Abstract
Introduction
The use of an ‘eversion’ technique is not unequivocally proven to be superior to carotid endarterectomy with patch angioplasty. An up-to-date systematic review is needed for evaluation of benefits and harms of these two techniques.
Methods
RCTs comparing eversion technique versus endarterectomy with patch angioplasty in patients with a symptomatic and significant (≥50 %) stenosis of the internal carotid artery were enrolled. Primary outcomes were all-cause mortality rate, health-related quality of life and serious adverse events. Secondary outcomes included 30-day stroke and mortality rate, (a) symptomatic arterial occlusion or restenosis, and adverse events not critical for decision making.
Results
Four RCTs were included with 1272 surgical procedures for carotid stenosis; eversion technique n = 643 and carotid endarterectomy with patch closure n = 629. Meta-analysis comparing both techniques showed, with a very low certainty of evidence, that eversion technique might decrease the number of patients with serious adverse events (RR 0.47; 95% CI 0.34 to 0.64; p ≤ 0.01). However, no difference was found on the other outcomes. TSA demonstrated that the required information sizes were far from being reached for these patient-important outcomes. All patient-relevant outcomes were at low certainty of evidence according to GRADE.
Conclusions
This systematic review showed no conclusive evidence of any difference between eversion technique and carotid endarterectomy with patch angioplasty in carotid surgery. These conclusions are based on data obtained in trials with very low certainty according to GRADE and should therefore be interpreted cautiously. Until conclusive evidence is obtained, the standard of care according to ESVS guidelines should not be abandoned.
Keywords: Carotid endarterectomy, Systematic review, Eversion technique, Patch, Stenosis, Blood pressure
Highlights
-
•
This review was conducted according to our published review protocol following the recommendations of the Cochrane Handbook.
-
•
TSA and GRADE of randomized clinical trials were conducted.
-
•
This review benefits from a comprehensive search strategy.
-
•
To reduce the risk of design error, one operative technique was compared to one reference technique.
Introduction
Carotid artery stenosis occurs due to atherosclerosis and was first described as a pathologic substrate for ischemic diseases of the ipsilateral brain and eye by C. Miller Fisher in 1951 [1]. Preventive management of asymptomatic carotid artery stenosis includes antiplatelet therapy, statins, antihypertensive medication, diabetic control, as well as healthy lifestyle modifications [[2], [3], [4]]. Carotid endarterectomy (CEA) is one of the preferred treatment modalities for patients with symptomatic stenosis of the internal carotid artery [5], primarily based on the European Carotid Surgery Trial (ECST) and the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [[6], [7], [8]].
Two frequently used surgical techniques in carotid surgery are: the eversion technique (ET) and the more traditional CEA using a longitudinal arteriotomy and patch angioplasty (CEAP). CEAP is suggested to reduce both the risks of restenosis and recurrent ipsilateral stroke [9]. After CEAP, restenosis > 50 % is seen in 6 %–36 % of patients during long-term follow-up (Range: 1–120 months) [[10], [11], [12], [13], [14]] compared with ET where restenosis > 50 % occurred in 1.7 %–2.5 % of patients during long-term follow-up of at least 12 months (Range: 12–40 months) [15]. The guideline of the ‘European Society of Vascular Surgery’ (ESVS) considers CEA with patch angioplasty as the reference technique or ‘gold standard’ [8,16]. The choice between eversion or patched endarterectomy should be left to the discretion of the operating surgeon. A disadvantage of the ET is the potential damage to the carotid sinus nerve branches resulting in loss of the baroreceptor reflex [17]. Loss of the baroreceptor reflex is associated with postoperative hypertension, a risk factor for cerebral hyper perfusion syndrome [17]. The sympathetic nerve trunk is another structure that may be at risk when performing eversion technique, damage may result in signs of Horner's syndrome (Fig. 1) [18]. Whereas CEAP using a longitudinal arteriotomy, the incision is in fact made parallel to these nerve branches, probably reducing the risk of transection of these nerve fibers.
Fig. 1.
Schematic anatomy of carotid artery in neck. Superior cervical ganglion (ganglion cervicale superius) lies at the level of the bifurcation of common carotid artery into the external carotid artery and the internal carotid artery. Illustration is re-used with permission of the publisher [18].
A meta-analysis by Antonopoulos, included 6 randomized clinical trials (RCTs) with 2790 operations in 2666 patients and compared ET with CEAP and concluded that ET may reduce the risks of perioperative stroke and long-term restenosis [19]. However, the observed differences in intervention effects could also be explained by several confounding factors and/or differential use of adjunctive techniques, such as the use of perioperative transcranial Doppler (TCD) monitoring, perioperative carotid pressure measurement, electroencephalographic monitoring, selected (or standard) use of shunting, regional or general anesthesia and variations in materials used for patching, such as: autologous vein patch, synthetic, and biological [[20], [21], [22], [23], [24], [25], [26], [27], [28]].
The current guideline (ESVS) recommends performing ET instead of primary closure of the arterial wall (class 1, Level A evidence). It is up to the surgeon's preference to perform ET or CEAP (class 1, Level A evidence). These two recommendations are based on one single review article by Cao et al. [15]. Patch closure of the arterial wall is recommended over primary closure in the ESVS guideline. Our previous review comparing carotid endarterectomy with patch angioplasty versus primary closure of the arterial wall showed no conclusive evidence of a difference between on all-cause mortality, <30-day mortality, <30-day stroke, or any other serious adverse events [29].
To determine which of the surgical technique offers (more) benefits and less harm, such as reduced mortality and stroke for patients with a symptomatic and significant (≥50 %) stenosis of the internal carotid artery after ET or CEAP. It is important that all available evidence is evaluated according to the risks of errors in a systematic review in line with the Cochrane Handbook for Systematic Reviews of Interventions [30,31]. This systematic review is needed because the different techniques are both used in current practice and the use of ET is not unequivocally proven to be superior to carotid endarterectomy with patch angioplasty. An addition to previous reviews is the use of Trial Sequential Analysis (TSA) to confirm or reject the meta-analyses results [32].
