Abstract
There is increasing interest in the long-term cardiovascular health of women with complicated pregnancies and their affected offspring. Emerging antenatal risk factors such as preeclampsia appear to increase the risk of hypertension and cardiovascular disease across the life course in both the offspring and women after pregnancy. However, the antenatal programming mechanisms responsible are complex and incompletely understood, with roots in alterations in the development, structure, and function of the kidney, heart, vasculature, and brain. The renin-angiotensin-aldosterone system is a major regulator of maternal-fetal health through the placental interface, as well as kidney and cardiovascular tissue development and function. Renin-angiotensin-aldosterone system dysregulation plays a critical role in the development of pregnancy complications such as preeclampsia and programming of long-term adverse cardiovascular health in both the mother and the offspring. An improved understanding of antenatal renin-angiotensin-aldosterone system programming is crucial to identify at-risk individuals and to facilitate development of novel therapies to prevent and treat disease across the life course. Given the inherent complexities of the renin-angiotensin-aldosterone system, it is imperative that preclinical and translational research studies adhere to best practices to accurately and rigorously measure components of the renin-angiotensin-aldosterone system. This comprehensive synthesis of preclinical and translational scientific evidence of the mechanistic role of the renin-angiotensin-aldosterone system in antenatal programming of hypertension and cardiovascular disease will help (1) to ensure that future research uses best research practices, (2) to identify pressing needs, and (3) to guide future investigations to maximize potential outcomes. This will facilitate more rapid and efficient translation to clinical care and improve health outcomes.
Keywords: AHA Scientific Statements, aldosterone, angiotensin-converting enzyme 2, hypertension, pre-eclampsia, pregnancy, renin-angiotensin system
The antenatal period, spanning conception to birth, is critical for maternal and fetal health. Exposure to adverse health conditions and environmental stressors during this time period can have long-term consequences on the mother and her offspring. Briefly stated, antenatal programming happens when exposures occur from conception through birth that alter structural, physiological, and metabolic fetal development and maternal health to improve short-term survival but at the expense of programmed adverse cardiovascular health in the long term (ie, developmental plasticity).1 For example, maternal hypertension, the most common medical comorbidity in pregnancy, is a major health concern and is associated with increased risks of short-term mortality and morbidity, as well as programmed chronic disease later in life in both the mother and the fetus.2 Offspring of women with pre-eclampsia have lower birth weight and higher blood pressure throughout childhood and young adulthood compared with unexposed offspring.3 Numerous preclinical models have confirmed this association,4 yet the exact mechanisms remain incompletely understood. Several of the major components of the renin-angiotensin-aldosterone system (RAAS) regulate several key physiological processes in both mother and fetus during pregnancy and the development and function of the kidney and cardiovascular system. Most notably, these include the angiotensin-converting enzyme (ACE)/angiotensin II (Ang II)/Ang II type 1 receptor (AT1R) and the ACE2/angiotensin-(1–7) (Ang-[1–7])/Mas receptor pathways. Dysregulation of the circulating and tissue-specific RAAS contributes to the pathogenesis of numerous antenatal conditions, including hypertensive disorders in pregnancy.5 RAAS dysregulation is one potential mechanism for the long-term programming of hypertension in offspring exposed to preeclampsia and other adverse antenatal factors.4,6
Greater risk of long-term hypertension and cardiovascular disease is also observed in women after preeclampsia, highlighting that the burden of programmed cardiovascular disease is not limited to the offspring.7 It is important to note that, despite decades of research, recommendations for preeclampsia treatment have not changed,8 and the prevalence of hypertension in pregnancy continues to increase.9 Treatment strategies vary considerably around the world, with significant disparities in the screening and follow-up for the development of hypertension and cardiovascular disease in affected women during the postpartum period and beyond, including in women from disenfranchised populations in the United States.10 In addition, emerging evidence indicates that pregnancy may place women at greater risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which in turn may increase the risk of several pregnancy complications, including preeclampsia and low birth weight, possibly related to altered ACE2 expression, the binding site for SARS-CoV-2.11,12 Thus, the short- and long-term increased risk of hypertension and cardiovascular disease in the mother and offspring attributable to pregnancy complications remains a critical health concern.
Despite decades of high-quality studies that have provided insights into the mechanisms responsible for the programming of cardiovascular disease associated with complicated pregnancies, there remains a crucial need in the field to further characterize dysregulatory events affecting the RAAS during this critical period of life. Primary reasons for persistent knowledge gaps include heterogeneity in the methods used in many preclinical models and the complex nature of the RAAS that makes accurate and reliable quantification challenging. Thus, the goal of this scientific statement is to summarize the current state of knowledge related to preclinical evidence of antenatal programming mechanisms of long-term maternal and offspring cardiovascular health as it relates to the role of several of the major RAAS pathways using well-characterized preclinical models of developmental programming. This scientific statement identifies gaps in knowledge that require further research. Moreover, this scientific statement emphasizes the importance of better understanding programming mechanisms for both investigators and clinicians to develop targeted interventions to prevent or mitigate the increased risk of hypertension and cardiovascular disease. This is the first step in an approach to reduce future cardiovascular risk in women with complicated pregnancies and their children.
A comprehensive literature search was conducted from approximately September 15, 2021, to November 15, 2021, that encompassed preclinical and clinical studies and reviews that were published in PubMed, Scopus, and other relevant databases using standardized methods. Key search words included but were not limited to pregnancy, preeclampsia, RAAS, high blood pressure, hypertension, cardiovascular, renal, brain, placental insufficiency, hypoxia, glucocorticoids, maternal undernutrition, offspring, and chronic health. The selection of writing group members was based on a wide range of expertise, including clinical and preclinical researchers representing different backgrounds, geographic regions, sexes, races, and ethnicities.
MATERNAL-PLACENTAL-FETAL INTERFACE AND THE RAAS
Maternal Cardiovascular Physiology During Pregnancy
Maternal cardiovascular and renal adaptations to pregnancy are essential to accommodate the physiological stress imparted by the growing fetus and placenta. Marked systemic vasodilation with decreased systemic vascular resistance and subsequent lower blood pressure characterizes early pregnancy starting at 4 to 6 weeks of gestation. This likely stimulates the maternal circulating RAAS by the end of the first trimester to retain sodium and fluid to increase plasma volume progressively throughout gestation, up to 40% to 50% higher than the prepregnancy baseline.13 Cardiac output, renal blood flow, and glomerular filtration rate increase to ≈50% over baseline; these changes are apparent by the second trimester and persist until term.
The RAAS in Normal Pregnancy Physiology
The RAAS, a crucial regulator of blood pressure and fluid-electrolyte balance, particularly in pregnant women and the fetus, is a key contributor to cardiovascular and kidney development (Figure 1A). Assessment of RAAS components in the maternal circulation during pregnancy suggests that overall activation that contributes to the aforementioned physiological cardiovascular changes. In normotensive, healthy pregnant women, blood pressure remains lower while plasma renin activity (PRA) and aldosterone remain elevated until late in pregnancy when blood pressure increases.14 Increased angiotensinogen production and PRA lead to increased angiotensin I concentrations, favoring augmented Ang II production that occurs despite reduced serum ACE activity, in part as a result of activation of additional RAAS pathways.15 Ang II–mediated increased aldosterone concentrations directly stimulate renal sodium and fluid retention to increase blood volume.
Figure 1. Physiological roles of the RAAS in healthy and pathological pregnancies.
