Abstract
Background
Endovascular baroreflex amplification with the MobiusHD, a self-expanding stent-like device that is implanted in the internal carotid artery, was designed to reduce the sympathetic overactivity that contributes to progressive heart failure with reduced ejection fraction.
Methods
Symptomatic patients (New York Heart Association class III) with heart failure with reduced ejection fraction (left ventricular ejection fraction [LVEF] ≤40%) despite guideline directed medical therapy and n-terminal pro-B type natriuretic peptide (NT-proBNP) levels ≥400 pg/mL in whom carotid ultrasound and computed tomographic angiography demonstrated absence of carotid plaque were enrolled. Baseline and follow-up measures included 6-minute walk distance (6MWD), Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ OSS), and repeat biomarkers and transthoracic echocardiography.
Results
Twenty-nine patients underwent device implantation. The mean age was 60.6 ± 11.4 years, and all had New York Heart Association class III symptoms. Mean KCCQ OSS was 41.4 ± 12.7, mean 6MWD was 216.0 ± 43.7 m, median NT-proBNP was 1005.9 pg/mL (894, 1294), and mean LVEF was 34.7 ± 2.9%. All device implantations were successful. Two patients died (161 days and 195 days) and one stroke occurred (170 days) during follow-up. For the 17 patients with 12-month follow-up, mean KCCQ OSS improved by 17.4 ± 9.1 points, mean 6MWD increased by 97.6 ± 51.1 meters, a mean 28.4% reduction from the baseline NT-proBNP concentration was found, and mean LVEF improved by 5.6% ± 2.9 (paired data).
Conclusion
Endovascular baroreflex amplification with the MobiusHD device was safe and effected positive changes in quality of life, exercise capacity, and LVEF, consistent with observed reductions in NT-proBNP levels.
Keywords: Chronic heart failure, Endovascular baroreceptor amplification, HFrEF
Highlights
-
•
The MobiusHD device is designed to reduce sympathetic overactivity in heart failure
-
•
It’s implanted in nondiseased carotid arteries and amplifies baroreceptor signaling
-
•
All patients underwent successful and uneventful device implantation
-
•
Patients experienced positive changes in quality of life and exercise capacity
-
•
NT-proBNP levels decreased concordantly during follow-up
Introduction
Heart failure (HF) is estimated to affect ∼26 million people worldwide, with approximately half of the cases associated with reduced ejection fraction (HFrEF).1 Over the last several decades, advances in pharmacological therapy and devices to address arrhythmias and secondary mitral regurgitation have significantly improved the prognosis of HFrEF.2, 3, 4 Despite these advances, however, many patients with HFrEF remain symptomatic, with more than 30% severely limited (New York Heart Association [NYHA] class III or IV).5 These patients have poor health status marked by worse quality of life (QOL) and compromised functional capacity with increased risks for hospitalization and mortality. As a result, HF is a leading public health problem with related global expenditures estimated to be $108B USD.6
In response to reduced cardiac output and elevated ventricular filling pressures, patients with HFrEF develop elevated sympathetic nervous system activity which further increases myocardial oxygen demand and afterload. Modulation of this autonomic imbalance may improve patient outcomes. While beta-blockers reduce sympathetic overdrive and improve prognosis, some patients cannot tolerate these agents, and many others remain incompletely responsive. This has led to alternative means to manipulate the sympathetic tone in those with HF. One such option, stimulation of carotid baroreceptors, results in a centrally mediated reduction of sympathetic outflow and increased parasympathetic activity, increasing arterial and venous compliance and reducing systemic arterial resistance.7 Sympathetic reduction with electrical baroreflex activation therapy has been shown to be safe and effective in HFrEF patients.7, 8, 9, 10
Baroreceptors are stimulated by the deformation of the wall of the carotid sinus in which they are located.11 Furthermore, the natural physiologic baroreceptor function and baroreflex stimulation are activated and sustained by pulsatile carotid body stretch rather than static pressure. Constant static pressure results in the resetting of the reflex creating only a transient effect.12,13
An increase in static pressure in the isolated carotid sinus causes abrupt inhibition of sympathetic activity which returns gradually toward the control level; hence, limited hemodynamic changes have been noted after carotid stenting. In contrast, an increase in the pulsatile pressure causes a relatively sustained inhibition of sympathetic activity.12 In response to sustained increases in static pressure (carotid sinus stretch), the baroreceptor afferent activities ‘adapt’ over time. This “resetting” of baroreceptors can be prevented or attenuated if the pressure is pulsatile rather than static.12
The self-expanding MobiusHD device is a carotid implant designed to augment carotid baroreceptor signaling and the baroreflex mechanism. Endovascular baroreflex amplification (EVBA) using the MobiusHD device relies on the passive activation of the carotid baroreceptor reflex by changing the geometric shape of the carotid body and increasing carotid sinus wall stretch while preserving pulsatility. This amplified signaling triggers a negative feedback response, decreasing sympathetic activity and increasing parasympathetic activity.
The sustained effect of the MobiusHD device has been demonstrated in previous clinical studies in patients with refractory hypertension, resulting in marked sustained reductions in blood pressure at 6 months and 1, 2, and 3 years follow-up.14,15 However, to date, it had not been implanted in patients with HFrEF, a high-risk cohort with a poor prognosis in whom the benefit/risk profile of a permanent carotid implant might be favorable.
Accordingly, we sought to evaluate the safety and the exploratory effectiveness of the MobiusHD system for the treatment of HFrEF among individuals with NYHA class III symptoms and elevated N-terminal pro-B type natriuretic peptide (NT-proBNP) levels. We hypothesized that in this first-in-human feasibility study, EVBA with the MobiusHD system would be safe and associated with favorable impact on patient-reported health status, functional capacity, NT-proBNP concentrations, and left ventricular ejection fraction (LVEF).
