This cohort study investigates differences in the effectiveness and treatment persistence between dimethyl fumarate, fingolimod, and ocrelizumab among patients with relapsing-remitting multiple sclerosis who switched from natalizumab.
Key Points
Question
Is there any difference in the effectiveness and treatment persistence between dimethyl fumarate, fingolimod, and ocrelizumab among patients with relapsing-remitting multiple sclerosis (RRMS) who switched from natalizumab?
Findings
In this cohort study, including 1386 patients with RRMS who ceased natalizumab, a switch to ocrelizumab was associated with the lowest annualized relapse rate and discontinuation rates and the longest time to first relapse compared with dimethyl fumarate and fingolimod.
Meaning
Using real-world data from the MSBase registry, this study provides an outcome comparison of 3 treatment choices after natalizumab cessation.
Abstract
Importance
Natalizumab cessation is associated with a risk of rebound disease activity. It is important to identify the optimal switch disease-modifying therapy strategy after natalizumab to limit the risk of severe relapses.
Objectives
To compare the effectiveness and persistence of dimethyl fumarate, fingolimod, and ocrelizumab among patients with relapsing-remitting multiple sclerosis (RRMS) who discontinued natalizumab.
Design, Setting, and Participants
In this observational cohort study, patient data were collected from the MSBase registry between June 15, 2010, and July 6, 2021. The median follow-up was 2.7 years. This was a multicenter study that included patients with RRMS who had used natalizumab for 6 months or longer and then were switched to dimethyl fumarate, fingolimod, or ocrelizumab within 3 months after natalizumab discontinuation. Patients without baseline data were excluded from the analysis. Data were analyzed from May 24, 2022, to January 9, 2023.
Exposures
Dimethyl fumarate, fingolimod, and ocrelizumab.
Main Outcomes and Measures
Primary outcomes were annualized relapse rate (ARR) and time to first relapse. Secondary outcomes were confirmed disability accumulation, disability improvement, and subsequent treatment discontinuation, with the comparisons for the first 2 limited to fingolimod and ocrelizumab due to the small number of patients taking dimethyl fumarate. The associations were analyzed after balancing covariates using an inverse probability of treatment weighting method.
Results
Among 66 840 patients with RRMS, 1744 had used natalizumab for 6 months or longer and were switched to dimethyl fumarate, fingolimod, or ocrelizumab within 3 months of natalizumab discontinuation. After excluding 358 patients without baseline data, a total of 1386 patients (mean [SD] age, 41.3 [10.6] years; 990 female [71%]) switched to dimethyl fumarate (138 [9.9%]), fingolimod (823 [59.4%]), or ocrelizumab (425 [30.7%]) after natalizumab. The ARR for each medication was as follows: ocrelizumab, 0.06 (95% CI, 0.04-0.08); fingolimod, 0.26 (95% CI, 0.12-0.48); and dimethyl fumarate, 0.27 (95% CI, 0.12-0.56). The ARR ratio of fingolimod to ocrelizumab was 4.33 (95% CI, 3.12-6.01) and of dimethyl fumarate to ocrelizumab was 4.50 (95% CI, 2.89-7.03). Compared with ocrelizumab, the hazard ratio (HR) of time to first relapse was 4.02 (95% CI, 2.83-5.70) for fingolimod and 3.70 (95% CI, 2.35-5.84) for dimethyl fumarate. The HR of treatment discontinuation was 2.57 (95% CI, 1.74-3.80) for fingolimod and 4.26 (95% CI, 2.65-6.84) for dimethyl fumarate. Fingolimod use was associated with a 49% higher risk for disability accumulation compared with ocrelizumab. There was no significant difference in disability improvement rates between fingolimod and ocrelizumab.
Conclusion and Relevance
Study results show that among patients with RRMS who switched from natalizumab to dimethyl fumarate, fingolimod, or ocrelizumab, ocrelizumab use was associated with the lowest ARR and discontinuation rates, and the longest time to first relapse.
Introduction
Natalizumab is a monoclonal antibody against α4 integrin that prevents the development of multiple sclerosis (MS) lesions by interfering with α4β1 and α4β7 integrin binding to the endothelial ligand vascular cell adhesion molecule 1.1,2 Three randomized trials3,4,5 have demonstrated the efficacy of natalizumab in controlling relapsing-remitting MS (RRMS). However, the long-term use of natalizumab is of concern due to an increased risk of progressive multifocal leukoencephalopathy (PML),6,7 an opportunistic brain infection caused by the JC virus (JCV).8,9 Therefore, in patients who are, or become, positive for anti-JCV antibody, natalizumab is often discontinued to mitigate PML risk.10,11,12 Patients are usually switched to an alternative disease-modifying therapy (DMT) after natalizumab cessation because this group is intrinsically at high relapse risk. One special concern after natalizumab cessation is rebound disease activity, which can occur around 12 weeks after cessation due to its relatively rapid offset of efficacy.13,14,15
Fingolimod, a functional antagonist of sphingosine-1-phosphate receptors that is effective in reducing relapse rate and improving magnetic resonance imaging (MRI) outcomes in RRMS,16,17 is commonly used after natalizumab cessation.10,12,18,19,20,21 Dimethyl fumarate22,23,24 and ocrelizumab are 2 other options.25,26 Dimethyl fumarate is effective in downregulating the proinflammatory responses of T cells, B cells, and myeloid cells.27,28 Ocrelizumab is a B-cell depleting humanized monoclonal antibody, which has shown a significant efficacy in reducing disease activity and MRI progression compared with interferon beta in 2 phase 3 trials.29,30 However, evidence has been inconsistent regarding the comparative efficacy of these 3 common DMTs after switching from natalizumab,22,23,31,32 and a direct comparison between them is lacking.
