Abstract
Background and Aim
Toxoplasmosis is the most widespread zoonotic disease that affects one‐third of the world's population, and imposes a major public health problem worldwide. This study aimed to assess the prevalence of toxoplasmosis among patients with neuropsychiatric patients.
Methods
Electronic databases PubMed, Google Scholar, Web of Science, Research Gate, and Scopus were thoroughly searched from February to March 2022 to identify all relevant studies. The quality of studies was evaluated using the Newcastle−Ottawa quality scale for case‐control and cross‐sectional studies. Statistical analysis was done using STATA version 12 software. A random effect model was used to compute the global pooled seroprevalence of Toxoplasma gondii infection. Heterogeneity was quantified by using I 2 value. Subgroup analysis was done, and publication bias was assessed using a funnel plot and Egger's test.
Result
Of 1250 studies, 49 containing 21,093 participants and conducted in 18 countries were included. The global pooled seroprevalence of T. gondii IgG antibody was 38.27% (95% CI: 32.04−44.9) among neuropsychiatric patients and 25.31% (95% CI: 21.53−29.08) in healthy controls with substantial heterogeneity of 98.3%. The prevalence of T. gondii IgG antibody was higher in males (17.52%) than in females (12.35%) neuropsychiatric patients. The highest pooled prevalence of T. gondii IgG antibody was in Europe (57%) followed by Africa (45.25%) and Asia (43%). Time based analysis showed the highest pooled prevalence of T. gondii IgG antibody in 2012−2016 (41.16%).
The global pooled seroprevalence T. gondii IgM antibody among neuropsychiatric patients and healthy controls was 6.78% (95% CI: 4.87−8.69) and 3.13% (95% CI: 2.02−4.24), respectively.
Conclusion
The pooled prevalence of chronic and acute T. gondii infection among neuropsychiatric patients was 38.27% and 6.78%, respectively. This showed a high burden of toxoplasmosis among neurological and psychiatric patients and urges routine screening of those patients and providing appropriate treatment. It also indicates the need for different stakeholders to develop targeted prevention and control strategies for T. gondii infection.
Keywords: global prevalence, neuropsychiatric disorder, psychiatric patients, Toxoplasma gondii, toxoplasmosis
1. INTRODUCTION
Toxoplasmosis is the most widespread zoonotic disease caused by Toxoplasma gondii, which is a single‐cell obligate intracellular apicomplexan protozoan parasite that can invade and replicate inside all nucleated cell types of warm‐blooded animals. T. gondii infects a one‐third of the world's population with a seroprevalence of ranging from 10% to more than 90%. 1 , 2 It causes potentially serious disease in human and animals resulting in a major public health and economic burden in the world. T. gondii is reported to have a wide spectrum of intermediate hosts, which includes human, sheep, pig, rodents, and birds and harbors the asexual tachyzoite which is the active and lytic form of the parasite that cause life‐threatening diseases. The tissue cyst forms of the parasite is a slow‐growing stage capable of building cysts mostly in the brain and muscle tissues. 3 , 4 , 5 Human beings acquire T. gondii infection through ingestion of the tissue cysts in raw or undercooked meat containing the latent cyst, sporulated oocysts in contaminated water or food, and congenitally from mother to child. 6 , 7 High prevalence of toxoplasmosis is reported in Africa, Southeast Asia, Middle East, Central/Eastern Europe, and Latin America. Variable prevalence of T. gondii infection was reported in Asia (13.3%−85.3%), Europe (40%−76%), Africa (21.74%−74.8%), North America (7.3%−26.5%). In immunocompetent individuals, T. gondii infection is usually asymptomatic and majority of the parasite is cleared during acute phase infection. Surviving parasites persist as slow‐growing bradyzoite tissue cysts, most abundant in tissues with limited immune surveillance, including brain, eye, cardiac, and skeletal muscle. 3 In symptomatic cases, T. gondii is associated with lymphadenopathy, non‐flue like symptoms, toxoplasmic retinochoroiditis, ocular toxoplasmosis, and toxoplasmic encephalitis. 7 It also causes sever opportunistic infection in pregnant and other immunocompromised patients due to reactivation of infection in the central nervous system (CNS). 8 The synergetic effect of parasite growth, tissue damage, inflammatory response, host and parasite genotype results in the severity of the diseases. 4
T. gondii is able to produce long‐lasting infection and persists in the CNS invading neurons, and functional glial cells leading to various neurological and mental disorders. 9 T. gondii pass the impermeable blood brain barrier (BBB) through several mechanisms such as monocyte and other infected myeloid derived cells extravasate from capillaries to the brain, trans‐endothelial migration through attachment of the parasite to CD11b/ICAM1integrins, paracellular entry of the parasite into the CNS through actin‐myosin motors, and endothelial lysis. 9 , 10
Several neurological and mental disorders have been reported in patients with toxoplasmosis such as Alzheimer's disease (AD), schizophrenia, bipolar disorders, generalized anxiety disorder (GAD), obsessive‐compulsive disorder, suicidality, Parkinson's disease, epilepsy, depression, dysphoria, and sexual promiscuity. 11 , 12 , 13 , 14 , 15 , 16
There are several postulates regarding the chronic neuropathologic mechanisms in toxoplasmosis. These could be downregulation of the glutamate receptor (GLT‐1) expression leading to increased level of extracellular glutamate and excitatory glutamatergic signaling and neural damage, and alteration of glutamate decarboxylase 67 (GAD67) which consequently leads to decreased GABAergic synaptic activities. In addition, parasite induced inflammation and overexpression of several cytokines by the infection can contribute to the onset of seizure in T. gondii infected individuals. 4 T. gondii infection may also lead to impaired catecholamine metabolism resulting psychological, behavioral, and motor changes in infected individuals. 17
Several studies reported that there is a significant association between T. gondii and the various psychiatric and neurological disorders. A recent systematic review reported schizophrenia to be the most frequently linked psychiatric disorder with T. gondii infection. 18 A meta‐analysis conducted on the effect of T. gondii on epilepsy reported T. gondii to the main risk factor for epilepsy. 19 Another systematic review and meta‐analysis that evaluated the association between T. gondii infection and Parkinson and Alzheimer diseases found a positive association between the parasite and the neuropsychiatric diseases. 20
Although several studies have reported the seroprevalence of T. gondii among neuropsychiatric patients in different parts of the world, studies reporting the pooled prevalence of T. gondii at the global level are lacking. Therefore, this systematic review and meta‐analysis was aimed to systematically review and determine the global burden and impact of T. gondii infection among patients with neuropsychiatric disorders and the importance of screening patients with neuropsychiatric disorders for toxoplasmosis to improve the quality of life of the patients.
2. MATERIALS AND METHODS
2.1. Search strategy and study selection
All articles regarding T. gondii infection were retrieved through systematic search of electronic databases such as PubMed/Central, Google Scholar, Web of Science, Research Gate, and Scopus from February to March 2022. The keywords used in this study includes; (1) Prevalence, seroprevalence, and magnitude, (2) Toxoplasmosis, T. gondii, (3) psychiatric disorder, mental disorder, neurologic disorder, (4) world, Africa, Asia, Europe, Middle East, America, Russia. These keywords were also used in combination for retrieving the studies. Cited sources from these papers were used as a resource for finding other related studies. Duplicates were removed and three independent reviewers (H. B., D. G. F., H. D.) continued to screen the title and abstract of all potentially eligible studies. Then the full text of potentially eligible studies that reported the prevalence of T. gondii infection among patients with neuropsychiatric disorders were added to the collections for extraction. Disagreements among authors during data extraction were resolved by discussion.
