Abstract
AIDS causes increasing mortality every year. With advancements in nanomedicine, different nanomaterials (NMs) have been applied to treat AIDS and overcome its limitations. Among different NMs, nanoparticles (NPs) can act as nanocarriers due to their enhanced solubility, sustained release, targeting abilities and facilitation of drug–dose reductions. This review discusses recent advancements in therapeutics for AIDS/HIV using various NMs, mainly focused on three classifications: polymeric, liposomal and inorganic NMs. Polymeric dendrimers, polyethylenimine-NPs, poly(lactic-co-glycolic acid)-NPs, chitosan and the use of liposomal-based delivery systems and inorganic NPs, including gold and silver NPs, are explored. Recent advances, current challenges and future perspectives on the use of these NMs for better management of HIV/AIDS are also discussed.
Keywords: antiretroviral therapy, liposomes, nano drug-delivery system, nanomedicine, polymeric and inorganic nanoparticles
Plain language summary
AIDS is a disease affecting many worldwide. Since it is difficult to cure AIDS, new therapies have been developed. Tiny materials called nanoparticles with promising features are used to carry different drugs to relevant organs in the body. There are various nanoparticles with different textures and shapes used in AIDS therapy. Branched nanoparticles, nanoparticles with repetitive building blocks and metal-based nanoparticles are three commonly used nanoparticles in AIDS treatment that are studied in this review. These tiny materials can find the exact place in the body to deliver drugs. They can also reduce the side effects of anti-AIDS drugs and help patients use fewer drugs while getting the same or better results.
Tweetable abstract
In this review, recent advances, current challenges and future perspectives in the use of liposomal-based delivery systems and inorganic nanoparticles (including gold and silver nanoparticles) for better management of HIV/AIDS are critically discussed.
Graphical abstract

AIDS is the regular loss of CD4+ lymphocytes due to infection by HIV, which remains a public health problem worldwide [1]. The disease can transmit from one person to another through blood transfusion, drug injection and birth from infected mothers. This global disease was reported for the first time by the CDC in 1981 and identified in 1983. The virus is a lentivirus in the family retroviridae, subfamily orthoretrovirinae [2,3]. With a genome size of less than 10 kb and few genes, HIV-1 (which is the most significant cause of HIV infection worldwide) can simultaneously use cellular pathways and hide from the immune system [3,4]. The worldwide spread of HIV-1 also shows the virus actively facing innate, adapted and intrinsic immunities [5]. Currently, HIV infection and AIDS remain one of the most important epidemic diseases worldwide. According to WHO, approximately 38 million people had HIV at the end of 2019, with 81% of them being aware of their disease [6].
HIV: a T-cell attacker
The HIV genome encodes eight viral proteins that are critical for its life cycle. The HIV capsid, which is composed of the viral protein p24, encircles its single-stranded RNA. AIDS symptoms start with a decrease in the number of CD4+ T-cells and an increase in p24 levels in the blood. In addition, the virus encodes Vif, Vpr, negative regulatory factor (Nef) and viral protease. Nef is one of its many virulence factors. The small-sided protein influences the human immune system, allowing the spread of the infection and, at the same time, the survival or replication of the pathogen, which is indispensable for HIV-1 replication. The virus also has an envelope glycoprotein (Env) at its surface. Env, which is formed by heterodimers of gp120 and gp41, is responsible for the virus binding to the human host cell receptor CD4 and its coreceptors (mainly CCR5 or CXCR4), resulting in viral entry into the target cells. Gp120 and gp41 are, therefore, considered the best targets for HIV vaccine endeavors [7,8]. HIV can only enter the host cells following the interaction between CD4 molecules on human cells and molecules on the virus surface. Gp120 molecules on the HIV surface initially attach to CD4; CCR5 and CXCR4 then help complete the process by making the attachment more effective, leading to disease progression (Supplementary Figure 1) [9].
Challenges in AIDS treatment
Despite highly active antiretroviral therapy (HAART) being effective in treating HIV patients and reducing morbidity and mortality rates, HIV infection remains a global concern. One of the most important ways of combating such a serious disease is the use of antiretroviral (ARV) drugs. Despite their serious side effects, they are reported to considerably increase the life span of HIV/AIDS patients. According to the National Health Service (NHS), ARV medications inhibit virus replication in the body, giving the immune system another chance to repair itself and prevent further damage. They play a critical role in the fight against HIV by inhibiting HIV protease, blocking HIV maturation, preventing HIV entry, disturbing reverse transcriptase and restraining integrase [10]. This is while most of the existing ARV drugs show undetectable delivery through the blood–brain barrier (BBB) [11]. In low-income countries, the main challenges remain the irregular or lack of access to drugs, poor drug adherence, drug toxicities and drug–food or drug–drug interactions [12]. Moreover, due to HIV's quick adaptation and resistance rate, a combination of HIV drugs is usually needed. It is, therefore, vital to find novel ways to improve AIDS treatment.
Nanomedicine: a potential treatment for AIDS
Although there is no cure or vaccine for HIV/AIDS, nanomedicine has shown promising results for its diagnosis, prevention and treatment [13]. Nanomedicine is the use of nanomaterials (i.e., nanoparticles [NPs]) to improve drug delivery to targeted cells [14]. NPs are generally made of organic or inorganic materials with a size distribution of mostly under 100 nm. NPs are good candidates for drug delivery, inducing immunity and virus detection. There are different types of nanostructure materials, including nanotubes and nanofibers [15]. Thus far, nanomaterials such as dendrimers, liposomes, inorganic NPs and polymeric NPs have been used in HIV disease treatment, whereas quantum dots, carbon-based nanomaterials and metal nanoclusters are applied for diagnosis [11,16]. Nano-sized particles have the ability to solve various challenges in the detection and treatment of HIV. They increase the efficiency of drug delivery by releasing the drugs in the target organ or tissue and increasing the solubility of hydrophobic drugs. Very low concentrations of drugs are therefore needed when NPs are applied. The sustained release and lower toxicity of these drugs (compared with free drugs) are among other advantages of these particles. Antibodies immobilized on the surface of NPs can detect low amounts of antigens, improving the efficiency of diagnostic kits. In this review, the most commonly used NPs in HIV diagnosis and treatment, their mechanisms of action as well as pros and cons are discussed [17].
This review aims to elaborate on the most extensively used NPs in HIV therapy and investigate the recent studies and applications in this area. Polymeric, liposomal and inorganic NPs and their main subtypes are fully discussed, and the most recent studies in this area are investigated. In a novel manner, the similarities and relationships between different NPs are reviewed and visualized as concept maps. Furthermore, clinical/preclinical studies and US FDA-approved NP-based HIV drugs, along with the challenges facing the use NPs, are addressed in this review, which makes it comprehensive and critical compared with previously written articles in the field of HIV therapy. This article can help scientists to better investigate and compare useful NPs for future studies and clinical trials.
Nanomaterials applied for AIDS diagnosis & treatment
Polymeric NPs
Polymeric NPs can be synthesized from either the breakdown of previously synthesized polymers (top-down) or by polymerization of monomers (bottom-up) [18]. Dendrimers and chitosan-based NPs can also be subcategorized as polymeric NPs. With their size varying between 1 and 1000 nm, these particles can be loaded with active compounds entrapped within or decorated on their surface [19]. Polymeric NPs have great potential for targeted drug delivery. These NPs, such as polyethylenimine (PEI) and poly(lactic-co-glycolic acid) (PLGA) NPs, have great potential in delivering nucleic acid-based drugs due to their structure and surface charge. Some authors describe them as having great potential for improving physiochemical drug features. In fact, enhancing drug solubility and stability as well as achieving sustained drug release are the main goals for applying such particles. Using polymeric NPs is a promising strategy to level up the efficiency and safety of ARV treatments [20]. They can play an important role in detecting and blocking gp120, working as a viral mimic (HIV model), carrying siRNAs and ARV drugs, increasing the uptake of drugs and immune response and inhibiting HIV entry and cell-to-cell fusion. Some of these features are discussed further [20,21]. For instance, in recent studies, lipid-wrapped polymeric NPs were used as a virus-mimicking model in macrophages to observe the effect of core stiffness on NP uptake and intracellular fate [22]. Also, mono-sialodihexosylganglioside (GM3)-presenting lipid-coated polymer NPs that mimic HIV-1 particles in macrophages were applied as carriers for delivery and sustained release of two ARVs, rilpivirine and cabotegravir [23]. In the following sections, studies of different polymeric NPs like dendrimers, PEI, PLGA and chitosan NPs are discussed.
Dendrimers
Dendrimers are 3D branched nanopolymers containing a core, several branches and internal cavities. They are known as a subcategory of polymeric NPs despite their unique structure, as they can be prepared in different generations (layers) and be associated with polymeric molecules to obtain specific aims [24,25]. These particles have several functional groups that can attach to pharmaceutical and viral components during the vaccine design process, and their internal cavities have the ability to encapsulate anti-HIV drugs [26]. Dendrimer-based NPs have shown great potential in solving some of the problems with ARV therapy. For example, in the case of latent HIV infection in the CD4+ T-cells that could not be solved by ARVs alone, polyanionic carbosilane dendrimers were used to reduce latent HIV-1 persistence [27]. Also, the use of dendrimers for efavirenz with a booster dose of ritonavir was helpful. This improved bioavailability and compensated for inadequate organ perfusion [28]. Another reason for the failure of most ARVs is the appearance of resistance. Studies on novel dendrimers helped researchers design new types of drugs that do not generate resistance mutations [29].
Poly(amidoamine) dendrimers
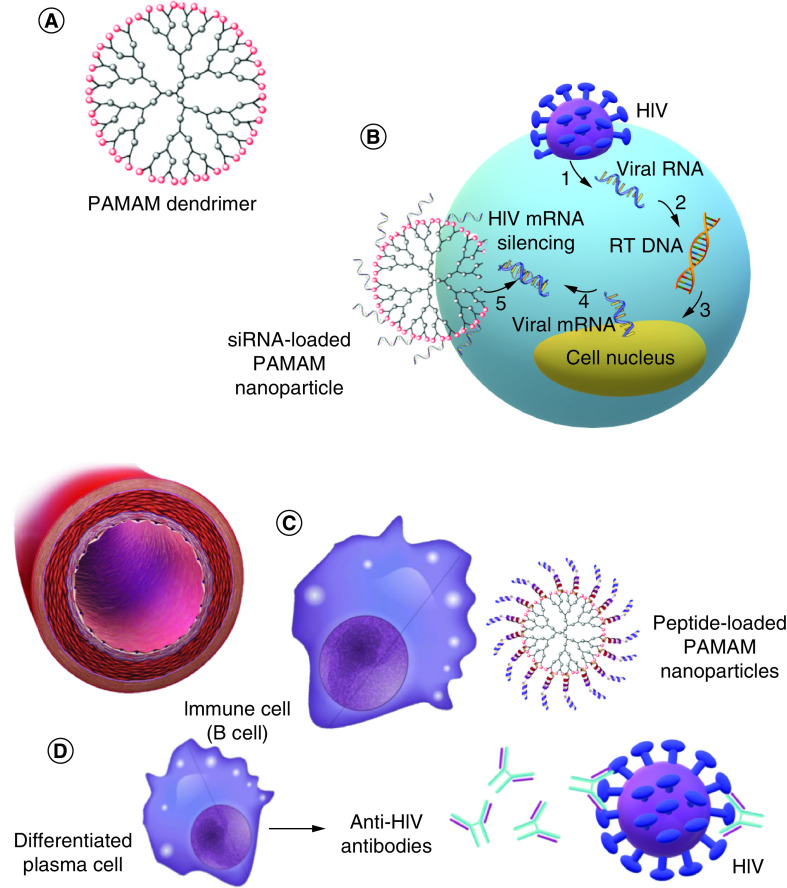
Poly(amidoamine) (PAMAM) is a branched-type dendrimer with a sphere-like shape and internal tree-like branching molecular architect (Figure 1A) [30]. It is mostly made of amides and amines [31,32]. In some studies, different generations of these molecules were designed as carriers, immunogens and adjuvants. PAMAMs are mainly used as recognizing or neutralizing agents against gp120 to stop HIV entry into CD4+ cells [33].
Figure 1. . Applications and properties of dendrimer nanoparticles in HIV therapy.
(A) PAMAM dendrimers, (B) siRNA-loaded PAMAM NPs that silence the HIV mRNA inside host cells to prevent HIV proliferation, (C) peptide-loaded PAMAM NPs that interact with immune cells and induce anti-HIV immune pathways and (D) induction of anti-HIV antibody production in plasma cells.
NP: Nanoparticle; PAMAM: Poly(amidoamine).
In addition to blocking entry, PAMAM NPs can enhance the immune system against HIV. In another study, intranasal immunizing G4-PAMAM was used to multiply antibody responses against HIV-1 gp120 peptide epitopes in BALB/cJ mice [34]. The treatment improved IgA and IgG responses (anti-gp120), suggesting its potential to be used as an adjuvant. The fourth generation of PAMAM dendrimers (G4-PAMAM) is capable of increasing immunoglobulin levels; they are thus the most applied such NPs. In Rodríguez-Fonseca et al.‘s study, two peptides were loaded on these particles to induce antibody production and neutralize gp-120 HIV type-1 (Figure 1C & D) [35]. The authors concluded that these NPs can act as nanocarriers for immunogenic peptides. PEGylated PAMAM NPs can also be used as carriers. They can prolong drug release while increasing safety and reducing toxicity risks by enhancing the encapsulation of HIV drugs. Surface alternation using PEG can lower toxicity and raise the circulation time, resulting in the accumulation of dendrimers at the target sites [36].
