Abstract
The key to stopping Alzheimer's disease (AD) lies in the pre‐dementia stages, with the goal to stop AD before dementia has started. We present the rationale and design of the ABOARD (A Personalized Medicine Approach for Alzheimer's Disease) project, which aims to invest in personalized medicine for AD. ABOARD is a Dutch public–private partnership of 32 partners, connecting stakeholders from a scientific, clinical, and societal perspective. The 5‐year project is structured into five work packages on (1) diagnosis, (2) prediction, (3) prevention, (4) patient‐orchestrated care, and (5) communication and dissemination. ABOARD functions as a network organization in which professionals interact cross‐sectorally. ABOARD has a strong junior training program “Juniors On Board.” Project results are shared with society through multiple communication resources. By including relevant partners and involving citizens at risk, patients, and their care partners, ABOARD builds toward a future with personalized medicine for AD.
Highlights
ABOARD (A Personalized Medicine Approach for Alzheimer's Disease) is a public–private research project executed by 32 partners that functions as a network organization.
Together, the project partners build toward a future with personalized medicine for Alzheimer's disease.
Although ABOARD is a Dutch consortium, it has international relevance.
ABOARD improves diagnosis, prediction, prevention, and patient‐orchestrated care.
Keywords: Alzheimer's disease, diagnosis, patient‐orchestrated care, personalized medicine, prediction, prevention
1. INTRODUCTION
Alzheimer's disease (AD) is among this century's major health challenges. An estimated 55 million individuals suffer from dementia, caused in roughly 70% by AD. 1 This number is expected to almost triple by 2050. AD comes with a huge burden, both on a personal level and on a socio‐economic level. 2 , 3
AD develops progressively over the course of 20 to 30 years. 4 , 5 To date, AD is mostly diagnosed in the dementia stage, with ill‐fitting care as a consequence. The stages before dementia offer a window of opportunity for intervention. Earlier intervention holds the promise that more cognitive function can be preserved, as less brain damage has occurred. Even small delays in onset and progression of AD and/or improved self‐management of patients could reduce the global burden of the disease. 6 Thus, prevention is key.
To prevent dementia, we need a paradigm shift. 7 We must focus on the disease stages before dementia and take differences between patients into account, both in biology and in preferences and needs. This will profoundly affect the patient journey. To optimize the effectiveness of preventive strategies, we need to identify those persons who benefit most in a timely way, underlining the need for a precise and molecular diagnosis. 8 , 9 When we diagnose AD in an early stage, the focus needs to shift toward optimizing prognosis. Prevention strategies by lifestyle modifications may be beneficial, but they have not been implemented in daily clinical practice. 10 , 11 In addition, secondary prevention in the form of disease‐modifying therapies aimed at delaying or stopping progression of the disease are under development. 12 , 13 , 14 , 15 , 16 Finally, because the needs of at‐risk individuals, patients, and their care partners differ, they should be encouraged to become involved in their (or their relative's) health and health care, to allow diagnosis, prediction, and prevention of AD to be patient orchestrated. Overall, many steps are needed to prepare for a future with personalized medicine for AD, which requires a broad and multidisciplinary collaboration. No party can do this alone—we need all aboard.
The ABOARD (A Personalized Medicine Approach for Alzheimer's Disease) project is a national public–private partnership (PPP) with 32 partners spanning the entire translational value chain (Figure 1). ABOARD is unique in that it connects all stakeholders from a scientific, clinical, and societal perspective: from fundamental research and clinical research and development (R&D) to applied research aiming at implementing novel personalized medicine tools and health‐care organizations, with a strong basis in society, putting citizens (at risk), patients, and care partners at the steering wheel. Because the ultimate aim is to transform patient care, and care systems vastly differ among countries, we initiated ABOARD as a Dutch consortium (with one Belgian partner). File S1 in supporting information provides an overview of all ABOARD partners.
FIGURE 1.
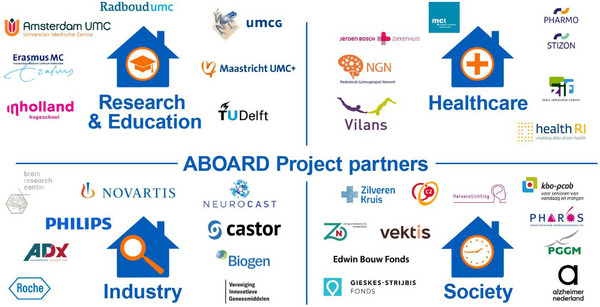
Overview of ABOARD project partners. The ABOARD consortium contains 32 public and private project partners from the four sectors of the quadruple helix: research & education, health care, society, and industry. ABOARD, A Personalized Medicine Approach for Alzheimer's Disease
ABOARD addresses the following broadly formulated hypotheses: that (1) an accurate and timely diagnosis benefits quality of care and quality of life and reduces care costs further on in the patient journey, (2) a well‐informed patient/individual at risk has the opportunity to make decisions that lead to better quality of life and reduce health‐care costs in the long run, and that (3) early preventive strategies contribute to more healthy years, reduced care costs, improved quality of life, and individuals with AD being more actively engaged in society for longer.
1.1. Objective
ABOARD aims to jumpstart personalized medicine for AD by preparing for and investing in effective, efficient, and patient‐orchestrated diagnosis, prediction, and prevention.
