Abstract
Gray zone lymphoma is a very rare liquid malignancy that possesses intersecting features between primary mediastinal B-cell lymphoma and classic Hodgkin Lymphoma. In the case presented and accompanying literature review, we will discuss a patient with a chief complaint of shortness of breath and was found to have a mediastinal mass with biopsy consistent with mediastinal gray zone lymphoma. Herein, we explore the historical and recently updated diagnostic criteria of gray zone lymphoma from 2022 as well as the pathophysiology as it pertains to gene expression, while also reviewing the histological findings, epidemiology and treatment modalities.
Keywords: Immunotherapy, Chemotherapy, Hodgkin, Mediastinum, Grey
1. Case presentation
A 30-year-old male with a past medical history significant for asthma presented to the emergency department with a chief complaint of shortness of breath. According to the patient, he had been experiencing worsening shortness of breath for the past few weeks which was initially present only on exertion but had recently progressed to being persistent at rest as well. The patient also complained of a concomitant non-productive cough during this time which was accompanied by intermittent febrile episodes. He also noted a 15-pound unintentional weight loss over the past two months. Vital signs on initial evaluation were significant for a temperature of 98.2 F, BP 153/89, HR 114 and sp02 95% on room air. Physical examination revealed an alert, cooperative male in mild respiratory distress, appearing his stated age, PERRL, conjunctiva/corneas clear, EOM's intact, fundi benign, no appreciable cervical/axillary/supraclavicular lymphadenopathy. Air entry was notably reduced in the right mid and lower chest region, regular, rate, and rhythm, S1 and S2 normal, no murmur, rub or gallop, abdomen was soft and non-tender, bowel sounds active in all four quadrants with no masses, no organomegaly. Lower extremities were without edema.
Labs were significant for WBC 15.62/nL, Hgb 13.4 g/dL, Platelets 428/nL, creatinine 0.94 mg/dL, AST 31, ALT 32, Na 137 mmol/L, LDH 817 U/L, Uric acid 5.4 mg/dL, ESR 28 mm/hr, CEA 0.9 ug/L, procalcitonin 0.06 ng/mL. CT angiogram of the chest was performed and showed a 20 × 7.8 cm predominantly low-density anterior mediastinal mass with no calcifications, seen in Fig. 1A. Some associated adenopathy in the precarinal space, 36 × 21 mm. Epicardial adenopathy was present measuring 31 × 22 mm. There was a also a small amount of pericardial fluid towards the cardiac apex. A large partially loculated right pleural effusion with areas of underlying consolidation and bilateral dependent changes was also seen and no filling defects in the central pulmonary arterial circulation that would suggest pulmonary embolism were appreciable. CT abdomen pelvis was also performed and showed no acute findings. Pulmonology was consulted for thoracentesis and Oncology was consulted for the mass seen on imaging. Echocardiogram was performed which was not suggestive of tamponade physiology and ejection fraction 60–65%. Thoracentesis revealed an exudative effusion with lymphocytic predominance, cytology was negative for malignant cells and flow cytometry revealed no immunophenotypic abnormalities. A mediastinal core needle biopsy was performed with findings of CD30 and CD15 positive lymphoid neoplasm, and negative for CD20 and EBV, consistent with Mediastinal Gray Zone Lymphoma. Additional serologic exams included an AFP <1.3 ng/mL, HCG <1.0 mIU/mL, Kappa Free light chain 1.18 mg/dL, Lambda free light chain 0.93 mg/dL, Kappa/Lambda ratio 1.27.
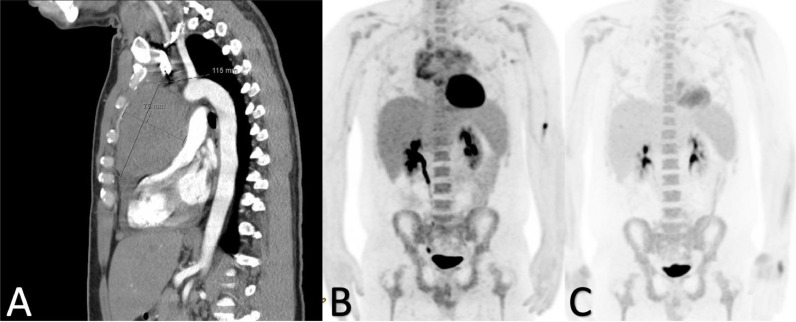
Fig. 1.