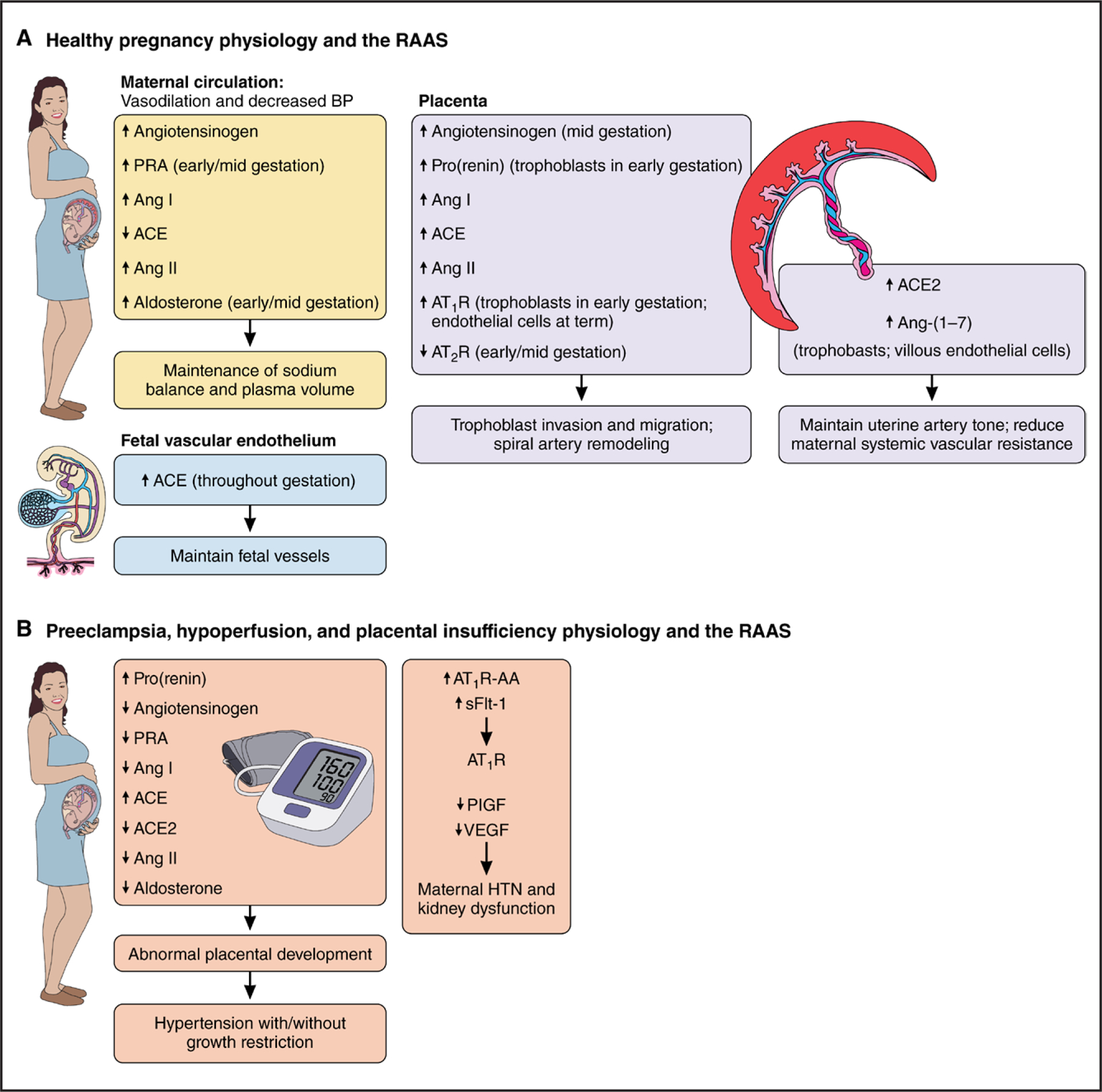
A, Expression and actions of the major RAAS components during healthy pregnancy. B, Expression and actions of the major RAAS components during pathological pregnancies, including preeclampsia and placental insufficiency. Ang indicates angiotensin; ACE, angiotensin-converting enzyme; AT1R, angiotensin type 1 receptor; AT2R, angiotensin type 2 receptor; AT1R-AA, angiotensin II type 1 receptor autoantibody; BP, blood pressure; HTN, hypertension; PlGF, placental growth factor; PRA, plasma renin activity; RAAS, renin-angiotensin-aldosterone system; sFlt-1, soluble fms-like tyrosine kinase 1; and VEGF, vascular endothelial growth factor.
Circulating and local tissue Ang II exerts key physiological functions in many crucial steps of placentation, including trophoblast invasion and migration, as well as spiral artery remodeling.16 RAAS components show a dynamic distribution throughout pregnancy. The AT1R is expressed in trophoblasts in early pregnancy but also in villous endothelial cells at term.17 Prorenin, (pro)renin receptor, AT1R, and Ang II type 2 receptor proteins are also expressed throughout gestation in trophoblasts at the maternal-fetal interface and in invasive trophoblasts, whereas ACE is concentrated predominantly in the fetal circulation, particularly in endothelial cells.18 The incremental ACE protein expression in fetal endothelial cells throughout pregnancy favors enhanced Ang II production in placental vessels from the fetal side, where angiogenesis, an essential process for maintaining fetal perfusion, continuously occurs.18 However, the expected increased Ang II production in the fetus and mother must be finely modulated to prevent excessive vasoconstriction and cardiovascular remodeling that could occur if Ang II concentrations increase above the expected physiological range.19
Pregnancy also stimulates the ACE2/Ang-(1–7) pathway to balance increased ACE/Ang II pathway activity and to contribute to maternal hemodynamic adaptations and placentation, trophoblast invasion, decidualization, and vascular remodeling.15,20 ACE2 breaks down Ang II, and Ang-(1–7), acting on its Mas receptor, antagonizes Ang II signaling through AT1R modulation.6 Estrogens regulate the progressive RAAS activation observed throughout gestation in part by directly stimulating angiotensinogen production and increasing ACE2 expression and activity in local tissue.21 In rats, renal ACE2 and Ang-(1–7) are progressively upregulated throughout pregnancy.22 ACE2 and Ang-(1–7) are also expressed in trophoblasts, villous vessel endothelial cells, primary villi vascular smooth muscle cells, and the syncytium and decidua.23 ACE2/Ang-(1–7) expression and activity in the placenta are dynamic, with greater concentrations in the decidua in early pregnancy that progressively change toward the placental villous endothelial cells and trophoblasts in late gestation.23 The presence of the ACE2/Ang-(1–7) pathway in invasive trophoblasts surrounding the spiral arteries, as well as in endothelial cells and vascular smooth muscle cells, suggests that the ACE2/Ang-(1–7) pathway helps regulate uterine artery tone and reduce maternal systemic vascular resistance.15 Therefore, ACE2-mediated conversion of Ang II into Ang-(1–7) and intracellular signaling between AT1R and Mas receptor are likely key factors regulating Ang II physiological effects during pregnancy.
Additional RAAS pathways contribute to the Ang II–Ang-(1–7) balance during pregnancy but are less well characterized; thus, their role in antenatal programming presents another important knowledge gap. The (pro) renin receptor is crucial for many developmental and physiological processes during pregnancy through several signaling pathways, including Wnt/b-catenin and mitogen-activated protein kinase.24 Neprilysin, another metallopeptidase that converts angiotensin I to Ang-(1–7), has unclear effects during pregnancy. Compared with pregnant women with healthy weight, pregnant women with overweight or obesity (body mass index ≥25 or ≥30 kg/m2, respectively) have lower endothelial cell neprilysin expression in the fetus and placenta, and fetal weight is associated inversely with circulating neprilysin levels in cord blood.25 Uterine mast cell and natural killer cell secretion of chymase, a serine protease that generates Ang II independently of ACE, may contribute to decidual vessel remodeling and subsequent fetal growth.26
The RAAS in Pregnancy Pathologies
Placental insufficiency is a hallmark of many adverse pregnancy events that program later disease in the offspring. Short- or long-term interruptions in the sufficient delivery of blood, oxygen, or nutrients to the fetus can alter fetal growth and organ development, leading to abnormal tissue structure and function, especially in the kidneys. Adequate fetal perfusion requires sufficient maternal cardiovascular and placental health; hence, interruptions in the maternal, placental, or fetal RAAS can potentially adversely affect fetal and maternal cardiovascular health in the short and long term (Figure 1B).
