Key Points
Question
Was the COVID-19 pandemic associated with changes in the rates of in-person visits and telemedicine use among patients with hematologic neoplasms receiving cancer therapy in 2020 to 2021?
Findings
In this cohort study with data from 24 261 patients with diverse hematologic neoplasms, time-series forecasting methods were used to compare actual vs forecasted visit rates. There was a significant relative reduction (21%) in in-person visits during the early months of the pandemic (March to May 2020) for oral and outpatient treatment types.
Meaning
These findings suggest that the COVID-19 pandemic was associated with significant changes in visit rates for care delivery among patients with hematologic neoplasms in 2020 to 2021.
This cohort study examines associations between the COVID-19 pandemic and in-person visits and telemedicine use among US patients receiving active treatment for hematologic neoplasms.
Abstract
Importance
The COVID-19 pandemic has led to a reduction in routine in-person medical care; however, it is unknown whether there have been any changes in visit rates among patients with hematologic neoplasms.
Objective
To examine associations between the COVID-19 pandemic and in-person visits and telemedicine use among patients undergoing active treatment for hematologic neoplasms.
Design, Setting, and Participants
Data for this retrospective observational cohort study were obtained from a nationwide electronic health record–derived, deidentified database. Data for patients with hematologic neoplasms who had received at least 1 systemic line of therapy between March 1, 2016, and February 28, 2021, were included. Treatments were categorized into 3 types: oral therapy, outpatient infusions, and inpatient infusions. The data cutoff date was April 30, 2021, when study analyses were conducted.
Main Outcomes and Measures
Monthly visit rates were calculated as the number of documented visits (telemedicine or in-person) per active patient per 30-day period. We used time-series forecasting methods on prepandemic data (March 2016 to February 2020) to estimate expected rates between March 1, 2020, and February 28, 2021 (if the pandemic had not occurred).
Results
This study included data for 24 261 patients, with a median age of 68 years (IQR, 60-75 years). A total of 6737 patients received oral therapy, 15 314 received outpatient infusions, and 8316 received inpatient infusions. More than half of patients were men (14 370 [58%]) and non-Hispanic White (16 309 [66%]). Early pandemic months (March to May 2020) demonstrated a significant 21% reduction (95% prediction interval [PI], 12%-27%) in in-person visit rates averaged across oral therapy and outpatient infusions. Reductions in in-person visit rates were also significant for all treatment types for multiple myeloma (oral therapy: 29% reduction; 95% PI, 21%-36%; P = .001; outpatient infusions: 11% reduction; 95% PI, 4%-17%; P = .002; inpatient infusions: 55% reduction; 95% PI, 27%-67%; P = .005), for oral therapy for chronic lymphocytic leukemia (28% reduction; 95% PI, 12%-39%; P = .003), and for outpatient infusions for mantle cell lymphoma (38% reduction; 95% PI, 6%-54%; P = .003) and chronic lymphocytic leukemia (20% reduction; 95% PI, 6%-31%; P = .002). Telemedicine visit rates were highest for patients receiving oral therapy, with greater use in the early pandemic months and a subsequent decrease in later months.
Conclusions and Relevance
In this cohort study of patients with hematologic neoplasms, documented in-person visit rates for those receiving oral therapy and outpatient infusions significantly decreased during the early pandemic months but returned to close to projected rates in the later half of 2020. There were no statistically significant reductions in the overall in-person visit rate for patients receiving inpatient infusions. There was higher telemedicine use in the early pandemic months, followed by a decline, but use was persistent in the later half of 2020. Further studies are needed to ascertain associations between the COVID-19 pandemic and subsequent cancer outcomes and the evolution of telemedicine use for care delivery.
Introduction
The COVID-19 pandemic has caused substantial disruptions in health care delivery in the US and the rest of the world. Several studies have shown a decline in hospitalizations and procedures for acute conditions such as stroke and myocardial infarction during the pandemic period.1,2 A decline in in-person visit rates was seen for ambulatory, preventive, and emergency care.3,4,5 The pandemic has had notable implications for cancer care due to increased risk of morbidity and mortality among individuals with cancer and COVID-19, thereby forcing health care facilities to create empirical guidelines focused on balancing risks of treatment delays against viral illness.6,7 Studies examining the association between the pandemic and cancer care delivery are limited. Some studies in solid organ neoplasms have suggested a trend toward delay in care and treatment modifications during the early pandemic period.8,9,10 However, how the pandemic affected in-person visit rates and telemedicine use among patients with hematologic neoplasms is unknown.