The specific objectives of ABOARD are:
To improve timely and molecular diagnosis by translating novel digital, proteomic, and genetic advances to daily practice, including the development of an easily applicable blood‐based biomarker test.
To set up a national infrastructure to recruit the ABOARD Cohort, an ongoing, person‐centered data collection allowing scale up of results to the national level, and to define individualized risk profiles with patient‐reported outcomes (PROs), for example, quality of life, as outcomes.
To pilot lifestyle prevention strategies and analyze system readiness for secondary prevention with disease‐modifying treatment to prepare for personalized prevention.
To engage citizens (at risk), patients, and their care partners throughout all aspects of their AD‐related health, disease management, and research.
To develop recommendations and e‐health tools to support patients and care partners as well as professionals in patient‐orchestrated diagnosis, prediction, and prevention of AD and implement these in regular health care.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed existing literature on personalized medicine for Alzheimer's disease (AD), regarding diagnosis, prediction, prevention, and patient‐orchestrated care. References are appropriately mentioned.
Interpretation: ABOARD (A Personalized Medicine Approach for Alzheimer's Disease) aims to jumpstart personalized medicine for AD by preparing for and investing in patient‐orchestrated diagnosis, prediction, and prevention. With a strong focus on patient and public involvement, training of future AD professionals, and on communication and dissemination of results to society, ABOARD builds toward a future with personalized medicine for AD.
Future directions: To prevent dementia, we need a paradigm shift. We must focus on the disease stages before dementia and take differences between patients into account, both in biology and in preferences and needs. This will profoundly affect the patient journey. Many steps are needed to prepare for a future with personalized medicine for AD, which requires a broad and multidisciplinary collaboration. No party can do this alone—we need all aboard.
2. METHODS
ABOARD is a 5‐year project that runs from 2021 to 2026, and consists of many related, yet independent, sub‐projects, each of which are crucial in the path toward a future with personalized medicine for AD. By combining all these independent projects of partners with diverse expertise in one consortium, we foster synergy and connection, with the ambition to maximally leverage our project results.
The project is structured into five work packages (WPs), each with their own objectives and approach, totaling 36 tasks. Tasks that contribute most explicitly to the patient journey of the future are displayed in Figure 2. An elaborate description of WP activities is provided in File S2 in supporting information.
FIGURE 2.
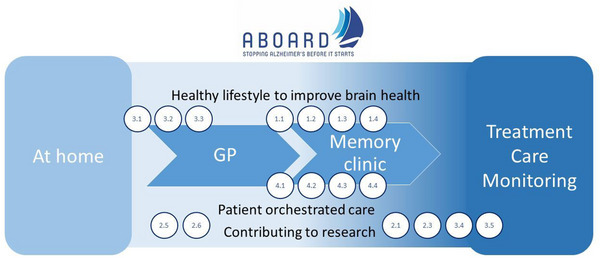
AD patient journey of the future. The patient journey starts with a citizen having concerns about their brain health at home, being referred to a general practitioner and subsequently to a memory clinic. Recommendations for patient management including (preventive) treatment, care, and monitoring are provided. Throughout the diagnostic trajectory, improving awareness about stimulating brain health, providing an environment and tools fostering patient‐orchestrated care, and information on participation in research are essential. ABOARD tasks with specific relevance to the patient journey are depicted in the figure: stimulating citizens at risk to engage in a healthy lifestyle (task 3.1, 3.2, 3.3), developing individual risk profiles (task 2.5, 2.6), developing diagnostic tests of the future in terms of digital biomarkers (task 1.1), blood‐based biomarkers (task 1.2), genetics and patient stratification (task 1.3, 1.4), stimulating patient‐orchestrated care by addressing ethical dilemmas (task 4.1), identifying perspectives and developing e‐tools to support diagnosis, prediction and prevention (task 4.2; 4.3), while fostering inclusive health care accessible for all (task 4.4). Finally, we provide the possibility to participate in research by developing recruitment strategies (3.4) and inviting all to participate in the ABOARD Cohort (2.1) as a means to empower citizens at risk. AD, Alzheimer's disease; ABOARD, A Personalized Medicine Approach for Alzheimer's Disease.
2.1. Work packages
WP1: Diagnosis. The first WP aims to ensure a future in which a timely, accurate, and precise diagnosis of AD is broadly available, as this is a key starting point for effective treatment. We need a more fine‐grained, precise, and molecular diagnosis in which cognitive and imaging markers are combined with a proteomics and genomics approach and cognitive functioning can be monitored continuously. We plan to develop a smartphone–based digital biomarker and conduct a small feasibility study and larger validation study comparing AD patients and controls to evaluate how such an innovation can be integrated into the diagnostic work‐up. Studies on blood‐based biomarkers and genetics are aligned with large‐scale (inter)national initiatives on these quickly developing topics and focus on how we can translate these innovations to clinical practice.
