Abstract
To generalize findings on the mechanisms and prognosis in Alzheimer's disease and related dementias (ADRD), it is critical for ADRD research to be representative of the population. Sociodemographic and health characteristics across ethnoracial groups included in the National Alzheimer's Coordinating Center sample (NACC) were compared to the nationally representative Health and Retirement Study (HRS).
Baseline NACC data (n = 36,639) and the weighted 2010 HRS wave (N = 52,071,840) were included. We assessed covariate balance by calculating standardized mean differences across harmonized covariates (i.e., sociodemographic, health).
NACC participants were older, more educated, with worse subjective memory and hearing, but endorsed fewer depressive symptoms compared to HRS participants. While all racial and ethnic groups in NACC differed from HRS participants in the same way overall, these differences were further amplified between racial and ethnic groups.
NACC participants do not represent the U.S. population in key demographic and health factors, which differed by race and ethnicity.
HIGHLIGHTS
We examined selection factors included in NACC studies compared to a nationally representative sample.
Selection factors included demographic and health factors and self‐reported memory concerns.
Results suggest that NACC participants are not representative of the U.S. population.
Importantly, selection factors differed across racial and ethnic groups.
Findings are suggestive of selection bias within NACC studies.
Keywords: Alzheimer's disease centers, generalizability, racial/ethnic disparities, recruitment
1. INTRODUCTION
By 2050, it is anticipated that 12 million people in the United States will have Alzheimer's disease and related dementias (ADRD). 1 In an effort to clarify mechanisms and identify optimal treatment targets, many public‐private partnerships have been created to promote the sharing of ADRD data. 2 These multi‐site collaborations and data repositories aim to expand our understanding of the factors associated with ADRD risk, onset, and progression by facilitating larger scale analyses of ADRD clinical and pathological factors than any one site could achieve alone.
Due to rising rates of racial and ethnic disparities in ADRD, future rates of ADRD cases are projected to increase most in minoritized groups. 3 Comprehensive data indicate the prevalence of known ADRD risk factors (e.g., cardiovascular disease, lower educational attainment) disproportionately impact Black and Latinx populations compared to their non‐Latinx White counterparts. 4 , 5 Despite this, underrepresentation of diverse populations in large scale studies of aging persists, bringing the generalizability of ADRD literature into question.
The National Alzheimer's Coordinating Center's (NACC) Uniform Data Set is an example of a large multi‐site collaboration that collects rich cognitive and biomarker data that is publicly available. 6 , 7 , 8 Studies using NACC data have been critical to the ADRD field by influencing the latest NIA‐AA Research Framework for AD, 9 as well as providing support for racial/ethnic differences in dementia prevalence and presentation, 10 neuropathologic burden, 11 and ADRD biomarkers. 12 , 13 While these studies have demonstrated key racial and ethnic differences in AD‐related predictors and outcomes, they have not examined if the multi‐site participant sample comprehensively reflects the demographic makeup of the national population. Delineating the similarity/dissimilarity of the NACC participants to a nationally representative sample of older adults is critical to understanding whether estimates obtained from clinical and biological studies can be accurately applied to a larger target population.
Recent studies utilizing NACC data have demonstrated that factors related to selection for enrollment into studies (i.e., selection factors), such as enrollment strategies and knowledge of family history of dementia, influence ADRD outcomes of interest. A recent study demonstrated that healthy controls recruited from a clinic setting showed a steeper rate of progression and a higher risk of developing mild cognitive impairment (MCI) compared to those recruited from the community. 14 Another study found that referral source (i.e., clinic vs. community) and prior knowledge of family history of dementia attenuated racial differences in incident MCI. 15 To clarify how racial and ethnic disparities impact aging and ADRD and the magnitude of these effects on incidence and progression, we must better understand how large, multi‐site study samples reflect the U.S. population. Determining which sociodemographic and health factors influence participation in clinical research can provide insight into sources of possible bias in large ADRD studies.
In this study, we compared NACC participants to the nationally representative Health and Retirement Study (HRS) sample. The HRS is a study of mortality and health among adults aged 51 and older. 16 By leveraging the nationally weighted HRS data, we sought to compare participants enrolled in the NACC to the U.S. population and determine how these groups differ in key sociodemographic (e.g., age, education, race, and ethnicity) and health (e.g., cardiovascular disease, memory concerns) selection factors. As such, throughout the paper we refer to the nationally representative weighted HRS sample as “U.S. population”. Due to reported differences in study design and recruitment strategies between the NACC and HRS, we hypothesized that compared to the older adult U.S. population, NACC participants would be younger, with higher educational attainment and fewer depressive symptoms, and they would endorse worse subjective cognition. Given known racial and ethnic disparities in ADRD research recruitment strategies, 17 , 18 we also hypothesized minoritized racial and ethnic groups would be underrepresented compared with non‐Latinx White NACC participants relative to the older adult U.S. population.
