Abstract
Background
Reshaping the dilated left ventricle using a surgically implanted papillary muscle sling has been shown to provide long-term improvement in cardiac function compared to annuloplasty alone in patients with systolic heart failure. A papillary muscle sling which can be implanted via a transcatheter approach has the potential to make this treatment more widely available to patients.
Methods
The Vsling transcatheter papillary muscle sling device was evaluated in a chronic animal model (sacrificed at 30 and 90 days), in a simulator, and in a human cadaver.
Results
The Vsling device was successfully implanted in 10 pigs, 6 simulator procedures, and 1 human cadaver. Procedure complexity and device usability were rated as reasonable or better by 6 interventional cardiologists. Gross and histological analysis in chronic pigs through 90 days demonstrated near-complete endothelial coverage with mild inflammation and small hematoma formation but without adverse tissue reactions, thrombi, or embolization.
Conclusions
Preliminary feasibility and safety of the Vsling implant and implantation procedure have been demonstrated. Human trials are planned to begin in the summer of 2022.
Keywords: Heart failure, Papillary muscle repositioning, Papillary muscle sling, Transcatheter, Ventricular reshaping
Highlights
-
•
Transcatheter papillary muscle sling implantation is successful in pigs and cadavers.
-
•
Vsling transcatheter papillary muscle sling is found safe in chronic swine study.
-
•
Vsling implantation procedure is rated as reasonable or better by users.
-
•
Vsling implantation is estimated to take 45 to 60 minutes based on pigs and cadavers.
Introduction
Heart failure with reduced ejection fraction is a disease of the left ventricle (LV) in which the predominant cause of symptoms is dilatation and remodeling of the LV.1,2 This remodeling reduces the effectiveness of ventricular contraction and, in some patients, distorts the geometric positioning of the papillary muscles, tethering the mitral valve leaflets to prevent effective coaptation, resulting in secondary or functional mitral regurgitation (FMR). Surgical papillary muscle repositioning (PMR) (also known as papillary muscle apposition) when combined with mitral annuloplasty has been shown to improve LV function and reduce FMR.3, 4, 5, 6, 7, 8 Ventricular reshaping, referring to therapies that correct, compensate for, and/or prevent ventricular remodeling, offers the potential to improve ventricular function and prognosis in patients with heart failure with reduced ejection fraction, with or without significant FMR. In a randomized trial, the surgically implanted Coapsys device (Myocor, Inc, Maple Grove, Minnesota), which reduces the diameter of the LV and the mitral annulus, improved survival and significantly decreased major adverse outcomes compared to control, without substantially reducing FMR.9 In uncontrolled studies, the AccuCinch device (Ancora Heart, Santa Clara, California), which reduces the size of the LV by cinching the ventricular wall below the mitral annulus, has been shown to reduce LV dimensions and improve quality of life and 6-minute walk distance at 12-month follow-up.10 Favorable 12-month results have also been reported with the Revivent TC device (BioVentrix, San Ramon, California) which reduces LV volumes by excluding infarcted segments of the anterior myocardium.11
Surgical implantation of a papillary muscle sling, while generally classified as a method of PMR, also results in LV reshaping. Unlike other methods of PMR which typically focus on repositioning the tips of the papillary muscles, the papillary muscle sling is placed around the bases of the papillary muscles where they attach to the LV wall.12,13 Tightening the sling pulls the myocardium inward, reducing the size of the ventricle. The addition of a papillary muscle sling during surgical annuloplasty has been shown to improve the long-term maintenance of LV diameter reduction.4
The papillary muscle sling procedure currently involves wrapping an expanded polytetrafluoroethylene (ePTFE) vascular graft around the base of the papillary muscles during an open or minimally invasive on-pump surgical procedure.12,13 Its use to date has thus been limited to patients who both require and are healthy enough to undergo open heart surgery. It is generally performed in patients with advanced coronary artery disease and severe FMR undergoing coronary artery bypass graft surgery and annuloplasty, and its outcomes as a stand-alone procedure have not been reported. This surgical procedure is not commonly performed due to its complexity and additional on-pump time that it requires. The ability to implant a papillary muscle sling via a transcatheter procedure would potentially reduce the morbidity of the procedure (potentially expanding its use earlier in disease progression) and otherwise make it available to patients with LV dysfunction with or without FMR who are at high or prohibitive risk for surgery.
We herein report on the design, feasibility testing, and histological assessment of the Vsling, a novel transcatheter papillary muscle sling device.
Materials and Methods
Vsling Device
The Vsling implant (Cardiac Success Ltd, Yokneam, Israel) is constructed of an ePTFE tube, identical in material and similar in dimensions to the vascular graft used in the surgical procedure.12,13 Additional components are located inside the ePTFE tube of the Vsling to enable transcatheter positioning, adjustment, locking, and detachment from the delivery catheter. Like the surgical sling, the Vsling is placed through the spaces among the trabeculae carneae between the papillary muscles and the LV wall, without penetrating the endocardium. The procedure can be aborted and the implant entirely removed until the implant is detached.