A recent systematic review of 62 studies suggested that there were delays or disruptions in routine activity of cancer services but included mostly single-institution studies, of which 5% focused on hematologic neoplasms.11 Early in the pandemic, many health systems incorporated virtual visits using telemedicine for nonurgent clinical care. Telemedicine can be a particularly attractive option for patients receiving oral antineoplastic drugs. However, many treatments for hematologic neoplasms (eg, outpatient or inpatient infusions) are administered in face-to-face settings and, therefore, potentially susceptible to being disrupted by stay-at-home orders or a decrease in hospital capacity. The incidence of hematologic neoplasms remained stable during the pandemic period compared with the preceding year12,13; therefore, a decline in treatment-related visits during this period would suggest disrupted cancer care delivery for this patient population. In this study, we sought to determine associations between the pandemic and in-person visit rates for various therapeutic subgroups with hematologic neoplasms in the US. We hypothesized that the early pandemic period was associated with a decline in in-person visit rates compared with preceding years. We also investigated telemedicine visit rates for various hematologic neoplasms and utilization patterns by treatment type.
Methods
Study Design
We used data from the nationwide electronic health record (EHR)–derived Flatiron Health Research Database to conduct this retrospective observational cohort study of patients with hematologic neoplasms. Institutional review board approval of the study protocol was obtained from the University of Alabama at Birmingham before study conduct and included a waiver of informed consent because deidentified data were used. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The Flatiron Health Research Database comprises longitudinal, deidentified, patient-level structured and unstructured data, curated via technology-enabled abstraction.14,15 A team of trained professionals (ie, oncology nurses, tumor registrars, and clinical research professionals) collects specific data elements across patient documents. All abstractors are trained to identify and extract relevant information by following policies and procedures tested and optimized for reliability and reproducibility through iterative processes, and oversight is provided by medical oncologists.15,16 Some data elements (eg, oral therapies, clinical study drugs) were abstracted by machine-learning models, which were trained from a manual abstraction standard and then optimized and validated.
During the study period, the data originated from approximately 280 US cancer clinics (approximately 800 sites of care). Most patients in the Flatiron Health Research Database originated from community oncology settings; relative community or academic proportions may vary depending on the study cohort. The study period extended from March 1, 2016, to February 28, 2021, and was divided into 2 time periods: (1) the 60-month span preceding the declaration of the COVID-19 pandemic as a public health crisis in the US (March 1, 2016, to February 28, 2020) and (2) the 12-month span following the declaration of the COVID-19 pandemic as a public health crisis (March 1, 2020, to February 28, 2021). The data cutoff date was April 30, 2021, when study analyses were conducted.
Data were included if patients met the following criteria: (1) had a confirmed diagnosis of acute myeloid leukemia, chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, or multiple myeloma; (2) were at least 18 years of age at the time of initial cancer diagnosis; and (3) received at least 1 systemic, nonmaintenance line of therapy (LOT) during the study period. Data for patients with more than 90 days between the initial diagnosis date and first subsequent structured activity in the EHR were excluded. Since the unit of analysis for this study was patient visits, visits that occurred outside of the study period or when the patient was not receiving active therapy (requiring ≥2 visits) were excluded. See the eFigure in Supplement 1 for the patient selection diagram.
Patient visits were further categorized into 3 treatment types: outpatient oral therapies, outpatient infusion therapies, and inpatient infusion therapies, including bone marrow transplantation (BMT) and chimeric antigen receptor T-cell therapy. The specific therapies or regimens categorized into each treatment type were unique to each disease and based on current National Comprehensive Cancer Network guidelines (eTable 1 in Supplement 1).17
Each eligible LOT received during the study period was categorized into a treatment type for the drugs given within the LOT. If the LOT included regimens from 2 different treatment types, it was assigned to the higher-intensity treatment type (ranked oral therapy, outpatient infusions, and inpatient infusions from lowest to highest). All visits were then mapped to their respective treatment type if the visit date occurred during a systemic LOT (inclusive of start and end dates). Since patients were counted multiple times if they had multiple treatment-visit types during the observation period, the total number of patients receiving oral therapy, outpatient infusions, and inpatient infusions reported was greater than the overall number of active patients included in the analysis.
The Flatiron Health database captures race and ethnicity in EHRs to enable studies into access to care and disparities research. Race and ethnicity was self-reported as Hispanic or Latino, non-Hispanic Asian, non-Hispanic Black or African American, or non-Hispanic White (hereinafter, Hispanic, Asian, Black, or White) or other (American Indian or Alaska Native, Hawaiian or Pacific Islander, or multiple) (missing data are listed as unknown).
Outcome Variables
This study had 2 main outcome measures of interest: monthly standardized (30 patient-day) rates of (1) in-person visits and (2) telemedicine visits. In-person visits were defined as documented visits in the EHR that did not occur on the same day as telemedicine visits. Telemedicine visits were defined using evaluation and management coding from structured EHR data, complemented by patient documents containing a telemedicine keyword. Visits were counted as the total number of in-person or telemedicine visits each calendar month. Standardized 30-day patient-days were calculated from the number of active patients each month multiplied by the number of days in that calendar month and divided by 30.