Human intrauterine growth restriction, a proxy for placental insufficiency, is associated with higher cord blood Ang II concentration but no difference in fetal-placental AT1R concentration compared with term pregnancies with delivery by elective cesarean section.27 Uterine vessel ligation or clamping during mid or late pregnancy as a model of placental insufficiency and maternal preeclampsia commonly induces reduced glomerular number and hypertension in the offspring. Uteroplacental insufficiency in the rat model of reduced uterine perfusion pressure at gestational day 14 is associated with attenuated intrarenal RAAS activity in neonatal rats.28
The apparent bidirectional relationship between preeclampsia and placental insufficiency remains a paradox in the field that can hinder accurate inferences about RAAS measurements observed in preclinical models and clinical studies. Placental insufficiency in preclinical models and in women may be a precursor to maternal hypertensive disorders. Preeclampsia, in turn, exacerbates placental insufficiency and may program adverse cardiovascular health in affected women and their offspring independently of placental insufficiency. Although the cause of preeclampsia is still unclear, intriguing preclinical and clinical evidence suggests that several potentially interrelated pathways are involved. Early-pregnancy placental ischemia is associated with release of soluble fms-like tyrosine kinase 1, a circulating soluble isoform of the vascular endothelial growth factor receptor that has antiangiogenic properties. It binds free vascular endothelial growth factor and placental growth factor, leading to an imbalance of antiangiogenic and proangiogenic factors, resulting in maternal endothelial dysfunction, hypertension, proteinuria, and glomerular endotheliosis.29
Increased levels of circulating AT1R autoantibodies in women with preeclampsia, first described in 1999,30 have been studied extensively both in preclinical models of placental hypoperfusion or preeclampsia and in clinical studies. These antibodies are directed to a specific epitope on the second extracellular loop of the AT1R and bind to human trophoblasts. Multiple studies suggest that the antibodies may play an important role in the pathophysiology of preeclampsia by inducing vasoconstriction, hypertension, and increased coagulation.31 Antibodies derived from women with preeclampsia induce placental soluble fms-like tyrosine kinase 1 production through AT1R activation in pregnant mice, human placental villous explants, and human trophoblast cells.32 Human studies have shown that these antibodies can be detected early in pregnancy in women who later develop preeclampsia and that, once preeclampsia develops, >95% of women have the antibodies, which also correlate with disease severity.33 However, significant limitations remain in measuring these autoantibodies accurately, and their clinical significance remains unclear.34
RAAS contributions to preeclampsia development and progression remain elusive as well, with conflicting findings and much debate resulting mainly from a lack of consensus of methodological rigor.35 It remains unknown whether RAAS dysregulation is a prerequisite for preeclampsia development or a consequence. Activation of tissue RAAS and, by extension, the circulating RAAS is necessary to meet and regulate the growing demands of the fetus to ensure a healthy pregnancy. Although there is consensus that placental alterations are a principal causal mechanism in preeclampsia, it remains unclear what role the RAAS has and whether the observed changes in the circulating RAAS in women with preeclampsia (eg, lower PRA) contribute to the dysregulation of maternal kidney and vascular function or are altered in response to changes induced by angiogenic factor dysregulation. Leading theories suggest that abnormal or shallow placentation with defective spiral arteriolar remodeling, reduced placental blood flow, and increased placental oxidative stress contributes to preeclampsia pathogenesis.35 This abnormal placental development may contribute to underexpression or overexpression of placental RAAS components and excess shedding of placental particles such as miRNA that target RAAS mRNA.36
Subsequent release of RAAS components into the maternal circulation from the fetal-placental unit (eg, increased Ang II, decreased Ang-[1–7]) may alter the systemic and tissue-specific maternal RAAS, including the intrarenal RAAS, subsequently reduce uteroplacental blood flow, and further drive placental damage.35,37 Women who develop preeclampsia demonstrate decreased glomerular filtration rate, PRA, and aldosterone before and at the time of diagnosis, consistent with a volume-expanded circulation.38 Placental and plasma soluble (pro)renin receptor levels are higher in women with preeclampsia compared with women with normal pregnancies.39 During preeclampsia, neprilysin levels are higher in the placenta and in circulating extracellular vesicles derived from syncytio-trophoblasts.40 Maternal vascular endothelial chymase expression is increased in preeclampsia.41 In addition, these RAAS components may lead to secondary changes in neurohormonal regulation of cardiovascular and kidney function that program hypertension and cardiovascular disease.42 However, the precise timing of these RAAS changes and their importance to later programmed disease remain unknown.
OVERVIEW OF MAJOR PRECLINICAL PROGRAMMING MODELS
Numerous preclinical models using different programming events in various animal species have demonstrated consistently that programmed RAAS alterations in the antenatal period can lead to hypertension development in women and their offspring (Figure 2). Among the most widely studied are models of placental insufficiency and maternal protein or global nutrition restriction or excess in rodents and sheep (Table 1). Within these models, specific antenatal events include maternal-fetal vascular supply disruptions, maternal stress, and exogenous exposures such as glucocorticoids. Although most models attempt to induce fetal growth restriction, it is well documented that programming mechanisms can occur independently of placental insufficiency or growth restriction. Because human nephrogenesis is complete by 36 weeks’ gestation, most preclinical models target equivalent windows in animals in which nephrogenesis continues after birth (eg, the first 10–12 days of postnatal life in rodents) to investigate the effects of exposures during organogenesis on long-term kidney function and cardiovascular health.
Figure 2. Conceptual causal model of representative antenatal exposures and their effects on various tissue programming mechanisms.
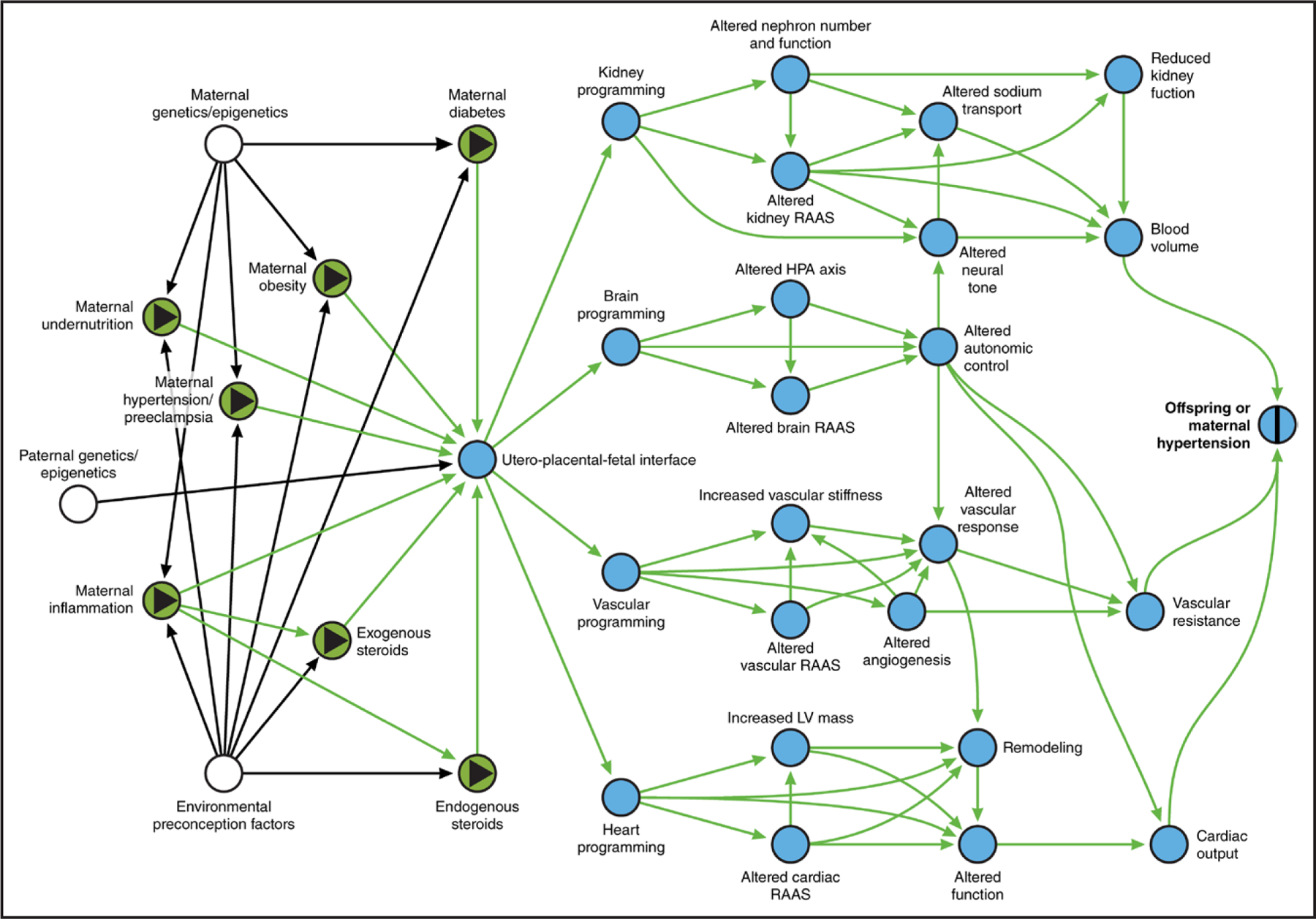
Directed acyclic graph with exposures noted as green ovals with black triangles and outcome as blue oval with black vertical bar. Intermediate mechanisms as mediators on the causal path are noted as blue ovals; causal paths are noted as green arrows. White ovals are unmeasured factors; black arrows are noncausal and nonbiasing paths. HPA indicates hypothalamic-pituitary axis; LV, left ventricular; and RAAS, renin-angiotensin-aldosterone system. Figure created with www.dagitty.net.
Table 1.
Overview of Preclinical Models of Programmed Hypertension Mediated Through Antenatal RAAS Dysregulation by Species
| Experimental model | Species | Antenatal programming exposure |
|---|---|---|
| Placental dysfunction | Rat | Reduced uterine perfusion pressure (35%–45%) |
| Uterine artery/vessel ligation | ||
| Maternal or fetal hypoxia (10.5%–12% oxygen or 25% reduction in fetal PaO2) | ||
| Sheep | Umbilico-placental embolization | |
| Removal of uterine/endometrial caruncles | ||
| Maternal hypoxia (25% reduction in fetal PaO2) | ||
| Natural twinning | ||
| Nutrient manipulation | Rat | Protein restriction (6%–18%) |
| Global undernutrition (40%–70%) | ||
| High-salt (8%–30%) or low-salt (0.03%) diet | ||
| High-fat (20%) diet | ||
| Mouse | Protein restriction (6%–12%) | |
| Global undernutrition (70%) | ||
| Sheep | Global undernutrition (85%) | |
| Pharmacological intervention | Rat | Diabetes induced by streptozotocin or glucose infusion |
| L-NAME–induced preeclampsia | ||
| Antenatal glucocorticoids: increased maternal endogenous glucocorticoids or clinical administration | ||
| Mouse | Antenatal glucocorticoids: increased maternal endogenous glucocorticoids | |
| Sheep | Antenatal glucocorticoids: increased maternal endogenous glucocorticoids or clinical administration | |
| Exogenous fetal or maternal cortisol infusion |
L-NAME indicates l-NG-nitro arginine methyl ester; and RAAS, renin-angiotensin-aldosterone system.
