Abstract
Background
Transcatheter aortic valve implantation is safe and effective for high-risk patients with bioprosthetic valve failure (BVF) but has not been studied in low- and intermediate-risk patients. One year outcomes of the PARTNER 3 Aortic Valve-in-valve (AViV) Study were evaluated.
Methods
This prospective, single-arm, multicenter study enrolled 100 patients from 29 sites with surgical BVF. The primary endpoint was a composite of all-cause mortality and stroke at 1 year. The key secondary outcomes included mean gradient, functional capacity, and rehospitalization (valve-related, procedure-related, or heart failure related).
Results
A total of 97 patients underwent AViV with a balloon-expandable valve from 2017 to 2019. Patients were 79.4% male with a mean age of 67.1 years and Society of Thoracic Surgeons score of 2.9%. The primary endpoint occurred in 2 patients (2.1%) who had strokes; there was no mortality at 1 year. Five patients (5.2%) had valve thrombosis events, and 9 patients (9.3%) had rehospitalizations, including 2 (2.1%) for strokes, 1 (1.0%) for heart failure, and 6 (6.2%) for aortic valve reinterventions (3 explants, 3 balloon dilations, and 1 percutaneous paravalvular regurgitation closure). From baseline to 1 year, New York Heart Association class III/IV decreased from 43.3% to 4.5%, mean gradient from 39.1 ± 18.2 mm Hg to 19.7 ± 7.6 mm Hg, and ≥moderate aortic regurgitation from 41.1% to 1.1%.
Conclusions
AViV with a balloon-expandable valve improved hemodynamic and functional status at 1 year and can provide an additional therapeutic option in selected low- or intermediate-risk patients with surgical BVF, although longer term follow-up is necessary.
Keywords: Aortic valve replacement, Bioprosthesis, Regurgitation, Stenosis, Transcatheter
Graphical abstract
Key outcomes from the PARTNER 3 Aortic Valve-in-Valve Study at 1 year. Rehospitalization was defined as valve or procedure related, including heart failure. Abbreviations: ID, internal diameter; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Highlights
-
•
Aortic valve-in-valve is safe and effective for patients with bioprosthetic valve failure at high risk for reoperative aortic valve replacement.
-
•
The PARTNER 3 Aortic Valve-in-Valve Study shows safety and efficacy in lower risk patients at 1 year.
-
•
Long-term follow-up will reveal impacts of postprocedural gradients and thrombosis.
Introduction
Bioprosthetic valves after surgical aortic valve replacement (AVR) are subject to bioprosthetic valve failure (BVF), most commonly caused by calcification or wear of the leaflets, termed structural valve deterioration, and less commonly caused by pannus ingrowth or valve thrombosis.1,2 Coupled with increased use of surgical bioprostheses over the past 2 decades,1 the number of patients with failing surgical bioprostheses is expected to increase, especially in younger patients.3
International guidelines now recommend transcatheter aortic valve implantation (TAVI) as a treatment option for high-risk patients with BVF4,5 based on data collected from studies in the patient subgroup.6, 7, 8 Aortic valve-in-valve (ViV) in patients with BVF who are at low or intermediate risk for reoperative AVR has not been studied and is currently not a labeled transcatheter heart valve indication for use. Here, we report the 1-year results of the PARTNER 3 Aortic ViV Study, which is the first to examine the safety and efficacy of aortic ViV with a balloon-expandable transcatheter heart valve in a low- or intermediate-risk cohort of patients.
Material and Methods
Study Population and Design
The PARTNER (Placement of Aortic Transcatheter Valves) 3 Trial included a ViV multicenter registry that prospectively enrolled patients at intermediate or lower surgical risk with failing aortic bioprosthetic valves (NCT03003299). The institutional review boards of all participating sites approved the study protocol before patient enrollment in compliance with the Declaration of Helsinki, and written informed consent was obtained for all patients. The study had 2 arms, one for failing surgical valves and the other for failing transcatheter valves. Our study will focus only on the failing surgical valve arm, which allowed for the enrollment of 100 patients.
Baseline imaging by computed tomography (CT) or transesophageal/transthoracic echocardiography was obtained for all patients. All echocardiograms and CT scans were interpreted at a core laboratory. Patients screened for the enrollment in the registry were assessed by a multidisciplinary heart team for key inclusion criteria, including a failing surgical aortic bioprosthetic valve demonstrating ≥moderate stenosis and/or regurgitation with a true internal diameter (ID) of 16.5-28.5 mm—as calculated by a ViV application by Bapat et al.9—and low and intermediate surgical risk defined as Society of Thoracic Surgeons (STS) score <8%. Selection was adjudicated by a case review board. The Vancouver method, which has been described previously,10 was used for predicting the risk of coronary occlusion. In brief, a virtual transcatheter valve (at the size of implant) was centered within the basal ring of each surgical valve, and the distance between the edge of the virtual transcatheter valve and the coronary ostia was measured.
