Abstract
Purpose:
Decisions for postoperative immobilization after bony hip reconstructive surgery in cerebral palsy are controversial in current practice. The aim of this study was to check if choosing not to use any kind of postoperative immobilization is a safe practice.
Methods:
A retrospective cohort study was conducted in a pediatric orthopedic tertiary referral center. The study included 148 patients (228 hips) with cerebral palsy, who had bony hip surgery. Medical records were reviewed for incidence of complications, methods of pain control, and length of hospital stay. Three radiographic measures (neck-shaft angle, Reimers migration index, and acetabular index) were performed on preoperative and postoperative X-rays. X-rays were also checked for mechanical failure of implant, recurrent dislocation/subluxation, and fractures in the first 6 months postoperatively.
Results:
In total, 94 (64%) were male and 54 (36%) were female. Seventy-seven (52%) were Gross Motor Function Classification System V, mean age at surgery was 8.6 years (2.5–18.4 years). Length of hospital stay was 6.25 days (SD 4.64 days). Medical complications that may have prolonged hospital stay occurred in 41 patients (27.7%). Radiological measurements showed significant improvement postoperatively (p = 0.001). Seven patients (4.7%) had another surgery in first 6 months (three for recurrent dislocation/subluxation, three for implant failure, and one for ipsilateral femur fracture).
Conclusion:
Avoiding postoperative immobilization following bony hip surgery in cerebral palsy is a safe practice and associated with reduced rate of medical and mechanical problems compared to the current literature. This approach should be utilized with optimal pain and tone management.
Keywords: Cerebral palsy, hip dysplasia, hip dislocation, hip spica, immobilization
Introduction
Hip dysplasia is the second most common musculoskeletal problem (after equinus) in children with Cerebral Palsy (CP) with an overall incidence of 35% (nearly one in three). 1 Its incidence has an incremental relationship with the level of gross motor function, ranging from 0% for children with GMFCS level I to 90% for those with GMFCS level V.1–3 The cause of hip displacement in CP is not entirely clear, but the hip is usually normal at birth. High hip joint reaction forces and abnormal orientation of force vectors are relevant and place an abnormal force on the acetabulum causing its deformation. Positioning particularly side-lying as well as impaired proprioception is also a contributing factor.2,4
If left untreated, the hip will exhibit progressive lateral displacement, subluxation, dislocation, and degenerative arthritis. Displaced hips ultimately result in sitting difficulty and pain. Other reported issues caused by untreated hip displacement are difficulty with perineal care and hygiene, pelvic obliquity, and scoliosis. 3 The decision for surgical intervention is based mainly on Reimers’ migration index (RMI) and acetabular index (AI). 5 Forty percent (40%) migration is considered by many surgeons as the threshold for intervention while 30%–40% is a controversial area. 6 Forty-six percent (46%) migration was defined by Wordie et al. 7 as “the point of no return” and considered as a strong indication for hip reconstructive surgery as the hip would not spontaneously regress. Most surgeons nowadays perform combined pelvic osteotomies with varus proximal femoral osteotomies to reduce recurrence of hip displacement as well as to facilitate remodeling and joint congruency, 8 while others base the decision for pelvic osteotomy either on arthrographic instability 9 or on an AI greater than 34°. 10 The decision to proceed to open reduction is another controversial area with some surgeons carrying out this routinely, 11 while others only when there is insufficient containment following a closed reduction. 12
There is currently no consensus in the literature about the optimal postoperative immobilization and rehabilitation after hip reconstructive surgery in CP.
In a recently published survey of 28 pediatric orthopedic surgeons from nine different countries about their preferred method of postoperative immobilization, 86% (24/28) recommended immobilization. Noteworthy, they reported use of seven different methods of immobilization, and they would choose increased immobilization with increased patient complexity, for example, Gross Motor Function Classification System (GMFCS) V, bilateral surgery, and open reduction. Similarly, return to weight bearing and physiotherapy recommendations were variable. 13 Methods of postoperative immobilization following reconstructive hip surgery includes hip spica cast—which is the most common method reported in the literature, short leg casts and anti-rotation bar, bilateral knee immobilizers with or without an abduction wedge, abduction wedge only, abduction brace, and abduction Petrie cast. 13
Although we know that the surgery itself has a positive influence on health-related quality of life, 14 the impact of the postoperative protocol is not well reported in the literature. Considering the variability in current practice and lack of evidence, we aimed to record the complication rate, either medical or mechanical, associated with immediate postoperative mobilization. We retrospectively reviewed our cohort of patients with neurodisability disorders who underwent bony hip reconstruction, followed by immediate postoperative mobilization (no hip spica, brace, abduction pillow, etc.) The primary objective was to record any mechanical failures of hip reconstruction (implant failure and subluxation) within the first 6 months postoperatively that could be attributed to lack of immobilization. Second aim was to document the rate of other complications and compare it to the current literature to see if they are within the expected rate, which is what we consider as “safe.” Our hypothesis is that immobilization is not indicated routinely after hip reconstructive surgery in patients with cerebral palsy, and this is considered to be a safe approach. In addition, immediate mobilization can avoid complications related to spica cast and other sorts of postoperative immobilization.