We, therefore, conducted a retrospective study to directly compare the treatment outcomes in participants treated with dimethyl fumarate, fingolimod, or ocrelizumab after natalizumab cessation using the MSBase registry data set.33 We evaluated relapse activity, disability accumulation and improvement, and persistence of the 3 therapies to inform the selection of the optimal DMT for patients with RRMS who discontinue natalizumab.
Methods
Patient data were obtained from the MSBase registry, an international observational cohort study of MS.33 Ethical approval for the MSBase registry was granted by the Alfred Health Human Research and Ethics Committee and the local ethics committees in participating centers. All enrolled patients provided written or verbal consent under local regulations. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Population
Patients were included if they were diagnosed with RRMS according to the McDonald criteria,32 had taken natalizumab monotherapy for 6 months or longer before discontinuation, and were switched to either dimethyl fumarate, fingolimod, or ocrelizumab with a minimum treatment persistence of 6 months. To minimize the loss of treatment effect and reduce the risk of contaminating the analysis with rebound relapse activity, we only included patients with a treatment gap of 3 months or less.10 Participants were also required to have at least 2 subsequent visits with a minimum 6-month gap and with recorded Expanded Disability Status Scale (EDSS; a nonlinear ordinal disability scale with range of 0-10) scores during the follow-up to allow for the ascertainment of disability accumulation or improvement. The MSBase study collected minimal participant data, the key data of which included relapse rates, relapse treatment, MS-specific therapies used, EDSS score, and diagnostic tests performed; race and ethnicity information was not collected.33 Patients were excluded if they had any missing data for sex, age, the date of starting and stopping natalizumab, and the date of starting and stopping a new treatment after natalizumab cessation. Patient data were recorded during routine clinic visits at participating centers via the locally installed iMed or MDS MSBase data entry systems and monitored through a series of procedures to maintain quality.34
Study End Points
The primary outcomes were annualized relapse rate (ARR), which was calculated by dividing the total number of relapses by the total number of person-years at risk, and time to first relapse. Secondary outcomes were confirmed disability accumulation events, confirmed disability improvement events, and treatment discontinuation.
The study baseline was defined as the date of new treatment commencement after natalizumab cessation. The EDSS score obtained within 6 months before or after baseline was chosen as the baseline EDSS score. We defined relapse as having new or recurrent neurologic symptoms lasting for 24 hours or more without fever or infection.35 Treatment discontinuation was defined as starting a new treatment. The primary reason for discontinuation was documented by the treating neurologist using predefined terms, but these data fell outside the MSBase minimum data set requirements and the percentage of missing data was high. Nonetheless, “scheduled stop” was the main selected reason for discontinuing natalizumab, accounting for 62% of discontinuations (eTable 1 in Supplement 1).
Disability accumulation was defined as the confirmed increase in EDSS score of 0.5 or more steps for patients with a baseline score greater than 5.5 (or 1.0 or more steps for those with a baseline score between 1.0 and 5.5) and 1.5 or more steps if the baseline score was 0. Disability improvement was defined as a decrease in EDSS score by 1 step for baseline EDSS scores of 2 to 5.5 (or 1.5 steps if baseline EDSS score was 1.5) and 0.5 steps if baseline EDSS score was higher than 5.5. EDSS scores obtained less than 30 days after the relapse onset date were excluded to mitigate the risk of early disease activity being labeled as the baseline EDSS score. Confirmed disability accumulation or improvement was defined as observed disability accumulation or improvement for 2 subsequent visits compared with baseline EDSS scores, with a minimum of 6 months between each assessment.
Statistical Analysis
Inverse Probability of Treatment Weighting
The inverse probability of treatment weighting (IPTW) was used to minimize baseline differences between the 3 treatment groups and selection bias.36 The weights were obtained by taking the inverse of the propensity scores, representing the probability of receiving a treatment conditional on observed covariates.37 A multinomial logistic regression model was used to estimate the propensity scores for each individual, with the treatment groups as a dependent variable and the baseline covariates as independent variables. To mitigate the influence of the extreme weights on the treatment effect, we stabilized the weights by multiplying the unstabilized weights by the proportion observed as treated or untreated in the data, respectively.38 An absolute standardized difference (ASD) was calculated to evaluate covariate balance, with an ASD greater than 0.1 indicating an imbalance.39
We used an IPTW-weighted negative binomial model to compare the ARRs between the treatment groups, with the natural logarithm of the follow-up time after the baseline as an offset term, the relapse count as a dependent variable, and the treatment groups as an independent variable. Hazard ratios (HRs) of time-to-event outcomes were estimated using an IPTW-weighted Cox proportional-hazards regression model with robust SEs. Kaplan-Meier cumulative hazard curves were used to show the cumulative risk for each individual outcome across treatment groups. The proportional-hazards assumption was checked by the Schoenfeld global test, and no violation was detected.40
Subgroup Analysis
We performed subgroup analyses by all baseline variables and the number of relapses 1 year before natalizumab commencement. Due to the small number of patients taking dimethyl fumarate, both dimethyl fumarate and fingolimod were grouped together into the moderate-efficacy category.