2.2. Eligibility criteria
Original articles that reported the seroprevalence of T. gondii infection among patients with any neuropsychiatric disorders were included. Studies reported only in English were included. However, non‐English articles which had abstracts in English that contained the required data for extraction were also included. On the other hand, studies reported the seroprevalence of T. gondii infection among non‐neuropsychiatric human study participants and nonhuman subjects (animals, rodents) were excluded. Furthermore, review articles, case reports, and letters to the editor were also excluded.
2.3. Outcome variables
The outcome variable for this study is the global pooled seroprevalence of T. gondii infection (T. gondii IgG and IgM antibodies) among neuropsychiatric patients.
2.4. Data extraction and quality assessment
Data from the eligible studies were extracted by three reviewers (H. B., N. M., and H. E.) independently in a Microsoft Excel sheet. The information extracted from each study includes name of the first author, publication year, country, continent, study design, sample size, number of male and female participants, diagnostic methods, form of neuro‐psychiatric disorder, prevalence of IgG, prevalence of IgM among case and control group. Quality of the included studies was assessed using the Newcastle−Ottawa quality scale for a case‐control and cross‐sectional studies. 21 , 22
2.5. Statistical analysis
The data extraction was done using Microsoft Excel worksheet and the meta‐analysis was done by using STATA version 12 software with the metan commands. The outcome of this study was reported as percentage prevalence and exact 95% confidence interval (CI). The point estimate and 95% CI of seroprevalence of T. gondii infection for all the included studies were calculated. Due to the high heterogeneity reported, the global pooled seroprevalence of T. gondii infection among neuropsychiatric patients and healthy controls was calculated using a random effect model. 23 The Cochrane's Q test and I 2 statistics provide an estimate of the percentage of variability in effect estimates that is due to heterogeneity rather than chance alone were used to assess the heterogeneity. The I 2 statistics (percentage of total variability due to heterogeneity) indicates the heterogeneity and its value of 25%, 50%, and 75% corresponds to low, moderate, and high heterogeneity, respectively. 24 Subgroup analysis for the primary outcome was performed in sex, region, country, and year of publication. Moreover, publication bias was assessed by visual observation of the symmetry of the funnel plot, and Egger's test statistics. 25 Sensitivity analysis was done to assess the impact of a single study on the overall pooled effect size.
3. RESULT
3.1. Selection and identification of studies
A total of 1250 articles were retrieved by systemic search and other methods. Of the total retrieved studies, 967 studies were removed due to duplication, reviews, case reports, letters to the editor, and meta‐analysis. Then the abstract and full text of 283 articles were evaluated in detail based on the eligibility criteria and 234 articles were removed due to failure to fulfill the inclusion criteria. Finally, 49 eligible studies were included in this systematic review and meta‐analysis. The preferred reporting items for Systematic Review and Meta‐analysis (PRISMA checklist 2009) was followed (Figure 1). 26
Figure 1.
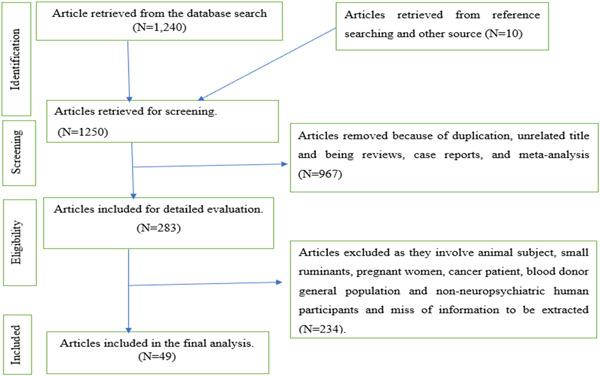
Flow diagram for the selection of eligible studies.
3.2. Study characteristics
In this systematic review and meta‐analysis, 49 studies conducted in 18 countries from the four continents (Asia, North America, Africa, and Europe) that consisted of 8592 neuropsychiatric patients and 12,501 healthy controls were included. The included studies were conducted between 2006 and 2022. The majority of the studies were from Iran (12 studies) and Mexico (10 studies). The number of studies was higher in Asia followed by North America and Africa. In terms of epidemiological design, majority of the studies (44/49) were case‐control. All the included studies used ELISA as their diagnostic method. Neurological and mental disorders such as autism, schizophrenia, suicide victims, suicidal ideation, depression, Parkinson's disease, anxiety, bipolar disorders, psychiatric, and neurodevelopmental disorders were reported to be associated with T. gondii infection in the included studies (Table 1).
Table 1.
Characteristics of the included studies.
| Author/year/reference | Country | Study design | Neuropsychiatric disorders | Cases | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | IgG case | IgG (%) | IgM case | IgM (%) | Sample size | IgG case | IgG (%) | IgM case | IgM (%) | ||||
| Alvarado‐Esquivel (2017) 27 | Mexico | Case control | Neurological disorder | 344 | 25 | 7.3 | 5 | 14.3 | 344 | 35 | 10.2 | NA | NA |
| Alvarado‐Esquivel (2016) 28 | Mexico | Case control | Depression | 89 | 11 | 12.4 | NA | NA | 356 | 22 | 6.2 | 4 | 1.1 |
| Daryani (2010) 29 | Iran | Case control | Schizophrenia | 80 | 28 | 35 | 9 | 11.2 | 99 | 25 | 25.3 | 11 | 11.1 |
| Chen (2019) 30 | China | Case control | Psychiatric disorders | 798 | 106 | 13.3 | 33 | 4.1 | 681 | 64 | 9.4 | 13 | 1.9 |
| Sapmaz (2019) 31 | Turkey | Case control | Depression | 37 | 8 | 21.6 | NA | NA | 36 | 2 | 5.6 | NA | NA |
| Akgul (2021) 32 | Turkey | Case control | Schizophrenia | 117 | 63 | 53.8 | NA | NA | 120 | 33 | 27.5 | NA | NA |
| Alvarado‐Esquivel (2006) 33 | Mexico | Case control | Psychiatric disorders | 137 | 25 | 18.2 | NA | NA | 180 | 16 | 8.9 | NA | NA |
| Huseein (2020) 34 | Egypt | Case control | Schizophrenia and bipolar disorder | 110 | 57 | 51.8 | NA | NA | 50 | 15 | 30 | NA | NA |
| Muflikhah (2018) 35 | Indonesia | Case control | Schizophrenia | 94 | 65 | 69.1 | NA | NA | 64 | 42 | 65.6 | NA | NA |
| Akaltun (2018) 36 | Turkey | Case control | OCD and GAD | 120 | 40 | 33.3 | NA | NA | 60 | 6 | 10 | NA | NA |