Patients and methods
We retrospectively searched the database of our pediatric orthopedic—tertiary referral center, looking specifically for neuromuscular patients who have had hip reconstructive surgery in the last 12 years (from August 2009 to August 2021). The inclusion criteria were as follows: (1) patients with confirmed diagnosis of CP or equivalent neurologic condition, (2) bony hip surgery in the form of proximal femoral osteotomy ± pelvic osteotomy ± open reduction in the specified period, (3) available medical and radiographic records, and (4) a minimum clinical and radiological follow-up of 6 months. Revision hip surgery, that was done more than 2 years after primary surgery, was considered to be a separate episode and was included in the study. We excluded patients with CP who had only soft tissue releases or salvage hip procedures and patients with diagnoses other than CP, for example, developmental dysplasia of the hip (either isolated or concurrent with CP), Legg–Calvé–Perthes disease, avascular necrosis of the hip, slipped upper femoral epiphysis, trauma, Down syndrome, and others.
Data were collected from a single pediatric orthopedic—tertiary referral center and operations were performed by three different full-time pediatric orthopedic surgeons. An open reduction of the femoral head, via Smith Peterson approach, was usually carried out when the RMI was greater than 90% (local criterion to define complete hip dislocations). Proximal femoral osteotomies were performed via lateral sub-vastus approach with varisation, shortening, and de-rotation of the proximal femur and fixed with pediatric hip locking plates from two different companies (Orthopediatrics, Warsaw, IN, USA or Synthes, West Chester, PA, USA). The surgeons (D.R., F.N.T., and M.K.) aimed for a final Neck-Shaft-Angle (NSA) of 110°, final Femoral-Neck-Anteversion-Angle of 0–10°, and shortening depending on the case. A Dega-like osteotomy (posterior acetabuloplasty) was the method of pelvic osteotomy performed. The main indications to proceed to a pelvic osteotomy were an AI > 27°, RMI > 50% or on-table instability. All operations were carried out under general anesthesia and additional epidural/caudal or nerve blocks and/or local anesthetic infiltration were also performed as decided by the anesthetist and surgeon on a case-by-case basis.
We reviewed the patient demographics (sex and age at time of surgery), GMFCS level, postoperative mobilization versus immobilization, length of hospital stay, and the intraoperative use of nerve blocks/local anesthetics. We were not able to assess patient’s postoperative pain objectively due to lack of thorough documentation (particularly in the patients treated in the early years of the studied period). Since patients were discharged from hospital when they deemed comfortable, we used the length of hospital stay as an indirect measure of pain management. We also investigated delayed discharges (more than 5 days) due to pain control issues. Complications in the immediate postoperative period (6 months following surgery) were also recorded.
Hip radiographs were done in a standardized position with pelvis and hips in a neutral position (neutral rotation and add/abduction), pillows and wedges were used for support. Three radiographic measurements were obtained for each hip including NSA, RMI, and AI preoperatively. 15 Postoperatively, NSA and RMI were measured for hips that had proximal femoral osteotomy. Likewise, AI was assessed postoperatively only for hips that had pelvic osteotomy. All measurements were made by a suitably trained pediatric orthopedic fellow (J.A.) on both pre- and postoperative standardized X-rays of both hips (AP view). Postoperative X-rays were usually taken in the first 2 weeks after surgery. Follow-up hip radiographs were also scrutinized for any evidence of metalwork failure and/or early recurrent dislocation/subluxation in the first 6 months postoperatively that required revision surgery.
