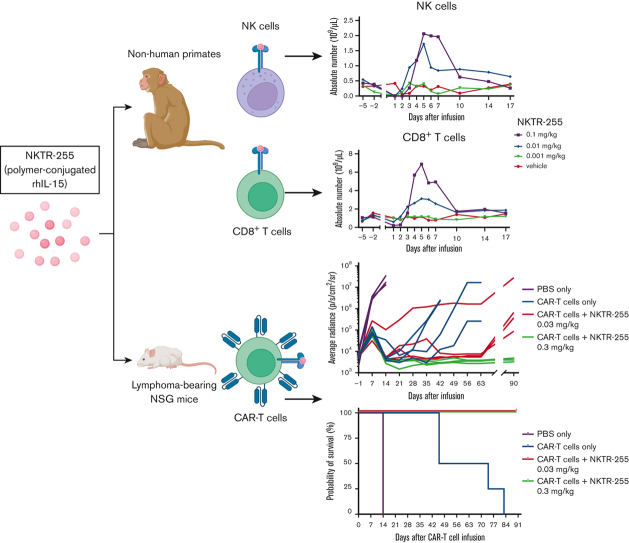
Key Points
-
•
Polymer-conjugated IL-15 (NKTR-255) induced CD8+ and CD4+ T-cell and natural killer cell proliferation in nonhuman primates.
-
•
NKTR-255 enhanced the efficacy of human CD19 CAR T cells in a xenogeneic lymphoma model.
Visual Abstract
Abstract
Chimeric antigen receptor (CAR)–modified T-cell therapies targeting CD19 represent a new treatment option for patients with relapsed/refractory (R/R) B-cell malignancies. However, CAR T-cell therapy fails to elicit durable responses in a significant fraction of patients. Limited in vivo proliferation and survival of infused CAR T cells are key causes of failure. In a phase 1/2 clinical trial of CD19 CAR T cells for B-cell malignancies (#NCT01865617), low serum interleukin 15 (IL-15) concentration after CAR T-cell infusion was associated with inferior CAR T-cell kinetics. IL-15 supports T-cell proliferation and survival, and therefore, supplementation with IL-15 may enhance CAR T-cell therapy. However, the clinical use of native IL-15 is challenging because of its unfavorable pharmacokinetic (PK) and toxicity. NKTR-255 is a polymer-conjugated IL-15 that engages the entire IL-15 receptor complex (IL-15Rα/IL-2Rβγ) and exhibits reduced clearance, providing sustained pharmacodynamic (PD) responses. We investigated the PK and immune cell PDs in nonhuman primates treated with NKTR-255 and found that NKTR-255 enhanced the in vivo proliferation of T cells and natural killer cells. In vitro, NKTR-255 induced dose-dependent proliferation and accumulation of human CD19 CAR T cells, especially at low target cell abundance. In vivo studies in lymphoma-bearing immunodeficient mice demonstrated enhanced antitumor efficacy of human CD19 CAR T cells. In contrast to mice treated with CAR T cells alone, those that received CAR T cells and NKTR-255 had markedly higher CAR T-cell counts in the blood and marrow that were sustained after tumor clearance, without evidence of persistent proliferation or ongoing activation/exhaustion as assessed by Ki-67 and inhibitory receptor coexpression. These data support an ongoing phase 1 clinical trial of combined therapy with CD19 CAR T cells and NKTR-255 for R/R B-cell malignancies.
Introduction
Chimeric antigen receptor (CAR)–modified T-cell therapy represents a new paradigm for the treatment of relapsed/refractory (R/R) B-lineage malignancies and shows promise for the treatment of other tumors. As of June 2022, there are 6 Food and Drug Administration–approved CAR T-cell products.1, 2, 3, 4, 5, 6, 7, 8 Although the outcomes in many studies of CAR T-cell therapy are encouraging, there remains a significant fraction of patients in whom CAR T-cell therapy fails to elicit a durable response.
Limited in vivo proliferation and survival of infused CAR T cells are key causes of failure in CAR T-cell therapy.9, 10, 11, 12, 13, 14, 15, 16 Lymphodepleting chemotherapy is typically administered before adoptively transferring T cells to reduce the levels of endogenous lymphocytes that compete with infused T cells for homeostatic cytokines that maintain T-cell survival.17 Interleukin 15 (IL-15) is one such homeostatic cytokine that promotes proliferation and survival of T cells.18 In patients with R/R B-cell malignancies treated with CD19 CAR T cells, high peak serum IL-15 concentration was associated with response.10,19 These results suggest that IL-15 supplementation could augment CAR T-cell proliferation and/or survival and improve outcomes.
Treatment with native IL-15 is problematic owing to its short half-life (t1/2), resulting in the need to administer high and frequent doses or give continuous infusions.20,21 PEGylation, which involves covalently attaching a polyethylene glycol (PEG) moiety to a molecule, has successfully improved the pharmacokinetics (PK) and pharmacodynamics (PD) of various drugs that are currently used in clinical settings.22,23 NKTR-255 is a novel clinical grade PEG-conjugated IL-15 agonist that engages the entire IL-15 receptor complex (IL-15Rα/IL-2Rβγ), resulting in downstream signaling effects similar to native IL-15 in human mononuclear cells.24 Compared with recombinant human IL-15 (rhIL-15) and precomplexed rhIL-15/IL-15Rα, single-agent NKTR-255 treatment of mice demonstrated enhanced activation of natural killer (NK) and CD8+ T cells and tumor eradication,24 supporting investigation of NKTR-255 for augmenting the antitumor efficacy of CAR T cell in humans. Here, we report our preclinical findings on the PK and immune cell PD of NKTR-255 in nonhuman primates (NHPs) and demonstrate the enhanced in vitro function and in vivo efficacy of human CD19 CAR T cells combined with NKTR-255 than CD19 CAR T cells alone.
Materials and methods
Analyses of clinical trial samples
Serum IL-15 concentrations (Luminex Corporation) and CAR T-cell persistence (quantitative reverse transcription polymerase chain reaction; FlapEF1α copies per μg DNA) were analyzed in 190 patients with R/R B-cell malignancies treated in a phase 1/2 clinical trial of CD19 CAR T cells (#NCT01865617), as previously described.9,15,16,25, 26, 27 The study was conducted according to the principles of the Declaration of Helsinki with approval by the Fred Hutchinson Cancer Center Institutional Review Board.
Reagents and cell lines
rhIL-15 and NKTR-255 were manufactured by Nektar Therapeutics (San Francisco, CA) and formulated in aqueous buffer at near-neutral pH. For in vivo experiments, NKTR-255 was administered in IL-15 protein equivalents, excluding the mass of PEG. Injection volumes were ∼2 mL/kg for cynomolgus monkeys or 5 mL/kg for mice. K562 cells stably expressing CD19 (K562-CD19+) and Raji cells stably expressing firefly luciferase and enhanced green fluorescent protein (eGFP) (Raji-ffluc-eGFP) were generated as previously described.28,29 The cell lines were cultured in RPMI 1640 with 10% fetal bovine serum, 0.71 mM L-glutamine, and 100 U/mL penicillin-streptomycin, and maintained at 37°C and 5% CO2.
NHP studies
NHP studies were conducted under accreditation by the Association for Assessment and Accreditation of Laboratory Animal Care or the Canadian Council on Animal Care. All studies met the ethical and humane criteria for transportation, housing, and care established by the US National Institutes of Health guidelines or Canadian animal care regulators. Young adult male and female cynomolgus monkeys weighing 2.0 to 3.5 kg and 2.2 to 2.5 kg, respectively, at treatment initiation were obtained from the spare colony at the International Toxicology Research Laboratories Canada Inc (Baie d’Urfe, Quebec, Canada). The animals were cohoused in pairs or groups, except during the initial dosing period. A standard diet and water were available ad libitum.
PK evaluation in NHPs
Cynomolgus monkeys received a single dose of NKTR-255 (0.1 mg/kg) either subcutaneous (SC) or IV (2 male animals per group) or a single IV dose of rhIL-15 (0.05 mg/kg) or NKTR-255 (0.1 mg/kg) (1 male and 1 female per group). Peripheral blood was collected at 0.033, 0.25, 1, 4, and 12 hours after rhIL-15 and NKTR-255 administration and at 1, 2, 3, 4, 5, 6, and 7 days after NKTR-255 infusion. Plasma IL-15 concentrations were measured using a qualified enzyme-linked immunosorbent assay method. PK parameters (t1/2, volume of distribution, and clearance) were determined by noncompartmental analysis using Phoenix WinNonlin (version 6.4; Certara Inc, Princeton, NJ).