Methods
Study Design and Population
The effect of the MobiusHD Device in Patients With Heart Failure [HF-FIM (registered at clinicaltrials.gov, NCT04590001)] study is a single-arm, open-label, prospective investigation that is enrolling up to 50 adults at multiple centers worldwide with symptomatic chronic HF with NYHA class II (if class II, must have been class III at any time within 3 months of screening) or III symptoms, LVEF ≤40%, and NT-proBNP concentrations ≥400 pg/mL. Additionally, study subjects were on stable guideline-directed medical therapy (GDMT) for HF for at least 4 weeks and had a 6-minute walk distance (6MWD) ≥150 m and ≤400 m. Carotid artery anatomy was assessed with noninvasive carotid duplex ultrasonography and computed tomography angiography. Patients with acceptable anatomy were enrolled for device implantation if carotid plaque was absent, as assessed at an independent imaging core laboratory. A complete list of inclusion and exclusion criteria is shown in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| 1. Provide written informed consent |
| 2. Age 18 y or above |
| 3. Currently New York Heart Association Class II or III heart failure; if Class II, must have been Class III at any time within 3 mo of screening |
| 4. Left ventricular ejection fraction ≤40% |
| 5. N-terminal-pro-B-type natriuretic peptide ≥ 400 pg/mL |
| 6. Prescribed optimally tolerated, stable, guideline-directed medical therapy (GDMT) per country-specific guidelines for the treatment of heart failure for at least 4 wk |
| 7. Six-minute hall walk (6MHW) distance of ≥150 m and ≤400 m |
| 8. Deemed an acceptable candidate by the investigator |
| 9. Adequacy of the carotid anatomy for treatment with the MobiusHD implant based on noninvasive carotid duplex and computated tomography angiography/magnetic resonance angiography imaging, and invasive carotid angiography |
| Exclusion criteria |
| 1. Known or clinically suspected baroreflex failure or autonomic neuropathy |
| 2. Currently implanted with a barostimulator device |
| 3. Received cardiac resynchronization therapy (CRT) within 6 months of implantation |
| 4. Currently have an indication for a CRT device according to American Heart Association/American College of Cardiology/European Society of Cardiology guidelines for the treatment of congestive heart failure |
| 5. Received a CardioMEMS device within 3 months of the Screening Visit |
| 6. Heart failure secondary to a reversible cause, such as cardiac structural valvular disease, acute myocarditis, and pericardial constriction |
| 7. Unacceptable arterial access for implantation |
| 8. History of major bleeding complications associated with anti-platelet therapy |
| 9. History of known uncorrected or uncorrectable bleeding diathesis |
| 10. History of heparin-induced thrombocytopenia unless bivalirudin will be used as the procedural anticoagulant |
| 11. Prior carotid surgery or stent placement, therapeutic radiation to the neck, or endovascular stent placement in the carotid region on the intended side |
| 12. History of stroke with permanent neurologic defect or any prior intracranial bleed or other serious brain injury |
| 13. Active infection within the last 30 d requiring oral or intravenous antibiotics |
| 14. Body mass index >45 kg/m2 |
| 15. Serum estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 |
| 16. Two or more occurrences of a resting heart rate of either <50 bpm or >100 bpm via clinic measurements within 45 d of the screening visit (Note: heart rate <50 bpm is not applicable for subjects with an implanted device capable of pacing) |
| 17. Two or more occurrences of symptomatic hypotension within 45 d |
| 18. Significant uncontrolled symptomatic bradyarrhythmias or unstable ventricular arrhythmia |
| 19. Subjects with any surgery that has occurred, or is planned to occur, within 45 d of the implantation procedure, including pacemaker or implantable cardioverter defibrillator implants or battery replacements |
| 20. Hospitalization or unscheduled clinic/urgent care visit resulting in need for IV diuretic within 30 d prior to screening |
| 21. History of myocardial infarction or unstable angina within 3 mo |
| 22. History of percutaneous coronary intervention (e.g., coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) within 3 mo |
| 23. History of sudden cardiac arrest |
| 24. Solid organ or hematologic transplant, or currently being actively evaluated for an organ transplant |
| 25. Has received or is receiving left ventricular assist device therapy |
| 26. Has received or is receiving chronic dialysis |
| 27. Infiltrative cardiomyopathy (e.g., cardiac amyloidosis) |
| 28. Severe chronic obstructive pulmonary disease or severe restrictive lung disease (e.g., requires chronic steroid use or home oxygen use) |
| 29. Active malignancy |
| 30. Current or planned treatment with intravenous positive inotrope therapy |
| 31. Life expectancy less than 1 y |
| 32. Unable or unwilling to fulfill the protocol medication compliance, testing, or follow-up requirements |
| 33. Enrolled and active in another (e.g., device, pharmaceutical, or biological) clinical trial |
| 34. History of allergy to nickel, to contrast media or study medications that cannot be managed medically |
| 35. Uncontrolled systemic disease |
| 36. Pregnant or lactating females. For females of child-bearing potential, a positive mandatory pregnancy test during screening or refusal to use a medically accepted method of birth control for the duration of the trial |
Baseline assessments included medical history, physical examination, blood/urine analysis, Kansas City Cardiomyopathy Questionnaire (KCCQ) questionnaire, 6MWD, NT-proBNP levels, echocardiogram, National Institutes of Health Stroke Scale (NIHSS) score, and cerebral magnetic resonance imaging (MRI). A carotid duplex and computed tomography angiography of the carotid (CTA) were conducted to assess the suitability of vessels for placement of the device. Follow-up assessments were done on the day of discharge, 7 days and 1, 3, 6, 12, 18, and 24 months. KCCQ overall summary score (OSS), 6MWD, NT-proBNP, and an echocardiogram were obtained at the 3, 6, 12, 18, and 24-month visits. Cerebral MRI was obtained at discharge, 1-week, 1-month, and 12-month visits. An NIHSS assessment was taken at all follow-up visits.