Results
Our retrospective search retrieved 197 patients. Forty-nine (49) patients were excluded, and 148 patients (228 hips) were included in the study, 80 bilateral and 68 unilateral. Causes of exclusions are highlighted in flowchart (Figure 1). Ninety-four patients (64%) were male and 54 (36%) were female. Mean age at the time of surgery was 8.6 years (range: 2.5–18.4 years). Bilateral cases had both hips treated in the same operating session. One-hundred-and-twenty-one (121) hips had isolated proximal femoral osteotomy, 56 hips had proximal femoral and pelvic osteotomies and 51 hips had open reduction, proximal femoral and pelvic osteotomies performed. One-hundred-and-thirty-one (131) patients (87.2%) had a formal diagnosis of cerebral palsy and 17 patients (12.8%) had other neurological conditions considered equivalent to CP with the list of diagnoses outlined in Table 1. The patients had a range of GMFCS levels, with the majority (52%) being GMFCS V. Additional anesthetic choices intra-operatively (in addition to general anesthetic) included regional anesthetic in 94/148 patients, local anesthetic infiltration in 10/148 patients and both in 6/148 patients. This information was unavailable in 33 cases (22.3%) due to anesthetic chart/the information not being recorded.
Figure 1.
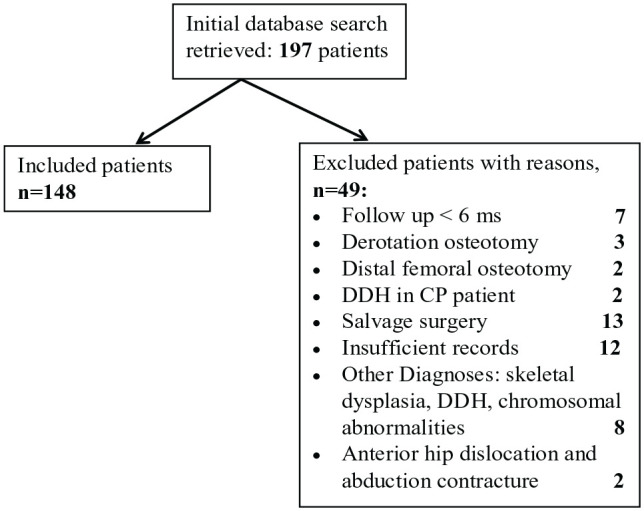
Flowchart of included and excluded cases.
Table 1.
Patient and disease demographics.
| Patients | No. (%) |
|---|---|
| Male | 94 (64%) |
| Female | 54 (36%) |
| Surgeries* | |
| PFO | 121 (53%) |
| PFO + PO | 56 (24.6%) |
| PFO + PO + OR | 51 (22.4%) |
| Condition† | |
| Formal diagnosis of CP | 131 (88.5%) |
| Other neurologic conditions | 17 (11.5%) |
| Hypoxic brain injury | 1 |
| Hypoxic ischemic encephalopathy | 1 |
| Hereditary spastic paraplegia | 1 |
| Corpus callosum agenesis | 1 |
| Muscle eye brain disease | 1 |
| “Undiagnosed neurological condition” | 2 |
| Bifrontal polymicrogyria | 1 |
| Generalized dystonia of congenital onset of unknown cause | 1 |
| Spinal muscular atrophy type 3 | 1 |
| Global developmental delay with four-limb motor disorder | 1 |
| Aicardi–Goutierres syndrome | 1 |
| Progressive leuko-dystrophy of unknown cause | 1 |
| Rhett’s syndrome | 1 |
| Angelman syndrome | 1 |
| Congenital muscular dystrophy with cerebral/cerebellar anomalies | 1 |
| Mitochondrial cytopathy | 1 |
| GMFCS level † | |
| I | 1 |
| II | 1 |
| III | 13 (8.8%) |
| IV | 37 (25%) |
| V | 77 (52%) |
| Not formally categorized | 19 (12.8%) |
Per hip (/228).
Per patient (/148).
PFO = proximal femoral osteotomy, PO = pelvic osteotomy, OR = open reduction.