Flow cytometry in NHPs
Male cynomolgus monkeys (3-4 per group) received a single IV dose of NKTR-255 (0.001, 0.01, or 0.1 mg/kg). Blood samples were collected before NKTR-255 infusion (days −5 and −2) and at multiple intervals following infusion to evaluate absolute cell counts and Ki-67 (BD Biosciences) and pSTAT5 (BD Biosciences) expression in NK cells and CD8+ and CD4+ T cells by flow cytometry. To evaluate IL-15 signaling, fresh whole blood samples from 3 animals were stimulated ex vivo for 20 minutes at 37°C with rhIL-15 or NKTR-255. pSTAT5 was assessed in CD3−CD56+ NK cells, CD3+CD8+ T cells, and CD3+CD4+ T cells by flow cytometry. Data were acquired using an LSRFortessa X-20 or FACSCanto II (BD Biosciences) and analyzed using the FlowJo software (BD Life Sciences).
Human CAR T-cell generation
CD8+ and CD4+ T cells were isolated from healthy donor leukaphereses using the EasySep Human T Cell Isolation Kit (STEMCELL Technologies). Isolated CD8+ or CD4+ T cells were activated using Dynabeads Human T-Activator CD3/CD28 (Thermo Fisher Scientific) and cultured in RPMI 1640 with 10% human serum, 3.51 mM L-glutamine, 0.05 mM β-mercaptoethanol, 100 U/mL penicillin-streptomycin, and 50 IU/mL IL-2 (PeproTech). After 24 hours, activated T cells were spinoculated at 800g and 32°C for 90 minutes with a lentivirus encoding a CAR comprising an FMC63-derived CD19-specific single-chain variable fragment (scFv), 4-1BB costimulatory domain, and CD3ζ signaling domain. A truncated cell-surface human epidermal growth factor receptor (EGFRt) was separated from the CAR using a ribosomal skip sequence to enable the detection of transgene expressing cells by flow cytometry. Dynabeads were removed on day 5. Transduced EGFRt+CD8+ and EGFRt+CD4+ T cells were flow-sorted (BD FACSAria or Sony MA900) on day 7 and expanded by coculture with irradiated allogeneic CD19+ lymphoblastoid cell line for 8 days. Media were changed every 2 days. CAR T cells were used for further experiments on days 14 to 16.
Flow cytometry of human CAR T cells
CAR T-cells or single-cell suspension of mouse bone marrow were stained with live dead fixable AmCyan or Violet (Life Technologies) in phosphate-buffed saline (PBS) at 4°C. Cells were washed with staining buffer (PBS/2% fetal bovine serum) and blocked with human FcR (Miltenyi Biotec) and/or mouse FcR (BioLegend). Surface staining (CD45, CD3, CD8, CD4, anti-EGFR, PD-1, and TIM-3) was performed using a Brilliant Stain Buffer (BD Biosciences). For intracellular staining, the cells were fixed and permeabilized using a BD Cytofix/Cytoperm Solution Kit (BD Biosciences) and stained with BCL-2 and Ki-67 antibodies (BD Biosciences). Cells were washed and resuspended in Perm/Wash Buffer containing Liquid Counting Beads (BD Biosciences). Data were acquired using LSR II, FACSymphony A5, or LSRFortessa X-50 (BD Biosciences) and analyzed using FlowJo software.
To assess STAT5 phosphorylation, CAR T cells were washed twice with PBS, resuspended in IL-2–free media at 2 × 106 cells per mL, and incubated overnight (humidified incubator, 37°C, and 5% CO2). Dead cells were removed by density gradient centrifugation using a Histopaque-1077 (Sigma-Aldrich). CAR T cells were cultured (20 minutes at 37°C) with rhIL-15 or NKTR-255, fixed with BD Cytofix Fixation Buffer (BD Biosciences) for 20 minutes at 37°C, washed with staining buffer, and permeabilized at −20 °C with 100% methanol before incubation (30 minutes at 4°C) with pSTAT5 antibody (BD Biosciences). Data were acquired using a FACSCelesta (BD Biosciences) and analyzed using FlowJo software.
Assessment of proliferation of human CAR T cells
CAR T cells were labeled with 0.5 μM carboxyfluorescein succinimidyl ester (CellTrace, Thermo Fisher Scientific) and cocultured with irradiated (15 000 cGy) K562-CD19+ cells at different effector to target (E:T) ratios and concentrations of NKTR-255. Proliferation was assessed using carboxyfluorescein succinimidyl ester dilution after 96 hours. Liquid counting beads were used to determine the absolute cell counts. Flow cytometry data were acquired using FACSCelesta and analyzed using FlowJo software.
Human CAR T-cell immunotherapy of lymphoma-bearing immunodeficient mice
Mouse experiments were approved by the Fred Hutchinson Cancer Center Institutional Animal Care and Use Committee. Six- to 8-week-old NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD/ severe combined immunodeficiency/γc−/−) (NSG) mice were bred in house. The mice were engrafted with 5 × 105 Raji-fflu-eGFP cells via tail vein injection. Six to 7 days later, tumor-bearing mice received 0.8 × 106 CD8+ and CD4+ CD19 CAR T cells at a 1:1 ratio. NKTR-255 or buffer was injected via the tail vein at the designated time points. Bioluminescence imaging was performed using a Xenogen IVIS imaging system and luciferase activity was analyzed using Living Image software.30 The dosing and frequency of NKTR-255 infusions were selected based on PK data in mice.24 Blood was collected via retro-orbital bleeding. Single-cell bone marrow suspensions were obtained by flushing bilateral femurs and tibias. Red blood cells were lysed with ACK Lysing Buffer (Thermo Fisher Scientific) and washed with PBS before immunophenotyping.
Statistical analysis
A proportional odds logistic regression was used for multivariable modeling of CAR T-cell kinetics according to peak serum IL-15 concentrations in patients treated with CD19 CAR T cells using RStudio software (RStudio, Boston, MA). Other statistical analyses were performed using Prism software (GraphPad, San Diego, CA). Half maximal effective concentration (EC50) values were calculated using 4-parameter nonlinear regression curves. The area under the curve (AUC) was calculated using the trapezoidal rule. Two-tailed Mann-Whitney, unpaired 2-tailed t test, or log-rank tests were used for comparisons. P < .05 was considered statistically significant.
Results
Serum IL-15 concentration is independently associated with longer CD19 CAR T-cell persistence in humans
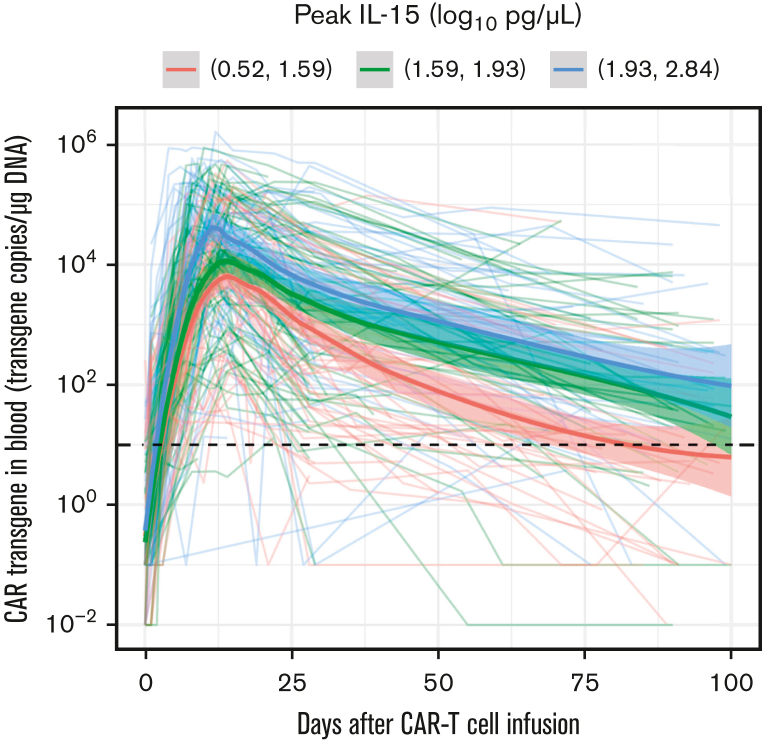
We and others have previously reported that a higher peak serum IL-15 concentration after CD19 CAR T-cell infusion was associated with better clinical outcomes in patients with R/R B-cell lymphomas in univariate analysis.10,16 Using multivariable modeling, adjusting for disease type, prelymphodepletion serum lactate dehydrogenase, and type of lymphodepletion, we found that a higher peak serum IL-15 concentration after CAR T-cell infusion correlated with a higher CAR transgene AUC from days 0 to 90 (AUC0-90; odds ratio, 4.88; P = .002) (Figure 1). The median time to peak serum IL-15 levels was 1 day after CAR T-cell infusion (range, 0-3 days). These data demonstrate an association between endogenous IL-15 concentration and in vivo CAR T-cell counts, providing a rationale for IL-15 supplementation to augment CD19 CAR T-cell efficacy.