The protocol was approved by the ethics committee of each participating site and all patients provided written informed consent.
Device and Mechanism of Action
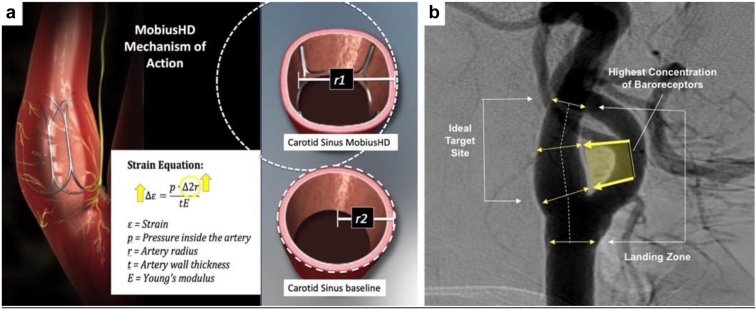
The MobiusHD (Vascular Dynamics Inc., Irvine, CA) device is a self-expanding rectangular-shaped nitinol implant, available in 3 sizes, that is implanted in non-atherosclerotic carotid arteries with internal lumen diameters ranging from 5.0 mm to 11.75 mm (Figure 1). The device may be implanted in either the right or left internal carotid artery depending on the patient’s anatomy. The implanted device reshapes the carotid artery wall without expansion while allowing the vessel to retain its pulsatility. The reshaping effect creates a larger effective arterial radius and thereby increases local carotid sinus wall stretch,16 dynamically leading to amplification of baroreceptor output to the central nervous system. The amplified baroreceptor signaling triggers a negative feedback response that decreases sympathetic activity and increases parasympathetic activity. The reduction of sympathetic activity is believed to prevent progression of HFrEF.17
Figure 1.
MobiusHD device and mechanism of action. (a) The MobiusHD rectangular-shaped nitinol carotid implant creates a larger effective radius (r1 vs. r2), increasing local arterial wall stretch resulting in baroreflex amplification. (b) As shown in this carotid angiogram, the device is deployed in the carotid sinus which has a high anticipated concentration of baroreceptors.
The MobiusHD system consists of 2 components: 1) the implant, and 2) the delivery catheter which is introduced via the femoral artery. The implant is advanced over a guidewire into the carotid sinus under fluoroscopic control. A 6 Fr guide sheath or 8 Fr guiding catheter is inserted via the femoral artery over a 0.035 inch (0.09 mm) guidewire and advanced to the carotid artery. Angiographic measurements are made to confirm the diameter of the carotid sinus and to select an appropriate implant size. A 0.014 inch (0.04 mm) guidewire is navigated into the distal internal carotid artery, the delivery catheter is introduced over the guidewire, and, when properly positioned, the delivery catheter protective sheath is retracted to allow expansion and deployment of the implant and apposition with the vessel intima. The catheter is then withdrawn, leaving the implant at the site of the carotid sinus. As the device is implanted in patients without carotid atherosclerosis, embolic protection devices are not utilized. A detailed description of the implantation procedure has been reported.14
Endpoints
Safety outcomes included the incidence and severity of adverse events and serious adverse events, including cardiovascular and neurological events. MRI prior to discharge was performed to assess asymptomatic cerebrovascular findings. Exploratory effectiveness outcomes were 1) change of functional parameters from baseline (NYHA class, 6MWD)15; 2) change in NT-proBNP levels from baseline; 3) change in QOL as measured by the KCCQ OSS from baseline16; and 4) change of cardiac function from baseline (LVEF measured by transthoracic echocardiography). Follow-up visits are planned at 1 week, and 1, 3, 6, 12, 18, and 24 months. Patients were treated with dual antiplatelet therapy with aspirin and a P2Y12 inhibitor prior to the procedure; aspirin was continued for the duration of the trial and the P2Y12 inhibitor for at least 30 days post procedure. Assessments of clinical events including adjudication of the relatedness of adverse events to the device and/or procedure were performed by an independent events committee.
Statistical Methods and Data Analysis
The safety and effectiveness outcomes for this first-in-human study were not powered. Descriptive statistics were used to tabulate and summarize baseline, procedural, and follow-up data. Categorical data are presented as numbers and percentages of total; continuous variables are shown as means with standard deviations or medians with 25th and 75th percentiles (Q1, Q3) for nonnormally distributed data. Differences across time intervals between groups for normally distributed continuous variables were determined using ANOVA, and pairwise comparisons using Tukey simultaneous tests for differences of means were performed. For nonnormally distributed continuous variables, Kruskal-Wallis test by ranks was used to determine differences across time intervals between groups, and Mann-Whitney U tests were performed for pairwise comparisons. All tests were two-sided and a p-value <0.05 was considered to be statistically significant.
Results
Patient Population
The present report includes the first 29 patients who were enrolled at 2 sites. To date, 25, 21, and 17 patients have completed 3-, 6-, and 12-month follow-up, respectively.