The key anatomic exclusion criteria included severe regurgitation (>3+) or stenosis of any other valve, ≥moderate aortic paravalvular regurgitation (PVR), increased risk of coronary obstruction by prosthetic leaflets of the failing valve, surgical or transcatheter valve in the mitral position (exception: mitral rings), known residual mean gradient >20 mm Hg at the end of the index surgical AVR procedure, severe left ventricular dysfunction (left ventricular ejection fraction <30%), and vascular anatomy not suitable for safe femoral access. The key clinical exclusion criteria included acute myocardial infarction within 1 month, stroke or transient ischemic attack within 90 days, untreated significant coronary artery disease requiring revascularization, renal insufficiency (estimated glomerular filtration rate <30 mL/min) and/or renal replacement therapy, hemodynamic or respiratory instability, and planned concomitant surgical or transcatheter ablation for atrial fibrillation (not including percutaneous coronary intervention). Follow-up was planned for 30 days, 6 months, and annually through 10 years (Figure 1).
Figure 1.
Design of the PARTNER 3 Aortic Valve-in-Valve Study. This registry was designed to evaluate outcomes for patients with a failing surgical aortic bioprosthesis at low or intermediate risk for surgery with anatomy suitable for TF access undergoing a valve-in-valve TAVI procedure.
Abbreviations: STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; TF, transfemoral; THV, transcatheter heart valve.
Outcomes
The primary outcome was a composite endpoint of all-cause mortality and stroke at 1-year post-procedure. The secondary endpoints at 1 year included rehospitalization (defined as valve related, procedure related, or heart failure related), mean and peak echocardiographic transaortic gradients, Kansas City Cardiomyopathy Questionnaire (KCCQ), New York Heart Association (NYHA) class, and 6-minute walk test. We also evaluated left ventricular mass regression by mean gradient at 1 year.
Follow-Up
All patients underwent baseline and 30-day neurological assessment. Patients with suspected neurological events received serial neurologist examinations and neurological imaging. All major endpoint events (using VARC-2 definitions when applicable, including for all-cause mortality, stroke/transient ischemic attack, coronary obstruction, and valve thrombosis) were adjudicated by a Clinical Events Committee. Clinical and echocardiography follow-up will be performed yearly in all patients through 10 years; all echocardiograms will be read by a core laboratory through 5 years, then at 7 and 10 years.
Statistical Analysis
The analysis was performed in the as-treated population, which included all enrolled patients for whom the index procedure began, regardless of whether the index procedure was completed. The results for the nonhierarchical composite primary endpoint and the individual components (all-cause mortality and all stroke) at 30 days and 1 year were summarized by Kaplan-Meier event rates and number of subjects with the event. Descriptive statistics were presented as continuous variables summarized by mean ± standard deviation. Categorical variables were summarized by count and percent. Analyses for echocardiographic, NYHA class, KCCQ and 6-minute walk test were performed in the valve implant population, which included patients in whom the intended valve was implanted. Changes from baseline were compared using the paired t-test. By convention, a p value of ≤0.05 was used to determine statistical significance. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina).
Results
Patient Characteristics
Of the 165 patients with a failing surgical bioprosthesis at low or intermediate risk who were screened for enrollment, 100 patients from 29 sites were enrolled. The most common reason for screen failure was an increased risk of coronary obstruction by the bioprosthetic leaflets of the failing valve, which was observed in 10.9% of patients screened (Supplemental Table 1). Enrolled patients underwent ViV TAVI between January 2017 and June 2019. The procedure was attempted in 97 patients, and valves were implanted in 97 patients; 92 patients were available for 1-year follow-up (Figure 2).
Figure 2.
Study flow and patient disposition for the PARTNER 3 Aortic Valve-in-Valve Study. The PARTNER 3 Aortic Valve-in-Valve Registry for failing surgical bioprosthesis enrolled a total of 100 patients at 29 sites between 2017 and 2019.
∗Detailed reasons for screen failures can be found in Supplemental Table 1.
†The same patient who missed their visit at 30 days also missed their 1-year visit.
Abbreviation: CMS, Centers for Medicare and Medicaid Services.
