Abstract
Objective
To evaluate associations between changes in weight, length, and weight/length ratio during infancy and outcomes later in life among individuals born extremely preterm.
Study design
Among participants in the Extremely Low Gestational Age Newborn (ELGAN) study, we measured weight and length at discharge from the neonatal intensive care unit (NICU) and at age 2 years and evaluated neurocognitive, psychiatric, and health outcomes at age 10 years and 15 years. Using multivariable logistic regression, we estimated associations between gains in weight, length, and weight/length ratio z-scores between discharge and 2 years and outcomes at 10 and 15 years. High gain was defined as the top quintile of change; low gain, as the bottom quintile of change.
Results
High gains in weight and weight/length were associated with greater odds of obesity at 10 years, but not at 15 years. These associations were found only for females. High gain in length z-score was associated with lower odds of obesity at 15 years. The only association found between high gains in growth measures and more favorable neurocognitive or psychiatric outcomes was between high gain in weight/length and lower odds of cognitive impairment at age 10 years.
Conclusions
During the 2 years after NICU discharge, females born extremely preterm with high gains in weight/length or weight have greater odds of obesity at 10 years, but not at 15 years. Infants with high growth gains in the 2 years after NICU discharge have neurocognitive and psychiatric outcomes in middle childhood and adolescence similar to those of infants with lower gains in weight and weight/length.
Monitoring growth is a central aspect of health care for infants born extremely preterm during and after their discharge from the neonatal intensive care unit (NICU).1,2 Whereas a majority of extremely preterm infants experience a decrease in weight z-score during their neonatal hospitalization,3,4 following discharge from the NICU, their weight z-scores typically increase,5–8 a change referred to as “catch-up” growth.9,10 By middle childhood, body mass index (BMI) z-scores of extremely preterm children are similar to those born at term,11 and about one-quarter of children born extremely preterm are overweight or obese at school age.12,13 Failure to exhibit catch-up growth in early childhood has been associated with worse neurodevelopmental outcome,14 suggesting that trade-offs might exist between beneficial effects of improved nutrition on brain growth and adverse effects on BMI.15 Clarifying these trade-offs is critical to informing diet-based and other interventions to promote optimal weight gain in extremely preterm infants after discharge from the NICU.
We used data from the Extremely Low Gestational Age Newborn (ELGAN) study to analyze associations of changes in weight, length, and weight-for-length z-score in the first 2 postnatal years after discharge from the NICU with neurocognitive, psychiatric, and health outcomes of extremely preterm children at age 10 years and 15 years.
Methods
All procedures for this study were approved by the Institutional Review Board of each of the 12 participating study sites.
Data were acquired from the ELGAN study, a prospective observational study of children born extremely preterm16 in which 1249 women and their 1506 newborns delivered before 28 weeks of gestational age were enrolled in 14 hospitals across 5 US states from 2002 to 2004. The Figure (available at www.jpeds.com) presents a flow diagram describing the derivation of the study sample.
Figure.
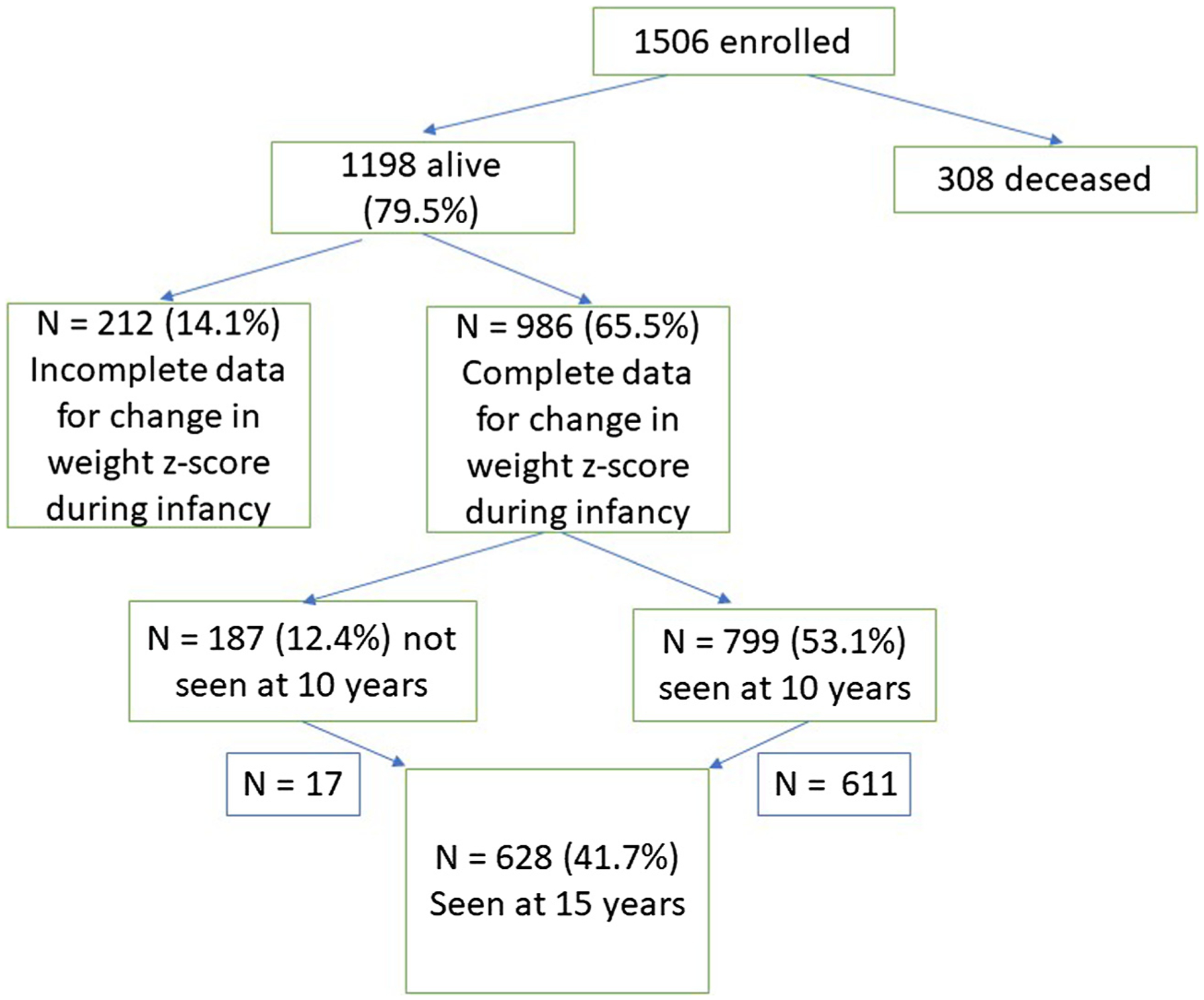
Derivation of the study sample.
Prenatal and Postnatal Data
Within a few days of delivery, a trained research coordinator interviewed the mother about demographic and prenatal factors and reviewed medical records to collect data on perinatal and neonatal factors.16 Birth weight z-scores were derived from reference data reported by Fenton et al.17 In prior publications, we presented definitions used in the ELGAN study for neonatal chronic lung disease,18 ultrasound-diagnosed cerebral white matter injury,19,20 neonatal bacteremia,21 spontaneous intestinal perforation and necrotizing enterocolitis,22,23 and severe retinopathy.24
Change in Weight and Length Z-Scores From NICU Discharge to Age 2 Years
Prior to discharge from the NICU, weight and length were measured by NICU staff following local practices. Follow-up visits occurred at around 2 years corrected age, calculated as actual age minus (40 - gestational age). At these visits, weight and length were measured by research coordinators, clinic nurses, or follow-up examiners. Weight and length z-scores for postmenstrual age at discharge were derived from reference data reported by Fenton et al17; weight and length z-scores at approximately 2 years corrected age were derived from World Health Organization reference data25 using macros published by the SAS Institute: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm for infants aged ≤731 days; https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm for infants aged >731 days. Weight/length ratio z-scores were derived from the study sample.
Outcomes at Age 10 Years
Weight and height were measured by research coordinators using standardized procedures, which included having the participants remove shoes and over garments, at age 10 years, and BMI percentiles were calculated based on measured weight and height and age- and sex-specific US growth standards.25 Obesity was defined as a BMI ≥95th percentile.13 The diagnosis of asthma was based on parent or guardian report of a health care provider’s diagnosis of asthma.26 General health was assessed with the following question, with the parent or guardian as respondent: “How would you describe your child’s health in general?” (excellent/very good/good/fair/poor).