Sensitivity Analysis
The robustness of the primary outcome results was tested with 6 sensitivity analyses. To assess the consistency of our results, we first repeated the analysis using a doubly robust standardization method.41,42 It combines the IPTW with the G-computation43 method to mitigate the risk of model misspecification and achieve the double robustness property. Second, we repeated the analysis by adding baseline cerebral MRI information (presence or absence of a contrast-enhancing lesion and the number of hyperintense T2 lesions) into the model for propensity score generation. MRI information was collected by practitioners in accordance with local MRI protocols and policies. Baseline MRI was defined as the most recent MRI undertaken 12 months before or 6 months after the start date of treatment. The multiple imputation method was used to deal with missing data (86% of patients missing data on contrast-enhancing lesion and 76% on hyperintense T2 information). Twenty imputed data sets were generated, and the estimates were computed separately and then combined using Rubin rules.44 We also introduced the intention-to-treat analysis to check the result consistency in the situation with censoring at either event occurrence or the end of the follow-up. We added country as a covariate to the propensity score model and redid the analyses using newly generated weights. Moreover, we evaluated the influence of different baseline EDSS on results by narrowing the time interval for obtaining baseline EDSS score to 6 months before or 1 month after the new treatment start. Lastly, we assessed potential bias introduced by the difference in disease activities between the treatment groups before the start of natalizumab. We added the number of relapses 1 year before natalizumab start as a covariate to the propensity score model and repeated the main analysis.
All statistical tests were 2-sided with a significance level defined as P < .05. All analyses were performed in R software, version 4.1.1. (R Foundation for Statistical Computing). Data were analyzed from May 24, 2022, to January 9, 2023.
Results
Among 66 840 patients with RRMS, 1744 had taken natalizumab for 6 months or longer and were switched to dimethyl fumarate, fingolimod, or ocrelizumab within 3 months of natalizumab discontinuation. Between June 15, 2010, and July 6, 2021, after fulfilling the selection criteria and excluding 358 patients without baseline data, a total of 1386 patients with RRMS (mean [SD] age, 41.3 [10.6] years; 990 female [71%]; 396 male [29%]) from 26 countries and 79 centers were included in the analysis (Figure 1). Among them, 138 patients (9.9%) were treated with dimethyl fumarate (median [IQR] follow-up, 2.0 [1.1-3.4] years), 823 patients (59.4%) were treated with fingolimod (3.5 [1.7-5.3] years), and 425 patients (30.7%) were treated with ocrelizumab (2.1 [1.2-3.1] years) (Table).
Figure 1. Flowchart of Patient Inclusion and Exclusion.

EDSS indicates Expanded Disability Status Scale.
Table. Characteristics of the Study Population by Baseline Switch in Disease-Modifying Therapy.
| Variable | Total No. (n = 1386) | Dimethyl fumarate (n = 138) | Fingolimod (n = 823) | Ocrelizumab (n = 425) | ASDa | |
|---|---|---|---|---|---|---|
| Before IPTW | After IPTW | |||||
| Age, mean (SD), y | 41.3 (10.6) | 44.0 (11.4) | 40.1 (10.0) | 42.8 (11.2) | 0.35 | 0.02 |
| Female, No. (%) | 990 (71) | 103 (75) | 581 (71) | 306 (72) | 0.04 | 0.02 |
| Male, No. (%) | 396 (29) | 35 (25) | 242 (29) | 119 (28) | ||
| Disease duration, median (IQR), y | 10.6 (6.2-16.1) | 10.0 (5.5-16.6) | 11.0 (6.4-16.1) | 10.1 (5.6-16.0) | 0.02 | 0.03 |
| Disability (EDSS) score, median (IQR) | 3.0 (1.5-4.5) | 3.0 (1.5-4.5) | 3.0 (1.5-4.5) | 3.0 (1.5-5.0) | 0.05 | 0.02 |
| No. of relapses 1 y before baseline, mean (SD) | 0.2 (0.6) | 0.1 (0.4) | 0.3 (0.6) | 0.2 (0.5) | 0.27 | 0.03 |
| No. of relapses 2 y before baseline, mean (SD) | 0.6 (1.0) | 0.5 (0.8) | 0.6 (1.0) | 0.5 (0.9) | 0.17 | 0.03 |
| Previous therapies, median (IQR)b | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (0-2) | 0.12 | 0.04 |
| Washout period after natalizumab discontinuation, median (IQR), d | 28 (0-49) | 0 (0-50) | 24 (0-55) | 32 (0-47) | 0.18 | 0.09 |
| Duration of natalizumab use, median (IQR), y | 2.6 (1.6-4.0) | 2.5 (1.7-4.4) | 2.6 (1.8-3.8) | 2.4 (1.3-4.4) | 0.10 | 0.03 |
Abbreviations: ASD, absolute standardized difference; EDSS, Expanded Disability Status Scale; IPTW, inverse probability of treatment weighting.