The Vsling implant provides size adjustability of the sling circumference over 18 mm, from a minimum circumference of 30 mm up to 48 mm, providing a wide range of anatomical variability, and can be produced in even smaller or larger lengths if necessary for extremely thin or large papillary muscles.
Vsling Procedure
A steerable introducer sheath is inserted via a transfemoral approach. The device includes 2 catheters that are inserted through the introducer sheath and passed retrograde across the aortic valve into the LV (Figure 1). The encircling catheter is used first for encircling the papillary muscles, defining the path that the implant will take through the spaces among the trabeculae. A wire is then left in the ventricle along the intended path of the implant, and the encircling catheter is removed. The sling insertion catheter is then used to insert the Vsling implant along the path defined by the wire and to adjust, lock, and deploy the Vsling implant.
Figure 1.
Vsling device catheters and Vsling implant, Vsling implant locked into a sling, and Vsling implant mounted on catheter. The encircling catheter includes a pair of nested catheter shafts which are optimized for encircling the papillary muscles. The pair of shafts are controlled in multiple degrees of freedom using a single catheter handle. The outer shaft of the pair has a preshaped distal tip and a highly flexible neck region designed for easy manipulation around the papillary muscles using only translation and rotation. This shaft is used for manipulation around the posterior papillary muscle, after which rotation of the shaft allows positioning of the tip relative to the anterior papillary muscle. The inner shaft of the pair can be translated, rotated, and deflected to allow manipulation around the anterior papillary muscle to complete the intended path for the Vsling implant. A wire is then passed through the catheter, grasped by a built-in snare located in a side lumen of the catheter, and left behind to define the path as the encircling catheter is removed. The sling insertion catheter is provided with the Vsling implant preloaded at its distal tip and is used to insert the implant through the introducer sheath and into the ventricle. One end of the implant is connected to the wire before insertion, and the wire is used to pull the implant into place along the predefined path around the papillary muscles. Once the implant is positioned around the papillary muscles, the sling insertion catheter handle is used to adjust the size of the sling, lock the size of the sling, and finally detach the sling from the insertion catheter. The insertion catheter and introducer sheath are then removed, leaving the Vsling locked around the bases of the papillary muscles.
LV and mitral valve function can be assessed in real time as the Vsling is positioned and adjusted, while the sling can still be tightened, loosened, moved, or even removed altogether. Once the Vsling position and size have been optimized, the Vsling is locked and detached from the implantation device. An animation of the Vsling implantation procedure is available on the journal website with the text of this article.
Chronic Animal Study
Previous studies in both sheep and pig, as well as descriptions of the ventricular anatomy of ovine, porcine, and canine hearts,14 were used to determine the most appropriate chronic animal model for the Vsling. None of the large animals typically used in medical research have ventricular anatomy similar enough to that of humans to allow for assessment of the Vsling in its intended anatomical configuration. Specifically, the large animal models do not have the spaces among the trabeculae between the papillary muscles and the ventricular wall through which the Vsling is intended to be implanted in the human heart. The Vsling was therefore implanted higher in the animal model, at the level of the tips of the papillary muscle rather than the base of the papillary muscle. To evaluate the tissue interaction with the Vsling implant, the Vsling was implanted snugly around a single papillary muscle in order to achieve the tight papillary muscle tissue contact that is expected in humans without inducing unrepresentative damage or interference with the functioning of the ventricle or the mitral valve. For this reason, the animal study was intended to assess the safety and feasibility of the implantation procedure and tissue interactions of the Vsling implant but was not able to assess the clinical effectiveness of the Vsling.
A chronic animal study approved by the Israeli National Council of Animal Experimentation was performed from August through November 2021 at Lahav CRO (Lahav, Israel) on female domestic pigs weighing 85 ± 5 kg. A total of 10 pigs were implanted, 4 intended for 30-day survival (group 1) and 6 intended for 90-day survival (group 2).
The Vsling implant was introduced using the Vsling delivery system through the femoral artery to the LV. The implantation procedure was performed under fluoroscopic and ultrasound guidance, as is intended for the procedure in humans. To simulate the transesophageal echocardiogram (TEE) probe position in humans, a TEE probe was inserted through an open thoracotomy and positioned inside the pericardium. Study animals underwent scheduled fluoroscopic and angiographic assessments, as well as echocardiographic assessments on the day of implantation (day 0, prior to and after implantation) and on the days of termination (days 30 ± 3 and days 90 ± 5 for groups 1 and 2, respectively). Blood was collected for standard biochemistry, hematology, coagulation, and free hemoglobin analysis on day 0 (prior to and after procedure) and the day of sacrifice. Upon termination, target organ samples were collected from the heart, kidneys, liver, spleen, and lungs and were sent for histopathology evaluation at the CVPath Institute (Maryland, USA).