As described in detail elsewhere, latent profile analysis was used to classify study participants’ level of cognitive function (normal, low-normal, moderate impairment, severe impairment) based on verbal and nonverbal IQ and 5 executive function measures from the Differential Ability Scales-II and a Developmental NEuroPSYchological Assessment.27 In this study, the outcome of cognitive impairment at age 10 years included individuals with either moderate or severe impairment of cognitive function, based on the aforementioned latent profile analysis. Psychiatric disorders were identified at age 10 years using the Child Symptom Inventory, Fourth Edition (CSI-4), a 97-item screening tool for emotional and behavioral disorders.28–30 For assessment of attention deficit hyperactivity disorder (ADHD), 3 contexts were considered: (1) the parent or caregiver completed the CSI-4, (2) the child’s current teacher completed the CSI-4 Teacher Checklist, and (3) information based on the parent’s indication of the child having been diagnosed previously by a clinician to have ADHD. Participants who met criteria in any 2 of these 3 contexts were classified with ADHD.31 Anxiety and depression also were identified with the CSI-4. Children were classified as having an anxiety disorder if they screened positive for social phobia, separation anxiety, or generalized anxiety disorder and were classified as having depression if they screened positive for either major depressive disorder or dysthymic disorder.30
Outcomes at Age 15 Years
Data on asthma, anthropometric measurements, and general health were collected at age 15 years using methods similar to those described above for data collection at 10 years, except that 3 measurements were obtained for weight and height, from which the mean was calculated.
Cognitive abilities were assessed at age 15 years with the Wechsler Abbreviated Scale of Intelligence Second Edition (WASI-II)32 and the National Institutes of Health Toolbox Cognition Battery (NTCB).33,34 Latent profile analysis was used to classify study participants’ level of cognitive functioning (normal, low-normal, impaired) based on WASI-II verbal IQ and nonverbal IQ and the 7 NTCB subtests. In this study, the outcome of cognitive impairment at age 15 years included individuals with impaired cognitive function, based on the latent profile analysis of WASI-II and NTCB scores just described. Across the 9 assessments used to characterize cognitive function, the mean z-scores for the group with impaired cognitive function ranged from −1 to −2.
Mental health outcomes at age 15 years were identified using the Mini International Neuropsychiatric Interview–Kid edition 7.0.2 (MINI-KID),35 a structured clinical diagnostic interview to identify current Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, in children aged 6–17 years.35 Participants with a full-scale IQ <50 or a verbal IQ <50 were evaluated with the parent version of the MINI-KID, and those with a verbal IQ of 50-<70 and/or full-scale IQ of 50-<70 were evaluated by interviewing the adolescent alongside their parent or guardian using the adolescent version of the interview. For the current study, we defined an anxiety disorder as including generalized anxiety disorder, panic disorder, agoraphobia, separation anxiety disorder, social anxiety disorder, and specific phobia and defined depression as a diagnosis of major depressive disorder. We considered only current diagnoses identified by the MINI-KID.
Statistical Analyses
For primary analyses of relationships between change in weight, length, or weight/length ratio z-scores between NICU discharge and 2 years corrected age, we classified z-score changes into quintiles. To analyze associations between changes in z-scores in the interval between NICU discharge and age 2 years and the 10-year and 15-year outcomes, we estimated unadjusted ORs and aORs and 95% CIs for 2 types of exposures: top quintile change in weight, length, or weight/length ratio z-scores and bottom quintile change in z-scores. Individuals with a z-score change in quintiles 2–4 served as the referent groups.
We created causal models (ie, directed acyclic graphs) to inform our analytic plan36,37 by identifying the minimally sufficient adjustment sets of variables to include in our multivariable regression models. All adjustment sets included gestational age, chronic lung disease, neonatal cerebral white matter abnormality, and an indicator of maternal socioeconomic status. Statistical significance was defined as a 2-sided P value <.05.
Results
Compared with all 1222 study participants who were discharged alive from the NICU, the subset of participants seen at the 10- and 15-year follow-up visits were less likely to have mothers who were unmarried or had no formal education beyond high school or were covered by Medicaid or a state-supported medical insurance program (Table I; available at www.jpeds.com).
Table I.
Comparison of ELGAN study participants at discharge alive from the NICU, at age 10 years, and at age 15 years
| Characteristics | Discharge alive from NICU (N = 1222), n (%) | Age 10 y (N = 889), n (%) | Age 15 y (N = 694), n (%) |
|---|---|---|---|
| Maternal age, y | |||
| <21 | 174 (14) | 115 (13) | 80 (12) |
| 21–35 | 820 (67) | 594 (67) | 445 (67) |
| >35 | 228 (19) | 180 (20) | 144 (22) |
| Unmarried mother | 533 (44) | 353 (40) | 235 (35) |
| Maternal education, y | |||
| ≤12 | 521 (44) | 367 (41) | 250 (37) |
| >12-<16 | 276 (24) | 210 (24) | 153 (23) |
| ≥16 | 378 (32) | 312 (35) | 266 (40) |
| Mother covered by Medicaid or other state-supported medical insurance | 483 (40) | 314 (35) | 217 (32) |
| Race | |||
| Asian, Native American, or mixed race | 153 (13) | 98 (11) | 61 (9) |
| Black | 336 (28) | 227 (26) | 159 (24) |
| White | 722 (60) | 562 (63) | 449 (67) |
| Hispanic | |||
| Yes | 147 (12) | 86 (9.7) | 57 (8.6) |
| No | 1068 (88) | 800 (90) | 609 (91) |
| Maternal prepregnancy BMI, kg/m2 | |||
| <18.5 | 91 (7.8) | 68 (7.9) | 47 (7.3) |
| 18.5–<30 | 824 (70) | 595 (69) | 444 (69) |
| ≥30 | 256 (22) | 194 (23) | 154 (24) |
| Cesarean delivery | 809 (66) | 590 (66) | 440 (66) |
| Sex | |||
| Male | 638 (52) | 455 (51) | 341 (51) |
| Female | 584 (48) | 434 (49) | 328 (49) |
| Multiple gestation | 365 (32) | 293 (35) | 236 (38) |
| Gestational age, wk | |||
| 23–24 | 251 (21) | 187 (21) | 144 (22) |
| 25–26 | 562 (46) | 400 (45) | 305 (46) |
| 27 | 409 (34) | 302 (34) | 220 (33) |
| Birth weight, g | |||
| ≤750 | 448 (37) | 332 (37) | 253 (38) |
| 750–1000 | 529 (43) | 382 (43) | 284 (43) |
| >1000 | 245 (20) | 175 (20) | 132 (20) |
| Birth weight z-score <−2 | 65 (5.3) | 53 (6.0) | 41 (6.1) |
| Average daily weight gain in NICU | |||
| Lowest quartile | 301 (25) | 207 (23) | 156 (23) |
| Highest quartile | 308 (25) | 225 (25) | 167 (25) |
| Bacteremia | 382 (31) | 279 (31) | 205 (30) |
| White matter injury on neonatal ultrasound | 246 (20) | 188 (21) | 138 (21) |
| Severe retinopathy of prematurity | 162 (14) | 118 (14) | 94 (14) |
| Medical necrotizing enterocolitis | 11 (0.9) | 8 (0.9) | 5 (0.7) |
| Surgical necrotizing enterocolitis | 45 (3.7) | 32 (3.6) | 25 (3.7) |
| Spontaneous intestinal perforation | 36 (2.9) | 29 (3.3) | 20 (3.0) |
| Chronic lung disease | 616 (50) | 461 (52) | 357 (51) |
Of study participants who contributed data to this analysis, 49% were female, and 21% were born at 23–24 weeks of gestation, 45% at 25–26 weeks, and 34% at 27 weeks. Fifty-one percent had neonatal chronic lung disease, 21% had ultrasound-identified cerebral white matter injury, and 3.3% had necrotizing enterocolitis necessitating surgery.
Correlates of Weight Z-Score and Length Z-Score Change Between NICU Discharge and Approximately 2 Years of Age
Quintiles for change in weight z-score between NICU discharge and approximately 2 years corrected age were derived as <−0.28, ≥−0.28 and <0.60, ≥0.60 and <1.29, ≥1.29 and <2.02, and ≥2.02 (Table II; available at www.jpeds.com). Quintiles for change in length z-score from NICU discharge until 2 years corrected age were <0.48, ≥0.48 and <1.28, ≥1.28 and <2.08, ≥2.08 and <2.98, and ≥2.98. Compared with infants in the highest quartile for weight gain during the NICU hospitalization, infants in the lowest quartile were more likely to be in the highest quintile for change in weight z-score or change in length z-score between NICU discharge and age 2 years.
Table II.