2. METHODS
2.1. Data sources
2.1.1. NACC
Data from the NACC Uniform Data Set (UDS) were used in this study (data extracted on 09/07/2021). NACC data consist of over 40,000 participants from the 30+ past and present Alzheimer's Disease Core Centers and Alzheimer Disease Research Centers (collectively referred here as ADRCs) funded by the National Institute on Aging. 19 NACC data are described as a case series; there is marked heterogeneity across ADRCs in terms of recruitment, clinical focus, and target populations. Data were collected from 2005 to 2021 by supporting ADRCs. We analyzed baseline visit data for all Latinx, non‐Latinx White, and non‐Latinx Black participants who were at least age 60 at their initial visit. We selected 60 as our age cut‐off due to the higher prevalence of atypical Alzheimer's disease presentations before that age. 20 Similarly, we excluded participants with genetic causes of dementia (autosomal dominant AD mutations, frontotemporal lobar degeneration mutations, Huntington's disease, Down syndrome). These criteria allowed for a total analytic sample of 36,639 individuals (Figure S1).
2.1.2. HRS
Data from the Health and Retirement Study (HRS) 2010 wave were used as the population‐representative sample. We chose the 2010 HRS wave as our target population for a couple of reasons. First, 2010 is near the middle of the enrollment period for the NACC data used in this analysis, making it a reasonable “benchmark” year. Second, the 2010 HRS wave is larger than adjacent waves because it included a replenishment sample, which allows us to produce more precise estimates. The HRS (funded by the National Institute on Aging and the U.S. Social Security Administration) is a longitudinal cohort of community‐dwelling U.S. adults age 51 and older and their spouses that seeks to examine economic, health, and demographic factors related to aging. 21 HRS was designed to be representative of all community‐residing adults in the contiguous United States and included supplemental oversamples of Latinx and Black individuals. We restricted the HRS sample to Latinx, non‐Latinx White, and non‐Latinx Black participants who were at least 60 years old at their 2010 visit to parallel the NACC data. We excluded HRS participants with a 2010 HRS sampling weight of zero (indicating non‐respondents, including those who died, and participants living in nursing homes), resulting in a final analytic sample of n = 12,074 (Figure S1). 22 We applied HRS sampling weights to weight the 2010 HRS up to the non‐institutionalized adult U.S. population ages 60+ (weighted n = 52,071,840).
RESEARCH IN CONTEXT
Systematic Review: The authors thoroughly reviewed the literature using PubMed. Recent studies using National Alzheimer's Coordinating Center (NACC) data have demonstrated that selection factors (i.e., recruitment strategies, family history of Alheimer's disease [AD]) can influence AD and related dementias (ADRD) outcomes. It is unclear the extent to which these selection factors impact the representativeness of NACC participants compared to the broad U.S. population.
Interpretation: Overall, standardized mean differences showed older age, higher educational attainment, more subjective memory concerns, and more hearing difficulties were strong selection factors into NACC. Importantly, these selection factors differed across racial and ethnic groups. Findings therefore suggest that NACC participants are not representative of the U.S. population across key sociodemographic and health factors.
Future Directions: Future studies should assess whether differences in the sociodemographic makeup of the NACC may bias interpretation of ADRD risk factors and outcomes. Studies should also consider mitigating these sources of bias through inclusive recruitment efforts across Alzheimer's Disease Centers to improve generalizability.
2.2. Harmonized selection factors
We harmonized NACC and HRS data using several sociodemographic and health variables comparable across datasets, allowing us to evaluate differences across studies. The following sections summarize our harmonization approach; Table S1 provides additional details.
2.2.1. Sociodemographic factors
Variables included self‐reported race and ethnicity (non‐Latinx White, non‐Latinx Black, Latinx), sex/gender (female, male), age (continuous years), educational attainment (continuous years), and marital status (married/living as married vs. not). As previously noted, these sociodemographic factors have been associated with both ADRD risk and participants’ decisions to enroll in ADRC studies. 14 , 15 , 23 , 24
2.2.2. Health factors
We examined self‐reported history of hypertension or high blood pressure, diabetes or high blood sugar, and hearing/visual functioning. These comorbidities may exclude potential participants from entry into clinical trials. 25 , 26 Harmonized history of hypertension/high blood pressure (“hypertension”) and diabetes/high blood sugar (“diabetes”) measures were derived as “Recent/active” or “Remote/inactive” (“yes” in HRS) versus “Absent” in NACC (“no” in HRS). Hearing and vision functioning variables were derived from multiple questions in NACC indicating “functionally normal” hearing/vision (with hearing aid[s] or corrective lenses, if subject uses them) versus “not functionally normal” hearing/vision. HRS participants rated their hearing and vision on a 5‐point scale ranging from “poor” to “excellent.” The HRS variable was dichotomized with “poor” responses (and “legally blind” for vision) representing difficulties with vision/hearing to be harmonized with NACC respondents reporting their hearing/vision was not functionally normal.