Methods
This review was conducted according to our published protocol [33] and was registered at PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019119361) [34], following the recommendations of the ‘Cochrane Handbook for Systematic Reviews of interventions’ [30] and is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org) [35] and Assessing the methodological quality of systematic reviews (AMSTAR) guidelines [36]. According to the AMSTAR 2 Checklist, (enclosed as supplementary material) this review may be considered as a high-quality review. The search was last updated on the 1st of April 2023.
Studies
Randomized clinical trials comparing ET versus CEAP, regardless of the type of patch material used, were included.
Patients
Patients with symptomatic and ≥50 % stenosis of the internal carotid artery, as measured by computed tomographic angiography, magnetic resonance angiography and/or duplex ultrasound, were evaluated for inclusion [[6], [7], [8]].
Experimental intervention
The experimental intervention was ET (Fig. 2A and B).
Fig. 2.
Ways of reconstructing the carotid artery (bifurcation). CCA: common carotid artery, STA: superior thyroid artery, ECA: external carotid artery, ICA: internal carotid artery.
A: Transection of the internal carotid artery.
B: Reconstruction after the eversion technique.
C: Longitudinal arteriotomy.
D: Reconstruction of the longitudinal arteriotomy with patch angioplasty.
Control intervention
The control intervention was CEAP with longitudinal incision in the carotid artery. (Fig. 2C and D) [37]. Studies including primary closure of the arterial wall in CEA patients were excluded.
Outcomes
The outcome measures were graded from the patients' perspective (GRADE Working Group 2008, Fig. 3) [38]. The number of patients with one or more complications were assessed rather than the number of events, depending on the availability of data (to reduce the risk for double counts).
Fig. 3.
Outcomes prioritized according to importance to patients (critical for decision making) undergoing carotid surgery for symptomatic carotid stenosis (GRADE 2008).
*<30 days and long term (>30 days). GRADE: Grading of Recommendations, Assessment, Development and Evaluation.
Primary outcomes were defined as, all-cause mortality, serious adverse events (SAE) and health-related quality of life. Secondary outcomes were defined as, <30-day mortality rate, <30-day stroke rate, (a)symptomatic (50 % to 99 %) arterial restenosis or occlusion, and non-serious adverse events. Exploratory outcomes were separately reported (non) SAE. A detailed description of the outcomes is found in the protocol.
Search strategy
The Cochrane Central Register of Controlled Trials (CENTRAL [https://www.cochranelibrary.com/central]) in The Cochrane Library, PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com) and other databases (such as Google Scholar (https://scholar.google.com/) were searched (Fig. 4). References of the identified trials were searched to identify any further relevant RCTs. We also searched online trial registries [32]. The detailed search strategy is added as Additional file 1. In each step of the selection, the publication was included in any case of doubt. Double publications of trial results were considered as one trial.
Fig. 4.
Flow diagram summarizing the search process and results of each phase of the systematic review. doi:https://doi.org/10.1371/journal.pmed1000097 (Moher 2009).
Data collection
Two authors independently performed the screening and selected the trials for inclusion. Additional (or missing) data of each trial were requested by contacting the authors repeatedly if needed. Excluded trials and studies were listed with reasons for exclusion. When disagreements occurred, a third author was approached to reconcile. If there were any unclear or missing data, the corresponding authors of the individual trials were contacted at least twice.
Risk of bias assessment
Two authors assessed the risks of bias, without masking for trial names [30,32]. When disagreements occurred, a co-author was approached to reconcile.
Differences between the published review protocol and this paper
Not all trials reported the numbers of patients with one or more complications or a number of patients in each intervention group, therefore meta-analysis was conducted based on the numbers of surgeries instead of numbers of patients. In this scenario, the number of surgeries were counted, hereby possibly underestimating the proportion of patients having a complication. Also, the degree of stenosis of each patient was not always specified in each trial. The lack of data and, unfortunate, the very low response (one reply) from the corresponding authors of the selected trial reports made us include all patients. This resulted in an undefined mix of symptomatic and asymptomatic patients that were included in this review. The threshold for surgery according to the guidelines is a >50 % stenosis of the internal carotid artery. This threshold was also used in all the trials that described asymptomatic patients [[39], [40], [41]]. The outcome: health related quality of life was not analyzed because none of the included the trials reported this outcome.
Statistical methods
Meta-analyses were performed according to the Cochrane Handbook for Systematic Reviews of Interventions [30]. The software package Review Manager (RevMan) version 5.4.1 (2020) was used [42]. Significance levels were adjusted due to multiplicity of several outcomes. The results of each outcome were determinative for the use of the intervention and requires an adjusted statistical significance level (threshold). An alfa of 0.05/ ((1 + 3)/2) = 0.025 was planned to use for the primary outcomes to keep the family wise error rate (FWER) < 0.05. Because health related quality of life was not analyzed in the included trial reports, we chose to adjust maximal type I error for each analyzed outcome to 0.05/ ((1 + 2)/2) = 0.033 % to preserve a FWER of 0.05. For the secondary outcomes we adjusted the maximal type I error allowable for each analyzed outcome to 0.017 = 0.05/ ((5 + 1)/2) [43,44]. For exploratory outcomes, we considered a p value < 0.05 as significant, because we viewed these outcomes as only hypothesis-generating outcomes and not decisive for which technique to recommend. For dichotomous variables, the risk ratio with TSA-adjusted confidence interval (CI) were calculated. For continuous variables, the mean difference (MD) or the standardized mean difference with 95 % CI were calculated.
Trial sequential analyses
Meta-analyses may result in type-I errors and type-II errors due to an increased risk of random error when sparse data are analyzed and due to repeated significance testing when a cumulative meta-analysis is updated with new trials [45,46]. To assess the risk of type-I and type-II errors, TSA was used. A detailed TSA description has been published in the protocol [32,[45], [46], [47]].
A random-effects model and a fixed-effect model were used for meta-analysis in the presence of two or more trials included under the outcomes. In case of discrepancy between the two models, both results were reported. Considering the anticipated abundant clinical heterogeneity, the random-effects model was emphasized except if one or two trials dominated the available evidence. The assumptions behind the two models are different. However, we seldom know which assumptions are correct in each specific case. We chose to present the random-effects model to reflect the weighted average between the results from different populations/trial methods and this average may not apply to all situations.
