Abstract
Heart failure with preserved ejection fraction is associated with elevated left atrial pressure during exercise. Sodium-glucose cotransporter-2 inhibitors have demonstrated the evidence of benefit in heart failure with preserved ejection fraction, but even with this treatment, heart failure hospitalizations remain high, and improvements in quality of life scores are modest. Thus, there is growing interest in nonpharmacological methods of limiting the rise in left atrial pressure during exertion. Creation of an interatrial shunt (IAS) may unload the left heart during exercise. Multiple implant or nonimplant IAS procedures are under investigation. Implantation of the most studied device results in 3 to 5 mm Hg decreases in pulmonary capillary wedge pressure during exercise, no increase in incidence of stroke, stable increases in Qp/Qs (1.2-1.3), and mild right heart enlargement without change in function out to at least a year after treatment. The findings from the first large randomized controlled trial of an atrial shunt have recently been published. For the population as a whole, implantation of the atrial shunt device appeared to be safe but did not provide clinical benefit. However, prespecified and post-hoc analyses have demonstrated that men, patients with larger right atrial volumes, and those with pulmonary artery systolic pressure >70 mm Hg at 20 W exercise had worse outcomes with IAS therapy, whereas those with peak exercise pulmonary vascular resistance <1.74 Wood units and absence of a pacemaker represented a potential responder group. Here, we summarize the results of the published data and the current IAS therapies under investigation. We also highlight unanswered questions in this field of inquiry.
Keywords: Exercise capacity, Exercise hemodynamics, Heart failure with preserved ejection fraction, Pulmonary capillary wedge pressure, Randomized controlled trial, Quality of life
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) can be difficult to diagnose because classical physical findings that are associated with clinical congestion such as peripheral edema, elevated jugular pressure, or pulmonary rales are often absent. In addition, serum natriuretic peptide levels may be normal or only mildly elevated. Invasive hemodynamic measurements during exercise are currently the favored method for confirming HFpEF, especially when other findings are equivocal.1 A rise in pulmonary capillary wedge pressure (PCWP) during supine exercise to ≥25 mm Hg is considered diagnostic of HFpEF.1 It has also been proposed that an excessively steep slope of the relationship between PCWP and cardiac output (CO) may be diagnostic of the condition, as it indicates a reduction in left heart compliance.2 In addition to the diagnostic uncertainties, treatment of patients with HFpEF, especially those without overt congestion, can be very challenging. Numerous clinical trials of pharmacological therapies in HFpEF have failed to demonstrate clinical benefit. Thus far, only sodium-glucose cotransporter-2 inhibitors (SGLT2i) have demonstrated clear evidence of benefit, but even with this treatment, heart failure hospitalizations remain high as shown in the EMPEROR-Preserved trial.3 Improvements in health status (quality of life and symptoms) in the relatively small PRESERVED-HF trial of dapagliflozin in HFpEF (n = 324) were clinically meaningful,4 but in the much larger EMPEROR-Preserved trial and 2 other smaller trials of SGLT2i (DETERMINE-Preserved [clinicaltrials.gov, NCT03877224] and EMPERIAL-Preserved), there was very modest or no benefit in health status.3,5 Therefore, there is still a critical unmet need to identify effective therapies for patients with HFpEF.
Compared with pharmacological therapies, atrial shunts have the theoretical advantages of (1) not causing dehydration, kidney injury, or electrolyte imbalances; (2) not lowering blood pressure or causing orthostatic hypotension; (3) avoiding polypharmacy (which is common in HFpEF and associated with adverse outcomes)6; and (4) producing less left-to-right shunting when left atrial (LA) pressures are normal, while allowing increased left-to-right shunting when LA pressures rise excessively, such as during exercise.
Existing Data on Atrial Shunts
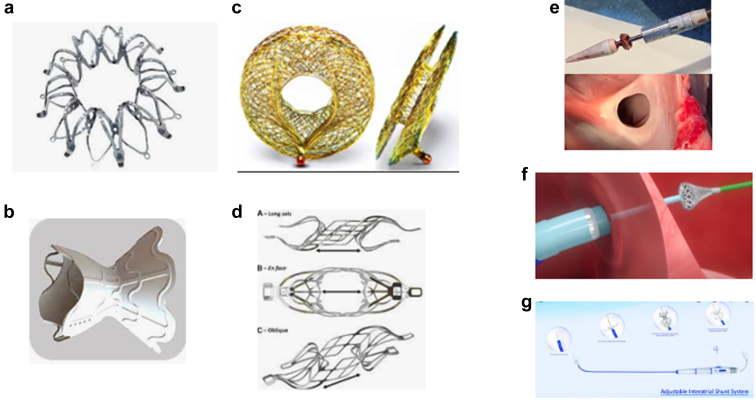
Corvia Atrial Shunt, Inter Atrial Shunt Device System II
The most widely studied device is made by Corvia Medical Inc. It consists of a nitinol frame with discs positioned on the left and right atrial (RA) surfaces of the atrial septum. The LA side of the shunt is flush with the interatrial septum to reduce thrombogenicity, whereas the RA side of the shunt has legs to ensure the stability of the device. There is an 8 mm central opening to allow shunting (Figure 1). The device is deployed via right femoral vein access with and positioned in the fossa ovalis using an atrial septal puncture. The timeline of development of the device has been summarized previously.7
Figure 1.
The various atrial shunt devices and shunts without implants currently under investigation for treatment of heart failure. (a) Corvia Atrial Shunt, IASD System II, (b) V-Wave Ventura Interatrial shunt, (c) Occlutech atrial flow regulator, (d) Edwards Life Sciences, (e) Alleviant, (f) Intershunt, (g) NoYa.
Feasibility Study
An open-label study in 11 patients showed that resting PCWP decreased from a mean of 19 mm Hg to 14 mm Hg in 10 of the 11 patients at 30 days following implantation.8 Compared with baseline, patients had a mean 32-m improvement in 6-minute walk (6MW) distance and a significant reduction of Minnesota Living with Heart Failure score at 1-year of follow-up (29 point improvement).
Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF) Study
REDUCE LAP-HF study was an open-label study in 64 patients with left ventricular (LV) ejection fraction (EF) ≥40%.9 At 6 months after the implant, there was an average reduction of ∼2 to 3 mm Hg in PCWP (from 32 to 29 mm Hg at 20 W and 34 to 32 mm Hg at peak exercise). Qp:Qs at rest averaged 1.27 at the 6-month follow-up. There was an ∼2 mm Hg increase in RA pressure (from 9 to 11 mm Hg). In this population, work-normalized peak PCWP decreased significantly from 89.1 to 70.5 mm Hg/Watts/kg at 6 months. Importantly, the study also showed that CO (Fick method) was not decreased at rest (4.8 vs. 5.1 L/min). This is significant as reduction in systemic flow could theoretically be an adverse effect of left-to-right shunting. At 1 year, PCWP reductions were sustained, and Qp:Qs values were stable compared with those measured at 6 months.10
In the 1-year outcomes report for the open-label REDUCE LAP-HF study, Kaye et al.10 reported no significant changes in RA or LA size, small reductions of LV end-diastolic volume, small increases in RV end-diastolic volume, no changes in LV EF, and increases in right ventricular (RV) EF. Those changes that were evident by 6 months following shunt implant did not change further from 6 to 12 months.
REDUCE LAP-HF I
The REDUCE LAP-HF I mechanistic trial was a multicenter, randomized, double-blind, sham-controlled study.11,12 Forty-four patients were randomized 1:1 to the atrial shunt or a sham control procedure. Patients had a clinical diagnosis of HF, EF ≥40%, elevated natriuretic peptide levels, New York Heart Association (NYHA) class III or ambulatory class IV symptoms, exercise PCWP ≥25 mm Hg and a PCWP-to-RA pressure gradient ≥5 mm Hg. At 1 month, there was a 3.2 ± 5.2 mm Hg reduction of exercise PCWP in the inter atrial shunt device (IASD) group compared with a 0.9 ± 5.1 mm Hg increase in the sham control group (primary endpoint, p = 0.03). There were trends toward improved exercise duration at 1 month (1.2 ± 3.7 vs. 0.4 ± 3.5 minutes), increased achieved work (1.5 ± 14.6 vs. ‒1.9 ± 10.8 Watts), workload-corrected PCWP (‒5.7 ± 27.3 vs. +10.3 ± 45.9) and reductions of pulmonary vascular resistance (PVR) index (−0.29 ± 1.22 vs. 0.31 ± 1.64 Wood units [WU], p = 0.051). At 1-year follow-up, there were no significant differences in major adverse cardiac, cerebral, and renal events, with trends in favor of the IASD for improvement in NYHA class and reduction in heart failure hospitalization.13 An independent echocardiographic core laboratory documented patency of the shunt at 1 year in all patients.
Obokata et al.14 evaluated the intermediate term (1-6 months) hemodynamic effects of interatrial shunt (IAS) treatment in patients with HFpEF/heart failure with mildly reduced ejection fraction (HFmrEF) from the earlier studies. They showed evidence that increases in pulmonary blood flow and oxygen content associated with IAS treatment were associated (on average) with improvements in pulmonary vascular function as assessed by reduction in PVR and pulmonary artery elastance and improvements in pulmonary artery (PA) compliance. Importantly, there was not a reduction in systemic blood flow at rest or during exercise, despite the presence of a left-to-right shunt. Patients with improvement in PA compliance derived greater benefit in exercise capacity, suggesting that individuals with greater pulmonary vascular reserve can accommodate increased flow and may represent an enhanced responder group.
REDUCE LAP-HF II
The REDUCE LAP-HF II pivotal trial was undertaken to determine whether placement of the Corvia Atrial Shunt would improve symptoms and quality of life and reduce HF events in patients with HF and LV EF ≥40%.15 Enrollment began in May 2017 and concluded in July 2020 with 626 patients randomized. Primary results have been reported up to 24 months of follow-up, and clinical follow-up is ongoing.16 All patients were required to meet invasive exercise hemodynamic criteria for the diagnosis of HFpEF (exercise PCWP ≥25 mm Hg). REDUCE LAP-HF II was also designed to be an enrichment trial (a type of precision medicine trial in which the trial is enriched for patients hypothesized to be most responsive to the tested treatment).17 Besides the requirement for elevated PCWP during exercise, patients were required to have a gradient of at least 5 mm Hg between PCWP and RA pressure to ensure left-to-right shunting would occur. Patients with severe HF (e.g., low cardiac index, requiring inotropes, Stage D HF) or evidence of right heart or pulmonary vascular abnormalities (e.g., greater than mild RV dysfunction, moderate or greater tricuspid regurgitation, RA pressure ≥14 mm Hg, or PVR >3.5 WU) were excluded. Notably, the exclusion criteria were based on resting findings, and not during exertion.
The primary endpoint was a hierarchical composite of cardiovascular death or nonfatal ischemic stroke at 12 months, rate of total heart failure events up to 24 months, and change in Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score at 12 months. Prespecified subgroup analyses were conducted for the HF event endpoint. Analyses of the primary endpoint, all other efficacy endpoints, and safety endpoints were conducted in the modified intention-to-treat population (n = 621), defined as all patients randomly allocated to receive treatment, excluding those found to be ineligible after randomization and therefore not treated.
Patients were closely matched in the control and treatment groups. The mean age was 72.0 years, 61.5% were female, the mean body mass index was 32.0 kg/m2, and 29% had an HF hospitalization in the prior 12 months. The mean brain natriuretic peptide was 180 pg/mL in those with sinus rhythm and 311 pg/mL in those with atrial fibrillation. The mean n-terminal pro brain natriuretic peptide was 575 pg/mL in those with sinus rhythm and 1597 pg/mL in those with atrial fibrillation. Comorbid conditions were common, with hypertension in 88%, diabetes in 37%, and atrial fibrillation in 52%. Blood pressure was relatively well controlled with the mean systolic blood pressure of 127 mm Hg. Most patients were NYHA class III (77%). The mean KCCQ overall summary score was 45.8, indicating very poor quality of life, and 6MWD was 301 m, which is markedly reduced. The mean MAGGIC HF mortality risk score18 was 22.5, also indicating a high-risk population.