ASD is the absolute difference in means or proportions divided by the SE. An imbalance was defined as an ASD greater than 0.10.
Natalizumab excluded.
Before weighting, the mean (SD) age of the 3 treatment groups differed significantly, with the dimethyl fumarate group being the oldest (44.0 [11.4] years), followed by the ocrelizumab group (42.8 [11.2] years) and the fingolimod group (40.1 [10.0] years). Patients who had experienced more relapses in the previous year were more likely to receive fingolimod, followed by ocrelizumab and dimethyl fumarate (Table). After weighting, all variables were balanced (ASDs ≤0.1) (eTable 2 in Supplement 1).
Effectiveness
The IPTW-weighted ARR was 0.06 (95% CI, 0.04-0.08) for patients taking ocrelizumab, 0.26 (95% CI, 0.12-0.48) for patients taking fingolimod, and 0.27 (95% CI, 0.12-0.56) for patients taking dimethyl fumarate. Ocrelizumab use was associated with a significant reduction in ARR compared with fingolimod (fingolimod vs ocrelizumab IPTW-weighted ARR ratio, 4.33; 95% CI 3.12-6.01) and dimethyl fumarate (dimethyl fumarate vs ocrelizumab ARR ratio, 4.50; 95% CI, 2.89-7.03). Similar results were found for the cumulative hazards of the first relapse. Patients taking fingolimod and dimethyl fumarate had a significantly higher risk of experiencing the first relapse than those taking ocrelizumab, with IPTW-weighted HRs of 4.02 (95% CI, 2.83-5.70) and 3.70 (2.35-5.84), respectively (Figure 2). No significant differences in any primary outcome were observed between fingolimod and dimethyl fumarate.
Figure 2. Annualized Relapse Rate (ARR) and the Hazards of Study Outcomes Weighted Using Inverse Probability of Treatment Weighting (IPTW).
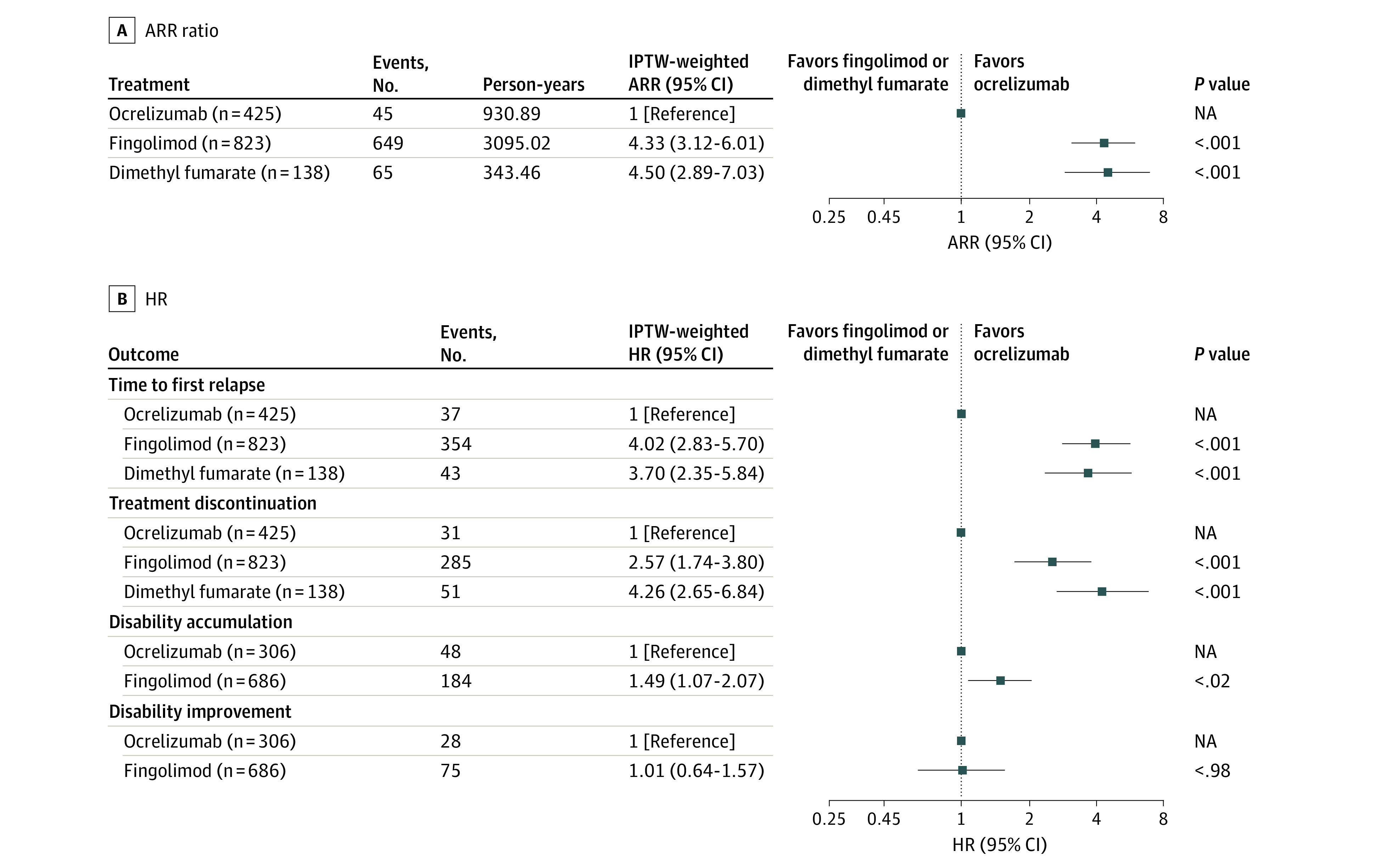
A, ARR ratio. B, Hazard ratio (HR). Baseline covariates used for generating weights in the propensity score models included age, sex, disease duration, disability, relapse 1 year before baseline, relapse 2 years before baseline, previous therapies, washout period after natalizumab discontinuation, and duration of natalizumab use. NA indicates not applicable.