Gene delivery is another method to combat HIV (Figure 1B), by targeting both viral and cellular transcripts without causing serious toxicity. Cationic PAMAM dendrimers can protect the body against viral-induced CD4+ T-cell depletion by controlling the transcription process in a RAGhu mouse model through siRNA delivery [37].
To summarize, preventing HIV entrance to CD4+ human cells, encapsulating anti-HIV drugs and peptides and cellular uptake enhancement are options by which PAMAM NPs are used in HIV treatment and delivery systems.
Poly(propyleneimine) dendrimers
Poly(propyleneimine) (PPI), an amine-terminated molecule, is another type of polymeric NP with a branched molecular structure and a discrete number of functional end groups [38,39]. PPI dendrimers as well as their modified forms have shown therapeutic effects. They have the potential to protect anti-HIV antisense oligonucleotides against nucleolytic digestion [40]. Interactions between anti-HIV antisense oligonucleotides and PPI have been monitored using dynamic light scattering and transmission electron microscopy. Zeta potential has been used to characterize various features of these NPs, confirming that the nature of the complex depends on the stoichiometry and concentration of DNA phosphate and dendrimer amines concentration as well as solvent properties and dynamics of mixing [41]. Their use might thus be challenging, as correct assessment of the stoichiometry of DNA phosphate, dendrimer amines and solvent properties is needed.
PPI-based NPs can be used as ARV drug carriers. Dendriplexes, anti-HIV oligonucleotides combined with PPI-Mal G4 (generation 4 PPI modified with maltose) and PPI-Mal III (maltotriose) G4 have the capability to self-assemble. These particles construct 1D and 3D nanostructures with therapeutic properties [42]. Again, gp120 detection plays an important role when it comes to PPI dendrimers. John et al. designed a novel HIV platform based on the detection of specific sequences of DNA and gp120 using G4-PPI/streptavidin. They characterized these particles using scanning electron microscopy, voltammetry and electrochemical impedance spectroscopy. In this platform, a single-stranded 5′-biotinylated probe DNA and B40 aptamer were used for DNA sequence and gp120 protein detection with great selectivity. The team also claimed that it can detect complementary DNA at picomolar concentrations. The detection and blocking of gp120 via PPI dendrimers can protect the cells against HIV and inhibit virus entry, respectively. Among different available PPI dendrimers, the fourth generation is more popular [43].
Other dendrimers
In various investigations, dendrimer NPs have been loaded with different types of drugs or antigens to achieve specific goals. Certain dendrimers have microbicide properties (even when not loaded with drug). They can stop HIV in various stages of infection including attachment, entry, latency and replication. One such dendrimer is SPL7013. It is potent against different strains of HIV, such as CXCR4-(X4) and CCR5-(R5). The complex uses a CXCR4 coreceptor to inhibit HIV entry [44]. In an in silico study, the ability of the dendrimer to attach to the gp120 binding site of CD4+ cells and block gp120-CD4 formation was shown [45]. It was therefore concluded that the dendrimer can prevent the entrance of the virus. It is known that G2-S16 protects the cell against host cell infection through viral attachment and acting directly against the virus; however, Rodríguez-Izquierdo et al. studied the chance of resistance mutation in HIV model cell lines treated with G1-S4 or G2-S16 dendrimers. G1-S4 induced three mutations while G2-S16 caused none, making it a safe HIV-entry inhibitor [46]. The comparison of different generations of anionic dendrimers showed G1C (first generation with carboxylated terminal groups) and G1S (first generation with sulfonate terminal groups) to be capable of effectively blocking HIV-1 from entering the host cells. They were shown to have antiviral properties in both acidic and basic pH environments without causing any noticeable inflammation in the vaginal epithelium [47].
Tyssen et al. used the second (SPL7115) and fourth generation (SPL7013) of DNA dendrimers in an in vivo study to optimize their activity against HIV-1 and herpes simplex virus 2 (HSV-2). SPL7013 was shown to be more effective against HSV and inhibited cell-to-cell fusion by blocking the HIV-1 envelope [48]. SB105-A10-based HIV-1 dendrimers synthesized on a lysine core inhibited HIV-1 infection on peripheral blood mononuclear cells by interfering with the attachment of CXCR4 (X4) and CCR5 (R5) to host cells and their early replication [49]. To study latent HIV-1 persistence, G1-S4, G2-S16 and G3-S16 polyanionic carbosilane dendrimers were combined with latency reversal agents showing a dramatic increase in GFP expression in model monocytes and reduced virus latency [50]. In another study, anti-HIV drugs loaded on the same NPs showed higher efficiency [51]. The second and third generations of polyanionic carbosilane dendrimers with metal cores showed anti-HIV-1 and anti-HIV-2 properties. Their cytotoxicity and antimicrobial features were evaluated in Briz et al.'s study using different anti-HIV drugs. The combination of anti-HIV drugs with dendrimer complexes has shown great synergistic effects against HIV.
To study the effects of X4-HIV-1 tropic cell-to-cell fusion, Guerrero-Beltran et al. investigated the TZM-bl, HEK-293T and HEK-293T cell lines with G1-S4, G2-S16 and G3-S16 dendrimers. They concluded the designed systems to be biocompatible and capable of inhibiting X4-HIV-1 infection. G2-S16 and G1-S4 noticeably decreased syncytia formation in HIV-1 Env-mediated cell-to-cell fusion models [52]. Carbosilane dendrimers are also reported to be able to inhibit viral infection at the entry step. They can inhibit the binding of the virus to the surface of target cells and impede membrane fusion by blocking gp120-CD4 interactions. In fact, polyanionic carbosilane dendrimers have the potential to prevent cell-to-cell HIV transmission [53].
To enhance the solubility and, consequently, cell uptake of the drug, Alfei et al. applied novel water-soluble NPs with low cytotoxicity. Thanks to their water solubility, few complexes permitted the drug to become bioavailable and they had a good pharmacokinetic profile [54]. As a result, they managed to solve the insolubility problem of ARV drugs. Nanocarriers are also used for drug cytotoxicity reduction while maintaining their ARV effects. Pargoo et al. developed a negatively charged nano-biopolymer linear globular G2 dendrimer loaded by two ARV drugs, lamivudine and efavirenz. Their study showed retroviral activity reduction without cell toxicity [55]. Different dendrimers used in HIV treatment are summarized in Table 1.
Table 1. . Dendrimers studied against HIV.
| PAMAM dendrimers | ||||
|---|---|---|---|---|
| Type of nanocarrier | Targeted cells | Cargo | Results and effects | Ref. |
| Sialic acid-PAMAM glycodendrimers | – | – | Sulfo-6 stopped all four HIV-1 strains examined in the low micromolar range | [56] |
| G4-PAMAM-HIV peptide complexes | Ab-secreting plasma cell precursors | pPGT122: DIIGDIRQAH and pVRC03: DGGANNTSNETFR | Immunogenic and effective in mucous membrane via increasing IgG and IgA levels | [34,35] |
| PEGylated PAMAM dendrimers | – | Lamivudine | Controlled and prolonged drug release, reduced toxicity and improved drug loading capacity in PEGylated PAMAM | [36] |
| Cationic PAMAM dendrimers | RAGhu mouse model | siRNAs | System suppressed HIV-1 infection and preserved against virally induced CD4+ cell depletion; inhibiting HIV-1 titers and accumulation of NPs in PBMCs | [57] |
| PPI dendrimers | ||||
|---|---|---|---|---|
| PPIG1-G4 | – | Anti-HIV antisense oligonucleotides: SREV, ANTI TAR, GEM91 | Improving drug delivery by protecting nucleic acid-based drugs via DNA folding | [41] |
| PPI-Mal G4 and PPI-Mal III G4 (modified PPI) | – | ANTI-TAR, GEM91, SREV | Focusing on comparative morphological assessment. Self-assemble of ANTI-TAR and GEM 91 into fibrils. On the contrary, SREV formed quadrilateral-like 3D nanostructures | [42] |
| G4-PPI/streptavidin | Sequence-specific DNA and gp120 | – | Detection of cDNA at picomolar concentrations; Outstanding selectivity in the presence of noncomplementary and mismatch DNA | [43] |
| Other dendrimers | ||||
|---|---|---|---|---|
| SPL7013 | TZM-bl | – | SPL7013 has HIV-1 virucidal properties against X4 and R5X4 but not R5 HIV-1 strains. Use of CXCR4 coreceptor for HIV entry inhibition | [58] |
| SPL7013-gp120-CD4 | In silico study | – | Dendrimer competes with gp120 to attach to gp120 binding region of CD4 | [45] |
| G2-S16 sulfonate dendrimer | MT-2 | – | G1-S4 induced three mutations while G2-S16 caused none, making it a safe alternative as a HIV entry inhibitor | [46] |
| G1C, G2C, G3C G1S, G2S and G3S | TZM.bl | – | Blocks entry of HIV-1 strain by G1C and G1S. Antiviral properties in both acidic and basic pH with no noticeable inflammation | [47] |
| SPL7115 and SPL7013 | MT-2 | – | Inhibiting cell-to-cell fusion by blocking HIV-1 envelope; inhibition of viral entry with parallel potency against X4 and R5 HIV-1 strains | [48] |
| Peptide-derivatized SB105-A10 dendrimer | PBMCs and cervicovaginal histocultures | – | Interfering with attachment and replication of X4 and R5 strains of HIV | [49] |
| Polyanionic carbosilane dendrimers | J89GFP lymphocyte and THP89GFP MDMs | Bryostatin, romidepsin or panobinostat | Increased GFP expression in monocytes and reduced latency persistence of virus by combining NPs with LRAs | [50] |
| G2 and G3 anionic carbosilane dendrimers | PBMCs, TZM-bl, HeLa | Primary R5- and X4-HIV-2 isolates | Inhibits replication of HIV-2 at early stages before integration of virus DNA into human genome | [51] |
| Anionic carbosilane dendrimers | TZM-bl, HEK-293T, HEK-293T | – | Biocompatible system, X4-HIV-1 infection inhibition, decrease in syncytia formation by G1-S4 | [52] |
| G3-S16 and G2-NF16 | PBMDC, MDM, DC, HeLa MAGI P4.R5, TZM.bl | X4-HIV-1NL4.3 or R5-HIV-1NL (AD8) | Aimed at various protein targets of viral envelope and host cells; inhibition of HIV infection | [53] |
| Polycationic polyester-based amino acids-modified hydrophilic and amphiphilic dendrimers | SKOV-3 and MCF7 | O-TC 1 | Bioavailable with a proper pharmacokinetic profile drug; high solubility in water | [54] |
| Negatively charged nano-biopolymer linear globular G2 dendrimer | HEK293 T-cells | Lamivudine and efavirenz | Decreased retroviral activity without cell toxicity | [55] |
BRY: Bryostatin; CD4: Cluster of differentiation 4, glycoprotein T-cell receptor; G1C: First generation with carboxylated terminal groups of dendrimers; G1S: First generation with sulfonate terminal groups of dendrimers; G4-PAMAM: Fourth generation of PAMAM dendrimers; gp120: Glycoprotein 120; PAMAM: Poly (amidoamine); PBMCs: Peripheral blood mononuclear cells; PNB: Panobinostat; PPI: Poly(propyleneimine) dendrimers; PPI-Mal G4: Generation 4 of poly(propyleneimine) modified with maltose; PPI-Mal III G4: Generation 4 of poly(propyleneimine) modified with maltose maltotriose; RMD: Romidepsin; SPL7013: G4 L-lysine dendrimer with DNAA surface groups; SPL7115: Second generation of L-lysine dendrimer with DNAA surface groups.
Poly(ethyleneimine)
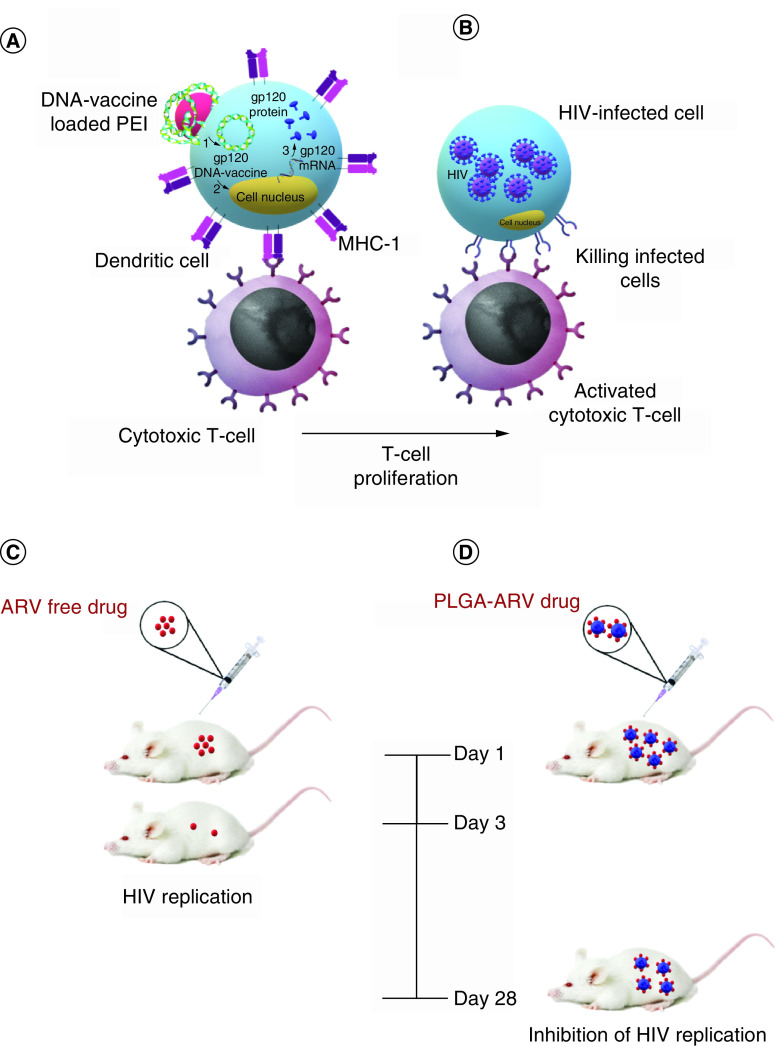
PEI is a polymer containing repeating amine groups with carbon aliphatic CH2CH2 spacers. Three forms of PEI, linear, branched and dendritic have been reported [59]. PEI has several advantages, including high charge density, chain flexibility and low immune response, over other polycations such as L-lysine [60]. PEI NPs, when used as drug carriers, have also helped increase drug uptake, enhance immune responses and improve transfection. Due to the positive charge of PEI NPs, they are promising carriers for delivering different nucleic acid-based drugs and DNA vaccines, since they can be attached electrostatically. The more potent to transfecting cells, the more immune responses in the case of immunotherapy or vaccination will be earned [61,62]. Modified PEI with His-tag peptides in Hauptmann's study increased their uptake in dendritic cells (Figure 2A & B) [63]. The system also facilitated the interaction between NPs and immature dendritic cells. It was thus described as a promising nontoxic carrier for antigen delivery. In another attempt, mannosylated PEI was used to enhance in vitro transfection efficiency in dendritic cells compared with naked DNA [64]. The polymer was therefore suggested as a promising compound in DNA vaccines, directed at the anti-HIV immune response.