By echocardiography, mean LV size, LV mass index, and LA volume index were all in the normal range. The mean site reported LV EF was 60%, and the vast majority of patients had HFpEF (EF ≥ 50%), and only 7% had HFmrEF (EF 40%-49%). The mean E/e’ was 12.5, the mean LV global longitudinal strain was normal (−17.7%), and LA reservoir strain was lower than normal at 20.3% (normal ∼35%19,20). Invasive hemodynamic measurement revealed mean resting RA pressure of 9.0 mm Hg, pulmonary artery systolic pressure 40.0 mm Hg, PCWP 18.0 mm Hg, CO 5.3 L/min and PVR 1.5 Wood units. At peak exercise (average 40 Watts), RA pressure rose to 18.0 mm Hg, pulmonary artery systolic pressure (PASP) to 70 mm Hg, PCWP to 34 mm Hg, CO to 7.6 L/min, and PVR decreased slightly to 1.4 Wood units.
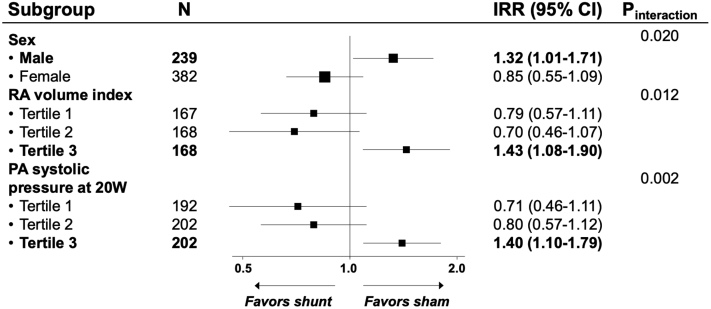
There were no differences between the shunt and control groups in the primary composite endpoint (win ratio 1.0 [95% confidence interval [CI] 0.8-1.2]; p = 0.85) or in the individual components of the primary endpoint.16 However, several prespecified subgroups demonstrated a differential effect of atrial shunt device treatment on heart failure events. These included pulmonary artery systolic pressure at 20 W of exercise (p interaction = 0.002 [>70 mm Hg associated with worse outcomes]), RA volume index (p interaction = 0.012 [≥29.7 mL/m2, worse outcomes]), and sex (p interaction = 0.02 [men, worse outcomes]; Figure 2). There were no differences in the composite safety endpoint between the 2 groups (n = 116 [38%] for shunt device vs. n = 97 [31%] for sham procedure; p = 0.11).
Figure 2.
Forest plot of treatment effect on recurrent heart failure events by prespecified subgroups. Modified from Figure 3 and Supplemental Figure 2, Shah et al.16
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; RA, right atrial.
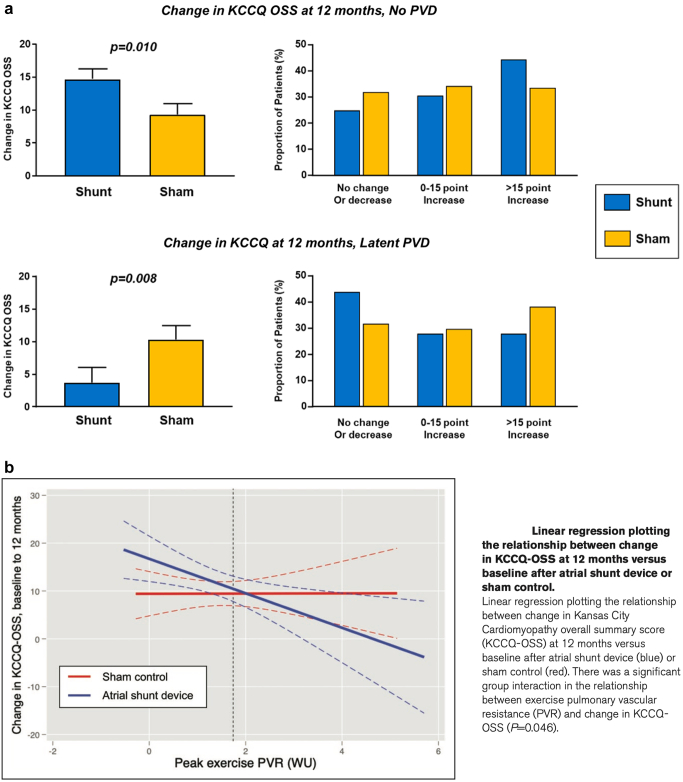
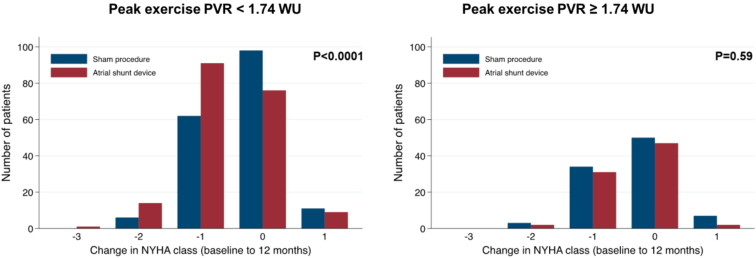
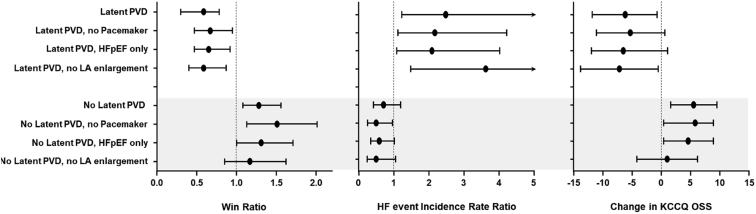
In a subsequent analysis of data from REDUCE LAP-HF II, Borlaug et al.21 tested the hypothesis that characteristics that reflect right heart and pulmonary vascular structure and function might modulate the effects of the atrial shunt. Latent pulmonary vascular disease (LPVD) was defined as PVR ≥1.74 WU at peak exercise (reflecting the highest tertile of peak exercise PVR), measured before randomization. Compared with patients without LPVD (n = 382), those with LPVD (n = 188) were older, had more atrial fibrillation, more right heart dysfunction, and were more likely to have elevated PCWP at rest. Shunt treatment was associated with worse outcomes in the patients with LPVD (win ratio 0.60 [95% CI 0.42, 0.86]; p = 0.005), and there was a signal of clinical benefit in patients without LPVD (win ratio 1.31 [95% CI 1.02, 1.68]; p = 0.038). Patients with larger RA volumes and men had worse outcomes with the device, and both groups were more likely to have implanted cardiac rhythm devices, HFmrEF, and increased LA volume. Patients without LPVD also appeared to derive greater benefit in health status (KCCQ overall summary score), whereas patients with latent LPVD had worse health status with the IAS device (Figure 3). In addition, patients without LPVD had greater improvement in NYHA class with treatment (Figure 4). For patients without LPVD or pacemaker (n = 313, 50% of randomized patients), shunt treatment resulted in a robust signal of clinical benefit (win ratio 1.51 [95% CI 1.14, 2.00]; p = 0.004; Figure 5).