Due to the small number of patients who took dimethyl fumarate meeting confirmed EDSS progression criteria, we only compared patients taking fingolimod with those taking ocrelizumab. Fingolimod use was associated with a 49% higher risk for disability accumulation compared with ocrelizumab (IPTW-weighted HR, 1.49; 95% CI, 1.07-2.07) (Figure 2). There was no difference in confirmed disability improvement rates (IPTW-weighted HR, 1.01; 95% CI, 0.64-1.57).
Results in the defined subgroups were generally consistent with the results in the main analysis. Ocrelizumab significantly interacted with baseline EDSS score and the number of previous therapies on the primary outcomes (P < .05 for interaction), with the greater association seen in patients with lower EDSS score (EDSS score <3; adjusted ARR, 9.73; 955 CI, 5.21-20.02; P =.004) and those with exposure to fewer DMTs (EDSS score <2; adjusted ARR, 7.79; 95% CI, 4.48-14.48; P = .02) before natalizumab (eTable 4 in Supplement 1).
Persistence
The most commonly reported reason for treatment discontinuation with fingolimod and dimethyl fumarate were lack of efficacy (137 of 285 [48%] and 16 of 51 [31%], respectively), and for ocrelizumab was adverse events (11 of 31 [35%]). The detailed reasons for treatment discontinuation are shown in eTable 3 in Supplement 1. Compared with ocrelizumab, fingolimod and dimethyl fumarate were associated with a significant increase in treatment discontinuation rates over time, with an IPTW-weighted HR of 2.57 (95% CI, 1.74-3.80) and 4.26 (95% CI, 2.65-6.84), respectively. Compared with dimethyl fumarate, patients taking fingolimod were less likely to discontinue the treatment (IPTW-weighted HR, 0.60; 95% CI, 0.44-0.84). The Kaplan-Meier cumulative hazard curves are presented in Figure 3.
Figure 3. Inverse Probability of Treatment Weighting (IPTW)–Weighted Cumulative Hazard of Primary and Secondary Outcomes.

Weighted Kaplan-Meier failure function was applied to present cumulative hazard of the time to the first relapse (A) and treatment discontinuation (B) in patients taking dimethyl fumarate, fingolimod, and ocrelizumab. For disability accumulation (C) and disability improvement (D), the plots were drawn from the weighted cumulative hazard models on the basis of separate propensity score models. Dimethyl fumarate was not included due to the insufficient number of participants. HR indicates hazard ratio.
Results from all sensitivity analyses were consistent with the main analysis results (eFigure 1, 2, 3, 4, 5, and 6 in Supplement 1). Details on the covariates balance after weighting between the treatment groups were reported along with the results.
Discussion
This retrospective cohort study compared the effectiveness and persistence between ocrelizumab, fingolimod, and dimethyl fumarate after natalizumab cessation in 1386 patients with RRMS. Compared with fingolimod and dimethyl fumarate, ocrelizumab use was associated with a significant reduction in ARR and time to first relapse. No significant difference in ARR and time to first relapse was found between fingolimod and dimethyl fumarate use. The discontinuation rate was significantly lower in patients taking ocrelizumab, followed by fingolimod and dimethyl fumarate. Due to a minority of patients taking dimethyl fumarate, we could only compare the disability accumulation and improvement between ocrelizumab and fingolimod. Ocrelizumab use was associated with a lower rate of sustained disability accumulation than fingolimod use, but there was no significant difference in disability improvement. Given that patients treated with natalizumab typically have high intrinsic relapse risk and natalizumab cessation is associated with risk of rebound as its biological effect declines quickly,15 our study is not, therefore, generalizable to other treatment switch scenarios or first-line therapy.
A previous study45 reported that fingolimod reduced the risk of relapse occurrence by 64% in comparison to interferon beta and glatiramer acetate after natalizumab cessation. Another cohort study10 found that fingolimod successfully controlled disease activity in patients who had stopped natalizumab, with an ARR of 0.38. Although the frequency of relapses was moderately higher than that during natalizumab treatment (ARR, 0.26), it was significantly lower than that before natalizumab was used (ARR, 1.54).10 We also found a relatively low relapse rate in patients treated with fingolimod after natalizumab discontinuation, with an ARR of 0.26. However, we found that ocrelizumab is a better choice of switch DMT after natalizumab cessation, with an ARR of 0.06. This observation might be explained by the intrinsic superiority of ocrelizumab over fingolimod in controlling disease activity rather than faster immunosuppression action, as both drugs lead to a fast lymphocyte depletion or redistribution.46 Natalizumab and fingolimod have similar therapeutic mechanisms, preventing immune cells from migrating to the central nervous system.1,16 However, ocrelizumab has a very different mechanism of action, depleting the CD20-expressing B cells.46 This superiority indicates that B-cell depletion could be advantageous over lymphocyte sequestration in rapidly controlling rebound after natalizumab discontinuation. In a recent observational study32 of 54 patients taking fingolimod and 48 patients taking ocrelizumab at 1 year after natalizumab cessation, those taking ocrelizumab had a significantly lower ARR than fingolimod (ARR, 0.12 vs 0.41), and the HR of relapse was 3.4 for fingolimod vs ocrelizumab. These results were consistent with ours.