All 29 patients had HFrEF with NYHA class III symptoms despite GDMT. The mean age was 60.6 ± 11.4 years and 31.0% were female; the mean time since HF diagnosis was 63.2 ± 49.0 months. Mean KCCQ OSS was 41.4 ± 12.7; mean 6MWD was 216.0 ± 43.7 m; median NT-proBNP was 1005.9 (894, 1294) pg/mL, and mean LVEF was 34.7 ± 2.9%. Other baseline features are shown in Table 2.
Table 2.
Demographic and baseline features in 29 patients
| Age (years) | 60.6 ± 11.4 |
| Female | 9 (31.0%) |
| Body mass index (kg/m2) | 30.3 ± 4.6 |
| Systolic blood pressure (mmHg) | 128.2 ± 7.2 |
| Diastolic blood pressure (mmHg) | 71.8 ± 5.4 |
| Estimated glomerular filtration rate (mL/min) | 63.0 ± 16.2 |
| Hemoglobin (dg/L) | 1.51 ± 0.22 |
| Heart failure hospitalization within the prior 6 mo | 9 (31.0%) |
| Medical history | |
| Coronary artery disease | 7 (24.1%) |
| Atrial fibrillation | 11 (37.9%) |
| Diabetes mellitus | 5 (17.2%) |
| Hypertension | 28 (96.6%) |
| Time since heart failure diagnosis (months) | 63.2 ± 49.0 |
| New York Heart Association class III | 29 (100%) |
| N-terminal-pro-B-type natriuretic peptide (pg/mL) | 1005.9 (894, 1294) |
| Transthoracic echocardiography | |
| Left ventricular end-diastolic volume (mL) | 166.9 ± 25.3 |
| Left ventricular end-systolic volume (mL) | 108.6 ± 18.7 |
| Left ventricular ejection fraction (%) | 34.7 ± 2.9 |
| Six-minute walk distance (m) | 216.0 ± 43.7 |
| Kansas City Cardiomyopathy Questionnaire overall summary score (points) | 41.4 ± 12.7 |
Data is displayed as mean ± standard deviation or median with interquartile ranges (Q1, Q3), depending on data distribution.
HF medications at baseline and during follow-up are shown in Table 3. The dosing of beta-blockers and other HF medications in this study was as tolerated by the patients, and up-titration was at the discretion of the treating physician during follow-up. All patients were taking diuretics, and a high proportion of patients were taking beta-blockers (96.6%), mineralocorticoid receptor antagonists (MRA) (86.2%), and inhibitors of the renin-angiotensin-aldosterone system (89.7%). More specifically, 10 of the 29 patients were taking ARNI medications. At baseline, 22 (75.9%) patients were on 3 of the major HF drug classes. While SGLT2 inhibitors were not included in their treatment, all patients in this study were required to be on stable GDMT before enrollment. The goal was to have stable GDMT throughout the study to allow for testing of the potential effectiveness of the device. Not all patients could tolerate optimal target doses but the level of dosing in this study compares favorably with reports from other studies. For instance, optimal dosing of beta-blockers in the COAPT study18 was 17.5% compared to 48.5% in this study. Medication use was largely unchanged during 12-month follow-up.
Table 3.
Heart failure medications for study population at baseline and during follow-up
| Drug class |
||||
|---|---|---|---|---|
| Diuretic | Beta-blocker | ACE-I/ARB/ARNI | MRA | |
| Baseline (n = 29) | ||||
| Taking drug class | 29 (100%) | 28 (96.6%) | 26 (89.7%) | 25 (86.2%) |
| Taking ≥50% of the target dose | - | 14 (48.3%) | 19 (65.5%) | 25 (86.2%) |
| 3-mo (n = 25) | ||||
| Taking drug class | 25 (100%) | 24 (96.0%) | 22 (88.0%) | 22 (88.0%) |
| Taking ≥50% of the target dose | - | 11 (44.0%) | 14 (56.0%) | 22 (88.0%) |
| 6-mo (n = 21) | ||||
| Taking drug class | 21 (100%) | 20 (95.2%) | 18 (85.7%) | 18 (85.7%) |
| Taking ≥50% of the target dose | - | 9 (42.0%) | 13 (61.9%) | 18 (85.7%) |
| 12-mo (n = 17) | ||||
| Taking drug class | 17 (100%) | 16 (94.1%) | 14 (82.4%) | 17 (100%) |
| Taking ≥50% of the target dose | - | 8 (47.1%) | 12 (70.6%) | 17 (100%) |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; ARNI, angiotensin-receptor neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist.
Target doses established from 2021 European Society of Cardiology Guidelines.19
Procedural Outcomes
Preprocedure planning allows for determination of the presence of plaque, assessment of the anatomy, appropriate device sizing, and determination of targeted implant placement. In the event that both internal carotids were suitable for implantation, the final determination is left to the discretion of the implanting physician. The MobiusHD device was successfully implanted in all patients at the preoperatively determined target site, either in the right (14 or 48.3%) or left (15 or 51.7%) internal carotid artery. Five (17.2%) patients received implant size A (5–7 mm), 14 (48.3%) patients received size B (6.25–9 mm), and 10 (34.5%) patients received size C (8–11.75 mm). Total procedure duration was 27.2 ± 11.8 minutes, device implantation time was 4.9 ± 1.9 minutes, and fluoroscopy time was 6.9 ± 5.5 minutes.
Safety Outcomes
There were no procedural or periprocedural serious adverse events. Predischarge MRI was performed in all 29 patients, 6 of whom (20.7%) were interpreted at an independent core laboratory to show new radiographic findings at discharge (Table 4). All 6 patients were clinically asymptomatic with NIHSS scores of zero at all follow-up visits.
Table 4.