| Elsaid (2014) 37 | Libya | Case control | Psychiatric disorders | 300 | 151 | 50.3 | NA | NA | 300 | 99 | 33 | NA | NA |
| Alvarado‐Esquivel (2011) 38 | Mexico | Case control | Schizophrenia | 50 | 10 | 20 | 2 | 4 | 150 | 8 | 5.3 | NA | NA |
| Alvarado‐Esquivel (2013) 39 | Mexico | Case control | Psychiatric disorders | 156 | 7 | 4.5 | 3 | 1.9 | 127 | 10 | 7.9 | 3 | 2.4 |
| Rashno (2016) 40 | Iran | Case control | Alzheimer's disease | 87 | 58 | 66.5 | NA | NA | 87 | 49 | 56.3 | NA | NA |
| Kheirandish (2016) 41 | Iran | Case control | Schizophrenia and bipolar disorder | 170 | 103 | 63.5 | 14 | 8.2 | 170 | 65 | 38.2 | 8 | 4.7 |
| Bakre (2015) 42 | Iraq | Case control | Schizophrenia | 93 | 30 | 32.3 | 9 | 9.7 | 93 | 4 | 4.3 | 1 | 1.1 |
| Dogruman (2009) 43 | Turkey | Case control | Schizophrenia | 88 | 42 | 47.7 | NA | NA | 88 | 19 | 21.6 | NA | NA |
| Mouhawess (2020) 44 | Lebanon | Case control | Schizophrenia | 150 | 56 | 37.5 | NA | NA | 150 | 1 | 0.7 | NA | NA |
| Esshili (2016) 45 | Tunisia | Case control | Schizophrenia | 246 | 184 | 74.8 | NA | NA | 117 | 63 | 53.8 | NA | NA |
| Fallahi (2017) 46 | Iran | Case control | Parkinson's disease | 115 | 61 | 53 | NA | NA | 115 | 64 | 55.6 | NA | NA |
| Stepanova (2019) 47 | Russia | Case control | NA | 115 | 62 | 40 | NA | NA | 152 | 39 | 25 | NA | NA |
| Alvarado‐Esquivel (2019) 48 | Mexico | Case control | Bipolar disorder | 66 | 6 | 9.1 | NA | NA | 396 | 22 | 5.6 | 4 | 1 |
| Hamdani (2013) 49 | France | Case control | Bipolar disorder | 110 | 85 | 76.9 | NA | NA | 106 | 51 | 48.2 | NA | NA |
| Hamidinejat (2010) 50 | Iran | Case control | Schizophrenia | 134 | 71 | 53 | 6 | 4.5 | 48 | 14 | 29.2 | 2 | 4.2 |
| Alipour (2011) 51 | Iran | Case control | Schizophrenia | 62 | 42 | 67.7 | NA | NA | 62 | 23 | 37.1 | NA | NA |
| Abdollahian (2017) 52 | Iran | Case control | Schizophrenia | 350 | 164 | 46.9 | 17 | 4.85 | 350 | 120 | 34.3 | 3 | 0.9 |
| James (2013) 53 | Nigeria | Case control | Psychiatric disorders | 140 | 43 | 30.7 | 10 | 7.14 | 140 | 25 | 17.9 | 12 | 8.6 |
| Abdelaal (2016) 54 | Egypt | Case control | Neuropsychiatric disorder | 230 | 50 | 21.7 | NA | NA | 60 | 7 | 11.7 | NA | NA |
| Zaki (2016) 55 | Saudi Arabia | Case control | Neuropsychiatric disorder | 162 | 58 | 35.8 | 10 | 6 | 162 | 24 | 14.8 | 6 | 3.7 |
| Khademvatan (2014) 56 | Iran | Case control | Schizophrenia | 100 | 34 | 34 | NA | NA | 200 | 53 | 26.5 | NA | NA |
| Alvarado‐Esquivel (2016) 57 | Mexico | Case control | Anxiety and depressive disorder | 65 | 15 | 23.1 | 4 | 6.2 | 260 | 18 | 6.9 | 10 | 3.8 |
| Kezai (2020) 58 | Algeria | Case control | Schizophrenia | 70 | 49 | 70 | NA | NA | 70 | 37 | 52.9 | NA | NA |
| Oskouei (2014) 59 | Iran | Case control | Parkinson's disease | 75 | 64 | 85.3 | NA | NA | 75 | 68 | 90.3 | NA | NA |
| Markovitz (2015) 60 | United state | Cross‐sectional | Anxiety disorders | 484 | 128 | 26.5 | NA | NA | NA | NA | NA | NA | NA |
| Grada (2022) 61 | Romania | Case control | Psychiatric disorders | 308 | 209 | 67.9 | NA | NA | 296 | 160 | 54.1 | NA | NA |
| Nasirpour (2020) 62 | Iran | Case control | Depression | 87 | 52 | 59.8 | NA | NA | 87 | 49 | 56.3 | NA | NA |
| Alvarado‐Esquivel (2021) 63 | Mexico | Case control | Suicidal ideation | 306 | 37 | 12.1 | 10 | 3.3 | 1739 | 134 | 7.7 | NA | NA |
| Alvarado‐Esquivel (2021) 64 | Mexico | Case control | Suicidal ideation | 224 | 34 | 15.2 | 5 | 2.23 | 1199 | 118 | 9.8 | NA | NA |
| Alvarado‐Esquivel (2021) 65 | Mexico | Cross‐sectional | Suicide victims | 87 | 7 | 8 | NA | NA | NA | NA | NA | NA | NA |
| Achaw (2019) 66 | Ethiopia | Case control | Psychiatric disorders | 152 | 51 | 33.6 | 1 | 1.3 | 152 | 25 | 16.4 | 6 | 3.9 |
| Cong (2015) 67 | China | Case control | Psychiatric disorders | 445 | 77 | 17.3 | 14 | 3.2 | 445 | 55 | 12.4 | 10 | 2.3 |
| Oana (2019) 68 | Romania | Case control | Schizophrenia and psychotic disorder | 91 | 40 | 44 | NA | NA | 206 | 73 | 35.4 | NA | NA |
| Olariu (2017) 69 | Romania | Cross‐sectional | Psychiatric disorders | 214 | 117 | 54.7 | NA | NA | NA | NA | NA | NA | NA |
| Shehata (2016) 70 | Egypt | Cross‐sectional | Neurodevelopmental disorder | 188 | 94 | 50 | 31 | 16.5 | NA | NA | NA | NA | NA |
| Ebadi (2014) 71 | Iran | Case control | Schizophrenia | 152 | 81 | 53.2 | 48 | 31.5 | 152 | 63 | 41.4 | 30 | 19.7 |
| Ansari‐Lari (2017) 72 | Iran | Case control | Schizophrenia | 99 | 42 | 42.4 | NA | NA | 152 | 41 | 27 | NA | NA |
| Xiao (2010) 73 | China | Case control | Psychiatric disorders | 547 | 62 | 11.3 | NA | NA | 2634 | 329 | 12.5 | NA | NA |
| Prandota (2015) 74 | Egypt | Cross‐sectional | Autism | 46 | 11 | 23.9 | NA | NA | NA | NA | NA | NA | NA |
| Esnafoglu (2017) 75 | Turkey | Case control | Autism | 102 | 3 | 2.9 | NA | NA | 51 | 1 | 2 | NA | NA |
Abbreviations: GAD, generalized anxiety disorder; NA, not available; OCD, obsessive‐compulsive disorder.
3.3. Seroprevalence of chronic T. gondii infection (IgG antibody) among neuropsychiatric patients and healthy controls
Overall, the seroprevalence of T. gondii IgG among patients with neuropsychiatric disorders was variable, ranged from 2.9% reported in Turkey 75 to 85.5% reported in Iran. 59 In this meta‐analysis, the global pooled seroprevalence of T. gondii IgG antibody among neuropsychiatric patients was 38.27% (95% CI: 32.04%−44.9%). There was substantial heterogeneity with I 2 of 98.3% (Figure 2). Comparatively, lower global pooled seroprevalence of T. gondii IgG antibody was found among health controls (25.31%; 95% CI: 21.53−29.08). The seroprevalence of T. gondii IgG among healthy controls ranged from 0.7% reported in Lebanon 2020 44 to 90% reported in Iran. 59 Significant heterogeneity was also observed (I 2 value of 97.9%) (Figure 3).
Figure 2.
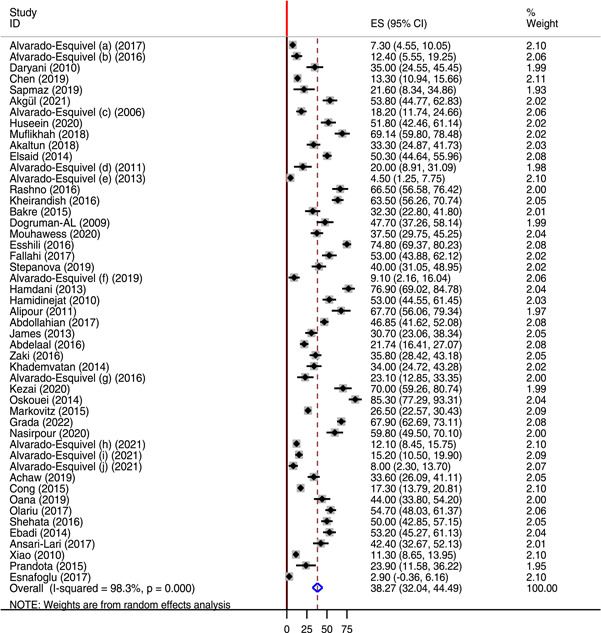
Forest plot showing the global pooled seroprevalence of Toxoplasma gondii IgG antibody among neuropsychiatric patients from 2006 to 2022.
