Abstract
The present study aims to validate the Gompertz model to predict the growth performance of chicken crosses according to growth curve parameters of the parental lines and the estimated heterosis for each curve parameter. A total of 252 one-day-old chicks of both sexes belonging to 6 genotypes, including Ross 308, Sassò (SA), Bionda Piemontese (BP), and Robusta Maculata (RM), and the crosses between these local breeds and SA (BP × SA and RM × SA) were randomly allocated in 18 pens (3 pens/genotype) in mixed-sex groups (14 animals/pen; 7 females and 7 males). The individual body weight (BW) of all birds was recorded once a week from hatching until slaughtering (81 d for Ross 308; 112 d for SA, 140 d for the other genotypes). We drew up our final dataset with 240 birds (40 birds/genotype; 20 females and 20 males). The growth curve of each genotype was described using the Gompertz model, and the heterosis for each growth curve parameter was calculated as the difference between F1 crosses and the average of parental breeds. The predicted growth curve parameters were evaluated by cross-validation. The Gompertz model accurately estimated the growth curves of all the genotypes (R2 > 0.90). Heterosis was significant for almost all growth curve parameters in both crosses (P < 0.05). Heterosis ranged from −13.0 to +11.5%, depending on parameters, but varied slightly between the crossbreeds (BP × SA and RM × SA). The predicted values of adult BW, weight at the inflection point, and maximum growth rate were overestimated for BP × SA and underestimated for RM × SA, with a mean error between observed and predicted values <│2.7│% for all the curve parameters. In conclusion, the growth performance of chicken crosses between local breeds and commercial strains can be accurately predicted with Gompertz parameters of the parental lines adjusting for heterosis.
Key words: poultry, genetic selection, hybrid vigor, conservation program
INTRODUCTION
Intensification of livestock production has led to the replacement of local breeds with crossbreds from highly selected genetic strains characterized by very favorable productive performances and feed efficiency. The resulting loss of biodiversity in farmed animal species is a well-known issue and is particularly severe in the poultry sector (Moula et al., 2009). In fact, the selection of chicken genotypes is managed by the international companies that provide chicks for the productive chain (Phocas et al., 2016). High-performance chicken genotypes often have several health and welfare issues, such as impaired locomotor ability, worsened immune response, increased susceptibility to biotic and abiotic stresses and diseases, and low meat quality (Soleimani et al., 2011; Petracci et al., 2015; Hartcher and Lum, 2020). On the other hand, local breeds can be considered part of the history of human populations as well as important genetic heritage and are classified as an important genetic resource in ensuring food security for countries around the world (Padhi, 2016; Boonkum et al., 2021). Such breeds have been subjected to selection by endemic diseases, climate conditions, feed availability, and other environmental-related factors. This has created a large diversity in morphological and physiological traits, providing a source of genes for future breeding and research purposes (Bianchi et al., 2012).
In Italy, as well as in other countries, there are numerous native chicken breeds that are characterized by marked explorative, kinetic, and foraging behavior (Dal Bosco et al., 2021), making them suitable for alternative low-input rearing systems (i.e., organic, free-range, agroforestry).
Considering the actual debate about the choice of suitable chicken genotypes for sustainable rearing systems, as also taken into account by the European Chicken Commitment, the safeguarding of local breeds with these distinctive features has become increasingly important. In Italy, 67% of the 53 recognized local chicken breeds are extinct, while the conservation status of 18 Italian chicken breeds has been classified as endangered or critically endangered (FAO, 2020). Despite national and international conservation programs, several studies have shown that local chicken breed populations are still endangered (Castillo et al., 2021; Franzoni et al., 2021). A recent survey conducted on 2 well-known Italian chicken breeds (i.e., Ancona and Livorno) in their area of origin confirmed that their rearing is confined to small-scale “hobby farms” (Cartoni Mancinelli et al., 2020). Compared to commercial hybrids, local breeds display very poor growth performance and feed efficiency, which are the main factors limiting their commercial use.
Crossbreeding between a local breed and a more productive chicken strain is a widespread strategy used to increase growth performance, egg production, gain desirable antioxidant properties, obtain more resistant animals with intermediate performance and perhaps to exploit hybrid vigor (i.e., heterosis) (Sungkhapreecha et al., 2022; Wang et al., 2022).
The magnitude of heterosis increases with increasing genetic distance of the parental lines (Wang et al., 2021), and its measurement is important to evaluate the improvement in crossbreed characteristics compared with their parents (Razuki and Al-Shaheen, 2011; Phocas et al., 2016; Soliman et al., 2020). Therefore, crossbreeding and heterosis represent an essential part of breeding strategies in chickens (Amuzu-Aweh et al., 2015). Indeed, several authors (Khawaja et al., 2012; Castellini et al., 2016) have shown that crossbred chickens have higher body weights, better feed efficiency, and lower mortality than purebred chickens. Most genetic approaches simply consider the final body weight (BW) of the parental lines, without analyzing the dynamics of their growth rate. However, the productive gap of local breeds is related not only to the low weight of adult chickens but also to low precocity and body structure. Thus, to make the crossbreeding strategy more efficient, it could be useful to characterize the growth curve of local breeds and commercial chicken genotypes. Using mathematical functions for describing the growth curve, it is possible to estimate the BW that chickens will reach at a specific age. Different nonlinear equations have been proposed for this purpose and, among these, logistic, Von Bertalanffy, Gompertz, and Richards models showed good fitness (Afrouziyeh et al., 2021; Narinç et al., 2017). In particular, the Gompertz model has been commonly used in poultry to compare the growth performance of slow-growing breeds (N'dri et al., 2018; Soglia et al., 2020), due to its low bias and the advantage of requiring only 3 parameters (Rizzi et al., 2013; Afrouziyeh et al., 2021; Narinç et al., 2017). Many authors (Aggrey, 2002; Wang and Zuidhof, 2004; Ersoy et al., 2006) have reported that using the shape of the Gompertz curve to calculate BW in relation to age would be an effective strategy for evaluating the productive traits of local breeds and their crossbreeds. The robustness of the ability of the Gompertz model to predict their growth pattern remains to be determined.
Thus, the aim of this study was to validate the Gompertz model as a tool to predict the growth performance of chicken crosses based on the growth curve traits of their parental lines and the estimated heterosis of the curve parameters. To do so, the growth curves of commercial genotypes (i.e., Ross 308 and Sassò), 2 Italian local breeds (i.e., Bionda Piemontese and Robusta Maculata) and their crosses with Sassò (SA), were analyzed using the Gompertz model; the heterosis for each growth curve parameter was calculated and used to predict the growth performance of crossbreds, and the estimates were evaluated through cross-validation.