All patients were allowed to mobilize immediately after surgery without any restriction, and we did not use any sort of immobilization (cast or brace). Patients were nursed in bed in the most comfortable position, and legs were supported with pillows if needed. They were allowed semi-setting and setting in bed, gentle turning on either side, as well as moving the hips for perineal hygiene. Physiotherapists and occupational therapists reviewed the patients while in ward to start practicing sliding transfer, mobilization from bed to chair, and hoisting. Ambulatory patients were allowed partial weight bearing or non-weight bearing according to the surgeon discretion. Community physiotherapists continued the same plan after discharge. According to bone healing after 6 weeks, ambulatory patients were allowed to progress in weight bearing while non-ambulators were allowed starting standing frames if they were using it preoperatively. Having no cast or brace allowed easier inspection of wounds, epidural entry sites, and pressure areas, as well as easy access to PEG (percutaneous endoscopic gastrostomy) for patients who used it for feeding. The pain management postoperatively included epidural infusion for the first 48 h followed by Patient- or Nurse-Controlled Analgesia, oral opiate analgesics, and/or paracetamol and non-steroidal-anti-inflammatories. This was our standardized protocol that was implemented and modified by our pain specialist if necessary, according to each case individually. Patients were discharged when they were comfortable sitting and transferring without opiates and medically stable. Patients were reviewed routinely by our pediatric neurologist preoperatively and postoperatively if needed, where tone management plan was usually developed—which may include adjustment of the rate of baclofen pump, epidural administration of fentanyl/clonidine, and use of additional antispasmodics perioperatively. Oral antispasmodics were added to pain killers on discharge whenever needed. The average length of hospital stay was 6.25 days (SD 4.64 days). Postoperative complications in 41 patients (27.7%) that may have caused prolonged hospital stay are summarized in Table 2.
Table 2.
Postoperative complications leading to prolonged hospitalization (>5 days) and their frequency.
| Complications postoperatively | No. of patients (%) |
|---|---|
| Chest infection | 13 (8.8%) |
| Urinary infection | 4 (2.7%) |
| Surgical site infection | 4 (2.7%) |
| Pressure sore/tissue viability issue | 2 (1.4%) |
| Pain control issues | 2 (1.4%) |
| Constipation/diarrhea/vomiting | 6 (4%) |
| Failed removal of catheter | 1 (0.7%) |
| Infection unknown source | 6 (4%) |
| Ileus | 2 (1.4%) |
| Anaphylaxis | 1 (0.7%) |
| Total | 41 (27.7%) |
Radiological measurements preoperatively were compared to postoperative values using Wilcoxon-signed rank test and all showed significant change postoperatively (p = 0.001). Minimum, maximum, median, and p-values are summarized in Table 3. Range of postoperative neck shaft angles is also illustrated in Table 4.
Table 3.
Pre- and Postoperative (first 2 weeks) “radiological” measurements of the affected hip joint in all included patients.
| Pre-op | Post-op | p-value | |||||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Median | Minimum | Maximum | Median | ||
| Neck-shaft angle right | 109 | 178 | 156 | 86 | 137 | 116 | 0.001 |
| Neck-shaft angle left | 93 | 179 | 156 | 80 | 144 | 114 | 0.001 |
| Acetabular index right | 9 | 50 | 26.5 | 11 | 37 | 20 | 0.001 |
| Acetabular index left | 8 | 50 | 26.5 | 11 | 37 | 20 | 0.001 |
| Reimer’s migration right | 0 | 100 | 43.5 | 0 | 68 | 0 | 0.001 |
| Reimer’s migration left | 0 | 100 | 42 | 0 | 75 | 0 | 0.001 |
Table 4.
Range of postoperative neck shaft angle (NSA).
| Postoperative NSA (°) | No of hips (%) |
|---|---|
| ≤100 | 31, 13.6% |
| 101 to ≤110 | 47, 20.6% |
| 111 to ≤120 | 77, 33.8% |
| 121 to ≤130 | 58, 25.4% |
| ≥131 | 15, 6.6% |
Seven patients (4.7%) required revision surgery for complications within the first 6 month postoperatively:
Three patients had revision surgery for recurrent dislocation in the first 6 weeks postoperatively. Two patients required revision surgery and hip spica application as the hip felt unstable intra-operatively, and the third patient also received revision surgery, but did not need a form of postoperative immobilization after surgery as the hip felt reduced and stable. Root cause analysis revealed inadequate surgical correction of NSA and/or AI to be the cause of recurrent dislocation.
Three patients required revision surgery for metalwork failure (screw breakage, plate pull-out) at 3 months follow-up, and all three of them had severe uncontrolled dystonia that was difficult to be managed medically. This number was not high enough for the authors to change their practice. However, spica cast immobilization for uncontrolled dystonic patients could be justified.
