Abstract
Background
Heart failure–related cardiogenic shock (HF-CS) is increasingly common. Moderate/severe functional mitral regurgitation (FMR) is commonly seen in patients presenting with decompensated heart failure and is associated with worse outcomes. Percutaneous mechanical circulatory support devices are increasingly used to provide hemodynamic support for ongoing CS. There is no description of the impact of Impella device on hemodynamic response when used in combination with preexisting FMR.
Methods
Retrospective review of patients aged ≥18 years, who underwent Impella 5.5 implant for HF-CS, and who had a transthoracic echocardiogram performed pre- and post-Impella.
Results
Of 24 patients, 33% had moderate-to-severe/severe FMR, 38% had mild-moderate/moderate FMR, and 29% had trace/mild FMR on pre-Impella transthoracic echocardiogram. Additional right ventricular assist device was simultaneously inserted in 3 patients, of whom 1 had severe, 1 had moderate, and another had mild FMR pre-Impella. Despite maximally tolerated Impella unloading, 6 patients (25%) had persistent moderate-severe/severe FMR, and 9 (37.5%) patients had persistent moderate FMR. Overall, however, there was a decrease in central venous pressure, pulmonary artery diastolic pressure, serum lactate, and vasoactive-inotrope score at 24 hours post-Impella, and survival was high at 83%.
Conclusions
In a retrospective cohort of patients admitted with HF-CS who underwent Impella 5.5 implant for hemodynamic support, Impella did not seem to acutely ameliorate FMR severity. Despite this, there was a significant improvement in hemodynamic response at 24 hours post-Impella. In carefully selected patients, especially those with isolated left ventricular failure, Impella 5.5 may provide adequate hemodynamic support even in the presence of higher severity FMR.
Keywords: Cardiogenic shock, Functional mitral regurgitation, Heart failure, Impella
Introduction
Over the last decade, acute decompensated heart failure (ADHF) has superseded acute coronary syndromes as the predominant cause of cardiogenic shock (CS).1 Moderate or severe functional mitral regurgitation (FMR) is commonly seen in patients presenting with ADHF and is associated with worse outcomes.2 Percutaneous mechanical circulatory support (MCS) devices are increasingly used to provide hemodynamic support for patients with CS.3 Recent experiences have shown the feasibility of the Impella 5.5 device as a bridge to recovery and/or to orthotopic heart transplant and durable left ventricular assist devices (LVADs).4 However, little is known about the interaction between preexisting FMR and percutaneous MCS devices. Preclinical studies demonstrated a reduction in FMR with the use of intra-aortic balloon pump (IABP),5 and clinical studies have found the presence of moderate or severe mitral regurgitation (MR) to be a predictor of hemodynamic response to IABP (such as improved cardiac output and reduced mean pulmonary arterial pressure).6,7 The Impella device (Abiomed, Danvers, Massachusetts), in contrast, has been shown not to impact the integrity of the mitral valve apparatus,8 but only a few cases of its use in the presence of acute, severe ischemic MR have been reported.9,10 There is no description of the impact of Impella device on hemodynamic response when used in combination with preexisting FMR. We sought to examine the hemodynamic response to Impella 5.5 in patients with heart failure–related CS (HF-CS) and concomitant FMR.
Methods
We performed a retrospective review of all patients aged ≥18 years, who underwent Impella 5.5 implantation for ongoing CS secondary to ADHF at our institution, and who had a transthoracic echocardiogram (TTE) performed before and after Impella implantation. Patients with primary mitral valve disease were excluded. Patient demographics were noted at baseline. Hemodynamic data and laboratory values were collected at 24 hours pre- and post-Impella implant. TTE studies performed in closest proximity to the Impella placement, both pre- and post-implant, were used. At our institution, the level of Impella support (performance level 1 through 9) is assessed daily to ensure maximally tolerated unloading. The performance (P) level of Impella support at the time of postimplant TTE was noted. Continuous data are presented as median (25th-75th interquartile range) or proportions. The chi-square test was used to compare proportions, and medians were compared using the Wilcoxon rank-sum test. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata version 15.1 (College Station, Texas). The study was approved by our center’s institutional review board.
