/fgene.2013.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Richards KE, Xiao W, Hill R, Team UPR. Cancer-associated fibroblasts confer gemcitabine resistance to pancreatic cancer cells through PTEN-targeting miRNAs in exosomes. Cancers (2022) 14(11):2812. doi: 10.3390/cancers14112812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, et al. PTEN loss accelerates kras G12D-induced pancreatic cancer development. Cancer Res (2010) 70(18):7114–24. doi: 10.1158/0008-5472.CAN-10-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang Y, Zhang J, Xu K, Xiao Z, Sun J, Xu J, et al. PTEN/PI3K/mTOR/B7-H1 signaling pathway regulates cell progression and immuno-resistance in pancreatic cancer. Hepato-gastroenterology (2013) 60(127):1766–72. [PubMed] [Google Scholar]
- 114. Hannafon BN, Ding W-Q. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci (2013) 14(7):14240–69. doi: 10.3390/ijms140714240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Uddin M, et al. Exosomal microRNA in pancreatic cancer diagnosis, prognosis, and treatment: from bench to bedside. Cancers (2021) 13(11):2777. doi: 10.3390/cancers13112777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J gastrointest Oncol (2012) 3(2):105. doi: 10.3978/j.issn.2078-6891.2011.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhao Z, Zhao G, Yang S, Zhu S, Zhang S, Li P. The significance of exosomal RNAs in the development, diagnosis, and treatment of pancreatic cancer. Cancer Cell Int (2021) 21(1):1–16. doi: 10.1186/s12935-021-02059-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fitts CA, Ji N, Li Y, Tan C. Exploiting exosomes in cancer liquid biopsies and drug delivery. Advanced healthcare materials (2019) 8(6):1801268. doi: 10.1002/adhm.201801268 [DOI] [PubMed] [Google Scholar]
- 119. Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J extracellular vesicles (2016) 5(1):31292. doi: 10.3402/jev.v5.31292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: challenges and opportunities. J Cell Physiol (2018) 233(9):6370–80. doi: 10.1002/jcp.26481 [DOI] [PubMed] [Google Scholar]
- 121. Nakamura S, Sadakari Y, Ohtsuka T, Okayama T, Nakashima Y, Gotoh Y, et al. Pancreatic juice exosomal microRNAs as biomarkers for detection of pancreatic ductal adenocarcinoma. Ann Surg Oncol (2019) 26(7):2104–11. doi: 10.1245/s10434-019-07269-z [DOI] [PubMed] [Google Scholar]
- 122. Sun H, Shi K, Qi K, Kong H, Zhang J, Dai S, et al. Natural killer cell-derived exosomal miR-3607-3p inhibits pancreatic cancer progression by targeting IL-26. Front Immunol (2019) 10:2819. doi: 10.3389/fimmu.2019.02819 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature (2015) 523(7559):177–82. doi: 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol (2010) 12(1):19–30. doi: 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- 125. Li Z, Jiang P, Li J, Peng M, Zhao X, Zhang X, et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene (2018) 37(28):3822–38. doi: 10.1038/s41388-018-0237-9 [DOI] [PubMed] [Google Scholar]
- 126. Kumar SR, et al. RNA Cargos in extracellular vesicles derived from blood serum in pancreas associated conditions. Sci Rep (2020) 10(1):1–10. doi: 10.1038/s41598-020-59523-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hu J, et al. A signal-amplifiable biochip quantifies extracellular vesicle-associated RNAs for early cancer detection. Nat Commun (2017) 8(1):1–11. doi: 10.1038/s41467-017-01942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kitagawa T, Taniuchi K, Tsuboi M, Sakaguchi M, Kohsaki T, Okabayashi T, et al. Circulating pancreatic cancer exosomal RNA s for detection of pancreatic cancer. Mol Oncol (2019) 13(2):212–27. doi: 10.1002/1878-0261.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, et al. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res (2018) 37(1):1–16. doi: 10.1186/s13046-018-0822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yonemori K, Kurahara H, Maemura K, Natsugoe S. MicroRNA in pancreatic cancer. J Hum Genet (2017) 62(1):33–40. doi: 10.1038/jhg.2016.59 [DOI] [PubMed] [Google Scholar]