The preoperative clinical and echocardiographic characteristics and information about the initial valve are listed in Table 1. Most patients were male (79.4%), with a mean age of 67.1 ± 11.7 years and STS score of 2.9 ± 1.8%. The average STS score was lower for the 77 male patients (2.5 ± 1.6%) compared with the 20 female patients (4.6 ± 1.8%). A total of 42 patients (43.3%) were in NYHA class III/IV at baseline. Aortic valve area was 1.1 ± 0.5 cm2, mean gradient was 39.1 ± 18.2 mm Hg, and the rate of ≥moderate aortic regurgitation was 41.1%. Of previously implanted valves, 59.8% were Edwards Lifesciences, 10.3% were Medtronic, and 29.9% other manufacturers. The most common reason for native valve replacement was stenosis (46.4%), with the remainder of indications equally divided between regurgitation (26.8%) and a combination of regurgitation and stenosis (26.8%). A mean of 11.4 ± 4.0 years had elapsed since the implantation of the original bioprosthetic valve. Labeled surgical valve sizes were most commonly 23 to 25 mm (56.7%) or >25 mm (26.8%). Surgical valve true IDs were most commonly <23 mm or 23 to 25 mm (both 47.2%). Only 5 (5.6%) patients had a true ID >25 mm, and they were all male. Of the 20 female patients, no true IDs were >21 mm (mean true ID was 19.7 ± 1.3 mm). The average true ID for the 69 male patients with available values was 23.1 ± 1.9 mm.
Table 1.
Baseline clinical, hemodynamic, and valve characteristics
| Characteristics∗ | N = 97† |
|---|---|
| Baseline demographics and comorbidities | |
| Age, y | 67.1 ± 11.7 |
| Sex | |
| Male | 77 (79.4) |
| Female | 20 (20.6) |
| Body mass index (kg/m2) | 29.8 ± 6.2 |
| Body surface area, m2 | 2.1 ± 0.3 |
| Society of Thoracic Surgeons score, % | 2.9 ± 1.8 |
| New York Heart Association class | |
| I | 0 (0) |
| II | 55 (56.7) |
| III | 41 (42.3) |
| IV | 1 (1.0) |
| Coronary artery disease | 23 (23.7) |
| Prior coronary artery bypass grafting | 18/95† (18.9) |
| Prior myocardial infarction | 9 (9.3) |
| Prior stroke or cerebrovascular accident | 4 (4.1) |
| Peripheral vascular disease | 7 (7.2) |
| Diabetes | 20 (20.6) |
| Chronic obstructive pulmonary disease | 8/96† (8.3) |
| Pulmonary hypertension | 10 (10.3) |
| Creatinine level >2 mg/dL | 2 (2.1) |
| Overall frailty‡ | 0 (0) |
| Atrial fibrillation | 31 (32.0) |
| Permanent pacemaker or defibrillator | 10 (10.3) |
| Left bundle branch block | 12 (12.4) |
| Right bundle branch block | 17 (17.5) |
| Baseline echocardiography and computed tomography characteristics | |
| Mean gradient, mm Hg | 39.1 ± 18.2 (95†) |
| Aortic valve area, cm2 | 1.1 ± 0.5 (92†) |
| Left ventricular ejection fraction, % | 59.4 ± 10.6 (95†) |
| Left ventricular mass index, g/m2 | 131.7 ± 37.0 (96†) |
| Dimensionless velocity index | 0.2 ± 0.1 (93†) |
| Moderate mitral regurgitation | 13/93† (14.0) |
| ≥Moderate aortic regurgitation | 39/95† (41.1) |
| Previous valve information | |
| Years since previous valve implant to the index procedure | 11.4 ± 4.0 |
| Reason for valve replacement | |
| Regurgitation only | 26 (26.8) |
| Stenosis only | 45 (46.4) |
| Mixed | 26 (26.8) |
| Labeled surgical valve size | |
| <23 mm | 12 (12.4) |
| 23-25 mm | 55 (56.7) |
| >25 mm | 26 (26.8) |
| Other§ | 4 (4.1) |
| Surgical valve true internal diameter (ID)‖ | |
| <23 mm | 42/89† (47.2) |
| 23-25 mm | 42/89† (47.2) |
| >25 mm | 5/89† (5.6) |
| Surgical valve manufacturer | |
| Edwards Lifesciences | 58 (59.8) |
| Medtronic | 10 (10.3) |
| Sorin | 3 (3.1) |
| St. Jude Medical | 7 (7.2) |
| Other | 19 (19.6) |
Values are presented as n (%) or mean ± SD (n).
Denominator provided if data were not available for all 97 patients at baseline.
Overall frailty is defined if the subject meets 3 or more of the following criteria: grip strength <18 kg; 5 m walk test >6 s; serum albumin <35 g/L; Katz total score of 4 or less.
These were homografts without a site-specified labeled surgical valve size.
True IDs were derived from the valve-in-valve application that was developed by UBQO and Bapat et al.9
Procedure
Procedure success, defined as absence of procedural death (within 72 hours) and correct positioning of a single prosthetic heart valve into the proper anatomical location, was 100% (Table 2). A majority of cases (68.0%) were performed under conscious sedation, and no patients underwent concomitant procedures. Balloon postdilation was performed in 29 patients (29.9%). Participating sites were advised against intentional fracture of the sewing ring because evidence was insufficient to support widespread adoption of this practice when the study was initiated, and long-term efficacy is still unknown3,11; however, valve fracture or remodeling was suspected in 4 of the postdilations that used a high-pressure balloon. All patients were discharged to home.