Results
Twenty-four patients underwent Impella 5.5 implant for HF-CS between December 2019 and September 2021. The median age was 60 years, and 42% were females. Three patients had newly diagnosed or de-novo HF, whereas the remaining 21 patients had presented with acute on chronic heart failure (Table 1). At baseline (before Impella 5.5 implant), 7 patients (29%) had trace/mild FMR, 9 patients (38%) had mild-moderate/moderate FMR, and 8 patients (33%) had moderate-to-severe/severe FMR. Because of concern for right ventricular (RV) failure (high central venous pressure [CVP] and severe tricuspid regurgitation), RV support with a Protek Duo RV assist device was instituted concurrently with Impella insertion in 3 patients, of whom 1 had severe FMR, 1 had moderate, and another had mild FMR at baseline.
Table 1.
Baseline demographics and pre- and post-Impella clinical characteristics
| Parameter | Preimplant |
Postimplant |
p-value |
|---|---|---|---|
| Median [IQR]/number (%) | Median [IQR]/number (%) | ||
| 1. Age | 60 [52-66] | NA | NA |
| 2. Females | 10 (42) | NA | NA |
| 3. Chronicity of HF | |||
| De-novo | 3 (12.5) | NA | NA |
| Acute on chronic | 21 (87.5) | ||
| 4. Mean arterial pressure (mm Hg)∗ | 73 [68-77] | 75 [69-80] | 0.6 |
| 5. Left ventricular end-diastolic dimension in mm | 62.5 [58-66] | 59.5 [55-68] | 0.4 |
| 6. FMR severity | |||
| a. Trace/mild | 7 (29) | 9 (38) | |
| b. Mild-moderate/moderate | 9 (38) | 9 (38) | |
| c. Moderate-severe/severe | 8 (33) | 6 (24) | |
| 7. Central venous pressure (mm Hg)∗ | 12 [8-14] | 9 [7-11] | 0.02 |
| 8. Pulmonary artery diastolic pressure (mm Hg)∗ | 25 [19-27] | 18 [14-21] | 0.002 |
| 9. Pulmonary capillary wedge pressure (mm Hg)∗ | 22 [16-26] | 17 [10-20] | 0.008 |
| 10. Cardiac index (liters per min/kg/m2)∗ | 1.7 [1.5-2.1] | 2.7 [2.5-3] | <0.001 |
| 11. Systemic vascular resistance (dynes/s/cm−5) | 1541 [1225-1914] | 960 [864-1183] | <0.001 |
| 12. Serum creatinine | 1.6 [1.1-1.9] | 1.4 [1-1.9] | 0.6 |
| 13. Serum lactate | 1.9 [1.2-2.7] | 1.3 [1-1.7] | 0.046 |
| 14. Serum alanine aminotransferase | 40 [22.5-164.5] | 32.5 [17.5-93.5] | 0.5 |
| 15. Serum aspartate transaminase | 46.5 [25.5-113.5] | 43.5 [31-95] | 0.8 |
| 16. Serum total bilirubin | 0.9 [0.5-1.6] | 1.3 [0.7-1.9] | 0.3 |
| 17. Vasoactive-inotropic score∗ | 12 [7-21] | 8 [4-12] | 0.01 |
| 18. Days on Impella support | NA | 13 [8-21] | NA |
Notes. Bold values indicate statistical significant.
FMR, functional mitral regurgitation; HF, heart failure; IQR, interquartile range; NA, not available.
Values measured at 24 h pre- and post-Impella 5.5 implant.