Histological Evaluation
The tissue samples were sent for histological evaluation packed in 10% neutral buffered formalin. The gross hearts underwent radiography and were photographed showing both the atrial and ventricular views. The base of the heart was opened in the direction of the blood flow after the apex of the right and LVs was cut parallel to the posterior atrial ventricular sulcus starting from the apex to the midportion of the ventricle near the takeoff of the papillary muscles. The area of the Vsling around the papillary muscle and adjoining LV wall, mitral annulus, and the attached atrial wall were separated and submitted for embedding in plastic (Spurr). The plastic block with the tissue embedded was sawed longitudinally, and 3 slices were prepared (ground by the Exakt method) and stained using hematoxylin and eosin. The remainder of the LV was sliced, and 3 to 5 sections were submitted for paraffin embedding from the anterior, lateral, posterior, and ventricular septum and the posterior wall of the right ventricle to determine presence of fibrosis, necrosis, or emboli. Sections from the kidneys, liver, spleen, and lungs were also submitted for paraffin embedding, sectioning, and staining with hematoxylin and eosin. All slides were assessed by light microscopy, and images at various levels of magnification and findings were recorded.
Simulator Usability Study
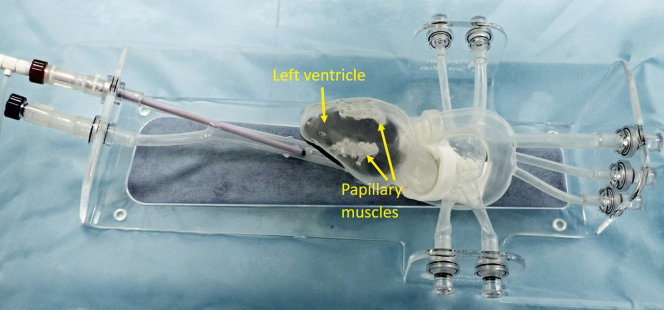
A Vsling implantation simulator was constructed (Pangolin Medical, Ein Carmel, Israel) to allow usability assessment and physician training (Figure 2). The simulator consists of an anatomically reasonable transparent silicon model of the vasculature from the femoral artery to the LV, as well as a dilated LV with papillary muscles and trabeculae. The model enables a full Vsling implantation procedure including adjustment, locking, and detachment of the Vsling implant. The initial usability assessment reported herein was performed in air under direct visualization, but the simulator also supports use in a water bath with fluoroscopy and ultrasound guidance for training purposes.
Figure 2.
Vsling training simulator. The Cardiac Success silicon training simulator (Pangolin Medical, Ein Carmel, Israel) includes the vasculature from the femoral artery up to the aortic arch and the left atrium and ventricle (the right chambers of the heart are not included). Within the left ventricle are 2 appropriately positioned papillary muscles connected to the ventricular walls by trabeculae. The simulator allows both direct visualization and fluoroscopic imaging. It can be used dry inflated with air or filled with water within a water bath to allow ultrasound guidance.
The full Vsling implantation procedure was performed by 6 interventional cardiologists of varying background and experience from 6 different institutions in 3 different countries. After a 15-minute hands-on training session, they each performed a complete implantation procedure. Following the procedure, they completed a usability questionnaire assessing the device performance, including the number of attempts, complexity, and time that it took to complete specific portions of the implantation procedure. In the questionnaire, the procedure was compared to existing complex structural heart procedures such as transcatheter aortic valve implantation and MitraClip (Abbott Laboratories, Abbott Park, Illinois) in order to provide an appropriate and consistent context for the terms used to describe the levels of difficulty in performing the procedure such as “straightforward”, “reasonable”, and “challenging”.
Feasibility Study in Human Cadavers
Human cadaver studies were performed to assess the feasibility of implanting the Vsling implant into the ventricle of a human heart via a transcatheter approach. A preliminary cadaver study was performed at the University of Washington (Seattle, Washington) in August 2019 using prototype catheters, and an additional cadaver study was performed at Biotech Farm (Mazkeret Batya, Israel) in April 2022 using the final design of the implantation device. In each study, full transcatheter implantation procedures were successfully performed on full-torso cadavers. The results of the April 2022 study are reported herein in which a transcatheter procedure was performed by an interventional cardiologist in a cadaver whose primary cause of death was “unspecified systolic congestive heart failure.” The procedure was performed under the guidance of fluoroscopic imaging and a single rigid borescope inserted through the aortic valve. Ultrasound imaging was not possible due to the large amount of air bubbles in the water within the heart. Data were collected on the timing and flow of the procedure and the performance of the implantation device.