Figure 2. . Applications and properties of polymeric nanoparticles in HIV therapy.
(A) DNA-loaded PEI NP as a positively charged polymeric NP for DNA-vaccine transfection into dendritic cells; gp120 DNA-vaccine is translated to gp120 proteins inside dendritic cells and presents to CD8+ T-cells (cytotoxic T-cells) through MHC-1 molecules. (B) After T-cell activation by dendritic cells, mature T-cells will proliferate and attack HIV-infected cells. (C) ARV free drug will circulate and be cleared in hu-CD3-NSG mice three days after injection. (D) PLGA-ARV polymeric NPs increase drug concentration in hu-CD3-NSG mice and delay its clearance until day 28.
ARV: Antiretroviral; NP: Nanoparticle; PEI: Poly(ethyleneimine); PLGA: Poly(lactic-co-glycolic acid).
PEI can also play a significant role in gene and protein delivery. In one example, they were used to deliver siRNA to infected microglial cells in mice, aiming to inactivate suppression of Beclin-1, a host autophagic protein. In fact, siBeclin1 could lower HIV replication and HIV-induced inflammatory responses by activating phosphatidylinositol-3 kinase (PI3KCIII/Vps34) complexes that can facilitate autophagosome fusion with lysosomes. PEI polymers have also been used as carriers for suppressing RNAs and have shown potential in silencing the HIV-infected brain [65].
Magnetic silica nano-complexes coated with PEI were used by Hu et al. to enhance multilayer attachment to NPs. The system was able to detect HIV DNA by delivering the NPs containing virus cDNA [66]. In another attempt, PEG-PEI dendrimers loaded with nucleoside reverse transcriptase inhibitors and delivered to monocyte-derived macrophages harbored in the brain showed high efficiency in inhibiting HIV-1 at very low concentrations [67].
It is possible to stop viral replication by gene suppressors. Using PEG to form PEG-PEI/siRNA, Weber et al. targeted Nef, the HIV gene, and knocked it down by siRNA. The PEG-PEI/siRNA complex was used for stability enhancement and cytotoxicity reduction. The complex knocked down the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), preventing HIV replication [68].
As previously described, increasing the solubility of hydrophobic anti-HIV drugs is one application of NPs. NPs coated with PEG are shown to have high suitability and low toxicity. PEGylated NPs, when used as siRNA carriers, have revealed promising potential in suppressing or knockdown of genes (like GAPDH) that mediate HIV replication. Similar to other NPs, they can be used as carriers of ARV drugs to achieve a more efficient delivery to the targeted organs. PEG dendrimers are also popular, accessible and commonly used in nanosystems [69].
Poly(lactic-co-glycolic acid)
PLGA is a copolymer produced by means of ring-opening copolymerization of two different monomers such as glycolic acid and lactic acid [70]. This polymer is broadly used as a carrier for several ARV drugs, antibodies and suppressing molecules. This is mainly because PLGA-based NPs have almost all the advantages of the previously discussed NPs, plus the capability to enhance binding to target cells. It can also help stop cell-to-cell transmission of the virus by enhancing the antiviral properties of ARV treatment and prolonging drug resistance at the target site.
Yang et al. compared the synergistic properties of two AVRs with PLGA as the carrier [71]. PLGA was loaded with griffithsin and dapivirine and showed strong synergistic properties against HIV-1 at a 1:1 ratio of IC50 values. The use of PLGA helped with the sustained release of drugs. Mandal et al. claimed that tenofovir alafenamide (TAF) and elvitegravi (EVG)-PLGA NPs could cause sustained protection in the mouse model of HIV [72]. They used TAF and EVG via subcutaneous delivery due to their prevention effects. They concluded that their long-acting nanoformulation caused high-level suppression of vaginal HIV-1 infection among hu-CD3-NSG mice (Figure 2C & D).
The combination of ARV-NPs has shown a dramatic increase in the antiviral properties of the drug, reducing its needed dose. This confirms the synergistic effects of these particles compared with free drugs [73]. The particles can be used to detect and combat HIV. PLGA NPs coated with CD7 antibodies were also used to target T-cells [74]. The evaluation of pemetrexed showed that the scFvCD7 and scFvCD7-conjugated particles have a high binding affinity to A3.01 cells. PLGA NPs resulted in no cytotoxicity. In another attempt, elvitegravir was loaded on PLGA-based NPs to suppress HIV in infected monocytes [75]. The results revealed enhanced drug uptake by the monocyte U1 cell line and superior viral suppression for a long time period. Furthermore, PLGA NPs can prevent HIV transmission. The incorporation of HIV-1 inhibitor peptide with PLGA NPs covered with glycol-chitosan can lead to better NP mobility to reach the HIV transmission site in the vaginal epithelium [76]. The combination of PLGA and combination ARV NPs (cART NPs), when loaded with efavirenz, has shown promising results in minimal cell viability and effective inhibition of HIV transduction, both necessary to stop HIV replication [77]. PLGA-ARV NPs loaded with ritonavir, lopinavir and efavirenz were also used for sustained monthly drug release and were effective in inhibiting HIV-1 replication [78]. Similar to other polymeric NPs, PLGA dendrimers can prolong drug release, making the treatment more efficient. Using PEG-PLGA-based NPs loaded with efavirenz, Nunes et al. reported enhanced colorectal availability of EFV, prolonged drug residence at the lower colon and possibly increased protection from HIV transmission (Table 2) [79].
Table 2. . Polymeric nanoparticles studied against HIV.
| PEI nanoparticles | ||||
|---|---|---|---|---|
| Type of nanocarrier | Targeted cells | Cargo | Results and effects | Ref. |
| Ni(II)-nitrilo (triacetic acid)-modified PEI-maltose | iDCs | His6-Gp160, His6-P24 and His6-HIV-TAT | Increase in peptide, no toxicity in iDCs | [63] |
| man-PEI/DNA complex/AdV | DC 2.4 (murine DCs) | DNA complex/AdV (pVAX1-HIV gag plasmid vector) | Higher IgG2a titer, nonsignificant titers of IgG and IgG1, enhanced anti-HIV immune response with lower doses of drug | [64] |
| PEI-FITCsiRNA nanoplexes | Adult C57BL/6 J mice and primary human microglia | siRNA against Beclin1 gene | Silencing Beclin1 gene and lowering expression of targeted protein in brain tissues | [65] |
| Nano-γ-Fe2O3-coated magnetic nanospheres | – | – | Detecting target with high sensitivity; convenient, fast, capable of high anti-interference detecting methods | [66] |
| PEG-cl-(ss) PEI (NG1) and pluronic F68-cl-(ss) PEI (NG2) | MDMs | 5′-TPs of antiviral nucleoside analogues | High efficiency of HIV-1 inhibition at very low concentrations of NTIRs | [67] |
| PLGA nanoparticles | ||||
|---|---|---|---|---|
| PLGA NPs | TZM-bl | Griffthsin and dapivirine | Sustained drug release, synergistic effects of the two drugs | [71] |
| PLGA-based TAF and EVG NPs | Hu-BLT and hu-CD34-NSG mouse model | TAF and EVG | Positive suppression of vaginal HIV-1 infection | [72] |
| ARV-PLGA-NP | TZM-bL | Maraviroc, etravirine, raltegravir | High antiviral properties. Reduced need for effective dosage and blocked virus transmission | [73] |
| ScFvCD7-PLGA/HDACi | A3.01-derived HIV infected cells, ACH2 and human PBMCs | PTX and DOX⋅HCl | More than 80% of binding ability on A3.01 cells, no cytotoxicity | [74] |
| PLGA-EVG | HIV-infected monocytic cell lines U1 and HIV-infected primary macrophages. | Elvitegravir | Concentration-dependent uptake of elvitegravir in monocytes, high suppression of virus compared with control for a long time period | [75] |
| NPsE2-FAM | Vaginal mucosa of swine | HIV-1 inhibitor peptide (E2) | Efficient inhibitory effects on HIV | [76] |
| PLGA-cART NPs | HeLa and H9 T | Efavirenz and boosted lopinavir | Minimal cell viability and effective inhibition of HIV transduction and replication | [77] |
| PLGA-fabricated AR NPs | BALB/c mice | Ritonavir, lopinavir, efavirenz | Sustained monthly treatment for AR drug delivery and inhibition of HIV-1 replication | [78] |
| PEG-PLGA NPs | Colorectal epithelial cell lines (Caco-2, HT29-MTX and SW480) and PBMCs | EFV | Enhanced colorectal availability of EFV, long drug presence at lower colon and possibly increased protection from HIV transmission | [79] |
| Chitosan NPs | ||||
|---|---|---|---|---|
| PLA/chitosan | Mouse fibroblast cell line | Lamivadine | Entrapping and protecting the drugs in acidic pH, sustained drug release in neutral pH | [80] |
| Chitosan-based nanoparticles | – | Zidovudine | By modulating the rate of loading and release of AZT through adjusting the AZT concentration, preparation conditions and molecular characteristics of chitosan | [81] |
| Multiwalled carbon nanotubes-chitosan | MCF7 | Tenofovir | Controlling and enhancing drug loading, its high dispersibility and stability of NPs in aqueous medium, decrease in vitro cytotoxicity of carbon nano tubes | [82] |
| Cs-DTG NPs | C8166 | Dolutegravir | Better therapeutic efficacy and lower cytotoxicity than pure drug, higher dissolution and minimal toxic effects | [83] |
| Chitosan-thioglycolic acid NPs | – | Tenofovir | Enhancement of mucoadhesion properties of the particles by increasing nanoparticle diameter | [84] |
| CsNPs | Macrophage RAW 264.7 and HEK293 | Anti-HIV siRNA | Enhanced siRNA delivery, no cytotoxicity or apoptosis inducing effect | [85] |
| PLGA-CS-LMV NPs and PLGA-CS-NVP NPs | – | Lamivudine and nevirapine | Affordable and accessible. At the end of the burst of the first ARV (LMV), burst of the second ARV (NVP) happens, which is a promising method of HIV-1 infection | [86] |
| Ab-Cs-NPs | HIV-infected brain astrocytes | SiRNA | Improved cell accumulation and gene silencing efficiency of Ab-CS-NPs in astrocytes | [86] |
| Saquinavir-Cs | Jurkat and CEM-CCR5 | Saquinavir | High solution efficiency and great cell targeting efficiency, great control on the virus proliferation in T-cells | [87] |
| Chitosan-O-isopropyl-50-O-d4T monophosphate | MT4 | – | Reduced NRTI side effects and prolonged drug release | [88] |
| CS-NPs | Human lymphocytes | HIV-1 P24 protein-derived peptides | High encapsulation efficiency (>96%), controlled and sustained release of peptide drugs, reduced toxicity and side effects of loaded peptide | [89] |
| Chondroitin-sulfate nanoPECs | PBMCs | Tenofovir | Dose-dependent reduction of HIV-1, decreased IC50 | [90] |
ART: Antiretroviral therapies; ARV: Anti-retroviral; ChonS: Chondroitin-sulfate; CS: Chitosan; CSNP: Chitosan-based nanoparticles; DOX⋅HCl: Doxorubicin hydrochloride; EFV: Efavirenz; ETR: Etravirine; iDC: Immature dendritic cells; LMV: Lamivudine; MDM: Monocyte-derived macrophages; MVC: Maraviroc; Ni-NTA-DG: Ni(II)-nitrilo (triacetic acid)-modified PEI-maltose; NP: Nanoparticle; NTIRs: Nucleoside reverse transcriptase Inhibitors; NVP: Nevirapine; PBMC: Peripheral blood mononuclear cell; PEI: Poly(ethyleneimine); PLA: Poly(lactic acid); PLGA: Poly(lactic-co-glycolic acid)/polylactic acid-polyglycolic acid; RAL: Raltegravir.