2.2.3. Depressive symptoms
Mood symptoms may also influence selection into NACC studies (e.g., via exclusion criteria related to psychiatric conditions or reduced willingness of potential participants with depression). 26 , 27 To assess depressive symptoms, NACC included the Geriatric Depression Scale—Short Form (GDS). 28 Scores range from 0 to 15 and higher scores indicate more depressive symptoms. HRS questionnaires included eight items from the Center for Epidemiologic Studies Depression Scale (CES‐D), with higher scores indicating more depressive symptoms. 29 A harmonized elevated depressive symptoms measure (yes vs. no; defined as moderate to severe for GDS, and evidence of clinical depression for CES‐D short form) was derived using each scale's cut‐off score for clinically concerning symptoms (≥9 for GDS scores and ≥4 CES‐D scores).
2.2.4. Subjective cognition
Subjective ratings of cognitive function were also a factor of interest due to evidence supporting difference in rates of help‐seeking, as well as conversion rates of those with concerns of memory decline. 14 NACC asked respondents to report a decline in memory relative to previously attained abilities (yes vs. no), and HRS asked respondents to rate their memory at present using a 5‐point scale. The HRS variable was dichotomized to match the NACC response type by treating “poor” and “fair” responses as endorsement of a decline in memory functioning.
2.3. Missing data
Multiple imputations with chained equations and predictive mean matching 30 was implemented to address covariate missingness, which ranged from 0%‐7.3% in NACC and 0%‐6.5% in HRS (Table S2).
2.4. Statistical analysis
Descriptive statistics included unweighted means and frequencies in the NACC sample, and weighted means and frequencies in the HRS sample weighted up to the 2010 U.S. population age 60+. To assess differences between the samples, standardized mean differences ([mean(NACC)‐mean(HRS)]/SD(HRS)) were calculated for NACC vs. weighted HRS overall and stratified by race and ethnicity. Standardized mean differences greater than +0.25 or less than −0.25 were considered strong selection factors into NACC. 31 However, because standardized mean differences are continuous and multiple threshold values are used, we also discuss relative strengths of standardized mean differences across covariates. Analyses were conducted in R version 4.0.4 with twang and mice packages. 32 , 33 Code for this project is available online: https://github.com/t‐mmobley/ADC‐sample‐representativeness.
3. RESULTS
Compared to the weighted HRS 2010 sample, NACC participants were older, more likely to be female, with more years of education, and more likely to report subjective cognitive concerns. NACC was also more racially and ethnically diverse than the weighted HRS (8.5% Latinx and 13.7% non‐Latinx Black vs. 7.7% and 9.7% in weighted HRS, respectively). Additionally, NACC participants were less likely to report history of hypertension, diabetes, and depressive symptoms compared to the weighted HRS. Lastly, 34% of NACC participants had a diagnosis of dementia at baseline.
Standardized mean differences between NACC and weighted HRS overall suggested older age, higher educational attainment, worse subjective cognition, greater hearing difficulties, and absence of self‐reported depressive symptoms were strong selection factors into NACC (Figure 1). Self‐reported history of hypertension and diabetes were less common in NACC compared with weighted HRS, though standardized mean differences were smaller.
FIGURE 1.
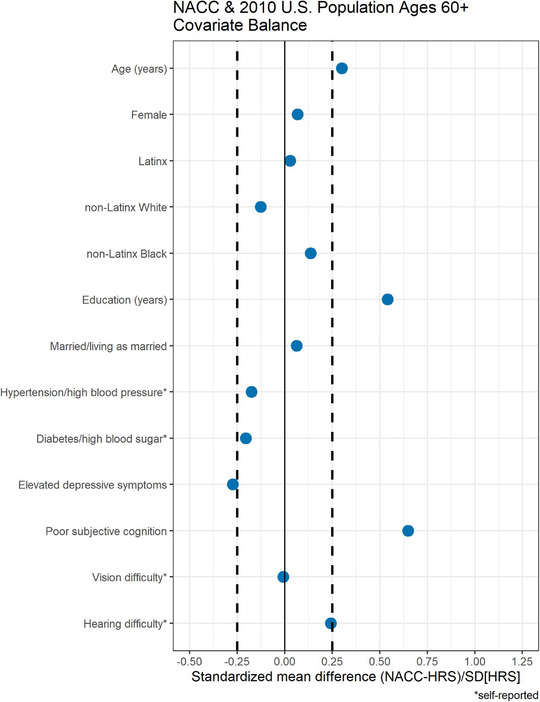
Covariate balance (standardized mean differences) between National Alzheimer's Coordinating Center (NACC) and the Health and Retirement Study (HRS) overall.
Stratified analyses suggested that all racial and ethnic groups in NACC were older, with higher years of education, worse subjective cognition, and absence of self‐reported depressive symptoms compared to the weighted HRS sample (Figure 2). Older age was a stronger selection factor among Latinx and non‐Latinx Black NACC participants compared to non‐Latinx White NACC participants. Higher educational attainment and subjective cognition were stronger selection factors among non‐Latinx White NACC participants compared to non‐Latinx Black and Latinx NACC participants. Differences from HRS‐derived expectations for hearing difficulty were slightly greater for non‐Latinx Black NACC participants, and differences for depressive symptoms were greater for Latinx and non‐Latinx Black NACC participants compared with differences for non‐Latinx White NACC participants. Conversely, differences in lower self‐reported cardiovascular risk factors were greater for non‐Latinx White NACC participants compared to Latinx and non‐Latinx Black NACC participants.