Results
Chronic Animal Study
A total of 10 animals underwent transcatheter implantation procedures. The Vsling delivery device was successfully used via a transfemoral approach to implant the Vsling around a single papillary muscle as planned in all cases. The Vsling implant and implantation device were visible under both fluoroscopy and ultrasound. Sample radiographic and echocardiographic images of the heart with the Vsling implantation device and the Vsling implant are shown in Figure 3.
Figure 3.
Sample fluoroscopic and 3D echocardiographic images. Fluoroscopy (left) and 3D echocardiography (right) of the Vsling implantation device (upper panels) and the Vsling implant (lower panels).
None of the 10 implanted animals experienced clinically significant adverse events during the implantation procedure. Three of the animals in group 1 died before the 30-day intended survival. The cause of death in all cases was attributed to complications resulting from the thoracotomy performed to insert the TEE probe, unrelated to the Vsling transcatheter implantation procedure or Vsling implant. Of the 7 surviving animals, 1 was sacrificed on day 33, and the remaining 6 were sacrificed on days 90 to 93.
A comparison of the echocardiography-based assessments between day 0 before and after implantation and sacrifice day demonstrated that Vsling implantation around 1 of the papillary muscles did not cause any mitral leaflet abnormalities or chordal damage or significant mitral regurgitation. Immediately after implantation, small thrombi were observed on the Vsling implant in 3 animals. However, no thrombi around the device or anywhere else were observed on the sacrifice day. Mild thickening of the chordae was found on the sacrifice day in 3 animals in which the Vsling was in contact with the chordae. No evidence of embolic material or embolized thrombus was found in downstream organs (heart, lungs, kidneys, liver, and spleen) in any of the animals.
Measurements of cardiac output between preimplantation and postimplantation stages were unchanged indicating that the implantation procedure had no detrimental effect on LV function. Indeed, cardiac output increased from implantation to sacrifice due to the significant increase in the size of the animals over 90 days (Table 1). Hematology, blood biochemistry (including liver transaminases), coagulation parameters, and free hemoglobin were within normal range during the 90-day study period.
Table 1.
Cardiac output measurements of the 90-day animals
| Parameter | Preprocedure, day 0 (group A) | Postprocedure, day 0 (group B) | Presacrifice, day 90 (group C) |
|---|---|---|---|
| Cardiac output (L/min), mean ± standard deviation | 4.05 ± 0.74 (n = 6) | 3.52 ± 0.75 (n = 6) | 6.40 ± 1.16 (n = 6) |
| p value | Group A vs. B: 0.32 | Group B vs. C: <0.01 | Group A vs. C: <0.01 |
In gross pathology evaluation of group 1 animals, the Vsling implants were observed to be wrapped around the papillary muscle. In the single animal that survived until scheduled termination at 33 days, the implant was almost fully covered with tissue, with no thrombus observed. In the 3 animals that died prematurely, bloody pericardial effusions were present, associated with the insertion of the ultrasound probe through the chest wall, the likely cause of death.
In gross pathology evaluation of group 2 animals at 90 days, the Vsling implants were observed to be wrapped around the papillary muscle. In 5 of 6 animals, the implants were fully covered with tissue, and in the sixth animal, only the tip of the implant was exposed (Figure 4). There was no evidence of thrombus. In 2 animals, small (3 mm in diameter) hematomas were observed on the tissue covering the implant. In 3 animals, a focal pale area of decolorization was observed on the external cortex of the kidneys. Histology demonstrated 2 of these to be mild chronic inflammation not related to the Vsling and the third to be embolic foreign material not originating from the Vsling device. All the organ findings sent for histopathology evaluation were determined to be not related to the Vsling device.
Figure 4.
(a) A typical Vsling wrapped around a single papillary muscle that is fully covered with glistening neoendocardial tissue. (b) A Vsling that was partially covered with endocardial tissue leaving the detachment tip bare.
Chronic Histology
The entire heart and sections from the kidneys, liver, spleen, and lungs of each of the 7 sacrificed animals were sent for histological evaluation. Radiographic images of the gross heart with the Vsling implant are shown in Figure 5. The area containing the Vsling implant was blocked, and sections for light microscopy were produced by cutting through the Vsling implant. Representative images at multiple levels of magnification are shown in Figure 6 and Figure 7.
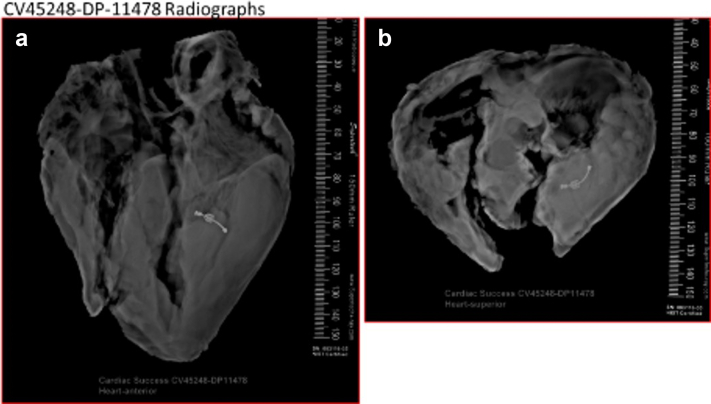
Figure 5.