Figure 3.
(a)Changes in Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 12 months based on the presence or absence of latent pulmonary vascular disease (PVD; exercise PVR >1.74 Wood units). (b) Relationship between exercise PVR at baseline and changes in KCCQ score over 12 months. Reproduced from Borlaug et al.21
Abbreviations: PVR, pulmonary vascular resistance; WU, wood units.
Figure 4.
Changes in New York Heart Association (NYHA) functional class from baseline to 12 months based on the absence or presence of latent pulmonary vascular disease (peak exercise pulmonary vascular resistance (PVR) <1.74 vs. ≥1.74 Wood units [WU]). Reproduced from Borlaug et al.21
Figure 5.
Forest plots showing primary outcome (win ratio), heart failure event rate and change in Kansas City Cardiomyopathy Questionnaire (KCCQ) score based on the presence or absence of latent pulmonary vascular disease, presence or absence of permanent cardiac rhythm device (pacemaker) and presence or absence of left atrial (LA) enlargement. Reproduced from Borlaug et al.21
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; PVD, pulmonary vascular disease.
Why Measure Exercise PVR as Opposed to Other Variables?
Measuring PVR during exercise can be challenging because PA pressure, PCWP, and CO are all needed for the calculation. Although it is more complex than simply measuring PA pressure, and not available in everyone, 570 of 626 (91%) subjects in REDUCE LAP-HF II had the data required for the calculation of exercise PVR. Multiple pieces of evidence suggest that exercise PVR gives additional insight into pulmonary vascular physiology above and beyond what we get from PASP alone (see above).21 Moreover, in REDUCE LAP-HF II, exercise PVR was related to outcomes, whereas rest PVR was not. In patients without LPVD, sex differences in response to the shunt were no longer evident. Thus, based on currently available data, it seems that the extra effort needed to calculate exercise PVR likely adds value and clinically relevant information.
V-Wave Ventura IAS
The V-Wave device is the second IASD to enter into a large, multicenter (120 international sites), randomized, controlled clinical trial with a primary endpoint of clinical events. The V-Wave device is an hourglass-shaped nitinol frame with ePTFE encapsulation (covered stent). The inner shunt diameter is 5.1 mm (Figure 1). The initial design of the device contained a valve to prevent right to left shunting.22 Although the device appeared to be associated with clinical benefit, the valve was prone to occlusion,23 and a second generation design without the valve was developed and evaluated in a small single-arm trial.24 The ongoing pivotal trial includes patients with both HFpEF and HFrEF. Patients are included based on resting hemodynamic evaluation with no exercise performed during the qualifying right heart catheterization. The pivotal trial is still enrolling at the time of this publication. The trial includes 97 unblinded “roll-in” patients and up to 500 randomized patients, with enrollment concluding in the fall of 2022. The endpoints will include effectiveness: (death, transplantation/LVAD, HF hospitalization, outpatient treatment of worsening HF, and quality of life); safety (device-related major complications at 30 days); and health economic metrics. Preliminary results from the roll-in cohort have been presented at the Technologies in Heart Failure Therapeutics (THT) meeting in 2022. Among the 97 roll-in patients, the mean age was 70 years, 29% were female (although 50% were female in the HFpEF cohort), body mass index was 32 kg/m2, and high proportions had atrial fibrillation (50%), hypertension (84%), and diabetes (55%). The mean LV EF was 49%, and 52% of the patients had EF ≥40%. The mean resting PCWP was 21 in the patients with HFrEF and 19 in those with HFpEF. The mean resting PVR was 2.6 WU in both groups. Device implantation was successful in 99%; there were no major adverse cardiac or neurological events at 30 days, and shunt patency was 100% at 30 days by TEE. The mean KCCQ improved from 45.9 to 58 at 1 month and remained stable to 12 months for the subgroup with that duration of follow-up (n = 67). An interim analysis by the data safety monitoring board in October 2021 recommended the continuation of the trial as planned.