Maternal and neonatal characteristics and child outcomes according to quintile of change in weight z-scores from NICU discharge to approximately age 2 years
| Characteristics/outcomes | Growth from NICU discharge to 2 y (change in weight z-score), n (%) | ||||
|---|---|---|---|---|---|
| Quintile 1 (<−0.28) (N = 152) |
Quintile 2 (≥−0.28; <0.60) (N = 170) |
Quintile 3 (≥0.60; <1.29) (N = 164) |
Quintile 4 (≥1.29; <2.02) (N = 150 |
Quintile 5 (≥2.02) (N = 163) |
|
| Maternal characteristics | |||||
| Age, y | |||||
| <21 | 17 (11) | 21 (12) | 19 (12) | 21 (14) | 23 (14) |
| 21–35 | 102 (67) | 118 (69) | 110 (67) | 103 (69) | 99 (61) |
| >35 | 33 (22) | 31 (18) | 35 (21) | 26 (17) | 41 (25) |
| Unmarried | 58 (38) | 51 (30) | 74 (45) | 70 (47) | 61 (37) |
| Education, y | |||||
| ≤12 | 59 (40) | 66 (40) | 71 (45) | 62 (42) | 58 (37) |
| >12–<16 | 33 (22) | 40 (24) | 35 (22) | 38 (26) | 31 (20) |
| ≥16 | 56 (38) | 60 (36) | 53 (33) | 47 (32) | 66 (43) |
| IQ | |||||
| ≤−2 | 8 (5.6) | 7 (4.2) | 8 (5.2) | 5 (3.4) | 4 (2.6) |
| >−2, ≤−1 | 8 (5.6) | 12 (7.2) | 10 (6.5) | 12 (8.3) | 11 (7.1) |
| >−1, ≤1 | 96 (68) | 123 (74) | 108 (70) | 108 (74) | 107 (69) |
| Medicaid or other state-supported medical insurance | 59 (40) | 54 (32) | 64 (40) | 49 (33) | 51 (32) |
| Race | |||||
| Asian, Native American, or mixed race | 11 (7.3) | 21 (13) | 21 (13) | 19 (13) | 14 (8.6) |
| Black | 34 (23) | 40 (24) | 43 (27) | 43 (29) | 41 (25) |
| White | 105 (70) | 107 (64) | 95 (60) | 88 (59) | 107 (66) |
| Hispanic | |||||
| No | 143 (95) | 152 (90) | 147 (90) | 135 (90) | 144 (89) |
| Yes | 8 (5.3) | 17 (10) | 17 (10) | 15 (10) | 18 (11) |
| Prepregnancy BMI, kg/m2 | |||||
| <18.5 | 22 (15) | 12 (7.3) | 12 (7.6) | 8 (5.4) | 6 (3.9) |
| 18.5–<30 | 101 (68) | 110 (67) | 111 (70) | 105 (71) | 111 (73) |
| ≥30 | 25 (17) | 43 (26) | 35 (22) | 34 (23) | 35 (23) |
| Histologic chorioamnionitis | 87 (57) | 87 (51) | 74 (45) | 65 (43) | 82 (50) |
| Indication for delivery | |||||
| Preterm labor | 69 (45) | 77 (45) | 77 (47) | 68 (45) | 77 (47) |
| Premature rupture of membranes | 34 (22) | 38 (22) | 40 (24) | 27 (18) | 34 (21) |
| Preeclampsia | 18 (12) | 21 (12) | 20 (12) | 25 (17) | 20 (12) |
| Placental abruption | 14 (9.2) | 21 (12) | 14 (8.5) | 13 (8.7) | 22 (13) |
| Cervical insufficiency | 10 (6.6) | 7 (4.1) | 4 (2.4) | 11 (7.3) | 5 (3.1) |
| Fetal indication | 7 (4.6) | 6 (3.5) | 9 (5.5) | 6 (4.0) | 5 (3.1) |
| Cesarean delivery | 98 (64) | 109 (64) | 116 (71) | 100 (67) | 108 (66) |
| Neonatal characteristics | |||||
| Sex | |||||
| Female | 85 (56) | 90 (53) | 74 (45) | 67 (45) | 76 (47) |
| Male | 67 (44) | 80 (47) | 90 (55) | 83 (55) | 87 (53) |
| Multiple gestation | 48 (33) | 61 (38) | 55 (36) | 47 (34) | 58 (38) |
| Gestational age, wk | |||||
| 23–24 | 35 (23) | 44 (26) | 22 (13) | 21 (14) | 40 (25) |
| 25–26 | 66 (43) | 74 (44) | 82 (50) | 76 (51) | 65 (40) |
| 27 | 51 (34) | 52 (31) | 60 (37) | 53 (35) | 58 (36) |
| Birth weight, g | |||||
| ≤750 | 59 (39) | 69 (41) | 45 (27) | 49 (33) | 67 (41) |
| 750–1000 | 62 (41) | 72 (42) | 73 (45) | 73 (49) | 67 (41) |
| >1000 | 31 (20) | 29 (17) | 46 (28) | 28 (19) | 29 (18) |
| Birth weight z-score | |||||
| <−2 | 5 (3.3) | 9 (5.3) | 5 (3.0) | 14 (9.3) | 13 (8.0) |
| ≥−2, <−1 | 18 (12) | 27 (16) | 17 (10) | 18 (12) | 24 (15) |
| ≥−1 | 129 (85) | 134 (79) | 142 (87) | 118 (79) | 126 (77) |
| Average daily weight gain in NICU | |||||
| Lowest quartile | 28 (18) | 31 (18) | 27 (16) | 34 (23) | 68 (42) |
| Highest quartile | 63 (41) | 56 (33) | 41 (25) | 24 (16) | 11 (6.7) |
| Bacteremia | 40 (26) | 49 (29) | 58 (35) | 47 (31) | 54 (33) |
| White matter injury on neonatal ultrasound | 43 (28) | 33 (19) | 27 (16) | 27 (18) | 35 (21) |
| Severe retinopathy of prematurity | 17 (11) | 27 (16) | 12 (7.5) | 19 (13) | 26 (16) |
| Necrotizing enterocolitis/SIP | |||||
| Medical | 1 (0.7) | 1 (0.6) | 2 (1.2) | 1 (0.7) | 3 (1.8) |
| Surgical | 2 (1.3) | 5 (2.9) | 4 (2.4) | 5 (3.3) | 11 (6.7) |
| Spontaneous intestinal perforation | 4 (2.6) | 4 (2.4) | 3 (1.8) | 3 (2.0) | 11 (6.7) |
| Chronic lung disease | 65 (44) | 98 (59) | 75 (46) | 71 (48) | 95 (58) |
| SNAP score | |||||
| <20 | 83 (55) | 81 (49) | 82 (52) | 85 (57) | 84 (52) |
| 20–29 | 35 (23) | 44 (26) | 44 (28) | 37 (25) | 34 (21) |
| 30+ | 32 (21) | 42 (25) | 33 (21) | 27 (18) | 43 (27) |
| Child outcomes at 10 y | |||||
| Cognitive impairment | 36 (24) | 36 (22) | 32 (20) | 37 (25) | 32 (20) |
| Anxiety | 19 (13) | 25 (15) | 32 (20) | 22 (15) | 21 (13) |
| ADHD | 21 (14) | 29 (17) | 34 (21) | 30 (21) | 22 (14) |
| Depression | 9 (6.1) | 10 (5.9) | 13 (8.0) | 12 (8.2) | 10 (6.3) |
| Obesity | 11 (7.4) | 18 (11) | 18 (11) | 19 (13) | 27 (17) |
| Asthma | 50 (33) | 63 (37) | 66 (40) | 50 (33) | 66 (40) |
| Fair/poor health by parent report | 6 (3.9) | 8 (4.7) | 6 (3.7) | 5 (3.3) | 8 (4.9) |
| One or more adverse outcomes† | 93 (61) | 105 (62) | 118 (72) | 105 (70) | 107 (66) |
| Child outcomes at 15 y | |||||
| Cognitive impairment | 18 (17) | 14 (11) | 15 (13) | 20 (18) | 16 (12) |
| Anxiety | 7 (6.5) | 9 (7.3) | 12 (10) | 10 (9.0) | 9 (7.0) |
| ADHD | 10 (9.3) | 6 (4.9) | 6 (5.1) | 7 (6.3) | 9 (7.0) |
| Depression | 5 (4.6) | 8 (6.5) | 7 (5.9) | 5 (4.5) | 6 (4.6) |
| Obesity | 18 (19) | 18 (17) | 22 (20) | 21 (20) | 26 (22) |
| Asthma | 28 (25) | 27 (22) | 33 (27) | 28 (25) | 32 (24) |
| Fair/poor health by parent report | 29 (26) | 29 (23) | 27 (22) | 23 (20) | 22 (17) |
| One or more adverse outcomes* | 61 (54) | 69 (55) | 83 (67) | 65 (57) | 79 (59) |
SIP, spontaneous intestinal perforation; SNAP, Score for Neonatal Acute Physiology.
