Abstract
Background
There are numerous risk-prediction models applied to acute myocardial infarction–related cardiogenic shock (AMI-CS) patients to determine a more accurate prognosis and to assist in patient triage. There is wide heterogeneity among the risk models including the nature of predictors evaluated and their specific outcome measures. The aim of this analysis was to evaluate the performance of 20 risk-prediction models in AMI-CS patients.
Methods
Patients included in our analysis were admitted to a tertiary care cardiac intensive care unit with AMI-CS. Twenty risk-prediction models were computed utilizing vitals assessments, laboratory investigations, hemodynamic markers, and vasopressor, inotropic and mechanical circulatory support available from within the first 24 hours of presentation. Receiver operating characteristic curves were used to assess the prediction of 30-day mortality. Calibration was assessed with a Hosmer-Lemeshow test.
Results
Seventy patients (median age 63 years, 67% male) were admitted between 2017 and 2021. The models' area under the curve (AUC) ranged from 0.49 to 0.79, with the Simplified Acute Physiology Score II score having the most optimal discrimination of 30-day mortality (AUC: 0.79, 95% confidence interval [CI]: 0.67-0.90), followed by the Acute Physiology and Chronic Health Evaluation-III score (AUC: 0.72, 95% CI: 0.59-0.84) and the Acute Physiology and Chronic Health Evaluation-II score (AUC: 0.67, 95% CI: 0.55-0.80). All 20 risk scores demonstrated adequate calibration (p > 0.05 for all).
Conclusions
Among the models tested in a data set of patients admitted with AMI-CS, the Simplified Acute Physiology Score II risk score model demonstrated the highest prognostic accuracy. Further investigations are required to improve the discriminative capabilities of these models or to establish new, more streamlined and accurate methods for mortality prognostication in AMI-CS.
Keywords: Acute myocardial infarction cardiogenic shock, Cardiogenic shock, Mortality, Risk prediction, Risk calculator
Introduction
Acute myocardial infarction–related cardiogenic shock (AMI-CS) is characterized by end-organ hypoperfusion as a result of a low cardiac output due to loss of contracting myocardium. This deficiency in end-organ perfusion is often characterized by hypotension, tachycardia, peripheral vasoconstriction, pulmonary and systemic venous congestion, decreased urine output, altered sensorium, acute kidney or liver injury, and lactic acidosis.1 Randomized clinical trials since the 1990s have consistently reported mortality rates between 40% and 60% for patients in AMI-CS.2, 3, 4, 5, 6 Despite the SHOCK trial (Should We Emergently Revascularize Occluded Coronaries for CS), which demonstrated survival benefits from early revascularization in AMI-CS, and the further advancements of primary percutaneous coronary interventions, AMI-CS mortality has remained high.3,7 In recent years, many tertiary care centers have developed treatment teams and algorithms specific for AMI-CS patients with promising results.8,9 Nevertheless, despite advances in reperfusion strategies and hemodynamic support, delayed recognition and regional disparities in care remain fundamental challenges in the treatment of AMI-CS.4,10 As such, tools that lead to effective, early risk stratification of AMI-CS patients may help guide therapy and ultimately reduce mortality.
There is wide heterogeneity in the studies that have evaluated predictors of mortality for AMI-CS; these studies vary on multiple levels including patient population, the nature of the predictors evaluated, and therapies utilized.11 Several scoring systems have been validated to predict the mortality of patients in medical intensive care units.12, 13, 14, 15 Attempts have been made to apply these scoring systems to a wide variety of medical indications including severe trauma, abdominal pathology, chronic obstructive pulmonary disease, acute pancreatitis, sepsis, and postcardiac surgery care.16, 17, 18, 19, 20, 21 A few of these risk scores, namely the Acute Physiology and Chronic Health Evaluation-II (APACHE-II), Acute Physiology and Chronic Health Evaluation-III (APACHE-III), and Simplified Acute Physiology Score II (SAPS-II) models, have been applied to the AMI-CS population and have demonstrated adequate predictive capacities albeit with varying individual results.22, 23, 24 Over the past decade, there has been elaboration of risk scores specific to the AMI-CS population. These risk scores, however, have not been robustly evaluated for their comparative prognostic performance and, at times, include measures that are not readily available at the time of patient presentation. As such, this makes their relative uses in clinical practice challenging.