Representative radiography images. Faxitron x-ray, (a) anterior and (b) superior views of the heart with a Vsling device within the left ventricle around the papillary muscle. The device is intact, and there is no visible calcification.
Figure 6.
Representative light microscopy images. Whole-mount histologic sections of the left atrium (LA), mitral annulus, mitral leaflet (ML), chordae tendineae, Vsling, anterior papillary muscle (APM), and a portion of the left ventricular wall. Each section shows the Vsling cut longitudinally, and therefore, 2 sections of the Vsling are seen, the locking mechanism, composed of metal, plastic, and ePTFE, and the other sling section, which is composed of ePTFE only. Note that the Vsling is around the APM and not around the chordae tendineae and that the locking mechanism and the sling are fully covered by endocardial tissue.
Abbreviations: ePTFE, expanded polytetrafluoroethylene; LV, left ventricle.
Figure 7.
Representative light microscopy images. Whole-mount section of A1.1 is shown in image a, and boxed areas are shown at higher power in images b-h. Image b shows the anterior papillary muscle with the Vsling surrounding the papillary muscle; note there is no fibrosis or necrosis of the intervening myocardium. Image d shows the Vsling fully covered by endocardium (the adjacent LV wall is artificially sheered along with the thickened endocardium from cutting during gross examination). Also, the inner region of the device shows focal presence of fibrin. Image c is a higher power image of the superior region of the Vsling device showing complete coverage by endocardial tissue of the ePTFE, with macrophages around the ePTFE (better seen in images e and f). Image e also shows the presence of an early lymphoid follicle. Image g is a high-power image of the inferior surface showing ePTFE lined by macrophages and surrounding smooth muscle cells in a proteoglycan collagen matrix. Image h is the lateral surface toward the LV wall, showing full endocardial coverage of the locking device.
Abbreviation: ePTFE, expanded polytetrafluoroethylene.
The 30-day animal showed the device located around the anterior papillary muscle. As compared to the 90-day animals, there was less healing, mostly with the presence of granulation tissue and organizing fibrin/hemorrhage. The 90-day animals had greater inflammation with a few small collections of lymphocytes including occasional lymphoid follicles around the device, especially in the superior and inferior surfaces. Healing was also complete, with most device surfaces fully covered by endocardial tissue. Two devices showed the presence of hematomas in the outer layer although the devices were covered by endocardial tissue. These 2 devices had excessive ePTFE wrapping that may have contributed to hematoma formation. All ePTFE outer surfaces showed palisading and nonpalisading macrophages, and a few of the devices at 90 days also had collections of giant cells around the ePTFE. Some giant cells showed the presence of foreign material, likely ePTFE. Overall, the findings were within the expected range, showing safety of the device.
Simulator Device Usability Study
The results of the usability questionnaire from the 6 cardiologists demonstrated acceptable usability of the Vsling implantation device (Figure 8).
Figure 8.
Responses to the usability questionnaire. The height of the bars indicates the number of interventional cardiologists that chose each of the possible answers to each of the questions.
Abbreviation: PM, papillary muscle.
Cadaver Implantation Feasibility Study
The Vsling was successfully implanted around the papillary muscles in a cadaver by an interventional cardiologist (Figure 9). All functional components of the implantation device including catheter rotation and deflection, snare positioning and locking, and clasp positioning, adjusting, locking, and detaching were used and functioned properly. The duration of each of the main components of the procedure was as follows:
Figure 9.
Vsling implantation procedure steps and acute implant. (a) The 4 main steps of the Vsling implantation procedure in the cadaver as seen on the boroscope image: (1) positioning of the introducer sheath and beginning of the encircling, (2) encircling the papillary muscles, (3) snaring the wire, and (4) adjusting the Vsling implant. (b) The acute Vsling implant around the bases of the papillary muscles in the explanted heart.
Introducer sheath insertion and positioning—Smooth insertion into the ventricle with a dilator over a 0.035” guidewire. Positioning within the ventricle with deflection and rotation. ∼5 minutes.
Encircling the papillary muscles—This is the most challenging part of the procedure, and for training purposes, it was attempted multiple times. In the cadaver, it is particularly challenging compared to a beating heart (based on live animal experience and experience with other devices in humans) due to significant friction and the lack of ultrasound guidance. Each attempt at encircling took ∼10 to 15 minutes. There were multiple failed attempts and 2 successful encircling procedures, which took 8 minutes and 10 minutes.