WP2: Prediction. Realizing that diagnosis increasingly takes place before the stage of dementia implies that diagnosis becomes prognosis. We need to move toward individualized risk profiling. 17 This requires large, truly longitudinal data sets, advanced statistical modeling, and careful development of prediction models. WP2 aims to improve personalized prediction of patient‐relevant outcomes, leveraging the Rotterdam Study 18 (community based) and on the Amsterdam Dementia Cohort 19 (memory clinic based), and available registry data of primary care, health‐care insurers, and Statistics Netherlands. A crucial part of WP2 is the initiation of the ABOARD Cohort (www.aboard‐cohort.nl). Including patients with AD, at prodromal stages, and at‐risk populations, the ABOARD Cohort is a societal initiative that aims to engage as many citizens as possible in AD research. The ABOARD Cohort lays the foundation for value‐based health care through its focus on collecting PROs. In addition to collecting PROs, we ask for consent to link to existing medical data, research data, and registries (e.g., Statistics Netherlands, general practitioner data, and health‐care insurance data), and for consent to approach individuals for novel studies for which they might be eligible. As such, the objectives of the ABOARD Cohort are (1) to provide insight into disease trajectories with a focus on outcomes that matter (PROs), while leveraging available data, (2) provide a dataset for external generalization of research cohorts in a real‐life setting, and (3) act as a catalyst for future research by laying a foundation to extrapolate research efforts to the national level. A unique feature of the ABOARD Cohort is its direct‐to‐participant recruitment strategy and the active involvement of the participant panel. At the time of writing, > 2000 individuals and 250 care partners have signed up for the ABOARD Cohort.
WP3: Prevention. We regard lifestyle intervention and prevention by disease‐modifying treatment as complimentary preventive strategies, rather than mutually exclusive. ABOARD takes several necessary steps for successful preparation for and implementation of primary and secondary preventive strategies. Studies included in this WP include adaptation of the existing Lifestyle for Brain Health (LIBRA) risk algorithm to an online prevention module for use in a clinical setting, piloting the first prototype for feasibility in the memory clinic setting (n = 30 patients) and subsequently evaluating the demonstration version of this tool for increase in awareness, willingness to change lifestyle, self‐efficacy, and coping in a proof‐of‐concept study among 150 memory clinic patients. We collect high‐frequency, digital data on the effect of the online intervention on patients’ emotional and psychosocial well‐being, physical activity, blood pressure, and contextual factors in the flow of daily life using the experience sampling method. Studies targeted at disease‐modifying treatment include the development of strategies to improve recruitment for trials and the evaluation of system readiness with regard to introduction of disease‐modifying treatment. Data collected in the ABOARD Cohort combined with health‐care costs data allow for the future testing of hypotheses regarding whether treatment strategies benefit quality of life, and are affordable and manageable.
WP4: Patient‐orchestrated care. Working toward personalized medicine, ABOARD takes as a starting point the notion that citizens (at risk), patients, and their families should be at the steering wheel of their own health and disease management. We intend to modify the patient journey so that patients and care partners can become more involved in decision making about diagnosis, prediction, and prevention. This WP harbors different studies focusing on ethical aspects of early diagnosis; on public–patient involvement in diagnosis, prediction, and prevention; and on diversity and inclusiveness. All studies have a mixed‐method design, making use of interviews, focus groups, and surveys. WP4 strongly reaches out to other WPs; an example is the DNA‐ABOARD, a study evaluating the views, considerations, and experiences of patients and families with DNA diagnosis as part of the diagnostic work‐up (cross‐link with WP1).
WP5: Communication, dissemination, implementation. Communication of scientific results to all societal stakeholders is a necessary step toward implementation into regular health care. We therefore make it a priority to communicate findings in an accessible way. We disseminate and promote the knowledge, materials, and tools to society, patients, and families and in various national and regional dementia networks. ABOARD ensures that the generated knowledge is applicable and applied with a firm focus on training and education. This WP involves diverse activities aimed at translation and implementation of generated knowledge in WPs 1 through 4 to patients, families, professionals, and society. This includes a social media campaign as a backbone for the direct‐to‐participant recruitment strategy of the ABOARD Cohort; deployment of tools developed in WPs 2, 3, and 4 to well‐frequented online platforms; and development of (blended) training modules for education of (future) professionals. Alzheimer Nederland (a Dutch patient organization) is in the lead of this WP to ensure optimal alignment with patient needs and maximal outreach.
2.2. Approach
ABOARD is the first research project launched as part of the Dutch National Dementia Strategy 2021–2030 of the Ministry of Health, Welfare and Sport. In addition, ABOARD gives further effect to Mission IV of the Knowledge and Innovation Agenda 2020–2023 of Health∼Holland (issued by the Ministry of Economic Affairs and Climate), in which public and private partners collaborate to improve the quality of life of people with dementia. As such, ABOARD receives funding from both the Dutch Ministry of Health, Welfare and Sport and the Ministry of Economic Affairs and Climate (via ZonMw [#73305095007] and Health∼Holland, Topsector Life Sciences & Health [PPP‐allowance; #LSHM20106]). Additionally, ABOARD receives in‐cash co‐financing by six consortium partners and from two foundations (Edwin Bouw Fonds and Gieskes‐Strijbisfonds). All partners contribute in kind to the project. The ABOARD project aligns with the overarching ambitions of the Dutch National Dementia Strategy, which include a tailored diagnosis and personalized medicine at the end of its term (2030). More specifically, there is alignment with the research focus: (1) diagnosis: translate biomarker and genetic knowledge to clinic (WP1); (2) risk reduction: a first attempt to translate lifestyle interventions to a clinical setting (WP3); (3) promising technology: development of innovative digital biomarkers based obtained from wearables (WP1); (4) integration research, care, education (WP4, 5).
2.2.1. Management structure
With >30 partners from all sectors of the Dutch health‐care landscape, ABOARD is broad in scope and large in number. To mitigate the risk of losing cohesion, the ABOARD consortium has a firm management structure (Figure 3). Management bodies include the General Assembly, the Executive Board fueled by the WPs, the Advisory Board, and the End User Committee, all supported by the Project Support Office.