All percentages are based on denominators that do not include participants with missing values.
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, and depression.
Associations Between Weight Z-Score Change Between NICU Discharge and Age 2 Years and Outcomes at 10 and 15 Years
No associations were found between either top or bottom quintile change in weight z-score between NICU discharge and approximately age 2 years and outcomes at age 10 or 15 years (Table III). In sex-stratified analyses, the aOR for the association between top quintile change in weight z-score and obesity at 10 years was 2.8 (95% CI, 1.3–5.9) in females and 1.0 (95% CI, 0.4–2.3) in males (Table IV). The aORs for the association between top quintile change in weight z-score and asthma at 10 years were 2.1 (95% CI, 1.2–3.7) in females and 0.8 (95% CI, 0.4–1.3) in males. aORs for the association between top quintile change in weight z-score and asthma at age 15 years were 2.3 (95% CI, 1.1–4.7) for females and 0.6 (95% CI, 0.3–1.3) for males.
Table III.
Associations between change in weight z-score from NICU discharge to age 2 years and outcomes at age 10 years and 15 years for individuals with lower gains in weight z-score (quintile 1) and individuals with higher gains in weight z-score (quintile 5) compared with the referent group (quintiles 2–4)
| Outcomes | Quintile 1 vs quintiles 2–4 | Quintile 5 vs quintiles 2–4 | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | aOR (95% CI) | Unadjusted OR (95% CI) | aOR (95% CI) | |
| Outcomes at 10 y (n = 799) | ||||
| Cognitive impairment | 1.1 (0.7–1.7) | 1.0 (0.6–1.6) | 0.9 (0.6–1.4) | 0.8 (0.5–1.4) |
| Anxiety | 0.7 (0.4–1.3) | 0.7 (0.4–1.2) | 0.8 (0.5–1.3) | 0.7 (0.4–1.3) |
| ADHD | 0.7 (0.4–1.1) | 0.6 (0.4–1.1) | 0.7 (0.4–1.1) | 0.7 (0.4–1.2) |
| Depression | 0.8 (0.4–1.7) | 0.9 (0.4–1.9) | 0.8 (0.4–1.8) | 1.0 (0.5–2.1) |
| Obesity | 0.6 (0.3–1.2) | 0.6 (0.3–1.2) | 1.5 (0.9–2.5) | 1.6 (0.9–2.7) |
| Asthma | 0.8 (0.6–1.2) | 0.9 (0.6–1.3) | 1.2 (0.8–1.7) | 1.2 (0.8–1.8) |
| Fair/poor health by parent report | 1.0 (0.4–2.6) | 0.9 (0.3–2.3) | 1.3 (0.5–2.9) | 1.5 (0.6–3.6) |
| One or more adverse outcomes* | 0.7 (0.5–1.1) | 0.7 (0.5–1.1) | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) |
| Outcomes at 15 y (n = 611) | ||||
| Cognitive impairment† | 1.2 (0.7–2.2) | 1.2 (0.6–2.4) | 0.9 (0.5–1.6) | 0.8 (0.4–1.5) |
| Anxiety‡ | 0.7 (0.3–1.7) | 0.8 (0.4–2.0) | 0.8 (0.4–1.7) | 0.8 (0.3–1.8) |
| ADHD‡ | 1.8 (0.8–4.0) | 1.6 (0.7–3.6) | 1.3 (0.6–3.0) | 1.5 (0.6–3.5) |
| Depression‡ | 0.8 (0.3–2.2) | 0.7 (0.2–2.1) | 0.8 (0.3–2.0) | 0.8 (0.3–2.1) |
| Obesity§ | 1.0 (0.5–1.7) | 1.1 (0.6–2.0) | 1.2 (0.7–2.0) | 1.1 (0.6–2.0) |
| Asthma¶ | 1.0 (0.6–1.7) | 1.0 (0.6–1.8) | 1.0 (0.6–1.6) | 1.1 (0.7–1.9) |
| Fair/poor health by parent report** | 1.2 (0.8–2.0) | 1.2 (0.7–2.0) | 0.7 (0.4–1.2) | 0.8 (0.5–1.4) |
| One or more adverse outcomes* | 0.8 (0.5–1.2) | 0.8 (0.5–1.2) | 1.0 (0.7–1.5) | 1.0 (0.6–1.5) |
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, and depression.
Adjusted for gestational age, maternal education, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal eligibility for Medicaid, maternal education, mother’s marital status, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal education, neonatal chronic lung disease, and white matter damage.
Adjusted for birth weight z-score, neonatal chronic lung disease, gestational age, public insurance, maternal education, unmarried mother, and white matter damage.
Adjusted for gestational age, maternal education, maternal Medicaid eligibility, mother marital status, neonatal chronic lung disease, and white matter damage.
Table IV.
Sex-stratified adjusted associations between change in weight z-score from NICU discharge to age 2 years and outcomes at age 10 years and 15 years for individuals with lower gains in weight z-score (quintile 1) and individuals with higher gains in weight z-score (quintile 5) compared with the referent group (quintiles 2–4)
| Outcomes | Quintile 1 vs quintiles 2–4, aOR (95% CI) | Quintile 5 vs quintiles 2–4, aOR (95% CI) | ||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Outcomes at 10 y (n = 799) | ||||
| Cognitive impairment | 0.9 (0.4–1.9) | 1.2 (0.6–2.4) | 0.8 (0.4–1.9) | 0.8 (0.4–1.5) |
| Anxiety | 0.9 (0.4–1.9) | 0.6 (0.2–1.3) | 1.1 (0.5–2.4) | 0.5 (0.2–1.2) |
| ADHD | 0.8 (0.4–1.9) | 0.6 (0.3–1.2) | 1.1 (0.5–2.6) | 0.5 (0.2–1.0) |
| Depression | 0.9 (0.3–2.9) | 0.9 (0.3–2.7) | 2.3 (0.9–6.1) | 0.3 (0.1–1.4) |
| Obesity | 0.4 (0.1–1.1) | 0.7 (0.3–2.0) | 2.8 (1.3–5.9) | 1.0 (0.4–2.3) |
| Asthma | 0.8 (0.4–1.3) | 1.0 (0.6–1.9) | 2.1 (1.2–3.7) | 0.8 (0.4–1.3) |
| Fair/poor health by parent report | 0.4 (0.1–1.9) | 2.1 (0.6–7.8) | 2.1 (0.6–7.3) | 1.1 (0.3–4.3) |
| One or more adverse outcomes* | 0.6 (0.4–1.1) | 0.8 (0.4–1.5) | 1.2 (0.6–2.2) | 0.6 (0.3–1.0) |
| Outcomes at 15 y (n = 611) | ||||
| Cognitive impairment† | 1.1 (0.4–3.4) | 1.4 (0.6–3.2) | 0.8 (0.2–2.4) | 0.7 (0.3–1.7) |
| Anxiety‡ | 0.6 (0.2–2.1) | 1.5 (0.4–6.3) | 0.9 (0.3–2.7) | 0.6 (0.1–2.8) |
| ADHD‡ | 1.4 (0.4–5.5) | 1.8 (0.6–5.8) | 2.9 (0.8–10.1) | 1.0 (0.3–3.4) |
| Depression‡ | 0.2 (0.0–1.5) | 2.7 (0.5–14.3) | 0.7 (0.2–2.6) | 1.3 (0.2–8.1) |
| Obesity§ | 1.2 (0.5–2.6) | 1.2 (0.5–3.3) | 1.1 (0.5–2.5) | 1.1 (0.5–2.5) |
| Asthma¶ | 0.8 (0.3–1.9) | 1.2 (0.6–2.6) | 2.3 (1.1–4.7) | 0.6 (0.3–1.3) |
| Fair/poor health by parent report** | 0.5 (0.2–1.2) | 2.3 (1.1–4.9) | 1.3 (0.6–2.6) | 0.5 (0.2–1.1) |
| One or more adverse outcomes* | 0.6 (0.3–1.2) | 0.9 (0.5–1.9) | 1.3 (0.7–2.6) | 0.7 (0.4–1.4) |
Bold type indicates OR significant at P < .05.
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, and depression.
Adjusted for gestational age, maternal education, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal eligibility for Medicaid, maternal education, mother’s marital status, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal education, neonatal chronic lung disease, and white matter damage.
Adjusted for birth weight z-score, neonatal chronic lung disease, gestational age, public insurance, maternal education, unmarried mother, and white matter damage.