FIGURE 2.
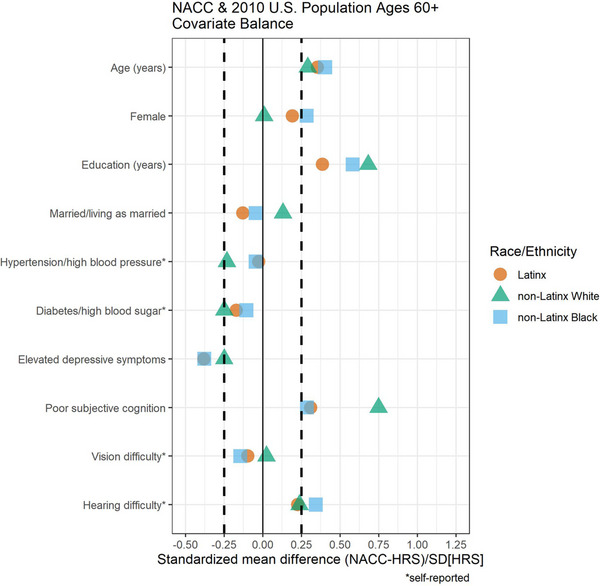
Covariate balance (standardized mean differences) between National Alzheimer's Coordinating Center (NACC) and the Health and Retirement Study (HRS) stratified by race/ethnicity.
4. DISCUSSION
The advent of large, multi‐site studies and data sharing repositories has improved the ADRD research community's ability to address disease‐specific questions using well‐powered, multi‐modal study designs. Important questions have been raised, however, regarding the representativeness of participant samples and the generalizability of results across racial and ethnic groups. We examined selection factors overall and across race/ethnicity included in the NACC compared to the 2010 nationally representative weighted sample of adults age 60+ in the HRS in order to make inferences to the U.S. population more broadly. NACC participants were typically older and more likely to have higher educational attainment, reported worse subjective cognition as well as greater hearing difficulties, but were less likely to report depressive symptoms and cardiovascular risk factors compared to the U.S. older adult population. When looking within each racial and ethnic group, standardized mean differences for age were larger for Latinx and non‐Latinx Blacks compared to non‐Latinx White participants. Furthermore, Latinx and non‐Latinx Black NACC participants reported fewer depressive symptoms compared to their national population counterparts. In contrast, differences between educational attainment, subjective cognition, and cardiovascular risk factors were larger for the non‐Latinx White participants relative to all other groups when compared to the U.S. population. Overall, our study suggests that NACC participants diverged from the U.S. population in several sociodemographic and health factors. However, it is important to explore each of these factors in detail to better understand their associations with study participation, ADRD risk and resilience, and late‐life cognitive decline.
The presence of older age, worse subjective cognition, and higher prevalence of self‐reported hearing difficulties is not surprising given that a goal of ADRCs is to study cognitive decline and ADRD and these three factors are associated with increased risk of ADRD. 34 The mechanistic link and directionality between sensory impairment and cognitive decline remains poorly understood. While there may be shared biological pathways and common etiologies that tie ADRD to sensory loss, studies have indicated that social (e.g., increased loneliness, isolation), mood (e.g., depression), cardiovascular (e.g., reduced physical activity), and neuroanatomical (e.g., diminished input into critical functional networks) factors may serve as underlying mediators of the observed association between sensory loss and cognitive decline. 35 , 36
NACC participants had higher levels of education relative to the U.S. population which aligns with the body of evidence indicating a positive association between years of formal education and participation in clinical and biomarker research studies. 37 Generally, higher education is associated with higher socioeconomic status, increased health literacy, and optimal healthcare access and utilization. 38 These socioeconomic factors associated with education may increase the possibility that older adults engage with healthcare facilities such as those in which the ADRCs are generally housed. Additionally, lower educational attainment is associated with increased barriers to engage with healthcare and to participate in research overall. 39 Differences in educational attainment between populations may potentially bias our understanding of ADRD disease progression since the relationship between years of formal education and cognitive outcomes in late life is complex. Several studies have reported increased dementia risk with lower education level. 40 Higher education may provide a buffer against cognitive decline, particularly in earlier disease stages; however, once cognitive reserve is “depleted” and more extensive and severe atrophy is present, higher education has been linked to a more rapid cognitive decline. 41 , 42 These considerations are important when interpreting clinical outcomes in large aging studies. Future studies should determine whether these findings suggest bias toward participants having higher cognitive reserve and, in turn, being further along the AD disease continuum compared to a national population. Moreover, the NACC is currently developing a social determinants of health module with which to characterize in greater detail these socioeconomic factors. Future studies could leverage these additional socioeconomic variables to replicate these analyses to fully tease the associations between education and healthcare utilization. Given the sociocultural, environmental, and economic implications of education level, our understanding of the modifying role of education on symptom severity and progression is likely limited given the narrow level of education represented in research.