Figure 1.
Higher peak of IL-15 is associated with a higher CD19 CAR T cell AUC0-90 in humans. The CAR transgene (FlapEF1⍺ copies per μg of DNA) is shown as a polynomial regression line using the locally estimated scatterplot smoothing method (bold lines) for 3 distinct tertiles of serum IL-15 peak concentration. The shaded areas represent the 95% confidence interval of the locally estimated scatterplot smoothing estimates. The dashed line indicates the limit of quantitation of the CAR transgene quantitative polymerase chain reaction assay.
NKTR-255 promotes T-cell and NK cell accumulation in NHPs
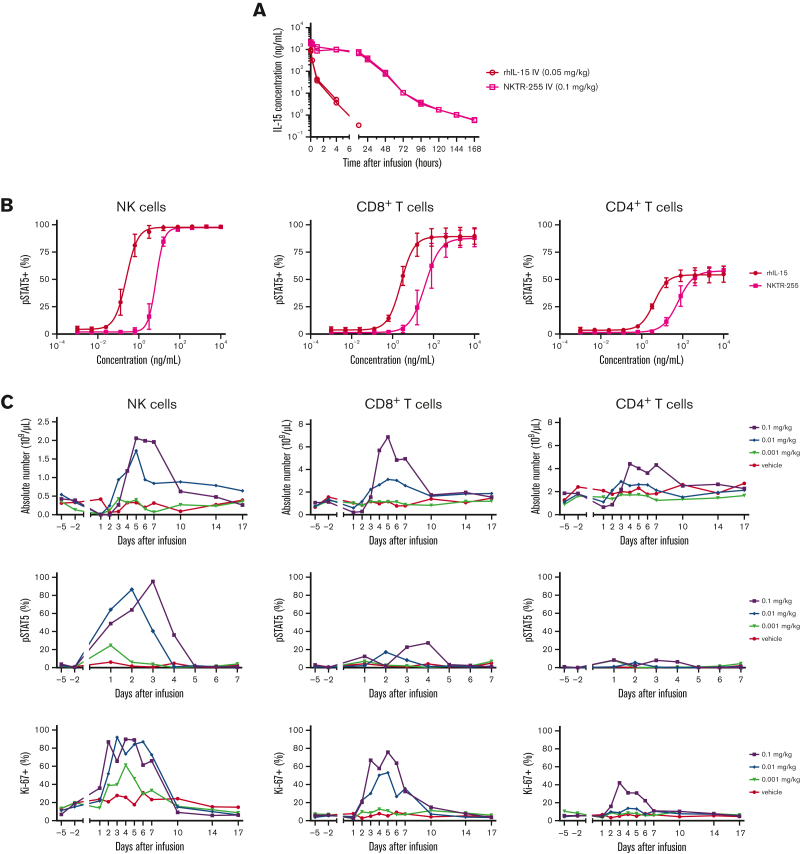
The short t1/2 of native IL-15 complicates its systemic administration.21 We investigated the PK and PD of NKTR-255 in NHPs, given that simian and human IL-15 share 97% sequence identity.31 We compared SC and IV administration of NKTR-255 in preclinical studies and observed injection site reactions with SC infusion. Furthermore, SC administration of NKTR-255 in cynomolgus monkeys was not associated with a clear PK benefit. Therefore, the IV administration route was selected for further studies. We treated cynomolgus monkeys (1 male and 1 female per group) with a single IV dose of rhIL-15 (0.05 mg/kg) or NKTR-255 (0.1 mg/kg) and evaluated plasma IL-15 concentrations at intervals after infusion. These dose levels were used because previous studies showed lower binding affinity for IL-15Rα of NKTR-255 relative to rhIL-15.24 In animals that received rhIL-15, we observed a transient increase in IL-15 concentration with rapid decline to undetectable levels 12 hours after infusion. In contrast, in animals that received NKTR-255, the levels of IL-15 were maintained in the first 12 hours after infusion and declined more slowly than rhIL-15 (Figure 2A). There was a longer IL-15 t1/2 (30.5 vs 1.2 hours), lower volume of distribution (72.8 vs 112.0 mL/kg), and slower clearance (4.3 vs 161.4 mL/h per kg) in NKTR-255–treated monkeys compared with those treated with rhIL-15. These data demonstrate the favorable PK of NKTR-255 compared with native IL-15 in cynomolgus NHPs.
Figure 2.
PK and PD of NKTR-255 in NHPs. (A) Cynomolgus monkeys (n = 2 per group) received a single IV dose of rhIL-15 at 0.05 mg/kg or NKTR-255 at 0.1 mg/kg. Blood samples were collected to determine plasma concentrations of IL-15 or NKTR-255 (IL-15 protein content, IL-15 equivalent). (B) Whole blood samples from cynomolgus monkeys (n = 3 per group) were incubated with the indicated concentrations of rhIL-15 or NKTR-255 for 20 minutes. Phosphorylation of STAT5 was measured by flow cytometry as the percentage of positive CD3−CD56+ NK cells (left), CD3+CD8+ T cells (middle), and CD3+CD4+ T cells (right). Mean ± standard error of the mean (SEM) is shown. (C) Cynomolgus monkeys (n = 3-4 per group) received a single IV dose of 0.001, 0.01, or 0.1 mg/kg of NKTR-255 or vehicle. Blood samples were collected before infusion and at the indicated time points to assess the absolute numbers of CD3−CD56+ NK cells (left), CD3+CD8+ T cells (middle), CD3+CD4+ T cells (right), percent of pSTAT5 positive cells, and percent of Ki-67+ cells using flow cytometry. Figures show mean values.
To determine whether NKTR-255 induces signaling in NHP immune cells, fresh whole blood from 3 animals was incubated with rhIL-15 or NKTR-255, and phosphorylation of STAT5 was evaluated in NK cells and CD8+ and CD4+ T cells. We observed dose-dependent STAT5 phosphorylation in each NHP cell subset (Figure 2B). The EC50 of STAT5 phosphorylation in NK cells and CD8+ and CD4+ T cells incubated with NKTR-255 was higher than that observed with rhIL-15, owing to the lower binding affinity of NKTR-255 for IL-15 receptor.24 To determine if systemic administration of NKTR-255 changes immune cell subset composition, we treated cynomolgus monkeys (n = 3-4 per group) with a single IV dose of NKTR-255 (0.001, 0.01, or 0.1 mg/kg) or vehicle. NKTR-255 induced a dose-dependent increase in the absolute numbers of NK cells and CD8+ and CD4+ T cells in the blood (Figure 2C) but did not significantly affect B-cell and monocyte counts (supplemental Figure 1). At the highest dose level (0.1 mg/kg), NK cells and T cells began to increase in the blood between 2 and 3 days after infusion, peaked around days 4 to 5, and then declined to baseline by day 10. Similar patterns were observed after NKTR-255 0.01 mg/kg dose, albeit with lower peak counts. The lowest dose (0.001 mg/kg) did not result in significant increases in NK cells and CD8+ or CD4+ T cells. We observed dose-dependent increases in the fraction of immune cells expressing pSTAT5 (Figure 2C), with the most significant changes observed in NK cells and CD8+ T cells. Dose-dependent increases in Ki-67 expression peaked 3 to 7 days after infusion, predominantly in NK cells and CD8+ T cells, consistent with in vivo proliferation (Figure 2C). Ki-67+ cells were found in the naïve and memory T-cell subsets (supplemental Figure 2). The fractions of NK cells and CD8+ and CD4+ T cells expressing Ki-67 returned to baseline ∼10 days after NKTR-255 infusion (0.1 mg/kg). These data indicate that NKTR-255 IV is associated with increased proliferation and absolute NK cell and CD8+ and CD4+ T-cell counts in cynomolgus NHP blood.