A-C. 1A: Sagittal view of CT angiogram of the chest revealing mediastinal mass. 1B: PET scan after 2 cycles of dose adjusted EPOCH. Notably with significant debulking of mediastinal mass. 1C: PET scan after 3 cycles of dose adjusted EPOCH. Complete metabolic response with a Deauville score of 2.
Abbreviations: CT: Computed Tomography, Dose adjusted EPOCH (Dose-Adjusted Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, prednisone), PET: Positron Emission Tomography
A bone marrow biopsy revealed a normocellular marrow with maturing trilineage hematopoiesis, negative for involvement by lymphoma. Concurrent flow cytometry was negative for lymphoma. The patient's clinical presentation was consistent with Stage 2, bulky disease. The patient proceeded with starting allopurinol for prevention of tumor lysis and began dose adjusted EPOCH. After 2 cycles, there was significant delbulking of his disease noted on PET scan seen in Fig. 1B and improvement of his symptoms. He continued with cycle 3/6 of dose adjusted EPOCH and was noted to have complete metabolic response with a Deauville Score of 2 seen in Fig. 1C.
2. Introduction
Lymphoma is an umbrella term used to describe neoplasms that result from a pathological clonal proliferation of B-cells, T-cells, or natural killer (NK) cells [1]. Lymphomas are broadly categorized into either Hodgkin or non-Hodgkin, with approximately 90% of lymphomas being of the non-Hodgkin subtype [2]. The principal differentiator between the two general types of lymphoma is the presence of transformed B cells that are large and multinucleated in appearance, known as Reed-Sternberg cells, which represent the hallmark characteristic of Hodgkin's lymphoma [3,4]. Meanwhile, Non-Hodgkin lymphomas possess an extensive variety of histological appearances and are the most common hematological malignancies worldwide [5,6]. Diffuse large B cell lymphoma (DLBCL) is the most prevalent sub-type of non-Hodgkin lymphoma worldwide, characterized histologically by a diffuse production of B cells with enlarged nuclei and characterized clinically by its somewhat aggressive nature with a variable response to treatment [7,8]. Gray zone lymphoma (GZL) is a term used to represent cases of malignant lymphoma which cannot be distinctively classified as either Hodgkin's or non-Hodgkin lymphoma and has been denoted as being an intermediate group between Primary Mediastinal B-cell Lymphoma (PMBL) and classical Hodgkin's lymphoma (CHL), nodular sclerosis subtype per the recent 5th edition World Health Organization (WHO) classification of lymphoid neoplasms from 2022. [9].