Differences between the NACC participant sample composition and the national population were further magnified when appraising how selection factors differ across racial and ethnic groups. We found that differences in age, depressive symptoms, and hearing difficulties were larger among Latinx and non‐Latinx Black participants compared to non‐Latinx White participants. These results suggest that Latinx and non‐Latinx Black participants in NACC were much older and reported fewer depressive symptoms compared to their U.S. population counterparts, and the non‐Latinx Black NACC participants had greater hearing difficulties than their U.S. representative Black counterparts. While differences in these factors were also observed among the non‐Latinx White participants, the magnitude of these differences was larger among these racial and ethnic groups. Conversely, while all racial/ethnic groups had higher educational attainment and more cognitive concerns compared to the U.S population, this difference was stronger among the non‐Latinx White participants, suggesting that non‐Latinx White participants had even higher years of education and worse self‐reported cognition compared to their national population counterparts.
These differences in the distribution of ADRD risk (i.e., older age, subjective cognition, hearing problems) and protective factors (i.e., higher educational attainment, fewer depressive symptoms) across ethnoracial groups may impact the inferences and generalizability of study findings. Similarly, these differences in sociodemographic and health factors can limit our understanding of the differential vulnerability to and resilience against ADRD within racial and ethnic groups. For instance, education may not confer the same degree of resilience across racial and ethnic groups 43 , 44 and environmental factors such as air pollution are associated with disproportionate ADRD risk among minoritized groups. 45 Although Black and Latinx populations are more likely to be adversely impacted by known risk factors for ADRD (e.g., cardiovascular disease, lower educational attainment) compared to their White counterparts; current and projected future prevalence of ADRD disproportionately impact these racial and ethnic groups. 4 , 5 Despite systematic population differences in known determinants of health, very few studies have examined how well diverse populations are represented in studies of ADRD. It is important to note that simply evaluating percentages (Table 1) might be misleading given that these metrics suggest that NACC could be considered unexpectedly more racially and ethnically diverse than the weighted HRS data (i.e., national population; NACC 14% non‐Latinx Black vs. 10% in weighted HRS). However, when moving beyond simple percentage comparisons and more deeply examining the demographics and health characteristics across racial and ethnic groups, the strength of selection factors varied markedly. Thus, although the ethnoracial composition of a participant sample may mirror demographic percentages of the national population, that does not mean that the participants are representative of the national population as our study results suggest.
TABLE 1.
Characteristics of NACC and 2010 HRS analytic samples.
| NACC | HRS | |
|---|---|---|
| (N = 36,639) | (N = 12,074) | |
| Age, years (mean [SD]) | 74.2 (8.0) | 71.5 (8.8) |
| Female (%) | 56.7 | 53.4 |
| Race/ethnicity (%) | ||
| Latinx | 8.5 | 7.7 |
| non‐Latinx White | 77.9 | 82.6 |
| non‐Latinx Black | 13.7 | 9.7 |
| Married/living as married (%) | 64.2 | 61.2 |
| Education, years (mean [SD]) | 14.5 (2.9) | 12.8 (3.1) |
| Hypertension/high blood pressure (%) | 53.2 | 61.7 |
| Diabetes/high blood sugar (%) | 13.6 | 22.2 |
| Elevated depressive symptoms (%) | 3.6 | 12.7 |
| Poor subjective cognition (%) | 57.7 | 28.4 |
| Self‐reported vision difficulty (%) | 6.0 | 6.2 |
| Self‐reported hearing difficulty (%) | 11.9 | 6.1 |
Note: Characteristics are averaged across 20 multiply imputed samples. HRS percentages are shown weighted to be representative of the 2010 adult US population aged 60+.
Abbreviations: HRS, Health and Retirement Study; NACC, National Alzheimer's Coordinating Center.
Our findings demonstrate the need to increase efforts for inclusive recruitment strategies to ensure adequate representation across and within racial and ethnic groups. Raman et al. (2021) demonstrated that site‐specific, rather than centralized, recruitment strategies were most successful in recruiting Black, Latinx, and Asian participants for a preclinical AD trial, whereas White participants were more likely to be recruited via media advertisement. 46 Site‐specific strategies may involve recruitment from internal sources (e.g., other studies, internal clinic referrals, local research registries) and community outreach. Increased trust established through community engagement is likely central to increasing ADRD research representation of marginalized communities. ADRCs across all sites may consider mitigating sources of selection bias by increasing community engagement through community lectures and presentations at local health centers, conferences, churches, and health fairs and establishing community/participant advisory boards to help guide recruitment and involve the community at all stages of research. 18 , 46 , 47
Dedicated funding to increase diversity and representativeness in ADRCs may also be helpful. As of this publication, all ADRCs are in major urban centers in the United States, with coastal metropolitan regions housing several ADRC sites. Entire regions of the country, however, are represented by having a single ADRC within hundreds of miles. For example, the only ADRC in the Great Plains regions is presently only in the Kansas City metropolitan area: https://www.nia.nih.gov/health/alzheimers‐disease‐research‐centers. The location of ADRCs can make it impractical for eligible participants to participate in ADRC studies and, consequently, be included in the NACC database. Moreover, other racial/ethnic groups (e.g., Asians, Indigenous Americans) are not well represented in the NACC. This limits our full understanding of the impact of ADRD across diverse populations. For instance, a recent study leveraging diverse and representative healthcare data of older adults residing in northern California found differences in dementia incidence between racial and ethnic groups such that Asian‐American had the lowest risk compared to all other groups. 48 Efforts should be dedicated to not just increase recruitment of these underrepresented populations, but to develop study materials and normative data needed for diagnosis of adults with diverse languages and cultural backgrounds.