Best-case scenario and worst-case scenario analysis
Some of the included trials did not specify in which group an event occurred. Worst-case/best-case scenarios for ET were made for the outcome ‘all-cause mortality’ and ‘asymptomatic (50 %–99 %) arterial restenosis or occlusion’. Best-case scenario ET is defined as all the events that occurred in the CEAP group. Worst-case scenario ET is defined as all the events occurred in the ET group.
GRADE
Summary of findings (SOF) Table (Additional file 2) were produced summarizing the results of the trials with overall low risk of bias and for all trials, separately. Reasons for downgrading the quality of the available evidence are: risk of bias evaluation of the included bias domains, publication bias, heterogeneity, imprecision, and indirectness (such as: length of hospital stay is a surrogate outcome measure) [[48], [49], [50]]. We compared the imprecision assessed according to GRADE with that of TSA [51]. No differences were found, and all (available) evidence is graded at very low certainty.
Patient and public involvement
Patients and/or public were not directly involved in this study.
Results
Study selection
The search resulted in 21,044 hits (Fig. 4). Based on titles and abstracts 21,007 publications could be excluded. A total of 37 publications remained for full text evaluation from which 33 were excluded based on the protocol criteria. Finally, four publications [[39], [40], [41],52] describing 4 RCTs were included, published in the period 1994 to 2000. None of the included trials used a quasi-randomized design. Three trials [[53], [54], [55]] were found and also used in previous published reviews, but were excluded from the current study due to the three-armed design. We have contacted the authors of the trials requesting data of the subgroup (eversion technique versus CEA with patch closure), but did not receive any response. Another paper [55] described ET versus conventional CEA in carotid surgery but used patch- and/or primary closure as one single arm. None of the included trials reported about funding.
Patient characteristics and trial designs
Overall, the four included trials randomized 1130 patients, in which 1272 surgical procedures for carotid stenosis were performed. There were 643 treated with ET 629 with CEAP. All four trials used similar inclusion criteria; the baseline characteristics of the populations were comparable. Concerning the grade of carotid stenosis, the trials reported inconsistently. The exclusion criteria were more clearly reported and included concomitant surgery such as coronary arterial bypass grafting, previous carotid surgery, small diameter of the internal carotid artery (ICA) (<4 mm), and abnormal anatomy of the ICA varied and were sometimes not described (63). Patient characteristics were not extensively described, but no imbalances in age or gender were found (Table 1, Table 2). The number of patients and procedures differed (within the trial) because some patients were operated on both carotid arteries, sometimes with different techniques on each side. The four included trials used a two-armed parallel group design (ET versus CEAP).
Table 1.
baseline characteristics of the randomized patients of all included trials.
| Author and year | Total ptns (n) | Number of procedures |
(A)symptomatic |
Total procedures |
Age (yr) |
Sex |
Period |
Country (m/s) |
Smoking |
Diabetes |
Hypertension |
Coronary disease |
PAD/previous vascular surgery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ET | CEAP | ET | CEAP | ET | CEAP | ET | CEAP | ET | CEAP | ET | CEAP | ET | CEAP | ET | CEAP | ET | CEAP | |||||
| VanMaele 1994 [38] | 170 | 102 | 98 (SV) | 24 (23 %) | 23 (23 %) | 200 (30 bilateral) | 66.3 (SD 8.05; R43–84) | 64.3 (SD7.23; R47–85) | 75%M | 79%M | 11-1988–11-1991 | Belgium (s) | U | U | U | U | U | |||||
| Balzer 2000 [51] | 564 | 286 | 278 | U | 564 | 64.2 | 68.5 | 68 % M | 64%M | 01-1991–03-1992 | Germany (s) | U | U | U | U | U | ||||||
| Ballotta 1999 [40] | 310 | 158 | 152 | n = 56 (35 %) | n = 53 (35 %) | 336 | 70.2 (SD 5.3; R42–89) | 70.9 (SD 4.3; R41–89) | 68%M | 69%M | 1 July 1992 to 30 June 1997 | Italy (s) | 106 (67 %) | 101 (66 %) | 55 (35 %) | 51 (34 %) | 89 (56 %) | 86 (57 %) | 84 (53 %) | 81 (53 %) | 95 (60 %) | 92 (61 %) |
| Ballotta 2000 [39] | 86 | 86 | 86 | 33 (38 %) | 30 (35 %) | 172 | 70.5 (R41–84) | 75 % M | 1 July 1992 to 30 June 1997 | Italy (s) | 66 (77 %) | 64 (74 %) | 21 (24 %) | 24 (28 %) | 60 (70 %) | 58 (67 %) | 46 (54 %) | 44 (51 %) | 35 (41 %) | 33 (38 %) | ||
Author = first author of paper, year and reference, s = single centre, m = multicentre, M = male, F = female, n = number of patients, SD = standard deviation, R= range, U = unknown, PAD = peripheral arterial disease, SV = saphenous vein patch. Ballotta 2000, 86 patients were bilateral operated on, ET on one side, CEAP on the other side.
Table 2.
perioperative characteristics of randomized CEA patients with eversion technique versus CEAP patients of all included trials.
| Author and year | Anesthesia | TCD | Pressure assessment | Shunt |
|---|---|---|---|---|
| VanMaele 1994 | General | U | U | Selective |
| Balzer 2000 | U | Used | U | Selective |
| Ballotta 1999 | General | Used (preoperative) | U | Selective |
| Ballotta 2000 | General | Used (preoperative) | U | Selective |
TCD = transcranial doppler. U = unknown.
Surgical interventions
All trials gave a description of the studied surgical techniques. ET versus CEAP were performed as described in line with the description given in our protocol [33,34].
Risk of bias
The risk of bias of the included trials were assessed (Fig. 5). None of the trials used any form of blinding, especially regarding outcome assessment. In all four trials, one or more out of seven bias components were scored as unclear or at high risk of bias. Therefore, all trials were classified at high risk of bias. All the available evidence was scored at very low certainty according to GRADE (Additional File 2).
Fig. 5.
risk of bias summary of all included trials, the eight criteria on the X-axis. Name of first author and year of trial on Y-axis. + = adequate. − = inadequate. ?-mark = unclear.