Figure 3.
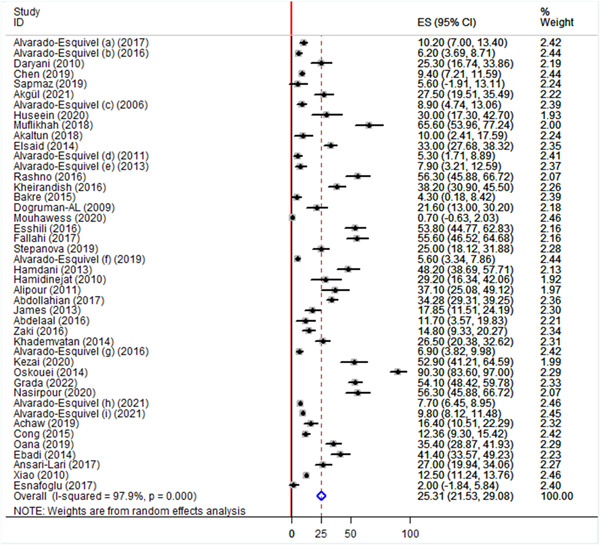
Forest plot showing the global seroprevalence of Toxoplasma gondii IgG antibody among apparently healthy control from 2006 to 2022.
3.4. Seroprevalence of acute T. gondii infection among neuropsychiatric patients and healthy controls
Of the included 49 studies, 18 studies reported the prevalence of T. gondii IgM antibodies among neuropsychiatric patients and 15 studies reported the prevalence of IgM antibodies among healthy controls. The global pooled seroprevalence of T. gondii IgM antibody among neuropsychiatric patients was 6.78% with a range varying between 1.3% and 31.5%. On the other hand, the global pooled seroprevalence of T. gondii IgM antibody among healthy controls was 3.13% with a range varying between 0.85% and 19.7%. There was significantly high heterogeneity with I 2 value of 93.7% among the cases. However, the heterogeneity was relatively lower in the healthy controls (I 2 value of 79.1%) (Figure 4).
Figure 4.
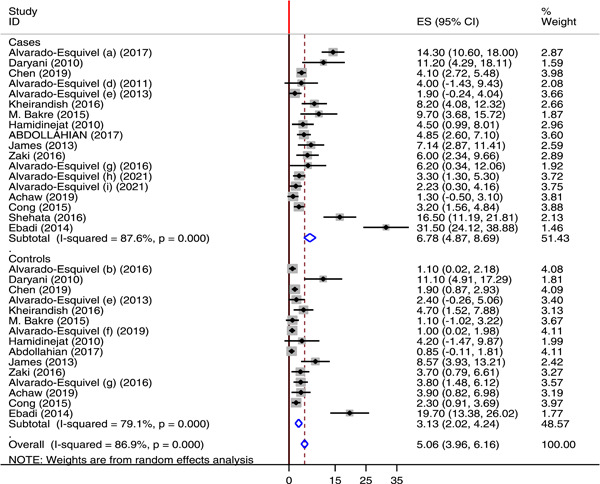
Forest plot showing the global seroprevalence of anti Toxoplasma gondii IgM among neuropsychiatric patients and healthy controls from 2010 to 2021.
3.5. Subgroup analysis
Subgroup analysis for sex, region, country, and publication year was done to investigate the source of heterogeneity across studies. With regard to sex of the study participants with neuropsychiatric disorders, 26 and 25 studies reported the prevalence of latent T. gondii infection among male and female patients, respectively. The prevalence of T. gondii IgG antibody was higher among males 17.52% (95% CI: 13.68−21.37) than in females 12.35% (95% CI: 9.65−15.04). In both cases, high heterogeneity was reported with I 2 of 96.4% and 92%, respectively (Figure 5). According to the continent, Europe contributed for the highest pooled seroprevalence of T. gondii IgG antibody (57%) followed by Africa (45.25%) and Asia (43%) (Figure 6). Another subgroup analysis was done for countries. According to this analysis, the highest pooled seroprevalence of T. gondii IgG antibody was found in Romania 56.1% followed by Iran (55.03%) and Egypt (36.93%). The least pooled prevalence was recorded in Mexico 11.85% (Table 2). Time based subgroup analysis showed the highest pooled prevalence of T. gondii IgG antibody in the year 2012−2016 (41.16%) than in 2006−2011 (36.6%) and 2017−2022 (35.82%) (Figure 7).
Figure 5.
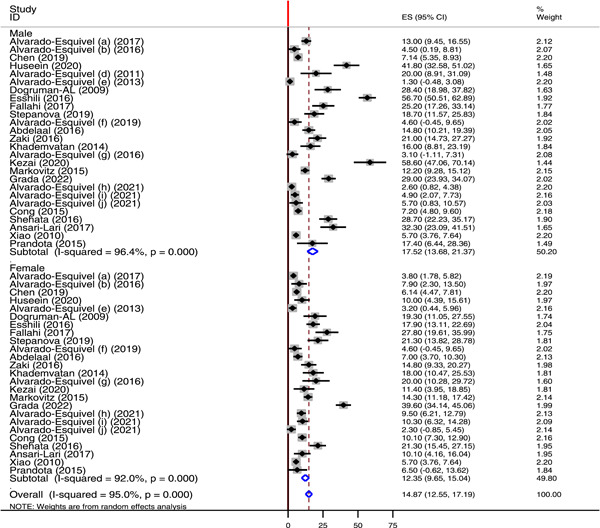
Forest plot showing the subgroup analysis Toxoplasma gondii IgG antibody seroprevalence by sex.
Figure 6.
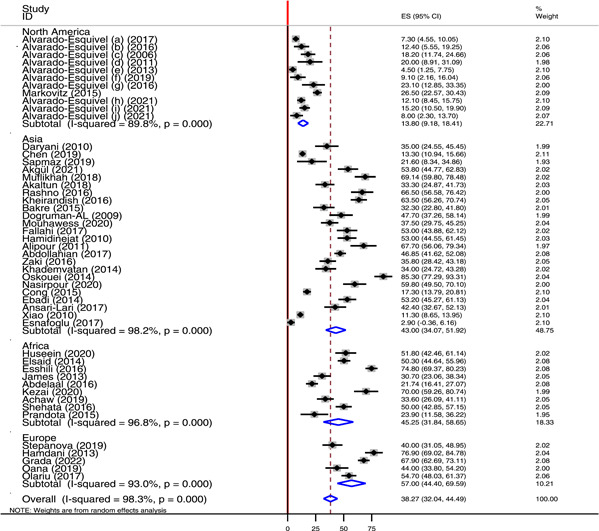
Forest plot showing the pooled prevalence of anti Toxoplasma gondii IgG in different region of the world.
Table 2.
Seroprevalence of anti Toxoplasma gondii IgG among different countries.
| Country | Region | Number of studies | Pooled prevalence of IgG (%) | 95% CI |
|---|---|---|---|---|
| Mexico | North America | 10 | 11.85 | 8.54−15.16 |
| Iran | Asia | 12 | 55.03 | 46.88−63.19 |
| China | Asia | 3 | 13.76 | 10.7−16.82 |
| Turkey | Asia | 5 | 31.74 | 8.31−55.18 |
| Egypt | Africa | 4 | 36.93 | 19.75−54.11 |
| Romania | Europe | 3 | 56.1 | 42.97−69.23 |
Figure 7.