FIGURE 3.
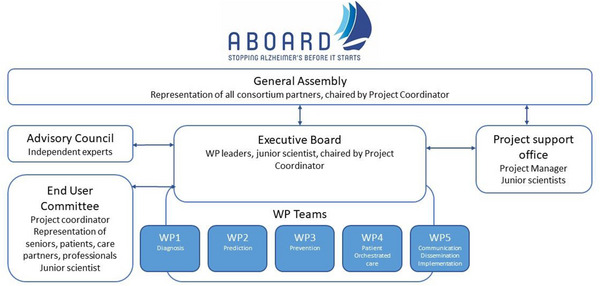
ABOARD Management structure. ABOARD has >30 partners from all sectors of the Dutch health‐care landscape and is broad in scope and large in number. To mitigate the risk of losing cohesion, the ABOARD consortium has a firm management structure. Management bodies include the GA, the EB fueled by the WPs, the Advisory Board and the EUC, all supported by the Project Support Office. ABOARD, A Personalized Medicine Approach for Alzheimer's Disease; EB, Executive Board; EUC, End User Committee; GA, General Assembly; WPs, work packages
The General Assembly is the decision‐making body for consortium‐overarching matters. The General Assembly consists of representatives; the Executive Board is responsible for the operational management of the ABOARD consortium, as a representation of the General Assembly. The Advisory Board (Figure 3) provides the consortium with strategic advice per request.
The End User Committee ensures that research activities are well aligned with the views of the intended end users. The End User Committee consists of representatives from citizens, patients and their caregivers, health‐care professionals, educational institutions, and business.
The Project Support Office is headed by a dedicated project manager, who is crucial for the smooth execution of ABOARD by investing in building relationships with all partners. Well‐established individual relationships are of high importance to have all partners on the same page and keep up the motivation. Considering the diverse nature, ambition, and motives of project partners, we put effort in the engagement of the entire “quadruple helix” of academia, health care, society, and business. To strengthen our network, we organize bi‐annual consortium meetings for all project members.
2.2.2. Societal outreach
ABOARD has the ambition to deploy research findings to drive innovation in health care. To make impact, we pay particular attention to communication and dissemination of results to society. Project partner Alzheimer Nederland (a patient organization) is in the lead regarding the communication and dissemination of findings and outcomes; this partner also hosts the ABOARD website (www.aboard‐project.nl). We have an active (social) media policy and have developed several communication products. At the launch of the project, we developed an animation video to introduce the goals and ambitions of the project (Figure 4). To foster dissemination of research results, (junior) researchers write blogs for the general audience about their scientific publications. Blogs are published on the website and serve as the basis for social media outreach. In addition, we launched a quarterly newsletter and a freely accessible webinar series, for the general audience with an interest in AD. Researchers from all WPs of the ABOARD project use this webinar series “Alzheimer's Explained” (Dutch: “Alzheimer Uitgelegd”) to share knowledge, results, and insights.
FIGURE 4.

ABOARD animation video. The animation video provides a brief overview of the ABOARD project, with its goals and scope. The video is used in presentations and displayed on the project website (www.aboard‐project.nl). Scan the QR code to watch the animation video. ABOARD, A Personalized Medicine Approach for Alzheimer's Disease
2.2.3. Juniors on board training program
Today's junior researchers are tomorrow's principal investigators, key opinion leaders, and health‐care transformers. To achieve the transformations and innovations needed, we need a new generation of collaborating professionals with broad knowledge of research and health care, as well as a truly translational way of working. For this reason, ABOARD has a strong focus on the training of junior researchers by means of a junior scientist training program, entitled Juniors On Board (JOB). A key point of this 5‐year program is building a network of junior scientists from all Dutch Alzheimer Centers, which provides important cross‐links on their research topics.
The main activity of JOB is a series of masterclasses and workshops, organized by (private and public) project partners to install knowledge and develop research skills. To stimulate personal development, we also encourage junior researchers to take an organizational role within the consortium, supporting the Executive Board, End User Committee, Communication, and JOB. Student chairs rotate on an annual basis. Furthermore, junior scientists are invited to participate in a cross‐sectoral mentoring program in which they are mentored by senior professionals from a different organization within the consortium.
2.2.4. Public patient involvement
Involvement of citizens (at risk) and patients is critical as we hope that project results are embraced and used to contribute to personalized medicine for AD. Public patient involvement (PPI) is organized at different levels throughout the consortium. First, Alzheimer Nederland represents AD patients and care partners in the Executive Board and leads WP5 (on communication, dissemination, and implementation), to guarantee that ABOARD results reach end users as quickly as possible.
Second, the End User Committee harbors a diverse palette of stakeholders and includes two care partners of patients living with dementia. Finally, for the ABOARD Cohort, which is part of WP2 and a crucial subtask of the project, we set up a panel of (potential) participants to provide advice (the ABOARD Cohort Panel). This panel is initiated and led by project partners Health‐RI (www.health‐ri.nl) and Hersenstichting (www.hersenstichting.nl), and executed by WP4 (patient‐orchestrated care). The ABOARD Cohort Panel is involved throughout the design and execution of the study and contributes to choices that must be made, such as how research results should be fed back to the participants.