MATERIALS AND METHODS
Ethics Statement
The Ethical Committee of the University of Perugia approved the experimental procedure (project ID: 62700, approved on 15/07/2020). All animals were raised, managed, and processed according to the regulation 2007/43/EC for the protection of chickens kept for meat production and the regulation 2010/63/EU for the protection of animals used for scientific purposes. Research staff involved in animal handling were animal specialists (PhD or MS in Animal Science) and veterinary practitioners.
Experimental Design
This study is part of a larger research project (PRIN LoChAl 2017) dealing with the characterization, conservation, and valorization of Italian local chicken breeds. Two Italian breeds were selected for this study: Bionda Piemontese (BP) and Robusta Maculata (RM). The BP and RM breeds are 2 slow-growing dual-purpose local chicken breeds. The BP breed (rooster average live weight = 2.5–2.8 kg; eggs produced per year = ∼190) originates from the Piemonte region (Northwest Italy); this breed is characterized by blond plumage and a black tail. The RM breed (rooster average live weight = 4.0–4.5 kg; mean eggs produced per year = ∼150) was selected in the middle of the last century in the Stazione Sperimentale di Pollicoltura, Veneto region (Northeast Italy) (Ferrante et al., 2016). The RM rooster is characterized by white plumage with irregular black spots, while the hens are characterized by white plumage with large dark gray to black spots spread irregularly over the whole body.
Parental breeds, such as the SA breed, are used for producing crossbred broilers with more favorable performance as a way to conserve these local breeds. SA is a fast-growing breed commonly used in crossbreeding programs with local breeds because of its recessive plumage characteristics. In particular, the SA T44 chosen in this study is a dual-purpose line characterized by red plumage.
A “high-performance” (both fast growth rate and high adult BW) chicken line was included in the experimental design as an external “control” line to test the growth modeling equation on a dataset characterized by great variability in growth rate, patterns of the growth curves, and somatic precocity. Moreover, Ross 308 was selected, as it is one of the fastest-growing chicken lines used in intensive broiler production worldwide. This genotype is characterized by a white plumage and reaches commercial weight (2.8–3 kg) in 42 to 45 d (performance objectives reported by the company).
The experimental design was then based on a comparison among 6 different chicken genotypes.
These 6 strains are very different from each other in terms of not only growth rate but also maturity precocity, which makes it very difficult to adopt a standard for determining the slaughtering age. This is one of the reasons why growth modeling was not based on “statistical” approaches maximizing the fitting ability of the experimental data but on a model based on few equation parameters easily interpreted from a biological/physiological point of view. The use of this type of equation allows users to compare strains tested at different end-point criteria. Therefore, the Gompertz curve was selected.
Facilities, Animals, and Recordings
This study was conducted at the experimental farm of the University of Perugia (Perugia, Italy) in a closed building with forced ventilation, radiant heating, and controlled lighting systems.
A total of 252 one-day-old chicks of both sexes belonging to 6 genotypes were used: 42 chicks of a high-producing commercial genotype, Ross 308 (Aviagen Group, Midlothian, United Kingdom) (21 females, 21 males); 42 chicks of SA (Sassò T44, Hendrix Genetics, Boxmeer, The Netherlands) (21 females, 21 males); 42 chicks of BP (21 females, 21 males); 42 chicks of RM (21 females, 21 males), 42 chicks of BP × SA (21 females, 21 males); and 42 chicks of RM × SA (21 females, 21 males). Reproducing females and males (144 breeder females and 18 roosters; 24 females and 3 males per genotype) of both local breeds and Sassò strain were reared at the experimental farm of the University of Perugia. Birds were reared in pens (2 m2/each; 3 pens/genotype) with 1 rooster and 8 females per pen under natural mating conditions. The crosses BP × SA and RM × SA were obtained by crossing roosters of the local breeds with SA breeder females. The eggs of BP, RM, BP × SA and RM × SA were collected in 1 wk and incubated under the same conditions in a commercial hatchery. The same hatchery provided and incubated the eggs of Ross 308.
Chicks were vaccinated at the hatchery (for coccidiosis, infectious bronchitis, Marek's, New-castle, and Gumboro disease) and then transported to the poultry house, individually identified through a numbered ring, and randomly allocated to 18 pens (2 m2/each; 3 pens/genotype) in mixed-sex groups (14 animals/pen; 7 females and 7 males). Each pen was equipped with 5 nipple drinkers and 1 circular feeder (diameter: 370 mm) for the manual distribution of feed. The pens had a concrete floor covered with wood shaving litter (depth 5 cm, 2.5 kg/m2). The relative humidity was maintained in the range of 65 to 70% throughout the rearing period; the temperature was set to 31°C to 30°C during the first week, decreased by 2°C to 3°C each week down to 23°C to 21°C at the end of the fourth week, and then maintained at 21°C to 20°C until the end of the trial. The photoperiod was set to 23L:1D during the first 2 d after chick arrival, and then the hours of light were progressively decreased to reach the 18L:6D-photoperiod from 12 d of age until the end of the rearing period. Water and feed were provided ad libitum.
Although the feed requirements of the local and commercial breeds are different, we used the same diets as in other similar previous experiments (Cartoni Mancinelli et al., 2022), which were formulated according to the nutritional recommendations for broiler chickens. Therefore, all the chicken strains were fed the same diets in crumble form as follows: starter (crude protein 20.80%, ether extract 5.60%, crude fiber 3.50%, ash 7.00%, lysine 1.27%, methionine 0.58%, calcium 1.10%, phosphorus 0.72%, sodium 0.15%) from 0 to 21 d; grower (crude protein 18.30%, ether extract 5.20%, crude fiber 3.60%, ash 6.50%, lysine 1.06%, methionine 0.49%, calcium 0.90%, phosphorus 0.70%, sodium 0.14%) from 22 d until slaughter.
The individual BW of all chickens was recorded at their arrival (0 d of age) and then once a week until slaughtering. Due to the different growth rates and precocity of the 6 genotypes, the slaughtering of the animals took place at different ages: Ross 308 at 12 wk (81 d); SA at 16 wk (112 d); RM, BP, RM × SA, and BP × SA at 20 wk (140 d).
Growth Modeling
At the end of the growth trial, the dataset was edited to remove, within each genotype, birds with a final BW (weight at slaughtering) outside the average value ±2.5 standard deviations. Thus, a final dataset was obtained including 40 birds/genotype (20 females and 20 males; 240 subjects in total).