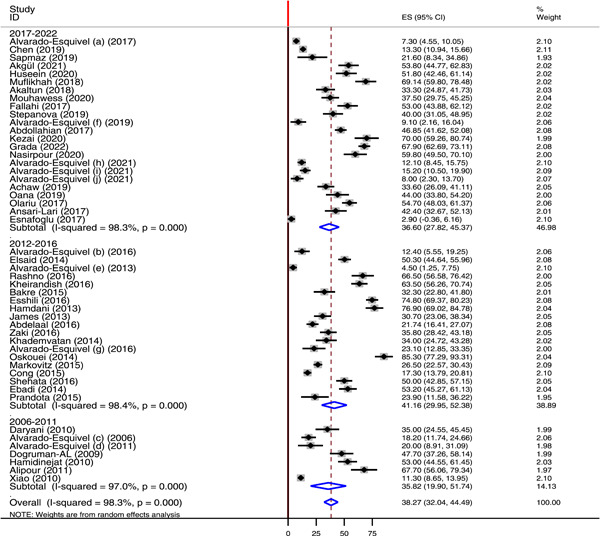
Forest plot showing the pooled seroprevalence of Toxoplasma gondii IgG antibody by publication year.
3.6. Publication bias and sensitivity analysis
In this study, the symmetry of the funnel plot indicated the absence of publication bias (Figure 8). Furthermore, the Egger's test statistics confirmed the absence of publication bias with p‐value of 0.765. According to sensitivity analysis, the pooled effect size when individual studies omitted lied within the 95% CI of the overall pooled effect size. This confirmed the absence of single study impact on the overall pooled seroprevalence of T. gondii infection.
Figure 8.
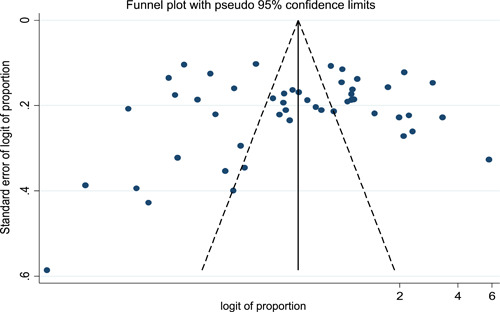
Funnel plot showing absence of publication bias.
4. DISCUSSION
The purpose of this systematic review and meta‐analysis was to determine the global seroprevalence of T. gondii infection among neuropsychiatric patients. To the best of our knowledge, our review represents the largest systematic review and meta‐analysis of the global pooled seroprevalence of T. gondii infection among neuropsychiatric patients including 21,093 study participants with 8592 neuropsychiatric patients and 12,501 healthy controls. People with neuropsychiatric disorders are highly affected by T. gondii infection proving the need for routine screening of neuropsychiatric patients for T. gondii infection. The present study revealed that the global pooled seroprevalence of chronic T. gondii infection was 38.27% (95% CI: 32.04−44.49) among neuropsychiatric patients and 25.31% (95% CI: 21.53−29.08) in apparently healthy controls with high heterogeneity (I 2 value of 98.3%). This finding is in line with the global pooled prevalence of T. gondii among HIV patients (35.8%; 95% CI: 30.8−40.7) 76 and the national overall pooled prevalence of human T. gondii infection in Nigeria (32.92% [95% CI: 27.89−38.37]). 77 Toxoplasma gondii induces a suite of behavioral and neurological changes in the infected individuals. There are pronounced behavioral changes in the individual infected with T. gondii. The parasite induced behavioral alteration include decreased cognitive function, prolonged reaction times, increased risk‐taking behavior, and suicidal tendencies in individuals with latent T. gondii infection. 32 , 78 , 79 The reason for the complexity in human behavior is not yet understood, but it is commonly seen in T. gondii seropositive individuals.
The global seroprevalence of T. gondii IgG antibody among neuropsychiatric patients obtained in this study was higher than the pooled prevalence of T. gondii infection in cancer patients (30.8%), 80 immunocompromised patients (35.9%), 81 and Ethiopian general population (34.59%). 82 On the other hand, the finding of this study was lower than the prevalence of T. gondii among the Iranian general population (39.3%). 83 The observed variability might be due to differences in the study group, number of studies included in the meta‐analysis, and the diagnostic methods employed.
The high burden of T. gondii infection among patients with neurological and mental disorders can be associated with different mechanisms employed by this neurotropic parasite. Once passing the BBB through paracellular, transcellular or stowaway mechanism, T. gondii infection results in different spectrums of neuropsychiatric disorders and achieves this through alteration of the level of neurotransmitters. Majority of the neurobehavioral and neurological disorders associated with chronic toxoplasmosis are due to an elevation of dopamine in the brain and alteration in the level of GABA, serotonin, glutamate, nitric oxide, noradrenaline, and kynurenic acid. 18 The neuropsychiatric disorders include; AD which is characterized by the abnormal configuration and excessive phosphorylation of amyloid deposition and neurofibrillary tangles in neurons. This is associated with T. gondii infection, due to the ability of the parasites to cause lower beta‐amyloid plaque deposition through inflammation induced beta‐amyloid phagocytosis and degradation. 84 Unbalanced excitatory and inhibitory neurotransmission due to toxoplasmosis can induce seizure in T. gondii infected mice. 4 Another neuropsychiatric disorder associated with T. gondii infection is bipolar disorder (manic depression), which encompasses a wide range of behavioral changes and involve reduction of corticosterone production due to latent T. gondii infection. Likewise, significantly higher prevalence of T. gondii infection was also reported among patients with schizophrenia. Toxoplasmosis patients are 2.73 times more likely to develop schizophrenia in their later life than healthy controls. 85 The presence of large amount of bradyzoite in the olfactory bulb and production of large amount of anti T. gondii IgG is associated with the development of anosmia. A compressive review that assessed the association between T. gondii and neurologic disorders found that the increased production of nitric oxide (NO2), enhanced the production of inflammatory cytokines (interferon gamma, tumor necrosis factor alpha, interleukin 1 reactive oxygen and nitrogen species), neurotic biomolecules, and altered the dopamine balance leading to olfactory impairment, migraine, Asperger's syndrome, autism, and schizophrenia. 86 , 87
According to subgroup analysis, the pooled seroprevalence of T. gondii IgG antibody was higher among males 17.52% than in females 12.35%. This finding was in agreement with the finding of a meta‐analysis study that assessed toxoplasmosis in Iranian population. 83 With regard to regional classification, a high burden of chronic T. gondii infection was reported in Europe (57%) followed by Africa (45.25%) and Asia (43%). The pooled prevalence of T. gondii IgG antibody in North America was 13.8%. The overall pooled seroprevalence of T. gondii IgG antibody among Iranian neuropsychiatric patient was 55.03% (95% CI: 46.88−63.19). This was higher than the prevalence of T. gondii among Iranian general population (39.3%), 83 Iranian blood donors (34.4%), 88 Iranian pregnant women (41%), 89 and immunocompromised patients in Iran (50.01%). 90 This study also showed 13.76% pooled seroprevalence of T. gondii IgG antibody among neuropsychiatric patients in China. This finding was higher than the prevalence of T. gondii among blood donors in China (6.26%). 91 The variability in the seroprevalence of chronic toxoplasmosis across the different regions of the world and countries might be due to different reasons including geographical variation, difference in climatic condition, implementation of prevention and control strategies, personal and environmental hygienic conditions, and animal contact behaviors. In addition, difference in life style and habit of having close contact with cat or other felids can also contribute to the variability in the prevalence of T. gondii across the different regions and countries throughout the world.
