Abstract
Autologous haematopoietic stem cell transplantation is now well-established as an effective treatment for severe systemic sclerosis with clear demonstration of favourable end-organ and survival outcomes. Treatment-related cardiotoxicity remains the predominant safety concern and contraindicates autologous haematopoietic stem cell transplantation in patients with severe cardiopulmonary disease. In this review, we describe the cardiovascular outcomes of autologous haematopoietic stem cell transplantation recipients, discuss the potential mechanisms of cardiotoxicity and propose future mitigating strategies.
Keywords: Scleroderma, systemic sclerosis, cardiac, stem cell transplantation, autologous haematopoietic stem cell transplantation, toxicity, safety
Introduction
Autologous haematopoietic stem cell transplantation (AHSCT) is an effective treatment for severe systemic sclerosis (SSc) as determined by phase II/III trials.1–3 Subsequently, AHSCT has been proposed as the gold standard treatment for severe disease. 4 Most SSc patients undergoing AHSCT have an uncomplicated transplant journey and exhibit a sustained disease response. Nonetheless, concerns regarding treatment-related toxicity remain, particularly acute cardiotoxicity, which is the leading cause of treatment-related mortality (TRM). 5 The heightened risk of poor acute cardiac outcomes suggests an interaction of prevalent underlying SSc with heart involvement (SSc-HI) or pulmonary arterial hypertension (PAH) with the physiological stress and/or inherent toxic effects of therapeutic agents used in the AHSCT process, particularly high-dose cyclophosphamide (CYC). While screening guidelines may help mitigate risk, outcomes such as acute CYC-cardiotoxicity are unlikely to be completely prevented with the current screening algorithm. 6 Strict cardiac exclusion criteria also leave a significant proportion of patients ineligible for AHSCT. Hence, there is a clear need to adopt evidence-based AHSCT protocols with an improved cardiac safety profile while maintaining the demonstrated efficacy of current therapy.
Cardiovascular outcomes in AHSCT for SSc
Cardiovascular events are the principal cause of treatment-related death
AHSCT offers significant end-organ and mortality benefits over conventional therapy in SSc with the majority of patients undergoing transplant without serious complications. Nonetheless, early toxicity due to AHSCT is a long-recognised issue.7,8 The reported frequency of death as a result of treatment (TRM), is highly variable; in the randomised trials of AHSCT in SSc, the rate is 0–10%1–3 and a recent meta-analysis estimated the rate of TRM at 6.3%. 5 Importantly, TRM has decreased over time.5,9
Cardiac events are the predominant cause of early treatment-related death. 5 Causes of cardiac death are variable and include cardiac arrest, cardiac failure, ischaemic events, tamponade and arrhythmias (Tables 1 and 2). CYC has a well-characterised potential to cause acute cardiotoxicity when used in high doses and CYC-cardiotoxicity has been cited as a cause of death in numerous studies of AHSCT for SSc (Table 2).8,10–12 Nonetheless, while the characteristic histopathological entity of CYC-induced haemorrhagic myopericarditis has been reported, direct pathological evidence of CYC-cardiotoxicity is not explicit in other studies. 13
Table 1.
Causes of cardiac morbidity/mortality and potential cardiovascular risk factors.
| Known causes of cardiac mortality/morbidity in AHSCT |
| Cyclophosphamide toxicity |
| Arrhythmia |
| Coronary artery disease |
| Pulmonary arterial hypertension |
| Constrictive pericarditis |
| Pericardial effusion/tamponade |
| Acute cardiac failure |
| Sudden cardiac arrest |
| Myocarditis |
| Cardiovascular risk factors associated with poor outcomes from AHSCT |
| Smoking (active or former) |
| Male gender |
| Baseline left ventricular dysfunction (LVEF < 50%) |
AHSCT Autologous haematopoietic stem cell transplantation; LVEF left ventricular ejection fraction.
Table 2.
Cardiovascular outcomes from studies of AHSCT for SSc.