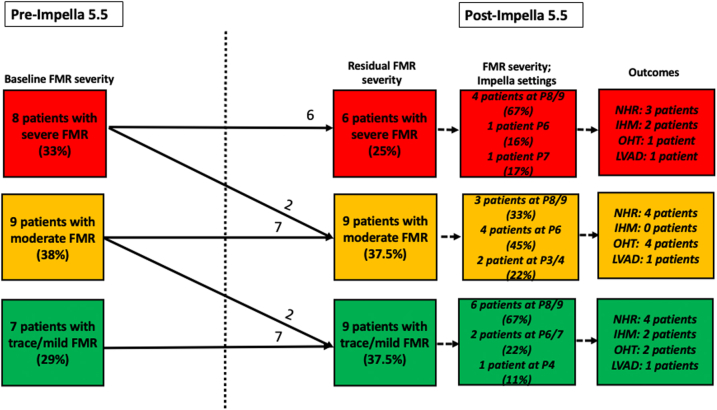
The median time to post-Impella implant TTE was 2 days [interquartile range (IQR) 1-5]. After Impella 5.5 implant, 6 of 8 patients (75%) had persistent moderate-severe/severe FMR, including the 1 patient who received concomitant RV support. Four of the 6 patients who had persistent moderate-severe/severe FMR were being maximally supported with Impella P level 8 or 9 (Figure 1). The remaining 2 patients with persistent FMR post-Impella implant were being supported with Impella P level 6 or 7 and had failed an increase in P level secondary to ventricular ectopy and worsening RV failure, respectively. Two (of 8) patients with moderate-severe/severe FMR at baseline improved slightly to moderate FMR at Impella P levels 9 and 3, respectively. A total of 9 patients had moderate FMR, and 9 had trace/mild FMR after Impella placement with maximally tolerated Impella support ranging from P level 4 to 9 (Figure 1). Overall, left ventricular end-diastolic dimension measured on the post-Impella implant TTE was not significantly different from that measured pre-Impella implant (Table 1).
Figure 1.
Distribution of functional mitral regurgitation severity pre- and post-Impella 5.5 implant with Impella performance level at the time of post-implant transthoracic echocardiogram as well as outcomes stratified by severity of residual FMR post-Impella implant.
Abbreviations: FMR, functional mitral regurgitation; IHM, in-hospital mortality; LVAD, left ventricular assist device; NHR, native heart recovery; OHT, orthotopic heart transplant; P level, performance level.
Baseline hemodynamics in patients with trace/mild FMR were not significantly different from patients with moderate and moderate-to-severe/severe FMR. At 24 hours post-Impella implant, there was a significant decrease in CVP (12 [8-14] to 9 [7-11], p = 0.012), a significant decrease in pulmonary artery diastolic pressure (PADP; 25 [19-27] to 18 [14-21], p = 0.002) and pulmonary capillary wedge pressure (22 [16-26] to 17 [10-20], p = 0.008), a significant drop in systemic vascular resistance (1541 [1225-1914] to 960 [864-1183], p ≤ 0.001), a significant decrease in serum lactate levels (1.9 [1.2-2.7] to 1.3 [1-1.7], p = 0.046), and a significant decrease in vasoactive-inotrope score (VIS; 12 [7-21] to 8 [4-12], p = 0.014) compared with 24 hours pre-Impella implant (Table 1). There was a significant increase in cardiac index (CI) at 24 hours post-Impella implant compared with 24 hours pre-Impella implant (1.7 [1.5-2.1] to 2.7 [2.5-3], p < 0.001). There were no significant changes in mean arterial pressure, serum creatinine, serum alanine aminotransferase, aspartate transaminase, and total bilirubin at 24 hours post-Impella implant compared with 24 hours pre-Impella implant (Table 1).
Of the 24 patients included in this study, 4 patients (16.7%) suffered in-hospital mortality, 10 patients (41.7%) experienced native heart recovery (NHR) and were weaned off MCS, 6 patients (25%) underwent orthotopic heart transplant, and 4 patients (16.7%) underwent durable LVAD implant. Patient outcomes stratified by residual FMR severity after Impella implant are shown in Figure 1 and did not seem to vary across groups. Three patients who had severe FMR at baseline went on to have NHR. Of them, 1 patient had a reduction in FMR severity to mild-to-moderate, whereas the remaining 2 patients had persistent severe FMR on a TTE performed after Impella removal. Overall survival was at 83%.
Discussion
In a retrospective cohort of patients admitted with HF-CS who underwent Impella 5.5 implant for hemodynamic support, Impella did not appear to acutely ameliorate preexisting severe FMR. However, there was an overall improvement in hemodynamic response at 24 hours post-implant, with increased CI and decreased CVP, PADP, VIS, and serum lactate levels. Overall survival was high at 83%.