2.1. Pathophysiology
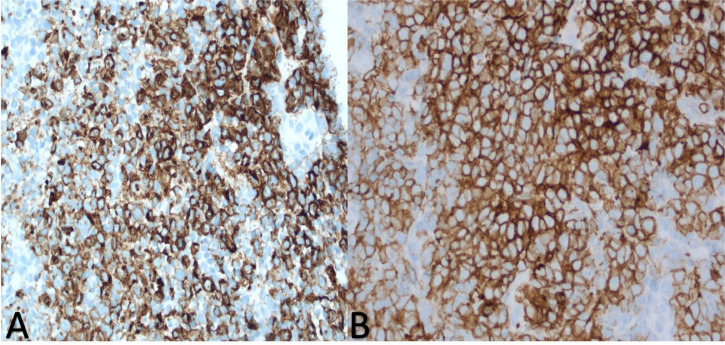
The WHO classification of lymphoid neoplasms officially recognized GZL in 2008 as a B-cell lymphoma, unclassifiable, with overlapping features of DLBCL and Hodgkin's lymphoma at that time [10]. However, the most recent 5th Edition from 2022 rectifies this definition by identifying Mediastinal Gray Zone Lymphoma (MGZL) as a B-cell lymphoma with overlapping features between PMBL and classic Hodgkin lymphoma, particularly the nodular sclerosis subtype. Topographically, GZL classically presents in the mediastinum as a large mass which has been thought to have originated from thymic B-cells, hence the denoted nomenclature of MGZL [11]. MGZL is unique in that its morphologic and immunophenotypic profile hedge on a spectrum of immunophenotypic and morphologic similarities between classic Hodgkin's lymphoma and PMBL. The characteristics of mediastinal classic Hodgkin's lymphoma and PMBL include preponderance to affect young females, have mature B cells with CD20 expression, amplification of the REL locus on chromosome 2p and the JAK2 locus on chromosome 9p, and both are also notorious for the absence of CD15. [12] The presence of Reed-Sternberg cells will elevate the likelihood that the diagnosis is that of classic Hodgkin's lymphoma. However, additional markers to differentiate classic Hodgkin's from PMBL include the B-cell associated transcription factors OCT-2 and BOB.1 whose roles in immunoglobulin regulation are strongly expressed in PMBL and absent in classic Hodgkin's. [12] CD30 will be expressed in both, however, it is expressed in a weaker more heterogenous fashion in PMBL compared to classic Hodgkin's lymphoma. Additionally, there are several genes that are expressed which are unique to PMBL, including MAL, CD23, Fig. 1, TARC, NFkB2 and PDL1/L2. MGZL was henceforth recognized in order to reflect the “gray zone” of diagnosis as it pertained to diagnosing either classic Hodgkin's lymphoma or PMBL that presented characteristically in a morphological fashion, but uncharacteristically in an immunophenotypic fashion, with the absence of CD20 or strong expression of CD15 as seen in Fig. 2A [12]. MGZL habitually expresses at least one B-cell marker in a collection of either CD20, CD79a, and PAX5 but also variably expresses PAX-5, BOB.1, and OCT-2 (Fig. 3) [13].
Fig. 2.
A-B. 2A-strong CD15 expression 2B-diffuse and strong expression of CD30 image obtained from Morristown Medical Center Pathology Department with consent obtained from patient.
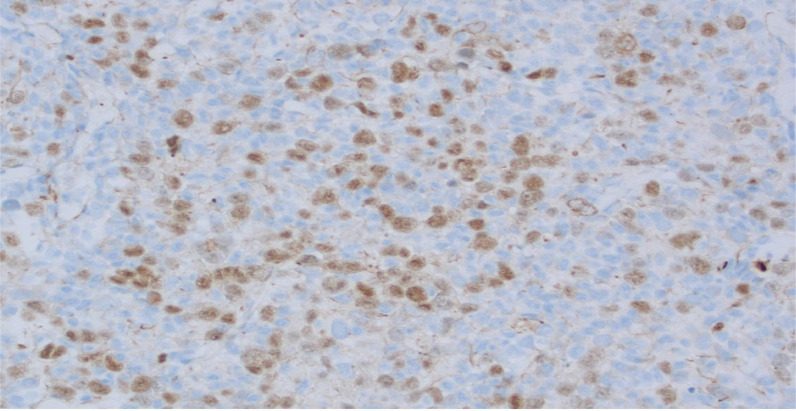
Fig. 3.
B-cell lineage confirmed by PAX5 expression; CD20 and CD79a stains negative. Image obtained from Morristown Medical Center Pathology Department with consent obtained from patient.