Our results showed a lower discontinuation rate for fingolimod than for dimethyl fumarate (IPTW-weighted HR, 0.60; 95% CI, 0.44-0.84). A retrospective study23 including 732 patients with RRMS (409 taking dimethyl fumarate and 323 taking fingolimod) showed similar results regarding the discontinuation between dimethyl fumarate and fingolimod (29.3% of patients discontinued dimethyl fumarate vs 20.7% in fingolimod; P = .008). However, the study investigators found that dimethyl fumarate was associated with an increased relapse risk compared with fingolimod, with an HR of 1.9 (95% CI, 1.4-2.6) and a higher relapse risk in patients taking dimethyl fumarate who were pretreated with natalizumab compared with those switching to fingolimod (HR, 4.5; 95% CI, 1.9-10.8). In our study, no significant difference in ARR and time to first relapse between fingolimod and dimethyl fumarate was observed.
The superiority of ocrelizumab to dimethyl fumarate and fingolimod might be explained by the different immunologic mechanisms of action between the treatment classes. This includes the onset of treatment effect and relative treatment effectiveness in patients at very high risk of relapse. However, in MSBase, we currently do not have enough patient medical records to examine whether our findings are a class effect (eg, other anti-CD20 DMTs, such as rituximab or ofatumumab, or other sphingosine-1 phosphate inhibitors beyond fingolimod or diroximel fumarate). Although our data are observational in nature, it is very unlikely that prospective randomized clinical trials for specific switch scenarios will be funded in the future. Observational data are increasingly important for refining approved treatment indications and funding decisions of payers. Our results could be incorporated into specific switch treatment guidelines and, together with other real-world observational studies, could provide an extended evidence base for regulators and payers.
Limitations
This study has several limitations. Although we applied the propensity score–based IPTW approach to balance baseline covariates and our study findings were robust in sensitivity analyses, potential bias caused by unmeasured and unobserved factors cannot be completely ruled out. Because the number of patients in the fingolimod and dimethyl fumarate groups declined dramatically since 2018 (the approximate year of release of ocrelizumab), we did not have sufficient patient numbers to assess data consistency for true contemporaneous use of the 3 treatments. In addition, because DMT approval dates vary from country to country, it may not be possible to have a choice of all 3 treatments in all sites and for all study dates. Both issues may lead to the failure of fulfilling the positivity assumption required by the propensity score method and introduce bias. To assess the impact of this bias, we repeated the analysis using a doubly robust standardization method, given that the doubly robust estimators are consistent when the positivity assumption holds or fails.42,47,48 Furthermore, we did not include MRI data in the primary propensity score model because only 34% of participants had this information. However, we performed a sensitivity analysis using MRI information using the multiple imputation method, and the results were consistent. Moreover, previous studies have demonstrated that MRI activity before the start of natalizumab is a strong predictor of future disease activity after natalizumab discontinuation.49 In our study, only 12.2% of patients (n = 201) had a record of prior MRI activity. Thus, we were unable to verify this in our cohort. Future studies are needed to provide useful evidence in this regard to guide personalized clinical decisions. Drug safety data are also important to adequately assess the risk-benefit profiles of different DMTs, especially when used over the long term. Currently, the safety data in the MSBase registry are largely missing, but we are engaged in improving the collection of this data through a specific program.
Conclusion
Results of this cohort study showed that among patients with RRMS who had discontinued natalizumab, ocrelizumab was associated with a significant reduction in the ARR and the hazard of the time to first relapse and a lower discontinuation rate compared with fingolimod and dimethyl fumarate. In addition, a switch to ocrelizumab was associated with a lower rate of confirmed disability progression events than a switch to fingolimod. Stopping natalizumab for PML risk management is common. Our findings can help inform subsequent DMT selection for these patients.