One patient had an ipsilateral supracondylar femoral fracture 5 months post-surgery that was managed with open reduction and internal fixation as the proximal fragment was tethering the skin.
Discussion
Although there is a relative agreement currently among surgeons on the surgical management of hip dysplasia in patients with cerebral palsy, postoperative protocols and in particular the need for immobilization after surgery are still debatable. Fully or partially removable immobilization may allow easier hygiene or permit the patient to sit and initiate rehabilitation therapy within days of surgery. 16 Proponents of hip spica may argue that it has advantages of surgical wound protection, reduced motion at the osteotomy site(s), mitigating hip flexion contractures, protection of osteotomies in patients with relative osteopenia, and possibly reducing postoperative pain. However, there is no strong evidence in the literature to support these benefits. Furthermore, hip spica is not without its own complications—which include skin sores, disuse-mediated osteopenia causing fractures, joint stiffness, among others. 16
Postoperative hip spica is considered mandatory after open reduction in DDH due to the inherent instability of the hip joint within the dysplastic acetabulum. 17 This is not the case in neurogenic hip dysplasia (NHD) due to different pathophysiology of the underlying condition. For children with CP, the acetabulum is usually capacious and should be reshaped and redirected to achieve on-table stability, whereas this is more difficult to achieve in DDH. Also, children with DDH are likely to try to get up. The children with CP are older and more cautious and frequently unable to move—either due to the underlying lack of active movement or the post-op spasticity and dystonia. With the increased awareness of hip spica cast complications in DDH, several studies reported protocols to either decrease the cast size or duration as an attempt to lessen these risks.18,19 Applying the same principles, and in view of the different patho-anatomy, we think it is in the patient’s best interests to avoid any sort of immobilization following hip surgery in patients with CP.
In our retrospective cohort study, we demonstrated that immediate mobilization postoperatively is a safe and effective approach. The complication rate is comparable or even less than other types of immobilization. Lubicky et al. 20 studied the difference in complication rate between casted and non-casted CP patients undergoing hip reconstruction, but the groups were not identical in number or in patient characteristics. They found the complication rate was higher in the casted group, but this did not reach statistical significance. They also reported skin sores in 43/286 (15%), in the casted group, that were managed conservatively, apart from three cases that needed hospitalization but not surgical management. 20 Ruzbarsky et al. 21 also reported decubitus ulcers in 3/61 (4.9%) and Pisecky et al. 22 counted superficial skin lesions in 7/83 (8.4%). When compared spica cast and abduction pillow, Vasconcellos et al. 23 reported incidence of pressure sore associated with abduction pillow especially over bony prominences and from pillow straps. In our series, we have had 2/148 (1.3%) rate of skin sores that were managed by tissue viability team and did not need any intervention. 4/148 (2.7%) developed superficial wound infection and all managed with wound care and antibiotics, and no cases needed open washout and drainage. Our results confirm that no immobilization is in fact protective to the skin against pressure sores.
In their cohort of 61 non-ambulatory CP patients (93 hips), Ruzbarsky et al. 21 found spica cast immobilization to be a risk factor to all complications. Decubitus ulcers, heterotopic ossification, and severe pain were directly related to the cast, but the distribution of femoral fractures was mostly equivalent among the spica and non-spica groups (four in each). 21 Nevertheless, other authors reported higher rates of fractures in patients managed in spica cast after hip surgery. Sturm et al. 24 reported 6/21 (29%), Mubarak et al. 25 reported 1/11 (10%), and Pritchett 26 reported 9/50 (18%) rate of fractures in the postoperative period which could be attributed to immobilization-related disuse osteopenia. However, Miller et al., 27 who allowed immediate mobilization, had only 2/50 (4%) fractures. In our cohort, we had only one patient (0.67%) sustained femur fracture 4 months after surgery. Our results, in line with the literature, can conclude that immediate mobilization is in fact protective against fractures.
Pisecky et al. retrospectively analyzed the complication profile in patients undergoing hip reconstructive surgery for DDH, NHD or Perthes disease and a spica cast postoperatively for 6 weeks. In NHD group, they reported spasticity of hip adductors in 3/23 (13%) and of knee flexors in 1/23 (4%) that was associated with the use of the spica cast. 22 In our series, spasticity was not recorded as a cause of prolonged hospitalization. Our patients may have had a degree of spasticity that was managed by medication according to our protocol. The authors think that spica cast or other immobilization devices may itself trigger spasms and dystonia.