Other Studies
There are at least 7 ongoing investigational approaches using some form of atrial shunting as a therapy for HF (mostly HFpEF and HFmrEF, but some trials including HFrEF; Figure 1). In addition to the 2 trials described earlier, multiple companies have pivotal trials that are in the final planning stages or are expected to begin enrolling patients in the near future (Table 1). Four of the companies in this space are using implanted devices (Corvia, V-Wave, Occlutech, and Edwards Life Sciences), and 3 are creating atrial shunts without an implant (Alleviant, NoYa, and Intershunt). Occlutech makes devices with 3 different orifice sizes: 6, 8, and 10 mm. The nonimplant approaches use radiofrequency energy (Alleviant, NoYa) or a cutting technique (Intershunt) to produce the shunt. The NoYa device has the ability to adjust the size of the defect that is created. The nonimplant approaches offer potential advantages, including lack of potential for device thrombosis, infection, or dislodgement; and simpler subsequent trans-septal access in the event of need for procedures such as placement of LA occlusion devices or atrial fibrillation ablation. As well, the ability to subsequently close the shunt is better; in the event, this is needed.
Table 1.
Overview of clinical trials of atrial shunts for treatment of heart failure
| Device | Study | Study design (key inclusion criteria) | N | Author | Year published | Time frame | Main efficacy findings∗ |
|---|---|---|---|---|---|---|---|
| Corvia Atrial Shunt | Feasibility | Single arm (EF >45%, NYHA II-IV, PCWP >15 mm Hg at rest or >25 mm Hg during exercise) | 11 | Søndergaard et al.8 | 2014 | 1 mo | PCWP at rest† ↓5 mm Hg |
| Malek et al.25 | 2015 | 12 mo | NYHA ↓0.5; MLWHFQ ↓29; 6MW ↑32 m | ||||
| REDUCE LAP-HF9 | Single arm (EF >40%, NYHA II-IV, PCWP >15 mm Hg at rest or >25 mm Hg during exercise) | 64 | Hasenfuß et al.26 | 2016 | 6 mo | Exercise PCWP∗,† ↓3 mm Hg Qp:Qs 1.27 Peak exercise PCWP/Watts/kg ↓ 15 mm Hg/W/kg |
|
| Kaye et al.10 | 2016 | 12 mo | NYHA median ↓ by 1 class; 6MW ↑32 m; MLWHFQ ↓15; patency confirmed in 98.5% | ||||
| Kaye et al.27 | 2018 | 36 mo | Observed mortality was 3.4/100 pt-yrs; lower than 10.2/100 pt-yrs predicted by baseline MAGGIC score (p = 0.02) | ||||
| REDUCE LAP-HF I Mechanistic study12 | Randomized, sham-controlled, double-blinded (NYHA III/IV, EF ≥40%, exercise PCWP ≥15 mm Hg) | 44 | Feldman et al.11 | 2018 | 1 mo | PCWP at peak exercise† ↓3 mm Hg Peak exercise PCWP/Watts/kg ↓ 16 mm Hg/W/kg |
|
| Shah et al.13 | 2018 | 12 mo | Yearly HF hospitalization rate ↓67% (p = 0.06); NYHA median ↓ by 1 class; 6MW no difference; KCCQ no difference. 100% shunt patency. | ||||
| V-Wave Gen 1 | Special access pilot (Canada) | Single arm (NYHA III/IV, EF ≤40%, PCWP ≥15 mm Hg) | 10 | Del Trigo et al.22 | 2016 | 3 mo | 8/9 showed improved NYHA; Duke activity status ↑ 11; KCCQ ↑ 35; 6MW ↑ 74 m; PCWP ↓6 mm Hg |
| Pilot | Single arm (NYHA III/IV; EF >15%; ≥1 HFH within 1 y) | 38 | Rodes-Cabau et al.23 | 2018 | 12 mo | NYHA Class I or II in 62%; KCCQ 73% improved ≥5 point improvement; 6MW ↑28 m; no significant change in PCWP, RAP or PAP; Qp:Qs 1.1; 14% of valves occluded and 36% stenotic by 12 mo; better hemodynamic responses in patients with patent shunt | |
| V-Wave Gen 2 | Pilot | Single arm (NYHA III or IV) | 6 | Guimaraes et al.24 | 2020 | 12 mo | NYHA Class I or II in 83%; Duke activity status ↑15; KCCQ ↑32; 6MW ↑64 m; Qp:Qs 1.16. |
| Occlutech AFR | AFR-PRELIEVE | Single arm (NYHA III or IV; EF ≥15%) | 36 | Paitazoglou et al.28 | 2019 | 3 mo | HFrEF/HFpEF, NYHA Class −1.4/−1.1; KCCQ ↑16/↑ 20; 6MW ↑ 30/↑ 26 m; PCWP ↓2/↓5 mm Hg; Qp:Qs 1.3/1.1 |
| Occlutech AFR | PROLONGER study, AFteR registry | Currently enrolling | |||||
| NoYa | Pilot | Single arm (EF >35%) | 10 | Lotan | 2019 | 1-3 mo | Shunt diameter 3-4 mm; 6MW ↑ 56 m; BNP ↓ 1878 pg/mL |
| NoYa | RAISE trial | Single arm | 10 | Sun et al.29 | 2022 | 3/10 shunts closed by 6 mo in pigs; 3/10 shunts closed at 6 mo in humans | |
| Alleviant | HF-1, HF-2, HFrEF | Single arm, 28 HFpEF, 5 HFrEF | 33 total | Preliminary data presented at THT, TVT and SCAI meetings | 2022 | 7 mm shunt; no implant; 100% patency 6 mo; ↓ ex PCWP; ↑6MW; Ongoing f/u. | |
| Edwards Life Sciences | ALt FLOW | Single arm | 15 | Recruiting | |||
| Pivotal trials | |||||||
| Corvia | REDUCE LAP-HF II | Double-blind RCT (EF >40%) | 626 | Shah et al.16 | 2022 | 5 y | Enrollment and 1 y f/u completed. No overall benefit, but potential responder subgroups identified. Follow-up ongoing. |
| Ongoing trials | Target enrollment | ||||||
| V-Wave | RELIEVE-HF | 100 roll-in (open label), remainder double-blind RCT, NYHA II-IVa, HFrEF, and HFpEF | 100 roll-in, up to 500 randomized (double bind) | Preliminary data from roll-in patients presented at THT meeting 2022 | 97 roll-in patients and up to 500 randomized. Enrollment completion expected October 2022. Preliminary results from roll-in patients: high success, good safety, improved KCCQ, and NHYA. Favorable cardiac remodeling. | ||
| Occlutech | FROST not yet started | Double blind, 6 mm, 8 mm or sham | 575 | ||||
| Edwards Lifesciences | ALt FLOW not yet started | ||||||
| Alleviant Medical | ALLAY-HF not yet started | ||||||
| NoYa | Not yet started | ||||||
| Intershunt | Not yet started | ||||||
| Corvia | RESPONDER-HF | Double-blind RCT exercise PVR <1.74 WU, no pacemaker, RA volume <29.7 mL/m2 | 250 |
Notes. Modified from Griffin et al.7
6MW, 6-minute walk; BNP, brain natriuretic peptide; EF, ejection fraction; f/u, follow-up; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure; MLWHF, Minnesota Living With Heart Failure; NYHA, New York Heart Association; PA, pulmonary artery; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RCT, randomized controlled trial; REDUCE LAP-HF, Reduce Elevated Left Atrial Pressure in Patients with Heart Failure; SCAI, society for cardiovascular angiography and interventions; THT, Technologies in Heart Failure Therapeutics; TVT, transcatheter valve therapies.