The aim of this analysis was to compare the predictive performance of a number of risk models in assessing the risk of 30-day, all-cause mortality in patients with AMI-CS treated at our medical center.
Methods
Patient Population
This was a retrospective study including patients who presented with AMI-CS to the Columbia University Irving Medical Center between 2017 and 2021. This study was approved by the Columbia institutional review board. As the study was retrospective, no informed consent was required. Clinical information including vital signs, invasive hemodynamic recordings, the use of inotropic or vasopressor support, the use of mechanical circulatory support, and laboratory investigations were used to identify patients with AMI-CS. Twenty risk-prediction models, listed in Supplemental Table 1 with their respective component variables, were calculated and assessed: APACHE-II, APACHE-III, APACHE-IV, Sequential Organ Failure Assessment (SOFA), SAPS-II, Morrow model, Global Registry of Acute Coronary Events Score, Zwolle model, Primary Angioplasty for the Treatment of Acute ST Elevated Myocardial Infarction Model, Klein model, Thrombolysis in ST-Elevation in Myocardial Infarction Score, Thrombolysis in Non-ST Elevation in Myocardial Infarction Score, Pathophysiology and Prognostic Factors in Cardiogenic Shock Score, Intraaortic Balloon Pump in Cardiogenic Shock Score, Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications, Assessment of Pexelizumab in Acute Myocardial Infarction, Modified Shock Index, SHOCK, Prediction of Cardiogenic Shock Outcome for AMI patients salvaged by Veno-Arterial Extracorporeal Membrane Oxygenation Score, and the Inova Heart and Vascular Institute score.12,13,15,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 The data entered into the risk score models were from the date of patient presentation.
Outcomes
The primary endpoint of our analysis was 30-day mortality. The secondary endpoints examined were 90-day and 1-year mortality.
Statistical Methods
Categorical variables are reported as frequencies and percentages; statistical differences were analyzed using chi-squared or Fisher exact test as appropriate. Continuous variables are reported as mean and standard deviation; statistical differences were analyzed using Student’s t-test. Receiver operating characteristic curves were constructed for each of the risk scores to assess the discriminative power of the scores for 30-day mortality prediction and to compare them. The calibration of the risk scores was assessed using the Hosmer-Lemeshow goodness-of-fit test. For all statistical tests, a p value of <0.05 was considered statistically significant. All analyses were performed using Stata version 12.1 (Stata Corp, College Station, Texas).
Results
Baseline Characteristics
The study cohort included a total of 70 patients with AMI-CS. Baseline characteristics are demonstrated in Table 1. The median age was 63 years (interquartile range 55-72), and 67.1% were male. The median body mass index was 31 (interquartile range 26-34). Of all the patients in the cohort, 78.6% had been previously diagnosed with hypertension, 67.1% with hyperlipidemia, and 48.6% with diabetes; 22.9% of the cohort had sustained a prior myocardial infarction.
Table 1.
Overall patient characteristics by 30-day survival vs. 30-day mortality
| Characteristics | All patients, N = 70 | 30-D survival group, N = 38 | 30-D mortality group, N = 32 | p Value |
|---|---|---|---|---|
| Age, y | 63 (55-72) | 62 (53-68) | 66 (60-73) | 0.47 |
| Male | 47 (67.1%) | 27 (75%) | 20 (62.5%) | 0.46 |
| BMI | 31.0 (26.5-34.0) | 31.0 (26.1-33.8) | 30.1 (26.8-34.5) | 0.54 |
| Hypertension | 55 (78.6%) | 27 (75.0%) | 28 (87.5%) | 0.09 |
| Hyperlipidemia | 47 (67.1%) | 24 (66.7%) | 23 (71.9%) | 0.44 |
| Diabetes | 34 (48.6%) | 15 (41.7%) | 19 (59.4%) | 0.10 |
| CAD | 36 (51.4%) | 16 (44.4%) | 20 (62.5%) | 0.09 |
| CKD | 10 (14.3%) | 2 (5.6%) | 8 (25.0%) | <0.05 |
| Prior MI | 16 (22.9%) | 8 (22.2%) | 8 (25.0%) | 0.70 |
| Prior CABG | 8 (11.4%) | 1 (2.8%) | 7 (21.9%) | <0.05 |
| CCU length of stay, d | 17 (7-25) | 19 (12-34) | 8 (4-19) | <0.001 |
| Hospitalization length, d | 20 (8-35) | 34 (18-45) | 8 (4-19) | <0.001 |
| Cardiac arrest at admission | 27 (41.5%) | 15 (41.7%) | 13 (40.6%) | 0.92 |
| STEMI at admission | 42 (60.0%) | 26 (76.5%) | 16 (50.0%) | 0.12 |
| Anterior MI at admission | 36 (51.4%) | 22 (61.1%) | 14 (43.8%) | 0.25 |
| Total proximal LAD at admission | 20 (28.6%) | 13 (36.1%) | 7 (21.9%) | 0.26 |
| Vasopressor/inotropic support | 65 (92.9%) | 35 (97.2%) | 30 (93.8%) | 0.40 |
| Any MCS | 67 (95.7%) | 36 (94.7%) | 31 (96.9%) | 0.16 |
| IABP support | 27 (38.6%) | 20 (55.6%) | 7 (21.9%) | <0.01 |
| Impella support | 42 (60.0%) | 19 (52.8%) | 23 (71.9%) | <0.05 |
| ECMO | 51 (72.9%) | 24 (66.7%) | 27 (84.4%) | <0.05 |
| Ventilation | 53 (75.7%) | 28 (77.8%) | 25 (78.1%) | 0.50 |
Notes. All data are presented as n (%) or median (interquartile range).
BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCU, coronary care unit; CKD, chronic kidney disease; ECMO, extracorporeal membrane oxygenation; IABP, intraaortic balloon pump; LAD, left anterior descending artery; MCS, mechanical circulatory support; MI, myocardial infarction; STEMI, ST-elevation myocardial infarction.
Primary Outcome: 30-Day Mortality
The 30-day mortality was 46% (n = 32). In an unadjusted analysis, patients with 30-day mortality had a higher incidence of chronic kidney disease (8 vs. 2 patients, p < 0.05) and were more likely to have had prior coronary artery bypass grafting (7 vs. 1, p < 0.05). Additionally, they were more likely to receive a percutaneous transvalvular left ventricular assist device (Impella) (23 vs. 19, p < 0.05) and/or extracorporeal membrane oxygenation (27 vs. 24, p < 0.05) than those who survived.
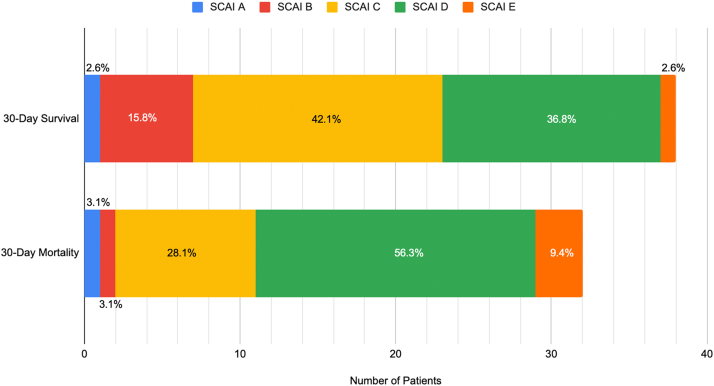
There were no significant differences between those who survived versus those who died in terms of the need for a vasopressor or inotropic support, rates of mechanical ventilation, incidence of ST-elevation myocardial infarction, or cardiac arrest at the time of presentation. When patients were stratified by SCAI classification at the time of presentation, a larger percentage of patients who survived to 30 days presented with SCAI shock classification A, B, or C compared with those who died by 30 days. As seen in Figure 1, a majority of patients who died by 30 days presented with SCAI shock classification D or E.43
Figure 1.
Thirty-day outcomes by SCAI classification. A larger percentage of patients who survived up to 30 days presented with SCAI Shock classification A–C compared to who died. Of those with 30-day mortality, the majority presented with SCAI Shock classification D or E. SCAI stage classification was determined post hoc based on the parameters defined by the most recent expert consensus review statement.42
Abbreviation: SCAI, Society of Cardiovascular Angiography and Interventions.
In terms of risk scores, as shown in Table 2, patients who died by 30 days had significantly higher APACHE II (15 vs. 12, p < 0.05), APACHE III (68 vs. 52, p < 0.01), SAPS-II (49 vs. 41, p < 0.001), SOFA (6 vs. 4, p < 0.05), Morrow (41.3 vs. 34.5, p < 0.05), Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (13 vs. 11, p < 0.05), Assessment of Pexelizumab in Acute Myocardial Infarction (152 vs. 133, p < 0.01), SHOCK Trial (43 vs. 29, p < 0.05), Intraaortic Balloon Pump in Cardiogenic Shock Score II (5 vs. 3, p < 0.05), and APACHE IV (78 vs. 78, p < 0.05) scores.