Clinically asymptomatic postprocedural magnetic resonance imaging findings
| Patient identifier | Discharge radiographic findings |
|---|---|
| 056-112 | On the discharge DWI scan, 2 new areas of ischemia in the cerebellum, and 2 within the right posterior cerebral territory, ipsilateral to the implant, were noted. No new changes were noted on 30-d and 12-mo scans. |
| 056-126 | On the discharge GRE scan, multiple bilateral emboli were suspected. |
| 056-128 | At discharge, bilateral ischemic areas were noted on the DWI scan. New microemboli were noted on the GRE scan performed at discharge, ipsilateral to the implant location. |
| 056-131 | Multiple ischemic changes ipsilateral to the implant were noted on the discharge DWI scans. |
| 057-112 | New ischemic areas were noted on the DWI scan at discharge ipsilateral to the implant. |
| 057-113 | New microemboli were noted on the discharge GRE scan at discharge ipsilateral to the implant. |
DWI, diffusion weight imaging; GRE, gradient recalled echo.
Two deaths (at 161 and 195 days) occurred during follow-up (Kaplan-Meier estimated 1-year mortality rate of 8.2%). One death occurred in a 70-year-old male with coronary artery disease, atrial fibrillation, diabetes, hypertension, and prior defibrillator implant. At 3 months post MobiusHD implant, the patient was doing well and showed an improvement in KCCQ OSS (by 10.7 points), 6MWD (by 54 m), a 378 pg/mL (30%) reduction in NT-proBNP from baseline, and an improved LVEF by 20%. For uncertain reasons, the patient subsequently discontinued his HF medications and died of decompensated HF 195 days after procedure. The second death occurred in a 56-year-old male with diabetes and HF since 2011. At 3 months post MobiusHD implant, the patient was doing well with an improvement in KCCQ OSS (by 1.8 points), 6MWD (by 25 m), and a 157 pg/mL (17%), a reduction in NT-proBNP from baseline, and improved LVEF (by 13%). The patient died suddenly at 161 days postprocedure of an unknown cause. At the time of his death, the family reported that the patient was compliant with his medications and in overall good health. Both deaths were adjudicated as unrelated to the device.
One patient, 86 years of age, experienced a stroke at 170 days postimplant; this patient had chronic atrial fibrillation and had discontinued oral anticoagulation for unclear reasons before the stroke. Bilateral neurological symptoms were noted including difficulty speaking and swallowing, headache, dizziness, dysphonia, blurred vision of the left eye, numbness, and motor limitations on the right side of the body. MRI demonstrated an occipital ischemic stroke ipsilateral to the implant, and thus the stroke was adjudicated as possibly related to the device. At discharge, the stroke symptoms had resolved and the NIHSS score was 0. However, at the patient’s 6-month visit, 40 days after the event, the patient had an NIHSS score of 1, with mild speech impairment.
There were no reports of procedural or late hypotension, and blood pressure and heart rate were substantially unchanged during the 12-month follow-up as compared with baseline (Table 5). The assessment of heart rate variability was not captured in this study.
Table 5.
Blood pressure (office visit) and heart rate changes over time
| Baseline (n = 29) | 3-mo follow-up (n = 25) | 6-mo follow-up (n = 21) | 12-mo follow-up (n = 17) | |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 128.2 ± 7.2 | 125.0 ± 7.8 | 125.0 ± 6.7 | 123.1 ± 5.3 |
| Diastolic blood pressure (mmHg) | 71.8 ± 5.4 | 73.0 ± 5.6 | 78.3 ± 4.4 | 75.9 ± 3.5 |