As described by Menchetti et al. (2020) and González Ariza et al. (2021a), the Gompertz model was used to analyze the growth curve of each animal according to the following equation:
where Y is the BW reached at age t; A is the upper asymptote or adult weight; t is the time; b is the parameter of the curve describing the proportion of the asymptotic adult weight to be gained after birth; and k is the instantaneous relative growth rate. Large k values indicate early maturing animals. Other parameters with biological meaning were derived by considering the time taken to reach 50, 70, and 99% of adult BW (T50, T70, and T99, respectively). Moreover, several parameters referring to the inflection point (ip), or the time at which the growth rate reached its maximum, were calculated. In particular, the BW at ip (g) was obtained as BWip = A/e, the age at ip (day) as tip = (ln(b))/k, and the maximum growth rate (MGR) or rate at ip (g/d) as BWip × k (González Ariza et al., 2021b).
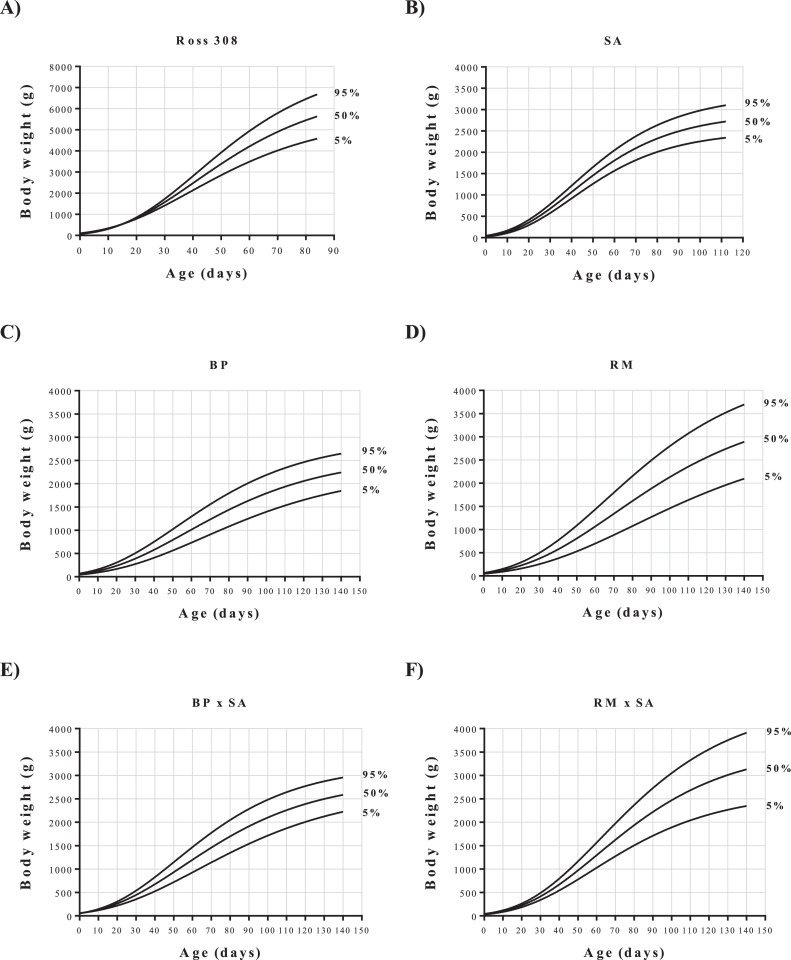
The goodness of fit was assessed using the R2 (coefficient of determination) and corrected Akaike's information criteria (AICc) (Menchetti et al., 2020). Finally, z scores were computed, and growth centile curves were built showing 5, 50, and 95% centiles for each genotype (Menchetti et al., 2020).
Statistical Analysis
The effect of genotype on the growth curve and derived indices was investigated by 1-way ANOVA. First, Levene's test and diagnostic charts were used to verify the assumptions. When the homogeneity of variance assumption was not met, logarithmic transformations and Welch's F were used. The results for unequal variances were used, as Levene's test was significant for all the parameters, and the logarithmic transformation did not significantly improve the homoscedasticity.
To compare the least squares means (LSM) of the 6 strains, the following orthogonal contrasts were performed:
-
(1)
Ross 308 vs. SA: comparison between the high-performance control strain and the fast-growing crossing strain
-
(2)
SA vs. [(BP + RM)/2]: comparison between the crossing strain and the local Italian breeds
-
(3)
BP vs. RM: comparison between the 2 Italian local breeds
-
(4)
BP × SA vs. [(BP + SA)/2]: heterosis quantification between SA and BP breeds
-
(5)
RM × SA vs. [(RM + SA)/2]: heterosis quantification between SA and RM breeds.
Recorded BW data were analyzed with a repeated measures model, which included the genotype, the age of the animals, and their interactions as fixed effects. An autoregressive model was chosen after checking the goodness-of-fit criteria compared to other covariance structures (Littell et al., 1998).
The heterosis (H%) was calculated according to the formula proposed by Fairfull (1990):
where F1 are the average values of traits of the crosses and Р1 and P2 are the average values of traits of the parental lines. Given that the diallel crossbreeding design was not complete, the H(%) was not corrected for the maternal effect.
The average growth curve parameters of F1 chickens (BP × SA and RM × SA) were predicted based on the average growth curve parameters of the parental lines corrected for heterosis according to the following formula:
where y is the Gompertz growth curve parameter; Р1 and P2 are the average values of the growth parameters of the parental lines; and H is the heterosis expressed in F1.
The predicted growth curve parameters for crossbreeds were evaluated by cross-validation. For the cross-validation, the main dataset was split into 2 subdatasets with 80% (32 subjects/genotype; 16 females and 16 males) and 20% (8 subjects/genotype; 4 females and 4 males) of the observations for the training and test datasets, respectively. The heterosis was calculated in the training dataset and applied in the test dataset to estimate all the growth curve parameters. The mean error between the observed and predicted values was calculated (as absolute value and percentage) and used to quantify the precision of the predictive model.
Statistical analyses were performed with SPSS Statistics version 25 (IBM, SPSS Inc., Chicago, IL) and GraphPad Prism, version 7.0 (GraphPad Software, San Diego, CA). The level of statistical significance was set at P < 0.05.
RESULTS
Growth Patterns
The Gompertz model accurately estimated the growth curves of the commercial genotypes (Ross 308 and SA), local chicken breeds (BP and RM), and their crosses with SA (Table 1). R2 values indicated that the growth curves explained more than 90% of the variance. The Ross 308 genotype showed the best-fitting values, as indicated by the lowest AICc, while the worst was found for RM.
Table 1.
Goodness of fit of weekly body weight of 6 chicken strains by Gompertz curves.
| Genotype |
||||||
|---|---|---|---|---|---|---|
| Parameter | Ross 308 | SA | BP | RM | BP × SA | RM × SA |
| R2 | 0.968 | 0.972 | 0.943 | 0.907 | 0.964 | 0.934 |
| AICc | 4875 | 5449 | 7009 | 7698 | 6972 | 7533 |
R2 = coefficient of determination; AICc = corrected Akaike's information criteria; BP = Bionda Piemontese; RM = Robusta Maculata; SA = Sassò; BP × SA = cross BP × SA; RM × SA = cross RM × SA.