For single-arm studies, values are changes relative to patients’ baseline values; for randomized studies, changes are relative to the control group.
Primary endpoint when one was declared prospectively.
Recently published preclinical (n = 11 pigs) and first in human data (n = 10) using the NoYa device showed 100% acute procedural success, no major safety events or thromboembolism, and clinical improvement in 8 patients at 6 months.29 This included a significant reduction in n-terminal pro brain natriuretic peptide, an 88 m increase in 6MW distance and improved NYHA class. However, 3 of 11 pigs and 3 of 10 humans had closure of the shunt by 6 months of follow-up.
Preliminary data (unpublished) from 3 studies using the Alleviant device (total n = 33) have been presented (Technologies in Heart Failure Therapeutics, transcatheter valve therapy, society for cardiovascular angiography and interventions meetings 2022; Table 1). These studies showed 100% procedural success, no MACCE events and improvement in exercise PCWP, KCCQ score, NYHA class, and 6MW distance. In 15 patients with 6-month echoes, all the shunts remained patent.
Discussion
There is growing interest in atrial shunt therapies for HF. The various approaches use different locations, sizes, and designs of the devices as well as some that do not use an implant. Enrollment criteria between the trials vary but largely are focusing on HF patients with class II-III symptoms, enrichment based on prior HF hospitalizations and/or elevated natriuretic peptide levels. Some trials are including patients with LV EF <40%. Following the publication of the results from REDUCE LAP-HF II, some of the newer trials will likely have inclusion and exclusion criteria that capitalize on the knowledge gained from the completed study. These may include cutoffs for right heart chamber sizes and function and measures of PVR at rest or during exercise.
Currently, invasive pressure measurement during supine cycle exercise is considered the optimal method for diagnosing HFpEF, particularly in the subgroup with unclear biochemical and clinical criteria for HF.1 These studies can be challenging to perform and interpret, and ergometry in the catheterization laboratory is not yet widely available or used at present. However, the successful invasive exercise pressure recordings in the large population of HFpEF and HFmrEF patients enrolled in REDUCE LAP-HF II at more than 100 centers in multiple countries help establish the generalizability of this approach. Nonetheless, it is currently uncertain whether exercise testing is necessary or if simpler and less invasive clinical scoring systems such as the H2FPEF score30 or approaches using resting or diastolic stress echocardiography31 would be suitable alternatives.
Medical therapy for HFpEF has been relatively ineffective to date. Most pharmacological interventions that have been evaluated either failed to show significant clinical benefit or the magnitude of benefits were relatively small or inconsistent.32 This includes the use of combined angiotensin/neprilysin inhibitors.33 More recently, SGLT2 inhibitors have been shown to be associated with reduced HF hospitalizations,3 with a larger effect size in patients with mildly reduced EF vs. those with HFpEF.3 The use of SGLT2 inhibitors was also associated with modestly improved quality of life metrics,34 but no improvement in total hospitalizations or survival.4,34 Most of the other pharmacological strategies studied to date involve some form of neurohormonal antagonism.33,35, 36, 37 Failure to show consistent beneficial effects may have occurred because many patients with HFpEF do not have overt volume or major neurohormonal activation. As such, these patients may be prone to develop dehydration, acute kidney injury, chronotropic incompetence, or orthostatic hypotension with the use of neurohormonal antagonists and/or diuretics.
A prior review on IAS devices highlighted a series of unanswered questions (below).7 Some of these can now be addressed.
-
1)
Are results of invasive exercise hemodynamics at baseline beneficial in selecting patients more likely to respond to IASDs? Exercise hemodynamic evaluation provides unique data including exercise PASP and PVR. Based on REDUCE LAP-HF II, these parameters appear to be useful in determining the likelihood of worsening or benefit from IAS.16,21 Exercise PASP and PVR are challenging to extrapolate from resting hemodynamic studies because the correlation between rest and exercise PVR is low. On the other hand, the ongoing RELIEVE-HF trial is not using hemodynamic assessment during exercise and preliminary results from the roll-in cohort of the trial suggest the efficacy of the shunt. Moreover, baseline PVR in the roll-in cohort from RELIEVE-HF was relatively high at 2.7 WU. Thus, the question of whether invasive hemodynamics during exercise is required, currently remains unanswered. As well, more data will be needed to determine if exercise echocardiography will be able to provide similar information to invasive pressure measurement during exercise. This is an important area for future investigation.