| Heart rate (bpm) | 82.9 ± 13.6 | 76.4 ± 12.5 | 75.3 ± 18.3 | 77.7 ± 14.8 |
Effectiveness Outcomes
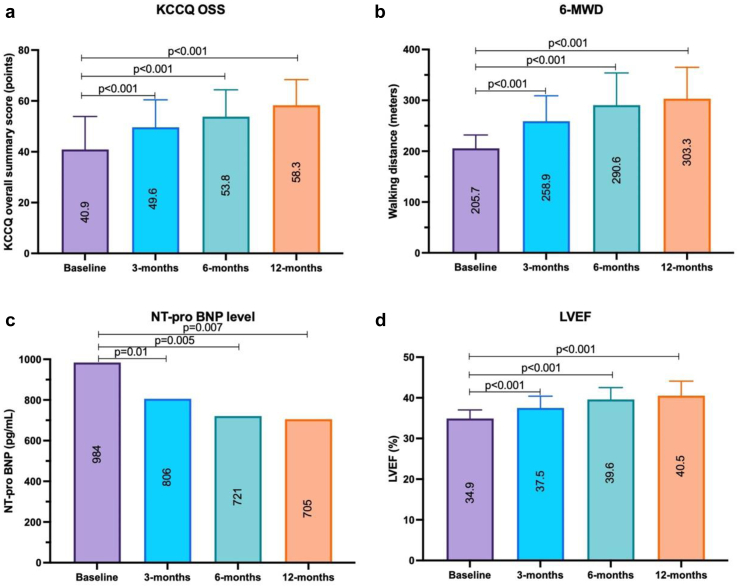
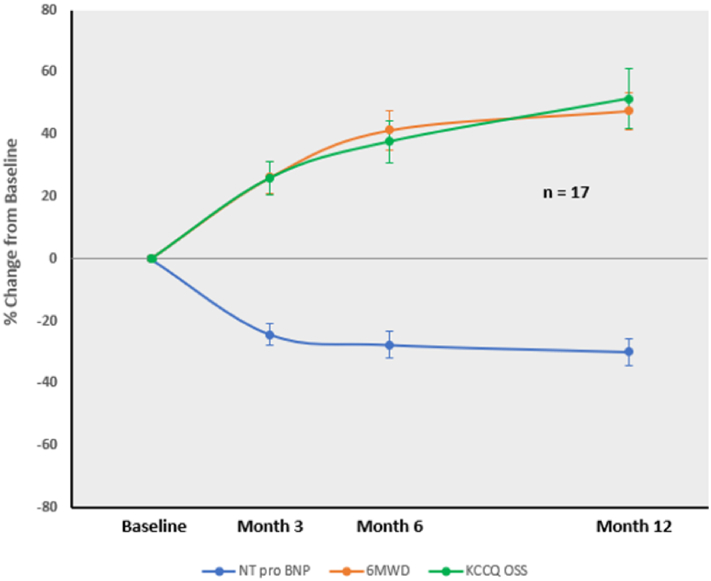
Summary effectiveness outcomes for all patients at 3-, 6-, and 12-month follow-up are shown in Table 6, and paired data for the 17 patients with 12-month outcomes are shown in Figures 2 and 3. Progressive improvements in KCCQ OSS, 6MWD, and LVEF were present over time, with reduced NT-proBNP levels. For the 17 patients with 12-month follow-up, mean KCCQ OSS improved by 17.4 points, mean 6MWD increased by 97.6 meters, mean LVEF improved by 5.6%, and in paired data a mean 28.4% reduction from the baseline in the mean of NT-proBNP concentration was found. NYHA class was improved to class II in 11 patients (64.7%) with 12-month follow-up, with the remainder staying in class III. For both the 6- and 12-month analyses in the present study, almost every patient showed improvement in quality of life, 6MWD, and NT-proBNP outcome assessed (Table 7). Only 1 patient was hospitalized for HF within 1 year (Kaplan-Meier estimated 1-year HF hospitalization rate of 4.3%).
Table 6.
Effectiveness outcomes
| Baseline (n = 29) | 3-mo follow-up (n = 25) | 6-mo follow-up (n = 21) | 12-mo follow-up (n = 17) | |
|---|---|---|---|---|
| NT-proBNP (pg/mL) | 1005.9 (894, 1294) | 806.0 (670, 1032) | 697.0 (623, 861) | 705.0 (631, 908) |
| KCCQ OSS (points) | 41.4 ± 12.7 | 48.3 ± 12.4 | 53.8 ± 12.0 | 58.3 ± 10.1 |
| 6MWD (m) | 216.0 ± 43.7 | 266.9 ± 46.6 | 298.6 ± 61.8 | 303.3 ± 61.8 |
| LVEF (%) | 34.7 ± 2.9 | 38.0 ± 3.5 | 39.1 ± 3.3 | 40.5 ± 3.6 |
| NYHA class | 29 (100%) class III | 8 (32%) class II 17 (68%) class III |
11 (52%) class II 10 (48%) class III |
11 (65%) class II 6 (35%) class III |
Data is displayed as mean ± standard deviation or median with interquartile ranges (Q1, Q3), depending on data distribution.
KCCQ OSS, Kansas City Cardiomyopathy Questionnaire overall summary score; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; NYHA, New York Heart Association; 6MWD, 6-min hall walk distance.
Figure 2.
Paired baseline, 3-, 6-, and 12-month data for 17 patients completing 12-month follow-up. (a) Mean KCCQ OSS; (b) mean 6MWD; (c) median NT-proBNP levels; and (d) mean LVEF. Abbreviations: 6MWD, 6-minute hall walk distance; KCCQ OSS, Kansas City Cardiomyopathy Questionnaire overall summary score; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-pro-B-type natriuretic peptide.
Figure 3.
Mean changes over time from baseline in 6MWD, KCCQ overall summary scores, and in NT-proBNP levels in 17 patients with 12-month follow-up (paired data). Abbreviations: 6MWD, 6-minute hall walk distance; KCCQ OSS, Kansas City Cardiomyopathy Questionnaire overall summary score; NT-proBNP, N-terminal-pro-B-type natriuretic peptide.
Table 7.
Response analysis at 12 mo follow-up
| Change from baseline to 12 mo (n = 17) |
|||
|---|---|---|---|
| Positive improvement | Clinically meaningful improvement |
||
| Moderate | Large | ||
| KCCQ OSS | 94% (16/17) | ≥5 pts: 94% (16/17) | ≥10 pts: 65% (13/17) |
| 6MWD | 100% (17/17) | ≥30m: 100% (17/17) | ≥50m: 65% (13/17) |
| NT-proBNP | 100% (17/17) | ≥10%: 82% (14/17) | ≥30%: 47% (8/17) |
KCCQ OSS, Kansas City Cardiomyopathy Questionnaire overall summary score; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; 6MWD, 6-min hall walk distance.
Discussion
In the present single-arm study, implantation of the MobiusHD system in patients with HFrEF was feasible and safely improved health status, exercise capacity, and LVEF, consistent with observed reductions in NT-proBNP levels during 12-month follow-up.