Chitosan
Chitosan is a linear polysaccharide made of randomly distributed D-glucosamine and N-acetyl-D-glucosamine units. It can be produced by treating the chitin shells of crustaceans with an alkaline substance [91]. Chitosan has recently gained great attention as an anticancer drug carrier because of its biocompatibility and biodegradability and simple preparation methods for drug-loaded NPs [92]. Chitosan can attach to anti-HIV drug-loaded NPs and control drug release while enhancing drug-loading efficacy. For instance, poly(lactic acid) (PLA)/chitosan loaded with lamivadine showed a lower rate of drug release in acidic pH. NPs based on PLA-chitosan are nontoxic in mouse fibroblast cells [80]. Chitosan-based NPs conjugated with tripolyphosphate are proper carriers for zidovudine. They provide continuous release of zidovudine in vitro and their physicochemical and biological features (specifically mucoadhesive properties) help with zidovudine delivery [81].
Adding chitosan to carbon nanotubes can help with the sustained release of tenofovir along with higher dispersibility and stability of the NPs in aqueous medium [93]. These NPs have also been used for food applications. Chitosan-based NPs loaded with dolutegravir were used as a food and milk admixture for children [83]. Chitosan can also increase the attachment of NPs to the target tissue. Chitosan-thioglycolic acid NPs loaded with tenofovir have shown high mucoadhesion properties when used as a noncytotoxic vaginal drug-delivery system. By increasing the NP diameter, the mucoadhesion properties of the particles were enhanced; the highest encapsulation efficiency belonged to the largest NPs [84].
By combining with other polymeric molecules, such as PEI and PLGA, chitosan can show additional features. The combination of chitosan and PEI enhanced siRNA delivery to model cell lines without causing any cytotoxicity or inducing apoptosis [85]. In another attempt, loading both hydrophobic and hydrophilic ARV treatments on PLGA chitosan-coated NPs resulted in an affordable and accessible nanoformulation (in resource-limited countries), which improved patient outcomes [86]. The sudden release of the second ARV drug (nevirapine) followed that of the first ARV drug (lamivudine), resulting in a promising method for HIV-1 treatment. The use of antibodies to modify chitosan NPs can result in the development of targeted nanodrug delivery systems and increase the drug concentration in the target area. In Gu et al.‘s study, Ab-Cs-NPs could deliver siRNA across the BBB and inhibit HIV replication [94]. Saquinavir-loaded chitosan NPs were studied in Ramana's investigation [87]. High solution and great cell targeting efficiency was shown to effectively control the proliferation of the virus in T-cells.
Chitosan-based NPs have also shown great potential in treating viral diseases by reducing the side effects and toxicity of common drugs. By enhancing the antiviral efficiency of nucleoside reverse transcriptase inhibitors, Yang et al. reduced their side effects and prolonged drug release. They designed nano side monophosphate polymer coated with chitosan and evaluated its anti-HIV activity and cytotoxicity in the MT4 cell line. These particles can be used as prodrugs to improve treatment and reduce side effects [95]. In addition, the toxicity and side effects of p24 were observed when combined with chitosan-based NPs [89]. Loading tenofovir on the particles caused no cytotoxicity to human peripheral blood. The reduction in HIV-1 infection was reported to be dose-dependent (Table 2) [90].
When it comes to biocompatible NPs, chitosan-based NPs are always a great choice for drug delivery. The use of chitosan enhances drug loading and drug-release rate [96]. As mentioned earlier, the attachment of NPs to target tissues is an important factor in increasing the chance of the exact delivery of the drug to the target tissue in the body. Chitosan-based NPs have shown great potential in this regard. Chitosan NPs also lower the toxicity of drugs and their side effects. PLGA dendrimers and chitosan NPs are both great options for drug delivery and both seem to be broadly used.
Nowadays, improvements in polymeric NPs have helped scientists overcome some of their drawbacks and develop some of their efforts as FDA-approved drugs [97]. Using multiple drugs for simultaneous delivery, application of conventional chemotherapeutics combined with other treatment modalities, delivery of drugs in association with photosensitizing agents, nucleic acids and antiangiogenic compounds can increase the efficacy of these systems and improve personalized therapy [98].
Supplementary Figure 2 illustrates a concept map to better review the effects of each polymeric NP on HIV treatment, pointing out those with similar therapeutic properties. For instance, PLGA, PAMAM and chitosan all prolong drug release; PLGA and PPI are both used as ARV drug carriers; chitosan inhibits the replication of HIV mediated by efficient delivery anti-HIV siRNAs while PEI acts via two sequential mechanisms, siRNAs delivery and knocking down Nef. PAMAM stops HIV entrance to the cells via binding to gp120, SPL7013 dendrimer using X4 and R5 HIV strains. G1C, G1S and carbosilane dendrimers, however, stop HIV entry directly.
Liposomes
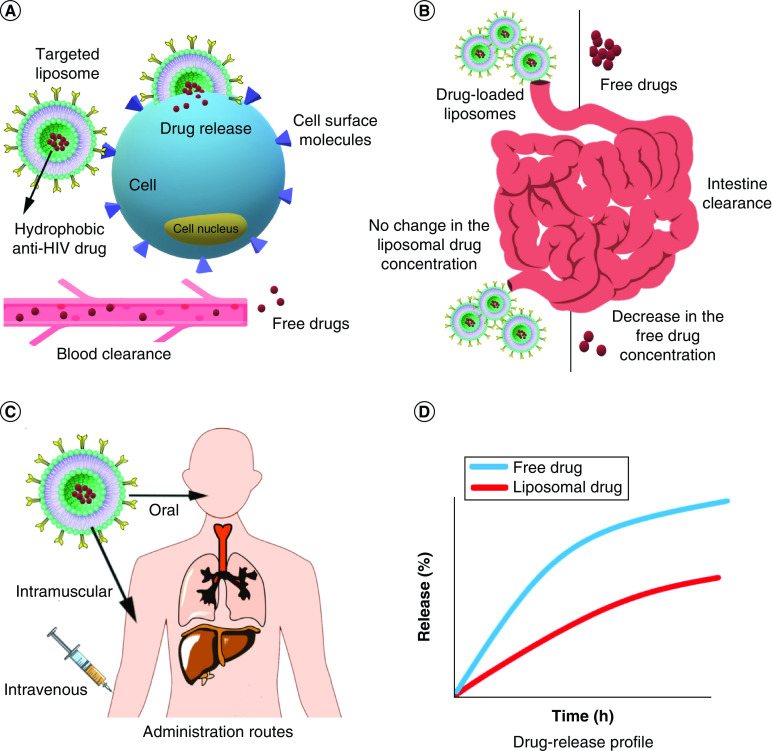
The spherical vesicles commonly used for drug-delivery purposes are called liposomes, as they contain lipid bilayers [12]. Liposomes enhance the efficacy of drug release. Nayak et al. designed a novel system using liposomes in which low doses of NPs (under the WHO recommended dosage) helped with the sustained release of stavudine for up to 12 h while increasing their chances of reaching the reservoir sites (Figure 3A & B) [99]. Magneto-plasmonic liposomes containing tenofovir disoproxil fumarate released 59% of the loaded drug, increased its transmigration efficiency in the BBB and, finally, reduced viral replication through delivering the anti-HIV drug to HIV-infected cells using the guidance of triple-modal imaging [100]. There are ways to control and prevent HIV at the early stages of infection. Zhao et al. studied the targeting of the lymphatic system and the competency of the N15P nano-liposome system (naLipo-N15P) to block HIV entrance based on the antagonistic role of N15P against CXCR4 in HIV infection. It has shown promising results, such as an increase in the specific binding to CXCR4, facilitation of cell targeting, enhancement of anti-HIV properties and blocking of HIV infection [101].
Figure 3. . Applications and properties of liposomal nanoparticles in HIV therapy.
(A) Targeted liposome NPs loaded with hydrophobic anti-HIV drugs increase the drug entrance into cells in two ways: more targeted and more concentrated drug delivery followed by less blood clearance. (B) Anti-HIV drugs' intestine enzyme degradation is much less, carrying in liposomal vehicles instead of being free delivered. (C) Different administration routes for liposomal formulations due to their stability and biocompatibility. (D) Comparison between drug-release profile of free and liposomal drugs.
NP: Nanoparticle.
Octyglyerol-loaded NPs were effective as a vaginal microbiocide against HIV. The system showed higher efficacy, better anti-HSV-1, HSV-2, and HIV activity and higher efficacy against N. gonorrhoeae than other groups. There are no reports of toxicity for the liposomal formulation or lactobacilli [102]. In another study, cell-derived liposomes with CCR5 molecules on their surface were shown to efficiently affect the infected cells, reducing the viability of the targeted cells (gp120-expressing HIV-infected cells) by 60% [103]. Therefore, liposomes can have different effects in terms of increasing or decreasing the toxicity response based on the design of the system.
A PEGylated niosomal formulation was introduced by Nikravesh et al. for tenofovir anti-HIV drug delivery. In this formulation, PEGylated niosomes were incorporated with TAT cell-penetrating peptide, which is the basic region of the trans-activating transcriptional activator protein from HIV-1 and is necessary for the replication of the virus. This nanoformulation increased cell entrance, enhanced the anti-HIV effect and improved the tenofovir release profile in vitro [104]. Lipid NPs can also be utilized to stabilize anti-HIV drugs. In work done by Chauhan et al., two different combinations of anti-HIV drugs with fixed dosages were encapsulated in b-casein micelles. Further encapsulation in industrial microparticles of Eudragit® L100 could successfully prevent these drugs from enzymatic digestion. Such stabilizing nano-micelles can be used in the oral delivery of anti-HIV drugs (Figure 3C & D) [105]. Nanococrystallization of two ARV drugs was done by Witika et al. They cocrystallized lamivudine and zidovudine using sodium lauryl sulfate as an anionic detergent and α-tocopherol polyethylene glycol succinate 1000 as vitamin E derivatives. The results showed less cytotoxicity of the nanoformulation compared with the free drugs alone [106]. These NPs can be effective against HIV through changing amounts of cytokines. Kim et al. have shown HIV-1 suppression and latency reversion by decorating CpG motifs on the surface of uracil-decorated liposomes. CpG oligonucleotides activated dendritic and natural killer cells and also increased both IFN-1 and IFN-γ through toll-like receptor 9 (TLR9) activation. This phenomenon led to anti-HIV responses [107]. Liposomes not only are used for ARV drug delivery but also are used as dual-loaded carriers. In research by Rojekar et al., both etravirine as an ARV drug and selenium NPs (SeNPs) were used as cargo. Loading these two materials together enhanced the intracellular antioxidant and anti-HIV effects by synergic mechanisms. Liposomes were synthesized by solvent evaporation and SeNPs were captured as the core of the whole NP while etravirine was loaded in the lipid shell [108].
Liposome NPs can also be applied for detection purposes. A novel detection method was introduced by Zhuang's team. They designed liposome-based NPs with antibodies against HIV-p24 antigen (p24) on the surface and containing alkaline phosphatase (ALP). The particles could detect p24 by releasing ALP. Adding ascorbic acid 2-phosphate (AA-p) as substrate resulted in the production of ascorbic acid, increasing the photocurrent signals. This method increased detecting signals, showing high sensitivity in detecting the p24 antigen of HIV [109]. Damhorst et al. set up an electrical sensing technique for the detection of biological molecules using ion-encapsulating liposome particles. HIV detection was completed by measuring the number of certain ions released by the liposome (Table 3) [110].
Table 3. . Liposome nanoparticles against HIV.
| Type of nanocarrier | Targeted cells | Cargo | Results and effects | Ref. |
|---|---|---|---|---|
| SG-LP | Raw 264.7 macrophages | Stavudine | Sustained release of stavudine for up to 12 h; increased chance of reaching reservoir sites | [99] |
| Magneto-plasmonic liposomes | Human microglia cell line (CHME-5) and primary human astrocytes | Tenfovir | 59% of drug release, increased transmigration efficiency in BBB and reduced in viral replication | [111] |
| NaLipo-N15P | PBMCs | – | Increase in specific binding to CXCR4, facilitated cell-targeting, enhanced anti-HIV properties and blocked HIV infection | [101] |
| OG liposomes | Vero and CV-1 cell model | Octylglycerol | Higher efficacy, better anti-HSV-1, HSV-2 and HIV activity, better efficacy against N. gonorrhoeae and no toxicity for the liposome against lactobacilli | [102] |
| Cell-derived liposomes | Namalwa or namalwa/ENV | Expressing CCR5 | 60% of viability reduction in targeted cells (gp120-expressing HIV-infected cells) | [103] |
| Lipid-modified uracil | U1 | CpG motifs | Suppressing HIV-1 spreading and latency reversion | [107] |
| Lipid emulsion | JC53-bl (HeLa derivative) | Etravirine and SeNPs | Intracellular antioxidant and anti-HIV effects | [108] |
| ALP-Ls | – | Alkaline phosphatase | Improved signal detection and high sensitivity in detecting p24 antigen of HIV | [109] |
| Ion-encapsulating dipalmitoyl phosphatidylcholine liposomes | – | Ion | No need for bulky and high-cost optical equipment; proper point of care tool and easy to use for detecting different pathogens | [110] |
| PEGylated noisome | HeLa and HEK-293 T-cells | Tenofovir and TAT peptide | Improved tenofovir release profile and lowered anti-Scr HIV effect | [104] |
| b-Casein micelles | – | Tipranavir-efavirenz and darunavir-efavirenz-ritonavir | Enhanced oral delivery of anti-HIV drugs combinations; protected drugs against enzymatic degradation | [105] |
| Nanococrystals | HeLa | Lamivudine and zidovudine | Less cytotoxicity than free drugs | [106] |
ALP: Alkaline phosphatase; bCN: b-Casein; CCR5 (CD195): HIV-co-receptor; CXCR4 (CD184): HIV-co-receptor; EFV: Efavirenz; gp120: Glycoprotein 120; HSV-2: Herpes simplex virus 2; naLipo-N15P: Nano-liposome system; OG: Octyglyerol; PBMC: Peripheral blood mononuclear cell; RTV: Ritonavir, SeNP: Selenium nanoparticle; TPV: Tipranavir.