Table 2 reports the growth curve parameters and the inflection-point traits of the different genotypes. Ross 308 chickens exhibited the highest adult BW and MGR. While the estimated adult BW of SA chickens was much lower than that of Ross 308 chickens (2,946 g vs. 6,909 g, respectively; P < 0.001), the precocity indices were similar between the 2 commercial genotypes: both had k = 0.036 and reached 50, 70, and 99% of their adult BW at approximately 51 d, 69 d, and 168 d, respectively. BP chickens had the lowest adult BW (approximately 2,600 g), BWip (approximately 960 g), and MGR (22.0 g/d). RM chickens had an adult BW greater than SA and BP but had the worst precocity indices, taking approximately 90 d, 120 d, and 300 d to reach 50, 70, and 99% of their adult BW, respectively. All the parameters of the SA growth curve significantly differed from those of BP and RM (P < 0.001; Table 2).
Table 2.
Estimated growth parameters, growth characteristics, and inflection points of different chicken genotypes (n = 32/genotype).
| Genotype |
Contrasts (P value) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Ross 308 | SA | BP | RM | BP × SA | RM × SA | Ross 308 vs. SA | SA vs. BP + RM | BP vs. RM | BP × SA vs. BP + SA | RM × SA vs. RM + SA | RMSE |
| A (g) | 6909 | 2946 | 2607 | 3672 | 2955 | 3485 | <0.001 | 0.001 | <0.001 | <0.001 | 0.135 | 473 |
| B | 4.41 | 4.36 | 3.70 | 4.03 | 4.13 | 4.36 | 0.156 | <0.001 | <0.001 | 0.091 | 0.027 | 0.35 |
| k | 0.036 | 0.036 | 0.023 | 0.020 | 0.026 | 0.024 | 0.813 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
| T50 (d) | 51 | 50 | 72 | 87 | 69 | 76 | 0.018 | <0.001 | <0.001 | <0.001 | <0.001 | 5 |
| T70 (d) | 69 | 69 | 101 | 120 | 95 | 103 | 0.028 | <0.001 | <0.001 | <0.001 | <0.001 | 8 |
| T99 (d) | 168 | 167 | 257 | 296 | 233 | 250 | 0.329 | <0.001 | <0.001 | 0.001 | <0.001 | 23 |
| tip (d) | 41 | 41 | 57 | 69 | 55 | 61 | 0.026 | <0.001 | <0.001 | <0.001 | <0.001 | 4 |
| BWip (g) | 2541 | 1084 | 959 | 1350 | 1087 | 1282 | <0.001 | 0.001 | <0.001 | <0.001 | 0.135 | 174 |
| MGR (g/d) | 92.0 | 39.4 | 22.0 | 27.3 | 28.1 | 31.2 | <0.001 | <0.001 | <0.001 | 0.013 | 0.064 | 6.7 |
BP = Bionda Piemontese; RM = Robusta Maculata; SA = Sassò; BP × SA = cross BP × SA; RM × SA = cross RM × SA.
A = adult body weight (g); B and k = parameters (large k values indicate early maturing animals); T50, T70, and T99 = time taken to reach 50, 70, and 99% of adult body weight, respectively; tip = age at inflection point; BWip = body weight at inflection point; MGR = maximum growth rate (i.e., growth rate at inflection point); RMSE = root mean square error.
Significant differences in all growth curve parameters were also found between the local breeds. In particular, BP had lower adult BW and MGR but higher k than RM (P < 0.001); as a result, T50, T70, and T99 values were lower in BP than in RM (P < 0.001). Regarding the crosses, the increase in adult BW and BWip was significant for BP × SA (+6.4% on average; P < 0.001) but less evident for RM × SA (+5.3% on average; P > 0.10) compared to the mean of the parental lines. For the large majority of parameters (8 out of 9 parameters for BP × SA and 6 out of 9 parameters for RM × SA), the growth curves of the crosses were significantly different from the expected intermediate values with respect to their parental breeds.
The average adult BW of crosses was greater than the average of their parental strains in both cases, but the difference was significant only for BP × SA (Table 2). Additionally, the b parameter was greater than expected in both crosses but significant only in RM × SA. In contrast, the k parameter was lower than the average of their parental strains in both crosses, which means they had a lower somatic precocity, confirmed by the longer time required to reach 50, 70, and 99% of adult BW. As a result of the lower than expected precocity, the MGR of crosses was also lower than the average MGR of their parental strains (Table 2).
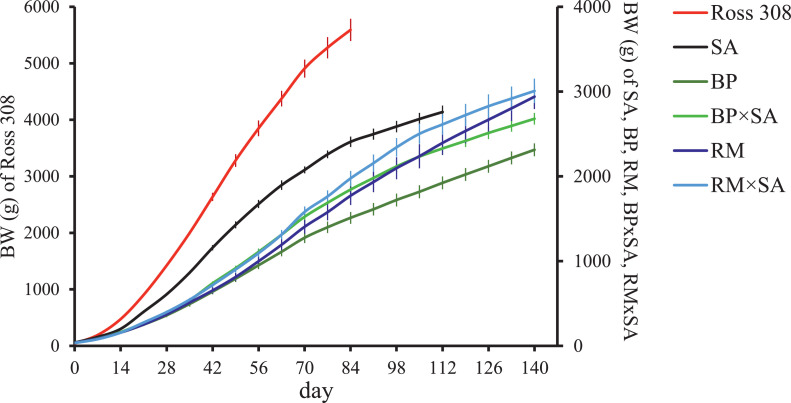
As expected, the growth patterns of the chicken genotypes showed that Ross 308 grew faster than other genotypes and had the highest BW at slaughtering (5,591 g at 81 d of age; approximately 80% of the adult BW). SA chickens reached a BW of 2,759 g (approximately 94% of the adult BW) at slaughtering (112 d of age), confirming their precocity. BP and RM chickens weighed 2,313 g (approximately 89% of the adult BW) and 2,938 g (approximately 80% of the adult BW), respectively, at 140 d of age (Figure 1). The cross of BP and RM males with SA females improved the precocity of the chickens, even though less than expected, and increased their BW at slaughtering compared to the local breeds. The increase in BW at slaughtering was particularly relevant for BP × SA (+367 g with respect to BP) and less evident for RM × SA (+69 g with respect to RM).
Figure 1.
Actual data of body weight (BW) trend (mean ± 95% confidential limits) of Ross 308, Sassò (SA), Bionda Piemontese (BP), Robusta Maculata (RM), and crossbreds (BP × SA and RM × SA) chickens from posthatching to slaughtering.