Primary outcomes
All-cause mortality
All trials reported on all-cause mortality. In the best-case-scenario, the number of patients who died were: 109 patients (or 115 patients in the worst-case scenario) for ET (eversion technique) (16.9 % to 17.9 %) compared with the CEAP group in which 111 patients (worst-case scenario CEAP) or 105 (best-case scenario CEAP) (17.6 % to 16.7 %) died (Fig. 6A). In the meta-analysis, a moderate heterogeneity was present (I2 43 % to 46 %; p = 0.16 and p = 0.13). The random-effects model did not show statistically significant differences between the ET and the CEAP group (RR 0.81; 95 % CI 0.48 to 1.38; p = 0.44) with very low CoE in the best-case scenario for patch angioplasty (Fig. 6B). In the worst-case scenario for patch angioplasty, also no significant difference was found (RR 0.96; 95 % CI 0.58 to 1.59; p = 0.88).
Fig. 6.
forest plot on all-cause mortality after ET or CEAP. Random-effects model.
A: forest plot on all-cause mortality. Best case scenario ET.
B: forest plot on all-cause mortality. Worst case scenario ET.
Serious adverse events
All trials reported serious adverse events after surgery. There were 56 SAEs reported (8.7 %) in the ET group versus 118 patients in the CEAP group (18.8 %). The meta-analysis (Fig. 7), showed a low heterogeneity (I2 7 %; p = 0.36), and the random-effects model showed statistically significant differences between the ET and the CEAP group (RR 0.47; 95 % CI 0.34 to 0.64; p ≤ 0.01) at very low CoE.
Fig. 7.
forest plot on serious adverse events after ET or CEAP. Random-effects model.
Health-related quality of life
None of the trials reported on quality of life.
Secondary outcomes
<30-day mortality rate
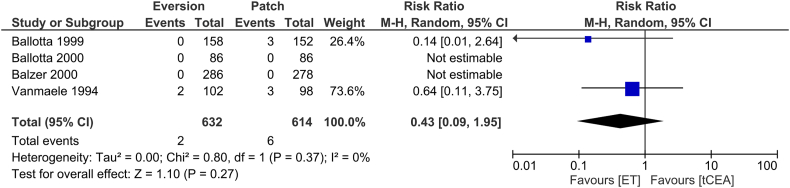
All trials reported on <30-day mortality after surgery. There were 2 deaths reported (0.3 %) in the ET group versus 6 patients (0.9 %) in the CEAP group. In meta-analysis (Fig. 8), low heterogeneity was present (I2 0 %; p = 0.15), and the random-effects model did not show a statistically significant difference between ET and CEAP (RR 0.43; 95 % CI 0.09 to 1.95; p = 0.27) at very low CoE.
Fig. 8.
forest plot on <30-day stroke after ET or CEAP. Random-effects model.
<30-day stroke rate
All trials reported on <30-day stroke after surgery. There were 3 strokes reported (0.5 %) in the ET group versus 8 patients with a stroke (1.3 %) in the CEAP group. In meta-analysis (Fig. 9), moderate heterogeneity was present (I2 47 %; p = 0.15), and the random-effects model did not show a statistically significant differences between ET and CEAP (RR 0.48; 95 % CI 0.06 to 4.07; p = 0.50) at very low CoE.
Fig. 9.
forest plot on <30-day (procedure related) mortality after ET or CEAP. Random-effects model.
Symptomatic (50 %–99 %) arterial restenosis or occlusion
Of the four trials included, the Vanmaele trial [41] described three acute postoperative internal carotid artery occlusions that were all symptomatic. Unfortunately, they did not describe to which group (ET or CEAP) these patients were allocated. The other three trials [39,40,52] did not describe any symptomatic arterial restenosis nor occlusions. Because there is only one trial that reported symptomatic occlusions postoperative and they did not describe in which group these occlusions occurred. It was not found useful to do a best- and worst-case scenario for three patients.
Asymptomatic (50 %–99 %) arterial restenosis or occlusion
All trials reported on asymptomatic (50 %–99 %) arterial restenosis or occlusion. In the best-case-scenario, 16 patients after ET (2.5 %) developed asymptomatic (50 %–99 %) carotid arterial restenosis or occlusion compared with 38 patients (6.0 %) in the CEAP group (worst-case scenario CEAP). In the worst-case-scenario, 18 patients (or 18 patients in the worst-case scenario) for ET (eversion technique) (2.8 %) had asymptomatic (50 %–99 %) arterial restenosis or occlusion compared with 36 patients in the CEAP group (5.7 %) (Fig. 10A). In meta-analysis, substantial heterogeneity was present (I2 67 % to 70 %; p = 0.03 and p = 0.02), the random-effects model did not show statistically significant difference between the ET versus the CEAP group (RR 0.27; 95 % CI 0.06 to 1.17; p = 0.08) with very low CoE according to GRADE in the best-case scenario for ET (Fig. 10B). In the worst-case scenario for ET, also no significant difference was found (RR 0.38; 95 % CI 0.10 to 1.43; p = 0.15) both outcomes at very low CoE.
Fig. 10.
forest plot on asymptomatic restenosis ≥ 50 % or occlusion after ET or CEAP. Random-effects model.
A: forest plot on asymptomatic restenosis ≥ 50 % or occlusion. Best case scenario ET.
B: forest plot on asymptomatic restenosis ≥ 50 % or occlusion. Worst case scenario ET.
Non-serious adverse events (exploratory outcomes)
None of the trials reported non-serious adverse events.
Funnel plots were not performed since <10 trials were included in the meta-analysis.
Trial sequential analysis (TSA)
When calculating the TSA scenarios for point-estimates obtained in the meta-analysis we found for all outcomes, except SAE, that none of the boundaries are surpassed. TSA for the evaluation of the effect on SAE clearly demonstrate evidence (disregarding risk of bias) for a 20 % RRI, as the z-curve breaks through the boundary of benefit of ET. So, there is evidence for a 20 % RRR, but the effect may be higher as the estimate suggests a RR of 0.47, which is more than a reduction to half of the SAEs using ET.
Subgroup analysis
None of the trials have overall low risk of bias and the subgroups describing different patch materials were not reported in detail, so it was unclear how many patients received autologous, synthetic or biological patches.