Table 2.
Risk scores by 30-day survival vs. 30-day mortality
| Risk model | Score bounds | All patients, N = 70 | 30-D survival group, N = 38 | 30-D mortality group, N = 32 | p Value |
|---|---|---|---|---|---|
| APACHE II | 0 to 71 | 14 (11-17) | 12 (10-15) | 15 (12-19) | <0.05 |
| APACHE III | 0 to 299 | 64 (48-72) | 52 (45-63) | 68 (51-74) | <0.01 |
| SAPS-II | 0 to 163 | 45 (37-50) | 41 (36-45) | 49 (45-54) | <0.001 |
| SOFA | 0 to 24 | 5 (3-6) | 4 (2-6) | 6 (4-7) | <0.05 |
| TIMI-STEMI | 0 to 14 | 7 (6-9) | 7 (6-8) | 8 (6-9) | 0.10 |
| TIMI-NSTEMI | 0 to 7 | 4 (3-5) | 4 (3-5) | 4 (3-5) | 0.50 |
| Morrow model | 0 to 100 | 37.1 (25.4-48.5) | 34.5 (24.3-44.1) | 41.3 (30.1-51.3) | <0.05 |
| GRACE | 1 to 372 | 223 (198.5-246) | 216 (198-246) | 222 (200-250) | 0.68 |
| Zwolle et al. | 0 to 16 | 13 (12-14) | 12 (11-13) | 13 (12 -14) | 0.20 |
| PAMI | 0 to 15 | 6 (4-7) | 6 (4-8) | 7 (4-9) | 0.72 |
| Klein et al. | 0 to 262 | 75 (50-99) | 75 (50-100) | 75 (68-100) | 0.11 |
| CADILLAC | 0 to 18 | 13 (11-14) | 11 (9-13) | 13 (10-15) | <0.05 |
| APEX-AMI | 0 to 239 | 152 (125-163) | 133 (105-155) | 152 (135-168) | <0.01 |
| Modified Shock Index | 0 to 3 | 1.27 (0.9-1.4) | 1.1 (0.9-1.4) | 1.2 (1.0-1.4) | 0.20 |
| CARD-SHOCK | 0 to 9 | 4 (3-5) | 4 (3-5) | 4 (3-5) | <0.05 |
| SHOCK Trial | 0 to 102 | 40 (25-55) | 29 (23-48) | 43 (28-63) | <0.05 |
| ENCOURAGE | 0 to 45 | 19 (17-24) | 19 (14-24) | 22 (19-24) | 0.07 |
| IABP-SHOCK II | 0 to 8 | 5 (3-5) | 3 (2-5) | 5 (4-6) | <0.05 |
| INOVA | 0 to 10 | 5 (3-6) | 4 (2-6) | 5 (3-6) | 0.09 |
| APACHE IV | 0 to 286 | 78 (72-78) | 78 (54-78) | 78 (78-78) | <0.05 |
Notes. All data are presented as median (interquartile range).
APACHE-II, Acute Physiology and Chronic Health Evaluation-II; APACHE-III, Acute Physiology and Chronic Health Evaluation-III; APACHE-IV, Acute Physiology and Chronic Health Evaluation-IV; APEX-AMI, Assessment of Pexelizumab in Acute Myocardial Infarction; CADILLAC, Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications; CARD-SHOCK, Pathophysiology and Prognostic Factors in Cardiogenic Shock; ENCOURAGE, Prediction of Cardiogenic shock Outcome for AMI Patients Salvaged by VA-ECMO Score; IABP-SHOCK II, Intraaortic Balloon Pump in Cardiogenic Shock Score; INOVA, Inova Heart and Vascular Institute Score; GRACE, Global Registry of Acute Coronary Events Score; PAMI, Primary Angioplasty for the Treatment of Acute ST Elevated Myocardial Infarction Model; SAPS-II, Simplified Acute Physiology Score; SHOCK, Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock Score; SOFA, Sequential Organ Failure Assessment; TIMI-STEMI, Thrombolysis in ST-Elevation in Myocardial Infarction Score; TIMI-NSTEMI, Thrombolysis in Non-ST Elevation in Myocardial Infarction Score.