All patients enrolled in the present study had NYHA class III HFrEF and remained symptomatic despite GDMT (with more than three-quarters of patients taking 3 of the major class I indicated drug classes for HFrEF, a higher proportion than in most studies).20 Such patients have a poor health status and inferior QOL, as reflected in their limited baseline 6MWD and low KCCQ scores, with high rates of 1-year mortality and HF hospitalizations.10 Performed by interventionalists experienced with carotid stenting, implantation of the MobiusHD device in the normal carotid vasculature was straightforward (mean procedural duration of 27 minutes), with no procedural complications. EVBA did not cause hypotension or bradycardia, either in the periprocedural period or during 1-year follow-up. Effectiveness outcomes through 12 months were favorable, with clinically meaningful improvements in KCCQ OSS and 6MWD, reflecting improvements in functional capacity and QOL patient-reported outcomes. Although these measures are subject to bias in an open-label trial, there were also objective changes in NT-proBNP levels and LVEF, with progressive improvements in these parameters during the 1-year follow-up period. These favorable biomarker changes have been associated with reduced rates of death and HF hospitalization in large outcomes studies.20, 21, 22, 23
The results of the present study are supportive of a potential beneficial impact of EVBA with the MobiusHD device in HFrEF. From prior studies, a 5 point increase in KCCQ, a 25-30 m increase in 6MWD, and a 10-30% reduction from baseline in NT-proBNP are considered clinically meaningful.23 In the present study, most of these measures improved to a greater degree than these benchmarks after treatment with the MobiusHD device (Table 7). The improvement in NT-proBNP over time was concordant with improvements in KCCQ OSS and 6MWD and (Figure 3), consistent with the findings from earlier studies with effective HFrEF therapies.20 The improvement in LVEF demonstrated in this study over time, likely due to LV unloading, is also reflected in reduced NT-proBNP levels during follow-up.
The MobiusHD device is a permanent carotid implant warranting careful examination of its safety profile, especially as regards cerebrovascular events. Of note, device use is restricted to patients without carotid atherosclerosis, which should minimize the risk of embolic events. No procedural strokes were observed. One patient did develop a late stroke at 6 months postimplant, a time period at which the device would be expected to be endothelialized. This patient had chronic atrial fibrillation, and the event was temporally related to their discontinuation of chronic oral anticoagulation. MRI demonstrated a new defect ipsilateral to the device, although in the posterior circulation, more consistent with a cardiac than a carotid embolic event. Nonetheless, a relationship between the device and this event cannot be excluded.
In addition, 6 patients (20.7%) had a diffusion weighted MRI defect noted on predischarge imaging. These events are most likely due to catheter-related embolized atherothrombotic debris from the aorta, and this rate is consistent with MRI findings reported for cerebral angiography and other endovascular procedures.24, 25, 26 No symptoms were noted in association with these findings in the present study, and NIHSS scores were zero for all these patients during follow-up. However, whether asymptomatic atheroemboli from cardiovascular procedures result in subtle chronic changes in cognitive function is unsettled.27, 28 Moreover, any such deleterious changes may be positively offset from improved mental functioning from increased cardiac output and cerebral perfusion. Nonetheless, future studies with the MobiusHD implant should include careful baseline and follow-up MRI assessments in all patients, as well as longitudinal measures of cognitive function.
The 1-year Kaplan-Meier estimated rates of death and HF hospitalization were 8.2% and 4.3%, respectively. Neither of the 2 deaths after MobiusHD treatment were attributed to the device. While no conclusions can be drawn given the uncontrolled nature of the present study, these rates compare favorably to other studies in NYHA class III HFrEF patients. For example, among control group patients enrolled in the CHAMPION trial (all of whom were NYHA class III), the 1-year rates of death and HF hospitalization were approximately 20% and 30%, respectively.17
Limitations
The present study is limited by its small sample size and open-label design without a concurrent control. Placebo effect and regression to the mean may have contributed to the improvements in the study group. Serial cognitive function was not assessed. While medical therapy was generally excellent, SGLT2 inhibitors were not used, which would have improved the prognosis of the study group.29,30
Conclusions
In the present interim analysis, MobiusHD device implantation in HFrEF patients who remained highly symptomatic despite GDMT was safe and resulted in clinically meaningful improvements in health status, functional capacity, NT-proBNP levels, and LVEF changes that emerged within 3 months after device implantation and were sustained through 1 year. Adequately powered, sham-controlled, randomized trials are warranted to evaluate the safety and effectiveness of EVBA with the MobiusHD in high-risk HFrEF patients who are refractory to contemporary pharmacotherapy and approved device interventions.
Ethics Statement
The research reported has adhered to the relevant ethical guidelines. The protocol was approved by the ethics committee of each participating site and all patients provided written informed consent.
Funding
The authors report no funding in support of this paper.
Disclosure statement
Dr. H. Sievert has received institutional honoraria, travel expenses, and consulting fees from 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, CardiacDimensions, Cardimed, Celonova, Comed B.V., Contego, CVRx, Dinova, Edwards Lifesciences, Endologix, Hemoteq, Hangzhou Nuomao Medtech, Holistick Medical, Lifetech, Maquet Getinge Group, Medtronic, Mokita, Occlutech, Recor, RenalGuard, Terumo, Vascular Dynamics, Vectorious Medtech, Venus, Venock, and Vivasure Medical. Dr. Januzzi is a Trustee of the American College of Cardiology, a Board member of Imbria Pharmaceuticals, has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, Jana Care, Novartis Pharmaceuticals, Prevencio and Roche Diagnostics, consulting income from Abbott, Janssen, Novartis, Prevencio, and Roche Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, Takeda, and Vifor. Dr. Lindenfeld receives grant funding from AstraZeneca, NovoNordisk, and Volumetrix and consulting fees from AstraZeneca, Abbott, Alleviant, Boston Scientific, Boehringer Ingelheim, CVRx, Edwards Lifesciences, Medtronic, Merck, TegoSense, Vascular Dynamics, and V-Wave. Dr. Rothmann is an employee of Vascular Dynamics. Dr. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, Gore, Amgen; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, Xenter. Dr. Stone’s daughter is an employee at Medtronic. Institutional disclosure: Dr. Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, and V-wave. All other authors have nothing to disclose.