Table 2.
Procedural and hospital findings
| Outcomes∗ | N = 97† |
|---|---|
| Conscious sedation | 66 (68.0) |
| Procedure time, min | 61.9 ± 50.3 |
| Fluoroscopy time, min | 18.8 ± 14.8 (95†) |
| Balloon aortic valvuloplasty | 5 (5.2) |
| Postdilatation | 29 (29.9) |
| Intensive care unit stay, d | 1.0 (0, 2.0) |
| Total length of stay, d | 2.0 (2.0, 3.0) |
| Discharge to home/self-care | 97 (100) |
| Concomitant procedures | 0 (0) |
| Procedure success‡ | 97 (100) |
Values are presented as n (%), mean ± SD (n), or median (IQR).
Denominator provided if data were not available for all 97 patients.
Procedure success was protocol defined as absence of procedural mortality (within 72 hours) and correct positioning of a single prosthetic heart valve into the proper anatomical location.
Endpoints
Kaplan-Meier rates for the primary outcome (composite of all-cause mortality or stroke) were 1.0% (n = 1) at 30 days and 2.1% (n = 2) at 1 year (Figure 3). Both events were disabling strokes; no deaths occurred through 1 year.
Figure 3.
Kaplan-Meier estimates for the primary endpoint. Kaplan-Meier plot for the primary endpoint of all-cause mortality and stroke at 1 year.
Abbreviations: AViV, aortic valve-in-valve; P3, PARTNER 3.
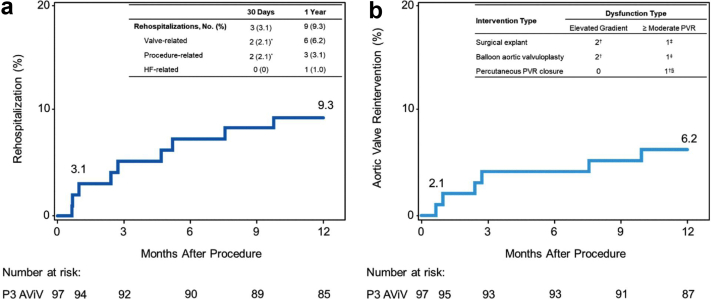
Among secondary endpoints, rehospitalization (valve related or procedure related, including heart failure) occurred in 9 patients (9.3%) through 1 year, 2 of which were procedure related for disabling strokes; 1 was for congestive heart failure (Figure 4a). The remaining 6 patients had valve-related rehospitalizations (Figure 4b and Table 3), requiring 7 aortic valve reinterventions: 3 surgical explants, 3 delayed balloon postdilations, and 1 percutaneous PVR closure with a vascular plug. At 1 year, there was no significant difference in aortic valve reintervention by sex (4 [5.2%] male patients vs. 2 [10.0%] female patients; p = 0.43).
Figure 4.
Kaplan-Meier estimates for occurrence of rehospitalization and AV reintervention. Kaplan-Meier plot for (a) rehospitalization and (b) AV reintervention through 1 year. Rehospitalization was defined as valve related or procedure related, including heart failure. All valve-related rehospitalizations through 1 year were for AV reinterventions. A total of 6 patients (2 female and 4 male) had 7 AV reinterventions.
∗1 patient’s event was classified as both valve related and procedure related.
†Three of 6 patients with AV reinterventions had valve thrombosis.
‡Patient had a prior balloon aortic valvuloplasty for PVR that was unsuccessful.
§Patient had a PVR closure for symptomatic PVR.
Abbreviations: AV, aortic valve; AViV, aortic valve-in-valve; HF, heart failure; P3, PARTNER 3; PVR, paravalvular regurgitation.
Table 3.
Additional outcomes of interest
| Outcomes∗ | 30 d | 1 y |
|---|---|---|
| Bleeding—life-threatening† | 0 (0) | 2 (2.1) |
| Major vascular complications | 0 (0) | 0 (0) |
| Acute kidney insufficiency—stage 2 or 3 | 0 (0) | NA |
| New permanent pacemaker insertion | 1 (1.1) | 2 (2.3) |
| New onset atrial fibrillation | 1 (1.5) | 1 (1.5) |
| Left bundle branch block (new or worsening) | 1 (1.0) | 3 (3.1) |
| Right bundle branch block (new or worsening) | 1 (1.0) | 2 (2.1) |
| Coronary obstruction requiring intervention‡ | 1 (1.0) | 1 (1.0) |
| Aortic valve reintervention | 2 (2.1) | 6 (6.2) |
| Endocarditis | 0 (0) | 0 (0) |
| Valve thrombosis§ | 1 (1.0) | 5 (5.2) |
Kaplan-Meier rates are presented as n (%).