Snaring the wire to complete the loop—Snaring was straightforward. ∼4 minutes.
Vsling implant insertion—The insertion of the implant along the path of the wire. ∼12 minutes.
Vsling implant positioning and adjusting. ∼4 minutes.
Vsling implant locking and detachment. ∼2 minutes.
Based on the timing of this cadaver procedure and experience with live animal implantations under ultrasound guidance, an uncomplicated Vsling implantation procedure in humans can be expected to take between 45 and 60 minutes (not including time for femoral artery access and closure and echocardiography) when performed by an experienced and trained interventional cardiologist.
Discussion
The present study has demonstrated the feasibility and safety of the transcatheter Vsling device and delivery system as a permanent cardiac implant in preclinical studies. Safety was demonstrated by evaluation of gross pathology and histopathology in a chronic swine model at 30 and 90 days. The implant was entirely covered by endocardium in 5 of 6 cases at 90 days, while in 1 case, only an uncovered tip protruded. An acceptable degree of inflammation was noted with small hematoma formation. Thrombus formation and embolization did not occur. Although mild thickening of the chordae was found on sacrifice day in 3 animals, this finding is not relevant for human implantation as the Vsling in humans will be positioned at the bases of the papillary muscles and will not contact the chordae.
The feasibility of the Vsling implantation procedure was demonstrated in a swine model, a silicon simulator, and human cadavers. Maneuvering catheters within the chambers of the heart may be challenging, but the Vsling implantation device has been optimized for positioning and implantation around the papillary muscles. The Vsling implant procedure was assessed as being reasonable relative to other transcatheter structural heart procedures. Other currently marketed and investigational devices such as MitraClip, Cardioband (Edwards Lifesciences Corp, Irvine, California), and AccuCinch (Ancora Heart) are at least as complex as the Vsling implantation procedure in terms of the requirements for positioning, the number and types of device manipulations necessary to perform the implantation procedure, and the length of the procedure.15, 16, 17, 18
The Vsling device has potential advantages compared with other ventricular reshaping devices undergoing investigation: (1) It does not penetrate the endocardium; (2) it has no interaction with the chordae or leaflets of the mitral valve; (3) the implantation procedure is completely reversible until after the sling is positioned, tested, and released; (4) the implantation procedure is 100% percutaneous; and (5) the Vsling directly targets both papillary muscle geometry and ventricular reshaping. Similar to surgical implantation of a papillary muscle sling, by repositioning the papillary muscles closer to their original location, the Vsling would be expected to reduce tethering of the mitral leaflets, thereby reducing FMR. However, because the Vsling directly reshapes the ventricle and not the mitral valve itself, it may not be sufficient as a stand-alone treatment for severe FMR. Human studies are required to determine the extent to which the Vsling as a stand-alone procedure reduces moderate or severe FMR. In this regard, the transcatheter Vsling implant may provide synergistic benefits in combination with transcatheter edge-to-edge treatment or annuloplasty in patients with severe FMR (consistent with surgical sling implantation4,5). Notably, the Vsling implant should not interfere with most future transcatheter interventions, including mitral valve replacement.
Due to its specific location around the bases of the papillary muscles, the Vsling is likely to be more effective at improving cardiac function in patients with specific types of ventricular dilatation. Nappi et al.19 reported that the amount of improvement associated with the surgical papillary muscle sling was dependent upon the region of LV wall motion abnormalities. While patients with impaired kinesis in the inferior and inferoposterior segments showed substantial improvements with the sling vs. annuloplasty alone, those with predominant anterolateral wall motion abnormalities showed smaller and less consistent benefits from the sling. The identification of patient subgroups for whom the Vsling provides greatest clinical benefits will need to be evaluated in clinical trials.
Limitations
Although the Vsling implantation device was found to be maneuverable in pig hearts, simulators, and cadavers, usability in humans remains to be demonstrated. The present study was also not designed to assess the effectiveness of the Vsling as there is no large animal model with separated trabecular bands at the level of the bases of the papillary muscles (as in humans), and the implantation in the present study was around a single papillary muscle only. LV geometry and function were thus not affected. In humans, both papillary muscles will be encircled, and the interpapillary muscle distance reduced, resulting in LV reshaping. The extent to which this will affect LV dimensions, ejection fraction, compliance, and severity of FMR can only be assessed in human studies. Finally, human studies are necessary to examine acute and long-term safety of the Vsling device and procedure after transcatheter implantation.
Conclusions
The Vsling device has been developed to accomplish LV reshaping in a similar manner to that achieved with surgical implantation of a papillary muscle sling but with a transcatheter delivery system. Transcatheter rather than surgical implantation is expected to enhance procedural safety and expand clinical eligibility to both surgically high-risk or ineligible patients and those not otherwise requiring an open-heart surgery. Preliminary safety and feasibility of the Vsling implant and implantation device have been demonstrated in an animal model, simulator, and human cadavers. Studies to examine the feasibility, safety, and effectiveness of Vsling implantation in humans are planned to begin in the summer of 2022.