NKTR-255 enhances proliferation and survival of human CAR T cells at low target cell abundance
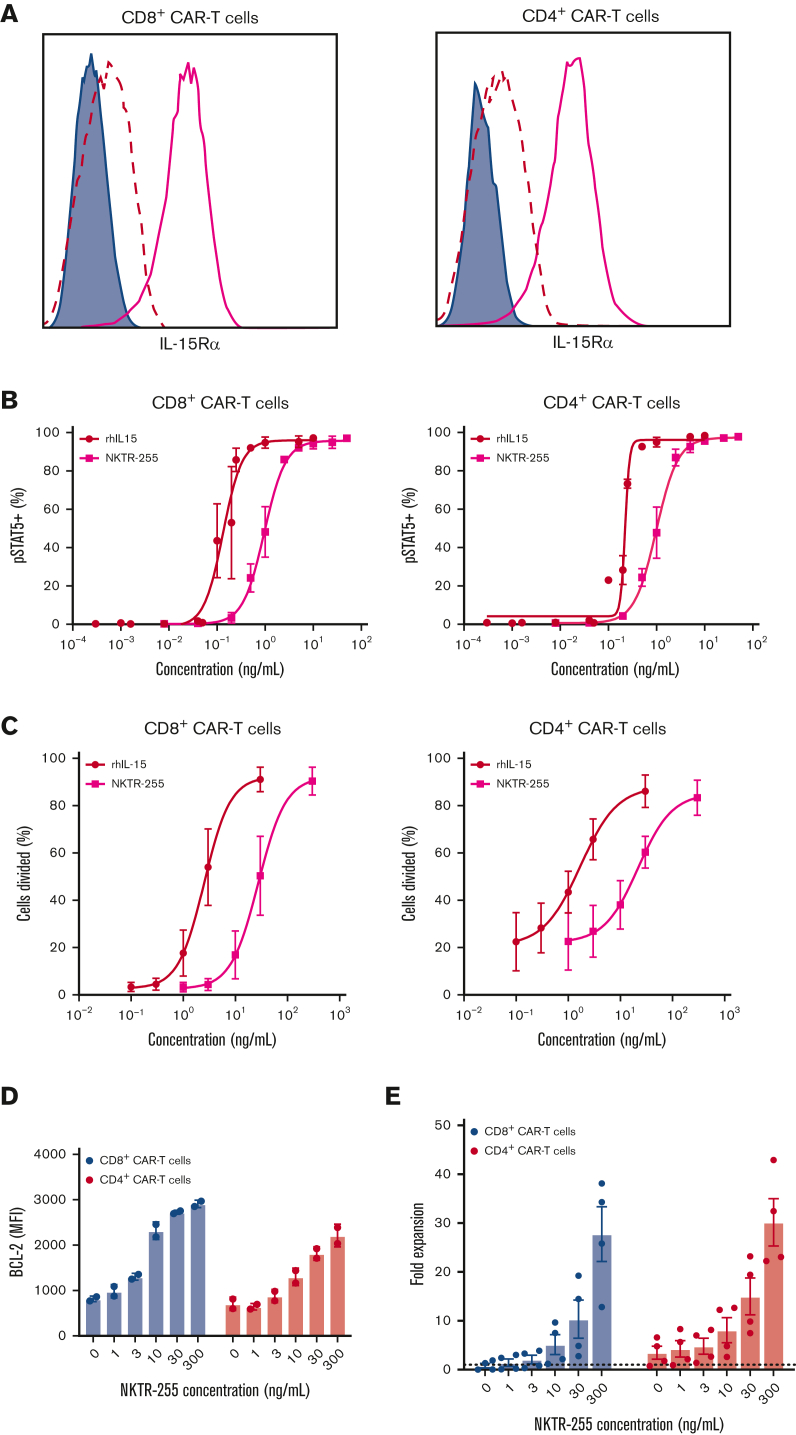
The favorable PK and immune cell PD observed after NKTR-255 treatment of NHPs suggests that NKTR-255 administration might enhance the efficacy of CD19 CAR T-cell therapy in humans. We first demonstrated IL-15Rα expression in human CD8+ and CD4+ CD19 CAR T cells (Figure 3A) and found a dose-dependent increase in STAT5 phosphorylation following incubation with NKTR-255 (Figure 3B). The EC50 of STAT5 phosphorylation in CAR T cells incubated with NKTR-255 was higher than that observed with rhIL-15, as seen in NHP T cells. NKTR-255 and rhIL-15 also induced the dose-dependent proliferation of human CD8+ and CD4+ CAR T cells (Figure 3C). Consistent with the lower affinity for IL-15Rα and pSTAT5 EC50 data, higher concentrations of NKTR-255 were required to achieve the same proliferation of CD8+ and CD4+ CAR T cells as seen with rhIL-15. NKTR-255 induced a dose-dependent increase in the expression of the prosurvival protein, BCL-2, in both CD8+ and CD4+ CAR T cells (Figure 3D). Consistent with the increased proliferation and expression of BCL-2, we observed a dose-dependent increase in the accumulation of CD8+ and CD4+ CAR T cells during in vitro culture (Figure 3E). These data indicate that in the absence of antigen, NKTR-255 induces a dose-dependent increase in proliferation, BCL-2 expression, and accumulation of CD8+ and CD4+ CAR T cells.
Figure 3.
Human CD19 CAR T cells exhibit a dose-dependent response to NKTR-255. Human CD19 CAR T cells were generated from healthy donors (n = 2-4) and assayed on days 14 to 16 after the start of manufacturing. (A) Representative surface expression of IL-15Rα (bold pink line) by flow cytometry on CD8+ (left) and CD4+ (right) CAR T cells. Filled histograms depict FMO and dashed lines represent isotype control. (B) CD8+ (left) and CD4+ (right) CAR T cells were incubated with the indicated concentrations of rhIL-15 or NKTR-255 for 20 minutes and phosphorylation of STAT5 was measured by flow cytometry. (C) CD8+ (left) and CD4+ (right) CAR T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and incubated with the indicated concentrations of rhIL-15 or NKTR-255 for 4 days. The percentage of divided cells was determined by CFSE dilution using flow cytometry. (D-E) CD8+ and CD4+ CAR T cells were incubated with the indicated concentrations of NKTR-255 for 4 days. (D) BCL-2 expression (mean fluorescence intensity) was measured using intracellular flow cytometry. (E) Fold expansion was determined by the fold change in absolute cell counts from days 0 to 4 by flow cytometry using counting beads. (B-E) Figures show the mean ± SEM. MFI, mean fluorescence intensity.