Future trials
Based on the results of the meta-analysis and trial sequential analysis recommendations for future trials are summed up in Table 3.
Table 3.
Checklist of recommendations for future randomized clinical trials comparing eversion technique (ET) versus carotid endarterectomy with patch angioplasty (CEAP) in patients with symptomatic and significant stenosis.
| Checklist of recommendations for future trial(s) comparing ET versus CEAP | |
|---|---|
| Item | Recommendation |
| To get the evaluation of serious adverse events (SAE) right | Count the number of patients with one or more SAE, and not just the total number of SAE. |
| To prevent design error | Compare one specific experimental intervention to one specific control intervention |
| To avoid bias | Future trials should be protocolized according to SPIRIT and be able to fulfill the CONSORT statements [55] |
| To minimize risk of random error | The sample size should exceed e.g., 2000a participants in one or more future trials. |
| Comparison | Outcome measures critical for decision making according to the GRADE [37]. |
In an attempt to bridge the information gap, a new trial should at least comprise as many patients as the hitherto largest and that preferably several new trials will be needed with at least as many patients as it takes to produce a boundary break through (boundary for benefit, harm or futility) in the Trial Sequential Analysis, or to close the gap between the required and the presently accrued information size.
Discussion
We found no conclusive evidence between ET and CEAP in patients with a (symptomatic and) significant (≥50 %) carotid stenosis on all primary- and secondary outcomes. In this review serious adverse events was the only outcome that showed a significant difference in favor for ET. All other investigated outcomes suggest a favorable, not significant trend towards ET. It needs to be emphasized that the outcomes, come with a very low CoE based on risk of bias and risk of random errors and should therefore be interpreted with care.
This finding fuels the need for one or more new RCTs with a low risk of bias and large sample size comparing ET versus CEAP. Our TSA analysis showed that none of the boundaries are crossed, except SAE, underlining the information size is extremely lower than required. This is an important conclusion, which the traditional meta-analyses were not able to draw.
Missing data occurred in some of the included trials, therefore best/worst case scenarios were used. For the outcome ‘all-cause mortality’ it was unclear for six patients who died, in which group they were allocated (ET or CEAP) [39]. For example, Vanmaele [41] described four patients (two in each group) who had a 20–59 % stenosis. The exact degree of stenosis was missing. In theory this could be a 21 % stenosis or both >50 % stenosis. For these missing data worst/best case scenarios were done. The results of both scenarios for both outcomes (all-cause mortality and re-stenosis) were not in favor of one of the two investigated techniques. For future RCTs it is recommended to fulfill the reporting guidelines such as Consolidated Standards of Reporting Trials statement (CONSORT) and reduce the incidence of missing data [56].
A recent review from Paraskevas [57] compared ET with CEAP. They concluded that ET is superior to CEAP on stroke, death, death/stroke and late restenosis. However, when looking at their subgroup analysis of RCT data comparing eversion technique with patch angioplasty only a significant difference was found in the outcome neck hematoma. A potential explanation for this difference between the review of Paraskevas and ours is that different RCTs were included, we excluded for example Markovic [54] due to the three-armed design in that study. They compared ET with conventional CEA but two different techniques were used within the conventional group (patch closure and primary closure) and there is no stratified data available. An assumption was made that the choice between the two techniques was done when the carotid artery was already exposed in the surgical field. Cao et al. [15] conducted another review and concluded in their sub analysis that there was not a difference between ET and CEAP except for the outcome arterial occlusion and restenosis. The incidence of these outcomes was less in the ET group. The rate of restenosis is lower, maybe due to shorter follow up within the ET group. In previous literature, the length of the follow up for ET (Range: 12–40 months) [15] is shorter compared with CEAP (Range: 1–120 months) [[10], [11], [12], [13], [14]]. We found that the majority of the patients, included in this review, suffered from restenosis or occlusion within the first 12 months after the surgery. The review of Antonopoulos [19] found that ET may be associated with a lower incidence in both short-term (perioperative stroke, perioperative mortality and stroke-related mortality) and long-term (late mortality and late carotid artery occlusion) outcomes compared with CEAP. The reason for the lower incidence could be explained by the fact that myointimal hyperplasia seems to be reduced when one oblique suture line is used in ET instead of prosthetic material and two suture lines with CEAP. ET could also offer a greater view of the interior of the ICA, by incision at the bulb, which is a wider part of the carotid artery, and this may be associated with decreased possibility for re-stenosis [19]. A recent paper of Nolde et al. [58], regarding the possible blood pressure (BP) changes after carotid surgery, described after ET [17], showed no statistically significant long term BP changes after ET of CEAP.
All included trials were conducted 22 to 26 years ago and some potential eligible RCTs were not included because there was uncertainty (e.g., three-armed designed study presented as a two-armed study, with two techniques mixed) about the data. Also, different co-interventions (statins, use of (different) platelet inhibitors), improvement of (digital) imaging techniques in the last decades and, not at least, the experience of the surgeon could have influenced the available outcomes. Despite contacting the (co)authors multiple times, no response was provided. We wanted to compare only one (intervention) technique (ET) with only one other control technique (CEAP). Including other RCTs despite these RCTs applying different (although related) techniques, would lead to heterogeneity regarding the cardinal question of which complication/outcome was (un)traceable to which patient and/or to which surgical technique. The lack of recent good quality RCTs comparing ET witch CEAP suggests there is some kind of consensus that these two techniques may continue to coexist in the guidelines.
The majority (54 %) of the included patients in our review came from the two trials of Ballotta et al. Ballotta compared the two techniques, ET on one side and CEAP on the other side in the same patient. Ballotta suggested, because of a significantly higher rate of unilateral recurrence, that local factors (technique) play a more important role than systemic factors (patients characteristics) in the occurrence of restenosis. This conclusion (surgical technique makes the difference) supports our hypothesis that at least more evidence is needed on this topic.
We suggest therefore conducting one or more new randomized clinical trials with a large sample size of patients comparing ET versus CEAP in symptomatic patients with an internal carotid artery stenosis of 50 % or more. TSA analysis showed this number of patients is minimal required to meet the information size. Such trials ought to be designed according to the Standard Protocol Items: Recommendations for Interventional Trials statement (SPIRIT) [59] and reported according to the Consolidated Standards of Reporting Trials statement (CONSORT) [56].