A large-scale DNA methylation analysis of tumor cells revealed further elucidated that there is a close relationship between MGZL, PBML and classic Hodgkin's lymphoma. The epigenetic profile of MGZL was found to share many similarities when compared to the profiles of PMBL and classic Hodgkin's lymphoma, however, MGZL did exhibit unique features. Specifically, the gene HOXA5 was observed to be hypomethylated in MGZL [14]. Modifications were reported in all three pathologies at the JAK2, CD274, and PDCD1LG2 locus at 9p24 and at the CIITA locus at 16p13.13. Gains were observed at the REL locus at 2p.16.1 in 33% of cases and gains at the MYC locus at 8q24 were observed in 27% of cases [13]. The aforementioned analysis ratifies the original WHO classification of MGZL from 2008, as there does exist an intertwinement of genetic parallels between MGZL, PMBL and classic Hodgkin's lymphoma, however, MGZL does have its own exclusive genetic modifications which renders it its own entity [13,15].
2.2. Histopathology
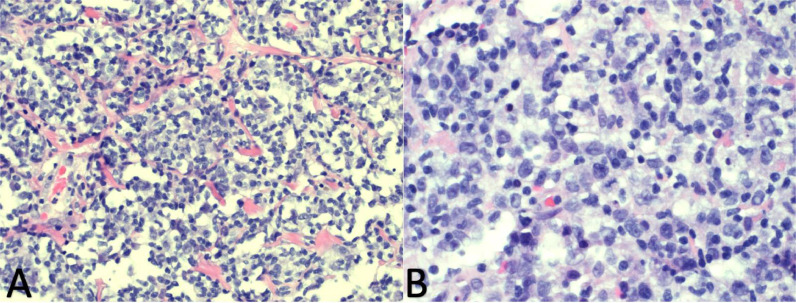
The histopathological make up of MGZL lies in a spectrum of what is archetypally seen in PMBL and Nodular Sclerosis classic Hodgkin's lymphoma. The morphological cellular make up in confirmed cases of GZL has been described as sheet-like with the tumor cells expressing a high degree of pleomorphism and with a diffusely appearing tumor architecture, which may be nodular or coarsely fibrotic [13,15]. The cells can exhibit a characteristic centroblastic or immunoblastic presence which is more in line with what is observed in PMBL, but with MGZL typically having larger cells, as seen in Fig. 4. Reed-Sternberg or Reed-Sternberg-like cells can sometimes be present in MGZL but are typically milder in size and possess nuclei that are not as prominently eosinophilic as compared to classic Hodgkin's lymphoma. Additionally, the inflammation seen in classic Hodgkin's lymphoma is seen to a milder extent with fewer eosinophils and fewer plasma cells seen in MGZL [13,16].
Fig. 4.
A-B. 4A- Diffuse proliferation of large neoplastic cells with variably fibrotic and finely vascular background and lack of pleomorphic inflammatory infiltrate. 4B- The neoplastic cells are large, with oval to lobulated nuclear contours, vesicular chromatin and conspicuous to prominent nucleoli. Image obtained from Morristown Medical Center Pathology Department with consent obtained from patient.
2.3. Descriptive epidemiology
Reported as early as 1998, then with more frequency in 2005, GZL was subsequently recognized by the World Health Organization (WHO) in 2008. Due to its rarity, the incidence cannot be estimated definitively, however Qasrawi et al. estimated an incidence rate of 0.53 per million person-years based on confirmed GZL between 2005 and 2016 with age-adjusted incidence rates according to the US Standard Population in the year 2000 [17]. Furthermore, GZL is both a diagnostic and clinical dilemma faced by pathologists for its morphologic and phenotypic complexities and by oncologists for its aggressive clinical course and poor guideline defined treatment options [13,18]. One study recognized 68 cases of GZL across 15 North American Academic Centers and after central pathologic review by 5 hematopathologists, it was determined that only 26 cases were confirmed GZL [19].
Additionally in 2008, GZL had further been characterized as Mediastinal Gray Zone Lymphoma (MGZL) as well as Non-Mediastinal Gray Zone Lymphoma (NMGZL). MGZL is the name applied to gray zone lymphoma that has similar morphologic and phenotypic features to both classic Hodgkin's lymphoma and PMBL, and is found in the mediastinum. Typically, it affects young men and children with a male to female predominance of 1.4:1, which is notably different than PMBL and classic Hodgkin's lymphoma which have a female predominance. The mean age is 32–37 years which is also similar to the age of incidence in both CHL and PMBL [20,21].