Ethics Statement
The study reported in this article was approved by the Israel National Council of Animal Experimentation and complies with all relevant ethical guidelines.
Funding
Funding for this study was provided by Cardiac Success Ltd, Yokneam, Israel.
Disclosure statement
Gregg W. Stone received speaker honoraria from Medtronic, Pulnovo, Infraredx; is a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, Gore, Amgen; has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, Xenter. Institutional disclosures: Dr Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, and V-wave. Family disclosure: Dr Stone’s daughter is an employee at Medtronic. Horst Seivert received study honoraria to institution, travel expenses, and/or consulting fees from 4Tech Cardio, Abbott, Ablative Solutions, Adona Medical, Akura Medical, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Cardiac Dimensions, Cardiac Success, Cardimed, Cardionovum, Celonova, Contego, Coramaze, Croivalve, CSL Behring LLC, CVRx, Dinova, Edwards, Endobar, Endologix, Endomatic, Esperion Therapeutics, Inc, Hangzhou Nuomao Medtech, Holistick Medical, Intershunt, Intervene, K2, Laminar, Lifetech, Magenta, Maquet Getinge Group, Metavention, Mitralix, Mokita, Neurotronic, NXT Biomedical, Occlutech, Recor, Renal Guard, Shifamed, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, Vivasure Medical, Vvital Biomed, and Whiteswell. Renu Virmani is a consultant of Abbott Vascular, Boston Scientific, Celonova, OrbusNeich Medical, Terumo Corporation, W. L. Gore, Edwards Lifesciences, Cook Medical, CSI, ReCor Medical, SinoMedical Sciences Technology, Surmodics, Bard BD and a scientific Advisory Board Member of Medtronic and Xeltis. Institutional disclosures: CVPath Institute received grant/research/clinical trial support from NIH-HL141425, Leducq Foundation Grant, 4C Medical, 4Tech, Abbott Vascular, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, AerWave Medical, Alivas, Amgen, Asahi Medical, Aurios Medical, Avantec Vascular, BD, Biosensors, Biotronik, Biotyx Medical, Bolt Medical, Boston Scientific, Canon USA, Cardiac Implants, Cardiawave, CardioMech, Cardionomic, Celonova, Cerus EndoVascular, Chansu Vascular Technologies, Childrens National Medical Center, Concept Medical, Cook Medical, Cooper Health, Cormaze Technologies GmbH, CRL/AccelLab, Croivalve, CSI, Dexcom, Edwards Lifesciences, Elucid Bioimaging, eLum Technologies, Emboline, Endotronix, Envision, Filterlex, Imperative Care, Innovalve, Innovative Cardiovascular Solutions, Intact Vascular, Interface Biolgics, Intershunt Technologies, Invatin Technologies, Lahav CRO, Limflow, L&J Biosciences, Lutonix, Lyra Therapeutics, Mayo Clinic, Maywell, MD Start, MedAlliance, Medanex, Medtronic, Mercator, Microport, Microvention, Neovasc, Nephronyx, Nova Vascular, Nyra Medical, Occultech, Olympus, Ohio Health, OrbusNeich, Ossio, Phenox, Pi-Cardia, Polares Medical, Polyvascular, PulseTherapeutics, Profusa, ProKidney LLC, Protembis, Pulse Biosciences, Qool Therapeutics, Recombinetics, Recor Medical, Regencor, Renata Medical, Restore Medical, Ripple Therapeutics, Rush University, Sanofi, Shockwave, Sahajanand Medical Technologies, SoundPipe, Spartan Micro, Spectrawave, Surmodics, Terumo Corporation, The Jacobs Institute, Transmural Systems, Transverse Medical, TruLeaf Medical, UCSF, UPMC, Vascudyne, Vesper, Vetex Medical, Whiteswell, WL Gore, Xeltis. Lea Waisman Shaler is employed by and owns stock options in Cardiac Success Ltd. Boaz Manash is employed by, owns stock options in, and an inventor on patents assigned to Cardiac Success Ltd. David Neustadter is employed by, owns stock in, founder and board member of, and an inventor on patents assigned to Cardiac Success Ltd.