Polymeric NPs include various types with different features. While they are mostly used for HIV treatment, liposome-based NPs are more efficient in HIV detection. Similar to chitosan NPs, liposomes are biocompatible. By adding antibodies against HIV antigen on the surface of liposomes, these NPs can detect the virus and show specific signals by releasing enzymes or dyes. From among polymeric NPs, PLGA dendrimers and chitosan NPs are the most used NPs in HIV treatment. Also, liposome-based NPs can be used as vaccine candidates or in HIV detection.
Some liposome nanoformulations, like doxil, have made their way to FDA approval. Microfluidic methods have also facilitated the large-scale production of liposomes with uniform size and consistent physicochemical properties. Today, antibody engineering has made multiple targeting possible, which can lead to precise selection in targeting genetically distinct tumor cells, immune cells and cancer stem cells, decreasing the risk of adverse side effects [112].
Inorganic NPs
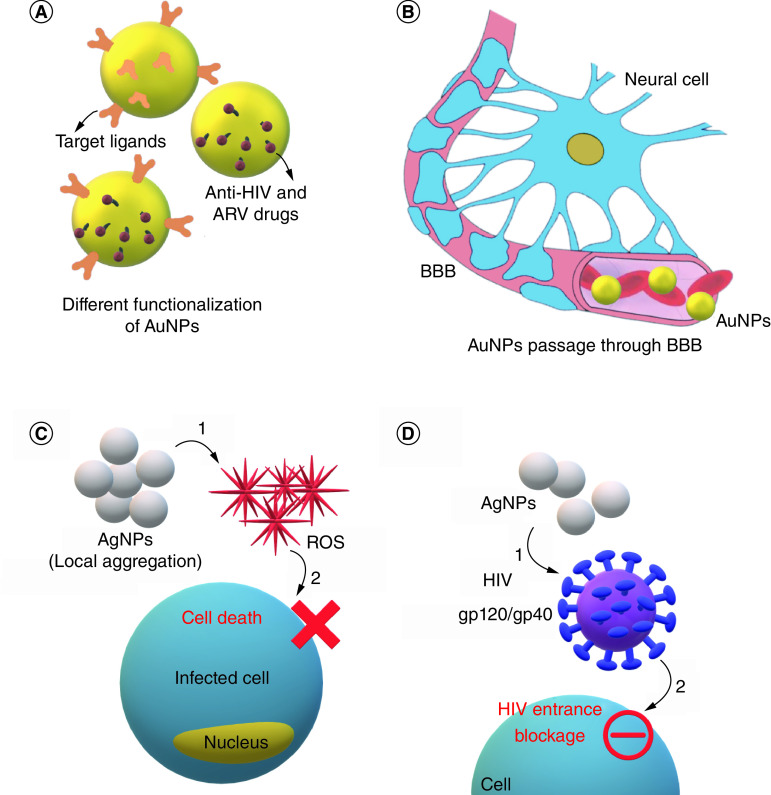
Inorganic NPs are synthetic particles used for different gene and protein delivery in the field of HIV treatment. They mostly consist of metal and metal oxide NPs in sub-100 nm diameters. Despite some concerns about their safety and accumulation in some organs, especially in high doses, they are considered safe and controllable NPs with good modification properties. Gold, silver, iron oxide (magnetic NPs) [113], zinc, nickle copper [114] and silica [115] are some of the inorganic NPs used in HIV treatment. Gold (Au) and silver (Ag) NPs are explained here as they are most common ones [116]. Figure 4 shows different mechanisms through which metal NPs inhibit microbial agents.
Figure 4. . Applications and properties of metal nanoparticles in HIV therapy.
(A) Inorganic NPs (AuNPs and AgNPs) loaded with anti-HIV drugs. (B) AuNPs passage through BBB as a promising drug-delivery vehicle. (C) Local aggregation of AgNPs causes reactive oxygen species formation and, subsequently, HIV-infected cell death. (D) AgNPs have antiviral effects through an unknown mechanism by affecting gp120 and gp40 HIV surface molecules.
AgNP: Silver nanoparticle; AuNP: Gold nanoparticle; BBB: Blood–brain barrier; NP: Nanoparticle; ROS: Reactive oxygen species;.
Gold NPs
AuNPs are versatile metal NPs, known as exciting candidates for diagnosis, as reported in many articles in the last decade [117,118]. This is mainly due to their unique optical and structural properties. AuNPs have increasingly been applied as an imaging tool or even for drug-delivery or therapeutic purposes (Figure 4A) [119,120]. As previously mentioned, despite the advantages of ARV therapies or combinational therapy in improving the quality of life of HIV-positive individuals through targeting the viral replication tactics and suppressing infection, some drawbacks, such as multidrug resistance and insufficient cellular uptake and pharmacokinetic disorders have made researchers look for novel drug-delivery systems. Remarkable size-dependent characteristics of AuNPs, such as their ability to cross the BBB, deep penetration and multivalency or ease of functionalization, which allow AuNPs to bind with a wide range of biomolecules and chemical groups and lower toxicity, have turned AuNPs into a promising drug-delivery tool (Figure 4B) [121–124].
Many studies have demonstrated the synergistic effects of AuNPs with antiviral drugs in developing the ARV medications effective on various pathways, such as replication blockage or viral cycle, viral entry, retrotranscription, integration and maturation. As an example, viral inhibition happens by suppressing the pivotal enzymes in the replication step like integrase inhibitors (raltegravir, peptide or small molecules), RTase (abacavir and lamivudine) and also those hampering virus entry (gp120 blockage agents). Researchers have demonstrated that using a particular hexapeptide, known as an anti-integrase, can affect the conformational changes in the active site of the integrase enzyme that led to hampering the enzyme's activity. On the other hand, antiviral drugs have some drawbacks, such as the inability to enter cells or high degradation, which limit their effectiveness. Some studies have shown that AuNPs are able to improve the pharmaceutical characteristics of drugs and the biostability of peptides against protease activities. The antagonistic interactions with cell receptors underlie the metallic NPs' antiviral effects. The interaction of the virus's surface components with ligands and proteins of the cellular membrane is what allows the virus to enter and adhere to host cells, which is what causes the viral infection. So, the best strategy for designing antiviral medication might be to interfere with the interactions, which will stop the virus from adhering to cells and entering them [125].
In research done by Kesarkar and colleagues, the anti-HIV activity of NPs was investigated. They worked on p-24 antigen inhibition by in vitro ELISA assay as an indication. The results suggested that AuNPs were more effective entry inhibitors than neutralizing agents. The complexity of viral structure could make it difficult to comprehend how NPs interact with dangerous viruses [126]. Some studies have also shown AuNPs and PEGylated AuNPs to have the potential to fight viruses and can thus be used as virus-neutralizing agents (Table 4) [127–132].
Table 4. . Inorganic nanoparticles against HIV.
| Inorganic nanoparticles | ||||
|---|---|---|---|---|
| Gold NPs | ||||
| Type of nanocarrier | Targeted cells | Cargo | Results and effects | Ref. |
| AuNPs-galectin1 nanocomplex | CHME-5/HIV (HIV-transfected human microglial cell cultures) | Galectin 1 | AuNP binding to gelactin-1 to increase the bioavailability and neurotherapeutic potential | [119] |
| Peptide-functionalized AuNPs | TZM-bl | Peptide bonded with hexa-peptide | Peptide-mediated inhibition of integrase | [128] |
| Glucose-coated gold NPs | TZM-bl-infected | Raltegravir | Multifunctional drug-delivery system to enhance integrase inhibitory effects | [129] |
| Dendronized AuNPs | TZM.bl cell line (HeLa, CD4 overexpressed, derivatives) | AuNPs bonded with polyanionic dendrons | Increased antiviral activity of dendrones through occupying binding sites of gp120 associated with targeted cell entry | [130] |
| PEGylated AuNPs | HIV seropositive patient peripheral blood mononuclear cells | – | Pegylated AuNPs act as an entry inhibitor rather than a virus neutralizer | [132] |
| β-diketo acid-coated AuNPs | In vitro study | Surface-grafted β-diketo acid to the AuNPs | Enhanced inhibitory effect of HIV-1 integrase catalytic activities vs free DKA ligands | [133] |
| Aptamers functionalized AuNPs | HEK 293T | RT1t49 (Aptpol) and ODN 93 (AptRH)-functinalized AuNPs | Great potential as HIV-1 RT inhibitor | [134] |
| Efaverinz-AuNP-loaded mannosylated niosomes | HeLa-R5-HIV-1Ba-L virus | AuNPs–EFV coloaded in noisome gel carrier | AuNP and EFV synergistic effects in inhibiting HIV dissemination in PBMCs host cells | [135] |
| Silver NPs | ||||
|---|---|---|---|---|
| AgNPs | HeLa-CD4-LTR-β-gal cells, MT-2 cells, HL2/3 cells, H9 cells, TZM-bl cells | AgNPs coated with PVP | AgNPs inhibit HIV-1 infection through preventing virus entrance to the target cells | [136] |
| AgNPs | U373-MAGI-CXCR4CEM cells | AgNPs with four monoclonal antibodies | Increased neutralizing potency of ABs when combined with AgNPs | [137] |
| AgNPs | cMAGI cells | AgNPs coated with PVP | AgNP binds to gp120 to inhibit HIV-1 entrance | [138] |
AgNP: Silver nanoparticle; AuNP: Gold nanoparticle; CD4: Cluster of differentiation 4, glycoprotein T-cell receptor; DKA: β-diketo acid; EFV: Efavirenz; gp120: Glycoprotein 120; ODN: Antisense oligonucleotide; PBMC: Peripheral blood mononuclear cell; PVP: Poly(N-vinyl-2-pyrrolidone).
Silver NPs
AgNPs have been used as both antibacterial and antiviral elements in subcytotoxic concentrations for a long time, especially by producing reactive oxygen species (ROS; Figure 4C). As for HIV, AgNPs bind to gp120 and gp41 and hamper virus entrance through CD4-dependent viral binding process (Figure 4D) [139]. Some AgNP-based HIV-associated therapeutic products have obtained FDA approval [69,140]. Furthermore, AgNPs are more efficient than silver ions in their antibacterial and antiviral potencies. The difference in the oxidation number of AgNPs (Ag0) and silver ions (Ag+1) makes AgNPs about 12-times more efficient in antibacterial applications. Although AgNPs negatively affect HIV-entrance, silver ions may influence the postentry levels of the HIV cycle in cells due to their electron donation ability [136]. Table 4 shows studies done on AgNPs in HIV treatment.
Nowadays, inorganic NPs such as AuNPs have been applied as contrast agents in bioimaging and radiotherapy. They have shown higher loading efficiency and targeted delivery and more efficient drug release [141]. However, there are several challenges that must be overcome. Despite presenting broad and efficient virus inhibition in both in vitro and in vivo models, accumulation of the Au and AgNPs in some organs like the liver and spleen is still challenging. Furthermore, the preservation of antiviral particle activity when binding to the virus is a significant issue. These problems must be solved in future studies [142].
Supplementary Figure 3 illustrates a concept map representing the effects of various liposomal and inorganic NPs in HIV treatment, displaying their similarities in therapeutic effects. This concept map helps summarize the concept, showing the cause and effect of different NPs in a single view. For instance, liposomes and AuNPs both have BBB transmigration capabilities; the liposomes prevent the entrance of HIV to the cells in two ways: attachment to gp120 and/or enhancing site-directed delivery. AgNPs do this by hampering the entrance mechanisms.
FDA-approved drugs based on nanodelivery systems
According to the guidelines of the US Department of Health and Human Services, 47 FDA-approved drugs exist for HIV treatment, none of which are NP-based [143]. Despite the absence of any approved nano-based drugs for AIDS, three approved nano-based drugs for HIV-related diseases are explained (Supplementary Table 1). Since the immune system of AIDS patients is under attack, diseases such as certain cancers and infections are more probable in these individuals. As a result, it is important to discover drugs to treat such HIV-related diseases.
Kaposi's sarcoma is a cancer caused by human herpesvirus 8 (HHV-8). Patients infected with HIV are at a high risk developing Kaposi's sarcoma. It is the second most prevalent tumor disease in HIV-positive patients in the United States and sub-Saharan Africa [144]. Doxil caelyx and DaunoXome are two drugs delivered by liposomal NPs to treat this disease. Doxorubicin and daunorubicin are the two cargos used in this regard, respectively [145]. Cryptococcal meningitis is a fungal infection mostly reported in patients suffering from immunodeficiency diseases like AIDS [127]. It is the reason for more than 100,000 deaths among HIV-positive patients worldwide [146]. AmBisome is a liposome NP-based drug that carries amphotericin B to treat HIV patients with cryptococcal meningitis [145].