However, the programming effects of antenatal exposures on the RAAS are heterogeneous, depending on the animal model (species, strain), exposure (timing, severity, duration), and tissue of interest. Choice of model, exposure, and timing often depends on whether the investigator is interested in a specific tissue (kidney, heart, brain, or vasculature) and sex- or age-specific effects on RAAS programming.43 Variability in the timing of programmed events has led to heterogeneity among reported results and contributed to paradoxes in the field. Early, mid, late, or pan-gestation exposures can cause discrepant findings even in the same animal model. For example, maternal protein restriction–induced hypertension severity is greatest in mid to late gestation.44 As presented in the following sections, the expression, concentration, and activity of ACE/ACE2, Ang II/Ang-(1–7), and AT1R/Ang II type 2 receptor/Mas receptor can be altered dramatically and permanently in different tissues to contribute to increased hypertension and cardiovascular risk.
EVIDENCE FOR RAAS-MEDIATED ANTENATAL PROGRAMMING
Maternal Health
Women who had preeclampsia have an increased risk of developing hypertension and cardiovascular disease at an earlier age.45 Many of the maternal cardiovascular and RAAS pathophysiological alterations that occur in the antenatal period may contribute to maternal programmed hypertension. However, the precise mechanisms involved remain incompletely understood in preclinical models and clinical studies.46 In the reduced uterine perfusion pressure model in the rat, affected females demonstrate salt-sensitive blood pressure 3 weeks postpartum independently of PRA or plasma aldosterone.47 In this model, affected females have worse glomerular filtration rate and left ventricular ejection fraction despite no differences in mean arterial pressure or kidney or heart structure.48 AT1R autoantibody–induced preeclampsia is associated with greater left ventricular mass index, left ventricular remodeling, and cardiac susceptibility to ischemia in rats 16 weeks postpartum.49 In addition, AT1R autoantibody blockade during pregnancy in the reduced uterine perfusion pressure model improves maternal blood pressure during pregnancy and maternal blood pressure, cardiac hypertrophy, and cardiac mitochondrial function 10 weeks postpartum.50 However, much more investigation is needed to better delineate the role of the RAAS in mediating maternal antenatal hypertension programming.
Offspring Health
The RAAS plays a major role in fetal kidney development. RAAS manipulation during gestation can have profound effects on the developing fetus. This is perhaps best illustrated by the fact that the use of RAAS inhibitors to manage maternal hypertension during pregnancy is strongly contraindicated because of a high risk of fetal birth defects, including renal agenesis, tubular dysgenesis, kidney failure, and fetal death. In addition to higher blood pressure, human adolescents born preterm have higher circulating RAAS activity toward the ACE/Ang II pathway and away from the ACE2/Ang-(1–7) pathway, associations that are magnified in female individuals and those with obesity.51 Human male adolescents born prematurely with very low birth weight who were exposed to preeclampsia have higher circulating aldosterone levels compared with those who were unexposed.52 However, a major paradox in the field is whether preterm birth and lower birth weight in and of themselves are necessary and sufficient to cause programming or the antecedent exposures (eg, preeclampsia) actually program future disease.1,53
Various genetically modified animal models could be used to study the effects of antenatal RAAS manipulations on offspring outcomes. However, most studies that use genetic manipulations have targeted the offspring’s genome rather than that of the parents. As a result, dissociating the effects of such manipulations, specifically during the antenatal period, to program adult cardiovascular function, versus the ongoing effects of genetic manipulations throughout the life course through epigenetic mechanisms, is difficult. Thus, most evidence comes from preclinical surgical, dietary, or pharmacological interventions commonly used to induce antenatal RAAS programming.
Antenatal dexamethasone programs increased blood pressure and increased renal renin and ACE expression in male rat offspring, and in utero delivery of antioxidants, dimethyl fumarate, or melatonin can prevent these effects.54,55 Antenatal dexamethasone exposure in sheep upregulates fetal pulmonary and circulating RAAS components and impairs cardiovascular function through RAAS-dependent mechanisms.56 In sheep, antenatal betamethasone induces sex-specific alterations in renal proximal tubule cell responses to Ang II and Ang-(1–7), cerebral RAAS expression and content, and RAAS-dependent impaired baroreflex and heart rate variability.57–59 Intracerebroventricular Ang-(1–7) administration can attenuate the deleterious effects of antenatal betamethasone on blood pressure and autonomic dysfunction in sheep.60 Antenatal betamethasone causes developmental stage-specific effects on renin expression in female sheep and increases blood pressure and renal sympathetic nerve activity, although administration of the AT1R blocker losartan immediately after birth does not attenuate this effect, which supports additional RAAS-independent effects.61
A maternal low-protein diet during pregnancy leads to several RAAS programming effects. In mice, these include sexually dimorphic blood pressure control and increased RAAS expression in the lungs, pancreas, and brain.62,63 In rats, maternal protein deprivation increases blood pressure in offspring through several RAAS-dependent mechanisms.64 Manipulating the maternal gut microbiota can attenuate maternal high-fat diet–induced increased blood pressure and RAAS alterations in male offspring.65
Various other antenatal experimental manipulations can induce deleterious phenotypes mediated through the RAAS. For example, antenatal nicotine exposure sensitizes male rat offspring to the hypertensive effects of Ang II, whereas antioxidants attenuate this effect.66 Maternal exogenous Ang II infusion during pregnancy sensitizes male rat offspring to kidney damage induced by a high-sodium diet.67 Antenatal hypoxia in rats causes sex-specific programming of blood pressure responses to Ang II.68 The reduced uterine perfusion pressure model of placental insufficiency and intrauterine growth restriction that mimics many of the maternal characteristics of preeclampsia also programs Ang II hypersensitivity and hypertension.69 Uteroplacental insufficiency in the rat model of reduced uterine perfusion pressure at gestational day 14 is associated with Ang II–dependent hypertension, with increased renal ACE activity and renin and angiotensinogen mRNA expression but no changes in Ang II or AT1R in adult offspring.28 In summary, a wide array of antenatal manipulations are used in rodent and sheep models to investigate RAAS programming and later offspring cardiovascular health. Collectively, these studies highlight the diverse array of mechanisms that alter the RAAS. However, they also prompt many questions about the utility of measuring and manipulating the RAAS in the antenatal period to develop novel diagnostic and therapeutic tools.
Second Hits: Additive and Multiplicative Programming Effects
Programmed RAAS modifications in the antenatal period can also sensitize both the mother and the offspring to subsequent programming stimuli that directly cause hypertension and other cardiovascular disease risk factors, often referred to as second hits.51 Indeed, it is conceivable that many antenatal programming factors require additional adverse exposures over the life course to unmask hypertension programming.53 These can include environmental exposures such as unmet social needs and adverse childhood experiences in humans and the development of other conditions such as obesity.51,70–73 During pregnancy, maternal Ang II infusion in the rat sensitizes offspring to later high-fat diet–induced hypertension.74 Conversely, a maternal high-fat diet during pregnancy sensitizes offspring rats to the pressor effects of Ang II.75 Adult sheep offspring exposed to antenatal betamethasone demonstrate sex- and obesity-dependent modulation of insulin sensitivity and related RAAS alterations, including increased ACE and ACE2 expression and an imbalance between the Ang II/AT1R and Ang-(1–7)/Mas receptor signaling pathways.76 Similarly, antenatal betamethasone modifies renal responses to exogenous RAAS peptides in a sex-dependent manner.77 When exposed to maternal dietary high-sodium load during gestation, rat offspring exhibit an array of cardiometabolic alterations that parallel the effect of exogenous Ang II.78 After birth, antenatal dexamethasone exposure in maternal rats is associated with weight gain and glucose intolerance 12 months after weaning.79 Thus, sensitization of both mother and offspring to subsequent stimuli represents a clinically significant yet underappreciated consequence of antenatal RAAS manipulation.
ADDITIONAL RAAS PROGRAMMING MECHANISMS
Our understanding of the mechanisms underlying RAAS programming in offspring has advanced significantly in recent years as a result of the development of innovative techniques and different approaches to identify genetic and epigenetic changes in target genes. For example, models of maternal nutrient deprivation or excess demonstrate major epigenetic alterations to several RAAS gene promoters. These alterations occur in various tissues that directly affect adaptive kidney and cardiovascular structure and function across the life course, with adverse effects that are likely perpetuated over multiple generations.