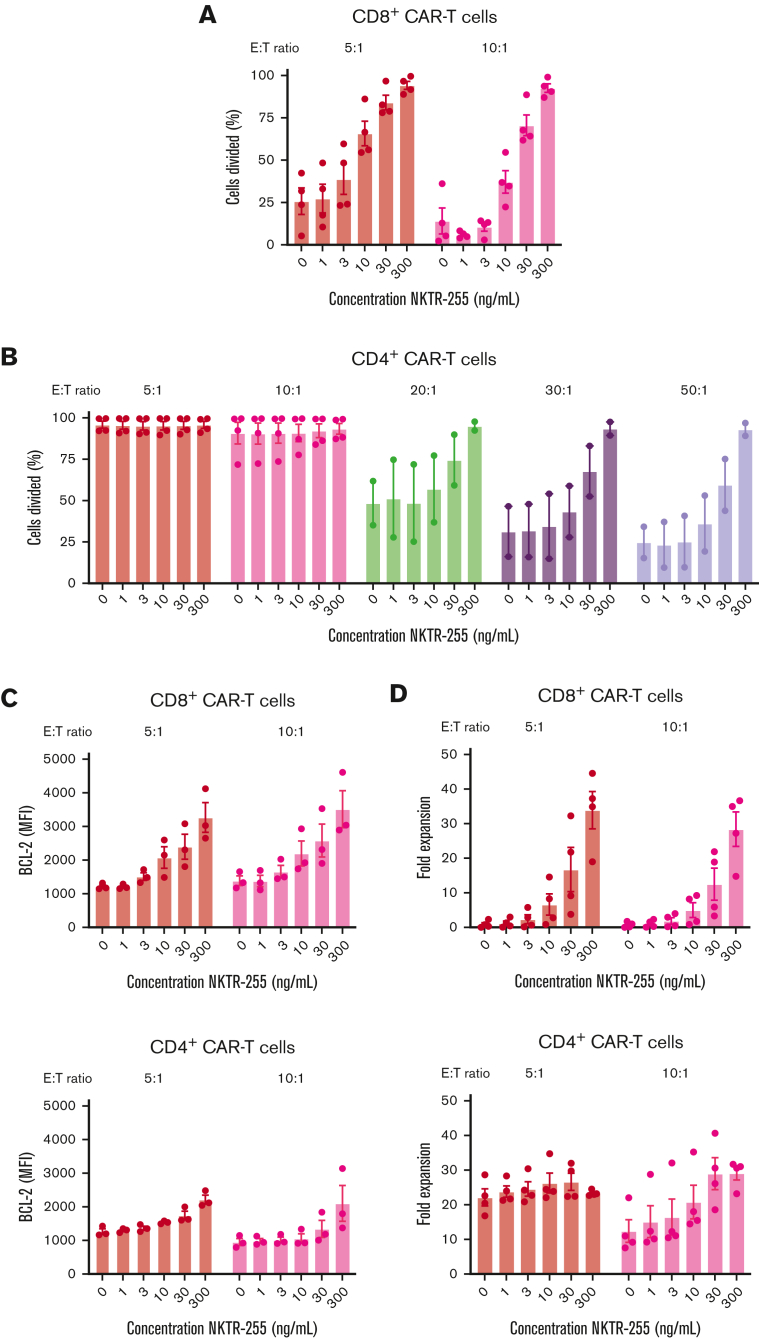
The extent of NKTR-255-induced augmentation of T-cell proliferation and accumulation may differ in the absence or presence of antigen. To address this, we cocultured CAR T cells with CD19-expressing K562 cells at different E:T ratios in the presence of increasing NKTR-255 concentrations. As expected, both CD8+ and CD4+ CAR T cells proliferated in response to the antigen, and the addition of NKTR-255 further enhanced the proliferation of CAR T cells in a dose-dependent manner (Figure 4A-B). This finding was more pronounced in the setting of lower target cell abundance, especially for CD4+ CAR T cells, in which the effect of NKTR-255 supplementation was more pronounced at high E:T ratios (Figure 4B). The increase in BCL-2 expression induced by increasing concentrations of NKTR-255 in the absence of antigen (Figure 3E) was also observed in the presence of antigen (Figure 4C). Consistent with the increased proliferation and BCL-2 expression (Figure 4A-C), we found a dose-dependent increase in the accumulation of CAR T cells with increasing NKTR-255 concentrations (Figure 4D), with the most marked increases observed with lower target cell abundance (high E:T ratio) and in the CD8+ CAR T cells. At a given E:T ratio, coculture of CD8+ and CD4+ CAR T cells with NKTR-255 in the presence of antigen did not significantly increase interferon gamma or tumor necrosis factor α (supplemental Figure 3A-B). The cytolytic activity of CD8+ CAR T cells cocultured with or without NKTR-255 against 51Cr-labeled CD19-expressing K562 cells analyzed by a standard 4-hour chromium release assay32 was also comparable (supplemental Figure 3C). These data indicate that NKTR-255 enhances the proliferation of CD8+ CAR T cells at high and low target cell burden and CD4+ CAR T cells mainly at low target cell burden, with limited effects on cytokine secretion and cytotoxicity.
Figure 4.
NKTR-255 increases the proliferation, survival, and expansion of CAR T cells in the presence of antigen. Human CD19 CAR T cells were generated from healthy donors (n = 2-4) and assayed on days 14 to 16 after start of manufacturing. CD8+ and CD4+ CAR T cells were independently cocultured with CD19-expressing K562 cells at the indicated E:T ratios and concentrations of NKTR-255 for 4 days. CD8+ (A) and CD4+ (B) CAR T cells were labeled with CFSE before coculture, and the percentage of divided cells was determined by flow cytometry using CFSE dilution. (C) BCL-2 expression (MFI) in CD8+ (top) and CD4+ (bottom) CAR T cells was measured by intracellular flow cytometry. (D) Fold expansion of CD8+ (top) and CD4+ (bottom) CAR T cells was determined by the fold change in absolute cell counts from days 0 to day 4 by flow cytometry using counting beads. (B-E) Figures show mean ± SEM.
NKTR-255 enhances accumulation and efficacy of human CD19 CAR T cells in lymphoma-bearing immunodeficient mice
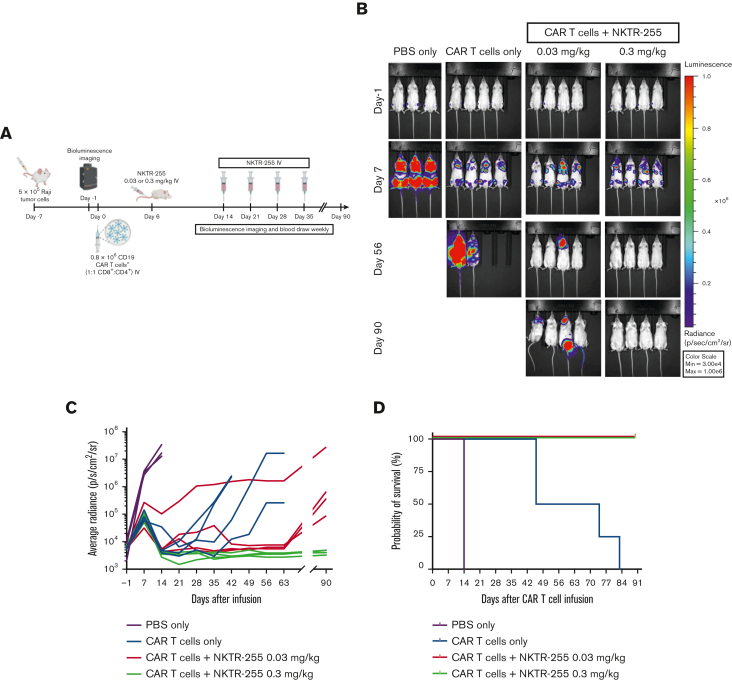
We next examined the effects of systemic administration of NKTR-255 on the in vivo efficacy of human CD19 CAR T cells in NSG mice with Raji lymphoma. Mice treated with PBS, NKTR-255 buffer, or NKTR-255 IV weekly starting 6 days after Raji inoculation showed similar tumor growth and short survival, indicating that neither NKTR-255 buffer nor NKTR-255 had a direct effect on tumor growth (supplemental Figure 4). In our phase 1/2 clinical trial of CD19 CAR T cells (#NCT01865617), the serum IL-15 concentration was elevated in the first week after CAR T-cell infusion owing to lymphodepletion chemotherapy, suggesting that early IL-15 supplementation might increase the risk of CAR T-cell–associated toxicities. Therefore, we decided to designate an initial NKTR-255 starting time of ∼1 week after CAR T-cell infusion. We first treated Raji tumor-bearing mice with a subtherapeutic dose of CD19 CAR T cells alone or supplemented with NKTR-255 (0.03 mg/kg or 0.3 mg/kg IV), starting on day 6 after CAR T-cell infusion and then weekly from day 14 (Figure 5A). One group of mice received PBS alone, without CAR T cells. Treatment with a subtherapeutic dose of CAR T cells alone increased survival compared with PBS alone, however, disease control was transient. In contrast, mice who received CAR T cells and NKTR-255 exhibited prolonged survival, with optimal tumor clearance observed in those that received NKTR-255 at 0.3 mg/kg compared with 0.03 mg/kg (Figure 5B-D).
Figure 5.
NKTR-255 increases the antitumor efficacy of human CD19 CAR T cells in vivo in a dose-dependent manner. NSG mice were injected IV with 5 × 105 Raji cells. Seven days later, tumor-bearing mice received 0.8 × 106 CD19 CAR T cells (1:1, CD8+:CD4+) IV. Cohorts of mice (n = 3-4 per group) received either PBS, NKTR-255 at 0.03 mg/kg, or NKTR-255 at 0.3 mg/kg IV starting on day 6 after CAR T-cell infusion followed by weekly doses from day 14. One control group received PBS without CAR T cells. (A) Schematic of the experimental setup. (B) Bioluminescence imaging of the Raji tumor burden at the indicated time points. (C) Average tumor radiance. (D) Kaplan-Meier survival curve. Max, maximum; Min, minimum.