NMGZL, which was previously thought to represent a heterogeneous subset of GZL, is no longer classified as a subset of GZL as of 2022. Cases with morphologic and immunophenotypic features similar to MGZL, but presenting outside and without involvement of the mediastinum should be classified as Diffuse Large B-cell Lymphoma not otherwise specified (DLBCL, NOS) [21,9].
2.4. Treatment modalities
Treatment options for Gray Zone Lymphoma have historically been similar to the treatment for PMBL [22]. This is due in part to the lack of standardized guidelines likely related to the rarity of the disease and its challenging diagnosis. The earliest reported treatment of 24 confirmed cases of MGZL previously untreated began in 2004 at the National Institutes of Health (NIH) during a prospective study of Dose-Adjusted Etoposide, Doxorubicin, Cyclophosphamide with Vincristine, prednisone and Rituximab (DA-EPOCH-R) [22]. The median follow up was 59 months, Event Free Survival (EFS) and Overall Survival (OS) were 62% and 74% respectively. Some years later, a retrospective by Pilichowska et al. would further identify 26 cases across 15 US and Canadian Academic Medical Centers with analysis of additional treatment options with equal or improved survival rates [19].
These regimens included Cyclophosphamide, doxorubicin, vincristine and prednisone +/-Rituximab (CHOP +/- R) which provided an Overall Response Rate (ORR) of 65% in 17/25 patients. Doxorubicin, bleomycin, vinblastine and dacarbazine +/- Rituximab (ABVD +/- R) which had an ORR of 60% in 6/25 patients and DA-EPOCH-R in 2/25 patients with an ORR of 70%. The median follow up was 44 months, 3 year Progression Free Survival (PFS) and OS were 39% and 95% respectively [19]. Due to the small samples among these groups though, no definitive management options could be derived without standardized clinical trials.
Studies from European centers have evaluated more dose intensive treatment regimens such as methylprednisolone, doxorubicin, cyclophosphamide, procarbazine, etoposide, bleomycin and vincristine (escBEACOPP) or doxorubicin, methylprednisolone, cyclophosphamide, bleomycin and vindesine (ACBVP) [23]. These regimens were studied in a sample of 99 patients with GZL with 3 year EFS being 73% and 70% respectively with the aforementioned regimens. Additionally, 3 year OS was for escBEACOPP and ACBVP were 94% and 86% respectively. 3 year EFS for both regimens was 74%. [23]. This study further provided additional treatment options but notably the patients studied had excellent performance statuses which could have contributed to their ability to tolerate those regimens.
Relapsed/Refractory (R/R) GZL signifies an even narrower definitive therapeutic approach. Historically, chemotherapy salvage regimens have been employed with studies such as Evens et al.’s, which included 112 patients treated with upfront therapy resulting in 65 relapses with a median time of 7 months and subsequently proceeding to salvage therapy. The regimens studied included Ifosfamide, carboplatin, etoposide (n = 39), etoposide, solumedrol, cytarabine and cisplatin (n = 8), gemcitabine-based (n = 3), ABVD (n = 2) and 4 cases treated with biologic therapy including brentuximab vedotin. The vast majority of these patients received hematopoietic stem cell transplant (HSCT), primarily being autologous. The 2 year OS for R/R GZL who underwent HSCT was 88% and 67% without HSCT. Even after multivariate analyses controlling for IPI and response to salvage chemo pre-HSCT these findings showed that the role for salvage chemo and HSCT was significant in patients with R/R GZL [24].