One of the life-threatening bleeds was a procedure-related tear of the aorta during redo-surgery; the other was a nonprocedure–related gastric bleed (both discharged in stable condition).
During reintervention for elevated mean gradient, left main obstruction by leaflet of prior surgical valve discovered (left main coronary height: 7.4 mm; right coronary artery height: 7.6 mm; sinutubular junction height: 16.2 mm).
None of the patients with confirmed valve thrombosis had a stroke.
There were 5 patients (5.2%) who had valve thrombosis events through 1 year (Table 3). Evidence of thrombosis was detected via CT in 3 patients, via transesophageal echocardiography during a PVR closure in 1 patient, and visually during an explant in 1 patient (Supplemental Table 2). All 5 patients received anticoagulation and experienced resolution of elevated gradients; none were associated with death or stroke.
Echocardiographic outcomes per core laboratory were available in 86 patients at 1-year follow-up (Table 4). The 3 patients who had surgical explants were censored. The mean gradient was 22.5 ± 8.8 mm Hg at 30 days and 19.7 ± 7.6 mm Hg at 1 year. At baseline, 41.1% of patients had ≥moderate aortic regurgitation; at 30 days and 1 year, aortic regurgitation was either absent or mild in 98.9% of patients (Figure 5a). Moderate or greater PVR was observed in 1 patient (1.2%) at 1 year. It is possible that this was PVR around the surgical valve that was mistaken for central aortic regurgitation at screening because of the eccentric nature of the patient’s PVR jet (Figure 5b).
Table 4.
Hemodynamic outcomes
| Outcomes∗ | Baseline | 30 d echo | p value† (Δ baseline to 30 d) | 1 y echo | p value† (Δ baseline to 1 y) |
|---|---|---|---|---|---|
| Mean gradient, mm Hg | 39.1 ± 18.2 (95) | 22.5 ± 8.8 (94) | <0.0001 | 19.7 ± 7.6 (86) | <0.0001 |
| Peak gradient, mm Hg | 65.2 ± 28.0 (95) | 39.0 ± 14.3 (94) | <0.0001 | 34.5 ± 12.8 (86) | <0.0001 |
| Aortic valve area, cm2 | 1.1 ± 0.5 (92) | 1.3 ± 0.3 (92) | <0.0001 | 1.4 ± 0.3 (81) | <0.0001 |
| Left ventricular ejection fraction, % | 59.4 ± 10.6 (95) | 58.1 ± 10.6 (92) | 0.12 | 60.8 ± 9.6 (80) | 0.15 |
| Dimensionless velocity index | 0.25 ± 0.10 (93) | 0.31 ± 0.06 (89) | <0.0001 | 0.32 ± 0.06 (80) | <0.0001 |
All values presented as mean ± SD (n). Echocardiography was not available for all patients with follow-up through 1 year. Of available echocardiography, not all hemodynamic outcomes were evaluable. The 3 patients with explanted valves were censored through 1 year.
p values from paired t-tests.
Figure 5.
Incidence of aortic and paravalvular regurgitation. Core laboratory adjudicated data for (a) total aortic regurgitation (includes paravalvular regurgitation) and (b) paravalvular regurgitation at 1 year. The 3 patients with explanted valves were censored at 1 year.
The mean gradients were also evaluated by true ID. Patients were grouped by large (>25 mm), medium (23-25 mm), and small (<23 mm) surgical valve true IDs at baseline. Patients with large true IDs had significantly lower mean gradients at 30 days (15.9 ± 4.6 mm Hg [n = 5]; p < 0.01) and at 1 year (17.0 ± 4.5 mm Hg [n = 5]; p = 0.04) compared with patients with medium (21.3 ± 8.3 mm Hg [n = 39] at 30 days; 18.4 ± 7.4 mm Hg [n = 36] at 1 year) and small (25.3 ± 9.3 mm Hg [n = 42] at 30 days; 22.0 ± 8.0 mm Hg [n = 38] at 1 year) surgical valve true IDs.
Table 5 compares left ventricular mass regression for patients with a mean gradient <20 mm Hg at 1 year (n = 51) to those with a mean gradient ≥20 mm Hg at 1 year (n = 35). For patients with mean gradients <20 mm Hg, left ventricular mass was significantly reduced by 8.6% from baseline to 30 days (p < 0.001) and 17.9% (p < 0.0001) from baseline to 1 year. Similarly, left ventricular mass was significantly reduced for patients with mean gradients ≥20 mm Hg from baseline to 30 days (10.2%; p = 0.02) and baseline to 1 year (19.2%; p < 0.001).
Table 5.