A critical factor to consider is that the NACC UDS is designed as a case series, but typically analyzed as a cohort sample. Each site is permitted to use different enrollment criteria that fit with their individual ADRC aims, goals, and budget, which may change over time and with each renewal cycle. Efforts could be directed toward creating a unified inclusion/exclusion criteria across sites. Enrollment criteria should consider selection factors, like those identified by the present study, that may disproportionately exclude minoritized groups. Prospective standardized collection and reporting of key sociodemographic factors 49 across sites would further help characterize selection factors and improve generalizability of results from large datasets like NACC. Future studies should also consider leveraging transportability methods such as weighting and outcome modeling aimed at generalizing findings to external populations. 50 , 51 , 52 , 53 These methods have important assumptions that limit their applicability, but there is some recent work in aging samples. 53 Last, future work evaluating updated recruitment strategies will also want to consider the impact of major historic events on research recruitment and enrollment, like the COVID‐19 pandemic. This may be particularly salient to questions related to selective sampling into studies given how the pandemic has exacerbated racial/ethnic health disparities. 54
Our retrospective study displayed numerous strengths, including the appraisal of a large, widely used, and well‐phenotyped database (NACC) and a nationally representative sample (HRS). By characterizing key racial and ethnic differences in selection factors, our study provides an important foundation for future investigations to assess whether these factors ultimately affect –and to what degree– estimates for clinical risk factors, biological and diagnostic outcomes, and rates of symptom progression in ADRD. There are also several limitations that are important to consider. To assess covariate balance across NACC and the weighted HRS data, harmonization of several key variables was conducted. While this is a necessary and well‐established method for comparing studies with distinct surveys/questionnaires, it remains possible that some measures may reflect slightly different aspects of a construct. It is also noteworthy that we did not have access to some sociodemographic factors that are known to impact ADRD outcomes (e.g., socioeconomic status). As such, it is likely there are other important convergent and/or divergent characteristics of these samples that could not be adequately assessed in the current study.
In summary, results suggest that participants in NACC are not representative of the U.S. population in key sociodemographic and health factors. Compared to the national population, NACC participants were typically older and more highly educated, reported worse self‐reported cognition but fewer depressive symptoms, and endorsed fewer vascular risk factors. Moreover, these selection factors differed across racial and ethnic groups. As large multi‐site data are becoming more readily available and used to develop conceptual frameworks for ADRD diagnosis and care, it is incumbent upon the field to assess for and mitigate selection factors through inclusive recruitment efforts. Selection factors that influence enrollment and retention in large scale studies can adversely impact the generalizability of study results to broader populations due to inaccurate estimates and impact co‐enrolling studies that use ADRC cohorts for recruitment purposes; as such, it is critical to assess the degree to which these factors may bias current findings and conceptualizations of ADRD clinical and biological outcomes.
CONFLICT OF INTEREST STATEMENT
Author disclosures are available in the supporting information.
CONSENT STATEMENT
All HRS participants provided written informed consent, and all study procedures were approved by the University of Michigan institutional review board. Regarding NACC participants, all contributing ADRCs are required to obtain informed consent from their participants and maintain their own separate IRB reviews and approvals from their institutions prior to submitting data to NACC.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA‐funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD). This work was supported by funding for the Advanced Psychometric Methods for Cognitive Aging Research Conference (R13 AG030995, PI Mungas). MAR is supported by the NIH/NIA (1K99AG066932; P30AG059303) and the Alzheimer's Association (AARFD‐18‐565015). T.M.M. is supported by the NIH (R56AG069126). L.D.M. is supported by NIH/NIA (R24AG065170) and Alzheimer's Association (2019‐AARGD‐642445). N.D.E. is supported by NIH/NIA (T32AG02049). JTFF is supported by NIH/NIA (1F31AG062158‐01A1). BMB is supported by NIH/NIA (R01AG058772) and the Department of Defense (W81XWH1910520). NIH/NIA: R13 AG030995, 1K99AG066932, P30AG059303, R24AG065170, T32AG02049, 1F31AG062158‐01A1, R01AG058772. Alzheimer's Association: AARFD‐18‐565015, 2019‐AARGD‐642445. Department of Defense: W81XWH1910520.