When assessing the role of novel biologics in GZL, the data is less robust with agents such as Immune Checkpoint Inhibitors and anti-body drug conjugates being of particular interest. Anti- CD30 antibody drug conjugates like Brentuximab vedotin are an appealing choice due to GZL's common expression of CD30 providing a targetable approach for therapy [25]. Chromosomal alterations of 9p24.1 have also been linked to the expression of programmed death (PD-1) ligand on classic Hodgkin's Lymphoma and PMBL further highlighting the possibility for tumor microenvironment manipulation in GZL due to the commonalities it shares with classic Hodgkin's lymphoma and PMBL [26]. These particular molecular features have led to the development of the phase 2 Checkmate 436 study which evaluated the PD-1 inhibitor Nivolumab in tandem with Brentuximab vedotin to fully elucidate survival outcomes in R/R MGZL. The study included 10 patients with a median of 2 prior lines of chemotherapy and none had received autologous HSCT. An ORR was 70% and Complete Remission of 50% was reached at 1.2–1.3 months [27]. Due to the favorable safety profile and excellent response rates, this regimen of novel agents continues to be explored and may one day solidify its place in the treatment regimen of GZL with further clinical trials.
3. Conclusion
While gray zone lymphoma by definition is classified as falling in-between two other types of malignancies, it should be viewed as a separate phenomenon. The importance of this distinction stems not necessarily from a diagnostic perspective, but primarily from a therapeutic one. Once a diagnosis is established through imaging and biopsy, the focus should be pivoted towards proper treatment. The most commonly used and provenly efficacious treatment outside of randomized clinical trial settings is EPOCH, which is the same treatment as for primary B-cell lymphoma. However, as interest has increased in gray zone lymphoma, different regimens and even different modalities, including antibody drug conjugates are progressively being studied and have shown favorable results. Although limited by its rarity, there is increasing hope and optimism that as more studies are conducted and as more data is accrued, there will be a marked increase in a targeted and effective treatment approach for gray zone lymphoma which will lead to improved outcomes in both frontline and relapsed settings.
4. Clinical practice points
The newest updates from the recent 5th edition World Health Organization (WHO) classification of lymphoid neoplasms from 2022 has updated the definition of Gray Zone Lymphoma from the prior iteration. Gene expressional differences, histologic findings and anatomical location presentation now differentiate Mediastinal Gray Zone Lymphoma from Diffuse Large B-Cell Lymphoma not otherwise specified. These updates will be reviewed in a comprehensive format in conjunction with a clinical case. This is the first review of literature to include the 5th edition and contribute a case to the literature under the updated diagnostic criteria.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Funding statement
No financial support was necessary for the preparation of this manuscript or acquiring data.
Submission declaration and verification
This article is not under consideration for publication elsewhere, its publication is approved by all authors and if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Patient consent statement
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
CRediT authorship contribution statement
Dariusz Uczkowski: Conceptualization, Writing – original draft, Data curation. Hamza Ashraf: Conceptualization, Writing – original draft. Mohamad Cherry: Supervision, Writing – review & editing. Nikolay Dimov: Resources, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
None.
References
- 1.Lymphoma - statpearls NCBI bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560826/. Accessed October 5, 2022.
- 2.Mugnaini E.N., Ghosh N. Lymphoma. Primary Care. 2016;43(4):661–675. doi:10.1016/j.pop.2016.07.012. [DOI] [PubMed]
- 3.Bräuninger A., Schmitz R., Bechtel D., Renné C., Hansmann M.-.L., Küppers R. Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int. J. Cancer. 2005;118(8):1853–1861. doi: 10.1002/ijc.21716. [DOI] [PubMed] [Google Scholar]
- 4.Küppers R., Hansmann M.-.L. The Hodgkin and reed/Sternberg cell. Int. J. Biochem. Cell Biol. 2005;37(3):511–517. doi: 10.1016/j.biocel.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Shankland K.R., Armitage J.O., Hancock B.W. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–857. doi: 10.1016/s0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 6.Thandra K.C., Barsouk A., Saginala K., Padala S.A., Barsouk A., Rawla P. Epidemiology of non-Hodgkin's lymphoma. Med. Sci. 2021;9(1):5. doi: 10.3390/medsci9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S., Young K.H., Medeiros L.J. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y., Pittaluga S., Jaffe E.S. The histological classification of diffuse large B-cell lymphomas. Semin. Hematol. 2015;52(2):57–66. doi: 10.1053/j.seminhematol.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alaggio R., Amador C., Anagnostopoulos I., et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi: 10.1038/s41375-022-01620-2. JulEpub 2022 Jun 22. PMID: 35732829; PMCID: PMC9214472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahluwalia A., Bondili L., Salamera J., Cholankeril M. Unraveling the mystery of gray zone lymphoma in human immunodeficiency virus-seropositive patients: two cases. J. Hematol. 2020;9(4):132–136. doi: 10.14740/jh554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grey Zone Lymphoma (GZL). Lymphoma Australia. https://www.lymphoma.org.au/types-of-lymphoma/non-hodgkin-lymphoma/aggressive-fast-growing-b-cell-nhl/grey-zone-lymphoma-gzl/. Published May 11, 2022. Accessed November 2, 2022.