Postoperative pain management in patients with CP can be a challenge, especially when the patient has communication difficulties. Pedersen et al. 28 found that epidural analgesia is superior to local anesthetic infiltration after unilateral hip reconstruction in CP. Our pain management protocol mentioned earlier seemed to be effective and to echo their results. In our series, two patients (1.3%) had problems with pain management that led to prolonged hospital stay. However, this result may not be an accurate measure of pain postoperatively as other patients may have had pain that was not severe enough to require prolonged hospitalization. In addition, patients who had prolonged stay for other reasons may have had concomitant pain as well. We believe that this percentage does not justify immobilization postoperatively.
We have had seven cases (4.7%) of mechanical failure needing revision surgery, which can be attributed to other factors, namely, inadequate surgical correction of NSA and/or AI and severe dystonia that was evident preoperatively and difficult to control medically, rather than the lack of immobilization. Our practice is similar to Beauchesne et al. 29 and Miller et al. 27 who allowed immediate mobilization after surgery and limited use of postoperative hip spicas (for concurrent DDH and unstable hips intra-operatively). Beauchesne et al. 29 reported two cases (2%) of mechanical failure which they attributed to technical errors. Both authors used AO fixed angle blade plate while we used proximal femoral locking plate. We assume therefore that fixed angle implant is mechanically stable and does not need protection by external support. Rutz and Brunner 30 found no significant difference between the AO blade plate and the proximal femoral locking plate regarding fixation and correction of the neck-shaft angle, apart from faster consolidation with proximal femoral locking plate. They used spica cast or abduction brace, only for 3 weeks, when open reduction is added to femoral and pelvic osteotomies and in patients with poor bone quality. Up to our knowledge, Miller et al., 27 Beauchesne et al., 29 and Rutz and Brunner 30 are the only studies in the literature that allowed immediate mobilization postoperatively.
In their case series, Tabaie et al. 31 compared the complication rate of three different types of immobilization after hip reconstruction in CP, namely, hip spica, Petrie cast, and abduction pillow. Likewise, Vasconcellos et al. 23 compared hip spica and abduction pillow. They both did not find a significant difference in complication rate between groups and recommended use of less restrictive types of immobilization. Interestingly, both studies found a significant difference in patient age, height, and weight between groups, which may reflect surgeon preference to avoid spica cast in older, taller, and heavier patients.
Our study is not without limitations. Its retrospective nature, being a single center study and lack of control group may make it open to criticism from proponents of hip spicas. Cost analysis and savings investigations were not done. No objective pain score was used, and we also did not obtain formal feedback about the experience of the parents/carers, the ward nurses, and allied health professionals. Although our radiological measurements showed significant change, which may confirm obtaining the desired surgical correction, but they were done by a single investigator, and we did not assess inter- and intra-observer reliability of measurements. Tone was not noted for study population as the information was lacking from our records. Our study also lacks long-term follow-up as it would be illogical to expect the effect of a hip spica to last more than 6 months. In fact, there are many confounding factors that contribute to failure of hip reconstruction in CP children, like age at index surgery, GMFCS level, preoperative migration percentage and neck-shaft angle, and concomitant correction of acetabular dysplasia. 32 Zakrzewski et al. 33 suggested over-containment for high-risk patients to decrease recurrence rate. In previous studies, postoperative immobilization was not considered as one of the factors contributing to long-term recurrence and we believe this needs to be addressed by future studies. A prospective randomized control trial would be needed to improve on our data. We do not however believe that there would be sufficient equipoise to justify it in the light of evidence that we present here.
Conclusion
In this article, we have demonstrated that immediate postoperative mobilization is a safe approach and can decrease the rate of complications for patients with CP and similar conditions. It also improves skin care and does not increase the likelihood of postoperative fractures. We recommend this protocol to be embarked on under umbrella of optimal tone and pain management.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, sj-xlsx-1-cho-10.1177_18632521231164983 for Bony hip reconstruction for displaced hips in patients with cerebral palsy: Is postoperative immobilization indicated? by John Amen, Oliver Perkins, Konstantinos Kafchitsas, Daniel Reed, Fabian Norman-Taylor and Michail Kokkinakis in Journal of Children's Orthopaedics
Acknowledgments
The authors thank Dr Tamer A El-Sobky for his help with manuscript review and advice in the stage of writing, as well as Dr George Nesr for his help with the statistical analysis.