Secondary Outcomes: 90-Day and 1-Year Mortality
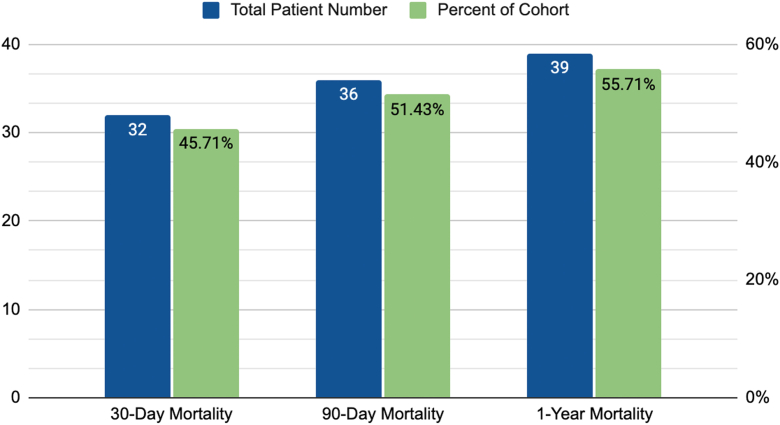
The 90-day mortality was 51% (n = 36), and the 1-year mortality was 56% (n = 39), as seen in Figure 2. Of those who died within 1 year, 82% of the deaths occurred in the first 30 days (32 of 39 patients).
Figure 2.
Thirty-day, 90-day, and 1-year mortality. The majority of patient deaths occurred within the first 30 days.
Receiver Operating Characteristic Curves
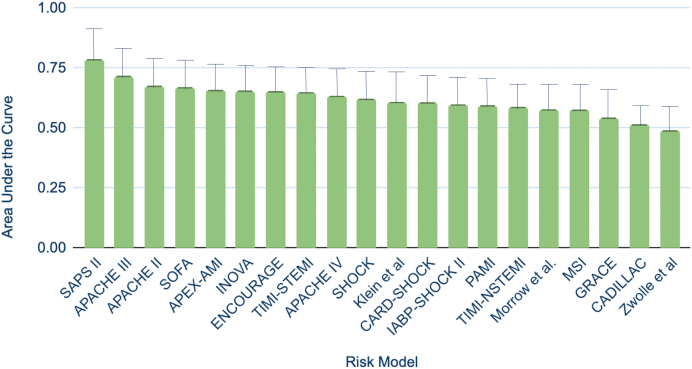
The individual risk score receiver operating characteristic analysis results are shown in Figure 3. The SAPS-II score (area under the curve [AUC]: 0.79, 95% confidence interval [CI]: 0.67-0.90) demonstrated the highest prognostic accuracy for 30-day mortality in this patient cohort, followed by the APACHE-III score (AUC: 0.72, 95% CI: 0.59-0.84) and the APACHE-II score (AUC: 0.67, 95% CI: 0.55-0.80). The poorest performer was the Zwolle model (AUC: 0.49, 95% CI: 0.41-0.56). The Hosmer-Lemeshow goodness-of-fit test demonstrated acceptable calibration for all risk scores (p > 0.05 for all).
Figure 3.
Receiver operating characteristic curve analysis for 30-day predicted mortality. The SAPS-II demonstrated the highest predictive ability for 30-day mortality (AUC: 0.79, 95% CI: 0.67-0.90), followed by the APACHE-III (AUC: 0.72, 95% CI: 0.59-0.84), the APACHE-II (AUC: 0.67, 95% CI: 0.55-0.80), and SOFA (AUC: 0.67, 95% CI: 0.54-0.79) scores.
Abbreviations: APEX-AMI, Assessment of Pexelizumab in Acute Myocardial Infarction; AUC, area under the curve; CADILLAC, Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications; GRACE, Global Registry of Acute Coronary Events Score; IABP, Intraaortic Balloon Pump; PAMI, Primary Angioplasty for the Treatment of Acute ST Elevated Myocardial Infarction; TIMI, Thrombolysis in ST-Elevation in Myocardial Infarction Score.
Discussion
This study utilized a real-world population of patients with AMI-CS presenting to a tertiary care center. The 30-day mortality of our cohort was 46%, which is similar to what has been seen in other studies examining the AMI-CS population.3,4,44,45 Our study demonstrated that multiple risk scores had similar, adequate predictive power for 30-day mortality. The AUCs for the risk scores ranged from 0.49 to 0.79, with the SAPS-II score having the most optimal discrimination followed by the APACHE-II, APACHE-III, and SOFA models.