The result of time‐based subgroup analysis demonstrated that the pooled prevalence of chronic T. gondii infection was higher in the year 2012−2016 (41.16%) compared to 2006−2011 (36.6%) and 2017−2022 (35.82%). The high prevalence in the year 2012−2016 might be due to climatic change, breaking of the prevention and control strategies, and environmental hygienic conditions.
The global pooled prevalence of acute T. gondii infection among neuropsychiatric patients and healthy controls was 6.78% (95% CI: 4.87−8.69) and 3.13% (95% CI: 2.02−4.24), respectively. This finding was higher than the global prevalence of acute toxoplasmosis among pregnant women (1.1%). 20 According to the symmetry of the funnel plot and the Eggers test statistics (p value of 0.765) there was no publication bias in this systematic review and meta‐analysis.
The result of sensitivity analysis proved that there is no single study that affects the pooled effect size. The overall pooled prevalence of anti T. gondii IgG was calculated by omitting each study sequentially and the computed pooled prevalence was within the 95% CI of the overall pooled prevalence.
This study has few important limitations. First, the included studies were conducted only in 18 countries from Asia, Africa, North America, and Europe. There were no studies in Middle East, Latin America, and other corners of the world. Next, there was substantial heterogeneity observed between studies that may affect the interpretation of the results. Finally, studies conducted in a language other than English were excluded which might lead to lose of some studies to be included.
5. CONCLUSION
This systematic review and meta‐analysis showed that patients with neurological and psychiatric disorders are facing infection with the global endemic neurotropic parasite T. gondii. The global pooled prevalence of T. gondii IgG antibody in those patients was 38.27%. This urges clinicians to consider T. gondii infection in these patients, request appropriate routine testing to confirm the infection and provide appropriate treatment to those with the infection. Moreover, it is also an alarm to international, continental, and nation health bureaus and other stakeholders to develop targeted prevention and control strategies of T. gondii infection. This review also provides valuable information to policy makers and different stack holders. Moreover, the information could be used for future complimentary research.
AUTHOR CONTRIBUTIONS
Habtye Bisetegn: Conceptualization; data curation; formal analysis; methodology; software; validation; visualization; writing—original draft. Habtu Debash: Methodology; resources; supervision; validation; visualization; writing—review and editing. Hussen Ebrahim: Formal analysis; investigation; methodology; project administration; validation; visualization; writing—review and editing. Naunian Mahmood: Formal analysis; methodology; software; supervision; visualization; writing—review and editing. Alemu Gedefie: Methodology; validation; visualization; writing—review and editing. Mihret Tilahun: Data curation; investigation; methodology; validation; visualization; writing—review and editing. Ermiyas Alemayehu: Conceptualization; investigation; methodology; validation; visualization; writing—review and editing. Ousman Mohammed: Conceptualization; formal analysis; investigation; methodology; software; writing—review and editing. Daniel Getacher Feleke: Formal analysis; investigation; methodology; validation; visualization; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Habtye Bisetegn affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors of this manuscript would like to acknowledge all the authors of the included study and their study participants. In addition, we want to thank all our friends and colleagues who helped us to complete this manuscript.
Bisetegn H, Debash H, Ebrahim H, et al. Global seroprevalence of Toxoplasma gondii infection among patients with mental and neurological disorders: a systematic review and meta‐analysis. Health Sci Rep. 2023;6:e1319. 10.1002/hsr2.1319
DATA AVAILABILITY STATEMENT
All data required for this research are available within the manuscript. If additional data are needed, it can be obtained from the corresponding authors upon request.
REFERENCES
- 1. Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ. 2013;91(7):501‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Safarpour H, Cevik M, Zarean M, et al. Global status of Toxoplasma gondii infection and associated risk factors in people living with HIV. AIDS. 2020;34(3):469‐474. [DOI] [PubMed] [Google Scholar]
- 3. Zhao XY, Ewald SE. The molecular biology and immune control of chronic Toxoplasma gondii infection. J Clin Invest. 2020;130(7):3370‐3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wohlfert EA, Blader IJ, Wilson EH. Brains and brawn: toxoplasma infections of the central nervous system and skeletal muscle. Trends Parasitol. 2017;33(7):519‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26(4):190‐196. [DOI] [PubMed] [Google Scholar]
- 6. Dubey JP. The history and life cycle of Toxoplasma gondii . Toxoplasma gondii. Elsevier; 2020:1‐19. [Google Scholar]
- 7. Al‐Malki ES. Toxoplasmosis: stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio‐economic status. Saudi J Biol Sci. 2021;28(1):962‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adekunle RO, Sherman A, Spicer JO, et al. Clinical characteristics and outcomes of toxoplasmosis among transplant recipients at two US academic medical centers. Tranpl Infect Dis. 2021;23(4):e13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ortiz‐Guerrero G, Gonzalez‐Reyes RE, de‐la‐Torre A, Medina‐Rincón G, Nava‐Mesa MO. Pathophysiological mechanisms of cognitive impairment and neurodegeneration by Toxoplasma gondii infection. Brain Sci. 2020;10(6):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Nat Rev Microbiol. 2021;19(7):467‐480. [DOI] [PubMed] [Google Scholar]
- 11. Severance EG, Xiao J, Jones‐Brando L, et al. Toxoplasma gondii—a gastrointestinal pathogen associated with human brain diseases. Int Rev Neurobiol. 2016:143‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearce BD, Kruszon‐Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the Third National Health and Nutrition Survey. Biol Psychiatry. 2012;72(4):290‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao J, Prandovszky E, Kannan G, et al. Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr Bull. 2018;44(5):983‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suvisaari J, Torniainen‐Holm M, Lindgren M, Härkänen T, Yolken RH. Toxoplasma gondii infection and common mental disorders in the Finnish general population. J Affect Disord. 2017;223:20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miman O, Kusbeci OY, Aktepe OC, Cetinkaya Z. The probable relation between Toxoplasma gondii and Parkinson's disease. Neurosci Lett. 2010;475(3):129‐131. [DOI] [PubMed] [Google Scholar]
- 16. Alvarado‐Esquivel C, Estrada‐Martínez S, Ramos‐Nevárez A, et al. Is Toxoplasma gondii infection associated with sexual promiscuity? A cross‐sectional study. Pathogens. 2021;10(11):1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fabiani S, Pinto B, Bruschi F. Toxoplasmosis and neuropsychiatric diseases: can serological studies establish a clear relationship? Neurol Sci. 2013;34(4):417‐425. [DOI] [PubMed] [Google Scholar]
- 18. Virus MA, Ehrhorn EG, Lui LM, Davis PH. Neurological and neurobehavioral disorders associated with Toxoplasma gondii infection in humans. J Parasitol Res. 2021;2021:6634807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ngoungou EB, Bhalla D, Nzoghe A, Dardé ML, Preux PM. Toxoplasmosis and epilepsy—systematic review and meta analysis. PLoS Neglected Trop Dis. 2015;9(2):e0003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bayani M, Riahi SM, Bazrafshan N, Ray Gamble H, Rostami A. Toxoplasma gondii infection and risk of Parkinson and Alzheimer diseases: a systematic review and meta‐analysis on observational studies. Acta Trop. 2019;196:165‐171. [DOI] [PubMed] [Google Scholar]
- 21. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. http://link.springer.com/10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, Connel DO, Peterson J, Welch V. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. scholar.archive.org; 2000.