Left ventricular mass regression by mean gradient at 1 year
| Left ventricular mass∗, g | Mean gradient at 1 y |
|||||
|---|---|---|---|---|---|---|
| Total (N = 86) | p value† (Δ from baseline) | <20 mm Hg (N = 51) | p value† (Δ from baseline) | ≥20 mm Hg (N = 35) | p value† (Δ from baseline) | |
| Baseline | 275.7 ± 88.0 (85) | 281.2 ± 93.6 (51) | 267.4 ± 79.5 (34) | |||
| 30 d | 251.4 ± 73.6 (85) | <0.0001 | 257.0 ± 81.2 (51) | <0.001 | 243.1 ± 60.6 (34) | 0.02 |
| 1 y | 226.8 ± 66.4 (84) | <0.0001 | 231.9 ± 72.4 (50) | <0.0001 | 219.3 ± 56.7 (34) | <0.001 |
All values presented as mean ± SD (n). Left ventricular mass could not be derived for all patients with available echocardiography through 1 year. The 3 patients with explanted valves were censored for 1 year comparison.
p values from paired t-tests.
Functional assessments included NYHA class and KCCQ Overall Summary score. At baseline, 43.3% of patients were in NYHA class III or IV. At 30 days, 98.9% of patients had improved to class I or II, which was maintained in 95.5% of patients at 1 year (Figure 6). The KCCQ Overall Summary score increased from 60.7 at baseline to 86.3 at 30 days and 88.8 at 1 year (p < 0.0001 for change from baseline to 30 days and 1 year). The 6-minute walk test increased from 321.1 m at baseline to 359.7 m at 30 days (p = 0.01) and 350.2 m at 1 year (p = 0.10).
Figure 6.
NYHA class through 1 year. Most patients had improved NYHA class from baseline to 1 year. The 3 patients with explanted valves were censored at 1 year.
Abbreviation: NYHA, New York Heart Association.
Discussion
At 30 days and 1 year, TAVI for BVF was associated with no mortality, improved valve hemodynamic status, and sustained excellent functional and quality of life outcomes. Valve thrombosis was observed in 5.2% of patients, and the need for aortic valve reintervention for transcatheter heart valve dysfunction was 6.2%. The early improvements associated with aortic ViV during the first 30 days after implantation were maintained for 1 year, with planned annual follow-up for 10 years. Comparative studies have demonstrated similar safety endpoints of mortality and morbidity between aortic ViV and reoperative AVR. The global Valve-in-Valve International Data registry showed that the survival rate at 1 year was 83.2% after the replacement of failed bioprosthetic aortic valves.6 A systematic review of clinical studies comparing aortic ViV with reoperative AVR found that transcatheter ViV implantation achieves similar operative mortality but lower risk of strokes and bleeding compared with reoperation.12 This study affirms the safety of aortic ViV in low- or intermediate-risk patients with BVF.
In terms of hemodynamic outcomes, differences between aortic ViV and reoperative AVR are less clear. Transcatheter ViV may achieve similar hemodynamic outcomes but is associated with higher PVR rates than reoperation.12 Recent comparative studies show that gradients after aortic ViV are comparable with reoperative AVR and are consistent with previous aortic ViV trials in high-risk patients.7,8,13 The resultant transvalvular gradient after aortic ViV is lower than baseline in all patients; and at 1 year, left ventricular mass regression was seen in all patients and was not impacted by residual gradients. Notably, the degree of improvement is dependent on the size of the original prosthetic. Larger prosthetics (true ID >25 mm) were associated with lower postprocedural gradient; and although the hemodynamic performance of smaller valves (true ID < 23 mm) was still favorable in our registry, aortic ViV may not be appropriate for patients with small bioprosthetic valves.14 Balloon valve fracture has emerged as a technique to improve postprocedural hemodynamic performance after ViV15 but with unknown long-term consequences. Our study did not allow for balloon valve fracture and this may explain why smaller prosthetics had a higher postprocedural gradient. Despite higher gradients in some patients, there was similar and significant left ventricular mass regression compared with patients with lower gradients.
As aortic ViV becomes more frequent and the demand for a bioprosthetic in younger patients increases, surgical planning at the time of the original AVR is increasingly important. If a bioprosthetic is placed in a younger patient who may outlive the prosthetic, a root enlargement to allow for placement of a prosthetic with the largest ID may be performed selectively. As the ID varies in prosthetics of the same valve size, the surgical prosthetic with the largest ID should be considered. The use of a prosthetic with an expandable valve frame (Inspiris Resilia, Edwards Lifesciences, Orange County, CA, USA) may be another alternative to facilitate ViV in the future. Planning for future ViV may also require consideration of the relationship of coronary heights to the surgical posts to minimize the risk of coronary obstruction, which was the most common reason for screen failure in this study. Screen failures are an impediment of valve choice today but may be mitigated by procedures such as BASILICA.16,17
Valve thrombosis event rates were higher than those reported in previous low-risk TAVI trials.18,19 The 1-year outcomes of the PARTNER 3 main trial18 detailed 5 cases (1.0%) of valve thrombosis in the TAVI cohort, none of which were associated with clinical sequelae. In this study, there were 5 events (5.2%) that were subclinical and were not associated with the primary endpoint of all-cause death or stroke. The routine use of anticoagulants or other antithrombotics remains an area of investigation, but anticoagulation has been shown to be effective in treating valve thrombosis when present.20 No comparative data on valve thrombosis are available for reoperative AVR.