Conclusion
HIV treatment has been a challenge in past decades. Recently, various NP-based treatment methods have been developed to improve the management of these patients. This is while antiviral drugs are still considered the most efficient HIV treatment option, despite their serious side effects, poor drug adherence, toxicity and unwanted interactions with other drugs. Therefore, stopping HIV from entering the body seems the safest option to stop the disease. Most NPs act in this very manner. This is while only NPs with negative charge can pass the skin barrier. It is therefore vital to keep the concentration of NPs' charge high enough to achieve the desired effects. PAMAM, Ag, liposomes and SLP7013 NPs are reported to inhibit the virus from entering the cells and also its proliferation. Early detection and neutralization of the virus are other effective ways to stop HIV before it damages the host cells. Again, PAMAM, AuNPs and AgNPs are shown to be efficient in this regard. The use of PLGA and PPI NPs in infected cells can enhance targeted ARV drug delivery. It is important to ensure these NPs do not cause toxicity in the cells. Some NPs can induce oxidative stress while others have antioxidative properties and thus are safer [147,148]. NPs such as AUNPs can be toxic at high concentrations. Thus, safer NPs like selenium NPs have gained more attention [149].
Toxicology, insufficient effectiveness and undesirable pharmacokinetics are frequently cited causes of phase I and phase II clinical trial failures. In a broader view, the drug leakage and limited capacity of loading in liposomes and hydrophobicity-dependent delivery of drugs delivered by micelles are other causes of their failure. Moreover, scale-up challenges for the industrial production of NPs, the probable toxicity of polymeric and inorganic NPs and inorganic NP accumulation in biological organs are other challenges facing the usage of NPs in HIV treatment. Also, physicochemical-dependent variability of NPs' behaviors like clearance, biodistribution and biocompatibility have been reported in studies that remained unanswered [150]. As a result, additional research must be conducted to address such concerns in the clinical and preclinical phases, followed by the feasibility of large-scale production in later steps.
Future perspective
The nanodelivery approach has been applied by some pharmaceutical companies to develop an effective vaccine against HIV. In this regard, ModernaTX, Inc. started a clinical trial for the first HIV mRNA vaccine in September 2021 [151]. Since mRNA vaccines have shown promising results for the SARS-CoV-2 virus as their first application, many are hopeful that an HIV mRNA vaccine can help defeat AIDS. According to recent developments, for oral administration of ARV medications, a variety of nanomedicine approaches are being investigated. Solid drug NP formulations are one of the current methods for enhancing the bioavailability of medicines that are poorly water-soluble and were recently used to create solid drug NPs of efavirenz compared with the normal Sustiva® product. Since 2011, rilpivirine, a non-nucleoside reverse transcriptase inhibitor, has been offered for oral dosing. Rilpivirine has been recently nanoformulated to create a long-acting injectable format. Rilpivirine concentrations were detected for up to two months in rats and six months in dogs after being injected subcutaneously or intramuscularly [152]. Recently, lopinavir solid drug NPs that have been pro-ethanol-free for pediatric administration have also been prepared in mono- and combinational-drug formulations [153].
In the case of anti-HIV nanodrugs, two recent preclinical cases have been investigated. A research group at Creighton University in 2018 and 2019 demonstrated the clinical effects of PLGA-bicteravir complex and PLGA-tenofovir@elvitegravir@emtricitabine complex. The clinical achievements revealed reliable outcomes including long-acting effects and lower toxicity of the noted drug-delivery system [154]. Also, in the most recent clinical study, long-acting injectable formats of cabotegravir and rilpivirine, using injectable NPs, are being studied in phase III (NCT02951052) [155]. Despite the amazing reported effects of NPs on HIV, most studies have remained theoretical and were never commercialized. This is because many factors should be taken into account before the NP is used in HIV treatment. For instance, changes in the NP size can change its features considerably. This suggests that more research on NPs and their characteristics are needed before these treatments are used in clinical practice.
Executive summary.
Background
AIDS is a worldwide disease with a high incidence rate.
HIV infects the CD4+ lymphocytes of humans.
AIDS is transmitted between humans by biological fluids.
The HIV genome is single-stranded RNA.
HIV has eight viral proteins.
Two of the main HIV proteins responsible for host-cell binding are gp120 and gp41.
Using antiretroviral drugs is the main AIDS therapy component.
The disadvantages of using antiretroviral drugs are their safety issues and poor permeability across the blood–brain barrier.
Nanotechnology is helping AIDS therapy fin terms of better safety and blood circulation and longer life span in HIV patients.
Nanoparticles (NPs) are made of organic or inorganic materials with a size distribution of mostly <100 nm.
NPs are widely used in drug and gene delivery.
Nano drug-delivery systems have advantages like low toxicity, ease of functionalization, high circulation rate, decrease drug dosage and high-efficiency drug/gene delivery.
Nanomaterials applied for AIDS diagnosis & treatment
Dendrimers, polyethylenimine (PEI), poly(lactic-co-glycolic acid) (PLGA) and chitosan are examples of the mostly used polymeric NPs in HIV therapy.
Polymeric NPs enhance drug solubility and stability and achieve sustained drug release.
Chitosan inhibits the replication of HIV, mediated by efficient delivery anti-HIV siRNAs.
Poly(amidoamine) (PAMAM) stops HIV entrance to the cells via binding to gp120 protein.
PLGA, PAMAM and chitosan all prolong drug release.
Dendrimers
Dendrimers are branched NPs with a high capacity to load drugs.
Polyanionic carbosilane dendrimers are used to reduce latent HIV-1 persistence.
PAMAM NPs can enhance the immune system against HIV.
PEGylated PAMAM NPs can enhance the encapsulation of HIV drugs.
Poly(propyleneimine) (PPI) NPs protect anti-HIV antisense oligonucleotides against nucleolytic digestion.
PPI-based NPs can be used as antiretroviral drug carriers.
Dendrimer-based anti-HIV drugs can stop HIV in various stages of infection including attachment, entry, latency and replication.
G2-S16 dendrimers protect the cell against host cell infection through viral attachment.
PEI has several advantages, including high charge density, chain flexibility and low immune response.
PEI is an important polymeric NP used in anti-HIV gene and drug delivery.
PLGA is a polymeric NP capable of increasing drug circulation in the body.
PLGA loaded with griffithsin and dapivirine has strong synergistic properties against HIV-1.
Chitosan has recently gained great attention as a nano drug carrier because of its biocompatibility and biodegradability.
Chitosan can be used for sustained release of drugs.
Liposomes
Liposomes enhance the efficacy of drug release.
By adding antibodies against HIV antigen on the surface of liposomes, these NPs can detect the virus.
Liposomes have good bioavailability and biocompatibility.
Liposome-based drugs have less clearance and enzymatic degradation.
Liposomes can be administered in different ways including oral, subcutaneous and intravenous routes.
Inorganic NPs
Inorganic NPs can be easily surface-modified.
Despite some concerns about inorganic NPs' safety and accumulation in some organs, especially in high doses, they are considered safe and controllable.
Gold NPs have the ability to cross the blood–brain barrier.
Local aggregation of silver NPs causes reactive oxygen species formation and, subsequently, HIV-infected cell death.
Silver NPs are more efficient than silver ions in their antibacterial and antiviral potencies.
Gold NPs have great optical, physical and structural properties.
Gold NPs can be used in anti-HIV targeted drug delivery.
Silver NPs have antibacterial and antiviral properties.
US FDA-approved drugs based on nanodelivery systems
There are 47 FDA-approved drugs for HIV treatment.
There are three approved nano-based drugs for HIV-related diseases.
Nanodrug delivery has good potential for future FDA-approved anti-HIV drugs.
Conclusion
NP-based treatment methods can help address challenges in HIV therapy.
Stopping HIV from entering the body seems the safest option to stop the disease.
PLGA and PPI NPs can enhance targeted antiretroviral drug delivery in HIV-infected cells.
Toxicity and tissue accumulation of some NPs must be considered when used as drug-delivery agents.
Future perspective
There are new and increasing clinical trials of NPs as drug carriers.
Solid drug NP formulations are one of the current methods for enhancing the bioavailability of medicines that are poorly water-soluble.
Investigation of NPs' surface properties is important for their safety and biological functions.
Supplementary Material
Acknowledgments
E Mostafavi would like to acknowledge support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035).
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/nnm-2022-0248
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Oti VB. Nanoparticles and its implications in HIV/AIDS therapy. Curr. Drug Discov. Technol. 17(4), 448–456 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Rodrigo MAM, Heger Z, Cernei N et al. HIV biosensors–the potential of the electrochemical way. Int. J. Electrochem. Sci. 9(7), 3449–3457 (2014). [Google Scholar]
- 3.Milowska K, Rodacka A, Melikishvili S et al. Dendrimeric HIV-peptide delivery nanosystem affects lipid membranes structure. Sci. Rep. 11(1), 16810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.German Advisory Committee Blood (Arbeitskreis Blut), Subgroup Assessment of Pathogens Transmissible by Blood. Human immunodeficiency virus (HIV). Transfus. Med. Hemother. 43(3), 203–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrouz R, Topel CH, Seifi A et al. Risk of intracerebral hemorrhage in HIV/AIDS: a systematic review and meta-analysis. J. Neurovirol. 22(5), 634–640 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Latest HIV Estimates and Updates on HIV Policies Uptake, July 2020 Global HIV, Hepatitis and STI Programmes. In: Global HIV, Hepatitis and STI Programmes. WHO, Geneva, Switzerland: (2020). https://cdn.who.int/media/docs/default-source/hiv-hq/presentation-international-aids-conference-2020_d5a25ad7-6dab-4600-81d8-e52d3ae66b83.pdf?sfvrsn=cbd9bbc_4 [Google Scholar]
- 7.Li G, De Clercq E. HIV genome-wide protein associations: a review of 30 years of research. Microbiol. Mol. Biol. Rev. 80(3), 679–731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seitz R. Human immunodeficiency virus (HIV). Transfus. Med. Hemother. 43(3), 203–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louboutin J-P, Strayer DS. Gene delivery of antioxidant enzymes in HIV-1-associated neurocognitive disorder. In: HIV/AIDS Oxidative Stress and Dietary Antioxidants. Preedy VR, Watson RR (Eds). Academic Press, MA, USA, 107–123 (2018). [Google Scholar]
- 10.Smith RL, de Boer R, Brul S, Budovskaya Y, van der Spek H. Premature and accelerated aging: HIV or HAART? Front. Genet. 3, 328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das MK, Sarma A, Chakraborty T. Nano-ART and NeuroAIDS. Drug Deliv. Transl. Res. 6(5), 452–472 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 58(14), 1532–1555 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Oti VB. Nanoparticles and its implications in HIV/AIDS therapy. Curr. Drug Discov. Technol. 17(4), 448–456 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Mostafavi E, Soltantabar P, Webster TJ. Nanotechnology and picotechnology: a new arena for translational medicine. In: Biomaterials in Translational Medicine. Yang L, Bhaduri SB, Webster TJ (Eds). Academic Press, MA, USA, 191–212 (2018). [Google Scholar]
- 15.Farzin L, Shamsipur M, Samandari L, Sheibani S. Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: a review. Microchimica Acta 185(5), 276 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Saravanan M, Asmalash T, Gebrekidan A et al. Nano-medicine as a newly emerging approach to combat human immunodeficiency virus (HIV). Pharm. Nanotechnol. 06, 17–27 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Magne TM, de Oliveira Vieira T, Alencar LMR et al. Graphene and its derivatives: understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostructure Chem. 12(5), 693–727 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Castro KC, Costa JM, Campos MGN. Drug-loaded polymeric nanoparticles: a review. Int. J. Polym. Mater. 71, 1–13 (2020). [Google Scholar]
- 19.Bhattacharjee S. Polymeric nanoparticles. In: Principles of Nanomedicine. Bhattacharjee S (Ed.). Jenny Stanford Publishing, NY, USA, 195–240 (2019). [Google Scholar]
- 20.Sadaquat H, Akhtar M, Nazir M, Ahmad R, Alvi Z, Akhtar N. Biodegradable and biocompatible polymeric nanoparticles for enhanced solubility and safe oral delivery of docetaxel: in vivo toxicity evaluation. Int. J. Pharm. 598, 120363 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Lin K, Kasko AM. Carbohydrate-based polymers for immune modulation. ACS Macro. Lett. 3, 652–657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshaghi B, Alsharif N, An X et al. Stiffness of HIV-1 mimicking polymer nanoparticles modulates ganglioside-mediated cellular uptake and trafficking. Adv. Sci. 7(18), 2000649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshaghi B, Fofana J, Nodder SB, Gummuluru S, Reinhard BM. Virus-mimicking polymer nanoparticles targeting CD169+ macrophages as long-acting nanocarriers for combination antiretrovirals. ACS Appl. Mater. Interfaces 14(2), 2488–2500 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golshan M, Rostami-Tapeh-Esmail E, Salami-Kalajahi M, Roghani-Mamaqani H. A review on synthesis, photophysical properties, and applications of dendrimers with perylene core. Eur. Polym. J. 137, 109933 (2020). [Google Scholar]
- 25.Akbarzadeh A, Khalilov R, Mostafavi E et al. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Exp. Oncol. 40(3), 178–183 (2018). [PubMed] [Google Scholar]
- 26.Chowdhury S, Toth I, Stephenson RJ. Dendrimers in vaccine delivery: recent progress and advances. Biomaterials 280, 121303 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Relaño-Rodríguez I, Juárez-Sánchez R, Pavicic C, Muñoz E, Muñoz-Fernández MÁ. Polyanionic carbosilane dendrimers as a new adjuvant in combination with latency reversal agents for HIV treatment. J. Nanobiotechnol. 17(1), 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharwade R, More S, Suresh E, Warokar A, Mahajan N, Mahajan U. Improvement in bioavailability and pharmacokinetic characteristics of efavirenz with booster dose of ritonavir in PEGylated PAMAM G4 dendrimers. AAPS PharmSciTech 23(6), 177 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Izquierdo I, Natalia C, García F, de Los Angeles Munoz-Fernandez M. G2-S16 sulfonate dendrimer as new therapy for treatment failure in HIV-1 entry inhibitors. Nanomedicine (Lond). 14(9), 1095–1107 (2019). 10.2217/nnm-2018-0364 [DOI] [PubMed] [Google Scholar]
- 30.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 6(8), 427–436 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Alibolandi M, Hoseini F, Mohammadi M et al. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int. J. Pharm. 549(1), 67–75 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Martins I, Tomás H, Lahoz F, Rodrigues J. Engineered fluorescent carbon dots and G4-G6 PAMAM dendrimer nanohybrids for bioimaging and gene delivery. Biomacromolecules 22(6), 2436–2450 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Akilesh MS, Wadhwani A. Novel applications of nanotechnology in controlling HIV and HSV infections. Curr. Drug Res. Rev. 13(2), 120–129 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Rolando Alberto RF, Martiniano B, Saúl RH et al. In silico and in vivo studies of gp120-HIV-derived peptides in complex with G4-PAMAM dendrimers. RSC Adv. 10(35), 20414–20426 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Fonseca RA, Bello M, de los Muñoz-Fernández MÁ et al. In silico search, chemical characterization and immunogenic evaluation of amino-terminated G4-PAMAM-HIV peptide complexes using three-dimensional models of the HIV-1 gp120 protein. Colloids Surf. B Biointerfaces 177, 77–93 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Kumar PD, Kumar PV, Anneer Selvam TP, Rao KS. Prolonged drug delivery system of PEGylated PAMAM dendrimers with a anti-HIV drug. Res. Pharm. 3(2), 8–17 (2013). [Google Scholar]; • This article showed that dendrimer nanoparticles surface modification with PEG could reduce the toxicity of nanoparticles in HIV therapy.