Statistical Analysis
In-Person Visit Rates
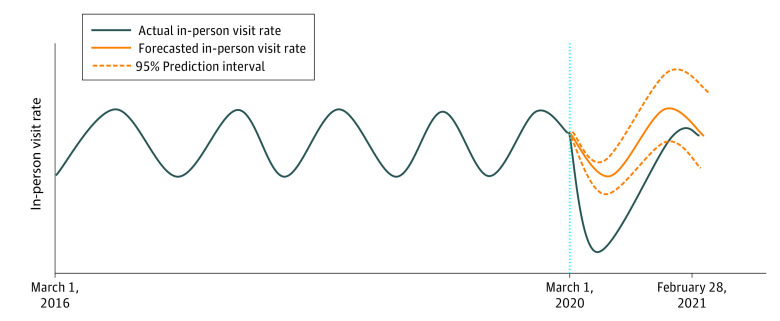
Autoregressive integrated moving average (ARIMA) models were used to measure the pandemic’s impact on monthly standardized (30 patient-day) in-person visit rates. Time-series forecasting based on ARIMA models has been used in the public health and health economics literature to evaluate associations between pandemics and health outcomes, including mortality and hospitalization rates.18,19 The ARIMA models were fitted to prepandemic data (March 2016 to February 2020) and used to estimate in-person visit rates expected to occur during the pandemic period (March 2020 to February 2021) and had there not been a pandemic (ie, the counterfactual), with specific focus on early pandemic months (March to May 2020) when most states had stay-at-home orders in place. The ARIMA models used a linear combination of lagged observations (autoregression) and a moving average of lagged errors to forecast future observations. That is, historical monthly visit rates were used to estimate future monthly visit rates. Figure 1 demonstrates a conceptual model of the ARIMA method.
Figure 1. Conceptual Demonstration of the Autoregressive Integrated Moving Average (ARIMA) Model.
The vertical blue dotted line indicates the onset of the COVID-19 pandemic.
The general model building procedure was as follows. First, because ARIMA models must fit to stationary data with constant mean, variance, and autocorrelation over time, stationarity of the time series was assessed by using augmented Dickey-Fuller tests and identifying appropriate orders of differencing.20 Second, the combination of autoregression and lagged error terms to incorporate to minimize the Akaike information criterion was estimated using autocorrelation and partial autocorrelation plots, respectively. Third, goodness of fit was tested using Ljung-Box Q tests of autocorrelation in the residuals.21
Seasonal components were added as needed to the ARIMA model (ie, SARIMA).21 Analyses were stratified by treatment type for all diseases combined and by each disease, resulting in the creation of 19 individual time series of monthly standardized (30 patient-day) rates of in-person visits for each subcohort. Separate (S)ARIMA models were fitted to each time series and used to forecast a projection (counterfactual) of expected monthly rates had the pandemic not occurred. Further details on the SARIMA model methods, all (S)ARIMA model parameters, and the results of Ljung-Box Q tests are found in the eMethods and eTables 2 and 3 in Supplement 1.
Months when the observed rates exceeded the 95% prediction interval (PI) surrounding the forecasted estimates were deemed to have been statistically significantly affected by the pandemic (Figure 1). The relative difference between the observed monthly rates of in-person visits and the forecasted estimates was calculated per month as follows:
| (Observed Visit Rate – Forecasted Visit Rate)/Forecasted Visit Rate |
Telemedicine Visit Rates
Data on prepandemic telemedicine visits were not available; therefore, we could not forecast nonpandemic monthly telemedicine visit rates. The telemedicine variable included audio and video visits but not other modes of virtual communication such as portal messages. We descriptively calculated the time series of monthly standardized (30 patient-day) rates of telemedicine visits stratified by disease and treatment type. All statistical analysis was performed using R, version 3.6.1 (R Core Team).
Results
This cohort study included data from 24 261 active patients, with a total of 1 107 457 visits (1 099 354 in-person and 8103 telemedicine) throughout the study period (March 2016 to February 2021). A total of 6737 patients received oral therapy, 15 314 received outpatient infusions, and 8316 received inpatient infusions. The median patient age was 68 years (IQR, 60-75 years), and the population comprised 14 370 men (58%) and 10 248 women (42%). Patients self-identified as Asian (391 [2%]), Black (2383 [10%]), Hispanic (1382 [6%]), White (16 309 [66%]), or other race and ethnicity (2231 [9%]). Race and ethnicity was unknown for 1924 patients (8%). The most common disease was multiple myeloma (7024 patients [29%]; Table 1). The distribution of patients by disease, treatment type, and visit type is found in Table 2.