Adjusted for gestational age, maternal education, maternal Medicaid eligibility, mother’s marital status, neonatal chronic lung disease, and white matter damage.
Associations Between Length Z-Score Change Between NICU Discharge and Approximately 2 Years of Age and Outcomes at 10 and 15 Years
Study participants in the top quintile for change in length z-score from NICU discharge to age 2 years had higher odds of cognitive impairment at 10 years of age (aOR, 1.9; 95% CI, 1.1–3.1) and lower odds of obesity at 15 years (Table V; available at www.jpeds.com). Among males, low gain in length z-score was associated with parent report of fair or poor health at 15 years (Table VI; available at www.jpeds.com).
Table V.
Associations between change in length z-score from NICU discharge to age 2 years and outcomes at age 10 years and 15 years for individuals with lower gains in length z-score (quintile 1) and individuals with higher gains in length z-score (quintile 5) compared with the referent group (quintiles 2–4)
| Outcomes | Quintile 1 vs quintiles 2–4 | Quintile 5 vs quintiles 2–4 | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | aOR (95% CI) | Unadjusted OR (95% CI) | aOR (95% CI) | |
| Outcomes at 10 y (n = 799) | ||||
| Cognitive impairment | 1.4 (0.8–2.2) | 1.4 (0.8–2.4) | 2.3 (1.5–3.6) | 1.9 (1.1–3.1) |
| Anxiety | 0.7 (0.4–1.4) | 0.7 (0.3–1.3) | 1.3 (0.8–2.2) | 1.2 (0.7–2.1) |
| ADHD | 1.0 (0.5–1.7) | 0.9 (0.5–1.7) | 1.0 (0.6–1.7) | 0.9 (0.5–1.5) |
| Depression | 0.4 (0.2–1.1) | 0.4 (0.2–1.1) | 0.5 (0.2–1.1) | 0.5 (0.2–1.2) |
| Obesity | 0.7 (0.4–1.4) | 0.7 (0.4–1.4) | 0.8 (0.5–1.5) | 0.9 (0.5–1.7) |
| Asthma | 1.0 (0.6–1.5) | 1.0 (0.6–1.6) | 1.2 (0.8–1.8) | 1.2 (0.8–1.8) |
| Fair/poor health by parent report | 0.6 (0.2–2.2) | 0.7 (0.2–2.5) | 1.2 (0.4–3.1) | 1.0 (0.3–2.9) |
| One or more adverse outcomes* | 0.8 (0.6–1.3) | 0.8 (0.5–1.2) | 1.5 (1.0–2.3) | 1.2 (0.7–1.9) |
| Outcomes at 15 y (n = 611) | ||||
| Cognitive impairment† | 1.0 (0.5–2.1) | 1.2 (0.6–2.5) | 1.7 (1.0–3.1) | 1.3 (0.7–2.6) |
| Anxiety‡ | 0.5 (0.2–1.3) | 0.5 (0.1–1.4) | 0.8 (0.4–1.9) | 0.9 (0.4–2.2) |
| ADHD‡ | 1.9 (0.8–4.8) | 1.9 (0.7–5.1) | 1.6 (0.7–4.1) | 1.3 (0.5–3.4) |
| Depression‡ | 0.3 (0.1–1.5) | 0.3 (0.1–1.5) | 0.7 (0.3–2.1) | 0.7 (0.2–2.2) |
| Obesity§ | 1.0 (0.5–1.7) | 1.0 (0.5–1.9) | 0.5 (0.3–1.0) | 0.5 (0.2–0.9) |
| Asthma¶ | 1.2 (0.7–2.1) | 1.6 (0.9–2.9) | 1.0 (0.6–1.8) | 1.2 (0.7–2.2) |
| Fair/poor health by parent report** | 1.3 (0.8–2.2) | 1.4 (0.8–2.5) | 0.8 (0.5–1.4) | 0.8 (0.4–1.5) |
| One or more adverse outcomes* | 1.1 (0.7–1.7) | 1.3 (0.8–2.2) | 1.1 (0.7–1.8) | 0.9 (0.5–1.4) |
Bold type indicates OR significant at P < .05.
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, and depression.
Adjusted for gestational age, maternal education, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal eligibility for Medicaid, maternal education, mother’s marital status, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal education, neonatal chronic lung disease, and white matter damage.
Adjusted for birth weight z-score, neonatal chronic lung disease, gestational age, public insurance, maternal education, unmarried mother, and white matter damage.
Adjusted for gestational age, maternal education, maternal Medicaid eligibility, mother marital status, neonatal chronic lung disease, and white matter damage.
Table VI.
Sex-stratified adjusted associations between the change in length z-score from NICU discharge to age 2 years and outcomes at 10 years and 15 years
| Outcomes | 10-y outcomes (n = 434) | 15-y outcomes (n = 300) | ||
|---|---|---|---|---|
| Quintile 1 vs quintiles 2–4, aOR (95% CI) |
Quintile 5 vs quintiles 2–4, aOR (95% CI) |
Quintile 1 vs quintiles 2–4, aOR (95% CI) |
Quintile 5 vs quintiles 2–4, aOR (95% CI) |
|
| Females | ||||
| Cognitive impairment* | 1.0 (0.4–2.6) | 2.0 (0.9–4.6) | 0.8 (0.2–3.4) | 1.8 (0.6–5.1) |
| Anxiety† | 0.7 (0.3–1.9) | 1.0 (0.4–2.5) | 0.6 (0.2–2.1) | 0.7 (0.2–2.4) |
| ADHD† | 0.7 (0.3–2.1) | 1.4 (0.6–3.5) | 0.9 (0.2–4.7) | 0.5 (0.1–3.3) |
| Depression† | 0.2 (0.0–1.8) | 0.8 (0.3–2.9) | 0.2 (0.0–1.5) | 0.7 (0.2–3.1) |
| Obesity‡ | 0.8 (0.3–2.0) | 1.2 (0.5–3.0) | 0.9 (0.4–2.2) | 0.4 (0.1–1.1) |
| Asthma§ | 0.7 (0.3–1.4) | 2.0 (1.0–3.7) | 1.9 (0.8–4.6) | 2.2 (0.9–5.2) |
| Fair/poor health by parent report¶ | 1.6 (0.3–10.4) | 0.6 (0.1–4.6) | 0.9 (0.4–2.0) | 0.5 (0.2–1.3) |
| One or more adverse outcomes** | 0.5 (0.3–1.0) | 1.5 (0.7–3.2) | 1.2 (0.6–2.7) | 0.8 (0.4–1.7) |
| Males | ||||
| Cognitive impairment* | 1.5 (0.8–3.1) | 1.6 (0.8–3.2) | 1.5 (0.6–3.7) | 1.1 (0.5–2.9) |
| Anxiety† | 0.6 (0.2–1.6) | 1.2 (0.6–2.6) | †† | 0.3 (0.1–2.0) |
| ADHD† | 0.9 (0.4–2.0) | 0.6 (0.3–1.2) | 2.9 (0.7–11.7) | 2.0 (0.5–8.5) |
| Depression† | 0.6 (0.2–1.9) | 0.2 (0.0–1.1) | 0.5 (0.1–4.8) | 0.4 (0.0–3.9) |
| Obesity‡ | 0.6 (0.2–1.5) | 0.6 (0.2–1.6) | 1.1 (0.4–2.8) | 0.7 (0.2–2.1) |
| Asthma§ | 1.2 (0.6–2.2) | 0.7 (0.4–1.3) | 1.3 (0.6–2.9) | 0.7 (0.3–1.8) |
| Fair/poor health by parent report¶ | 0.3 (0.0–2.9) | 1.0 (0.3–3.9) | 2.4 (1.1–5.3) | 1.1 (0.5–2.7) |
| One or more adverse outcomes** | 1.2 (0.6–2.2) | 0.7 (0.4–1.3) | 1.3 (0.6–2.9) | 0.7 (0.3–1.8) |
Bold type indicates OR significant at P < .05.
Adjusted for gestational age, maternal education, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal eligibility for Medicaid, maternal education, mother’s marital status, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal education, neonatal chronic lung disease, and white matter damage.
Adjusted for neonatal chronic lung disease, gestational age, public insurance, maternal education, maternal single, and white matter damage.
Adjusted for birth weight z-score, gestational age, maternal education, maternal prepregnancy body mass index, neonatal chronic lung disease, and white matter damage.
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, and depression.
The low weight gain group had too few instances of anxiety to estimate associations.