- 23. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ. 1997;315(7121):1533‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 27. Alvarado‐Esquivel C, Rico‐Almochantaf YR, Hernández‐Tinoco J, et al. Toxoplasma gondii exposure and neurological disorders: an age‐ and gender‐matched case‐control pilot study. Eur J Microbiol Immunol. 2017;7(4):303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alvarado‐Esquivel C, Sánchez‐Anguiano LF, Hernández‐Tinoco J, et al. Toxoplasma gondii infection and depression: a case—control seroprevalence study. Eur J Microbiol Immunol. 2016;6(2):85‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daryani A, Sharif M, Hosseini SH, Karimi SA, Gholami S. Serological survey of Toxoplasma gondii in schizophrenia patients referred to Psychiatric Hospital, Sari City, Iran. Trop Biomed. 2010;27(3):476‐482. [PubMed] [Google Scholar]
- 30. Chen X, Chen B, Hou X, et al. Association between Toxoplasma gondii infection and psychiatric disorders in Zhejiang, Southeastern China. Acta Trop. 2019;192:82‐86. [DOI] [PubMed] [Google Scholar]
- 31. Yalın Sapmaz Ş, Şen S, Özkan Y, Kandemir H. Relationship between Toxoplasma gondii seropositivity and depression in children and adolescents. Psychiatry Res. 2019;278:263‐267. [DOI] [PubMed] [Google Scholar]
- 32. Akgül Ö, Demirel ÖF, Aksoy Poyraz C, et al. Toxoplasma gondii infection by serological and molecular methods in schizophrenia patients with and without suicide attempts: an age‐sex‐matched case‐control study. Int J Clin Pract. 2021;75(8):e14449. [DOI] [PubMed] [Google Scholar]
- 33. Alvarado‐Esquivel C, Alanis‐Quiñones OP, Arreola‐Valenzuela MÁ, et al. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. 2006;6(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huseein EAM, Khalifa H, Ramadan GK, Hassaan SH, Shaaban I, Haiam F. Seroprevalence of Toxoplasma gondii among patients with schizophrenia and bipolar disorder in upper Egypt: a comparative study with a control group. Ann Parasitol. 2020;66(2):183‐192. [DOI] [PubMed] [Google Scholar]
- 35. Muflikhah ND, Supargiyono S, Artama WT. Seroprevalence and risk factor of toxoplasmosis in schizophrenia patients referred to Grhasia Psychiatric Hospital, Yogyakarta, Indonesia. Afr J Infect Dis. 2018;12:76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akaltun İ, Kara SS, Kara T. The relationship between Toxoplasma gondii IgG antibodies and generalized anxiety disorder and obsessive‐compulsive disorder in children and adolescents: a new approach. Nord J Psychiatry. 2018;72(1):57‐62. [DOI] [PubMed] [Google Scholar]
- 37. Elsaid MMA, Azbedah AG, Dia Eddin E, EL‐Alem DEE, Alkout A. The prevalence of Toxoplasma gondii infection in psychiatric patients in Tripoli, Libya. J Am Sci. 2014;10(5):135‐140. [Google Scholar]
- 38. Alvarado‐Esquivel C, Urbina‐Álvarez JD, Estrada‐Martínez S, et al. Toxoplasma gondii infection and schizophrenia: a case control study in a low toxoplasma seroprevalence Mexican population. Parasitol Int. 2011;60(2):151‐155. [DOI] [PubMed] [Google Scholar]
- 39. Alvarado‐Esquivel C, Sánchez‐Anguiano LF, Arnaud‐Gil CA, et al. Toxoplasma gondii infection and suicide attempts: a case‐control study in psychiatric outpatients. J Nervous Mental Dis. 2013;201(11):948‐952. [DOI] [PubMed] [Google Scholar]
- 40. Menati Rashno M, Fallahi S, Kheirandish F, Bagheri S, Kayedi MH, Birjandi M. Seroprevalence of Toxoplasma gondii infection in patients with Alzheimer's disease. Arch Clin Infec Dis. 2016;11(3):e37205. [Google Scholar]
- 41. Kheirandish F, Nazari H, Mahmoudvand H, et al. Possible link between Toxoplasma gondii infection and mood disorders in Lorestan Province, Western Iran. Arch Clin Infec Dis. 2016;11(4):e36602. [Google Scholar]
- 42. Bakre H, Hussain S, Ali S. Toxoplasma gondii infection in patients with schizophrenia. Zanco J Med Sci. 2015;19(1):874‐879. [Google Scholar]
- 43. Dogruman‐Al F, Aslan S, Yalcin S, Kustimur S, Turk S. A possible relationship between Toxoplasma gondii and schizophrenia: a seroprevalence study. Int J Psychiatry Clin Pract. 2009;13(1):82‐87. [DOI] [PubMed] [Google Scholar]
- 44. El Mouhawess A, Hammoud A, Zoghbi M, et al. Relationship between Toxoplasma gondii seropositivity and schizophrenia in the Lebanese population: potential implication of genetic polymorphism of MMP‐9. BMC Psychiatry. 2020;20(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esshili A, Thabet S, Jemli A, et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatry Res. 2016;245:327‐332. [DOI] [PubMed] [Google Scholar]
- 46. Fallahi S, Rostami A, Birjandi M, Zebardast N, Kheirandish F, Spotin A. Parkinson's disease and Toxoplasma gondii infection: sero‐molecular assess the possible link among patients. Acta Trop. 2017;173:97‐101. [DOI] [PubMed] [Google Scholar]
- 47. Stepanova EV, Kondrashin AV, Sergiev VP, et al. Toxoplasmosis and mental disorders in the Russian Federation (with special reference to schizophrenia). PLoS One. 2019;14(7):e0219454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarado‐Esquivel C, Estrada‐Martínez S, Pérez‐Alamos AR. A case–control seroprevalence study on the association between Toxoplasma gondii infection and bipolar disorder. Front Psychiatry. 2019;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hamdani N, Daban‐Huard C, Lajnef M, et al. Relationship between Toxoplasma gondii infection and bipolar disorder in a French sample. J Affect Disord. 2013;148(2–3):444‐448. [DOI] [PubMed] [Google Scholar]
- 50. Hamidinejat H, Ghorbanpoor M, Hosseini H, et al. Toxoplasma gondii infection in first‐episode and inpatient individuals with schizophrenia. Int J Infect Dis. 2010;14(11):e978‐e981. [DOI] [PubMed] [Google Scholar]
- 51. Alipour A, Shojaee S, Mohebali M, Tehranidoost M, Abdi Masoleh F, Keshavarz H. Toxoplasma infection in schizophrenia patients: a comparative study with control group. Iran J Parasitol. 2011;6(2):31‐37. [PMC free article] [PubMed] [Google Scholar]
- 52. Abdollahian E, Shafiei R, Mokhber N, Kalantar K, Fata A. Seroepidemiological study of Toxoplasma gondii infection among psychiatric patients in Mashhad, Northeast of Iran. Iran J Parasitol. 2017;12(1):117‐122. [PMC free article] [PubMed] [Google Scholar]
- 53. James BO, Agbonile IO, Okolo M, Lawani AO, Omoaregba JO. Prevalence of Toxoplasma gondii infection among individuals with severe mental illness in Nigeria: a case control study. Pathog Glob Health. 2013;107(4):189‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abdelaal N, Saber M, Fawzy N, et al. Seroprevalence of anti Toxoplasma gondii antibodies among patients with neuropsychiatric disorders: epilepsy and depression. J Egypt Soc Parasitol. 2016;46(3):729‐736. [PubMed] [Google Scholar]
- 55. Zaki WM, Hofdi RY, Shebiley AA, et al. Seroprevalence of Toxoplasma gondii infection and its associated risk factors in neuropsychiatric patients in Jazan Province, Saudi Arabia. J Egypt Soc Parasitol. 2016;46(3):467‐474. [PubMed] [Google Scholar]
- 56. Khademvatan S, Saki J, Khajeddin N, et al. Toxoplasma gondii exposure and the risk of schizophrenia. Jundishapur J Microbiol. 2014;7(11):e12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alvarado‐Esquivel C, Sanchez‐Anguiano LF, Hernandez‐Tinoco J, et al. Toxoplasma gondii infection and mixed anxiety and depressive disorder: a case‐control seroprevalence study in Durango, Mexico. J Clin Med Res. 2016;8(7):519‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kezai AM, Lecoeur C, Hot D, Bounechada M, Alouani ML, Marion S. Association between schizophrenia and Toxoplasma gondii infection in Algeria. Psychiatry Res. 2020;291:113293. [DOI] [PubMed] [Google Scholar]