Epigenetic mechanisms, including DNA methylation, posttranslational histone modification, and noncoding RNA, are biological processes that modify gene expression without altering the DNA sequence. Activation of epigenetic mechanisms attributable to changes in maternal nutrition during pregnancy suggests that metabolic alterations trigger adaptive cardiovascular and renal processes mediated through the RAAS during placentation and fetal development. As an example, changes in methylation of the promoter for the AT1R gene, AGTR1, are widely studied and concisely reproduced in various models. Maternal low-protein diet results in AT1bR subtype promoter undermethylation and early adrenal overexpression in affected offspring,80 which is further shown to be associated with maternal glucocorticoid levels in early pregnancy. DNA methylation is found at AT1bR CpG sites in the fetal heart of offspring exposed to maternal high-salt diet.81 DNA methylation and histone modification of the AGTR1 promoter are observed in both the aorta and mesenteric arteries of offspring exposed to maternal high-sucrose diet during gestation.82 These findings suggest that AT1R is one of the primary RAAS components that undergo epigenetic modification as a potential programming pathway. However, an important limitation for translating these findings to humans is that, although rodents have 2 subtypes of genes transcribing AT1R, humans only have 1 AT1R gene, AGTR1.83 Studies using selective knockout have identified AT1aR as the closest homolog to human AT1R.84 Therefore, further studies are needed to confirm whether in humans AGTR1 is a major RAAS gene that antenatal events target epigenetically and whether these changes are transmitted across generations in a clinically meaningful manner. Although this is encouraging and in line with several clinical and epidemiological studies, further investigations are warranted to better elucidate which mechanisms are truly inherited through epigenetic processes and which are actually attributable to shared environmental exposures between parents and offspring. Indeed, this line of investigation is especially interesting as a mechanism for how social determinants of health such as unmet social needs and adverse childhood experiences may contribute to RAAS programming and the long-term effects of health disparities in perinatal care.
There is emerging evidence that exosomes and microRNA may in part mediate RAAS dysregulation–induced signaling.5,36 Last, alterations to the maternal microbiota and related metabolites during pregnancy are associated with RAAS dysregulation and subsequent programmed hypertension and hence have been studied as therapeutic targets during pregnancy.85
METHODOLOGICAL LIMITATIONS AND BEST RESEARCH PRACTICES
Manipulating the RAAS during specific phases of gestation is difficult for many biological and technical reasons. Complex pharmacokinetic changes occur during pregnancy, including expansion of specific fluid compartments and subsequent modifications in volume distribution, compound-specific placental transport, and potentially lethal outcomes for the offspring resulting from, for example, failed kidney development. Dietary manipulations can be difficult because the experimenter must demonstrate that the mother has an overall nutritional balance of key nutrients and experiences the intended consequence (eg, sodium depletion) within a carefully defined and targeted time line. Thus, in the study of the effects of antenatal manipulations on offspring biology, use of cross-fostering may be warranted. Genetic manipulations to specifically target the antenatal period can be complicated because of the potential expression of targeted genes in maternal tissues before pregnancy and during lactation versus in the offspring. Creative application of conditional manipulations (eg, Cre-Lox, tetracycline sensitive) can overcome these limitations, but careful validation of such models is required. Last, although commonly used to great effect, rodents exhibit importantly different placentation and fetal developmental biology compared with humans. Thus, results from rodent studies must be interpreted appropriately. Sheep provide an exceptional model given that their placentation biology is similar to that of humans, but sheep models involve substantially higher (and potentially prohibitive) costs and facility considerations that are much less commonly available. Ultimately, experimental RAAS manipulations during the antenatal period are possible but require consideration of many additional variables to achieve sufficient scientific rigor.
Accurate and reliable quantification of all relevant RAAS components requires well-validated methods to achieve appropriate rigor and reproducibility and to enable appropriate data comparisons across studies (Table 2). Limitations for angiotensin peptide measurement include naturally low concentrations (femtomole per milliliter), ongoing metabolism (degradation and generation), interfering substances, and poor specificity and cross-reactivity attributable to sequence homogeneity. Samples for plasma analysis should be collected in EDTA tubes containing the appropriate protease inhibitor cocktail (especially for renin) that is validated for the intended assay, whereas urine samples often require acidification or inhibitors. Samples should immediately be frozen or undergo extraction for purification. Various drugs can interfere with RAAS measurement, including common sedatives and paralytics used in preclinical studies. To date, there are no validated, reliable, commercially available ELISAs to quantify angiotensin peptides.86 Currently accepted best practices recommend assays such as mass spectrometry, high-performance liquid chromatography, and liquid chromatography–mass spectrometry; assays such as radioimmunoassays can have value if they have been validated with these aforementioned gold-standard techniques.34 Quantification of RAAS enzymatic activity and content also requires rigorous collection and processing methods. One must differentiate between quantifying full-length and soluble enzyme forms. Serum is generally preferred over plasma for enzymatic analysis given the risk of assay interference. These methods and assays are thus costly and labor intensive and require measurement in established laboratories with extensive experience measuring RAAS components.
Table 2.
Summary of Methodological Limitations in RAAS Quantification: Considerations for Rigor and Reproducibility
| Renin-angiotensin-aldosterone component | Weakness or limitation | Best practice consideration |
|---|---|---|
| Peptides | Improper collection practices Improperly validated ELISAs Naturally low concentrations (femtomole/milliliter) Ongoing metabolism Interfering substances Poor specificity and cross-reactivity Interference from sedatives and paralytics |
Design experiments with appropriate quantification methods a priori Collect plasma in EDTA with appropriate protease inhibitor cocktail validated for intended assay Extract for purification Use mass spectrometry, high-performance liquid chromatography, or well-validated RIAs |
| Enzymes | Improper collection and processing Interfering substances |
Use serum over plasma Use validated assays |
| Receptors | Poor specificity of antibodies for use in Western blots and immunohistochemistry | Use radiolabeled peptide binding Use proper positive and negative controls |
| mRNA or protein expression | Differential expression of mRNA vs protein, transcriptional regulation, turnover, and activity | Combine several methods |
| Interpretation | Difficult-to-interpret values without normative ranges in isolation | Ensure that values are biologically plausible Assess multiple components simultaneously Interpret in the context of both major pathways Consider tissue expression when available |
RAAS indicates renin-angiotensin-aldosterone system; and RIA, radioimmunoassay.
RAAS component tissue expression and quantification can be estimated with established techniques with appropriate rigor such as immunohistochemistry, Western blotting, and tissue enzymatic assays. The AT1R is expressed broadly throughout the body, whereas the Ang II type 2 receptor may have relatively lower expression in various cardiovascular and renal tissue. Mas receptor expression generally mirrors that of the Ang II receptors. When investigating tissue and whole-cell expression, one must consider that many RAAS components are also expressed in mitochondria and nuclei.87,88 Receptor measurement has been particularly challenging, in part because commercially available antibodies have poor specificity, leading to concerns about data obtained from immunolabeling techniques such as Western blot and immunohistochemistry.89 It is highly recommended that investigators use proper negative and positive controls. Alternatively, reverse transcription–polymerase chain reaction can assess gene expression of human AGTR1 or both murine AT1aR and AT1bR.84 However, it is important to note that, depending on the method used, the data may provide differential regulatory information, including differential expression of mRNA versus protein, transcriptional regulation, turnover, and activity. Therefore, combining several methods is usually preferred to provide more robust insights into receptor regulation and signaling.
Last, it is critical to perform a comprehensive analysis of the major RAAS pathway components simultaneously to infer pathophysiological changes appropriately because the analysis of isolated markers may result in misinterpretation of the functional status of the system. In addition, the circulatory RAAS and several epigenetic markers are not tissue specific even in preclinical studies; thus, changes in RAAS components in the blood cannot be ascribed to changes in any particular tissue. Although normative values of RAAS components do not exist in animals or humans, verified content across numerous sample sources and species is established.90 This same RAAS methodological rigor applies to translational and clinical studies. Thus, because the interpretation of RAAS status can be even more complex in humans, it is extremely important that samples are collected, processed, and analyzed with the use of best practices.12,34 Clinical studies should obtain tissue samples whenever feasible and ethical to better determine tissue-specific RAAS alterations, especially from participants at baseline at disease diagnosis or before treatment initiation. This approach allows a better understanding of the incidence, rather than the prevalence, of RAAS alterations and can mitigate sources of confounding bias and time-dependent bias.91
CONCLUSIONS AND FUTURE DIRECTIONS
Despite persistently increased perinatal mortality risk and long-term cardiovascular risk that antenatal events confer to women and their offspring, standard practices for screening pregnant women remain underused.92 In addition, Black women have a higher prevalence of preeclampsia and worse pregnancy outcomes, with disparities in access to adequate prenatal care being a critical factor.93 This gap in health care has become even more relevant during the coronavirus disease 2019 (COVID-19) pandemic. However, the role of RAAS programming in these health disparities is not known. Little is known about how environmental exposures and social determinants of health may additionally program the RAAS to increase the risk of subsequent hypertension and cardiovascular disease. Investigation is urgently needed into how unmet social needs and adverse childhood experiences may contribute to RAAS programming of disease through epigenetic pathways and, in particular, how novel primordial prevention and therapeutic strategies can be developed to target these emerging mechanisms.53
Our understanding of the mechanisms responsible for antenatal programming of hypertension remains limited despite decades of high-quality research in this area. Although RAAS dysregulation is established as an important driver of antenatal programming, crucial gaps in the field remain, in part because methodological limitations and a lack of consensus have significantly hindered translation to clinical practice. RAAS programming provides an opportunity to establish novel diagnostic and prognostic biomarkers and to develop new approaches to improve short- and long-term maternal and child cardiovascular health, including mitigating health disparities pertaining to personalized medicine and nutritional interventions.