Associations Between Weight/Length Ratio Z-Score Change Between NICU Discharge and Age 2 Years and Outcomes at 10 and 15 Years
Top quintile of change in weight/length ratio z-score was associated with higher odds of obesity at 10 years of age (aOR, 2.1; 95% CI, 1.2–3.6) (Table VII) but not at age 15 years. Sex-stratified analyses identified this association only in females (Table VIII). The top quintile of change in weight/length ratio z-score was associated with lower odds of cognitive impairment among males at age 10 years (aOR, 0.4; 95% CI, 0.2–0.9) and ADHD (aOR, 0.4; 95% CI, 0.2–0.9). The lowest quintile of change in weight/length ratio z-score was associated with higher odds of parent-reported fair or poor health among males at age 15 years (aOR, 2.6; 95% CI, 1.2–5.9) and with higher odds of anxiety among females at age 15 years (aOR, 3.7; 95% CI, 1.1–12.1).
Table VII.
Associations between change in weight/length ratio z-score from NICU discharge to age 2 years and outcomes at age 10 years and 15 years for individuals with lower gains in weight/length ratio z-score (quintile 1) and individuals with higher gains in weight/length ratio z-score (quintile 5) compared with the referent group (quintiles 2–4)
| Outcomes | Quintile 1 vs quintiles 2–4 | Quintile 5 vs quintiles 2–4 | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | aOR (95% CI) | Unadjusted OR (95% CI) | aOR (95% CI) | |
| Outcomes at 10 y (n = 799) | ||||
| Cognitive impairment | 2.1 (1.4–3.2) | 1.4 (0.9–2.2) | 0.5 (0.3–0.9) | 0.7 (0.4–1.2) |
| Anxiety | 1.1 (0.7–2.0) | 0.8 (0.4–1.5) | 1.0 (0.5–1.7) | 1.3 (0.7–2.3) |
| ADHD | 1.1 (0.7–1.9) | 0.9 (0.5–1.5) | 0.5 (0.3–0.9) | 0.6 (0.3–1.1) |
| Depression | 0.8 (0.3–1.8) | 0.8 (0.3–2.0) | 1.2 (0.6–2.4) | 1.3 (0.6–2.9) |
| Obesity | 0.8 (0.4–1.7) | 0.8 (0.4–1.6) | 2.1 (1.2–3.5) | 2.1 (1.2–3.6) |
| Asthma | 1.1 (0.7–1.6) | 1.0 (0.7–1.6) | 1.0 (0.7–1.5) | 1.0 (0.7–1.6) |
| Fair/poor health by parent report | 2.1 (0.8–5.2) | 1.6 (0.6–4.6) | 1.0 (0.3–3.2) | 1.6 (0.5–5.3) |
| One or more adverse outcomes* | 1.6 (1.0–2.5) | 1.4 (0.8–2.3) | 0.9 (0.6–1.3) | 1.0 (0.6–1.5) |
| Outcomes at 15 y (n = 611) | ||||
| Cognitive impairment† | 2.8 (1.6–4.8) | 1.8 (1.0–3.4) | 0.8 (0.4–1.5) | 1.2 (0.6–2.5) |
| Anxiety‡ | 1.9 (0.8–4.3) | 2.5 (1.0–6.2) | 1.9 (0.9–4.2) | 1.5 (0.7–3.4) |
| ADHD‡ | 2.8 (1.2–6.3) | 2.4 (0.9–5.9) | 1.0 (0.3–2.8) | 1.4 (0.5–4.2) |
| Depression‡ | 0.8 (0.3–2.6) | 0.9 (0.3–2.9) | 1.1 (0.4–2.9) | 1.1 (0.4–3.2) |
| Obesity§ | 0.8 (0.4–1.5) | 0.9 (0.4–1.8) | 1.6 (0.9–2.8) | 1.5 (0.8–2.6) |
| Asthma¶ | 1.3 (0.8–2.2) | 1.1 (0.6–2.1) | 1.3 (0.8–2.2) | 1.3 (0.8–2.4) |
| Fair/poor health by parent report** | 1.9 (1.1–3.2) | 1.7 (1.0–3.1) | 1.5 (0.9–2.4) | 1.6 (0.9–2.9) |
| One or more adverse outcomes* | 2.1 (1.3–3.5) | 1.6 (0.9–2.9) | 1.1 (0.7–1.7) | 1.5 (0.9–2.5) |
Bold type indicates OR significant at P < .05.
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, depression.
Adjusted for gestational age, maternal education, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal eligibility for Medicaid, maternal education, mother’s marital status, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal education, neonatal chronic lung disease, and white matter damage.
Adjusted for birth weight z-score, neonatal chronic lung disease, gestational age, public insurance, maternal education, unmarried mother, and white matter damage.
Adjusted for gestational age, maternal education, maternal Medicaid eligibility, mother marital status, neonatal chronic lung disease, and white matter damage.
Table VIII.
Sex-stratified adjusted associations between change in weight/length ratio z-score from NICU discharge to age 2 years and outcomes at age 10 years and 15 years for individuals with lower gains in weight/length ratio z-score (quintile 1) and individuals with higher gains in weight/length ratio z-score (quintile 5) compared with the referent group (quintiles 2–4)
| Outcomes | Quintile 1 vs quintiles 2–4, aOR (95% CI) | Quintile 5 vs quintiles 2–4, aOR (95% CI) | ||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Outcomes at 10 y (n = 799) | ||||
| Cognitive impairment | 0.9 (0.4–1.9) | 1.9 (1.0–3.8) | 1.1 (0.4–2.8) | 0.4 (0.2–0.9) |
| Anxiety | 0.9 (0.3–2.3) | 0.9 (0.4–2.0) | 1.2 (0.5–3.2) | 1.3 (0.6–2.8) |
| ADHD | 1.3 (0.5–3.2) | 0.7 (0.3–1.5) | 0.8 (0.3–2.3) | 0.4 (0.2–0.9) |
| Depression | 1.5 (0.4–5.5) | 0.5 (0.1–1.9) | 2.6 (0.8–8.4) | 0.8 (0.3–2.3) |
| Obesity | 0.3 (0.1–1.3) | 1.0 (0.4–2.5) | 3.2 (1.4–7.3) | 1.4 (0.6–3.4) |
| Asthma | 0.9 (0.4–1.7) | 1.3 (0.7–2.5) | 1.2 (0.6–2.3) | 0.9 (0.5–1.7) |
| Fair/poor health by parent report | 1.6 (0.3–9.0) | 1.8 (0.5–7.3) | 1.4 (0.2–9.0) | 1.2 (0.2–6.6) |
| One or more adverse outcomes* | 1.3 (0.6–2.8) | 1.6 (0.7–3.4) | 1.7 (0.8–3.4) | 0.7 (0.4–1.2) |
| Outcomes at 15 y (n = 611) | ||||
| Cognitive impairment† | 1.8 (0.7–5.1) | 1.9 (0.8–4.3) | 1.0 (0.3–3.7) | 1.1 (0.4–2.8) |
| Anxiety‡ | 3.7 (1.1–12.1) | 1.7 (0.3–8.3) | 2.6 (0.9–7.6) | 0.5 (0.1–3.1) |
| ADHD‡ | 4.1 (0.9–18.4) | 1.3 (0.4–4.6) | 3.2 (0.6–17.4) | 0.8 (0.1–3.9) |
| Depression‡ | 1.4 (0.3–6.2) | 0.7 (0.1–7.8) | 0.9 (0.2–3.8) | 1.3 (0.2–8.1) |
| Obesity§ | 0.6 (0.2–1.8) | 1.4 (0.5–4.0) | 1.5 (0.7–3.5) | 1.4 (0.6–3.2) |
| Asthma¶ | 2.0 (0.8–5.0) | 0.8 (0.3–1.9) | 1.4 (0.6–3.3) | 1.1 (0.5–2.5) |
| Fair/poor health by parent report** | 1.1 (0.4–2.7) | 2.6 (1.2–5.9) | 1.5 (0.7–3.4) | 1.8 (0.8–4.1) |
| One or more adverse outcomes* | 1.4 (0.6–3.3) | 2.1 (0.9–4.8) | 1.7 (0.8–3.7) | 1.3 (0.7–2.7) |
Bold type indicates OR significant at P < .05.
Adverse outcomes that were assessed: bilateral blindness, hearing impairment requiring amplification, cerebral palsy, asthma, obesity, epilepsy, autism spectrum disorder, cognitive impairment, ADHD, anxiety, and depression.
Adjusted for gestational age, maternal education, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal eligibility for Medicaid, maternal education, mother’s marital status, neonatal chronic lung disease, and neonatal ultrasound-identified white matter abnormality.
Adjusted for birth weight z-score, gestational age, maternal education, neonatal chronic lung disease, and white matter damage.
Adjusted for birth weight z-score, neonatal chronic lung disease, gestational age, public insurance, maternal education, unmarried mother, and white matter damage.