Conclusions
This systematic review showed no conclusive evidence of any difference between eversion technique and carotid endarterectomy with patch angioplasty in carotid surgery. These conclusions are based on data obtained from trials with very low certainty according to GRADE and should therefore be interpreted cautiously. Until conclusive evidence is obtained, the standard of care according to ESVS guidelines should not be abandoned.
Abbreviations
- CCA
Common Carotid Artery
- CEA
Carotid Endarterectomy
- CEAP
traditional carotid endarterectomy (Carotid EndArterectomy with Patch closure)
- CI
Confidence Interval
- CoE
Certainty of Evidence
- CONSORT
Consolidated Standards Of Reporting Trials Statement
- ECA
External Carotid Artery
- ECST European Carotid Surgery Trial
- ESVS
European Society of Vascular Surgery
- ET
Eversion Technique
- FWER
Family Wise Error Rate
- GRADE Grading of Recommendations Assessment, Development and Evaluation
- ICA
Internal Carotid Artery
- MD
Mean Difference
- NASCET-North American Symptomatic Carotid Endarterectomy Trial
- PAD
Peripheral Arterial Disease
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- PTFE
PolyTetraFluoroEthylene
- RCT
Randomized Clinical Trial
- RR
Relative Risk
- SAE
Serious Adverse Events
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials statement
- STA
Superior Thyroid Artery
- SV
Saphenous Vein
- TCD
Transcranial Doppler
- TSA
Trial Sequential Analysis
The following are the supplementary data related to this article.
Search strategy.
GRADE summary of findings tables.
Review protocol.
Additional File 4: AMSTAR2 Checklist.
Assistance
The authors would like to thank Mrs. L.W.M. Boerboom, MSc, medical information specialist (Medical Library, Elisabeth-Tweesteden Hospital, Tilburg, the Netherlands) for her assistance with building the search strategy. We would also thank Mrs. K. Monk for her assistance with checking the spell and grammar of the manuscript.
Credit authorship contribution statement
Idea: MSM, GGK. Conceived and designed the protocol: MSM, JW, FK, GGK. Performed the search: MSM, GGK. Analysed the data: MSM, JW, FK, GGK. Contributed reagents/materials/analysis tools/advice/comments: MSM, JW, AKhJ, FLM, FK, MMPJR, GGK. Wrote the paper: MSM, JW, FK, GGK. Contributed to high standard of performing a protocol based systematic review with Trial Sequential Analyses: MSM, JW, PWHEV, RLAWB, AKhJ, FLM, FK, MMPJR, GGK. Supervisor: GGK.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval and patient consent to participate
As a review article, this is not applicable.
Consent for publication
All authors approved this version of the manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Permission to reproduce material from other sources
We received permission from author and publisher to re-use, in this manuscript, Fig. 1.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
We have no conflicts of interest to disclose apart from the following: Jørn Wetterslev, MD, PhD was a member of the taskforce at Copenhagen Trial Unit (CTU) to develop theory and software for doing Trial Sequential Analysis (TSA) currently freeware available including a manual at www.ctu/tsa.
Footnotes
-
-Protocol registration number: PROSPERO CRD42019119361 https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=119361
-
-Review protocol publication 2020 DOI: https://doi.org/10.1136/bmjopen-2019-030503
-
-This study was presented at Charing Cross on April 26th 2022, London, United Kingdom.
Contributor Information
Martijn S. Marsman, Email: martijn.marsman@radboudumc.nl.
Jørn Wetterslev, Email: joern.wetterslev@gmail.com.
Patrick W.H.E. Vriens, Email: pwhe.vriens@etz.nl.
Ronald L.A.W. Bleys, Email: r.l.a.w.bleys@umcutrecht.nl.
Abdelkarime Kh. Jahrome, Email: a.k.jahrome@znb.nl.
Frans L. Moll, Email: f.l.moll@umcutrecht.nl.
Frederik Keus, Email: f.keus@umcg.nl.
Michel M.P.J. Reijnen, Email: m.reijnen@rijnstate.nl.
Giel G. Koning, Email: giel.koning@euregio-klinik.de.
References
- 1.Fisher M. Occlusion of the internal carotid artery. AMA Arch Neurol Psychiatry. 1951;65(3):346–377. doi: 10.1001/archneurpsyc.1951.02320030083009. [DOI] [PubMed] [Google Scholar]
- 2.Raman G., Moorthy D., Hadar N., Dahabreh I.J. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Ann Intern Med. 2013;158(9):676–685. doi: 10.7326/0003-4819-158-9-201305070-00007. Available from: [DOI] [PubMed] [Google Scholar]
- 3.Abbott A.L. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40(10):573–584. doi: 10.1161/STROKEAHA.109.556068. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 4.Constantinou J., Jayia P., Hamilton G. Best evidence for medical therapy for carotid artery stenosis. J Vasc Surg. 2013;58(4):1129–1139. doi: 10.1016/j.jvs.2013.06.085. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 5.Cina C.S., Clase C.M., Haynes R.B., Orrapin S., Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2017;(2) doi: 10.1002/14651858.CD001081. Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warlow C. MRC European carotid surgery trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991;337(8752):1235–1243. [PubMed] [Google Scholar]
- 7.Collaborators NASCET Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 8.Naylor A. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;55(1):1–79. doi: 10.1016/j.ejvs.2017.06.021. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 9.Rerkasem K., Rothwell P.M. Patch angioplasty versus primary closure for carotid endarterectomy (review) Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD000160.pub3. http://doi10.1002/14651858.CD000160.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein E.F., Torem S., Dilley R.B. Does carotid restenosis predict an increased risk of late symptoms, stroke, or death? Ann Surg. 1990;212(5):629–636. doi: 10.1097/00000658-199011000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen L., Sillesen H., Schroeder T., Buchardt Hansen H.J. Eight to ten years follow-up after carotid endarterectomy: clinical evaluation and Doppler examination of patients operated on between 1978-1980. Eur J Vasc Surg. 1990;4(3):259–264. doi: 10.1016/s0950-821x(05)80204-x. [DOI] [PubMed] [Google Scholar]