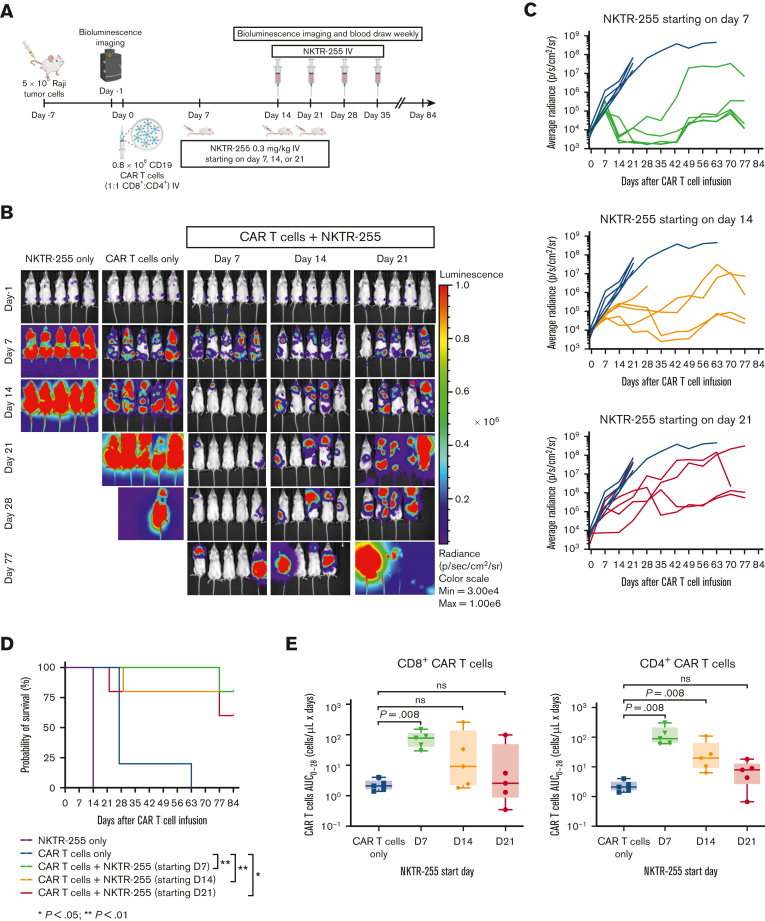
We then examined whether efficacy was affected by different NKTR-255 starting days beyond the first week after CAR T-cell infusion. We evaluated NKTR-255 at 0.3 mg/kg IV weekly starting on days 7, 14, or 21 after CAR T-cell infusion (Figure 6A). Days 14 and 21 were chosen to examine NKTR-255 supplementation starting at the CAR T-cell peak of expansion and contraction, respectively. Optimal tumor control was observed in mice receiving NKTR-255 starting on day 7 after CAR T-cell infusion (day 7 compared with day 21) (Figure 6B-C); and mice that received CAR T cells and NKTR-255 had longer survival compared with mice that received CAR T cells alone (day 7 vs CAR T cells only, P = .002; day 14 vs CAR T cells only, P = .007; and day 21 vs CAR T cells only, P = .04) (Figure 6D). Mice that received CAR T cells and NKTR-255 starting on day 7 had higher AUC0-28 of CD8+ and CD4+ CAR T-cell counts in blood compared with mice that received CAR T cells alone (P = .008 for both) (Figure 6E). The AUC0-28 of CD4+ CAR T cells was also higher in mice starting NKTR-255 on day 14 compared with CAR T cells alone (P = .008) (Figure 6E). These data indicate higher CAR T-cell counts in the blood and better tumor control in mice receiving CAR T cells followed by NKTR-255 starting on day 7 after CAR T-cell infusion.
Figure 6.
The starting day of NKTR-255 affects in vivo efficacy of human CD19 CAR T cells. NSG mice were injected IV with 5 × 105 Raji cells. Seven days later, tumor-bearing mice received 0.8 × 106 CD19 CAR T cells (1:1, CD8+:CD4+) IV. Cohorts of mice (n = 5 per group) received either CAR T cells only, or CAR T cells and NKTR-255 at 0.3 mg/kg IV starting on day 7, 14, or 21 after CAR T-cell infusion followed by weekly doses. One control group received NKTR-255 without CAR T cells. (A) Schematic of the experimental setup. (B) Bioluminescence imaging of Raji tumor burden at indicated time points. (C) Average tumor radiance grouped by the NKTR-255 start day. (D) Kaplan-Meier survival curve. Log-rank (Mantel-Cox tests) were used to compare differences between groups. (E) AUC of CD8+ (left) or CD4+ (right) CAR T-cell counts obtained by flow cytometry from days 0 to 28. Mann-Whitney tests were used to compare the differences between the groups. ns, not significant.
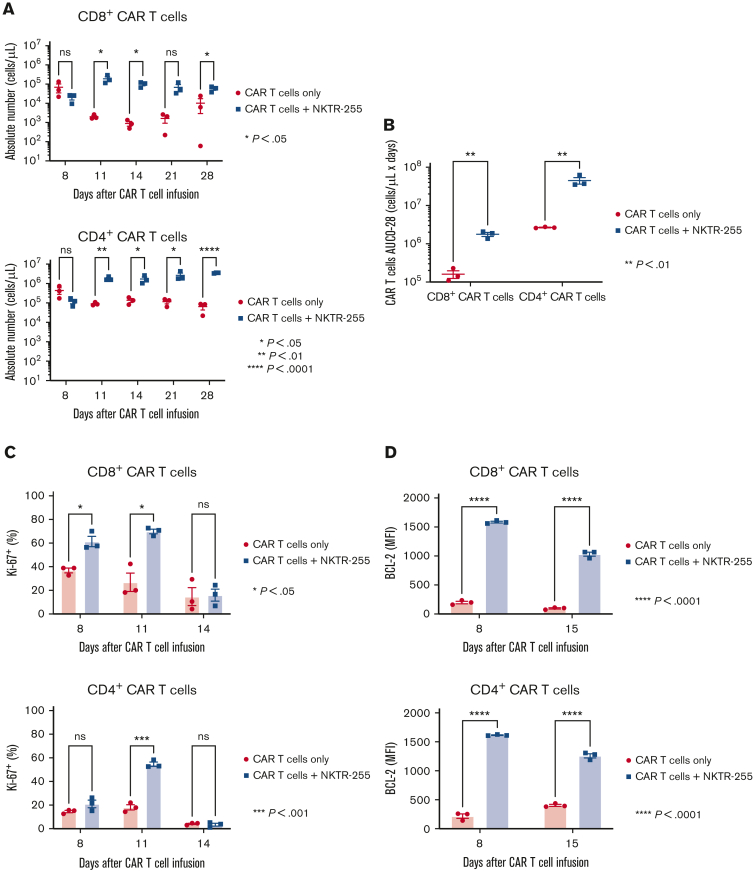
To determine whether NKTR-255 affected the accumulation of CAR T cells in bone marrow, cohorts of mice receiving CAR T cells and NKTR-255 at 0.3 mg/kg IV weekly starting on day 7 after CAR T-cell infusion were euthanized and CAR T cells were assessed in the bone marrow. Administration of NKTR-255 was associated with a higher percentage of Ki-67+ CD8+ and CD4+ CAR T cells in the first 8 to 11 days after CAR T-cell infusion (Figure 7A). By day 14, the fraction of Ki-67+ CAR T cells declined in the CAR T cells/NKTR-255 cohort, consistent with the rapid tumor elimination in this cohort (supplemental Figure 5; Figure 7A). Importantly, BCL-2 expression was higher in CD8+ and CD4+ CAR T cells in the CAR T cells/NKTR-255 cohort at all time points (Figure 7B), even after tumor clearance, resulting in persistently higher numbers of CD8+ and CD4+ CAR T cells in the bone marrow through day 28 after CAR T-cell infusion (Figure 7C-D). Despite profoundly higher numbers of CD8+ and CD4+ CAR T cells in the bone marrow on day 28 of mice in the CAR T cells/NKTR-255 cohort than in the CAR T cells alone cohort (CD8+ CAR T cells, 5.6-fold; CD4+ CAR T cells, 54.1-fold), evidence of ongoing activation/exhaustion by PD-1 and TIM-3 inhibitory receptor coexpression was not identified in CAR T cells from the CAR T cells/NKTR-255 cohort compared with the CAR T cell alone cohort (supplemental Figure 6A). CAR T cell samples from both cohorts showed similar fractions of interferon gamma and tumor necrosis factor α coexpression (supplemental Figure 6B).
Figure 7.