The SAPS-II was originally developed for mortality estimation for medical and surgical intensive care unit patients in North America and Europe.26 Paradoxically, in its original conception, it excluded patients with burn injuries, coronary care unit patients, and/or cardiac surgical patients from the analysis. Nevertheless, despite the relative paucity of hemodynamic parameters included in the calculation, the SAPS-II utility in mortality discrimination has been previously observed.46 Kellner et al. evaluated 41 patients with AMI-CS and found that the mean admission APACHE-II, APACHE-III, SAPS-II, and SOFA scores were higher in nonsurvivors vs. survivors and had modest predictive performance (APACHE-II AUC 0.691, APACHE-III AUC 0.786, and SAPS-II AUC 0.790). The maximum score AUCs demonstrated superior performance for prediction of mortality.23 More recently, in a 2019 analysis of a subset of patients with refractory AMI-CS (53.9%), the SAPS-II demonstrated superior mortality-prediction capacity in patients on VA-ECMO when compared to age, SOFA score, and pH alone.47
When considering the parameters employed by the highest-performing indices in our analysis—the SAPS-II, APACHE-II, APACHE-III, and SOFA models—all incorporated the Glasgow Coma Score, the fraction of inspired oxygen, creatinine output, and/or urine output into their respective calculations. Other than the APACHE-IV model, the Glasgow Coma Score was not used in the other 16 indices. The SAPS-II score also included blood urea nitrogen, urine output, and serum bicarbonate concentrations, potentially reflecting a sensitivity toward worsening renal function more than the other scores. The SOFA score was unique among the top performers in that it did not incorporate a measure of chronic health conditions nor the type of hospital admission.
Despite the relative success of the SAPS-II scoring system in our analysis, there was no clear superior model between all 20 analyzed. This is likely best explained by the heterogeneity of the CS population and the inherent challenge of patient generalizability. Furthermore, as mentioned, some scores analyzed were derived and validated in AMI-CS populations, while others in broader cardiogenic shock or septic shock populations. Finally, all risk score models were calculated using single, early, data points within the patients’ clinical courses. Given the multifaceted clinical trajectory of this heterogenous patient population, it may not be possible to utilize a single score to capture a process that inherently has multiple time horizons.
In this vein, there are several limitations to the aforementioned AMI-CS risk models that warrant attention. A significant portion of the scores studied, namely the APACHE II-IV, SHOCK, and Prediction of Cardiogenic shock Outcome for AMI Patients Salvaged by VA-ECMO Score scores, are widely regarded as too complex to calculate at the bedside, hindering their clinical utility. The SHOCK score was also developed from a cohort that preceded the widespread use of primary percutaneous coronary intervention and may not reflect current practice. Finally, none of these scores reflect the importance of time to support in AMI-CS patient outcomes.48,49 As such, when considering a theoretical “ideal” AMI-CS scoring system, such a model should be rigorous enough to incorporate universally available initial metrics at patient presentation such as vitals, physical findings, and cardiometabolic data but also be malleable enough to integrate advanced, hemodynamic parameters such as invasive monitoring, MCS specifications including time to and time on support, and responses to initial therapies into its global assessment.50 Scores should be contemporary, reflecting improvements in standards of care over time, and applicable to specific subgroups of patients, such as those with ST-elevation myocardial infarction or those placed on specific types of MCS.
Limitations
Our study has a few limitations that should be noted. First, this study was conducted at a single academic institution, limiting its generalizability. Second, as this was a retrospective analysis of previously collected data, there may have been variation in available data for risk score calculation. As such, these data need to be prospectively validated in a larger patient population.
Conclusions
In this study, the SAPS-II, APACHE-II, APACHE-III, and SOFA risk score models demonstrated the highest prognostic accuracy for 30-day mortality when applied to a population of AMI-CS patients. Further investigations are required to improve the discriminative capabilities of these models or to establish new, more-streamlined, and accurate methods for mortality prognostication in patients at AMI-CS.