The percentile growth curve revealed that of the commercial genotypes, Ross 308 was less homogeneous than SA. On the other hand, among local breeds and their crosses with SA, RM, and RM × SA showed the highest variability (Figure 2).
Figure 2.
Growth chart percentiles of chickens Ross 308, Sasso (SA), Bionda Piemontese (BP), Robusta Maculata (RM), and crossbreds BP × SA and RM × SA.
Heterosis of Growth Curve Parameters and Validation of the Estimated Parameters
Table 3 shows the heterosis as calculated for the training dataset (80% of the original data) and the error resulting from applying the heterosis-based model to predict the growth curve parameters in the test dataset (20% of the original data). The heterosis varied from −13.0% (parameter k) to +11.5% (parameter tip). Parameters A, BWip, and MGR were overestimated for BP × SA, whereas they were underestimated for RM × SA. However, these differences were nearly irrelevant from a productive point of view (i.e., lower than 40 g, 15 g, and 0.5 g/d for A, BWip, and MGR, respectively). Moreover, BP × SA and RM × SA had similar values of heterosis for all the parameters.
Table 3.
Heterosis of the F1 chickens calculated in the training dataset and the error between observed and predicted values in the test dataset.
| Training dataset |
Test dataset |
Test error (absolute) |
Test error (percentage) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Heterosis (%) |
Observed |
Predicted |
|||||||
| BP × SA | RM × SA | BP × SA | RM × SA | BP × SA | RM × SA | BP × SA | RM × SA | BP × SA | RM × SA | |
| A (g) | 7.10⁎⁎⁎ | 5.06 | 3017 | 3509 | 3053 | 3484 | −37 | 25 | −1.22 | 0.72 |
| b | 4.26 | 4.31⁎ | 4.19 | 4.51 | 4.19 | 4.44 | 0.00 | 0.07 | 0.05 | 1.56 |
| k | −12.59⁎⁎⁎ | −13.46⁎⁎⁎ | 0.025 | 0.024 | 0.025 | 0.024 | 0.001 | 0.001 | 0.85 | −1.21 |
| T50 (d) | 12.63⁎⁎⁎ | 8.82⁎⁎⁎ | 72 | 78 | 73 | 76 | −1 | 2 | −2.39 | 2.51 |
| T70 (d) | 11.89⁎⁎⁎ | 8.03⁎⁎⁎ | 99 | 105 | 101 | 103 | −2 | 2 | −2.38 | 2.30 |
| T99 (d) | 10.74⁎⁎⁎ | 6.77⁎⁎⁎ | 243 | 253 | 249 | 248 | −6 | 5 | −2.35 | 1.94 |
| tip (d) | 13.34⁎⁎⁎ | 9.58⁎⁎⁎ | 57 | 62 | 58 | 61 | −1 | 1 | −2.40 | 2.70 |
| BWip (g) | 7.10⁎⁎⁎ | 5.06 | 1110 | 1291 | 1123 | 1282 | −13 | 9 | −1.22 | 0.72 |
| MGR (g/d) | −7.72⁎⁎ | −6.34 | 28.2 | 31.1 | 28.1 | 31.4 | 0.1 | −0.3 | 0.39 | −1.04 |
BP × SA = crossbred Bionda Piemontese × Sasso; RM × SA = crossbred Robusta Maculata × Sasso; A = adult body weight (g); B and k = parameters (large k values indicate early maturing animals); T50, T70, and T99 = time taken to reach 50, 70, and 99% of adult body weight, respectively; tip = age at inflection point; BWip = body weight at inflection point; MGR = maximum growth rate (i.e., growth rate at inflection point).
The asterisks indicate a significant effect of the heterosis.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
DISCUSSION
The present study shows the Gompertz model to be a valid tool for predicting the performance of chicken strains belonging to very different genotypes with great variability in adult BW and in somatic precocity. It also demonstrated that the Gompertz model could be used for predicting the growth curves of crossbred chickens, provided that the equation parameters of the parental breeds and the specific heterosis are known.
Such a tool could be important for crossbreeding programs aimed at improving productivity while maintaining the robustness and resilience of the local breeds. Indeed, indigenous chicken breeds are more tolerant of local and stressful conditions (Soleimani et al., 2011) and show positive adaptation to alternative rearing systems (Ferrante et al., 2016) but have low BW and/or low precocity (Bilalissi et al., 2022). Specifically, comparing the 2 pure breeds used in our study, BP exhibited the lowest BW and MGR, whereas RM had the lowest precocity. On the other hand, the Ross 308 genotype reached the highest BW in the shortest rearing period, confirming the efficacy of commercial genetic selection for faster growth rates (Zuidhof et al., 2014; Tallentire et al., 2018), provided they are kept in high-input farming systems.
In this study, the SA breed crossed with 2 Italian local chicken breeds (BP and RM) was shown to be a good candidate for crossbreeding, with a considerable adult BW (approximately 3,000 g) and precocity traits similar to Ross 308.
As in the present trial, several authors have used SA chickens to produce F1 with improved growth rate, feed intake, and hatchability compared to pure lines (Alemneh et al., 2021; Bilalissi et al., 2022). Our findings showed that SA differed from BP and RM for all the growth curve parameters (A, b, and k) and derived indices as well as for the BW from posthatching to slaughtering. However, the effect of crossbreeding varied markedly depending on the trait analyzed and on the characteristics of the parental line. Specifically, only BP crossbreeds significantly improved in terms of both precocity and BW at slaughtering compared to purebred BP, suggesting that this F1 could be reasonable from a commercial point of view. On the other hand, RM × SA was found to be more precocious than RM, reaching a higher BW from 70 d to 105 d of age. However, with increasing age, the difference in BW was gradually less relevant (it disappeared at 119 d), thus limiting the benefits of crossing RM with SA. Furthermore, as demonstrated by the growth chart percentiles, RM also showed high heterogeneity that could be explained by high sexual dimorphism (Soglia et al., 2020). Moreover, although the trend of BW was more homogeneous in SA than in RM, their cross did not reduce heterogeneity.
Through the estimation of the growth curve with the Gompertz model, it would be possible to identify the weakest parameters of local chickens (i.e., A, b, and k) and consequently establish the main traits to improve, thus permitting a choice of the most suitable combinations between different lines. For a better estimation, the growth curve parameters of a genetic strain, calculated as the mean of the parent traits, must be corrected for heterosis. Such a correction must be applied by considering that heterosis: i) is proportional to the genetic distance and the degree of heterozygosity of parental lines resulting from nonadditive genetic effects; ii) can be either positive or negative depending on the trait; and iii) is not permanent (Williams et al., 2002; Sutherland et al., 2018a).