NKTR-255 increases human CAR T-cell accumulation and survival in the bone marrow of lymphoma-bearing mice. NSG mice were injected IV with 5 × 105 Raji cells. Seven days later, tumor-bearing mice received 0.8 × 106 CD19 CAR T cells (1:1, CD8+:CD4+) IV. Cohorts of mice (n = 3 per group) received either buffer or NKTR-255 at 0.3 mg/kg IV weekly starting on day 7 and euthanized on days 8, 11, 14, 21, and 28. Mice euthanized on days 14, 21, and 28 did not receive second, third, and fourth dose of NKTR-255, respectively. Single-cell suspensions from the bone marrow were analyzed by flow cytometry. (A) Percentage of Ki-67+ CD8+ (left) and CD4+ (right) CAR T cells in the bone marrow at the indicated time points. (B) BCL-2 expression (MFI) in CD8+ (left) and CD4+ (right) CAR T cells at indicated time points. (C) Absolute numbers of CD8+ (left) and CD4+ (right) CAR T cells at the indicated time points. (D) AUC0-28 of CD8+ and CD4+ CAR T-cell counts in the bone marrow. The figures show the mean ± SEM. An unpaired t test with a false discovery rate of 1% using the 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli were used to compare differences between groups.
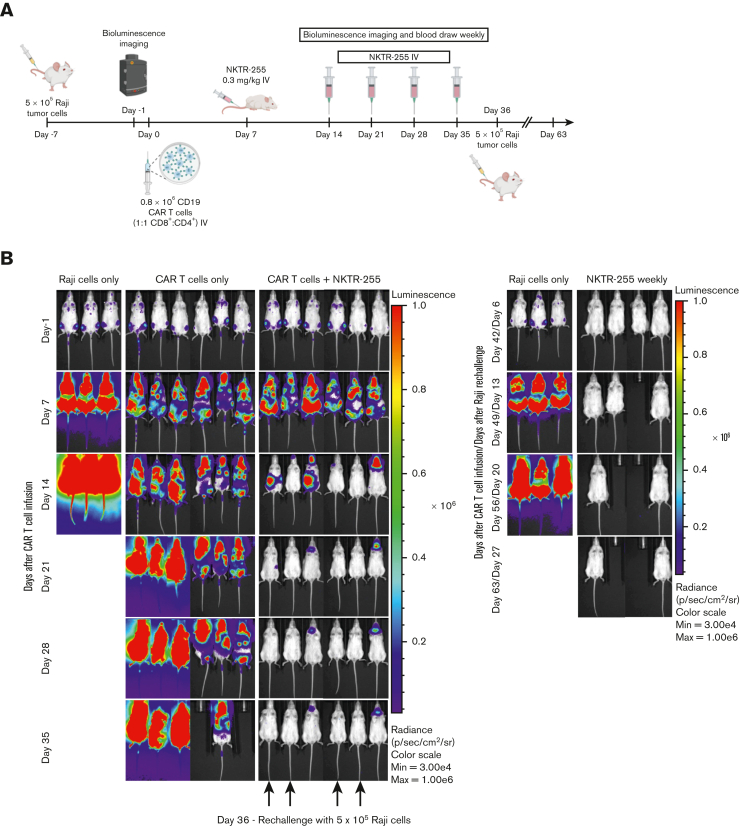
To investigate whether persistent CAR T cells after NKTR-255 administration could protect mice from tumor rechallenge, mice that cleared the tumor after receiving CAR T cells and NKTR-255 at 0.3 mg/kg IV weekly starting on day 7 after CAR T-cell infusion were rechallenged with Raji tumor cells on day 36 after CAR T-cell infusion (rechallenged mice) (Figure 8A). A separate cohort of mice with no prior CAR T cells or NKTR-255 treatment (naïve mice) was inoculated with Raji tumor cells alone. All rechallenged mice rejected the tumor 6 days after the rechallenge and remained tumor free (Figure 8B). Two rechallenged mice died of probable xenogeneic graft-versus-host disease without any evidence of tumor. In contrast, all naïve mice developed marked early tumor progression by day 13 and died. Together, these data show that NKTR-255 administration resulted in enhanced and early antitumor efficacy of human CD19 CAR T cells in lymphoma-bearing NSG mice and robust and sustained persistence of functional CAR T cells after tumor clearance.
Figure 8.
NKTR-255–treated mice are protected against tumor rechallenge. NSG mice were injected IV with 5 × 105 Raji cells. Seven days later, tumor-bearing mice received 0.8 × 106 CD19 CAR T cells (1:1 CD8+:CD4+) IV. Cohorts of mice (n = 6 per group) received either buffer or NKTR-255 at 0.3 mg/kg IV weekly starting on day 7. One control group did not receive CAR T cells. Mice that cleared the tumor were rechallenged with 5 × 105 Raji cells on day 36. A separate cohort of mice with no previous treatment was also injected with Raji cells on the same day as a control group (n = 3). (A) Schematic of experimental setup. (B) Bioluminescence imaging of Raji tumor burden at indicated time points.
Discussion
Peak serum IL-15 was associated with a higher probability of response in previous CD19 CAR T-cell studies10,19,33 and we demonstrated that peak serum IL-15 after CAR T-cell infusion was independently associated with CAR T-cell counts in the blood. In conjunction with the previously described effects of IL-15 on T-cell proliferation and survival,18 these data suggest that IL-15 supplementation may improve the efficacy of CAR T-cell therapy.
To enable clinical IL-15 supplementation, engineered IL-15–based molecules have recently been developed.24,34, 35, 36 We found that, compared with the administration of native IL-15, NKTR-255, a novel PEGylated form of IL-15, resulted in an extended IL-15 t1/2 (30.5 vs 1.2 hours) and increased NK cells and CD8+ T cells in NHP blood. NKTR-255 also increased the proliferation, accumulation, and antitumor efficacy of human CD19 CAR T cells. A first-in-human phase 1 clinical trial of single-agent NKTR-255 in patients with R/R hematologic malignancies (#NCT04136756) is currently enrolling,37,38 and preliminary results show a promising toxicity profile with no dose limiting toxicities and only transient elevations of cytokine release syndrome (CRS)–related cytokines.39 In this study, an increase in CAR T cells after NKTR-255 treatment was observed in all patients who had received and progressed after prior CAR T-cell therapy and had detectable CAR T cells in blood at the time of the first NKTR-255 infusion.40 These data provide strong support to test the combination of NKTR-255 and CAR T cells in human clinical trials.
The time to start IL-15 supplementation in relation to CAR T-cell infusion may affect efficacy and toxicity. Our previous clinical trial data suggested that adequate IL-15 concentration shortly after CAR T-cell infusion is associated with robust CAR T-cell accumulation. The concentrations of cytokines increase in response to lymphodepletion administered before CAR T-cell infusion,10,16 and adequate IL-15 concentrations at or shortly (within a week) after CAR T-cell infusion can be achieved following the administration of appropriate lymphodepletion chemotherapy. However, early exogenous IL-15 supplementation might improve CAR T-cell counts and/or function, especially in patients with preexisting T-cell dysfunction owing to disease or previous therapies or an inadequate response to lymphodepletion. The possibility that early NKTR-255 supplementation could enable a decreased lymphodepletion intensity is intriguing and needs to be further tested in suitable models.
Particularly in patients with normal T-cell function and/or high tumor burden, early IL-15 supplementation may increase the risk of toxicities such as CRS and/or immune effector cell–associated neurotoxicity syndrome (ICANS) owing to rapid and enhanced CAR T-cell proliferation. Approaches are still in development to consistently and prospectively characterize CAR T-cell potency to identify patients who could benefit from early IL-15 supplementation and those in whom early supplementation may increase the risk of CRS and/or ICANS. Patients who ultimately develop severe CRS/ICANS usually present within the first week after CAR T-cell infusion.41 Therefore, we considered that the earliest suitable time after CAR T-cell infusion to start IL-15 supplementation in initial clinical trials would be from around days 7 to 14, to allow deferral of patients with severe CRS/ICANS early after CAR T-cell infusion and decrease potential toxicities from IL-15 supplementation.