Table 1. Patient Characteristics.
| Characteristic | Overall cohort (N = 24 620)a | Prepandemic (n = 20 495) | Pandemic (n = 14 248) |
|---|---|---|---|
| Age at initial diagnosis, yb | |||
| Median (IQR) | 68 (60-75) | 69 (60-76) | 68 (59-75) |
| ≤49 | 1940 (8) | 1639 (8) | 1213 (9) |
| 50-64 | 7005 (28) | 5909 (29) | 4270 (30) |
| 65-74 | 8268 (34) | 6892 (24) | 4852 (34) |
| ≥75 | 7407 (30) | 6055 (30) | 3913 (28) |
| Sex | |||
| Women | 10 248 (42) | 8495 (41) | 5966 (42) |
| Men | 14 370 (58) | 11 998 (59) | 8282 (58) |
| Unknown | <5 (<1) | <5 (<1) | 0 |
| Race and ethnicity | |||
| Hispanic or Latino | 1382 (6) | 1173 (6) | 788 (6) |
| Non-Hispanic Asian | 391 (2) | 327 (2) | 219 (2) |
| Non-Hispanic Black or African American | 2383 (10) | 2002 (10) | 1441 (10) |
| Non-Hispanic White | 16 309 (66) | 13 769 (67) | 9342 (66) |
| Otherc | 2231 (9) | 1745 (9) | 1331 (9) |
| Unknown | 1924 (8) | 1479 (7) | 1127 (8) |
| Disease type | |||
| Acute myeloid leukemia | 4556 (19) | 3719 (18) | 1973 (14) |
| Chronic lymphocytic leukemia | 5052 (21) | 4191 (20) | 3720 (26) |
| Diffuse large B-cell lymphoma | 3861 (16) | 3146 (15) | 2431 (17) |
| Follicular lymphoma | 2135 (9) | 1772 (9) | 1234 (9) |
| Mantle cell lymphoma | 1992 (8) | 1666 (8) | 955 (7) |
| Multiple myeloma | 7024 (29) | 6001 (29) | 3935 (28) |
Unless indicated otherwise, values are presented as No. (%) of patients. The N value is the number of unique patients in the cohort or specified period (there is an overlap of patients in the prepandemic and pandemic periods).
Some patients had multiple hematologic neoplasms and thus had different ages at initial diagnosis depending on the disease. These patients were counted multiple times for this characteristic, resulting in a total N value for the characteristic that is greater than that for the cohort.
Specific race and ethnicity categories with lower representation in the US population were grouped as other for deidentification purposes and may include American Indian or Alaska Native, Hawaiian or Pacific Islander, or multiple races and ethnicities.
Table 2. Patient-Visit Counts During the Pandemic Period (March 1, 2020, to February 28, 2021).
| Treatment and visit type | No. of patient visits (%) | ||||||
|---|---|---|---|---|---|---|---|
| Overall (N = 34 179) | AML (n = 5421) | CLL (n = 7085) | DLBCL (n = 4948) | FL (n = 2592) | MCL (n = 2929) | MM (n = 11 204) | |
| Oral therapy | |||||||
| In person | 6734 (19.7) | 560 (10.3) | 2786 (39.3) | 81 (1.6) | 46 (1.8) | 544 (18.6) | 2717 (24.3) |
| Telemedicine | 1047 (3.1) | 57 (1.1) | 544 (7.7) | 12 (<1.0) | 6 (<1.0) | 75 (2.6) | 353 (3.2) |
| Outpatient infusion | |||||||
| In person | 15 440 (45.2) | NAa | 3332 (47.0) | 2999 (61.0) | 1676 (64.7) | 1447 (49.4) | 5986 (53.4) |
| Telemedicine | 1773 (5.2) | NA | 423 (6.0) | 398 (8.0) | 189 (7.3) | 108 (3.7) | 655 (5.8) |
| Inpatient infusion | |||||||
| In person | 8387 (24.5) | 4391 (81.0) | NA | 1246 (25.0) | 607 (23.4) | 716 (24.4) | 1427 (12.7) |
| Telemedicine | 798 (2.3) | 413 (7.6) | NA | 212 (4.3) | 68 (2.6) | 39 (1.3) | 66 (<1.0) |
Abbreviations: AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; NA, not applicable.
For certain diseases, some treatment types were not applicable and thus were not categorized. These included outpatient infusions for AML and inpatient infusions for CLL.
In-Person Visit Rates
Early pandemic months experienced significant reductions in in-person visit rates for oral therapy (March to May 2020) and outpatient infusions (April to May 2020) subgroups over all diseases combined (Figure 2). During this period, the forecasted, nonpandemic in-person visit rate averaged across both treatment types (ie, the expected in-person visit rate if no pandemic had occurred in 2020-2021, based on historical data) was 1.6 visits (95% PI, 1.4-1.7 visits) per patient per month. The actual in-person visit rate was 1.3 visits per patient per month. This was a reduction of 21% (95% PI, 12%-27%).
Figure 2. Visit Rates by Treatment Type During the Prepandemic (March 2019 to February 2020) and Pandemic (March 2020 to February 2021) Periods.