- 59. Mahami Oskouei M, Hamidi F, Talebi M, et al. The correlation between Toxoplasma gondii infection and Parkinson's disease: a case‐control study. J Parasitic Dis. 2014;40(3):872‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Markovitz AA, Simanek AM, Yolken RH, et al. Toxoplasma gondii and anxiety disorders in a community‐based sample. Brain Behav Immun. 2015;43:192‐197. [DOI] [PubMed] [Google Scholar]
- 61. Grada S, Mihu AG, Petrescu C, et al. Toxoplasma gondii infection in patients with psychiatric disorders from Western Romania. Medicina. 2022;58(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nasirpour S, Kheirandish F, Fallahi S. Depression and Toxoplasma gondii infection: assess the possible relationship through a seromolecular case–control study. Arch Microbiol. 2020;202(10):2689‐2695. [DOI] [PubMed] [Google Scholar]
- 63. Alvarado‐Esquivel C, Estrada‐Martínez S, Ramos‐Nevárez A, et al. Association between Toxoplasma gondii exposure and suicidal behavior in patients attending primary health care clinics. Pathogens. 2021;10(6):677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alvarado‐Esquivel C, Estrada‐Martínez S, Pérez‐Álamos AR, et al. Toxoplasma gondii infection and suicidal behavior in people with alcohol consumption. Pathogens. 2021;10(6):734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alvarado‐Esquivel C, Mendoza‐Larios LA, García‐Dolores F, et al. Association between Toxoplasma gondii infection in brain and a history of depression in suicide decedents: a cross‐sectional study. Pathogens. 2021;10(10):1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Achaw B, Tesfa H, Zeleke AJ, et al. Sero‐prevalence of Toxoplasma gondii and associated risk factors among psychiatric outpatients attending University of Gondar Hospital, Northwest Ethiopia. BMC Infect Dis. 2019;19(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cong W, Dong W, Bai L, et al. Seroprevalence and associated risk factors of Toxoplasma gondii infection in psychiatric patients: a case‐control study in eastern China. Epidemiol Infect. 2015;143(14):3103‐3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oana C, Alina LM, Rareş OT. Seroprevalence of Toxoplasma gondii antibodies in Romanian patients with schizophrenia and acute and transient psychotic disorder. Acta Medica Marisiensis. 2019;65. [Google Scholar]
- 69. Olariu TR, Capraru ID, Papava I, Romosan R, Dehelean L, Lupu MA. Seroprevalence of Toxoplasma gondii in Romanian psychiatric patients. Eur Psychiatry. 2017;41(S1):s825. [Google Scholar]
- 70. Shehata AI, Hassanein FI, Abdul‐Ghani R. Seroprevalence of Toxoplasma gondii infection among patients with non‐schizophrenic neurodevelopmental disorders in Alexandria, Egypt. Acta Trop. 2016;154:155‐159. [DOI] [PubMed] [Google Scholar]
- 71. Ebadi M, Akhlaghi H, Zamani MM, et al. The correlation between toxoplama gondii infection and schizophrenia: a comparative study with family members (control group). Scimetr. 2014;2(1). 10.5812/scimetr.15386 [DOI] [Google Scholar]
- 72. Ansari‐Lari M, Farashbandi H, Mohammadi F. Association of Toxoplasma gondii infection with schizophrenia and its relationship with suicide attempts in these patients. Trop Med Int Health. 2017;22(10):1322‐1327. [DOI] [PubMed] [Google Scholar]
- 73. Xiao Y, Yin J, Jiang N, et al. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis. 2010;10(1):4. https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Prandota J, Abdel Fattah Elleboudy N, Ahmed Ismail K, Kamal Zaki O, Hussein Shehata H. Increased seroprevalence of chronic toxoplasmosis in autistic children: special reference to the pathophysiology of IFN‐γ and NO overproduction: Prandota J, et al. Seroprevalence of chronic toxoplasmosis in autistic children. Int J Neurol Res. 2015;1(3):102‐122. [Google Scholar]
- 75. Esnafoglu E, Yancar Demir E, Cetinkol Y, et al. The seroprevalence of antibodies to Toxoplasma gondii among children with autism. Dusunen Adam: J Psychiatr Neurol Sci. 2017;30(4):309‐315. [Google Scholar]
- 76. Wang ZD, Wang SC, Liu HH, et al. Prevalence and burden of Toxoplasma gondii infection in HIV‐infected people: a systematic review and meta‐analysis. Lancet HIV. 2017;4(4):e177‐e188. [DOI] [PubMed] [Google Scholar]
- 77. Karshima SN, Karshima MN. Human Toxoplasma gondii infection in Nigeria: a systematic review and meta‐analysis of data published between 1960 and 2019. BMC Public Health. 2020;20:877‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gajewski PD, Falkenstein M, Hengstler JG, Golka K. Toxoplasma gondii impairs memory in infected seniors. Brain Behav Immun. 2014;36:193‐199. [DOI] [PubMed] [Google Scholar]
- 79. Flegr J, Havlícek J, Kodym P, Malý M, Smahel Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case‐control study. BMC Infect Dis. 2002;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anvari D, Sharif M, Sarvi S, et al. Seroprevalence of Toxoplasma gondii infection in cancer patients: A systematic review and meta‐analysis. Microb Pathog. 2019;129:30‐42. [DOI] [PubMed] [Google Scholar]
- 81. Wang ZD, Liu HH, Ma ZX, et al. Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta‐analysis. Front Microbiol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gebremedhin EZ, Tadesse G. A meta‐analysis of the prevalence of Toxoplasma gondii in animals and humans in Ethiopia. Parasit Vectors. 2015;8(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Daryani A, Sarvi S, Aarabi M, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta‐analysis. Acta Trop. 2014;137:185‐194. [DOI] [PubMed] [Google Scholar]
- 84. Inceboz M, Inceboz T. Toxoplasmosis and neuropsychological effects. Turkish J Parasitol. 2021;45(1):49‐55. [DOI] [PubMed] [Google Scholar]
- 85. Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta‐analysis. Schizophr Bull. 2007;33(3):729‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Prandota J. Possible link between Toxoplasma Gondii and the anosmia associated with neurodegenerative diseases. Am J Alzheimer's Dis Other Dementiasr. 2014;29(3):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Elsheikha HM, Zhu XQ. Toxoplasma gondii infection and schizophrenia: an inter‐kingdom communication perspective. Curr Opin Infect Dis. 2016;29(3):311‐318. [DOI] [PubMed] [Google Scholar]
- 88. Mansouri A, Adhami Mojarad MR, Badfar G, et al. Epidemiology of Toxoplasma gondii among blood donors in Iran: a systematic review and meta‐analysis. Transfus Apher Sci. 2017;56(3):404‐409. [DOI] [PubMed] [Google Scholar]
- 89. Foroutan‐Rad M, Khademvatan S, Majidiani H, Aryamand S, Rahim F, Malehi AS. Seroprevalence of Toxoplasma gondii in the Iranian pregnant women: a systematic review and meta ‐analysis. Acta Trop. 2016;158:160‐169. [DOI] [PubMed] [Google Scholar]
- 90. Ahmadpour E, Daryani A, Sharif M, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta‐analysis. J Infec Developing Countries. 2014;8(12):1503‐1510. [DOI] [PubMed] [Google Scholar]
- 91. Wang T, Han Y, Pan Z, Wang H, Yuan M, Lin H. Seroprevalence of Toxoplasma gondii infection in blood donors in mainland China: a systematic review and meta‐analysis. Parasite. 2018;25:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data required for this research are available within the manuscript. If additional data are needed, it can be obtained from the corresponding authors upon request.