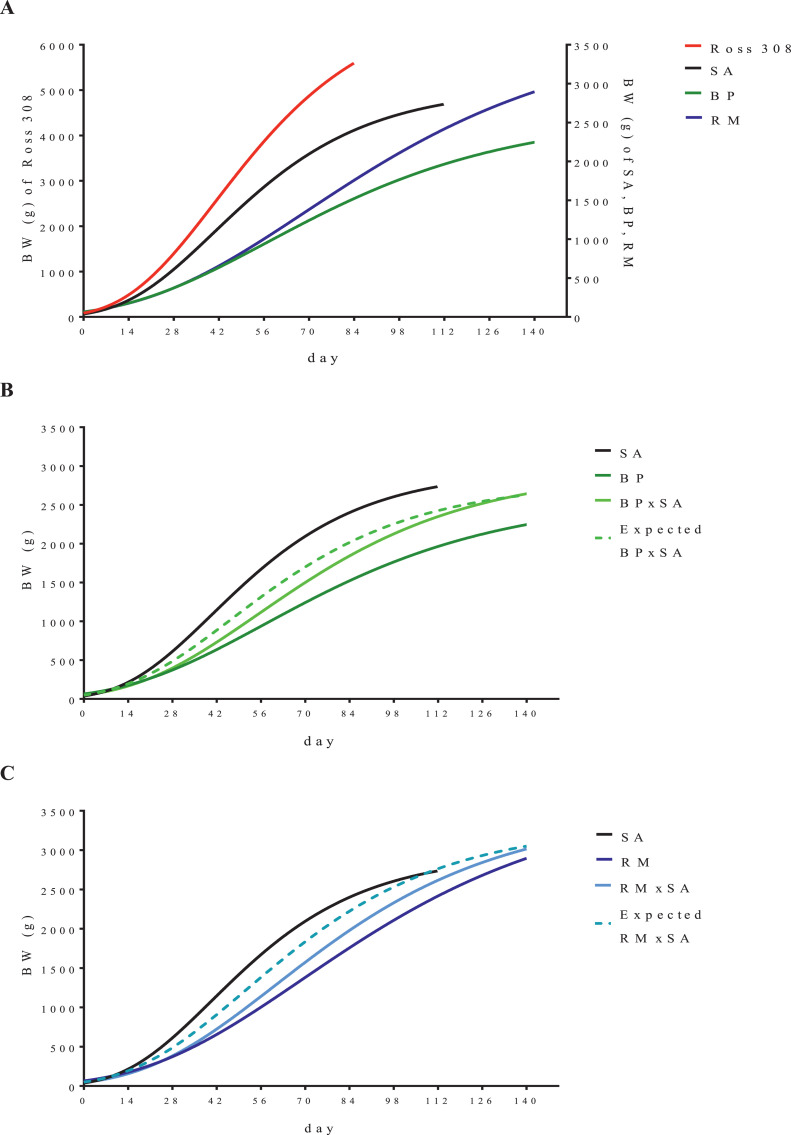
Moreover, the heterosis of chicken BW can vary according to their age (Iraqi et al., 2011; Lalev et al., 2014) and is generally lower than that of reproductive traits (Fairfull, 1990). Our results show that the growth curves of crossbreeds were significantly different from the expected growth curves calculated as the mean of the respective parental lines (Figure 3), which confirms the need to consider the heterosis effect to obtain reliable estimates.
Figure 3.
Gompertz growth curve of purebreds (Panel A), and of pure breeds with their respective crosses and expected curves (average values of the growth parameters of the parental lines, dashed line; Panel B and C) from posthatching to slaughtering calculated on training dataset.
Our findings suggest that heterosis values of 6, 4, and −13% for parameters A, b, and k, respectively, could be adopted to estimate the growth performance of F1 obtained by crossing roosters of pure local breeds with SA breeder females. Indeed, in agreement with our results, Lalev et al. (2014) estimated heterosis of 4 to 10 to 6% for BW at 18, 26, and 30 wk of age, respectively, by crossing 2 White Plymouth Rock lines. Similarly, reciprocal crosses between Rhode Island Red and White Leghorn chickens showed a heterosis of 8% on average for BW at 18 wk of age (Isa et al., 2020). A slightly higher heterosis (11% on average) for adult BW was observed by crossing red jungle fowl with a line of White Plymouth Rock chickens selected for low BW (Sutherland et al., 2018b). The latter study reported a negative heterosis for the MGR (−8% on average), in agreement with the present trial (−7% on average). Thus, crossdressing seems to be particularly useful to improve the adult BW and the BWip by having a positive heterosis effect. On the other hand, little information is available about the heterosis estimation of the precocity (k parameter). In our study, it was negative, which means that the precocity of the crossbreeds was lower than expected (mean of the parental lines). However, in both crossbreeds, the k value was improved with respect to the local breeds and, albeit to a lesser extent than expected, it could be interesting from a commercial point of view. To the best of our knowledge, this is the first study validating the use of a predictive model in crossbreeding programs for improving the growth performance of local chicken breeds. Moreover, thanks to the Gompertz function, not only the adult BW and the MGR but also the entire growth trend were predicted. Monitoring and forecasting the BW of chickens each week can assist in choosing the most convenient slaughtering age for the different genotypes. The accuracy of this approach is confirmed by the error between the observed and predicted values of the cross-validation which was lower than │2.7│% for all the growth curve parameters. In the present study, the percentile curves were also calculated. They could be a useful tool for farmers and geneticists to monitor growth and optimize animal management and feeding practices (Menchetti et al., 2020).
Despite the low level of incertitude of our results, it would also be possible to estimate the growth performances of backcrosses (F2), an approach frequently used in selective programs for the improvement of native chicken breeds, maintaining high allelic richness and reducing inbreeding, which is critical in small populations (Zanetti et al., 2011; Özdemir et al., 2016). For example, in the present study, the projections of the growth performance of the backcross BP × SA × BP (considering the heterosis as half of that expressed in F1) would improve all the growth curve parameters with respect to the pure local breed. Instead, the cross RM × SA followed by a backcross toward RM would lead to F2 with a growth curve very close to that of the local breed, thus reducing the meaning of this program and suggesting that another crossline having both higher precocity and BW would have been more suitable.
Further studies with larger datasets and different pure lines, reciprocal crosses and backcrosses are required to confirm the robustness of the approach proposed in the present work. Moreover, testing over several seasons and different environmental conditions would improve the reliability of the predictive model.