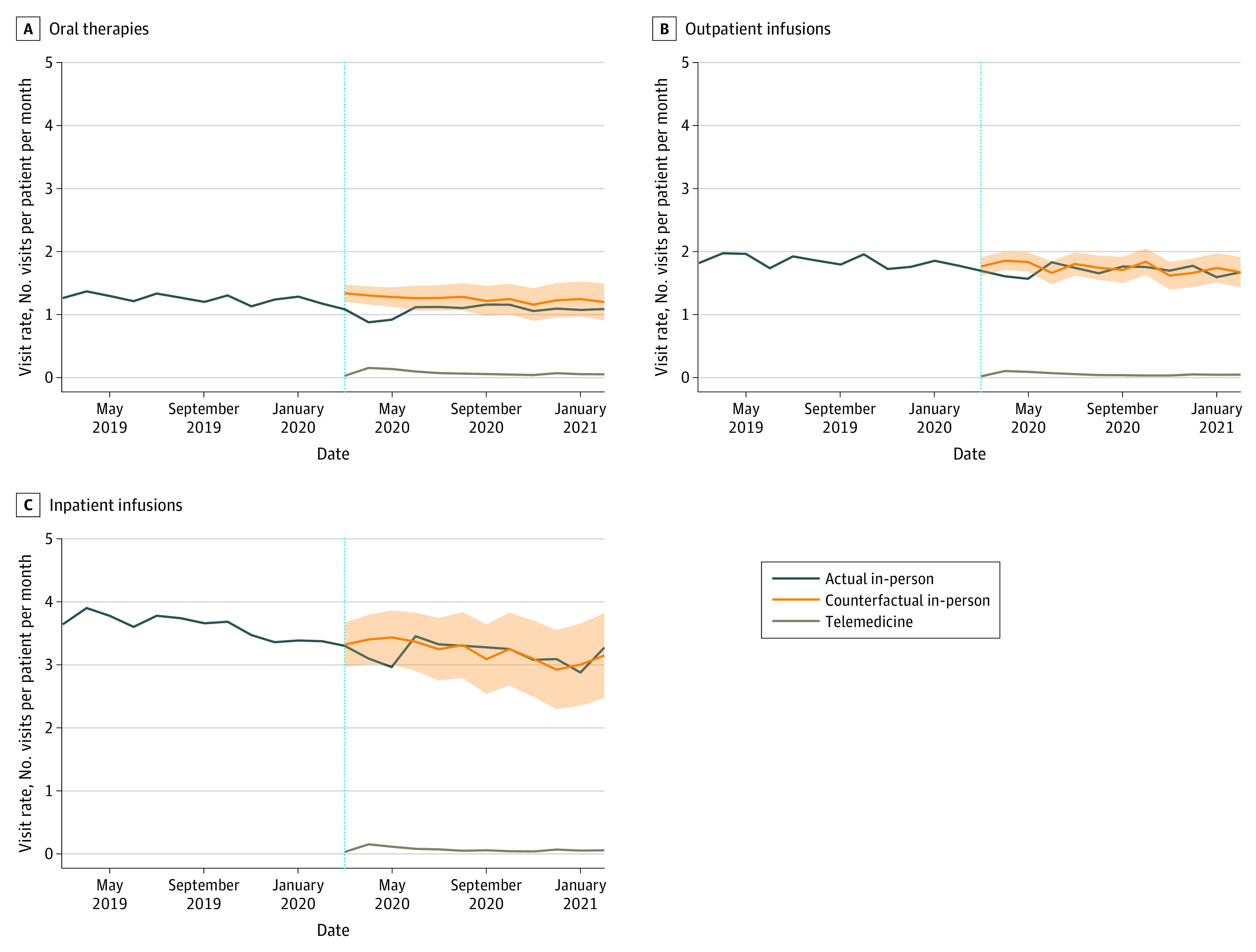
A to C, Actual in-person, forecasted (counterfactual) in-person, and telemedicine visit rates for oral therapies (A), outpatient infusion treatments (B), and inpatient infusion treatments (C). The vertical blue dotted lines indicate the onset of the COVID-19 pandemic; shaded areas indicate the 95% prediction interval.