Arce Rentería M, Mobley TM, Evangelista ND, et al. Representativeness of samples enrolled in Alzheimer's disease research centers. Alzheimer's Dement. 2023;15:e12450. 10.1002/dad2.12450
REFERENCES
- 1. Alzheimer's Association Facts and Figures. Accessed October 1, 2022. https://www.alz.org/media/Documents/alzheimers‐facts‐and‐figures.pdf
- 2. Toga AW, Neu SC, Bhatt P, Crawford KL, Ashish N. The Global Alzheimer's association interactive network. Alzheimer's Dement. 2016;12(1):49‐54. doi: 10.1016/j.jalz.2015.06.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged >/= 65 years. Alzheimer's Dement. 2019;15(1):17‐24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vila‐Castelar C, Fox‐Fuller JT, Guzman‐Velez E, Schoemaker D, Quiroz YT. A cultural approach to dementia ‐ insights from US Latino and other minoritized groups. Nat Rev Neurol. 2022;18(5):307‐314. doi: 10.1038/s41582-022-00630-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aranda MP, Kremer IN, Hinton L, et al. Impact of dementia: health disparities, population trends, care interventions, and economic costs. J Am Geriatr Soc. 2021;69(7):1774‐1783. doi: 10.1111/jgs.17345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91‐101. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐216. doi: 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- 8. The National Alzheimer's Coordinating Center (NACC). Accessed March 1, 2022. https://www.alz.washington.edu/
- 9. Jack CR Jr, Bennett DA, Blennow K, Research Framework NIA‐AA. Toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14(4):535‐562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennon JC, Aita SL, Bene VAD, et al. Black and White individuals differ in dementia prevalence, risk factors, and symptomatic presentation. Alzheimer's Dement. 2021. doi: 10.1002/alz.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graff‐Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer's coordinating center. Alzheimer's Dement. 2016;12(6):669‐677. doi: 10.1016/j.jalz.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264‐273. doi: 10.1001/jamaneurol.2018.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiong C, Luo J, Coble D, Agboola F, Kukull W, Morris JC. Complex interactions underlie racial disparity in the risk of developing Alzheimer's disease dementia. Alzheimer's Dement. 2020;16(4):589‐597. doi: 10.1002/alz.12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y, Denny KG, Harvey D, et al. Progression from normal cognition to mild cognitive impairment in a diverse clinic‐based and community‐based elderly cohort. Alzheimer's Dement. 2017;13(4):399‐405. doi: 10.1016/j.jalz.2016.07.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gleason CE, Norton D, Zuelsdorff M, et al. Association between enrollment factors and incident cognitive impairment in Blacks and Whites: data from the Alzheimer's disease center. Alzheimer's Dement. 2019;15(12):1533‐1545. doi: 10.1016/j.jalz.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576‐585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green‐Harris G, Coley SL, Koscik RL, et al. Addressing disparities in Alzheimer's disease and African‐American participation in research: an asset‐based community development approach. Front Aging Neurosci. 2019;11:125. doi: 10.3389/fnagi.2019.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751‐770. doi: 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249‐258. doi: 10.1097/WAD.0b013e318142774e [DOI] [PubMed] [Google Scholar]
- 20. Graff‐Radford J, Yong KX, Apostolova LG, et al. New insights into atypical Alzheimer's disease in the era of biomarkers. The Lancet Neurology. 2021;20(3):222‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher GG, Ryan LH. Overview of the health and retirement study and introduction to the special issue. Work Aging Retire. 2018;4(1):1‐9. doi: 10.1093/workar/wax032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. HRS . Sampling Weights: Revised for Tracker 2.0 & Beyond. 2019. Accessed August 1, 2021. https://hrs.isr.umich.edu/publications/biblio/10018
- 23. Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD. African Americans are less likely to enroll in preclinical Alzheimer's disease clinical trials. Alzheimers Dement (N Y). 2017;3(1):57‐64. doi: 10.1016/j.trci.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jefferson AL, Lambe S, Chaisson C, Palmisano J, Horvath KJ, Karlawish J. Clinical research participation among aging adults enrolled in an Alzheimer's Disease Center research registry. J Alzheimers Dis. 2011;23(3):443‐452. doi: 10.3233/JAD-2010-101536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimers Res Ther. 2010;2(6). 10.1186/alzrt58. doi:ARTN 34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Indorewalla KK, O'Connor MK, Budson AE, Guess DiTerlizzi C, Jackson J. Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in Alzheimer's disease research. J Alzheimers Dis. 2021;80(3):927‐940. doi: 10.3233/JAD-201081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson MG, Heller K, Rody CA. Recruitment challenges in studying late‐life depression: do community samples adequately represent depressed older adults? Psychol Aging. 1994;9(1):121‐125. doi: 10.1037//0882-7974.9.1.121 [DOI] [PubMed] [Google Scholar]