CONCLUSIONS
The present study confirms that the growth performance of chicken crosses can be predicted using the Gompertz growth curves of the parental lines adjusting for heterosis. Indeed, while the Gompertz model accurately estimated the growth curves of all the tested genotypes, the heterosis varied slightly in F1 chickens obtained by roosters of the 2 local breeds crossed with SA breeder females. Regarding the genotypes tested here, the analysis of the growth curves suggests that the cross between BP and SA yields better results in terms of improved precocity, growth rate, and adult BW than RM × SA. However, the heterosis values indicated that the improvement related to precocity and MGR was less than expected. The growth curve of chicken crosses can be predicted with a mean error lower than 2.7%, indicating that the model could be applied in future crossbreeding programs as a powerful tool to conserve and improve local chicken breeds. Further studies are needed to better establish the relationship between heterosis and growth curve traits.
ACKNOWLEDGMENTS
The authors would like to thank Giovanni Migni for technical support.
Funding: This work was supported by PRIN2017, grant number 2017S229WC.
DISCLOSURES
The authors declare that they have no conflicts of interest.
REFERENCES
- Afrouziyeh M., Kwakkel R.P., Zuidhof M.J. Improving a nonlinear Gompertz growth model using bird-specific random coefficients in two heritage chicken lines. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggrey S.E. Comparison of three nonlinear and spline regression models for describing chicken growth curves. Poult. Sci. 2002;81:1782–1788. doi: 10.1093/ps/81.12.1782. [DOI] [PubMed] [Google Scholar]
- Alemneh W., Berihun K., Melesse A. Comparative study on carcass quality characteristics of indigenous chickens and their F1-crosses with the Sasso chicken breed in Sheka zone, South Western Ethiopia. Int. J. Food Sci. Agric. 2021;5:692–697. [Google Scholar]
- Amuzu-Aweh E.N., Bovenhuis H., de Koning D.J., Bijma P. Predicting heterosis for egg production traits in crossbred offspring of individual White Leghorn sires using genome-wide SNP data. Genet. Sel. Evol. 2015;47:27. doi: 10.1186/s12711-015-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C.N., Morri C., Chiantore M., Montefalcone M., Parravicini V., Rovere A. In: Pages 1–55 in Life in the Mediterranean Sea: A Look at Habitat Changes. Stambler N., editor. Bar Ilan University; Ramat Gan, Israel: 2012. Mediterranean Sea biodiversity between the legacy from the past and a future of change. [Google Scholar]
- Bilalissi A., Lombo Y., Kossoga K.A., Tona K., Batimsoga B.B., Voemesse K., Tare T.P., Bagna B., Oke O.E. Influence of crossbreeding of Sasso and Faso chickens on some hatching events and post-hatch performances. Int. J. Food Sci. Agric. 2022;6:228–236. [Google Scholar]
- Boonkum W., Duangjinda M., Kananit S., Chankitisakul V., Kenchaiwong W. Genetic effect and growth curve parameter estimation under heat stress in slow-growing Thai native chickens. Vet. Sci. 2021;8:297. doi: 10.3390/vetsci8120297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni Mancinelli A., Di Veroli A., Mattioli S., Cruciani G., Dal Bosco A., Castellini C. Lipid metabolism analysis in liver of different chicken genotypes and impact on nutritionally relevant polyunsaturated fatty acids of meat. Sci. Rep. 2022;12:1–12. doi: 10.1038/s41598-022-05986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni Mancinelli A., Franzoni A., Dal Bosco A., Schiavone A., Mannelli F., Marzoni M., Castellini C. Distribution and consistency of Ancona and Livorno poultry breed in Central Italy. Ital. J. Anim. Sci. 2020;19:1297–1303. [Google Scholar]
- Castellini C., Mugnai C., Moscati L., Mattioli S., Guarino Amato M., Cartoni Mancinelli A., Dal Bosco A. Adaptation to organic rearing system of eight different chicken genotypes: behaviour, welfare and performance. Ital. J. Anim. Sci. 2016;15:37–46. [Google Scholar]
- Castillo A., Gariglio M., Franzoni A., Soglia D., Sartore S., Buccioni A., Mannelli F., Cassandro M., Cendron F., Castellini C., Cartoni Mancinelli A., Iaffaldano N., Di Iorio M., Marzoni M., Salvucci S., Cerolini S., Zaniboni L., Schiavone A. Overview of native chicken breeds in Italy: conservation status and rearing systems in use. Animals. 2021;11:490. doi: 10.3390/ani11020490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bosco A., Mattioli S., Cartoni Mancinelli A., Cotozzolo E., Castellini C. Extensive rearing systems in poultry production: the right chicken for the right farming system. A review of twenty years of scientific research in Perugia University, Italy. Animals. 2021;11:1281. doi: 10.3390/ani11051281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersoy I.E., Mendeş M., Aktan S. Growth curve establishment for American Bronze turkeys. Arch. Anim. Breed. 2006;49:293–299. [Google Scholar]
- Fairfull R. In: Pages 913–933 in Poultry Breeding and Genetics. Crawford R.D., editor. Elsevier Science; Amsterdam, The Netherlands: 1990. Heterosis. [Google Scholar]
- Ferrante V., Mugnai C., Ferraria L., Marelli S.P., Spagnoli E., Lolli S. Stress and reactivity in three Italian chicken breeds. Ital. J. Anim. Sci. 2016;15:303–309. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). 2020. Domestic Animal Diversity Information System (DAD-IS). FAO, Rome, Italy. Accessed Dec. 2022. http://www.fao.org/dad-is/en.