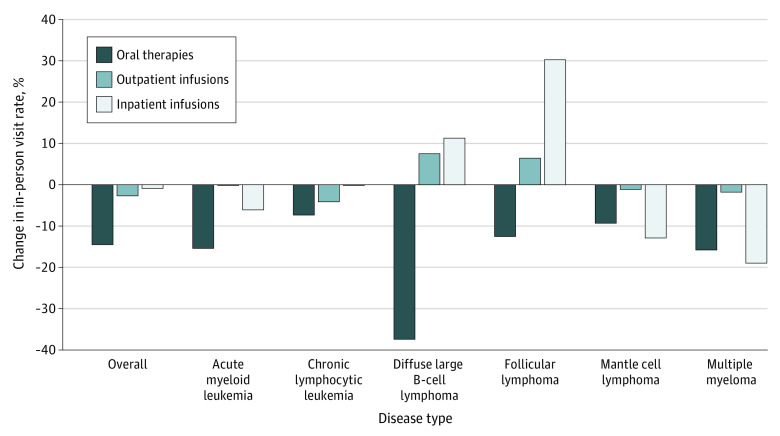
When stratified by disease, patients with multiple myeloma had significant reductions within each treatment type (Figure 3). Between March and May 2020, the observed in-person visit rate for patients receiving oral therapy was 29% lower (95% PI, 21%-36%) than the forecasted visit rate (P = .001). Between April and May 2020, the observed in-person visit rate for patients receiving outpatient infusions was 11% lower (95% PI, 4%-17%) than the forecasted visit rate during the same time period (P = .002). During April and May 2020, the observed in-person visit rate for patients receiving inpatient infusions was 55% lower (95% PI, 27%-67%) than the forecasted visit rate (P = .005). Additionally, significant reductions were observed for patients with chronic lymphocytic leukemia who were receiving oral therapies (28% [95% PI, 12%-39%] reduction, April to May 2020; P = .003) and outpatient infusion therapies (20% [95% PI, 6%-31%] reduction, April 2020; P = .002). Last, patients with mantle cell lymphoma receiving outpatient infusion therapies had a 38% (95% PI, 6%-54%) reduction in in-person visit rates in April 2020 (P = .003).
Figure 3. Percentage Change in In-Person Visit Rates Across an Entire Pandemic Year (March 1, 2020, to February 28, 2021).
The 95% prediction intervals can be found in eTable 4 in Supplement 1.
In-person visit rates did not change substantially during the later pandemic months (June 2020 to February 2021) compared with the forecasted nonpandemic visit rates over all diseases combined across treatment types. During this period, in-person visit rates returned to close to nonpandemic rates for many diseases and treatment types (Figure 2 and eTables 4 and 5 in Supplement 1). Only patients with multiple myeloma exhibited significant reductions in in-person visits related to oral therapy (16% [95% PI, 2%-27%] reduction) from July to August 2020 and in January 2021 and in in-person visits related to outpatient infusions (1% [95% PI, −9% to 9%] reduction) in June 2020 and January 2021.
Telemedicine Visit Rates
During the pandemic period (March 2020 to February 2021), mean (SD) telemedicine visit rates were greatest for patients who received oral therapy (0.07 [0.03] visits per patient per month), followed by inpatient infusions (0.07 [0.03] visits per patient per month) and then outpatient infusions (0.05 [0.01] visits per patient per month) over all diseases combined. Furthermore, we observed higher telemedicine use in the early pandemic months, followed by a decline but persistent nonzero visit rate in pandemic months over all diseases. This trend persisted across all treatment types (Figure 2 and eTable 6 in Supplement 1). This finding is particularly notable for patients receiving oral therapy, among whom in-person visits in the later pandemic period remained lower than forecasted while telemedicine use remained persistent (although lower than during the early pandemic).
Discussion
This cohort study used patient data from a nationwide, EHR-derived deidentified clinical database to assess associations between the COVID-19 pandemic and in-person and telemedicine visit rates among patients with a broad range of indolent and aggressive hematologic neoplasms. Although several studies have evaluated changes in cancer care delivery during the pandemic, most have focused on solid organ tumors and did not evaluate visit rates in relation to therapy type.8,9,10 We observed a significant decline in in-person visit rates for oral therapy and outpatient infusions during the early pandemic period in all hematologic neoplasm subtypes, which later returned to forecasted rates after June 2020. Our results mirror the findings from population-based and registry studies in other ambulatory care settings6,22,23 and reflect disruptions from the stay-at-home orders that were in place in most of the US during that period. A recent study examined trends in utilization of 6 ambulatory care services during the pandemic period and found an initial decrease in care visits, followed by an increase in visit rates.5
We did not observe a similar decline in in-person visits for inpatient infusions for all hematologic neoplasms combined. The latter finding could be explained by the need for patients to see health care providers in person before hospitalization for inpatient administration of antineoplastic therapy. When evaluated by disease, only multiple myeloma had a significant reduction in in-person visits for inpatient infusions during the early pandemic period. This finding is consistent with most treatments for multiple myeloma being administered in the outpatient setting except for cellular therapies, specifically autologous BMT. At the pandemic onset in March 2020, national guidelines were formulated that recommended deferral of elective BMT procedures,24 which may have affected inpatient infusion visit rates for multiple myeloma.
During the pandemic, the US Centers for Medicare and Medicaid Services liberalized reimbursement regulations for telehealth,22 enabling its use for broad patient populations. Our data suggest that telemedicine visit rates were more prevalent for oral therapy compared with outpatient infusions or inpatient infusions. However, overall telemedicine utilization was quite low for this cohort of patients with hematologic neoplasms. The low overall utilization of telemedicine observed here warrants further investigation, especially of socioeconomic barriers to utilization in the community. Recent studies have highlighted that the introduction of telemedicine capabilities may widen disparities to health care access, with disproportionately low utilization among vulnerable groups based on race and ethnicity, sex, and socioeconomic status.23,25 Although telemedicine use declined in the later pandemic months (June 2020 onward), it remained persistent, especially for patients receiving oral therapy. A recent survey-based study showed that patients who initiated telemedicine during routine surveillance visits for gastrointestinal malignant tumors were much more likely to continue using it than those who met their oncologist for the first time on a telemedicine platform.26 Telemedicine use is particularly attractive for patients receiving oral therapeutic agents, but whether it leads to patient satisfaction and delivers similar quality of care for hematologic neoplasms deserves further research.