Adjusted for gestational age, maternal education, maternal Medicaid eligibility, mother marital status, neonatal chronic lung disease, and white matter damage.
Discussion
In a multicenter cohort of individuals born extremely preterm, we evaluated associations between gains in weight, length, and weight/length ratio during the first 2 years after discharge from the NICU and developmental and health outcomes at age 10 and 15 years. Of the outcomes analyzed, only obesity was less prevalent among children born preterm compared with children born at term.26,30,38–43 We identified associations between a top quintile gain in weight z-score and weight/length ratio z-score and obesity at age 10 years, but these associations were found only in females and were not found at age 15 years. Also associated with top quintile weight gain in females, but not in males, was a higher odds of asthma, which is more prevalent among children with obesity. These results suggest that among females born extremely preterm, high gains in weight/length ratio and weight might increase the likelihood of obesity and asthma, at least in middle childhood. A lower odds of obesity at age 15 years was associated with high gains in length during the first 2 years after discharge from the NICU. Because we evaluated a large number of associations, it is likely that some of the statistically significant associations are attributable to chance; however, associations between gains in weight, length, and weight/length ratio early in life and subsequent BMI are biologically plausible and are consistent with findings from a recent systematic review.44
In our analyses, the only finding consistent with the possibility that extremely preterm infants who exhibit top quintile growth gains during infancy might have better neurodevelopmental outcomes was the association between high gain in weight/length ratio and lower odds of cognitive impairment and ADHD, but this was found only in males and only at age 10 years. Overall, our findings suggest that within the range of weight, length, and weight/length gains experienced by the ELGAN cohort, greater gains in weight and weight/length ratio are not associated with more favorable neurodevelopmental outcomes but might contribute, in females only, to obesity in middle childhood but not to obesity in adolescence.
After NICU discharge, nutritional modifications for extremely preterm infants can increase the rate of weight and length gain45,46 but are of uncertain benefit for neurodevelopment.14,47 Our finding that greater gains in weight and length were not associated with improved neurocognitive or psychiatric outcomes are in contrast to those from the Infant Health and Development Program (IHDP) cohort of children born preterm with low birth weight, in whom a 1-unit increase in weight z-score gain from term to 12 months was associated with an 1.9-point increase in IQ,48 and more rapid linear growth from term to 4 months and more rapid BMI gain from 4 to 12 months were associated with a lower odds of IQ <85.15 Comparing IHDP and ELGAN cohorts, the years of birth differed by 17–18 years (1985–1986 for IHDP versus 2002–2004 for ELGAN), an interval during which numerous nutritional practices for preterm infants changed, including fortification of human milk,49 micronutrient supplementation,50 protein and energy intake,51 and nutrient-enriched formula for preterm infants after NICU discharge.47 Changes also occurred in rates of obesity and cultural norms around food.52
Consistent with prior studies of growth after preterm birth,12–14,53 obesity was found more frequently among females in the top quintile of change in weight and weight/length ratio z-scores. In the ELGAN cohort54 and studies of children not selected on gestational age,55–59 obesity has been associated with asthma. In the IHDP cohort, more rapid BMI gain in the first year of life was associated with asthma at age 8 years.60 These associations between greater weight gain and obesity warrant further study, and it is possible that limiting “catch-up” growth might confer health benefits to subgroups of individuals born extremely preterm. The potential importance of timing of high weight gain is suggested by one study that found no association between weight gain in the first 12 weeks after NICU discharge and obesity,61 in contrast to another study that found an association between weight gain at 2.5–6 years and higher BMI.62
Limitations of our study that could introduce bias include sample attrition, which was approximately 50% greater in children whose mothers were Medicaid-eligible at the time of delivery. In addition, we lacked data on specific aspects of study participants’ nutrition, such as the relative proportions of human milk and formula, which could affect weight gain,63 and on genetic and environment factors associated with both postdischarge weight gain and the risk of adverse outcomes. Also, fat-free mass might be a more informative indicator of nutrient accretion than weight, length, or weight/length ratio.64 Furthermore, standardized procedures were not used for measurement of weight and length at the time of NICU discharge and at age 2 years. Related to the outcomes that we studied, BMI is an imperfect measure of adiposity, and parental report of physician-diagnosed asthma is less valid than objective assessment of lung function.65
Regarding the generalizability of our findings, we focused on gains in weight, length, and weight/length in the first 2 years after discharge from the NICU, so our conclusions about the relationship of infant growth to later outcomes might not apply to growth between birth and NICU discharge, an interval during which nutritional fortification66 and greater growth velocity in infants born very preterm or with extremely low birth weight67,68 has been associated with better neurodevelopmental outcomes at age 18–22 months. In addition, we focused on individuals born extremely preterm whose caregivers, owing to differing perceptions of weight and health, might feed their children differently from children born at term.69 Importantly, the distributions of gains in weight, length, and weight/length found in the ELGAN cohort might differ considerably from those of other samples of individuals born extremely preterm.
Strengths of the study include the relatively large sample size, the use of gestational age rather than birthweight to select the study sample,70 and outcome assessments by examiners who were unaware of participants’ growth data.
During the 2 years after NICU discharge, extremely preterm female infants with high gains in weight/length or weight had higher odds of obesity at 10 years, but not at 15 years. Infants with high growth gains in the 2 years after NICU discharge had neurocognitive and psychiatric outcomes similar to those of infants with lower gains in weight and weight/length.
Acknowledgments
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (5U01 NS040069-05, to A.L. and 2R01 NS040069-06A2, to K.K.); the Office of the National Institutes of Health Director (UH3OD023348, to T.O.), National Institute of Child Health and Human Development (5R01 HD092374-05, to T.O. and R.F.); and National Heart, Lung, and Blood Institute (K23 HL148394, to A.S., L40 HL148910, to A.S., and R01 HL146818, to L.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
Acknowledgment information is available at www.jpeds.com.
Glossary
- ADHD
Attention deficit hyperactivity disorder
- BMI
Body mass index
- CSI-4
Child Symptom Inventory 4
- ELGAN
Extremely Low Gestational Age Newborn
- IHDP
Infant Health and Development Program
- MINI-KID
Mini International Neuropsychiatric Interview–Kid Edition
- NICU
Neonatal intensive care unit
- NTCB
National Institutes of Health Toolbox Cognition Battery
- WASI-II
Wechsler Abbreviated Scale of Intelligence, Second Edition
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Zhang X, Donnelly B, Thomas J, Sams L, O’Brien K, Taylor SN, et al. Growth in the high-risk newborn infant post-discharge: results from a neonatal intensive care unit nutrition follow-up clinic. Nutr Clin Pract 2020;35:738–44. [DOI] [PubMed] [Google Scholar]
- 2.Ruys CA, van de Lagemaat M, Rotteveel J, MJJ Finken, Lafeber HN. Improving long-term health outcomes of preterm infants: how to implement the findings of nutritional intervention studies into daily clinical practice. Eur J Pediatr 2021;180:1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986–90. [DOI] [PubMed] [Google Scholar]
- 4.Fenton TR, Nasser R, Creighton D, Tang S, Sauve R, Bilan D, et al. Weight, length, and head circumference at 36 weeks are not predictive of later cognitive impairment in very preterm infants. J Perinatol 2021;41:606–14. [DOI] [PubMed] [Google Scholar]
- 5.Hack M, Schluchter M, Margevicius S, Andreias L, Taylor HG, Cuttler L. Trajectory and correlates of growth of extremely-low-birth-weight adolescents. Pediatr Res 2014;75:358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res 2006;60:751–8. [DOI] [PubMed] [Google Scholar]
- 7.Nash A, Dunn M, Asztalos E, Corey M, Mulvihill-Jory B, O’Connor DL. Pattern of growth of very low birth weight preterm infants, assessed using the WHO Growth Standards, is associated with neurodevelopment. Appl Physiol Nutr Metab 2011;36:562–9. [DOI] [PubMed] [Google Scholar]
- 8.Belfort MB, Kuban KC, O’Shea TM, Allred EN, Ehrenkranz RA, Engelke SC, et al. Weight status in the first 2 years of life and neurodevelopmental impairment in extremely low gestational age newborns. J Pediatr 2016;168:30–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hack M, Merkatz IR, McGrath SK, Jones PK, Fanaroff AA. Catch-up growth in very-low-birth-weight infants. Clinical correlates. Am J Dis Child 1984;138:370–5. [DOI] [PubMed] [Google Scholar]
- 10.Prader A, Tanner JM, von Harnack G. Catch-up growth following illness or starvation. An example of developmental canalization in man. J Pediatr 1963;62:646–59. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Growth in 10- to 12-year-old children born at 23 to 25 weeks’ gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics 2006;118:e1452–65. [DOI] [PubMed] [Google Scholar]