CS secondary to ADHF, also referred to as HF-CS, is increasingly recognized as a distinct clinical entity.11 In a single-center retrospective analysis of patients admitted with ADHF and left ventricular systolic dysfunction, more than half of all patients had at least moderate FMR, which was associated with worse outcomes.2 Initial analyses from the multicenter Cardiogenic Shock Working Group registry show that >77% of all patients admitted with HF-CS undergo percutaneous MCS device implantation with IABP being the most used device and Impella being the second most commonly used device.11,12 Given the increasing use of percutaneous MCS devices in these patients, choosing the right device for the right patient has never been more important.
FMR is often dynamic in response to changes in afterload. The use of an IABP results in lower aortic impedance and systemic vascular resistance and therefore reduces left ventricular afterload.7 Preclinical studies have demonstrated a reduction in MR and improvement in forward flow with the use of IABP.5 Clinical studies of IABP in patients with CS have found moderate-to-severe FMR to be a predictor of hemodynamic response to IABP.6 Single-center studies of durable LVADs raised some concern that residual MR after LVAD implant may adversely impact hemodynamics and RV function.13 However, a post-hoc analysis of the MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 trial showed that hemodynamic unloading with both axial and centrifugal durable LVADs ameliorated significant MR to a clinically insignificant degree within 1-month of support. This study also found that neither baseline nor residual MR affected survival at 24 months post-LVAD implant.13
The Impella 5.5 can provide flows comparable to durable LVADs, but only small case series have examined the use of Impella in the setting of CS with concomitant severe MR.9,10 In our small, retrospective cohort, we make 3 important observations: (1) the Impella 5.5 did not appear to acutely (within 24 hours) ameliorate moderate or greater FMR; (2) Impella 5.5 did provide significant left ventricular unloading as evidenced by a reduction in intracardiac filling pressures and lactate clearance as well as a significant decrease in systemic vascular resistance and vasoactive-inotrope score (Table 1); and (3) a significant proportion of patients in our cohort (42%) experienced NHR on Impella 5.5 support, regardless of baseline/residual FMR severity (Figure 1). These observations were made across the entire cohort, regardless of baseline severity of FMR. Overall, patient outcomes did not seem to differ across residual FMR severity (Figure 1). The Impella percutaneous catheters have evolved over time as has the Impella learning curve.14 In a retrospective, registry-based study, the Impella CP was shown to be associated with improved survival in patients with acute myocardial infarction low LVEF (without CS) undergoing high-risk percutaneous coronary intervention when compared with the less powerful Impella 2.5 and IABP used in a historic registry-based cohort.14 It remains to be seen whether the Impella 5.5, a device capable of delivering much higher flows, confers an increased chance of myocardial recovery in patients with HF-CS.
In our series, 2 patients with moderate or greater FMR demonstrated signs of RV failure and required addition of an RV assist device despite Impella 5.5 implantation. The Impella 5.5, by reducing pulmonary capillary wedge pressure, decreases RV afterload and can improve RV function.15 It has also been suggested that assessment of RV adaptation to left-sided continuous flow by the Impella device may predict RV failure after durable LVAD implant.16 More data are needed to determine which patients with CS would benefit from RV support in addition to Impella insertion, especially those with preexisting RV dysfunction and moderate/severe MR.
The limitations of our study include retrospective analysis in a small cohort at a single center. TTE beyond 24 hours post-Impella implant was not uniformly available to assess whether FMR decreased with longer duration of Impella support. Persistent severe FMR might have been due to low Impella speed in some patients, although 8 patients (33.33%) still had moderate or greater FMR despite high P levels (P7 or greater). This series supports the continued use of the Impella 5.5 in carefully selected patients presenting with HF-CS. Additional research in larger prospective cohorts is needed to inform better patient selection.
Conclusions
In a small retrospective cohort, the Impella 5.5 did not appear to acutely ameliorate preexisting severe FMR. Despite this, there were significant improvements in hemodynamic response at 24 hours post-implant, with increased CI and decreased CVP, PADP, VIS, and serum lactate levels. Overall survival was high at 83%. Collectively, these findings suggest that despite a lack of reduction in baseline FMR, the Impella 5.5 can provide adequate hemodynamic support in certain carefully selected patients, especially those with isolated left ventricular failure.