Strengths and Limitations
Our study has several strengths. We used a large national cohort of clinical patient data through the Flatiron Health Research Database, which included data from a mix of academic and community-based practices. Another strength of the database is the use of technology-enabled abstraction from EHRs, which provides more granular data and minimal data lag compared with other registries or claims-based databases.14,15 Therefore, this data set is positioned to address the questions of pandemic-related changes in patterns of clinical care within a relatively short time frame of the pandemic start. We also used a robust method to forecast visit rates based on the 5-year period preceding the pandemic instead of relying solely on the previous 1-year period. This approach helps overcome any outliers that could arise due to volume variations by chance. Compared with other methods to forecast pandemic mortality rates, ARIMA models have been found to best deal with large data sets and have flexibility to additionally account for seasonal variations.19
Our cohort study also has some limitations. There is expected missingness and loss to follow-up when analyzing clinical observational data, including data for patients who received care outside of the clinic network and the potential for misclassifications in LOT. We did not track the volume of health care practices joining and leaving the care network during the study period, which could potentially affect the number of new patient visits. However, such variation would not affect our primary outcome measure of visit rates, which is a ratio of the visit type to the number of active patients. We did not examine the changes in specific treatments associated with the pandemic. If a telemedicine visit was not documented or coded as such, this could potentially underreport telemedicine visits and mischaracterize these as in-person visits. The method used for time-series forecasting (ARIMA) for this study requires time series to be stationary and thus can have difficulty fitting to data that do not follow a repeated pattern (eg, those with many outliers or structural breaks). However, statistical tests confirmed stationarity before use of ARIMA models. Other time-series models may be more appropriate for data sets that are nonstationary. We did not have data on telemedicine use before the pandemic, which precluded comparison with telemedicine visit rates during the pandemic period. Although we restricted the analysis to patients receiving active therapy only during the study period, we could not rule out the potential difference in telemedicine use between new patients and existing patients. We also did not examine the correlation between decreased visit rates and outcomes, thereby limiting the implications toward policy decisions. Last, our study lacked data on some key factors that are associated with health care and technology access, including the adverse social determinants of health that perpetuate cancer health inequities.27
Conclusions
In summary, the findings of this cohort study of patients with hematologic neoplasms suggest that in-person visit rates for oral therapy and outpatient infusions declined significantly during the early pandemic period, with no significant change in inpatient infusions. In-person visit rates returned close to forecasted rates during the latter part of the study period (June 2020 onward). Additionally, we observed small but nonnegligible utilization of telemedicine during the pandemic, predominantly for patients receiving oral therapy. The reasons for the decline in in-person visits during the pandemic are likely manifold, partly due to changes in national guidelines at the pandemic onset and partly due to the strain on health care systems. Future research is needed to ascertain whether the decline in in-person visits led to adverse patient outcomes. An understanding of how global health crises like pandemics can affect health care delivery models and the role of tools such as telemedicine is critical to minimize future disruptions in oncologic care.
eMethods. (S)ARIMA Models
eFigure. Patient Selection Diagram for the Cohort With Hematologic Malignancies
eTable 1. Regimen Classification Based on National Comprehensive Cancer Network (NCCN) Guidelines as of April 2021, and Also by Consensus of the Authorship Team
eTable 2. ARIMA Model Parameters, (p,d,q)(P,D,Q)m
eTable 3. Q Statistics and P Values for All Ljung-Box Q Tests of the Autocorrelation of ARIMA Model Residuals
eTable 4. Percent Change in In-Person Visit Rates Across Entire Pandemic Year (March 1, 2020, to February 28, 2021)
eTable 5. In-Person Visit Rates in Year Before vs During Pandemic Over All Diseases Combined and by Disease and Treatment Type
eTable 6. Telemedicine Visit Rates During Early vs Later Pandemic Months Over All Diseases Combined and by Disease and Treatment Type
Data Sharing Statement
References
- 1.Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. doi: 10.1056/NEJMc2014816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon MD, McNulty EJ, Rana JS, et al. The COVID-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. doi: 10.1056/NEJMc2015630 [DOI] [PubMed] [Google Scholar]