- 12.Ouriel K., Green R.M. Clinical and technical factors influencing recurrent carotid stenosis and occlusion after endarterectomy. J Vasc Surg. 1987;5(5):702–706. [PubMed] [Google Scholar]
- 13.Volteas N., Labropulos N., Leon M., Kalodiki E., Chan P., Nicolaides N.A. Risk factors associated with recurrent carotid stenosis. Int Angiol. 1994;13(2):143–147. [PubMed] [Google Scholar]
- 14.Zierler R.E., Bandyk D.F., Thiele B.L., Strandness D.E. Carotid artery stenosis following endarterectomy. Arch Surg. 1982;117(11):1408–1415. doi: 10.1001/archsurg.1982.01380350016003. [DOI] [PubMed] [Google Scholar]
- 15.Cao P., De Rango P., Zannetti S. Eversion vs conventional carotid endarterectomy: a systematic review. Eur J Vasc Endovasc Surg. 2002;23(3):195–201. doi: 10.1053/ejvs.2001.1560. [DOI] [PubMed] [Google Scholar]
- 16.Liapis C.D., Bell S.P.R.F., Mikhailidis D., Sivenius J., Nicolaides A., Fernandes e Fernandes J., et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37(4 SUPPL):1–19. doi: 10.1016/j.ejvs.2008.11.006. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 17.Demirel S., Goossen K., Bruijnen H., Probst P. Systematic review and meta-analysis of postcarotid endarterectomy hypertension after eversion versus conventional carotid endarterectomy. J Vasc Surg. 2017;65(3):868–882. doi: 10.1016/j.jvs.2016.10.087. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 18.Koning G.G., Lüning T.H., ter Bruggen J.P. Een vrouw met linkszijdig retrobulbaire pijn, fotofobie en hornersyndroom. Ned Tijdschr Geneeskd. 2007;10(3):55–57. [Google Scholar]
- 19.Antonopoulos C.N., Kakisis J.D., Sergentanis T.N., Liapis C.D. Eversion versus conventional carotid endarterectomy: a meta-analysis of randomised and non-randomised studies. Eur J Vasc Endovasc Surg. 2011;42(6):751–765. doi: 10.1016/j.ejvs.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Bass A., Krupski W.C., Schneider P.A., Otis S.M., Dilley R.B., Bernstein E.F. Intraoperative transcranial Doppler: limitations of the method. J Vasc Surg. 1989;10(5):549–553. doi: 10.1067/mva.1989.15567. [DOI] [PubMed] [Google Scholar]
- 21.Gnanadev D.A., Wang N., Comunale F.L., Reile D.A. Carotid artery stump pressure: how reliable is it in predicting the need for a shunt? Ann Vasc Surg. 1989;3(4):313–317. doi: 10.1016/S0890-5096(06)60152-0. [DOI] [PubMed] [Google Scholar]
- 22.Kresowik T.F., Worsey M.J., Khoury M.D., Krain L.S., et al. Limitations of electroencephalography for cerebral ischemia during carotid endarterectomy. J Vasc Surg. 1991;13(3):439–443. doi: 10.1067/mva.1991.26500. [DOI] [PubMed] [Google Scholar]
- 23.Kearse L.A., Brown E.N., McPeck K. Somatosensory evoked potentials sensitivity relative to electroencephalography for cerebral ischemia during carotid endarterectomy. Stroke. 1992;23(4):498–505. doi: 10.1161/01.str.23.4.498. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin M.E., Silva M.B.J.R., Watt C., McCaffrey M.T., Burford-Froggs A., Flinn W.R. 114(4) 1993. Awake patient monitoring to determine the need for shunting during carotid endarterectomy; pp. 673–679. (discussion 679–681) [DOI] [PubMed] [Google Scholar]
- 25.Rerkasem K., Bond R., Rothwell P.M. Local versus general anaesthesia for carotid endarterectomy (review) Cochrane Collab. 2005;2 doi: 10.1002/14651858.CD000126.pub2. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Rerkasem K., Rothwell P.M. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting) Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD000190.pub2. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 27.Rerkasem K., Rothwell P.M. Patches of different types for carotid patch angioplasty. Cochrane Database Syst Rev. 2010:3. doi: 10.1002/14651858.CD000071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarides M.K. Editor ’ s choice – network meta-analysis of carotid endarterectomy closure techniques. Eur J Vasc Endovasc Surg. 2021;61(2):181–190. doi: 10.1016/j.ejvs.2020.10.009. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 29.Marsman M.S., Wetterslev J., Jahrome A.Kh., Gluud C., Moll F.L., Keus F., et al. Carotid endarterectomy with patch angioplasty versus primary closure in patients with symptomatic and significant stenosis: a systematic review with meta-analyses and trial sequential analysis of randomized clinical trials. BMC Syst Rev. 2021;10(139):1–17. doi: 10.1186/s13643-021-01692-8. https://systematicreviewsjournal.biomedcentral.com/track/pdf/10.1186/s13643-021-01692-8.pdf Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J., Green S. The Cochrane Collaboration; 2011. Cochrane handbook for systematic review of intervention version 5.1.0 [internet] www.Cochrane-handbook.org Available from:
- 31.Keus F., Wetterslev J., Gluud C., et al. Evidence at a glance: error matrix approach for overviewing available evidence. BMC Med Res Methodol 2010;10:90. Available from: 10.1186/1471-2288-10-90. [DOI] [PMC free article] [PubMed]
- 32.Marsman M.S., Wetterslev J., Jahrome A.Kh., Gluud C., Moll F.L., Karimi A., et al. Carotid endarterectomy with primary closure versus patch angioplasty in patients with symptomatic and significant stenosis: protocol for a systematic review with meta-analyses and trial sequential analysis of randomized clinical trials. BMJ Open. 2019;9(e026419):1–7. doi: 10.1136/bmjopen-2018-026419. Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsman M.S., Wetterslev J., Vriens P.W.H.E., Bleys R.L.A.W., Jahrome A.Kh., Moll F.L., et al. Eversion technique versus conventional endarterectomy with patch angioplasty in carotid surgery: protocol for a systematic review with meta- analyses and trial sequential analysis of randomised clinical trials. BMJ Open. 2020;10(e030503):1–9. doi: 10.1136/bmjopen-2019-030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsman M.S., Wetterslev J., Vriens P.W.H.E., Bleys R.L.A.W., Moll F.L., Jahrome A.Kh., et al. Eversion technique versus conventional endarterectomy with patch angioplasty in carotid surgery: protocol for a systematic review with meta-analyses and trial sequential analysis of randomized clinical trials [Internet] 2019. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019119361 p. PROSPERO 2019 CRD42019119361. Available from: [DOI] [PMC free article] [PubMed]