3. CURRENT STATE OF ABOARD
3.1. The ABOARD network
ABOARD was launched in April 2021. Two years into the project, ABOARD functions as a professional network organization, in which public and private partners find each other for collaboration on shared interests and expertise. ABOARD works as a flywheel: it fosters collaborations, attracts additional grants and new co‐financing from project partners. The strong and cohesive network is perhaps the main result and a prerequisite for working together toward the AD health‐care system of the future.
3.2. JOB
To date, 15 PhD students have been appointed throughout the Netherlands to work on the ABOARD project. Masterclasses organized by diverse project partners in the first year of JOB included effective communication, promoting diversity and inclusivity in research, and agile working. In May 2022, PhD students attended a retreat, with a focus on personal development through several workshops, and on strengthening the network of young dementia researchers. JOB contributes to group formation and connects PhD students on a national level. The cross‐sectoral mentoring program started in September 2022.
3.3. Communication activities
As of now (April 2023) we have > 350 subscribers for our newsletter (in Dutch). The quarterly webinar series “Alzheimer's Explained,” which started in April 2022, is attended by 150 people on average per live webinar. After the live session, the webinars can be watched on our YouTube channels, which attract > 100 people per webinar.
3.4. Scientific output
All research activities ultimately aim to impact the future diagnostic patient journey (Figure 2). The diagnostic journey starts with a citizen having concerns about their brain health at home, being referred to a general practitioner and subsequently to a memory clinic, patient management including (preventive) treatment, care, and monitoring. Throughout this diagnostic trajectory, we see that patient‐orchestrated care, improving awareness about stimulating brain health, and the possibility of participation in research are of importance.
In the first 2 years of the project, six main articles have been published. Two projects on the genetic underpinnings were conducted in the context of strong international collaborations. From WP1 (diagnosis; task 1.3), a paper on the genetic underpinnings of amyloid beta (Aβ) and tau concentrations in cerebrospinal fluid (CSF) was published. 20 In the context of the international European Alzheimer & Dementia Biobank collaboration, we conducted the largest genome‐wide analysis of CSF biomarkers to date and found that in addition to apolipoprotein E, also CR1 was associated with an increased risk of reduced Aβ1‐42, and BIN1 was associated with an increased risk of elevated CSF tau. A second paper based on the largest whole‐exome sequencing analysis in AD to date (task 1.4) identified two novel variants in ATP8B4 and ABCA1 associated with AD risk, and a suggestive signal in ADAM10, next to known variants in TREM2, SORL1, and ABCA7. 21 These results provide additional evidence for a major role for Aβ precursor protein processing, Aβ aggregation, lipid metabolism, and microglial function in AD. As a next step, we are currently studying how genetics can be implemented in routine diagnosis.
In WP2, we studied the hypothesis that a more precise diagnosis—operationalized by means of amyloid positron emission tomography (PET)—would lead to a more benign disease trajectory in terms of institutionalization, mortality, and health‐care costs. 22 Using propensity score matching to compare groups with and without amyloid PET as part of their diagnostic work‐up, we found that those with a more precise diagnosis had a lower risk of institutionalization and mortality and lower health‐care costs. This is one of the first studies to show clinical utility of novel diagnostic tests, even in the absence of disease‐modifying treatment.
Three other papers originate from WP4 (patient‐orchestrated care). In a review on theoretical considerations regarding the broad impact of a biomarker‐based diagnosis, we found 26 diverse and opposing considerations, related to a clinical, personal, or societal context, which are relevant to diagnosing AD before dementia (task 4.1). 23 The next step is to collect empirical evidence on the impact of a biomarker‐based diagnosis of AD, by conducting interviews to hear patients’ motives, views, and considerations. Analyzing data from an audiotape study of doctor–patient first consultations, we studied motivations of patients and their care partners to visit the memory clinic (task 4.3). 24 Most patients reported seeking a cause for symptoms or to confirm/exclude a (dementia) diagnosis, yet one out of five reported another motivation: (more) information, care access, or treatment/advice. Motivations were often only expressed in the survey afterward, and not voiced during the consultation itself. This shows there is room for improvement of doctor–patient communication, to ensure that the diagnostic work‐up aligns with the preferences and needs of patients and caregivers. Next steps include development of tools and trainings to support doctor–patient communication.
In a first attempt to empirically derive best practices for disclosure of a positive amyloid PET scan to patients with mild cognitive impairment, we conducted a large randomized controlled study using a video‐vignette design (task 4.3). The resulting paper showed that risk communication best practices, actively attending to emotions, and the teach‐back technique enhance information recall of amyloid PET results and/or could contribute to positive evaluations of the clinician and the provided care. 25
3.5. ABOARD Cohort
In September 2022, the ABOARD Cohort was launched (www.aboard‐cohort.nl). As of April 2023, > 2000 participants and 250 care partners have signed up via an online portal, and consequently received an invitation to fill in online questionnaires to assess PROs. The sign‐up portal and questionnaires are hosted on separate platforms by trusted third parties, that is, Motivaction (www.motivaction.nl) and Castor (www.castoredc.com), respectively. Participants optionally provide informed consent for their study data to be linked to ongoing research projects in which they participate, as well as Statistics Netherlands, health insurance data, general practitioner data, and hospital data. The ABOARD Cohort Panel provides input on the set‐up of the study via surveys, which is used to optimize study procedures. A social media campaign inviting potential study participants along the entire AD spectrum was launched in Spring 2023. The narrative of this campaign is that participants can contribute an hour of their time to help AD research (slogan: “Uurtje voor Alzheimer”; “one hour for AD”). As such, the ABOARD Cohort aims to build a national infrastructure between data registries that allows add‐on studies, helps with the national roll‐out of new studies and innovations, and may ultimately help to harmonize the trajectory of diagnosis and treatment between health‐care providers.