This study has helped us understand the risk of early aortic valve reintervention in aortic ViV. Smaller valve sizes appear to be at most risk of significant stenosis and should be considered in patient selection. However, aortic valve reinterventions did not result in any mortality or significant morbidity in short-term follow-up. Longer term follow-up is required to determine the impact of residual valve gradients and valve thrombosis on durability and the risk of aortic valve reintervention.
Study Limitations
There are several limitations in this multicenter, prospective study that need to be addressed. First, these results reflect only 1-year outcomes; long-term assessment of structural valve deterioration is needed. A 10-year clinical and echocardiographic follow-up is planned in all patients. In addition, this was a controlled, single-arm, clinical study with no comparator group, so our results apply only to the highly-selected enrolled population who met all inclusion and exclusion criteria (39.4% of subjects screened were not eligible for the study), and the 1-year outcomes reported here may not be generalizable to other patients with BVF. Furthermore, postsurgical mean gradients were not available, so it is possible some patients may have been included with patient-prosthesis mismatch and/or high gradients.
Another limitation is that the vast majority of patients enrolled (79.4%) were male. One possible reason for this is that female patients have a higher surgical risk profile. This has led to a larger proportion of women enrolled in extreme- and high-risk TAVI trials than men; the opposite occurred in low-risk trials.18,19,21, 22, 23 In addition, criteria for inclusion (true surgical valve ID of 16.5-28.5 mm) and exclusion (known bioprosthesis with residual mean gradient >20 mm Hg at the end of the index surgical procedure) were more likely to exclude patients with small annuli, which likely would exclude more females than males. Finally, the number of women undergoing surgical AVR in large consecutive series ranges from 27% to 34%, raising the possibility of disproportionate access to care.24,25 Additional studies with a larger sample size and more balanced distribution or a dedicated cohort are needed in female patients, particularly considering the small size aortic annulus in this patient population.
Conclusions
Transcatheter aortic ViV with a balloon-expandable transcatheter heart valve in failed surgical bioprostheses is safe in patients at low to intermediate risk for reoperative AVR. The procedure is associated with improved hemodynamic status and functional improvement. Residual valve gradients and valve thrombosis remain concerns for durability and need for aortic valve reintervention. Aortic ViV is an additional therapeutic option in selected, low- to intermediate-risk patients with failed bioprosthetic aortic valves, but comparative effectiveness studies with reoperative AVR with longer term follow-up and studies in smaller bioprosthetic valves and female patients are required.
Ethics Statement
The research reported has adhered to all relevant ethical guidelines. The Institutional Review Boards of all participating sites approved the study protocol before patient enrollment in compliance with the Declaration of Helsinki, and written, informed consent was obtained for all patients.
Funding
The PARTNER 3 Aortic Valve-in-Valve Study was sponsored by Edwards Lifesciences, Irvine, California.
Disclosure statement
Dr Malaisrie discloses a financial relationship with Edwards Lifesciences, Medtronic, Abbott, Terumo, Cryolife, and Baxter. Dr Zajarias discloses a financial relationship with Edwards Lifesciences, Medtronic, Boston Scientific, and Admedus. Dr Leon discloses a financial relationship with Edwards Lifesciences, Medtronic, Boston Scientific, Abbott, Gore & Associates, and Meril Lifescience. Dr Mack discloses a financial relationship with Edwards Lifesciences, Abbott, and Medtronic. Dr Pibarot discloses a financial relationship with institutional Echocardiography Core Lab agreements with Edwards Lifesciences. Dr Hahn discloses a financial relationship with Edwards Lifesciences, Philips Healthcare, Abbott Structural, Boston Scientific, Gore & Associates, Medtronic, and Navigate. Dr Wong discloses a financial relationship with Medtronic, Boston Scientific, Edwards Lifesciences, Abbott, and Boston Scientific. Dr Oldemeyer has no financial relationships to disclose. Dr Shang is an employee of Edwards Lifesciences. Dr Leipsic is a consultant and holds stocks options in CIRCLE CVI and holds institutional core laboratory agreements with Edwards Lifesciences, Medtronic, Abbott, Boston Scientific, MVRX, and Pi Cardia. Dr Blanke discloses a financial relationship with CIRCLE CVI, Edwards Lifesciences, Medtronic, Abbott, Boston Scientific, and Gore & Associates. Dr Guerrero discloses a financial relationship with Edwards Lifesciences and Abbott. Dr Brown has nothing to disclose.