- 131. Que R, Ding G, Chen J, Cao L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J Surg Oncol (2013) 11(1):1–9. doi: 10.1186/1477-7819-11-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ali S, Dubaybo H, Brand RE, Sarkar FH. Differential expression of microRNAs in tissues and plasma co-exists as a biomarker for pancreatic cancer. J Cancer Sci Ther (2015) 7(11):336. doi: 10.4172/1948-5956.1000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kawamura S, Iinuma H, Wada K, Takahashi K, Minezaki S, Kainuma M, et al. Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients. J Hepato-Biliary-Pancreatic Sci (2019) 26(2):63–72. doi: 10.1002/jhbp.601 [DOI] [PubMed] [Google Scholar]
- 134. Wu L, Zhou WB, Zhou J, Wei Y, Wang HM, Liu XD, et al. Circulating exosomal microRNAs as novel potential detection biomarkers in pancreatic cancer. Oncol Lett (2020) 20(2):1432–40. doi: 10.3892/ol.2020.11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (2020) 43(1):123–36. doi: 10.1007/s13402-019-00476-6 [DOI] [PubMed] [Google Scholar]
- 136. Pu X, Ding G, Wu M, Zhou S, Jia S, Cao L. Elevated expression of exosomal microRNA−21 as a potential biomarker for the early diagnosis of pancreatic cancer using a tethered cationic lipoplex nanoparticle biochip. Oncol Lett (2020) 19(3):2062–70. doi: 10.3892/ol.2020.11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lai X, Wang M, Mcelyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett (2017) 393:86–93. doi: 10.1016/j.canlet.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Madhavan B, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer (2015) 136(11):2616–27. doi: 10.1002/ijc.29324 [DOI] [PubMed] [Google Scholar]
- 139. Li Z, Tao Y, Wang X, Jiang P, Li J, Peng M, et al. Tumor-secreted exosomal miR-222 promotes tumor progression via regulating P27 expression and re-localization in pancreatic cancer. Cell Physiol Biochem (2018) 51(2):610–29. doi: 10.1159/000495281 [DOI] [PubMed] [Google Scholar]
- 140. Takahasi K, Iinuma H, Wada K, Minezaki S, Kawamura S, Kainuma M, et al. Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J Hepato-Biliary-Pancreatic Sci (2018) 25(2):155–61. doi: 10.1002/jhbp.524 [DOI] [PubMed] [Google Scholar]
- 141. Mikamori M, et al. MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci Rep (2017) 7(1):1–14. doi: 10.1038/srep42339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Machida T, Tomofuji T, Maruyama T, Yoneda T, Ekuni D, Azuma T, et al. miR−1246 and miR−4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol Rep (2016) 36(4):2375–81. doi: 10.3892/or.2016.5021 [DOI] [PubMed] [Google Scholar]
- 143. Xu J, Xu J, Liu X, Jiang J. The role of lncRNA-mediated ceRNA regulatory networks in pancreatic cancer. Cell Death Discovery (2022) 8(1):1–11. doi: 10.1038/s41420-022-01061-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Goodman KA, Hajj C. Role of radiation therapy in the management of pancreatic cancer. J Surg Oncol (2013) 107(1):86–96. doi: 10.1002/jso.23137 [DOI] [PubMed] [Google Scholar]
- 145. Matsuno S, Egawa S, Arai K. Trends in treatment for pancreatic cancer. J hepato-biliary-pancreatic Surg (2001) 8(6):544–8. doi: 10.1007/s005340100023 [DOI] [PubMed] [Google Scholar]
- 146. Hazrati A, Soudi S, Hashemi SM. Wharton's jelly mesenchymal stem cells-derived exosomes and imipenem in combination reduce apoptosis and inflammatory responses in e. coli-infected HepG2 cells. Iranian J Allergy Asthma Immunol (2022) 21(3):273. doi: 10.18502/ijaai.v21i3.9801 [DOI] [PubMed] [Google Scholar]
- 147. Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther (2011) 19(10):1769–79. doi: 10.1038/mt.2011.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xu Z, et al. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer (2020) 19(1):1–16. doi: 10.1186/s12943-020-01278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Motallebnezhad M, Omraninava M, Ghaleh HEG, Jonaidi-Jafari N, Hazrati A, Malekpour K, et al. Potential therapeutic applications of extracellular vesicles in the immunopathogenesis of COVID-19. Pathology-Research Pract (2022) p:154280. doi: 10.1016/j.prp.2022.154280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tran T-H, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol (2015) 160(1):46–58. doi: 10.1016/j.clim.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 151. Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Trans Med (2012) 10(1):1–12. doi: 10.1186/1479-5876-10-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhao X, et al. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother (2020) 128:110237. doi: 10.1016/j.biopha.2020.110237 [DOI] [PubMed] [Google Scholar]