4. DISCUSSION
ABOARD aims to jumpstart personalized medicine for AD by preparing for and investing in effective, efficient, and patient‐orchestrated diagnosis, prediction, and prevention. We take the preparatory steps for a future with personalized medicine for AD, with the ultimate aim to stop AD before dementia has started.
In ABOARD, all partners work on the aspect of AD research that they excel in, while agreeing to share results and attempt to mutually strengthen each other. In that sense, ABOARD is similar to a flotilla: a fleet of individual ships sailing in the same direction, together more certain of reaching the horizon than each one on their own.
Although ABOARD is a Dutch consortium, it has international relevance. Several dementia research groups and governments from around the world have shown their interest in a national PPP of this size, that spans the entire knowledge chain. PPPs are fostered by the Dutch government, to promote research and development, make sure that new knowledge fits with the needs of end users, and guarantee that this new knowledge generated by academia finds its way to society. ABOARD is funded by both the Ministry of Health, Welfare and Sport and the Ministry of Economic Affairs and Climate. In addition, PPPs are supported by technology transfer offices that are available at each university. All (academic) project partners participate in international collaborations, and specific tasks, such as those related to genetics, are conducted in an international context. In addition, project results are disseminated on a European level, as Alzheimer Europe is involved in the Advisory Council.
ABOARD being rooted in the Netherlands may imply that results have limited generalizability, as they are based on a relatively homogenous population from one country with one health‐care system. Particularly, preferences and wishes of citizens and patients, and constraints implied by the health‐care system differ between countries. In taking this national perspective, ABOARD may set an example for other countries of how perspectives on diagnosis, prediction, and prevention can be studied. Nonetheless, we also argue that the population of the ABOARD cohort will be far less homogenous than research cohorts normally are. The initiation of the ABOARD Cohort stems from the notion that prediction models are generally based on selected research populations. The same holds for evidence for disease‐modifying treatments, which invariably stems from phase III trials in highly selected research populations. The ABOARD Cohort aims for a real‐life population. It has wide inclusion criteria and invites all Dutch citizens with or at risk of AD to participate. The direct‐to‐participant recruitment strategy is intended to minimize the burden on local professionals, who mostly have only very limited time to participate in research projects. The ABOARD project dedicates an entire task to diversity and inclusiveness of research. One of the project partners is Pharos, the national knowledge center with expertise on reducing health‐care inequities. By including this partner and a dedicated task, we aim to promote an inclusive cohort. As such, the ambition for the ABOARD Cohort is to provide a real life and diverse population, which may be of service for evaluating generalizability of results obtained in more controlled settings. With future market access of disease‐modifying therapies, this type of research infrastructure and of data collection could be essential for evaluating of their effectiveness in a real life setting.
ABOARD has a strong focus on patient‐orchestrated care. It would be naïve to think that this would solve all challenges in AD health care. It is questionable whether all citizens want to be in control of their own health/disease management. Nor will every patient make the choices best for them to optimize their quality of life. Nonetheless, there is an increasing body of evidence illustrating that care aligned with wishes and needs of patients and their families is ultimately better care. This may entail shared decision making, but could also imply better information provision. Including research participants in the choices we make and asking for their feedback can only benefit the project. In addition, health‐care costs are increasing to such an extent that strong measures have to be taken to keep health care affordable. One of the strategies needed is to make sure that when possible, citizens do what they can to keep their own health in order. In a world with an increasing number of (expensive) options for diagnosis and treatment, it is essential to only provide diagnosis and treatment to those who may benefit from it, and not those who would not. Informing patients and their families about these options is crucial. It is our ambition that ABOARD can provide evidence and tools to help at least a proportion of patients/families to have more control of the management of their health and disease. These tools could also help professionals to find strategies to more actively engage patients and their families. Whether such tools actually have clinical utility needs to be studied in future, prospective studies that could be developed in the ABOARD framework.
4.1. Strengths and limitations
While PPPs are by no means unique for ABOARD, as many great examples (e.g., Alzheimer's Disease Neuroimaging Initiative; Australian Imaging, Biomarker & Lifestyle Study; European Prevention of Alzheimer's Dementia; and many others) have gone before, ABOARD has some unique strengths that make it stand out. First, the consortium is rooted in society, and aims to leverage the momentum felt by all participating individuals and organizations to act now. Rather than one, focused research goal, we aim together to build toward AD health care of the future, in which each organization is eager to contribute their part and expertise and realizes that all need to work together to ultimately have innovations land in society. Unique features intended to contribute to this goal are the strong focus on societal outreach, our strategy for public–patient involvement, and the dedicated resources for the junior training program.
The broad scope and size of the consortium also comes with challenges. A PPP of a size this large is not desirable for all research projects but proves to be useful to intensify the cross‐sectoral network. To start up a successful research structure, sufficient means are required. Building up the network takes time and dedication. A consistent investment in all individual partners and partner‐to‐partner relationships is essential. Furthermore, not all partners are equally motivated to actively participate in the project, despite their initial commitment. Solid stakeholder management is necessary to keep all partners engaged and to keep the overall project goals in mind. Sufficient allocated budget is needed for support staff and/or dedicated project management. Despite these challenges, the broad scope of the consortium is also its main strength, as we need all ABOARD to ultimately make the necessary changes to health care.