Acknowledgments
The authors acknowledge the staff of Lahav CRO (Lahav, Israel) for their contribution to the animal study presented in this article.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Brener M.I., Uriel N., Burkhoff D. Left ventricular volume reduction and reshaping as a treatment option for heart failure. Struct Heart. 2020;4(4):264–283. doi: 10.1080/24748706.2020.1777359. [DOI] [Google Scholar]
- 2.Murphy S.P., Ibrahim N.E., Januzzi J.L. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488–504. doi: 10.1001/jama.2020.10262. [DOI] [PubMed] [Google Scholar]
- 3.Mihos C.G., Xydas S., Yucel E., et al. Mitral valve repair and subvalvular intervention for secondary mitral regurgitation: a systematic review and meta-analysis of randomized controlled and propensity matched studies. J Thorac Dis. 2017;9(Suppl 7):S582–S594. doi: 10.21037/jtd.2017.05.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nappi F., Lusini M., Spadaccio C., et al. Papillary muscle approximation versus restrictive annuloplasty alone for severe ischemic mitral regurgitation. J Am Coll Cardiol. 2016;67(20):2334–2346. doi: 10.1016/j.jacc.2016.03.478. [DOI] [PubMed] [Google Scholar]
- 5.Hvass U., Joudinaud T. The papillary muscle sling for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2010;139(2):418–423. doi: 10.1016/j.jtcvs.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Padala M. Papillary muscle approximation is an anatomically correct repair for ischemic mitral regurgitation. J Am Coll Cardiol. 2016;68(10):1146–1147. doi: 10.1016/j.jacc.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Mihos C.G., Larrauri-Reyes M., Santana O. A meta-analysis of ring annuloplasty versus combined ring annuloplasty and subvalvular repair for moderate-to-severe functional mitral regurgitation. J Card Surg. 2016;31(1):31–37. doi: 10.1111/jocs.12662. [DOI] [PubMed] [Google Scholar]
- 8.Rama A., Praschker L., Barreda E., Gandjbakhch I. Papillary muscle approximation for functional ischemic mitral regurgitation. Ann Thorac Surg. 2007;84(6):2130–2131. doi: 10.1016/j.athoracsur.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Grossi E.A., Patel N., Woo Y.J., et al. Outcomes of the RESTOR-MV trial (randomized evaluation of a surgical treatment for off-pump repair of the mitral valve) J Am Coll Cardiol. 2010;56(24):1984–1993. doi: 10.1016/j.jacc.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 10.Jorde U. AccuCinch: Outcomes of transcatheter LV volume reduction in HFrEF with a transcatheter ventricular restoration system. https://www.tctmd.com/slide/accucinch-outcomes-transcatheter-lv-volume-reduction-hfref-transcatheter-ventricular
- 11.Klein P., Anker S.D., Wechsler A., et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail. 2019;21(12):1638–1650. doi: 10.1002/ejhf.1669. [DOI] [PubMed] [Google Scholar]
- 12.Hvass U., Tapia M., Baron F., Pouzet B., Shafy A. Papillary muscle sling: a new functional approach to mitral repair in patients with ischemic left ventricular dysfunction and functional mitral regurgitation. Ann Thorac Surg. 2003;75(3):809–811. doi: 10.1016/S0003-4975(02)04678-7. [DOI] [PubMed] [Google Scholar]
- 13.Lamelas J., Mihos C., Santana O. Surgical technique: papillary muscle sling for functional mitral regurgitation during minimally invasive valve surgery. Heart Surg Forum. 2013;16(5):E295–E297. doi: 10.1532/hsf98.2013209. [DOI] [PubMed] [Google Scholar]
- 14.Iaizzo P. Atlas of human cardiac anatomy. http://www.vhlab.umn.edu/atlas/comparative-anatomy-tutorial/ventricles.shtml
- 15.Eleid M.F., Reeder G.S., Malouf J.F., et al. The learning curve for transcatheter mitral valve repair with MitraClip. J Interv Cardiol. 2016;29(5):539–545. doi: 10.1111/joic.12326. [DOI] [PubMed] [Google Scholar]
- 16.Körber M.I., Landendinger M., Gerçek M., et al. Transcatheter treatment of secondary tricuspid regurgitation with direct annuloplasty: results from a multicenter real-world experience. Circ Cardiovasc Interv. 2021;14(8) doi: 10.1161/CIRCINTERVENTIONS.120.010019. [DOI] [PubMed] [Google Scholar]
- 17.Nickenig G., Hammerstingl C., Schueler R., et al. Transcatheter mitral annuloplasty in chronic functional mitral regurgitation: 6-month results with the Cardioband Percutaneous Mitral Repair System. JACC Cardiovasc Interv. 2016;9(19):2039–2047. doi: 10.1016/j.jcin.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Reisman M., Wudel J., Martin S., et al. TCT-88 6-month outcomes of an early feasibility study of the AccuCinch left ventricular repair system in patients with heart failure and functional mitral regurgitation. J Am Coll Cardiol. 2019;74(13) doi: 10.1016/j.jacc.2019.08.129. [DOI] [Google Scholar]
- 19.Nappi F., Spadaccio C., Nenna A., et al. Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? Subanalysis of the papillary muscle approximation trial. J Thorac Cardiovasc Surg. 2017;153(2):286–295.e2. doi: 10.1016/j.jtcvs.2016.09.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.