- 3.Zachrison KS, Yan Z, Schwamm LH. Changes in virtual and in-person health care utilization in a large health system during the COVID-19 pandemic. JAMA Netw Open. 2021;4(10):e2129973. doi: 10.1001/jamanetworkopen.2021.29973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina M, Evans J, Montoy JC, et al. Analysis of emergency department encounters among high users of health care and social service systems before and during the COVID-19 pandemic. JAMA Netw Open. 2022;5(10):e2239076. doi: 10.1001/jamanetworkopen.2022.39076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mafi JN, Craff M, Vangala S, et al. Trends in US ambulatory care patterns during the COVID-19 pandemic, 2019-2021. JAMA. 2022;327(3):237-247. doi: 10.1001/jama.2021.24294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172(11):756-758. doi: 10.7326/M20-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184(1):249-254. doi: 10.1007/s10549-020-05828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkons NR, Kim C, Boedec C, et al. Quantifying the impact of the COVID-19 pandemic on gastrointestinal cancer care delivery. Cancer Rep (Hoboken). 2022;5(1):e1427. doi: 10.1002/cnr2.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawrot K, Shulman LN, Bleiweiss IJ, et al. Time to treatment initiation for breast cancer during the 2020 COVID-19 pandemic. JCO Oncol Pract. 2021;17(9):534-540. doi: 10.1200/OP.20.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riera R, Bagattini ÂM, Pacheco RL, Pachito DV, Roitberg F, Ilbawi A. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311-323. doi: 10.1200/GO.20.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. Preprint posted online January 13, 2020. doi: 10.48550/arXiv.2001.09765 [DOI]
- 15.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. Preprint posted online May 30, 2020. doi: 10.1101/2020.03.16.20037143 [DOI]
- 16.Zhao Y, Howard R, Amorrortu RP, et al. Assessing the contribution of scanned outside documents to the completeness of real-world data abstraction. JCO Clin Cancer Inform. 2023;7:e2200118. doi: 10.1200/CCI.22.00118 [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network . National Comprehensive Cancer Network website. Accessed March 31, 2023. https://www.nccn.org
- 18.Lau K, Hauck K, Miraldo M. Excess influenza hospital admissions and costs due to the 2009 H1N1 pandemic in England. Health Econ. 2019;28(2):175-188. doi: 10.1002/hec.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3(1):37-49. doi: 10.1111/j.1750-2659.2009.00073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung YW, Lai KS. Lag order and critical values of the augmented Dickey–Fuller test. J Bus Econ Stat. 1995;13(3):277-280. doi: 10.2307/1392187 [DOI] [Google Scholar]
- 21.Box G, Jenkins GM, Reinsel GC, Ljung GM. Time Series Analysis: Forecasting and Control. 5th ed. Wiley; 2015. [Google Scholar]
- 22.American Academy of Family Physicians . Using telehealth to care for patients during the COVID-19 pandemic. Updated 2020. Accessed March 31, 2023. https://www.aafp.org/family-physician/patient-care/current-hot-topics/recent-outbreaks/covid-19/covid-19-telehealth.html
- 23.Katz AJ, Haynes K, Du S, Barron J, Kubik R, Chen RC. Evaluation of telemedicine use among US patients with newly diagnosed cancer by socioeconomic status. JAMA Oncol. 2022;8(1):161-163. doi: 10.1001/jamaoncol.2021.5784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waghmare A, Abidi MZ, Boeckh M, et al. Guidelines for COVID-19 management in hematopoietic cell transplantation and cellular therapy recipients. Biol Blood Marrow Transplant. 2020;26(11):1983-1994. doi: 10.1016/j.bbmt.2020.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao CC, McLeod MC, Gleason LT, et al. Inequity in telemedicine use among patients with cancer in the Deep South during the COVID-19 pandemic. Oncologist. 2022;27(7):555-564. doi: 10.1093/oncolo/oyac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasson SP, Waissengrin B, Shachar E, et al. Rapid implementation of telemedicine during the COVID-19 pandemic: perspectives and preferences of patients with cancer. Oncologist. 2021;26(4):e679-e685. doi: 10.1002/onco.13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borno HT, Idossa D, Gomez SL. Policy and health: leveraging a social determinants of health framework to alleviate the impact of the COVID-19 pandemic on patients with cancer. JCO Oncol Pract. 2021;17(3):121-124. doi: 10.1200/OP.20.00822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. (S)ARIMA Models
eFigure. Patient Selection Diagram for the Cohort With Hematologic Malignancies
eTable 1. Regimen Classification Based on National Comprehensive Cancer Network (NCCN) Guidelines as of April 2021, and Also by Consensus of the Authorship Team
eTable 2. ARIMA Model Parameters, (p,d,q)(P,D,Q)m
eTable 3. Q Statistics and P Values for All Ljung-Box Q Tests of the Autocorrelation of ARIMA Model Residuals
eTable 4. Percent Change in In-Person Visit Rates Across Entire Pandemic Year (March 1, 2020, to February 28, 2021)
eTable 5. In-Person Visit Rates in Year Before vs During Pandemic Over All Diseases Combined and by Disease and Treatment Type
eTable 6. Telemedicine Visit Rates During Early vs Later Pandemic Months Over All Diseases Combined and by Disease and Treatment Type
Data Sharing Statement