eTable 1. Reported Reasons for Natalizumab Cessation Based on Treatments
eTable 2. IPTW-Weighted Characteristics of the Study Population by Baseline Switch DMT
eTable 3. Reported Reasons for Treatment Discontinuation
eTable 4. Subgroup Analysis of the Efficacy Between Moderate-Efficacy Therapies (Dimethyl Fumarate and Fingolimod) and Ocrelizumab for the Primary Outcomes by Baseline Characteristics
eFigure 1. Sensitivity Analysis Using a Doubly Robust Standardization Approach
eFigure 2. Sensitivity Analysis Using Propensity Scores Model With MRI Data (Missing Handled by Multiple Imputations)
eFigure 3. Sensitivity Analysis Using Intention-to-Treat (ITT) Approach
eFigure 4. Sensitivity Analysis: Propensity Score Model Further Adjusting for Country
eFigure 5. Sensitivity Analysis: Baseline EDSS Obtained Within 6 Months Before or 1 Month After the New Treatment
eFigure 6. Sensitivity Analysis: Propensity Score Model, in Which the Number of Relapses in the Year Before Natalizumab Start Was Added as a Covariate
Nonauthor Collaborators. MSBase Study Group
Data Sharing Statement
References
- 1.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356(6364):63-66. doi: 10.1038/356063a0 [DOI] [PubMed] [Google Scholar]
- 2.Kent SJ, Karlik SJ, Cannon C, et al. A monoclonal antibody to alpha 4 integrin suppresses and reverses active experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58(1):1-10. doi: 10.1016/0165-5728(94)00165-K [DOI] [PubMed] [Google Scholar]
- 3.Miller DH, Khan OA, Sheremata WA, et al. ; International Natalizumab Multiple Sclerosis Trial Group . A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15-23. doi: 10.1056/NEJMoa020696 [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, O’Connor PW, Havrdova E, et al. ; AFFIRM Investigators . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899-910. doi: 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 5.Rudick RA, Stuart WH, Calabresi PA, et al. ; SENTINEL Investigators . Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911-923. doi: 10.1056/NEJMoa044396 [DOI] [PubMed] [Google Scholar]
- 6.Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol. 2018;17(5):467-480. doi: 10.1016/S1474-4422(18)30040-1 [DOI] [PubMed] [Google Scholar]
- 7.Butzkueven H, Kappos L, Wiendl H, et al. ; Tysabri Observational Program (TOP) Investigators . Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660-668. doi: 10.1136/jnnp-2019-322326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870-1880. doi: 10.1056/NEJMoa1107829 [DOI] [PubMed] [Google Scholar]
- 9.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93(15):e1452-e1462. doi: 10.1212/WNL.0000000000008243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jokubaitis VG, Li V, Kalincik T, et al. ; MSBase Study Group . Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 2014;82(14):1204-1211. doi: 10.1212/WNL.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76(6):802-812. doi: 10.1002/ana.24286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M, Maillart E, Tourbah A, et al. ; Club Francophone de la Sclérose en Plaques Investigators . Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol. 2014;71(4):436-441. doi: 10.1001/jamaneurol.2013.6240 [DOI] [PubMed] [Google Scholar]
- 13.Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10(8):745-758. doi: 10.1016/S1474-4422(11)70149-1 [DOI] [PubMed] [Google Scholar]
- 14.Naismith RT, Bourdette D. Interruption of natalizumab therapy for multiple sclerosis: what are the risks? Neurology. 2011;76(22):1854-1855. doi: 10.1212/WNL.0b013e31821d7553 [DOI] [PubMed] [Google Scholar]
- 15.Fox RJ, Cree BA, De Sèze J, et al. ; RESTORE . MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology. 2014;82(17):1491-1498. doi: 10.1212/WNL.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappos L, Radue E-W, O’Connor P, et al. ; FREEDOMS Study Group . A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387-401. doi: 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 17.Kappos L, Radue EW, Comi G, et al. ; TOFINGO study group . Switching from natalizumab to fingolimod: A randomized, placebo-controlled study in RRMS. Neurology. 2015;85(1):29-39. doi: 10.1212/WNL.0000000000001706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comi G, Gold R, Dahlke F, et al. Relapses in patients treated with fingolimod after previous exposure to natalizumab. Mult Scler. 2015;21(6):786-790. doi: 10.1177/1352458514549404 [DOI] [PubMed] [Google Scholar]
- 19.Sempere AP, Martín-Medina P, Berenguer-Ruiz L, et al. Switching from natalizumab to fingolimod: an observational study. Acta Neurol Scand. 2013;128(2):e6-e10. doi: 10.1111/ane.12082 [DOI] [PubMed] [Google Scholar]
- 20.Leurs CE, van Kempen ZL, Dekker I, et al. Switching natalizumab to fingolimod within 6 weeks reduces recurrence of disease activity in MS patients. Mult Scler. 2018;24(11):1453-1460. doi: 10.1177/1352458517726381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guger M, Enzinger C, Leutmezer F, et al. ; Austrian MS Treatment Registry (AMSTR) . Switching from natalizumab to fingolimod treatment in multiple sclerosis: real life data from the Austrian MS Treatment Registry. J Neurol. 2019;266(11):2672-2677. doi: 10.1007/s00415-019-09464-0 [DOI] [PubMed] [Google Scholar]
- 22.Calabrese M, Pitteri M, Farina G, et al. Dimethyl fumarate: a possible exit strategy from natalizumab treatment in patients with multiple sclerosis at risk for severe adverse events. J Neurol Neurosurg Psychiatry. 2017;88(12):1073-1078. doi: 10.1136/jnnp-2017-316236 [DOI] [PubMed] [Google Scholar]