In our preclinical in vivo studies, we studied the starting days from 7 to 21 after CAR T-cell administration and found that the timing of NKTR-255 after CAR T-cell infusion was an important determinant of efficacy. NKTR-255 starting 7 days after CAR T-cell infusion was associated with increased CAR T-cell survival and accumulation (AUC0-28) in the blood and tissue and better disease control. The effects of NKTR-255 on human T cells and CAR T-cell efficacy were dose dependent. However, the enhanced proliferation of CAR T cells was mainly evident under conditions of low target cell abundance. At a high target cell burden, proliferation and cytokine secretion were mainly antigen dependent. The dichotomy of NKTR-255–mediated enhanced CAR T-cell proliferation with limited effects on effector functions is intriguing and requires further investigation. The limited augmentation of effector functions by increasing doses of NKTR-255 under conditions of high antigen burden might limit toxicity, providing additional support for starting NKTR-255 7 to 14 days after CAR T-cell infusion, when the tumor burden is lower. Importantly, tumor control was rapid and high CAR T-cell counts were sustained after tumor elimination in NKTR-255–treated mice. Although additional studies are required to definitively exclude exhaustion and demonstrate functional quiescence in CAR T cells in NKTR-255–treated mice, we did not observe evidence of ongoing activation/exhaustion by increased inhibitory receptor expression in persistent CAR T cells in these mice, in contrast to mice that did not receive NKTR-255. Our data does not demonstrate that NKTR-255 prevents exhaustion; however, NKTR-255–treated mice were protected against tumor rechallenge, indicating that persistent CAR T cells retained their functional capacity.
Our results show that NKTR-255 enhances endogenous T cells and NK cells in NHP and improves the efficacy of human CD19 CAR T cells in a xenogeneic lymphoma model. A phase 1 clinical trial of NKTR-255 and CD19 CAR T-cell combination therapy in patients with R/R large B-cell lymphoma (#NCT05359211) is ongoing.
Conflict-of-interest disclosure: A.V.H. reports honoraria from Bristol Myers Squibb (BMS) and Novartis and receives research funding from Juno Therapeutics (a BMS company) and Nektar Therapeutics. C.K.C. is an employee and stockholder of Genentech/Roche. T.M. is an employee and stockholder of Nektar Therapeutics. J.G. reports honoraria from EUSA Pharma, JMP, Larvol, and Multerra Bio; serves on scientific advisory boards for Legend Biotech, Janssen, Kite (a Gilead company), and MorphoSys; and receives research funding from Sobi, Juno Therapeutics, Celgene (a BMS company), and Angiocrine Bioscience. S.F. reports grants from BMS and other support in the form of pending equity from Link Immunotherapeutics outside of the submitted work; and has issued patents for PCT/US2021/025255 and PCT/US2021/025248d, and a patent for PCT/US2021/025260 issued, licensed, and with royalties paid from BMS. W.W.O. is an employee and stockholder of Nektar Therapeutics. S.R.R. is a cofounder and adviser to Lyell Immunopharma; has research funding from and intellectual property licensed to Lyell Immunopharma; was a cofounder of Juno Therapeutics; is an inventor of patents licensed to Juno Therapeutics; and served as an adviser to Juno Therapeutics and Adaptive Biotechnologies. M.Q.M. is an employee and stockholder of Nektar Therapeutics. C.J.T. has received research funding from Juno Therapeutics and Nektar Therapeutics; serves on scientific advisory boards for Precision BioSciences, Eureka Therapeutics, Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, and Century Therapeutics; has stock/options in Precision BioSciences, Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, and ArsenalBio; serves on a data safety monitoring board for Kyverna; has the right to receive royalties from Fred Hutchinson Cancer Center as an inventor on patents related to chimeric antigen receptor T-cell therapy; and served on ad hoc advisory boards or as a consultant (last 12 months) for Allogene, Sobi, Decheng Capital, GlaxoSmithKline, and Nektar Therapeutics. The remaining authors declare no competing financial interests.
The current affiliation for C.J.T. is Faculty of Medicine and Health, The University of Sydney, Camperdown, NSW, Australia.
Acknowledgments
The authors acknowledge the Fred Hutchinson Cancer Center Shared Resources, including the Comparative Medicine, Preclinical Imaging, and Flow Cytometry Cores.
This work was supported by grants from the National Institutes of Health, National Cancer Institute (T32CA009351 and 5K12CA076930-20) (C.K.C.), and in part by research funding from Nektar Therapeutics (C.J.T.).
Some figures were created with BioRender.com.
Authorship
Contribution: A.V.H., C.K.C., T.K., and J.G. collected the research data; C.K.C., R.N.S., H.A.D., S.P.F., and R.M.H. performed the experiments; A.V.H., C.K.C., and S.F. analyzed and interpreted the data; S.R.R., M.Q.M., and C.J.T. designed the experiments; A.V.H., C.K.C., and C.J.T. wrote and edited the manuscript; and all authors reviewed and edited the final version of the manuscript.
Footnotes
∗A.V.H. and C.K.C. contributed equally to this study.
Data are available on request from the corresponding author, Alexandre V. Hirayama (ahirayama@fredhutch.org).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 4.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi NC, Anderson LD, Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 7.Shah BD, Bishop MR, Oluwole OO, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood. 2021;138(1):11–22. doi: 10.1182/blood.2020009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 9.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):1–12. doi: 10.1126/scitranslmed.aaf8621. 355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130(21):2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi J, Paczkowski P, Shen YW, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018;132(8):804–814. doi: 10.1182/blood-2018-01-828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finney OC, Brakke HM, Rawlings-Rhea S, et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest. 2019;129(5):2123–2132. doi: 10.1172/JCI125423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–1663. doi: 10.1182/blood-2018-11-883710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirayama AV, Gauthier J, Hay KA, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876–1887. doi: 10.1182/blood-2018-11-887067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilipow K, Roberto A, Roederer M, Waldmann TA, Mavilio D, Lugli E. IL15 and T-cell stemness in T-cell-based cancer immunotherapy. Cancer Res. 2015;75(24):5187–5193. doi: 10.1158/0008-5472.CAN-15-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier J, Chou C, Hirayama AV, et al. High IL-15 serum concentrations are associated with response to CD19 CAR T-cell therapy and robust in vivo CAR T-cell kinetics. Blood. 2020;136(suppl 1):37–38. [Google Scholar]
- 20.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlon KC, Potter EL, Pittaluga S, et al. IL15 by continuous intravenous infusion to adult patients with solid tumors in a phase I trial induced dramatic NK-cell subset expansion. Clin Cancer Res. 2019;25(16):4945–4954. doi: 10.1158/1078-0432.CCR-18-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105(2):460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Hussain Z, Khan S, Imran M, Sohail M, Shah SWA, de Matas M. PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res. 2019;9(3):721–734. doi: 10.1007/s13346-019-00631-4. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki T, Maiti M, Hennessy M, et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J Immunother Cancer. 2021;9(5):e002024. doi: 10.1136/jitc-2020-002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauthier J, Hirayama AV, Purushe J, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650–1660. doi: 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119(1):72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3(2):125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19(12):3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science (New York, NY) 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 32.Hudecek M, Lupo-Stanghellini M-T, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19(12):3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alizadeh D, Wong RA, Yang X, et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol Res. 2019;7(5):759–772. doi: 10.1158/2326-6066.CIR-18-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romee R, Cooley S, Berrien-Elliott MM, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. IL-15 in the combination immunotherapy of cancer. Front Immunol. 2020;11:868. doi: 10.3389/fimmu.2020.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Buhtoiarov IN, Guo H, Cheung NV. A novel multimeric IL15/IL15Ralpha-Fc complex to enhance cancer immunotherapy. OncoImmunology. 2021;10(1):1893500. doi: 10.1080/2162402X.2021.1893500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah N, Tan A, Budde L, et al. First-in-human phase I study of NKTR-255 in patients with relapsed/refractory hematologic malignancies. J Immunotherapy Cancer. 2020;8(3):A216–A217. [Google Scholar]
- 38.Shah N, Perales MA, Turtle CJ, et al. Phase I study protocol: NKTR-255 as monotherapy or combined with daratumumab or rituximab in hematologic malignancies. Future Oncol. 2021;17(27):3549–3560. doi: 10.2217/fon-2021-0576. [DOI] [PubMed] [Google Scholar]
- 39.Shah N, Tan A, Budde E, et al. Safety, tolerability, PK/PD and preliminary efficacy of NKTR-255, a novel IL-15 receptor agonist, in patients with relapsed/refractory hematologic malignancies. Blood. 2021;138(suppl 1):3134. 3134. [Google Scholar]
- 40.Turtle CJ, Budde E, Patel KK, et al. Pharmacodynamic analysis of CAR-T cell persistence in patients with hematologic malignancies treated with NKTR-255, an IL-15 receptor agonist that enhances CD8 + T-cells: preliminary results from a phase 1 study. Blood. 2021;138(suppl 1):2815. 2815. [Google Scholar]
- 41.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.