Ethics Statement
The research reported has adhered to all relevant ethical guidelines and was approved by our center's institutional review board.
Funding
The authors have no funding to report.
Disclosure statement
The authors report no conflict of interest.
References
- 1.Jentzer J.C., Ahmed A.M., Vallabhajosyula S., et al. Shock in the cardiac intensive care unit: changes in epidemiology and prognosis over time. Am Heart J. 2021;232:94–104. doi: 10.1016/j.ahj.2020.10.054. [DOI] [PubMed] [Google Scholar]
- 2.Kataria R., Castagna F., Madan S., et al. Severity of functional mitral regurgitation on admission for acute decompensated heart failure predicts long-term risk of rehospitalization and death. J Am Heart Assoc. 2022;11(1) doi: 10.1161/JAHA.121.022908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rihal C.S., Naidu S.S., Givertz M.M., et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt A.M., Potapov E., Schibilsky D., et al. First in men multicenter experience with the Impella 5.5. J Heart Lung Transplant. 2020;39(4):S99. doi: 10.1016/j.healun.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Dekker A.L., Reesink K.D., van der Veen F.H., et al. Intra-aortic balloon pumping in acute mitral regurgitation reduces aortic impedance and regurgitant fraction. Shock. 2003;19(4):334–338. doi: 10.1097/00024382-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Huang A.L., Fried J.A., Malick W., et al. Predictors of hemodynamic response to intra-aortic balloon pump therapy in patients with acute decompensated heart failure and cardiogenic shock. J Invasive Cardiol. 2021;33(4):E275–E280. doi: 10.25270/jic/20.00473. [DOI] [PubMed] [Google Scholar]
- 7.Baldetti L., Pagnesi M., Gramegna M., et al. Intra-aortic balloon pumping in acute decompensated heart failure with hypoperfusion: from pathophysiology to clinical practice. Circ Heart Fail. 2021;14(11) doi: 10.1161/CIRCHEARTFAILURE.121.008527. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein J.A., Dixon S.R., Douglas P.S., et al. Maintenance of valvular integrity with Impella left heart support: results from the multicenter PROTECT II randomized study. Catheter Cardiovasc Interv. 2018;92(4):813–817. doi: 10.1002/ccd.27242. [DOI] [PubMed] [Google Scholar]
- 9.Imaoka S., Kainuma S., Toda K., et al. Impella support as a bridge to surgery for severe mitral regurgitation with cardiogenic shock. Circ Rep. 2021;3(3):178–181. doi: 10.1253/circrep.CR-21-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenbriele C., Balthazar T., Wilson J., et al. Left Impella®-device as bridge from cardiogenic shock with acute, severe mitral regurgitation to MitraClip®-procedure: a new option for critically ill patients. Eur Heart J Acute Cardiovasc Care. 2020;10(4):415–421. doi: 10.1093/ehjacc/zuaa031. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Montfort J., Sinha S.S., Thayer K.L., et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ Heart Fail. 2021;14(5) doi: 10.1161/CIRCHEARTFAILURE.120.007924. [DOI] [PubMed] [Google Scholar]
- 12.Thayer K.L., Zweck E., Ayouty M., et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9) doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanwar M.K., Rajagopal K., Itoh A., et al. Impact of left ventricular assist device implantation on mitral regurgitation: an analysis from the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39(6):529–537. doi: 10.1016/j.healun.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill W.W., Anderson M., Burkhoff D., et al. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices: Impella-supported high-risk percutaneous coronary intervention in patients with severely depressed LVEF. Am Heart J. 2022;248:139–149. doi: 10.1016/j.ahj.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Everett K.D., Jain P., Botto R., et al. Acute effects of left ventricular support with Impella 5.5 on biventricular hemodynamics. Circ Heart Fail. 2021;14(9) doi: 10.1161/CIRCHEARTFAILURE.121.008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsi B., Joseph D., Trachtenberg B., et al. Degree of change in right ventricular adaptation measures during axillary Impella support informs risk stratification for early, severe right heart failure following durable LVAD implantation. J Heart Lung Transplant. 2022;41(3):279–282. doi: 10.1016/j.healun.2021.11.007. [DOI] [PubMed] [Google Scholar]