- 12.Quintanilla-Martinez L., Fend F. Mediastinal gray zone lymphoma. Haematologica. 2011;96(4):496–499. doi: 10.3324/haematol.2011.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan C., Pittaluga S. Into the gray-zone: update on the diagnosis and classification of a rare lymphoma. Expert Rev. Hematol. 2019;13(1):1–3. doi: 10.1080/17474086.2020.1696186. [DOI] [PubMed] [Google Scholar]
- 14.Eberle F.C., Rodriguez-Canales J., Wei L., et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin's lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011;96(4):558–566. doi: 10.3324/haematol.2010.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilichowska M., Pittaluga S., Ferry J.A., et al. Clinicopathologic consensus study of Gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv. 2017;1(26):2600–2609. doi: 10.1182/bloodadvances.2017009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkozy C., Molina T., Ghesquières H., et al. Mediastinal gray zone lymphoma: clinico-pathological characteristics and outcomes of 99 patients from the Lymphoma Study Association. Haematologica. 2017;102(1):150–159. doi: 10.3324/haematol.2016.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qasrawi Ayman, et al. Trends and outcomes of gray zone lymphoma in the United States: a population-based registry study. Biol. Blood Marrow Transplant. 2020;26(3):S230. [Google Scholar]
- 18.Swerdlow Steven H., et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, J. Am. Soc. Hematol. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilichowska Monika, Kritharis Athena, Evens Andrew M. Gray zone lymphoma: current diagnosis and treatment options. Hematol./Oncol. Clin. 2016;30(6):1251–1260. doi: 10.1016/j.hoc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Quintanilla-Martinez Leticia, Fend Falko. Mediastinal gray zone lymphoma. Haematologica. 2011;96(4):496. doi: 10.3324/haematol.2011.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch-Schips Jan, et al. The grey zones of classic Hodgkin lymphoma. Cancers (Basel) 2022;14(3):742. doi: 10.3390/cancers14030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson Wyndham H., et al. A prospective study of mediastinal gray-zone lymphoma. Blood, J. Am. Soc. Hematol. 2014;124(10):1563–1569. doi: 10.1182/blood-2014-03-564906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkozy Clémentine, et al. Mutational landscape of gray zone lymphoma. Blood. 2021;137(13):1765–1776. doi: 10.1182/blood.2020007507. [DOI] [PubMed] [Google Scholar]
- 24.Evens A.M., Kanakry J.A., Sehn L.H., et al. Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: characteristics, outcomes, and prognostication among a large multicenter cohort. Am. J. Hematol. 2015;90(9):778–783. doi: 10.1002/ajh.24082. [DOI] [PubMed] [Google Scholar]
- 25.Kritharis Athena, Pilichowska Monika, Evens Andrew M. How I manage patients with grey zone lymphoma. Br. J. Haematol. 2016;174(3):345–350. doi: 10.1111/bjh.14174. [DOI] [PubMed] [Google Scholar]
- 26.Green Michael R., et al. Integrative analysis reveals selective 9p24. 1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood, J. Am. Soc. Hematol. 2010;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoro Armando, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory mediastinal gray zone lymphoma: primary efficacy and safety analysis of the phase 2 CheckMate 436 study. Blood. 2020;136:44–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.