Ethics Statement
This study was approved by the Columbia Institutional Review Board. As the study was retrospective, no informed consent was required.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure statement
Dr. Amirali Masoumi receives Honoria from Abiomed. The other authors had no conflicts to declare.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Henry T.D., Tomey M.I., Tamis-Holland J.E., et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143(15):e815–e829. doi: 10.1161/CIR.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 2.Kapur N.K., Thayer K.L., Zweck E. Cardiogenic shock in the setting of acute myocardial infarction. Methodist Debakey Cardiovasc J. 2020;16(1):16–21. doi: 10.14797/mdcj-16-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochman J.S., Sleeper L.A., Webb J.G., et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 4.Thiele H., Zeymer U., Neumann F.J., et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 5.Kolte D., Khera S., Aronow W.S., et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. doi: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aissaoui N., Puymirat E., Delmas C., et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail. 2020;22(4):664–672. doi: 10.1002/ejhf.1750. [DOI] [PubMed] [Google Scholar]
- 7.Babaev A., Frederick P.D., Pasta D.J., et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448–454. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 8.Tehrani B., Truesdell A., Singh R., Murphy C., Saulino P. Implementation of a cardiogenic shock team and clinical outcomes (INOVA-SHOCK Registry): observational and retrospective study. JMIR Res Protoc. 2018;7(6):e160. doi: 10.2196/resprot.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basir M.B., Schreiber T., Dixon S., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the detroit cardiogenic shock initiative. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2018;91(3):454–461. doi: 10.1002/ccd.27427. [DOI] [PubMed] [Google Scholar]
- 10.Werdan K., Gielen S., Ebelt H., Hochman J.S. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35(3):156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 11.Acharya D. Predictors of outcomes in myocardial infarction and cardiogenic shock. Cardiol Rev. 2018;26(5):255–266. doi: 10.1097/CRD.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 13.Knaus W.A., Wagner D.P., Draper E.A., et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 14.Jentzer J.C., Bennett C., Wiley B.M., et al. Predictive value of the Sequential organ failure assessment score for mortality in a contemporary cardiac intensive care Unit population. J Am Heart Assoc. 2018;7(6) doi: 10.1161/JAHA.117.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge R., Fakhry S., Rutherford E., Muakkassa F., Meyer A. Comparison of APACHE II, Trauma Score, and Injury Severity Score as predictors of outcome in critically injured trauma patients. Am J Surg. 1993;166(3):244–247. doi: 10.1016/s0002-9610(05)80966-3. [DOI] [PubMed] [Google Scholar]
- 17.Bohnen J.M., Mustard R.A., Schouten B.D. Steroids, APACHE II score, and the outcome of abdominal infection. Arch Surg Chic Ill 1960. 1994;129(1):33–37. doi: 10.1001/archsurg.1994.01420250045005. discussion 37-38. [DOI] [PubMed] [Google Scholar]
- 18.Goel A., Pinckney R.G., Littenberg B. APACHE II predicts long-term survival in COPD patients admitted to a general medical ward. J Gen Intern Med. 2003;18(10):824–830. doi: 10.1046/j.1525-1497.2003.20615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tee Y.S., Fang H.Y., Kuo I.M., Lin Y.S., Huang S.F., Yu M.C. Serial evaluation of the SOFA score is reliable for predicting mortality in acute severe pancreatitis. Medicine (Baltimore) 2018;97(7):e9654. doi: 10.1097/MD.0000000000009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werdan K., Pilz G., Bujdoso O., et al. Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med. 2007;35(12):2693–2701. [PubMed] [Google Scholar]
- 21.Werdan K., Pilz G., Müller-Werdan U., et al. Immunoglobulin G treatment of postcardiac surgery patients with score-identified severe systemic inflammatory response syndrome--the ESSICS study. Crit Care Med. 2008;36(3):716–723. doi: 10.1097/01.CCM.0B013E3181611F62F. [DOI] [PubMed] [Google Scholar]
- 22.Chatzis G., Markus B., Syntila S., et al. Comparison of mortality risk models in patients with postcardiac arrest cardiogenic shock and percutaneous mechanical circulatory support. J Interv Cardiol. 2021;2021:8843935. doi: 10.1155/2021/8843935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellner P., Prondzinsky R., Pallmann L., et al. Predictive value of outcome scores in patients suffering from cardiogenic shock complicating AMI: APACHE II, APACHE III, Elebute-Stoner, SOFA, and SAPS II. Med Klin Intensivmed Notfallmedizin. 2013;108(8):666–674. doi: 10.1007/s00063-013-0234-2. [DOI] [PubMed] [Google Scholar]