There is emerging interest in the role that the RAAS could theoretically play in SARS-CoV-2 infection and COVID-19–related adverse maternal and offspring outcomes, given that ACE2 is the binding site for SARS-CoV-2 and is expressed in the placenta, as well as the lungs, heart, vasculature, brain, and kidneys. Virus-induced ACE2 downregulation could lead to local ACE/Ang II pathway upregulation at the expense of ACE2/Ang-(1–7) downregulation, leading to more Ang II–mediated vasoconstriction and inflammation in the placenta and cardiovascular tissues generally.12 In particular, proinflammatory and prothrombotic signaling may result in systemic endothelial and microvascular dysfunction, increasing the risk for embolization and immune system dysregulation. However, there remains a lack of robust clinical data to support preclinical evidence of these mechanisms. SARS-CoV-2–related RAAS dysregulation during pregnancy could theoretically contribute to adverse perinatal outcomes, including preeclampsia, preterm birth, and growth restriction, and program adverse cardiovascular changes that may increase the risk of developing hypertension and cardiovascular disease, but more preclinical and clinical data are necessary to support these hypotheses.94 Indeed, ongoing preclinical and clinical studies will provide important data supporting or refuting these interesting theories.
Based on these mechanisms, promising therapeutics include novel AT1R-selective agonists (TRV027), exogenous Ang-(1–7) and its analogs (TXA127), and soluble ACE2 (APN01). Theoretically, soluble ACE2 or ACE2/Ang-(1–7)–derived compounds would be of interest in restoring the balance between the ACE/Ang II and ACE2/Ang-(1–7) pathways in the placenta, given that Ang II is necessary for placental function and fetal development and that traditional RAAS-blocking medications are contraindicated in pregnancy and the first month of life in infants. Future translational studies and clinical trials should consider the unique opportunity that the maternal-fetal dyad offers in preventing or mitigating antenatal programming of cardiovascular disease.
Preclinical studies have provided crucial insight into adverse perinatal and long-term cardiovascular outcomes in women and their offspring, but much more work is needed to translate those findings to clinical practice more efficiently. RAAS programming has enormous potential to inform the development of diagnostic and prognostic biomarkers and novel therapeutics to improve perinatal outcomes in the short- and long-term cardiovascular health in women and their offspring. Consensus on RAAS methods is imperative to foster more rapid translation into clinical research and practice.
Writing Group Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Barbara T. Alexander | University of Mississippi Medical Center | NIH† | None | None | None | None | None | None |
| Andrew M. South | Wake Forest University, School of Medicine | NIH (grants)† | None | None | None | None | None | None |
| Phyllis August | Weill Cornell Medical College Hypertension Center | None | None | None | None | None | None | None |
| Mariane Bertagnolli | McGill University School of Physical and Occupational Therapy (Canada) | None | None | None | None | None | None | None |
| Erin P. Ferranti | Emory University | None | None | None | None | None | None | None |
| Justin L. Grobe | Medical College of Wisconsin | NIH (grants)†; AHA† | None | None | None | None | None | None |
| Emily J. Jones | University of Oklahoma Health Sciences Center | None | None | None | None | None | None | None |
| Analia S. Loria | University of Kentucky | None | None | None | None | None | None | None |
| Basmah Safdar | Yale University | NHLBI (ACTIV-4d)†; NHLBI (ACTIV-3)† | None | None | None | None | None | None |
| Maria Luisa Soledad Sequeira-Lopez | University of Virginia School of Medicine | NIH (2 R01s 1P50)† | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Tarek F. Antonios | St George’s, University of London (United Kingdom) | None | None | None | None | None | None | None |
| Michel Baum | University of Texas Southwestern Medical Center at Dallas | None | None | None | None | None | None | None |
| Hui Hu | Brigham and Women’s Hospital | None | None | None | None | None | None | None |
| Maris Laan | University of Tartu (Estonia) | Estonian Research Agency (grant EAG112 “Maternal Serum Based Multimarker Test for the Risk Assessment to Develop Preeclampsia: Translation to the Clinic [ESTPRE study]”)† | None | None | None | None | None | None |
| Anne M. Nuyt | Université de Montréal (Canada) | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Significant.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
Publisher's Disclaimer: ARTICLE INFORMATION
Publisher's Disclaimer: This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on December 5, 2022, and the American Heart Association Executive Committee on January 24, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
Publisher's Disclaimer: The American Heart Association requests that this document be cited as follows: Alexander BT, South AM, August P, Bertagnolli M, Ferranti EP, Grobe JL, Jones EJ, Loria AS, Safdar B, Sequeira-Lopez MLS; on behalf of the American Heart Association Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; and Council on Lifestyle and Cardiometabolic Health. Appraising the preclinical evidence of the role of the renin-angiotensin-aldosterone system in antenatal programming of maternal and offspring cardiovascular health across the life course: moving the field forward: a scientific statement from the American Heart Association. Hypertension. 2023;80:e75–e89. doi: 10.1161/HYP.0000000000000227
Publisher's Disclaimer: The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
REFERENCES
- 1.South AM. Antenatal programming of blood pressure. In: Flynn JT, Ingelfinger JR, Brady TM, eds. Pediatric Hypertension Springer, Cham; 2022. [Google Scholar]
- 2.Gestational hypertension and preeclampsia: ACOG Practice Bulletin, number 222. Obstet Gynecol 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 3.Hoodbhoy Z, Mohammed N, Nathani KR, Sattar S, Chowdhury D, Maskatia S, Tierney S, Hasan B, Das JK. The impact of maternal preeclampsia and hyperglycemia on the cardiovascular health of the offspring: a systematic review and meta-analysis [published online May 3, 2021]. Am J Perinatol doi: 10.1055/s-0041-1728823. 10.1055/s-0041-1728823 [DOI] [PubMed] [Google Scholar]
- 4.Davis EF, Newton L, Lewandowski AJ, Lazdam M, Kelly BA, Kyriakou T, Leeson P. Pre-eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin Sci (Lond) 2012;123:53–72. doi: 10.1042/CS20110627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumbers ER, Delforce SJ, Arthurs AL, Pringle KG. Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front Endocrinol (Lausanne) 2019;10:563. doi: 10.3389/fendo.2019.00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell MC. Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci 2019;133:55–74. doi: 10.1042/CS20171550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. doi: 10.1007/s10654-013-9762-6 [DOI] [PubMed] [Google Scholar]
- 8.Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, Rana S, Vermunt JV, August P; on behalf of the American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease, Kidney in Heart Disease Science Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association [published correction appears in Hypertension. 2022;79:e70]. Hypertension 2022;79:e21–e41. doi: 10.1161/HYP.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananth CV, Duzyj CM, Yadava S, Schwebel M, Tita ATN, Joseph KS. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension 2019;74:1089–1095. doi: 10.1161/HYPERTENSIONAHA.119.12968 [DOI] [PubMed] [Google Scholar]
- 10.Jones EJ, Hernandez TL, Edmonds JK, Ferranti EP. Continued disparities in postpartum follow-up and screening among women with gestational diabetes and hypertensive disorders of pregnancy: a systematic review. J Perinat Neonatal Nurs 2019;33:136–148. doi: 10.1097/JPN.0000000000000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narang K, Enninga EAL, Gunaratne M, Ibirogba ER, Trad ATA, Elrefaei A, Theiler RN, Ruano R, Szymanski LM, Chakraborty R, et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc 2020;95:1750–1765. doi: 10.1016/j.mayocp.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.South AM, Diz D, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol 2000;24:11–14. doi: 10.1016/s0146-0005(00)80047-6 [DOI] [PubMed] [Google Scholar]
- 14.Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 2014;306:R91–R101. doi: 10.1152/ajpregu.00034.2013 [DOI] [PubMed] [Google Scholar]
- 15.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine 2002;18:239–245. doi: 10.1385/ENDO:18:3:239 [DOI] [PubMed] [Google Scholar]
- 16.Hering L, Herse F, Geusens N, Verlohren S, Wenzel K, Staff AC, Brosnihan KB, Huppertz B, Luft FC, Muller DN, et al. Effects of circulating and local uteroplacental angiotensin II in rat pregnancy. Hypertension 2010;56:311–318. doi: 10.1161/HYPERTENSIONAHA.110.150961 [DOI] [PubMed] [Google Scholar]