- 12.Vohr BR, Heyne R, Bann CM, Das A, Higgins RD, Hintz SR, et al. Extreme preterm infant rates of overweight and obesity at school age in the SUPPORT neuroimaging and neurodevelopmental outcomes cohort. J Pediatr 2018;200:132–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood CT, Linthavong O, Perrin EM, Leviton A, Allred EN, Kuban KCK, et al. Antecedents of obesity among children born extremely preterm. Pediatrics 2018;142:e20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong KK, Kennedy K, Castañeda-Gutiérrez E, Forsyth S, Godfrey KM, Koletzko B, et al. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr 2015;104:974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr 2013;163:1564–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KCK, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev 2009;85:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527–32. [PubMed] [Google Scholar]
- 19.Kuban K, Adler I, Allred EN, Batton D, Bezinque S, Betz BW, et al. Observer variabiity assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol 2007;37:1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Shea TM, Allred EN, Kuban KCK, Hirtz D, Specter B, Durfee S, et al. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol 2012;27:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leviton A, O’Shea TM, Bednarek FJ, Allred EN, Fichorova RN, Dammann O. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr 2012;101:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin CR, Bellomy M, Allred EN, Fichorova RN, Leviton A. Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal Pediatr Pathol 2013;32:222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CR, Dammann O, Allred EN, Patel S, O’Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr 2010;157: 751–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolsma KW, Allred EN, Chen ML, Duker J, Leviton A, Dammann O. Neonatal bacteremia and retinopathy of prematurity: the ELGAN study. Arch Ophthalmol 2011;129:1555–63. [DOI] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 2002;11:1–190. [PubMed] [Google Scholar]
- 26.Jackson WM, O’Shea TM, Allred EN, Laughon MM, Gower WA, Leviton A. Risk factors for chronic lung disease and asthma differ among children born extremely preterm. Pediatr Pulmonol 2018;53:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Cognitive functioning at the age of 10 years among children born extremely preterm: a latent profile approach. Pediatr Res 2017;82:614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadow KD, Srafkin J. Adolescent Symptom Inventory - 4R (ASI-4R) Norms manual. Stony Brook (NY): Checkmate Plus; 2014. [Google Scholar]
- 29.Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc Psychol 2002;31:513–24. [DOI] [PubMed] [Google Scholar]
- 30.Dvir Y, Frazier JA, Joseph RM, Mokrova I, Moore PS, O’Shea TM, et al. Psychiatric symptoms: prevalence, co-occurrence, and functioning among extremely low gestational age newborns at age 10 years. J Dev Behav Pediatr 2019;40:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott MN, Hunter SJ, Joseph RM, O’Shea TM, Hooper SR, Allred EN, et al. Neurocognitive correlates of attention-deficit hyperactivity disorder symptoms in children born at extremely low gestational age. J Dev Behav Pediatr 2017;38:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wechsler D Wechsler abbreviated scale of intelligence second edition (WASI-II). San Antonio (TX): Pearson; 2011. [Google Scholar]
- 33.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013;80(11 Suppl 3):S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weintraub S, Bauer PJ, Zelazo PD, Wallner-Allen K, Dikmen SS, Heaton RK, et al. I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr Soc Res Child Dev 2013;78:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 2010;71:313–26. [DOI] [PubMed] [Google Scholar]
- 36.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen JB, D’Agostino McGowan L, Jensen ET, Rigdon J, South AM. Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: beyond confounding. J Hypertens 2021;39:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph RM, O’Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, et al. Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics 2016;137:e20154343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zablotsky B, Black LI, Maenner MJ, Schieve LA, Danielson ML, Bitsko RH, et al. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 2019;144: e20190811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance—United States, 2006–2018. MMWR Surveill Summ 2021;70:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebrun-Harris LA, Ghandour RM, Kogan MD, Warren MD. Five-year trends in US children’s health and well-being, 2016–2020. JAMA Pediatr 2022;176:e220056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. Mental health surveillance among children—United States, 2013–2019. MMWR Suppl 2022;71:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr 2019;206:256–67.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halilagic A, Moschonis G. The effect of growth rate during infancy on the risk of developing obesity in childhood: a systematic literature review. Nutrients 2021;13:3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aimone A, Rovet J, Ward W, Jefferies A, Campbell DM, Asztalos E, et al. Growth and body composition of human milk-fed premature infants provided with extra energy and nutrients early after hospital discharge: 1-year follow-up. J Pediatr Gastroenterol Nutr 2009;49:456–66. [DOI] [PubMed] [Google Scholar]
- 46.Lucas A, Fewtrell MS, Morley R, Singhal A, Abbott RA, Isaacs E, et al. Randomized trial of nutrient-enriched formula versus standard formula for postdischarge preterm infants. Pediatrics 2001;108:703–11. [DOI] [PubMed] [Google Scholar]
- 47.Young L, Embleton ND, McGuire W. Nutrient-enriched formula versus standard formula for preterm infants following hospital discharge. Cochrane Database Syst Rev 2016;12:CD004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belfort MB, Martin CR, Smith VC, Gillman MW, McCormick MC. Infant weight gain and school-age blood pressure and cognition in former preterm infants. Pediatrics 2010;125:e1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuschel CA, Harding JE. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Database Syst Rev 2004;1: CD000343. [DOI] [PubMed] [Google Scholar]
- 50.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med 1999;340:1962–8. [DOI] [PubMed] [Google Scholar]
- 51.Young A, Beattie RM, Johnson MJ. Optimising growth in very preterm infants: reviewing the evidence. Arch Dis Child Fetal Neonatal Ed 2022. [DOI] [PubMed] [Google Scholar]
- 52.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018;141:e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaskins RB, LaGasse LL, Liu J, Shankaran S, Lester BM, Bada HS, et al. Small for gestational age and higher birth weight predict childhood obesity in preterm infants. Am J Perinatol 2010;27:721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linthavong O, O’Shea TM, Allred E, Perrin E, Bauserman M, Joseph RM, et al. Neurocognitive and health correlates of overweight and obesity among ten-year-old children born extremely preterm. J Pediatr 2018;200:84–90.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang JE, Bunnell HT, Hossain MJ, Wysocki T, Lima JJ, Finkel TH, et al. Being overweight or obese and the development of asthma. Pediatrics 2018;142:e20182119. [DOI] [PubMed] [Google Scholar]
- 56.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Contreras ZA, Chen Z, Roumeliotaki T, Annesi-Maesano I, Baiz N, von Berg A, et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur Respir J 2018;52:1800504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med 2017;195: 1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cockrell Skinner A, Perrin EM, Steiner MJ. Healthy for now? A cross-sectional study of the comorbidities in obese preschool children in the United States. Clin Pediatr (Phila) 2010;49:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belfort MB, Cohen RT, Rhein LM, McCormick MC. Preterm infant growth and asthma at age 8 years. Arch Dis Child Fetal Neonatal Ed 2016;101:F230–4. [DOI] [PubMed] [Google Scholar]
- 61.Embleton ND, Korada M, Wood CL, Pearce MS, Swamy R, Cheetham TD. Catch-up growth and metabolic outcomes in adolescents born preterm. Arch Dis Child 2016;101:1026–31. [DOI] [PubMed] [Google Scholar]
- 62.Ni Y, Beckmann J, Hurst JR, Morris JK, Marlow N. Size at birth, growth trajectory in early life, and cardiovascular and metabolic risks in early adulthood: EPICure study. Arch Dis Child Fetal Neonatal Ed 2021;106:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate 1998;74:94–105. [DOI] [PubMed] [Google Scholar]
- 64.Bua J, Risso FM, Bin M, Vallon F, Travan L, Paviotti G. Association between body composition at term equivalent age and Bayley scores at 2 years in preterm infants. J Perinatol 2021;41:1852–8. [DOI] [PubMed] [Google Scholar]
- 65.Yang CL, Simons E, Foty RG, Subbarao P, To T, Dell SD. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol 2017;52:293–302. [DOI] [PubMed] [Google Scholar]
- 66.Walsh V, Brown JVE, Askie LM, Embleton ND, McGuire W. Nutrient-enriched formula versus standard formula for preterm infants. Cochrane Database Syst Rev 2019;7:CD004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117:1253–61. [DOI] [PubMed] [Google Scholar]
- 68.Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 2011;128:e899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy LT, Skinner AC, Check J, Warner DD, Perrin EM. Parental perceptions of weight status in preterm compared with term infants. Am J Perinatol 2016;33:1371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arnold CS, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol 1991;134:604–13. [DOI] [PubMed] [Google Scholar]