- 28. Hughes‐Morley A, Young B, Waheed W, Small N, Bower P. Factors affecting recruitment into depression trials: systematic review, meta‐synthesis and conceptual framework. J Affect Disord. 2015;172:274‐290. doi: 10.1016/j.jad.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 29. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: J Aging and Mental Health. 1986;5(1‐2):165‐173. [Google Scholar]
- 30. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385‐401. [Google Scholar]
- 31. van Buuren S. Flexible Imputation of Missing Data. 2nd ed. Chapman & Hall/CRC; 2018. [Google Scholar]
- 32. Stuart EA, Lee BK, Leacy FP. Prognostic score‐based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8):S84‐S90 e1. doi: 10.1016/j.jclinepi.2013.01.013 Suppl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. twang: Toolkit for Weighting and Analysis of Nonequivalent Groups . Version R package version 2.5. 2021. Accessed October 1, 2022. https://cran.r‐project.org/web/packages/twang/index.html
- 34. van Buuren S, mice Groothuis‐OudshoornK. Multivariate Imputation by chained equations in R. J Statistical Software. 2011;45(3):1‐67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 35. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu C, Nagarajan N, Assi L, et al. Assessment of hearing and vision impairment in cohort studies collecting cognitive data in older adults. Alzheimer's Dement. 2022. doi: 10.1002/alz.12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitson HE, Cronin‐Golomb A, Cruickshanks KJ, et al. American geriatrics society and national institute on aging bench‐to‐bedside conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc. 2018;66(11):2052‐2058. doi: 10.1111/jgs.15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blazel MM, Lazar KK, Van Hulle CA, et al. Factors associated with lumbar puncture participation in Alzheimer's disease research. J Alzheimers Dis. 2020;77(4):1559‐1567. doi: 10.3233/JAD-200394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zajacova A, Lawrence EM. The relationship between education and health: reducing disparities through a contextual approach. Ann Rev Public Health. 2018;39:273‐289. doi: 10.1146/annurev-publhealth-031816-044628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio‐demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24‐33. doi: 10.1016/j.cdp.2005.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53(9):1942‐1947. [DOI] [PubMed] [Google Scholar]
- 42. Zahodne LB, Mayeda ER, Hohman TJ, et al. The role of education in a vascular pathway to episodic memory: brain maintenance or cognitive reserve? Neurobiol Aging. 2019;84:109‐118. doi: 10.1016/j.neurobiolaging.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, Reed B. Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiol Aging. 2018;68:142‐150. doi: 10.1016/j.neurobiolaging.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farina MP, Hayward MD, Kim JK, Crimmins EM. Racial and educational disparities in dementia and dementia‐free life expectancy. J Gerontology: Series B. 2020;75(7):e105‐e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avila JF, Arce Rentería M, Jones RN, et al. Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimer's Dement. 2021;17(1):70‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Younan D, Wang X, Gruenewald T, et al. Racial/ethnic disparities in Alzheimer's disease risk: role of exposure to ambient fine particles. J Gerontology: Series A. 2022;77(5):977‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raman R, Quiroz YT, Langford O, et al. Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Netw Open. 2021;4(7):e2114364. doi: 10.1001/jamanetworkopen.2021.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams MM, Meisel MM, Williams J, Morris JC. An interdisciplinary outreach model of African American recruitment for Alzheimer's disease research. Gerontologist. 2011;51(1):S134‐S141. doi: 10.1093/geront/gnq098 Suppl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12(3):216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Medina LD, Torres S, Gioia A, Ochoa Lopez A, Wang J, Cirino PT, et al. Reporting of demographic variables in neuropsychological research: an Update of O'Bryant et al.’s trends in the current literature. J Int Neuropsychol Soc. 2021;27(5):497‐507. doi: 10.1017/S1355617720001083 [DOI] [PubMed] [Google Scholar]
- 51. Lesko CR, Buchanan AL, Westreich D, Edwards JK, Hudgens MG, Cole SR. Generalizing study results: a potential outcomes perspective. Epidemiology. 2017;28(4):553‐561. doi: 10.1097/EDE.0000000000000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Westreich D, Edwards JK, Lesko CR, Stuart E, Cole SR. Transportability of trial results using inverse odds of sampling weights. Am J Epidemiol. 2017;186(8):1010‐1014. doi: 10.1093/aje/kwx164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dahabreh IJ, Robertson SE, Steingrimsson JA, Stuart EA, Hernan MA. Extending inferences from a randomized trial to a new target population. Stat Med. 2020;39(14):1999‐2014. doi: 10.1002/sim.8426 [DOI] [PubMed] [Google Scholar]
- 54. Hayes‐Larson E, Mobley TM, Mungas D, et al. Accounting for lack of representation in dementia research: generalizing KHANDLE study findings on the prevalence of cognitive impairment to the California older population. Alzheimer's Dement. 2022. doi: 10.1002/alz.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chowkwanyun M, Reed Jr AL. Racial health disparities and Covid‐19—caution and context. New England J Medicine. 2020;383(3):201‐203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