- 17.Williams PJ, Mistry HD, Innes BA, Bulmer JN, Broughton Pipkin F. Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta 2010;31:448–455. doi: 10.1016/j.placenta.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 18.Pringle KG, Tadros MA, Callister RJ, Lumbers ER. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta 2011;32:956–962. doi: 10.1016/j.placenta.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 19.Yart L, Roset Bahmanyar E, Cohen M, Martinez de Tejada B. Role of the uteroplacental renin-angiotensin system in placental development and function, and its implication in the preeclampsia pathogenesis. Biomedicines 2021;9:1332. doi: 10.3390/biomedicines9101332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neves LAA, Stovall K, Joyner J, Valdés G, Gallagher PE, Ferrario CM, Merrill DC, Brosnihan KB. ACE2 and ANG-(1–7) in the rat uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol 2008;294:R151–R161. doi: 10.1152/ajpregu.00514.2007 [DOI] [PubMed] [Google Scholar]
- 21.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 2008;93:658–664. doi: 10.1113/expphysiol.2007.041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brosnihan KB, Neves LAA, Joyner J, Averill DB, Chappell MC, Sarao RUA, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of Ang-(1–7) and ACE2 during pregnancy. Hypertension 2003;42:749–753. doi: 10.1161/01.HYP.0000085220.53285.11 [DOI] [PubMed] [Google Scholar]
- 23.Valdés G, Neves LAA, Anton L, Corthorn J, Chacón C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J, et al. Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 24.Morosin SK, Lochrin AJ, Delforce SJ, Lumbers ER, Pringle KG. The (pro) renin receptor ((P)RR) and soluble (pro)renin receptor (s(P)RR) in pregnancy. Placenta 2021;116:43–50. doi: 10.1016/j.placenta.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 25.Weiß E, Berger HM, Brandl WT, Strutz J, Hirschmugl B, Simovic V, Tam-Ammersdorfer C, Cvitic S, Hiden U. Maternal overweight downregulates MME (neprilysin) in feto-placental endothelial cells and in cord blood. Int J Mol Sci 2020;21:834. doi: 10.3390/ijms21030834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer N, Woidacki K, Knöfler M, Meinhardt G, Nowak D, Velicky P, Pollheimer J, Zenclussen AC. Chymase-producing cells of the innate immune system are required for decidual vascular remodeling and fetal growth. Sci Rep 2017;7:45106. doi: 10.1038/srep45106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kingdom JC, McQueen J, Connell JM, Whittle MJ. Fetal angiotensin II levels and vascular (type I) angiotensin receptors in pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol 1993;100:476–482. doi: 10.1111/j.1471-0528.1993.tb15276.x [DOI] [PubMed] [Google Scholar]
- 28.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 2007;293:R804–R811. doi: 10.1152/ajpregu.00725.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 2019;15:275–289. doi: 10.1038/s41581-019-0119-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999;103:945–952. doi: 10.1172/JCI4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal S, Makris A, Hennessy A. Linking the old and new: do angiotensin II type 1 receptor antibodies provide the missing link in the pathophysiology of preeclampsia? Hypertens Pregnancy 2015;34:369–382. doi: 10.3109/10641955.2015.1051227 [DOI] [PubMed] [Google Scholar]
- 32.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, Cattaneo R, Crowley SD, Dell’Italia LJ, Ford AL, et al. Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices. Hypertension 2020;76:1350–1367. doi: 10.1161/HYPERTENSIONAHA.120.15948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gathiram P, Moodley J. The role of the renin-angiotensin-aldosterone system in preeclampsia: a review. Curr Hypertens Rep 2020;22:89. doi: 10.1007/s11906-020-01098-2 [DOI] [PubMed] [Google Scholar]
- 36.Arthurs AL, Lumbers ER, Delforce SJ, Mathe A, Morris BJ, Pringle KG. The role of oxygen in regulating microRNAs in control of the placental renin-angiotensin system. Mol Hum Reprod 2019;25:206–217. doi: 10.1093/molehr/gaz004 [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens 2003;21:1897–1903. doi: 10.1097/00004872-200310000-00017 [DOI] [PubMed] [Google Scholar]
- 38.Lindheimer MD, August P. Aldosterone, maternal volume status and healthy pregnancies: a cycle of differing views. Nephrol Dial Transplant 2009;24:1712–1714. doi: 10.1093/ndt/gfp093 [DOI] [PubMed] [Google Scholar]
- 39.Narita T, Ichihara A, Matsuoka K, Takai Y, Bokuda K, Morimoto S, Itoh H, Seki H. Placental (pro)renin receptor expression and plasma soluble (pro) renin receptor levels in preeclampsia. Placenta 2016;37:72–78. doi: 10.1016/j.placenta.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 40.Gill M, Motta-Mejia C, Kandzija N, Cooke W, Zhang W, Cerdeira Ana S, Bastie C, Redman C, Vatish M. Placental syncytiotrophoblast-derived extracellular vesicles carry active NEP (neprilysin) and are increased in preeclampsia. Hypertension 2019;73:1112–1119. doi: 10.1161/HYPERTENSIONAHA.119.12707 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Gu Y, Alexander JS, Lewis DF. Histone deacetylase inhibition disturbs the balance between ACE and chymase expression in endothelial cells: a potential mechanism of chymase activation in preeclampsia. Hypertens Res 2019;42:155–164. doi: 10.1038/s41440-018-0150-1 [DOI] [PubMed] [Google Scholar]
- 42.Sandgren JA, Scroggins SM, Santillan DA, Devor EJ, Gibson-Corley KN, Pierce GL, Sigmund CD, Santillan MK, Grobe JL. Vasopressin: the missing link for preeclampsia? Am J Physiol Regul Integr Comp Physiol 2015;309:R1062–R1064. doi: 10.1152/ajpregu.00073.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Sex and age differences of renal function in rats with reduced ANG II activity during the nephrogenic period. Am J Physiol Renal Physiol 2007;293:F506–F510. doi: 10.1152/ajprenal.00066.2007 [DOI] [PubMed] [Google Scholar]
- 44.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci 1996;91:607–615. doi: 10.1042/cs0910607 [DOI] [PubMed] [Google Scholar]
- 45.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J 2007;335:974. doi: 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 2010;55:1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuura T, Shinohara K, Iyonaga T, Hirooka Y, Tsutsui H. Prior exposure to placental ischemia causes increased salt sensitivity of blood pressure via vasopressin production and secretion in postpartum rats. J Hypertens 2019;37:1657–1667. doi: 10.1097/HJH.0000000000002091 [DOI] [PubMed] [Google Scholar]
- 48.Paauw ND, Joles JA, Spradley FT, Bakrania B, Zsengeller ZK, Franx A, Verhaar MC, Granger JP, Lely AT. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats. Am J Physiol Regul Integr Comp Physiol 2017;312:R664–R670. doi: 10.1152/ajpregu.00510.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang HP, Zhang WH, Wang XF, Zhu J, Zheng YQ, Xia Q, Zhi JM. Exposure to AT1 receptor autoantibodies during pregnancy increases susceptibility of the maternal heart to postpartum ischemia-reperfusion injury in rats. Int J Mol Sci 2014;15:11495–11509. doi: 10.3390/ijms150711495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Booz GW, Kennedy D, Bowling M, Robinson T, Azubuike D, Fisher B, Brooks K, Chinthakuntla P, Hoang NH, Hosler JP, et al. Angiotensin II type 1 receptor agonistic autoantibody blockade improves postpartum hypertension and cardiac mitochondrial function in rat model of preeclampsia. Biol Sex Differ 2021;12:58. doi: 10.1186/s13293-021-00396-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, Shaltout HA, O’Shea TM, Washburn LK. Association between preterm birth and the renin–angiotensin system in adolescence: influence of sex and obesity. J Hypertens 2018;36:2092–2101. doi: 10.1097/hjh.0000000000001801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Washburn LK, Brosnihan KB, Chappell MC, Diz DI, Gwathmey TM, Nixon PA, Russell GB, Snively BM, O’Shea TM. The renin–angiotensin–aldosterone system in adolescent offspring born prematurely to mothers with preeclampsia. J Renin Angiotensin Aldosterone Syst 2015;16:529–538. doi: 10.1177/1470320314526940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.South AM, Allen NB. Antenatal programming of hypertension: paradigms, paradoxes, and how we move forward. Curr Hypertens Rep 2022;24:655–667. doi: 10.1007/s11906-022-01227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu CN, Lin YJ, Yu HR, Lin IC, Sheen JM, Huang LT, Tain YL. Protection of male rat offspring against hypertension programmed by prenatal dexamethasone administration and postnatal high-fat diet with the Nrf2 activator dimethyl fumarate during pregnancy. Int J Mol Sci 2019;20:3957. doi: 10.3390/ijms20163957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tain YL, Chen CC, Sheen JM, Yu HR, Tiao MM, Kuo HC, Huang LT. Melatonin attenuates prenatal dexamethasone-induced blood pressure increase in a rat model. J Am Soc Hypertens 2014;8:216–226. doi: 10.1016/j.jash.