- 23.Diem L, Daponte A, Findling O, et al. Dimethyl fumarate vs fingolimod following different pretreatments: a retrospective study. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e660. doi: 10.1212/NXI.0000000000000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurawski J, Flinn A, Sklover L, Sloane JA. Relapse frequency in transitioning from natalizumab to dimethyl fumarate: assessment of risk factors. J Neurol. 2016;263(8):1511-1517. doi: 10.1007/s00415-016-8162-8 [DOI] [PubMed] [Google Scholar]
- 25.Mancinelli CR, Scarpazza C, Cordioli C, et al. Switching to ocrelizumab in RRMS patients at risk of PML previously treated with extended interval dosing of natalizumab. Mult Scler. 2021;27(5):790-794. doi: 10.1177/1352458520946017 [DOI] [PubMed] [Google Scholar]
- 26.Toorop AA, van Lierop ZYG, Strijbis EEM, et al. Mild progressive multifocal leukoencephalopathy after switching from natalizumab to ocrelizumab. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e904. doi: 10.1212/NXI.0000000000000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BAC, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e76. doi: 10.1212/NXI.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross CC, Schulte-Mecklenbeck A, Klinsing S, Posevitz-Fejfár A, Wiendl H, Klotz L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;3(1):e183. doi: 10.1212/NXI.0000000000000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators . Ocrelizumab vs interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 30.Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators . Ocrelizumab vs placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220. doi: 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- 31.Hersh CM, Harris H, Conway D, Hua LH. Effect of switching from natalizumab to moderate- vs high-efficacy DMT in clinical practice. Neurol Clin Pract. 2020;10(6):e53-e65. doi: 10.1212/CPJ.0000000000000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bigaut K, Kremer L, Fabacher T, et al. Ocrelizumab vs fingolimod after natalizumab cessation in multiple sclerosis: an observational study. J Neurol. 2022;269(6):3295-3300. doi: 10.1007/s00415-021-10950-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler. 2006;12(6):769-774. doi: 10.1177/1352458506070775 [DOI] [PubMed] [Google Scholar]
- 34.Kalincik T, Kuhle J, Pucci E, et al. ; MSBase Scientific Leadership Group and MSBase Study Group . Data quality evaluation for observational multiple sclerosis registries. Mult Scler. 2017;23(5):647-655. doi: 10.1177/1352458516662728 [DOI] [PubMed] [Google Scholar]
- 35.Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122(122):552-568. doi: 10.1111/j.1749-6632.1965.tb20235.x [DOI] [PubMed] [Google Scholar]
- 36.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706-710. doi: 10.1093/biomet/87.3.706 [DOI] [Google Scholar]
- 38.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273-277. doi: 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67:145-153. doi: 10.1093/biomet/67.1.145 [DOI] [Google Scholar]
- 41.Vansteelandt S, Keiding N. Invited commentary: G-computation—lost in translation? Am J Epidemiol. 2011;173(7):739-742. doi: 10.1093/aje/kwq474 [DOI] [PubMed] [Google Scholar]
- 42.Chatton A, Borgne FL, Leyrat C, Foucher Y. G-computation and doubly robust standardisation for continuous-time data: a comparison with inverse probability weighting. Stat Methods Med Res. 2022;31(4):706-718. doi: 10.1177/09622802211047345 [DOI] [PubMed] [Google Scholar]
- 43.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173(7):731-738. doi: 10.1093/aje/kwq472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin DB. Multiple Imputation for Survey Nonresponse. Wiley; 1987. [Google Scholar]
- 45.Iaffaldano P, Lucisano G, Pozzilli C, et al. ; Italian iMed-Web database . Fingolimod vs interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain. 2015;138(Pt 11):3275-3286. doi: 10.1093/brain/awv260 [DOI] [PubMed] [Google Scholar]
- 46.Genovese MC, Kaine JL, Lowenstein MB, et al. ; ACTION Study Group . Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(9):2652-2661. doi: 10.1002/art.23732 [DOI] [PubMed] [Google Scholar]
- 47.Neugebauer R, van der Laan M. Why prefer double robust estimators in causal inference? J Stat Plan Inference. 2005;129:405-426. doi: 10.1016/j.jspi.2004.06.060 [DOI] [Google Scholar]
- 48.Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res. 2012;21(1):31-54. doi: 10.1177/0962280210386207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Postnatalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809. doi: 10.1177/1756286419837809 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Reported Reasons for Natalizumab Cessation Based on Treatments
eTable 2. IPTW-Weighted Characteristics of the Study Population by Baseline Switch DMT
eTable 3. Reported Reasons for Treatment Discontinuation
eTable 4. Subgroup Analysis of the Efficacy Between Moderate-Efficacy Therapies (Dimethyl Fumarate and Fingolimod) and Ocrelizumab for the Primary Outcomes by Baseline Characteristics
eFigure 1. Sensitivity Analysis Using a Doubly Robust Standardization Approach
eFigure 2. Sensitivity Analysis Using Propensity Scores Model With MRI Data (Missing Handled by Multiple Imputations)
eFigure 3. Sensitivity Analysis Using Intention-to-Treat (ITT) Approach
eFigure 4. Sensitivity Analysis: Propensity Score Model Further Adjusting for Country
eFigure 5. Sensitivity Analysis: Baseline EDSS Obtained Within 6 Months Before or 1 Month After the New Treatment
eFigure 6. Sensitivity Analysis: Propensity Score Model, in Which the Number of Relapses in the Year Before Natalizumab Start Was Added as a Covariate
Nonauthor Collaborators. MSBase Study Group
Data Sharing Statement