- 37.Zhou J, Neff CP, Liu X et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 19(12), 2228–2238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agashe HB, Dutta T, Garg M, Jain NK. Investigations on the toxicological profile of functionalized fifth-generation poly(propylene imine) dendrimer. J. Pharm. Pharmacol. 58(11), 1491–1498 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Idris AO, Mamba B, Feleni U. Poly(propylene imine) dendrimer: a potential nanomaterial for electrochemical application. Mater. Chem. Phys. 244, 122641 (2020). [Google Scholar]
- 40.Drzewińska J, Appelhans D, Voit B, Bryszewska M, Klajnert B. Poly(propylene imine) dendrimers modified with maltose or maltotriose protect phosphorothioate oligodeoxynucleotides against nuclease activity. Biochem. Biophys. Res. Commun. 427(1), 197–201 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Pedziwiatr-Werbicka E, Ferenc M, Zaborski M, Gabara B, Klajnert B, Bryszewska M. Characterization of complexes formed by polypropylene imine dendrimers and anti-HIV oligonucleotides. Colloids Surf. B Biointerfaces 83(2), 360–366 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Maly J, Pedziwiatr-Werbicka E, Maly M et al. Highly organized self-assembled dendriplexes based on poly(propylene imine) glycodendrimer and anti-HIV oligodeoxynucleotides. Curr. Med. Chem. 19(27), 4708–4719 (2012). [DOI] [PubMed] [Google Scholar]
- 43.John SV, Rotherham LS, Khati M, Mamba BB, Arotiba OA. Towards HIV detection: novel poly(propylene imine) dendrimer-streptavidin platform for electrochemical DNA and gp120 aptamer biosensors. Int. J. Electrochem. Sci. 9(10), 5425–5437 (2014). [Google Scholar]
- 44.Khan T, Mayuresh Patkar M, Momin M, Omri A. Macrophage targeted nanocarrier delivery systems in HIV therapeutics. Expert Opin. Drug Deliv. 17(7), 903–918 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Saurabh S, Sahoo AK, Maiti PK. Energetics of dendrimer binding to HIV-1 gp120-CD4 complex and mechanismic aspects of its role as an entry-inhibitor. J. Phys. Conf. Ser. 759(1), 012020 (2016). [Google Scholar]
- 46.Rodríguez-Izquierdo I, Natalia C, García F, de los Ángeles Muñoz-Fernandez M. G2-S16 sulfonate dendrimer as new therapy for treatment failure in HIV-1 entry inhibitors. Nanomedicine 14(9), 1095–1107 (2019). [DOI] [PubMed] [Google Scholar]
- 47.MacIel D, Guerrero-Beltrán C, Ceña-Diez R, Tomás H, Muñoz-Fernández MÁ, Rodrigues J. New anionic poly(alkylideneamine) dendrimers as microbicide agents against HIV-1 infection. Nanoscale 11(19), 9679–9690 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Tyssen D, Henderson SA, Johnson A et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLOS ONE 5(8), e12309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bon I, Lembo D, Rusnati M et al. Peptide-derivatized SB105-A10 dendrimer inhibits the infectivity of R5 and X4 HIV-1 strains in primary PBMCs and cervicovaginal histocultures. PLOS ONE 8(10), 1–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Relaño-Rodríguez I, Juárez-Sánchez R, Pavicic C, Muñoz E, Muñoz-Fernández MÁ. Polyanionic carbosilane dendrimers as a new adjuvant in combination with latency reversal agents for HIV treatment. J. Nanobiotechnol. 17(1), 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briz V, Sepúlveda-Crespo D, Diniz AR et al. Development of water-soluble polyanionic carbosilane dendrimers as novel and highly potent topical anti-HIV-2 microbicides. Nanoscale 7(35), 14669–14683 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Guerrero-Beltran C, Rodriguez-Izquierdo I, Serramia MJ et al. Anionic carbosilane dendrimers destabilize the GP120-CD4 complex blocking HIV-1 entry and cell to cell fusion. Bioconjug. Chem. 29(5), 1584–1594 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Vacas-Córdoba E, Maly M, De la Mata FJ, Gómez R, Pion M, Muñoz-Fernández MÁ. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-I. Int. J. Nanomed. 11, 1281–1294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alfei S, Catena S, Ponassi M, Rosano C, Zoppi V, Spallarossa A. Hydrophilic and amphiphilic water-soluble dendrimer prodrugs suitable for parenteral administration of a non-soluble non-nucleoside HIV-1 reverse transcriptase inhibitor thiocarbamate derivative. Eur. J. Pharm. Sci. 124, 153–164 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Pargoo EM, Aghasadeghi MR, Parivar K, Nikbin M, Rahimi P, Ardestani MS. Lamivudine-conjugated and efavirenz-loaded G2 dendrimers: novel anti-retroviral nano drug delivery systems. IET Nanobiotechnol. 15(7), 627–637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clayton R, Hardman J, Labranche CC, McReynolds KD. Evaluation of the synthesis of sialic acid-PAMAM glycodendrimers without the use of sugar protecting groups, and the anti-HIV-1 properties of these compounds. Bioconjug. Chem. 22(10), 2186–2197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou J, Neff CP, Liu X et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 19(12), 2228–2238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Telwatte S, Moore K, Johnson A et al. Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1. Antiviral Res. 90(3), 195–199 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yemul O, Imae T. Synthesis and characterization of poly(ethyleneimine) dendrimers. Colloid Polym. Sci. 286(6–7), 747–752 (2008). [Google Scholar]
- 60.Zakeri A, Kouhbanani MAJ, Beheshtkhoo N et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon. Nano Rev. Exp. 9(1), 1488497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Y, Wu F, Duan W et al. Engineering a “PEG-g-PEI/DNA nanoparticle-in- PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 106, 110294 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Huang Y, Huang H et al. Farnesylthiosalicylic acid-derivatized PEI-based nanocomplex for improved tumor vaccination. Mol. Ther. Nucleic Acids 26, 594–602 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauptmann N, Pion M, Muñoz-Fernández MÁ et al. Ni(II)-NTA modified poly(ethylene imine) glycopolymers: physicochemical properties and first in vitro study of polyplexes formed with HIV-derived peptides. Macromol. Biosci. 13(5), 531–538 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Li M, Jiang Y, Xu C, Zhang Z, Sun X. Enhanced immune response against HIV-1 induced by a heterologous DNA prime-adenovirus boost vaccination using mannosylated polyethyleneimine as DNA vaccine adjuvant. Int. J. Nanomed. 8, 1843–1854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez M, Lapierre J, Ojha CR et al. Intranasal drug delivery of small interfering RNA targeting beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation. Sci. Rep. 7(1), 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu J, Wen CY, Zhang ZL et al. Optically encoded multifunctional nanospheres for one-pot separation and detection of multiplex DNA sequences. Anal. Chem. 85(24), 11929–11935 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Vinogradov SV, Poluektova LY, Makarov E, Gerson T, Senanayake MT. Nano-NRTIs: efficient inhibitors of HIV type-1 in macrophages with a reduced mitochondrial toxicity. Antivir. Chem. Chemother. 21(1), 1–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weber ND, Merkel OM, Kissel T, Muñoz-Fernández MÁ. PEGylated poly(ethylene imine) copolymer-delivered siRNA inhibits HIV replication in vitro. J. Control. Rel. 157(1), 55–63 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Mamo T, Moseman EA, Kolishetti N et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine 5(2), 269–285 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 17(3), 247–289 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Yang H, Li J, Patel SK, Palmer KE, Devlin B, Rohan LC. Design of poly(lactic-co-glycolic acid) (plga) nanoparticles for vaginal co-delivery of griffthsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics 11(4), 1–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mandal S, Prathipati PK, Kang G et al. Tenofovir alafenamide and elvitegravir loaded nanoparticles for long-acting prevention of HIV-1 vaginal transmission. AIDS 31(4), 469 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article proved the long-acting mechanism of poly(lactic-co-glycolic acid) polymeric nanoparticles, their long circulation time and low blood clearance.