- 24.Popovic B., Fay R., Cravoisy-Popovic A., Levy B. Cardiac power index, mean arterial pressure, and simplified acute physiology score II are strong predictors of survival and response to revascularization in cardiogenic shock. Shock. 2014;42(1):22–26. doi: 10.1097/SHK.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 26.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 27.Morrow D.A., Antman E.M., Giugliano R.P., et al. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: an InTIME II substudy. Lancet Lond Engl. 2001;358(9293):1571–1575. doi: 10.1016/S0140-6736(01)06649-1. [DOI] [PubMed] [Google Scholar]
- 28.Fox K.A.A., Dabbous O.H., Goldberg R.J., et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333(7578):1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Luca G., Suryapranata H., van ’t Hof A.W.J., et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109(22):2737–2743. doi: 10.1161/01.CIR.0000131765.73959.87. [DOI] [PubMed] [Google Scholar]
- 30.Addala S., Grines C.L., Dixon S.R., et al. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score) Am J Cardiol. 2004;93(5):629–632. doi: 10.1016/j.amjcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Klein L.W., Shaw R.E., Krone R.J., et al. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 2005;96(1):35–41. doi: 10.1016/j.amjcard.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 32.Morrow D.A., Antman E.M., Charlesworth A., et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 33.Antman E.M., Cohen M., Bernink P.J., et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 34.Harjola V.P., Lassus J., Sionis A., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 35.Pöss J., Köster J., Fuernau G., et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913–1920. doi: 10.1016/j.jacc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Halkin A., Singh M., Nikolsky E., et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol. 2005;45(9):1397–1405. doi: 10.1016/j.jacc.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 37.Stebbins A., Mehta R.H., Armstrong P.W., et al. A model for predicting mortality in acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: results from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. Circ Cardiovasc Interv. 2010;3(5):414–422. doi: 10.1161/CIRCINTERVENTIONS.109.925180. [DOI] [PubMed] [Google Scholar]
- 38.Abreu G., Azevedo P., Galvão Braga C., et al. Modified shock index: a bedside clinical index for risk assessment of ST-segment elevation myocardial infarction at presentation. Rev Port Cardiol. 2018;37(6):481–488. doi: 10.1016/j.repc.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Sleeper L.A., Reynolds H.R., White H.D., Webb J.G., Dzavík V., Hochman J.S. A severity scoring system for risk assessment of patients with cardiogenic shock: a report from the SHOCK Trial and Registry. Am Heart J. 2010;160(3):443–450. doi: 10.1016/j.ahj.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller G., Flecher E., Lebreton G., et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42(3):370–378. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 41.Tehrani B.N., Truesdell A.G., Sherwood M.W., et al. Standardized Team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73(13):1659–1669. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 42.Naidu S.S., Baran D.A., Jentzer J.C., et al. SCAI SHOCK stage classification expert consensus Update: a review and incorporation of validation studies: this statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for acute Cardiovascular care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical care medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. 2022;79(9):933–946. doi: 10.1016/j.jacc.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Naidu S.S., Baran D.A., Jentzer J.C., et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Am Coll Cardiol. 2022 doi: 10.1016/j.jacc.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Thiele H., Akin I., Sandri M., et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 45.Schrage B., Ibrahim K., Loehn T., et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 46.Metnitz P.G., Valentin A., Vesely H., et al. Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study. Simplified Acute Physiology Score. Intensive Care Med. 1999;25(2):192–197. doi: 10.1007/s001340050815. [DOI] [PubMed] [Google Scholar]
- 47.Lee H.S., Kim H.S., Lee S.H., et al. Clinical implications of the initial SAPS II in veno-arterial extracorporeal oxygenation. J Thorac Dis. 2019;11(1):68–83. doi: 10.21037/jtd.2018.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito M.L., Kapur N.K. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res. 2017;6:737. doi: 10.12688/f1000research.11150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellumkonda L., Gul B., Masri S.C. Evolving concepts in diagnosis and management of cardiogenic shock. Am J Cardiol. 2018;122(6):1104–1110. doi: 10.1016/j.amjcard.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 50.Kalra S., Ranard L.S., Memon S., et al. Risk prediction in cardiogenic shock: current state of knowledge, challenges and Opportunities. J Card Fail. 2021;27(10):1099–1110. doi: 10.1016/j.cardfail.2021.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.