- 153. Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol Ther (2017) 25(6):1269–78. doi: 10.1016/j.ymthe.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics (2021) 11(7):3183. doi: 10.7150/thno.52570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Calmeiro J, Carrascal MA, Tavares AR, Ferreira DA, Gomes C, Falcão A, et al. Dendritic cell vaccines for cancer immunotherapy: the role of human conventional type 1 dendritic cells. Pharmaceutics (2020) 12(2):158. doi: 10.3390/pharmaceutics12020158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pitt JM, et al. Dendritic cell–derived exosomes as immunotherapies in the fight against cancer. J Immunol (2014) 193(3):1006–11. doi: 10.4049/jimmunol.1400703 [DOI] [PubMed] [Google Scholar]
- 157. Oliveira C, Calmeiro J, Carrascal MA, Falcão A, Gomes C, Neves BM, et al. Exosomes as new therapeutic vectors for pancreatic cancer treatment. Eur J Pharmaceutics Biopharmaceutics (2021) 161:4–14. doi: 10.1016/j.ejpb.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 158. Tian H, Li W. Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges. Ann Trans Med (2017) 5(10):174–91. doi: 10.21037/atm.2017.02.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, et al. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol (2006) 36(6):1598–607. doi: 10.1002/eji.200535501 [DOI] [PubMed] [Google Scholar]
- 160. Jones S, Zhang X, Parsons DW, Lin JCH, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. science (2008) 321(5897):1801–6. doi: 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature (2015) 518(7540):495–501. doi: 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Eser S, Schnieke A, Schneider D, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer (2014) 111(5):817–22. doi: 10.1038/bjc.2014.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kamerkar S, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature (2017) 546(7659):498–503. doi: 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol pharmaceutics (2018) 16(1):24–40. doi: 10.1021/acs.molpharmaceut.8b00901 [DOI] [PubMed] [Google Scholar]
- 165. Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett (2019) 442:351–61. doi: 10.1016/j.canlet.2018.10.039 [DOI] [PubMed] [Google Scholar]
- 166. Han T, Yi XP, Liu B, Ke MJ, Li YX. MicroRNA-145 suppresses cell proliferation, invasion and migration in pancreatic cancer cells by targeting NEDD9. Mol Med Rep (2015) 11(6):4115–20. doi: 10.3892/mmr.2015.3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Huang H, Sun P, Lei Z, Li M, Wang Y, Zhang HT, et al. miR-145 inhibits invasion and metastasis by directly targeting Smad3 in nasopharyngeal cancer. Tumor Biol (2015) 36(6):4123–31. doi: 10.1007/s13277-015-3046-6 [DOI] [PubMed] [Google Scholar]
- 168. Hu H, Xu Z, Li C, Xu C, Lei Z, Zhang HT, et al. MiR-145 and miR-203 represses TGF-β-induced epithelial-mesenchymal transition and invasion by inhibiting SMAD3 in non-small cell lung cancer cells. Lung Cancer (2016) 97:87–94. doi: 10.1016/j.lungcan.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 169. McAndrews KM, Xiao F, Chronopoulos A, Lebleu VS, Kugeratski FG, Kalluri R. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Sci alliance (2021) 4(9). doi: 10.26508/lsa.202000875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hazrati A, Malekpour K, Soudi S, Hashemi SM. CRISPR/Cas9-engineered mesenchymal stromal/stem cells and their extracellular vesicles: a new approach to overcoming cell therapy limitations. Biomed Pharmacother (2022) 156:113943. doi: 10.1016/j.biopha.2022.113943 [DOI] [PubMed] [Google Scholar]