5. CONCLUSION
ABOARD is a Dutch PPP of > 30 partners, connecting all stakeholders from a scientific, clinical, and societal perspective. The ultimate goal is to stop AD before dementia has started. ABOARD aims for patient‐orchestrated diagnosis, prediction, and prevention of AD. To attain a future with personalized medicine for AD, we need all aboard. As such, the project functions as a network organization. With a strong focus on patient and public involvement, training of the generation of future AD professionals, and on communication and dissemination of results to society, ABOARD builds toward a future with personalized medicine of AD, which will entail tailored combinations of lifestyle interventions and medication.
CONFLICT OF INTEREST STATEMENT
Wiesje M. van der Flier: Research programs of WF have been funded by ZonMW, NWO, EU‐FP7, EU‐JPND, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health∼Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes‐Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc, Novartis‐NL, Life‐MI, AVID, Roche BV, Eisai, Fujifilm, Combinostics. WF holds the Pasman chair. WF is recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). WF has performed contract research for Biogen MA Inc, and Boehringer Ingelheim. All funding is paid to her institution. WF has been an invited speaker at Biogen MA Inc, Danone, Eisai, Novonordisk, WebMD Neurology (Medscape), Springer Healthcare, European Brain Council. WF is consultant to Oxford Health Policy Forum CIC, Roche, Eisai, and Biogen MA Inc. WF participated on advisory boards of Biogen MA Inc, Roche, and Eli Lilly. All funding is paid to her institution. WF is a member of the steering committee of PAVE, and Think Brain Health. WF was associate editor of Alzheimer, Research & Therapy in 2020/2021. WF is associate editor at Brain. At the time of publication, Anne Dreves has changed jobs and now works full time at Vereniging Innovatieve Geneesmiddelen (VIG). Charlotte E. Teunissen: Research of CET is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant no 831434), EPND (IMI 2 Joint Undertaking [JU], grant No. 101034344) and JPND (bPRIDE), National MS Society (Progressive MS alliance), Alzheimer Association, Health Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands. CT is recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). CET has a collaboration contract with ADx Neurosciences, Quanterix, and Eli Lilly; performed contract research or received grants from AC‐Immune, Axon Neurosciences, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, PeopleBio, Roche, Siemens, Toyama, Vivoryon. CET serves on editorial boards of Medidact Neurologie/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation. CET had speaker contracts for Roche, Grifols, Novo Nordisk. All funding is paid to her institution. Marjolein E. de Vugt, Ellen M.A. Smets, Janne M. Papma, Argonde C. van Harten, Sebastian Köhler and Madison I. J. Honey report no disclosures. Marco M. Blom is scientific director of Alzheimer Nederland. Leonie N.C. Visser (LNCV) has been an invited speaker by the Schwabe Group, fees were paid to her institution. Hanneke Rhodius‐Meester is recipient of the Memorabel Dementia Fellowship 2021 (ZonMw projectnumber 10510022110004). HR performs contract research for Combinostics, all funding is paid to her institution. Minke Kooistra is a full‐time employee of Alzheimer Nederland. Author disclosures are available in the supporting information.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
A Personalized Medicine Approach for Alzheimer's Disease (ABOARD) is a public–private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). Partners in ABOARD are Amsterdam UMC, locations VUmc and AMC, MUMC+, ErasmusMC, UMC Radboud, UMCG, TU Delft, InHolland, Vilans, Pharos, HealthRI, Jeroen Bosch ziekenhuis, Medisch Centrum Leeuwarden, Zorg Innovatie Forum (ZIF), Pharmo/STIZON, Alzheimer Nederland, Hersenstichting, KBO‐PCOB, PGGM, Zorgverzekeraars Nederland, CZ, Zilveren Kruis, Neurocast, Philips, ADx Neuroscience, Castor, Vereniging Innovatieve Geneesmiddelen, Roche NL, Biogen NL, Novartis NL, Brain Research Center. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes‐Strijbisfonds. We thank the members of the ABOARD consortium. Alzheimer Center and Department of Neurology, Amsterdam Neuroscience, VU University Medical Center: Wiesje M. van der Flier, PhD; Anne Dreves, MSc; Sven van der Lee, MD, PhD; Jort Vijverberg, MD, PhD. Department of Epidemiology and Biostatistics, Amsterdam Neuroscience, VU University Medical Center: Hans Berkhof, PhD. Neurochemistry laboratory, Department of Clinical Chemistry, Amsterdam Neuroscience, VU University Medical Center: Charlotte E. Teunissen, PhD; Madison Honey, MSc. Department of Internal medicine, Geriatric Medicine section, Vrije Universiteit Amsterdam, Amsterdam UMC: Hanneke Rhodius‐Meester. Genomics of Neurodegenerative Diseases and Aging, Human Genetics, VU University Medical Center: Henne Holstege, PhD. Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University: Marjolein de Vugt, PhD; Sebastian Koehler, PhD. Department of Neurology and Alzheimer Center Erasmus MC, Erasmus MC University Medical Center, Rotterdam: Janne Papma, PhD. Department of Radiology Erasmus MC, Erasmus MC University Medical Center, Rotterdam: Meike Vernooij, MD, PhD. Radboudumc: Jurgen Claassen, MD, PhD; Rianne de Heus, PhD. UMCG: Barbara van Munster, MD, PhD. Department of Medical Psychology, University of Amsterdam, Academic Medical Center: Ellen M. Smets, PhD; Leonie N.C. Visser, PhD. TU Delft: Marcel Reinders, PhD. InHolland: Robbert Gobbens, MSc. Vilans, Center of Expertise: Karlijn Kwint, PhD. Pharos: Jennifer van den Broeke, PhD. HealthRI: Jan‐Willem Boiten, PhD. Jeroen Bosch Ziekenhuis: Astrid van Strien, MD, PhD. Medisch Centrum Leeuwarden: Liesbeth Hempenius, MD. Zorg Innovatie Forum: Karin Kalverboer, MSc. PHARMO Institute: Ron Herings, PhD. Alzheimer Nederland: Marco Blom, MSc. Hersenstichting: Dirk‐Jan Saaltink, PhD. KBO‐PCOB: Marielle van Oort, Msc. PGGM: Chris Limbach, MSc. Zorgverzekeraars Nederland: Jan Reitsma, PhD. CZ: Mijke Buijs, MSc. Zilveren Kruis: Jeroen Kemperman, MSc. Neurocast: Erwin Redeman, MSc. Philips: Hans Hofstraat, PhD. ADx Neurosciences: Erik Stoops, PhD. Castor: Derk Arts, MD, PhD. Vereniging Innovatieve Geneesmiddelen: Carla Vos, PhD, MD. Roche NL: Wendy Maas. Biogen NL: Hans van Loenen, PhD. Novartis: Jurre Keijzers. Brain Research Center: Niels Prins, MD, PhD.