- Franzoni A., Gariglio M., Castillo A., Soglia D., Sartore S., Buccioni A., Manelli F., Cassandro M., Cedron F., Castellini C., Cartoni Mancinelli A., Cerolini S., Abdel Sayed A., Iaffaldano N., Di Lorio M., Marzoni M., Salvucci S., Schiavone A. Overview of native chicken breeds in Italy: small scale production and marketing. Animals. 2021;11:629–642. doi: 10.3390/ani11030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Ariza A., Arando Arbulu A., Navas González F.J., Nogales Baena S., Delgado Bermejo J.V., Camacho Vallejo M.E. The study of growth and performance in local chicken breeds and varieties: a review of methods and scientific transference. Animals. 2021;11:2492. doi: 10.3390/ani11092492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Ariza A., Nogales Baena S., Lupi T.M., Arando Arbulu A., Navas González F.J., León Jurado J.M., Delgado Bermejo J.V., Camacho Vallejo M.E. Characterisation of biological growth curves of different varieties of an endangered native hen breed kept under free range conditions. Ital. J. Anim. Sci. 2021;20:806–813. [Google Scholar]
- Hartcher K.M., Lum H.K. Genetic selection of broilers and welfare consequences: a review. Worlds Poult. Sci. 2020;76:154–167. [Google Scholar]
- Iraqi M.M., Hanafi M.S., EL-Moghazy G.M., El-Kotait A.H., Abdel A'al M.H. Estimation of crossbreeding effects for growth and immunological traits in a crossbreeding experiment involving two local strains of chickens. Livest. Res. Rural Dev. 2011;23:82. http://www.lrrd.org/lrrd23/4/iraq23082.htm Accessed Dec. 2022. [Google Scholar]
- Isa A.M., Sun Y., Shi L., Jiang L., Li Y., Fan J., Wang P., Ni A., Huang Z., Ma H., Li D., Chen J. Hybrids generated by crossing elite laying chickens exhibited heterosis for clutch and egg quality traits. Poult. Sci. 2020;99:6332–6340. doi: 10.1016/j.psj.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja T., Khan S.H., Mukhtar N., Parveen A. Comparative study of growth performance, meat quality and haematological parameters of Fayoumi, Rhode Island Red and their reciprocal crossbred chickens. Ital. J. Anim. Sci. 2012;11:e39. [Google Scholar]
- Lalev M., Mincheva N., Oblakova M., Hristakieva P., Ivanova I. Estimation of heterosis, direct and maternal additive effects from crossbreeding experiment involving two White Plymouth Rock lines of chickens. Biotech. Anim. Husb. 2014;30:103–114. [Google Scholar]
- Littell R.C., Henry P.R., Ammerman C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Menchetti L., Padalino B., Brasileiro Fernandes F., Nanni Costa L. Comparison of nonlinear growth models and factors affecting body weight at different ages in Toy Poodles. Ital. J. Anim. Sci. 2020;19:792–802. [Google Scholar]
- Moula N., Antoine-Moussiaux N., Farnir F., Leroy P. Evaluation of the production performances of an endangered local poultry breed, the Famennoise. Int. J. Poult. Sci. 2009;8:389–396. [Google Scholar]
- Narinç D., Öksüz Narinç N., Aygün A. Growth curve analyses in poultry science. Worlds Poult. Sci. J. 2017;73:395–407. [Google Scholar]
- N'dri A.L., Koua B.H.W., Ahouchi V.S., Adepo-Gourene A.B. Body weight and growths curve parameters evaluation of three chicken genotypes (Gallus gallus domesticus) reared in claustration. J. Adv. Vet. Anim. Res. 2018;5 [Google Scholar]
- Özdemir D., Maretto F., Cassandro M. Comparison of genetic diversity of Turkish and Italian local chicken breeds for further conservation strategies. Eur. Poult. Sci. 2016;80:143–157. [Google Scholar]
- Padhi M.K. Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica. 2016;2016 doi: 10.1155/2016/2604685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. 2015;71:363–374. [Google Scholar]
- Phocas F., Belloc C., Bidanel J., Delaby L., Dourmad J.Y., Dumont B., Ezanno P., Fortun-Lamothe L., Foucras G., Frappat B., González-García E., Hazard D., Larzul C., Lubac S., Mignon-Grasteau S., Moreno C.R., Tixier-Boichard M., Brochard M. Towards the agroecological management of ruminants, pigs and poultry through the development of sustainable breeding programmes: I-selection goals and criteria. Animal. 2016;10:1749–1759. doi: 10.1017/S1751731116000926. [DOI] [PubMed] [Google Scholar]
- Razuki W.M., Al-Shaheen S.A. Use of full diallel cross to estimate crossbreeding effects in laying chickens. Int. J. Poult. Sci. 2011;10:197–204. [Google Scholar]
- Rizzi C., Contiero B., Cassandro M. Growth patterns of Italian local chicken populations. Poult. Sci. 2013;92:2226–2235. doi: 10.3382/ps.2012-02825. [DOI] [PubMed] [Google Scholar]
- Soglia D., Sartore S., Maione S., Schiavone A., Dabbou S., Nery J., Zaniboni L., Marelli S., Sacchi P., Rasero R. Growth performance analysis of two Italian slow-growing chicken breeds: Bianca di Saluzzo and Bionda Piemontese. Animals. 2020;10:969. doi: 10.3390/ani10060969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani A.F., Zulkifli I., Omar A.R., Raha A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011;90:1435–1440. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- Soliman M.A., Khalil M.H., El-Sabrout K., Shebl M.K. Crossing effect for improving egg production traits in chickens involving local and commercial strains. Vet. World. 2020;13:407–412. doi: 10.14202/vetworld.2020.407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkhapreecha P., Chankitisakul V., Duangjinda M., Boonkum W. Combining abilities, heterosis, growth performance, and carcass characteristics in a diallel cross from Black-bone chickens and Thai native chickens. Animals. 2022;12:1602. doi: 10.3390/ani12131602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D.A.T., Honaker C.F., Dorshorst B., Andersson L., Brisbin I.L., Siegel P.B. Growth patterns for three generations of an intercross between red jungle fowl and chickens selected for low body weight. J. Appl. Gen. 2018;135:300–310. doi: 10.1111/jbg.12336. [DOI] [PubMed] [Google Scholar]
- Sutherland D.A.T., Honaker C.F., Dorshorst B., Andersson L., Siegel P.B. Asymmetries, heterosis, and phenotypic profiles of red jungle fowl, White Plymouth Rocks, and F1 and F2 reciprocal crosses. J. Appl. Gen. 2018;59:193–201. doi: 10.1007/s13353-018-0435-8. [DOI] [PubMed] [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Artifcial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 2018;8:1168. doi: 10.1038/s41598-018-19231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sun Y., Ni A., Li Y., Yuan J., Ma H., Wang P., Shi L., Zong Y., Zhao J., Bian S., Chen J. Research Note: Heterosis for egg production and oviposition pattern in reciprocal crossbreeds of indigenous and elite laying chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Yan T., Long Z., Huang L.Y., Zhu Y., Xu Y., Chen X., Pak H., Li J., Wu D., Xu Y., Hua S., Jiang L. Prediction of heterosis in the recent rapeseed (Brassica napus) polyploid by pairing parental nucleotide sequences. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zuidhof K.J. Estimation of growth parameters using a nonlinear mixed Gompertz model. Poult. Sci. 2004;83:847–852. doi: 10.1093/ps/83.6.847. [DOI] [PubMed] [Google Scholar]
- Williams S.M., Price S.E., Siegel P.B. Heterosis of growth and reproductive traits in fowl. Poult. Sci. 2002;81:109–1112. doi: 10.1093/ps/81.8.1109. [DOI] [PubMed] [Google Scholar]
- Zanetti E., De Marchi M., Abbadi M., Cassandro M. Variation of genetic diversity over time in local Italian chicken breeds undergoing in situ conservation. Poult. Sci. 2011;10:2195–2201. doi: 10.3382/ps.2011-01527. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]