-
2)
Is the magnitude of the PCWP-RAP pressure gradient an important determinant of clinical effectiveness of IAS? Although the PCWP-RA gradient is a determinant of flow through the shunt, this variable was studied at rest and at 20 W exercise as prespecified treatment modifier in REDUCE LAP-HF II, and there was no interaction with HF outcomes.16 The RELIEVE-HF study does not have a requirement for a specific PCWP-RA pressure gradient. The preliminary results of that study suggest benefit of the shunt. Taken together, it now seems likely that documenting such a gradient (at a single point in time with a certain set of loading conditions) is not necessary. Although left-to-right shunting is the mechanism that accounts for LA decompression, it is possible that too much shunting will be detrimental. Thus, there might be an optimal window for the PCWP-RA gradient to achieve optimal effects. Ongoing and future studies will likely shed more light on this question.
-
3)
Should the size of the IAS be optimized for individual patients, and, if so, what factors are critical for such a determination? This question remains unanswered. Comparison of findings from REDUCE LAP-HF II (8 mm shunt) and the ongoing RELIEVE-HF trial (5.1 mm shunt) may provide insights into this issue. In addition, there are planned studies of devices with variable-size shunts (e.g., Occlutech).
-
4)
Can we identify degrees of cardiac remodeling or myocardial dysfunction that would minimize the clinical benefits of IAS or be associated with harm? In REDUCE LAP-HF II, patients with HFmrEF and worse LV global longitudinal strain (indicative of worse LV systolic dysfunction) appeared to do worse with the device in prespecified subgroup analyses, but the interaction p-values did not achieve statistical significance (interaction p = 0.20 and 0.37, respectively). Indeed, the number of patients with LV EF of 40% to 49% in the REDUCE LAP-HF II trial was too small (7% of the total population) to definitively answer this question. Some of the ongoing trials with other devices are enrolling patients with a wider range of LV EF. Most trials are excluding patients with significant RV dysfunction due to concerns that this would limit efficacy or cause harm. Patients treated with the Corvia device have shown early increases in RV size, with some decreases in LV size following shunt therapy,10,21 but the impact of baseline structure and treatment response is not well understood.
-
5)
Does atrial fibrillation or LA or RA myopathy impact the effectiveness of IASs? Although it was not statistically significant (p = 0.09) in REDUCE LAP-HF II, there was a trend favoring device therapy in patients without atrial flutter or fibrillation (unpublished, presented at ESC HFA, 2022). Subsequent analyses (unpublished) suggest that patients with atrial fibrillation derive benefit from IAS if they have other characteristics associated with a favorable response (lack of cardiac rhythm device and exercise PVR <1.74 WU). Increased RA volume was shown to be associated with worse outcomes in response to IAS with the Corvia device.16 There does not appear to be any significant, independent effect of LA enlargement on response to IAS.21
-
6)
Aside from hemodynamic factors, what other clinical characteristics are important for identifying patients most likely to benefit from IASs? The finding that patients with cardiac rhythm devices fared worse with the device was somewhat unexpected. Theories about why pacemakers may have a negative impact include higher degrees of tricuspid valve regurgitation (possibly due to pacemaker lead impingement of the tricuspid valve leaflets), lower chronotropic reserve, and need for pacemaker being a marker of other myopathic features or more advanced heart or pulmonary vascular disease. Data from REDUCE LAP II suggest that men fare worse than women.16 The reasons for this are unknown, but hypothetically might be due to differences in body size, heart size, and cardiac output with resultant mismatch of shunt size and hemodynamic parameters. However, men without LPVD or pacemakers appear to benefit equally to women with IAS.21
-
7)
Will IASs serve as a viable treatment option for patients with HFrEF or valvular heart disease? Data from REDUCE LAP II suggest lower effectiveness of shunt therapy in HFmrEF. However, the HFmrEF subgroup was only 7% of the total population. Preliminary data from RELIEVE-HF roll-in cohort, in which nearly half of the patients have HFrEF, have shown comparable improvements in KCCQ score in HFrEF vs. HFmrEF and HFpEF. Patients with significant valvular heart disease have generally been excluded from the ongoing and planned trials. Ongoing studies will help to address the question of whether LV EF modulates the response to shunting.
-
8)
Will any safety issues arise during long-term follow-up (e.g., impact on RV and RA size and function, impact on pulmonary vasculature, right to left shunting with paradoxical stroke, and atrial arrhythmias)? The published data to date with the Corvia device show stable degrees of right to left shunting at 1 year with initial increases in RA and RV sizes, but these changes appear to be relative stable after 6 months.10 Interestingly, unpublished data from the RELIEVE roll-in cohort (61 patients with paired baseline and 12-month echocardiograms) suggest reductions in RV size with improvement in both RV and LV function. No long-term safety concerns have emerged at this time with either the Corvia or V-Wave devices, particularly the risk of stroke or atrial arrhythmias.
Conclusions
In conclusion, there is growing excitement about the use of atrial shunt therapy for the treatment of HF. The use of exercise in combination with invasive hemodynamic studies is feasible in multicenter clinical trials, suggesting that this tool could be more widely implemented in clinical practice. Subgroups of patients identified using clinical, echocardiographic, and hemodynamic phenotyping may identify patients who are more likely to derive benefit from this therapy while providing necessary insights into who might be best excluded based on the potential for harm. Future studies and ongoing analyses will hopefully allow us to offer more personalized therapeutics for the treatment of both HFpEF and HFrEF.
Funding
The authors have no funding to report.
Disclosure statement
S.E.L. is on an events adjudication committee for CVRx and is a consultant for Axon Therapies, Novartis, Alleviant, Eli Lilly, and Occlutech. He has received research funding (administered through institution) from Corvia. B.A.B. has received research grants from AstraZeneca, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, Tenax Therapeutics, and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. J.K. is an employee of Corvia. S.J.S. has received research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics.