- 35.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88(105906) doi: 10.1016/j.ijsu.2021.105906. Available from: [DOI] [PubMed] [Google Scholar]
- 36.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2 : a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;21(358):1–9. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Bakey M.E., Crawford E.S., Cooley D.A., Morris G.C., Jr. Surgical considerations of occlusive disease of innominate, carotid, subclavian, and vertebral arteries. Ann Surg. 1959;149(5):690–710. doi: 10.1097/00000658-195905000-00010. http://www.ncbi.nlm.nih.gov/pubmed/13637687%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1451085 Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guyatt G.H., Oxman A.D., Kunz R., Vist G.E., Falck-Ytter Y., Schünemann H.J., et al. What is “‘quality of evidence’” and why is it important to clinicians? BMJ. 2008;336(7651):995–998. doi: 10.1136/bmj.39490.551019.BE. Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballotta E., Renon L., Da Giau G., Toniato A., Baracchini C., et al. A prospective randomized study on bilateral carotid endarterectomy: patching versus eversion. Ann Surg. 2000;232(1):119–125. doi: 10.1097/00000658-200007000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballotta E., Da Giau G., Saladini M., Abbruzzese E., Renon L., Toniato A. Carotid endarterectomy with patch closure versus carotid eversion endarterectomy and reimplantation: a prospective randomized study. Surgery. 1999;125(3):271–279. [PubMed] [Google Scholar]
- 41.Vanmaele R.G., Van Schil P.E., Demaeseneer M.G., Meese G., Lehert P., Van Look R.F. Division-endartereetomy-anastomosis of the internal carotid artery: a prospective randomized comparative study. Cardiovasc Surg. 1994;2(5):573–581. [PubMed] [Google Scholar]
- 42.Review Manager (RevMan) [Internet] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2014. https://community.cochrane.org/help/tools-and-software/revman-5 Available from:
- 43.Jakobsen J.C., Wetterslev J., Lange T., Gluud C. Editorial - viewpoint: taking into account risks of random errors when analysing multiple outcomes in systematic reviews. Cochrane Libr. 2016:2–7. doi: 10.1002/14651858.ED000111. Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakobsen J.C., Wetterslev J., Winkel P., Lange T., Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14(1):1–13. doi: 10.1186/1471-2288-14-120. Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wetterslev J., Thorlund K., Brok J., Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. doi: 10.1016/j.jclinepi.2007.03.013. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 46.Brok J., Thorlund K., Wetterslev J., Gluud C. Apparently conclusive meta-analyses may be inconclusive - trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38(1):287–298. doi: 10.1093/ije/dyn188. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 47.Turner R.M., Bird S.M., Higgins J.P.T. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8(3):1–8. doi: 10.1371/journal.pone.0059202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guyatt G.H., Oxman A.D., Kunz R., Brozek J., Alonso-Coello P., Rind D., et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 49.Savović J., Jones H.E., Altman D.G., Harris R.J., Juni P., Pildal J., et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess (Rockv) 2012;16(35):1–81. doi: 10.3310/hta16350. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 50.Savovic J., Jones H.E., Altman D.G., Harris R.J., Pildal J., Als-nielsen B., et al. Research and reporting methods influence of reported study design characteristics on intervention. Ann Intern Med. 2012;157(6):429–438. doi: 10.7326/0003-4819-157-6-201209180-00537. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 51.Castellini G., Bruschettini M., Gianola S., Gluud C., Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and trial sequential analysis. Syst Rev. 2018;7(1):1–10. doi: 10.1186/s13643-018-0770-1. Available from: Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balzer K., Guds I., Heger J., Jahnel B. Konventionelle Thrombendarteriektomie mit Carotis-patch-Plastik vs. Eversionsendarteriektomie. Zentralbl Chir. 2000;125:228–238. [PubMed] [Google Scholar]
- 53.Cao P., Giordano G., De Rango P., Zannetti S., Chiesa R., Coppi G., et al. Original articles a randomized study on eversion versus standard carotid endarterectomy: study design and preliminary results: The Everest Trial. [DOI] [PubMed]
- 54.Markovic D.M., Davidovic L.B., Cvetkovic D.D., Maksimovic Z.V., Markovic D.Z, Jadranin D.B. Single-center prospective, randomized analysis of conventional and eversion carotid endarterectomy. J Cardiovasc Surg. 2008;49(5):619–625. [PubMed] [Google Scholar]
- 55.Kasprzak P., Raithel D. Eversionsendarteriektomie der A. carotis versus konventionelle TEA. Ergebebnisse einer prospektiven randomisierten Studie. VASA. 1992;35:86–87. [Google Scholar]
- 56.Schulz K.F., Altman D.G., Moher D., CONSORT Group . 7(3) 2010. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. [Google Scholar]
- 57.Paraskevas K.I., Robertson V., Saratzis A.N., Naylor A.R. An updated systematic review and meta-analysis of outcomes following eversion vs. conventional carotid endarterectomy in randomised controlled trials and observational studies. Eur J Vasc Endovasc Surg. 2018:1–9. doi: 10.1016/j.ejvs.2017.12.025. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 58.Nolde J.M., Cheng S.F., Richards T., Schlaich M.P. No evidence for long term blood pressure differences between eversion and conventional carotid endarterectomy in two independent study cohorts. Eur J Vasc Endovasc Surg. 2022;63(1):33–42. doi: 10.1016/j.ejvs.2021.09.005. Available from: Available from: [DOI] [PubMed] [Google Scholar]
- 59.Chan A., Tetzlaff J.M., Altman D.G., Laupacis A., Gøtzsche P.C., Hro A., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;1(158):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.
GRADE summary of findings tables.
Review protocol.
Additional File 4: AMSTAR2 Checklist.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.