Acknowledgments
Marissa Gunnarsson, an employee of Edwards Lifesciences, and Gayle Scott, a former employee of Edwards Lifesciences, created the tables and figures and provided technical review of the article.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Capodanno D., Petronio A.S., Prendergast B., et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2017;52(3):408–417. doi: 10.1093/ejcts/ezx244. [DOI] [PubMed] [Google Scholar]
- 2.Dvir D., Bourguignon T., Otto C.M., et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137(4):388–399. doi: 10.1161/CIRCULATIONAHA.117.030729. [DOI] [PubMed] [Google Scholar]
- 3.Edelman J.J., Khan J.M., Rogers T., et al. Valve-in-valve TAVR: state-of-the-art review. Innovations (Phila) 2019;14(4):299–310. doi: 10.1177/1556984519858020. [DOI] [PubMed] [Google Scholar]
- 4.Otto C.M., Nishimura R., Bonow R., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 6.Dvir D., Webb J.G., Bleiziffer S., et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312(2):162–170. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- 7.Deeb G.M., Chetcuti S.J., Reardon M.J., et al. 1-Year results in patients undergoing transcatheter aortic valve replacement with failed surgical bioprostheses. JACC Cardiovasc Interv. 2017;10(10):1034–1044. doi: 10.1016/j.jcin.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Webb J., Murdoch D., Alu M., et al. 3-Year outcomes after valve-in-valve transcatheter aortic valve replacement for degenerated bioprostheses. J Am Coll Cardiol. 2019;73(21):2647–2655. doi: 10.1016/j.jacc.2019.03.483. [DOI] [PubMed] [Google Scholar]
- 9.Bapat V.N., Attia R., Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve. JACC Cardiovasc Interv. 2014;7(2):115–127. doi: 10.1016/j.jcin.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Blanke P., Weir-McCall J.R., Achenbach S., et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR) JACC Cardiovasc Imaging. 2019;12(1):1–24. doi: 10.1016/j.jcmg.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Allen K.B., Chhatriwalla A.K., Cohen D.J., et al. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann Thorac Surg. 2017;104(5):1501–1508. doi: 10.1016/j.athoracsur.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Phan K., Zhao D.-F., Wang N., Huo Y.R., Di Eusanio M., Yan T.D. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: a systematic review. J Thorac Dis. 2016;8(1):E83–E93. doi: 10.3978/j.issn.2072-1439.2016.01.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeling W.B., Beckerman Z., Wei J., Binongo J., Leshnower B.G., Chen E.P. Benchmarking outcomes: reoperation for aortic valve patient-prosthesis mismatch. Ann Thorac Surg. 2021;111(5):1472–1477. doi: 10.1016/j.athoracsur.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Bonow R., Brown A., Gillam L., et al. ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS 2017 appropriate use criteria for the treatment of patients with severe aortic stenosis. J Am Coll Cardiol. 2017;70(20):2566–2598. doi: 10.1016/j.jacc.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Allen K.B., Chhatriwalla A.K., Saxon J.T., et al. Bioprosthetic valve fracture: technical insights from a multicenter study. J Thorac Cardiovasc Surg. 2019;158(5):1317–1328.e1. doi: 10.1016/j.jtcvs.2019.01.073. [DOI] [PubMed] [Google Scholar]
- 16.Lederman R.J., Babaliaros V.C., Rogers T., et al. Preventing coronary obstruction during transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12(13):1197–1216. doi: 10.1016/j.jcin.2019.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark A., Malaisrie S.C. Failed bioprosthetic valve approaches: transcatheter aortic valve replacement approach. J Thorac Cardiovasc Surg. 2022;163(5):1795–1798. doi: 10.1016/j.jtcvs.2020.12.148. [DOI] [PubMed] [Google Scholar]
- 18.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 19.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 20.Makkar R.R., Fontana G., Jilaihawi H., et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373(21):2015–2024. doi: 10.1056/NEJMoa1509233. [DOI] [PubMed] [Google Scholar]
- 21.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 22.Popma J.J., Adams D.H., Reardon M.J., et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972–1981. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 23.Feldman T.E., Reardon M.J., Rajagopal V., et al. Effect of mechanically expanded vs self-expanding transcatheter aortic valve replacement on mortality and major adverse clinical events in high-risk patients with aortic stenosis: the REPRISE III randomized clinical trial. JAMA. 2018;319(1):27. doi: 10.1001/jama.2017.19132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaker Z., Badhwar V., Alqahtani F., et al. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. 2017;6(9) doi: 10.1161/JAHA.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrei A.-C., Yadlapati A., Malaisrie S.C., et al. Comparison of outcomes and presentation in men-versus-women with bicuspid aortic valves undergoing aortic valve replacement. Am J Cardiol. 2015;116(2):250–255. doi: 10.1016/j.amjcard.2015.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.