References
- 1.Jain C.C., Borlaug B.A. Performance and interpretation of invasive hemodynamic exercise testing. Chest. 2020;158:2119–2129. doi: 10.1016/j.chest.2020.05.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisman A.S., Shah R.V., Dhakal B.P., et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 4.Nassif M.E., Windsor S.L., Borlaug B.A., et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham W.T., Lindenfeld J., Ponikowski P., et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700–710. doi: 10.1093/eurheartj/ehaa943. [DOI] [PubMed] [Google Scholar]
- 6.Minamisawa M., Claggett B., Suzuki K., et al. Association of hyper-polypharmacy with clinical outcomes in heart failure with preserved ejection fraction. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.008293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin J.M., Borlaug B.A., Komtebedde J., et al. Impact of interatrial shunts on invasive hemodynamics and exercise tolerance in patients with heart failure. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondergaard L., Reddy V., Kaye D., et al. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796–801. doi: 10.1002/ejhf.111. [DOI] [PubMed] [Google Scholar]
- 9.Hasenfuss G., Gustafsson F., Kaye D., et al. Rationale and design of the reduce elevated left atrial pressure in patients with heart failure (reduce LAP-HF) trial. J Card Fail. 2015;21:594–600. doi: 10.1016/j.cardfail.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Kaye D.M., Hasenfuss G., Neuzil P., et al. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(12) doi: 10.1161/CIRCHEARTFAILURE.116.003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman T., Mauri L., Kahwash R., et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [reduce elevated left atrial pressure in patients with heart failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137:364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 12.Feldman T., Komtebedde J., Burkhoff D., et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE elevated left atrial pressure in heart failure (REDUCE LAP-HF I) Circ Heart Fail. 2016;9(7) doi: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 13.Shah S.J., Feldman T., Ricciardi M.J., et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the reduce elevated left atrial pressure in patients with heart failure (REDUCE LAP-HF I) trial. JAMA Cardiol. 2018;3:968–977. doi: 10.1001/jamacardio.2018.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obokata M., Reddy Y.N.V., Shah S.J., et al. Effects of interatrial shunt on pulmonary vascular function in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74:2539–2550. doi: 10.1016/j.jacc.2019.08.1062. [DOI] [PubMed] [Google Scholar]
- 15.Berry N., Mauri L., Feldman T., et al. Transcatheter InterAtrial shunt device for the treatment of heart failure: rationale and design of the pivotal randomized trial to REDUCE elevated left atrial pressure in patients with heart failure II (REDUCE LAP-HF II) Am Heart J. 2020;226:222–231. doi: 10.1016/j.ahj.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Shah S.J., Borlaug B.A., Chung E.S., et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399:1130–1140. doi: 10.1016/S0140-6736(22)00016-2. [DOI] [PubMed] [Google Scholar]
- 17.Shah S.J. Innovative clinical trial designs for precision medicine in heart failure with preserved ejection fraction. J Cardiovasc Transl Res. 2017;10:322–336. doi: 10.1007/s12265-017-9759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong C.M., Hawkins N.M., Petrie M.C., et al. Heart failure in younger patients: the meta-analysis global group in chronic heart failure (MAGGIC) Eur Heart J. 2014;35:2714–2721. doi: 10.1093/eurheartj/ehu216. [DOI] [PubMed] [Google Scholar]
- 19.Nemes A., Kormanyos A., Domsik P., Kalapos A., Lengyel C., Forster T. Normal reference values of three-dimensional speckle-tracking echocardiography-derived left atrial strain parameters (results from the MAGYAR-healthy study) Int J Cardiovasc Imaging. 2019;35:991–998. doi: 10.1007/s10554-019-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathan F., D'Elia N., Nolan M.T., Marwick T.H., Negishi K. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30:59–70.e8. doi: 10.1016/j.echo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Borlaug B.A., Blair J., Bergmann M.W., et al. Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation. 2022;145(21):1592–1604. doi: 10.1161/CIRCULATIONAHA.122.059486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Trigo M., Bergeron S., Bernier M., et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet. 2016;387:1290–1297. doi: 10.1016/S0140-6736(16)00585-7. [DOI] [PubMed] [Google Scholar]
- 23.Rodes-Cabau J., Bernier M., Amat-Santos I.J., et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-wave system. JACC Cardiovasc Interv. 2018;11:2300–2310. doi: 10.1016/j.jcin.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes L., Bergeron S., Bernier M., et al. Interatrial shunt with the second-generation V-wave system for patients with advanced chronic heart failure. EuroIntervention. 2020;15:1426–1428. doi: 10.4244/EIJ-D-19-00291. [DOI] [PubMed] [Google Scholar]
- 25.Malek F., Neuzil P., Gustafsson F., et al. Clinical outcome of transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel implant. Int J Cardiol. 2015;187:227–228. doi: 10.1016/j.ijcard.2015.03.198. [DOI] [PubMed] [Google Scholar]
- 26.Hasenfuß G., Hayward C., Burkhoff D., et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298–1304. doi: 10.1016/S0140-6736(16)00704-2. [DOI] [PubMed] [Google Scholar]
- 27.Kaye D.M., Petrie M.C., McKenzie S., et al. Impact of an interatrial shunt device on survival and heart failure hospitalization in patients with preserved ejection fraction. ESC Heart Fail. 2019;6:62–69. doi: 10.1002/ehf2.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paitazoglou C., Ozdemir R., Pfister R., et al. The AFR-PRELIEVE trial: a prospective, non-randomised, pilot study to assess the atrial flow regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention. 2019;15:403–410. doi: 10.4244/EIJ-D-19-00342. [DOI] [PubMed] [Google Scholar]
- 29.Sun W., Zou H., Yong Y., et al. The RAISE trial: a novel device and first-in-man trial. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.008362. [DOI] [PubMed] [Google Scholar]
- 30.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obokata M., Kane G.C., Reddy Y.N., Olson T.P., Melenovsky V., Borlaug B.A. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borlaug B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 33.Solomon S.D., McMurray J.J.V., Anand I.S., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 34.Butler J., Filippatos G., Siddiqi T.J., et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-preserved trial. Circulation. 2022;145(3):184–193. doi: 10.1161/CIRCULATIONAHA.121.057812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S., Pfeffer M.A., Swedberg K., et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 36.Massie B.M., Carson P.E., McMurray J.J., et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 37.Pitt B., Pfeffer M.A., Assmann S.F., et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]