- 73.Jiang Y, Cao S, Bright DK et al. Nanoparticle-based ARV drug combinations for synergistic inhibition of cell-free and cell-cell HIV transmission. Mol. Pharm. 12(12), 4363–4374 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Choi S, Lee J, Kumar P, Lee KY, Lee SK. Single chain variable fragment CD7 antibody conjugated PLGA/HDAC inhibitor immuno-nanoparticles: developing human T cell-specific nano-technology for delivery of therapeutic drugs targeting latent HIV. J. Control. Rel. 152(Suppl. 1), e9–10 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Gong Y, Chowdhury P, Midde NM, Rahman MA, Yallapu MM, Kumar S. Novel elvitegravir nanoformulation approach to suppress the viral load in HIV-infected macrophages. Biochem. Biophys. Rep. 12, 214–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ariza-Sáenz M, Espina M, Bolaños N et al. Penetration of polymeric nanoparticles loaded with an HIV-1 inhibitor peptide derived from GB virus C in a vaginal mucosa model. Eur. J. Pharm. Biopharm. 120, 98–106 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Shibata A, McMullen E, Pham A et al. Polymeric nanoparticles containing combination antiretroviral drugs for HIV type 1 treatment. AIDS Res. Hum. Retroviruses 29(5), 746–754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly (DL-lactide-co-glycolide) nanoparticles in mice. J. Antimicrob. Chemother. 65(10), 2183–2187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nunes R, Araújo F, Barreiros L et al. Noncovalent PEG coating of nanoparticle drug carriers improves the local pharmacokinetics of rectal anti-HIV microbicides. ACS Appl. Mater. Interfaces 10(41), 34942–34953 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Dev A, Binulal NS, Anitha A et al. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym. 80(3), 833–838 (2010). [Google Scholar]
- 81.Dahmane EM, Rhazi M, Taourirte M. Chitosan nanoparticles as a new delivery system for the anti-HIV drug zidovudine. Bull. Korean Chem. Soc. 34(5), 1333–1338 (2013). [Google Scholar]
- 82.Afshari R, Mazinani S, Abdouss M. Nanohybrid nanoparticles based on chitosan/functionalized carbon nanotubes as anti-HIV nanocarrier. Nano 10(1), 1–12 (2015). [Google Scholar]
- 83.Priya Dharshini K, Fang H, Ramya Devi D et al. pH-sensitive chitosan nanoparticles loaded with dolutegravir as milk and food admixture for paediatric anti-HIV therapy. Carbohydr. Polym. 256, 117440 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Meng J, Sturgis TF, Youan BBC. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. Eur. J. Pharm. Sci. 44(1–2), 57–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iranpur Mobarakeh V, Modarressi MH, Rahimi P et al. Optimization of chitosan nanoparticles as an anti-HIV siRNA delivery vehicle. Int. J. Biol. Macromol. 129, 305–315 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Makita-Chingombe F, Kutscher HL, DiTursi SL, Morse GD, Maponga CC. Poly(lactic-co-glycolic) acid-chitosan dual loaded nanoparticles for antiretroviral nanoformulations. J. Drug Deliv. 2016, 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramana LN, Sharma S, Sethuraman S, Ranga U, Krishnan UM. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys. Acta Gen. Subj. 1840(1), 476–484 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Yang L, Chen L, Zeng R et al. Synthesis, nanosizing and in vitro drug release of a novel anti-HIV polymeric prodrug: chitosan-O-isopropyl-5′-O-d4T monophosphate conjugate. Bioorg. Med. Chem. 18(1), 117–123 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Alamdaran M, Movahedi B, Mohabatkar H, Behbahani M. In-vitro study of the novel nanocarrier of chitosan-based nanoparticles conjugated HIV-1 P24 protein-derived peptides. J. Mol. Liq. 265(2017), 243–250 (2018). [Google Scholar]
- 90.Wu D, Ensinas A, Verrier B et al. Zinc-stabilized chitosan-chondroitin sulfate nanocomplexes for HIV-1 infection inhibition application. Mol. Pharm. 13(9), 3279–3291 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Suner SS, Sahiner M, Sengel SB, Rees DJ, Reed WF, Sahiner N. Responsive biopolymer-based microgels/nanogels for drug delivery applications. In: Woodhead Publishing Series in Biomaterials. Makhlouf ASH, Abu-Thabit NYBT (Eds). Woodhead Publishing, Sawston, UK, 453–500 (2018). [Google Scholar]
- 92.Zhang E, Xing R, Liu S, Qin Y, Li K, Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 222, 115004 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Afshari R, Mazinani S, Abdouss M. Nanohybrid nanoparticles based on chitosan/functionalized carbon nanotubes as anti-HIV nanocarrier. Nano 10(1), 1–12 (2015). [Google Scholar]
- 94.Gu J, Al-Bayati K, Ho EA. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood–brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 7(4), 497–506 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Yang L, Chen L, Zeng R et al. Synthesis, nanosizing and in vitro drug release of a novel anti-HIV polymeric prodrug: chitosan-O-isopropyl-5′-O-d4T monophosphate conjugate. Bioorg. Med. Chem. 18(1), 117–123 (2010). [DOI] [PubMed] [Google Scholar]
- 96.Karimifard S, Rezaei N, Jamshidifar E et al. pH-responsive chitosan-adorned niosome nanocarriers for co-delivery of drugs for breast cancer therapy. ACS Appl. Nano Mater. 5(7), 8811–8825 (2022). [Google Scholar]
- 97.Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J. Control. Rel. 160(2), 117–134 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Gagliardi A, Giuliano E, Venkateswararao E et al. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 12, 17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nayak D, Boxi A, Ashe S, Thathapudi NC, Nayak B. Stavudine loaded gelatin liposomes for HIV therapy: preparation, characterization and in vitro cytotoxic evaluation. Mater. Sci. Eng. C 73, 406–416 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Tomitaka A, Arami H, Huang Z et al. Hybrid magneto-plasmonic liposomes for multimodal image-guided and brain-targeted HIV treatment. Nanoscale 10(1), 184–194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Y, Sun H, Li X, Mo X, Zhang G. Anti-HIV effect of liposomes bearing CXCR4 receptor antagonist N15P. Trop. J. Pharm. Res. 12(4), 503–509 (2013). [Google Scholar]
- 102.Wang L, Sassi AB, Patton D et al. Development of a liposome microbicide formulation for vaginal delivery of octylglycerol for HIV prevention. Drug Dev. Ind. Pharm. 38, 995–1007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bronshtein T, Toledano N, Danino D, Pollack S, Machluf M. Cell derived liposomes expressing CCR5 as a new targeted drug-delivery system for HIV infected cells. J. Control. Rel. 151(2), 139–148 (2011). [DOI] [PubMed] [Google Scholar]
- 104.Yadavar-Nikravesh M-S, Ahmadi S, Milani A et al. Construction and characterization of a novel tenofovir-loaded PEGylated niosome conjugated with TAT peptide for evaluation of its cytotoxicity and anti-HIV effects. Adv. Powder Technol. 32(9), 3161–3173 (2021). [Google Scholar]
- 105.Singh Chauhan P, Abutbul Ionita I, Moshe Halamish H, Sosnik A, Danino D. Multidomain drug delivery systems of β-casein micelles for the local oral co-administration of antiretroviral combinations. J. Colloid Interface Sci. 592, 156–166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Authors studied a combination of different anti-HIV drugs in micelles and precisely assessed its mechanisms. They developed a flexible and modular technology platform for the oral delivery of fixed-dose combinations.
- 106.Witika BA, Smith VJ, Walker RB. Top-down synthesis of a lamivudine-zidovudine nano co-crystal. Crystals 11(1), 33 (2021). [Google Scholar]
- 107.Kim H, Zhang W, Hwang J et al. Carrier-free micellar CpG interacting with cell membrane for enhanced immunological treatment of HIV-1. Biomaterials 277, 121081 (2021). [DOI] [PubMed] [Google Scholar]
- 108.Rojekar S, Pai R, Abadi LF et al. Dual loaded nanostructured lipid carrier of nano-selenium and Etravirine as a potential anti-HIV therapy. Int. J. Pharm. 607, 120986 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Zhuang J, Han B, Liu W et al. Liposome-amplified photoelectrochemical immunoassay for highly sensitive monitoring of disease biomarkers based on a split-type strategy. Adv. Biosens. Bioelectron. 99, 230–236 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Damhorst GL, Smith CE, Salm EM. A liposome-based ion release impedance sensor for biological detection. Biomed. Microdevices 15(5), 895–905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tomitaka A, Arami H, Huang Z et al. Hybrid magneto-plasmonic liposomes for multimodal image-guided and brain-targeted HIV treatment. Nanoscale 10(1), 184–194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhattacharya S, Saindane D, Prajapati BG. Liposomal drug delivery and its potential impact on cancer research. Anticancer Agents Med. Chem. 22(15), 2671–2683 (2022). [DOI] [PubMed] [Google Scholar]
- 113.Melidis L, Styles IB, Hannon MJ. Targeting structural features of viral genomes with a nano-sized supramolecular drug. Chem. Sci. 12(20), 7174–7184 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khorsandi Z, Metkazini SFM, Heydari A, Varma RS. Visible light-driven direct synthesis of ketones from aldehydes via CH bond activation using NiCu nanoparticles adorned on carbon nano onions. Mol. Catal. 516, 111987 (2021). [Google Scholar]
- 115.Peterhoff D, Thalhauser S, Sobczak JM et al. Augmenting the immune response against a stabilized HIV-1 clade C envelope trimer by silica nanoparticle delivery. Vaccines (Basel) 9(6), 642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jayant RD, Atluri VSR, Tiwari S et al. Novel nanoformulation to mitigate co-effects of drugs of abuse and HIV-1 infection: towards the treatment of NeuroAIDS. J. Neurovirol. 23(4), 603–614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mostafavi E, Zarepour A, Barabadi H, Zarrabi A, Truong LB, Medina-Cruz D. Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: beginning a new era in cancer theragnostic. Biotechnol. Rep. 34, e00714 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jahangiri-Manesh A, Mousazadeh M, Taji S et al. Gold nanorods for drug and gene delivery: an overview of recent advancements. Pharmaceutics 14(3), 664 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gulati S, Singh P, Diwan A, Mongia A, Kumar S. Functionalized gold nanoparticles: promising and efficient diagnostic and therapeutic tools for HIV/AIDS. RSC Med. Chem. 11(11), 1252–1266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barabadi H, Webster TJ, Vahidi H et al. Green nanotechnology-based gold nanomaterials for hepatic cancer therapeutics: a systematic review. Iran. J. Pharm. Res. 19(3), 3–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aalinkeel R, Mangum CS, Abou-Jaoude E et al. Galectin-1 reduces neuroinflammation via modulation of nitric oxide-arginase signaling in HIV-1 transfected microglia: a gold nanoparticle-galectin-1 “nanoplex” a possible neurotherapeutic? J. Neuroimmune Pharmacol. 12(1), 133–151 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Bowman M-C, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 130(22), 6896–6897 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garrido C, Carrie A, Dahl NP et al. Gold nanoparticles to improve HIV drug delivery. Future Med. Chem. 7, 1097–1107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mostafavi E, Medina-Cruz D, Vernet-Crua A et al. Green nanomedicine: the path to the next generation of nanomaterials for diagnosing brain tumors and therapeutics? Expert Opin. Drug Deliv. 18(6), 715–736 (2021). [DOI] [PubMed] [Google Scholar]
- 125.Hameed S, Rehman S. Nanotechnology for Infectious Diseases. Springer, NY, USA: (2022). [Google Scholar]
- 126.Kesarkar R, Oza G, Pandey S et al. Gold nanoparticles: effective as both entry inhibitors and virus neutralizing agents against HIV. J. Microbiol. Biotech. Res. 2(2), 276–283 (2012). [Google Scholar]
- 127.Tenforde MW, Shapiro AE, Rouse B et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst. Rev. 2018(7), CD005647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh L, Kruger HG, Maguire GEM, Govender T, Parboosing R. Development and evaluation of peptide-functionalized gold nanoparticles for HIV integrase inhibition. Int. J. Pept. Res. Ther. 25(1), 311–322 (2019). [Google Scholar]
- 129.Chiodo F, Marradi M, Calvo J, Yuste E, Penadés S. Glycosystems in nanotechnology: gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein J. Org. Chem. 10, 1339–1346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peña-González CE, García-Broncano P, Ottaviani MF et al. Dendronized anionic gold nanoparticles: synthesis, characterization, and antiviral activity. Chemistry 22(9), 2987–2999 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Chiodo F, Enríquez-Navas PM, Angulo J, Marradi M, Penadés S. Assembling different antennas of the gp120 high mannose-type glycans on gold nanoparticles provides superior binding to the anti-HIV antibody 2G12 than the individual antennas. Carbohydr. Res. 405, 102–109 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Res JMB, Kesarkar R, Oza G et al. Gold nanoparticles: effective as both entry inhibitors and virus neutralizing agents against HIV. Jmbronline Com. 2(2), 276–283 (2012). [Google Scholar]
- 133.Sanna V, Youssef MF, Pala N et al. Inhibition of human immunodeficiency virus-1 integrase by β-diketo acid coated gold nanoparticles. ACS Med. Chem. Lett. 11(5), 857–861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shiang YC, Ou CM, Chen SJ et al. Highly efficient inhibition of human immunodeficiency virus type 1 reverse transcriptase by aptamers functionalized gold nanoparticles. Nanoscale 5(7), 2756–2764 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Malik T, Chauhan G, Rath G, Kesarkar RN, Chowdhary AS, Goyal AK. Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif. Cells Nanomed. Biotechnol. 46(Suppl. 1), 79–90 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 8(1), 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Elucidates the mode of antiviral action of silver nanoparticles against HIV-1 using a panel of different in vitro assays. Few studies are available in this area.
- 137.Lara HH, Ixtepan-Turrent L, Garza Treviño EN, Singh DK. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J. Nanobiotechnol. 9(1), 38 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elechiguerra JL, Burt JL, Morones JR et al. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 3(1), 1–10 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malik T, Chauhan G, Rath G, Murthy RSR, Goyal AK. “Fusion and binding inhibition” key target for HIV-1 treatment and pre-exposure prophylaxis: targets, drug delivery and nanotechnology approaches. Drug Deliv. 24(1), 608–621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dunn K, Edwards-Jones V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns 30, S1–S9 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Nejati K, Dadashpour M, Gharibi T, Mellatyar H, Akbarzadeh A. Biomedical applications of functionalized gold nanoparticles: a review. J. Clust. Sci. 33(1), 1–16 (2022). [Google Scholar]
- 142.Chen L, Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 112, 110924 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.HIVinfo.NIH.gov. FDA-approved HIV medicines (2023). https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines
- 144.Gonçalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS 31(14), 1903 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4(3), e10143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review of the history, current clinical landscape and clinical challenges of nanoparticle delivery systems.
- 146.Ruschel MAP, Thapa B. Cryptococcal meningitis. StatPearls Publishing, FL, USA: (2022). www.ncbi.nlm.nih.gov/books/NBK525986/ [PubMed] [Google Scholar]
- 147.European Commission. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) modified opinion (after public consultation) on the appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious. European Commission, Strasbourg, France: (2006). https://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_003b.pdf [Google Scholar]
- 148.Surnar B, Shah AS, Park M et al. Brain-accumulating nanoparticles for assisting astrocytes to reduce human immunodeficiency virus and drug abuse-induced neuroinflammation and oxidative stress. ACS Nano 15(10), 15741–15753 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Davarani Asl F, Mousazadeh M, Azimzadeh M, Ghaani MR. Mesoporous selenium nanoparticles for therapeutic goals: a review. J. Nanopart. Res. 24(10), 1–14 (2022). [Google Scholar]
- 150.Kumar L, Verma S, Prasad DN et al. Nanotechnology: a magic bullet for HIV AIDS treatment. Artif. Cells Nanomed. Biotechnol. 43(2), 71–86 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Laufer D. A phase 1 study to evaluate the safety and immunogenicity of eOD-GT8 60mer mRNA vaccine (mRNA-1644) and Core-g28v2 60mer mRNA vaccine (mRNA-1644v2-Core) (2021). https://clinicaltrials.gov/ct2/show/NCT05001373?term=mrna+vaccine&cond=Human+Immunodeficiency+Virus&draw=2&rank=1
- 152.van’t Klooster G, Hoeben E, Borghys H et al. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob. Agents Chemother. 54(5), 2042–2050 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kraft JC, Mcconnachie LA, Koehn J et al. Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma. AIDS 31(6), 765–770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mandal S, Kang G, Prathipati PK, Fan W, Li Q, Destache CJ. Long-acting parenteral combination antiretroviral loaded nano-drug delivery system to treat chronic HIV-1 infection: a humanized mouse model study. Antiviral Res. 156, 85–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.ClinicalTrials.gov. Study to evaluate the efficacy, safety, and tolerability of long-acting intramuscular cabotegravir and rilpivirine for maintenance of virologic suppression following switch from an integrase inhibitor in HIV-1 infected therapy naive participants (2023). https://beta.clinicaltrials.gov/study/NCT02938520
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.