Footnotes
Author contributions: John Amen: study design, collection and analysis of data, manuscript preparation, performed the measurements.
Oliver Perkins: study design, collection and analysis of data, manuscript preparation.
Konstantinos Kafchitsas: statistical analysis, manuscript preparation and revision.
Daniel Reed: study design, data analysis, manuscript revision, performed surgeries.
Fabian Norman-Taylor: study design, data analysis, manuscript revision and approval of the version to be published, performed surgeries.
Michail Kokkinakis: study design, data analysis, manuscript revision and approval of the version to be published, performed surgeries.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Upon consultation with our institutional review board/ethics committee, approval for the study was not needed and study was classified as review of service.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Consent not required as study was an anonymized review of service.
ORCID iD: Konstantinos Kafchitsas  https://orcid.org/0000-0003-2750-2310
https://orcid.org/0000-0003-2750-2310
Supplemental material: Supplemental material for this article is available online.
References
- 1.Soo B, Howard JJ, Boyd RN, et al. Hip displacement in cerebral palsy. J Bone Joint Surg 2006; 88(1): 121–129. [DOI] [PubMed] [Google Scholar]
- 2.Davids JR. Management of neuromuscular hip dysplasia in children with cerebral palsy: lessons and challenges. J Pediatr Orthop 2018; 38(Suppl. 1): S21–S27. [DOI] [PubMed] [Google Scholar]
- 3.Hosseinzadeh P, Baldwin K, Minaie A, et al. Management of hip disorders in patients with cerebral palsy. JBJS Rev 2020; 8(3): e0148. [DOI] [PubMed] [Google Scholar]
- 4.Robb JE, Hägglund G. Hip surveillance and management of the displaced hip in cerebral palsy. J Child Orthop 2013; 7(5): 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sobky TA, Fayyad TA, Kotb AM, et al. Bony reconstruction of hip in cerebral palsy children Gross Motor Function Classification System levels III to V: a systematic review. J Pediatr Orthop B 2018; 27(3): 221–230. [DOI] [PubMed] [Google Scholar]
- 6.Hägglund G, Lauge-Pedersen H, Persson M. Radiographic threshold values for hip screening in cerebral palsy. J Child Orthop 2007; 1(1): 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wordie SJ, Bugler KE, Bessell PR, et al. Hip displacement in children with cerebral palsy: the point of no return. Bone Joint J 2021; 103(2): 411–414. [DOI] [PubMed] [Google Scholar]
- 8.Givon U. Management of the spastic hip in cerebral palsy. Curr Opin Pediatr 2017; 29(1): 65–69. [DOI] [PubMed] [Google Scholar]
- 9.Huh K, Rethlefsen SA, Wren TA, et al. Surgical management of hip subluxation and dislocation in children with cerebral palsy: isolated VDRO or combined surgery? J Pediatr Orthop 2011; 31(8): 858–863. [DOI] [PubMed] [Google Scholar]
- 10.Ha M, Okamoto T, Fukuta T, et al. Preoperative radiologic predictors of successful soft tissue release surgery for hip subluxation among cerebral palsy patients: a STROBE compliant study. Medicine 2018; 97(33): e11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Root L, Laplaza FJ, Brourman SN, et al. The severely unstable hip in cerebral palsy. Treatment with open reduction, pelvic osteotomy, and femoral osteotomy with shortening. J Bone Joint Surg Am 1995; 77(5): 703–712. [DOI] [PubMed] [Google Scholar]
- 12.Min JJ, Kwon SS, Sung KH, et al. Remodelling of femoral head deformity after hip reconstructive surgery in patients with cerebral palsy. Bone Joint J 2021; 103-B(1): 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller SD, Juricic M, Fajardo N, et al. Variability in postoperative immobilization and rehabilitation following reconstructive hip surgery in nonambulatory children with cerebral palsy. J Pediatr Orthop 2021; 41(7): e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiFazio R, Shore B, Vessey JA, et al. Effect of hip reconstructive surgery on health-related quality of life of non-ambulatory children with cerebral palsy. J Bone Joint Surg 2016; 98(14): 1190–1198. [DOI] [PubMed] [Google Scholar]
- 15.Pons C, Rémy-Néris O, Médée B, et al. Validity and reliability of radiological methods to assess proximal hip geometry in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2013; 55(12): 1089–1102. [DOI] [PubMed] [Google Scholar]