References
- 1.Ponikowski P., Anker S.D., AlHabib K.F., et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 2.Fudim M., Abraham W.T., von Bardeleben R.S., et al. Device therapy in chronic heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(9):931–956. doi: 10.1016/j.jacc.2021.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton G., Masters J., Cowburn P.J. Multidisciplinary team approach to heart failure management. Heart. 2018;104(16):1376–1382. doi: 10.1136/heartjnl-2016-310598. [DOI] [PubMed] [Google Scholar]
- 4.Seferovic P.M., Ponikowski P., Anker S.D., et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 5.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 6.Cook C., Cole G., Asaria P., Jabbour R., Francis D.P. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Abraham W.T., Zile M.R., Weaver F.A., et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail. 2015;3(6):487–496. doi: 10.1016/j.jchf.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Gronda E., Seravalle G., Brambilla G., et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16(9):977–983. doi: 10.1002/ejhf.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver F.A., Abraham W.T., Little W.C., et al. Surgical experience and Long-term results of baroreflex activation therapy for heart failure with reduced ejection fraction. Semin Thorac Cardiovasc Surg. 2016;28(2):320–328. doi: 10.1053/j.semtcvs.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Zile M.R., Lindenfeld J., Weaver F.A., et al. Baroreflex activation therapy in patients with heart failure with reducd ejection fraction. JACC. 2020;76(1):1–13. doi: 10.1016/j.jacc.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Davos C.H., Davies L.C., Piepoli M. The effect of baroreceptor activity on cardiovascular regulation. Hellenic J Cardiol. 2002;43:145–155. [Google Scholar]
- 12.Chapleau M.W., Hajduczok G., Abboud F.M. Peripheral and central mechanisms of baroreflex resetting. Clin Exp Pharmacol Physiol Suppl. 1989;15:31–43. doi: 10.1111/j.1440-1681.1989.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 13.Chapleau M.W., Hajduczok G., Abboud F.M. New insights into the influence of pulsatile pressure on the arterial baroreceptor reflex. Clin Exp Hypertens. 1988;A10(Suppl 1):179–191. doi: 10.3109/10641968809075971. [DOI] [PubMed] [Google Scholar]
- 14.Spiering W., Williams B., Van der Heyden J., et al. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet. 2017;390(10113):2655–2661. doi: 10.1016/S0140-6736(17)32337-1. [DOI] [PubMed] [Google Scholar]
- 15.van Kleef M., Devireddy C.M., van der Heyden J., et al. Treatment of resistant hypertension with endovascular baroreflex amplification: 3-year results from the CALM-FIM study. JACC Cardiovasc Interv. 2022;15(3):321–332. doi: 10.1016/j.jcin.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Peter D.A., Alemu Y., Xenos M., et al. Fluid structure interaction with contact surface methodology for evaluation of endovascular carotid implants for drug-resistant hypertension treatment. J Biomech Eng. 2012;134(4):041001. doi: 10.1115/1.4006339. [DOI] [PubMed] [Google Scholar]
- 17.Triposkiadis F., Karayannis G., Giamouzis G., Skoularigis J., Louridas G., Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Zalawadiya S., Cox Z., Simonato M., et al. Reasons for less than maximal target doses of heart failure medications in patients enrolled in the COAPT trial. J Am Coll Cardiol. 2021;78(19 Suppl):B115–B116. [Google Scholar]
- 19.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 20.Abraham W.T., Adamson P.B., Bourge R.C., et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt G.H., Sullivan M.J., Thompson P.J., et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 22.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 23.Zile M.R., Claggett B.L., Prescott M.F., et al. Prognostic implications of changes in N-Terminal Pro-B-Type Natriuretic Peptide in patients with heart failure. JACC. 2016;68(22):2425–2436. doi: 10.1016/j.jacc.2016.09.931. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira J.P., Duarte K., Graves T.L., et al. Natriuretic peptides, 6-min walk test, and quality-of-life questionnaires as clinically meaningful endpoints in HF trials. JACC. 2016;68(24):1–51. doi: 10.1016/j.jacc.2016.09.936. [DOI] [PubMed] [Google Scholar]
- 25.Weber M., Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savarese G., Orsini N., Hage C., et al. Utilizing NT-proBNP for eligibility and nrichment in trials in HFpEF, HFmrEF, and HFrEF. J Am Coll Cardiol. 2018;6:246–256. doi: 10.1016/j.jchf.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Chuah K., Stuckey S., Berman I. Silent embolism in diagnostic cerebral angiography: Detection with diffusion-weighted imaging. Australas Radiol. 2004;48(2):133–138. doi: 10.1111/j.1440-1673.2004.01273.x. [DOI] [PubMed] [Google Scholar]
- 28.Bendszus M., Koltzenburg M., Burger R., Warmuth-Metz M., Hofmann E., Solymosi L., et al. Silent Embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354(9190):1594–1597. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 29.Pina I.L., Camacho A., Ibrahim N.E., et al. Improvement of health status following initiation of sacrubitril/valsartan in heart failure and reduced ejection fraction. JACC. 2021;9(1):42–51. doi: 10.1016/j.jchf.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Sato M., Nakai Y., Tsurushima H., Shiigai M., Masumoto T., Matsumura A., et al. Risk factors of ischemic lesions related to cerebral angiography and neuro interventional procedures. Neurol Med Chir (Tokyo) 2013;53(6):381–387. doi: 10.2176/nmc.53.381. [DOI] [PubMed] [Google Scholar]