Dreves MAE, van Harten AC, Visser LNC, et al. Rationale and design of the ABOARD project (A Personalized Medicine Approach for Alzheimer's Disease). Alzheimer's Dement. 2023;9:e12401. 10.1002/trc2.12401
REFERENCES
- 1. World Health Organization . For a safer, healthier and fairer world: Results report Programme budget 2020‐2021. 2021. World Health Organization. [Google Scholar]
- 2. GBD 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105‐e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rigby T, Johnson DK, Taylor A, Galvin JE. Comparison of the caregiving experience of grief, burden, and quality of life in dementia with Lewy Bodies, Alzheimer's disease, and Parkinson's disease dementia. J Alzheimers Dis. 2021;80(1):421‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jack CR, Therneau TM, Weigand SD, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the national institute on Aging‐Alzheimer's Association Research Framework. JAMA Neurol. 2019;76(10):1174‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnett JH, Lewis L, Blackwell AD, Taylor M. Early intervention in Alzheimer's disease: a health economic study of the effects of diagnostic timing. BMC Neurol. 2014;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Flier WM, de Vugt ME, Smets EMA, Blom M, Teunissen CE. Nat Aging. 2023;3(5):494‐505. 10.1038/s43587-023-00404-2 [DOI] [PubMed] [Google Scholar]
- 8. Tijms BM, Gobom J, Reus L, et al. Pathophysiological subtypes of Alzheimer's disease based on cerebrospinal fluid proteomics. Brain. 2020;143(12):3776‐3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graff‐Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 11. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two Randomized Phase 3 studies of Aducanumab in Early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197‐210. [DOI] [PubMed] [Google Scholar]
- 13. Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double‐blind, phase 2b proof‐of‐concept clinical trial in early Alzheimer's disease with lecanemab, an anti‐Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691‐1704. [DOI] [PubMed] [Google Scholar]
- 15. Van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. [DOI] [PubMed] [Google Scholar]
- 16. Cummings J, Lee G, Nahed P, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimers Dement (N Y). 2022;8(1):e12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Maurik IS, Vos SJ, Bos I, et al. Biomarker‐based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol. 2019;18(11):1034‐1044. [DOI] [PubMed] [Google Scholar]
- 18. Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. European Journal of Epidemiology. 2020;35(5):483‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Der Flier WM, Scheltens P. Amsterdam dementia Cohort: performing research to optimize care. J Alzheimers Dis. 2018;62(3):1091‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jansen IE, Van Der Lee SJ, Gomez‐Fonseca D, et al. Genome‐wide meta‐analysis for Alzheimer's disease cerebrospinal fluid biomarkers. Acta Neuropathol. 2022;144(5):821‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holstege H, Hulsman M, Charbonnier C, et al. Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer's disease. Nature Genetics. 2022;54(12):1786‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Maurik IS, Broulikova HM, Mank A, et al. A more precise diagnosis by means of amyloid PET contributes to delayed institutionalization, lower mortality and reduced care costs in a tertiary memory clinic setting. Alzheimers Dement. 2023;19(5):2006‐2013. 10.1002/alz.12846 [DOI] [PubMed] [Google Scholar]
- 23. van der Schaar J, Visser LNC, Bouwman FH, et al. Considerations regarding a diagnosis of Alzheimer's disease before dementia: a systematic review. Alzheimers Res Ther. 2022;14(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Visser LNC, Fruijtier A, Kunneman M, et al. Motivations of patienst and their care partners for visiting a memory clinic. A qualitative study. Patient Educ Couns. 2023;111:107693. 10.1016/j.pec.2023.107693 [DOI] [PubMed] [Google Scholar]
- 25. Fruijtier AD, van der Schaar J, van Murrik IS, et al. Identifying best practices for disclosure of amyloid imaging results: a randomized controlled trial. Alzheimers Dementia. 2023;19(1):285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