- 16.Truong U, Sylvanus T, Koester TM, et al. A comparison of hip spica casting to short leg casts and bar after hip reconstruction in cerebral palsy. Cureus 2020; 12(5): e8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaquero- Picado A, González-Morán G, Garay EG, et al. Developmental dysplasia of the hip: update of management. EFORT Open Rev 2019; 4(9): 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zhou Q, Liu Y, et al. Closed reduction and dynamic cast immobilization in patients with developmental dysplasia of the hip between 6 and 24 months of age. Eur J Orthop Surg Traumatol 2019; 29(1): 51–57. [DOI] [PubMed] [Google Scholar]
- 19.Emara K, Kersh MAA, Hayyawi FA. Duration of immobilization after developmental dysplasia of the hip and open reduction surgery. Int Orthop 2019; 43(2): 405–409. [DOI] [PubMed] [Google Scholar]
- 20.Lubicky JP, Bernotas S, Herman JE. Complications related to postoperative casting after surgical treatment of subluxed/dislocated hips in patients with cerebral palsy. Orthopedics 2003; 26(4): 407–411. [DOI] [PubMed] [Google Scholar]
- 21.Ruzbarsky JJ, Beck NA, Baldwin KD, et al. Risk factors and complications in hip reconstruction for nonambulatory patients with cerebral palsy. J Child Orthop 2013; 7(6): 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisecky L, Großbötzl G, Gahleitner M, et al. Results after spica cast immobilization following hip reconstruction in 95 cases: is there a need for alternative techniques? Arch Orthop Trauma Surg 2022; 142: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasconcellos AL, Tagawa AS, Rhodes JT, et al. Postoperative immobilization after hip reconstruction in cerebral palsy: no difference between hip spica and abduction pillow. Front Surg 2022; 9: 863287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturm PF, Alman BA, Christie BL. Femur fractures in institutionalized patients after hip spica immobilization. J Pediatr Orthop 1993; 13(2): 246–248. [PubMed] [Google Scholar]
- 25.Mubarak SJ, Valencia FG, Wenger DR. One-stage correction of the spastic dislocated hip. Use of pericapsular acetabuloplasty to improve coverage. J Bone Joint Surg Am 1992; 74(9): 1347–1357. [PubMed] [Google Scholar]
- 26.Pritchett JW. Treated and untreated unstable hips in severe cerebral palsy. Dev Med Child Neurol 1990; 32(1): 3–6. [DOI] [PubMed] [Google Scholar]
- 27.Miller F, Girardi H, Lipton G, et al. Reconstruction of the dysplastic spastic hip with peri-ilial pelvic and femoral osteotomy followed by immediate mobilization. J Pediatr Orthop 1997; 17(5): 592–602. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen LK, Nikolajsen L, Rahbek O, et al. Epidural analgesia is superior to local infiltration analgesia in children with cerebral palsy undergoing unilateral hip reconstruction. Acta Orthop 2016; 87(2): 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beauchesne R, Miller F, Moseley C. Proximal femoral osteotomy using the AO fixed-angle blade plate. J Pediatr Orthop 1992; 12(6): 735–740. [PubMed] [Google Scholar]
- 30.Rutz E, Brunner R. The pediatric LCP hip plate for fixation of proximal femoral osteotomy in cerebral palsy and severe osteoporosis. J Pediatr Orthop 2010; 30(7): 726–731. [DOI] [PubMed] [Google Scholar]
- 31.Tabaie S, Sadur A, Shah A. Evaluating postoperative immobilization following hip reconstruction in children with cerebral palsy. Cureus 2022; 14(10): e30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minaie A, Gordon JE, Schoenecker P, et al. Failure of hip reconstruction in children with cerebral palsy: what are the risk factors? J Pediatr Orthop 2022; 42(1): e78–e82. [DOI] [PubMed] [Google Scholar]
- 33.Zakrzewski AM, Bryant AJ, McCarthy JJ. Can over-containment prevent recurrence in children with cerebral palsy and hip dysplasia undergoing hip reconstruction? J Pediatr Orthop 2022; 42(6): 300–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, sj-xlsx-1-cho-10.1177_18632521231164983 for Bony hip reconstruction for displaced hips in patients with cerebral palsy: Is postoperative immobilization indicated? by John Amen, Oliver Perkins, Konstantinos Kafchitsas, Daniel Reed, Fabian Norman-Taylor and Michail Kokkinakis in Journal of Children's Orthopaedics