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 56.Forhead AJ, Jellyman JK, De Blasio MJ, Johnson E, Giussani DA, Broughton Pipkin F, Fowden AL. Maternal dexamethasone treatment alters tissue and circulating components of the renin-angiotensin system in the pregnant ewe and fetus. Endocrinology 2015;156:3038–3046. doi: 10.1210/en.2015-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaltout HA, Rose JC, Chappell MC, Diz DI. Angiotensin-(1–7) deficiency and baroreflex impairment precede the antenatal betamethasone exposure-induced elevation in blood pressure. Hypertension 2012;59:453–458. doi: 10.1161/HYPERTENSIONAHA.111.185876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bi J, Contag SA, Carey LC, Tang L, Valego NK, Chappell MC, Rose JC. Antenatal betamethasone exposure alters renal responses to angiotensin-(1–7) in uninephrectomized adult male sheep. J Renin Angiotensin Aldosterone Syst 2013;14:290–298. doi: 10.1177/1470320312465217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendricks AS, Lawson MJ, Figueroa JP, Chappell MC, Diz DI, Shaltout HA. Central ANG-(1–7) infusion improves blood pressure regulation in antenatal betamethasone-exposed sheep and reveals sex-dependent effects on oxidative stress. Am J Physiol Heart Circ Physiol 2019;316:H1458–H1467. doi: 10.1152/ajpheart.00497.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connors N, Valego NK, Carey LC, Figueroa JP, Rose JC. Fetal and postnatal renin secretion in female sheep exposed to prenatal betamethasone. Reprod Sci 2010;17:239–246. doi: 10.1177/1933719109351752 [DOI] [PubMed] [Google Scholar]
- 62.Goyal R, Van-Wickle J, Goyal D, Longo LD. Antenatal maternal low protein diet: ACE-2 in the mouse lung and sexually dimorphic programming of hypertension. BMC Physiol 2015;15:2. doi: 10.1186/s12899-015-0016-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goyal R, Wong C, Van Wickle J, Longo LD. Antenatal maternal protein deprivation: sexually dimorphic programming of the pancreatic renin-angiotensin system. J Renin Angiotensin Aldosterone Syst 2013;14:137–145. doi: 10.1177/1470320312456329 [DOI] [PubMed] [Google Scholar]
- 64.Pladys P, Lahaie I, Cambonie G, Thibault G, Lê NLO, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36 [DOI] [PubMed] [Google Scholar]
- 65.Hsu CN, Hou CY, Chan JYH, Lee CT, Tain YL. Hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy. Nutrients 2019;11:2908. doi: 10.3390/nu11122908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao D, Huang X, Li Y, Dasgupta C, Wang L, Zhang L. Antenatal antioxidant prevents nicotine-mediated hypertensive response in rat adult offspring. Biol Reprod 2015;93:66. doi: 10.1095/biolreprod.115.132381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svitok P, Okuliarova M, Varga I, Zeman M. Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Exp Biol Med 2019;244:923–931. doi: 10.1177/1535370219851110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao D, Huang X, Xue Q, Zhang L. Antenatal hypoxia induces programming of reduced arterial blood pressure response in female rat offspring: role of ovarian function. PLoS One 2014;9:e98743. doi: 10.1371/journal.pone.0098743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 2010;298:R1421–R1427. doi: 10.1152/ajpregu.00096.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia Stress and Heart study. Circulation 2015;131:1674–1681. doi: 10.1161/CIRCULATIONAHA.114.013104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.South AM, Palakshappa D, Brown CL. Relationship between food insecurity and high blood pressure in a national sample of children and adolescents. Pediatr Nephrol 2019;34:1583–1590. doi: 10.1007/s00467-019-04253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, Shaltout HA, O’Shea TM, Washburn LK. Renal function and blood pressure are altered in adolescents born preterm. Pediatr Nephrol 2019;34:137–144. doi: 10.1007/s00467-018-4050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Shaltout HA, O’Shea TM, Washburn LK. Obesity is associated with higher blood pressure and higher levels of angiotensin II but lower angiotensin-(1–7) in adolescents born preterm. J Pediatr 2019;205:55–60.e1. doi: 10.1016/j.jpeds.2018.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xue B, Yu Y, Beltz TG, Guo F, Felder RB, Wei SG, Johnson AK. Maternal angiotensin II-induced hypertension sensitizes postweaning high-fat diet-elicited hypertensive response through increased brain reactivity in rat offspring. J Am Heart Assoc 2021;10:e022170. doi: 10.1161/JAHA.121.022170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang XF, Li JD, Huo YL, Zhang YP, Fang ZQ, Wang HP, Peng W, Johnson AK, Xue B. Blockade of angiotensin-converting enzyme or tumor necrosis factor-α reverses maternal high-fat diet-induced sensitization of angiotensin II hypertension in male rat offspring. Am J Physiol Regul Integr Comp Physiol 2020;318:R351–R359. doi: 10.1152/ajpregu.00200.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Massmann GA, Zhang J, Seong WJ, Kim M, Figueroa JP. Sex-dependent effects of antenatal glucocorticoids on insulin sensitivity in adult sheep: role of the adipose tissue renin angiotensin system. Am J Physiol Regul Integr Comp Physiol 2017;312:R1029–R1038. doi: 10.1152/ajpregu.00181.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 2009;296:R309–R317. doi: 10.1152/ajpregu.90645.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cabral EV, Vieira LD, Sant’Helena BRM, Ribeiro VS, Farias JS, Aires RS, Paz ST, Muzi-Filho H, Paixão AD, Vieyra A. Alpha-tocopherol during lactation and after weaning alters the programming effect of prenatal high salt intake on cardiac and renal functions of adult male offspring. Clin Exp Pharmacol Physiol 2019;46:1151–1165. doi: 10.1111/1440-1681.13161 [DOI] [PubMed] [Google Scholar]
- 79.Gomes PR, Graciano MF, Pantaleão LC, Rennó AL, Rodrigues SC, Velloso LA, Latorraca MQ, Carpinelli AR, Anhê GF, Bordin S. Long-term disruption of maternal glucose homeostasis induced by prenatal glucocorticoid treatment correlates with miR-29 upregulation. Am J Physiol Endocrinol Metab 2014;306:E109–E120. doi: 10.1152/ajpendo.00364.2013 [DOI] [PubMed] [Google Scholar]
- 80.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding Y, Lv J, Mao C, Zhang H, Wang A, Zhu L, Zhu H, Xu Z. High-salt diet during pregnancy and angiotensin-related cardiac changes. J Hypertens 2010;28:1290–1297. doi: 10.1097/HJH.0b013e328337da8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu L, Shi A, Zhu D, Bo L, Zhong Y, Wang J, Xu Z, Mao C. High sucrose intake during gestation increases angiotensin II type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides 2016;86:133–144. doi: 10.1016/j.peptides.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 83.Nuyt AM, Lenkei Z, Corvol P, Palkovits M, Llorens-Cortés C. Ontogeny of angiotensin II type 1 receptor mRNAs in fetal and neonatal rat brain. J Comp Neurol 2001;440:192–203. doi: 10.1002/cne.1379 [DOI] [PubMed] [Google Scholar]
- 84.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu CN, Chan JYH, Wu KLH, Yu HR, Lee WC, Hou CY, Tain YL. Altered gut microbiota and its metabolites in hypertension of developmental origins: exploring differences between fructose and antibiotics exposure. Int J Mol Sci 2021;22:2674. doi: 10.3390/ijms22052674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chappell MC, Pirro NT, South AM, Gwathmey TM. Concerns on the specificity of commercial ELISAs for the measurement of angiotensin-(1–7) and angiotensin II in human plasma. Hypertension 2021;77:e29–e31. doi: 10.1161/HYPERTENSIONAHA.120.16724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson BA, Nautiyal M, Gwathmey TM, Rose JC, Chappell MC. Evidence for a mitochondrial angiotensin-(1–7) system in the kidney. Am J Physiol Renal Physiol 2015;310:F637–F645. doi: 10.1152/ajprenal.00479.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 2013;61:253–258. doi: 10.1161/HYPERTENSIONAHA.112.203679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 2016;310:H137–H152. doi: 10.1152/ajpheart.00618.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen JB, D’Agostino McGowan L, Jensen ET, Rigdon J, South AM. Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: beyond confounding. J Hypertens 2021;39:795–805. doi: 10.1097/HJH.0000000000002706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 2014;63:1815–1822. doi: 10.1016/j.jacc.2014.02.529 [DOI] [PubMed] [Google Scholar]
- 93.Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia screening: evidence report and systematic review for the US Preventive Services Task Force. J Am Med Assoc 2017;317:1668–1683. doi: 10.1001/jama.2016.18315 [DOI] [PubMed] [Google Scholar]
- 94.Azinheira Nobrega Cruz N, Stoll D, Casarini DE, Bertagnolli M. Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility. Clin Sci 2021;135:1805–1824. doi: 10.1042/CS20210284 [DOI] [PMC free article] [PubMed] [Google Scholar]