- 171. Zhao X, Liu L, Lang J, Cheng K, Wang Y, Li X, et al. A CRISPR-Cas13a system for efficient and specific therapeutic targeting of mutant KRAS for pancreatic cancer treatment. Cancer Lett (2018) 431:171–81. doi: 10.1016/j.canlet.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 172. Xu L, Faruqu FN, Lim YM, Lim KY, Liam-Or R, Walters AA, et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials (2021) 264:120369. doi: 10.1016/j.biomaterials.2020.120369 [DOI] [PubMed] [Google Scholar]
- 173. He L-F, Xu HW, Chen M, Xian ZR, Wen XF, Chen MN, et al. Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget (2017) 8(11):17573. doi: 10.18632/oncotarget.7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zhou Y, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B (2020) 10(8):1563–75. doi: 10.1016/j.apsb.2019.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials (2021) 268:120546. doi: 10.1016/j.biomaterials.2020.120546 [DOI] [PubMed] [Google Scholar]
- 176. Bonomi A, Sordi V, Dugnani E, Ceserani V, Dossena M, Coccè V, et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy (2015) 17(12):1687–95. doi: 10.1016/j.jcyt.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 177. Zhao Y, Zheng Y, Zhu Y, Zhang Y, Zhu H, Liu T. M1 macrophage-derived exosomes loaded with gemcitabine and deferasirox against chemoresistant pancreatic cancer. Pharmaceutics (2021) 13(9):1493. doi: 10.3390/pharmaceutics13091493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Osterman CJD, Lynch JC, Leaf P, Gonda A, Ferguson Bennit HR, Griffiths D, et al. Curcumin modulates pancreatic adenocarcinoma cell-derived exosomal function. PloS One (2015) 10(7):e0132845. doi: 10.1371/journal.pone.0132845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M, et al. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Controlled Release (2015) 220:727–37. doi: 10.1016/j.jconrel.2015.09.031 [DOI] [PubMed] [Google Scholar]
- 180. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. New Engl J Med (2018) 379(25):2395–406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 181. Li Y-J, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta biomaterialia (2020) 101:519–30. doi: 10.1016/j.actbio.2019.10.022 [DOI] [PubMed] [Google Scholar]
- 182. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 183. Chen SL, Ma M, Yan L, Xiong S, Liu Z, Li S, et al. Clinical significance of exosomal miR-1231 in pancreatic cancer. Zhonghua zhong liu za zhi [Chinese J oncology] (2019) 41(1):46–9. doi: 10.3760/cma.j.issn.0253-3766.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 184. Zhao R, Chen X, Song H, Bie Q, Zhang B. Dual role of MSC-derived exosomes in tumor development. Stem Cells Int (2020) 2020. doi: 10.1155/2020/8844730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Shang S, Wang J, Chen S, Tian R, Zeng H, Wang L, et al. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med (2019) 8(18):7728–40. doi: 10.1002/cam4.2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Cetin Z, Saygili EI, Görgisen G, Sokullu E. Preclinical experimental applications of miRNA loaded BMSC extracellular vesicles. Stem Cell Rev Rep (2021) 17(2):471–501. doi: 10.1007/s12015-020-10082-x [DOI] [PubMed] [Google Scholar]
- 187. Zuo L, Tao H, Xu H, Li C, Qiao G, Guo M, et al. Exosomes-coated miR-34a displays potent antitumor activity in pancreatic cancer both in vitro and in vivo. Drug Design Dev Ther (2020) 14:3495. doi: 10.2147/DDDT.S265423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Antonsson B, Martinou J-C. The bcl-2 protein family. Exp Cell Res (2000) 256(1):50–7. doi: 10.1006/excr.2000.4839 [DOI] [PubMed] [Google Scholar]
- 189. Alemar B, Izetti P, Gregório C, Macedo GS, Castro M, Osvaldt AB, et al. miRNA-21 and miRNA-34a are potential minimally invasive biomarkers for the diagnosis of pancreatic ductal adenocarcinoma. Pancreas (2016) 45(1):84–92. doi: 10.1097/MPA.0000000000000383 [DOI] [PubMed] [Google Scholar]
- 190. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Controlled release (2014) 192:262–70. doi: 10.1016/j.jconrel.2014.07.042 [DOI] [PubMed] [Google Scholar]
- 191. Alqahtani FY, Aleanizy FS, El Tahir E, Alkahtani HM, Alquadeib BT. Paclitaxel. In: Profiles of drug substances, excipients and related methodology. Elsevier; (2019). p. 205–38. [DOI] [PubMed] [Google Scholar]