| Author | Year | Mobilisation | Conditioning regimen | Patients n = | TRM n = (%) | Cardiac death n = (%) | Types of cardiac death (time point post-AHSCT) (mob = mobilisation; d = day) |
Other acute cardiovascular events | Long-term cardiovascular outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Binks et al. 14 † | 2001 | CYC 4 g/m2 ± G-CSF or G-CSF alone | CYC 120–200 mg/kg ± ATG ± radiation ± anti-CD52 ± busulphan; carmustine/fludarabine/thiotepa | 41 | 7 (17.1)† | 3 (7.3)† | Ischaemic heart disease (mob), cardiac failure (mob), myocarditis (mob)† | Stable LVEF post-AHSCT Stable pulmonary pressures on echocardiogram Death from PAH (n = 1)† |
|
| Farge et al. 15 | 2002 | CYC 4 g/m2 + G-CSF (n = 1 BM stem cells) | CYC 200 mg/kg* | 11 | 1 (9.1) | 0 (0) | Transient cardiac failure due to hyperhydration (mob) Grade IV cardiac toxicity (n = 1) |
LVEF stable post-AHSCT | |
| McSweeney et al. 16 | 2002 | G-CSF | CYC 120 mg/kg + TBI + ATG | 19 | 3 (15.8) | 0 (0) | Transient fall in LVEF at 3 months post-AHSCT | ||
| Farge et al. 17 † | 2004 | CYC 4 g/m2 + G-CSF or G-CSF alone (n = 1 BM stem cells) | CYC 150–200 mg/kg ± irradiation ± ATG ± anti-CD52 BEAM; fludarabine; carmustine |
57 | 5 (8.8)† | 3 (5.3)† | Cardiac failure (d30), myocarditis (d8)† | Death from PAH (n = 1)† | |
| Nash et al. 18 | 2007 | G-CSF | CYC 120 mg/kg + TBI + ATG | 34 | 8 (23.5) | 1 (2.9) | Arrhythmia(0.5 months) | Supraventricular arrhythmias (n = 2), heart failure (n = 2) | Small decline in LVEF at 5–8 years |
| Oyama et al. 19 | 2007 | CYC 2 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 10 | 0 (0) | 0 (0) | Fluid overload (n = 3), acute left ventricular failure (n = 1) | Stable LVEF and pulmonary pressures post-AHSCT | |
| Farge et al. 20 † | 2010 | CYC 1.5-4 g/m2 + G-CSF or G-CSF alone (n = 4 BM stem cells) | CYC 150-200 mg/kg; busulfan; BEAM ± ATG; TBI | 175 | 12 (6.9) | 1 (0.6) | Cardiac toxicity | ||
| Burt et al. 1 | 2011 | CYC 2 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 10 | 0 (0) | 0 (0) | Arrhythmias(n = 2), volume overload (n = 2) | ||
| Henes et al. 21 | 2012 | CYC 2 g/m2 + G-CSF (n = 1 BM stem cells) | CYC 200 mg/kg # + ATG | 26 | 3 (11) | 0 (0) | Fatal arrhythmia at 23 months (n = 1) | ||
| Moore et al. 22 | 2012 | CYC 2 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 10 | 0 (0) | 0 (0) | |||
| Burt et al. 23 | 2013 | CYC 2 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 90 | 5 (5.6) | 4 (4.4) | Cardiac arrest (mob), cardiac failure (n = 2), constrictive pericarditis (during transplantation) | Volume overload (15.5%) | |
| Henes et al. 24 | 2014 | CYC 2 g/m2 + G-CSF | CYC 100 mg/kg + thiotepa 2x 5 mg/kg + ATG | 6 | 0 (0) | 0 (0) | Stable LVEF and troponin post-AHSCT Progressive PAH (n = 1) |
||
| Van Laar et al. 3 | 2014 | CYC 4 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 79 | 8 (10.1) | 3 (3.8) | Cardiac failure (n = 2; d11, d35), myocardial infarction (d14) | No difference in LVEF at 2 years between AHSCT and CYC arms No cardiac death or cardiac failure in HSCT arm Grade III cardiac event (16.4%) in HSCT arm |
|
| Del Papa et al. 25 | 2017 | CYC 4 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 18 | 1 (5.6) | 0 (0) | Transient fall in LVEF (n = 1) | Fatal arrhythmia at 34 months (n = 1) | |
| Sullivan et al. 2 | 2018 | G-CSF | CYC 120 mg/kg + TBI + ATG | 36 | 2 (6) | 0 (0) | ⩾Grade III cardiac disorders (8.8% in AHSCT vs 16.2% CYC arm, p = 0.1) | ||
| Nakamura et al. 26 | 2018 | CYC 4 g/m2 + G-CSF | CYC 200 mg/kg | 14 | 1 (7.1) | 1 (7.1) | Cardiac failure with cardiopulmonary arrest (during conditioning) | Cardiac failure (n = 1) | |
| Nair et al. 27 | 2018 | CYC 2 g/m2 + G-CSF | CYC 60 mg/kg + fludarabine 30 mg x2 + ATG | 4 | 0 (0) | 0 (0) | Stable LVEF post-AHSCT | ||
| Van Bijnen et al. 9 | 2020 | CYC 4 g/m2 + G-CSF | CYC 200 mg/kg + ATG | 92 | 10 (10.9) | 9 (9.8) | Not specified | Cardiac failure (n = 1) | |
| Henrique-Neto et al. 10 | 2021 | CYC 2 g/m2 + G-CSF | CYC 200 mg/kg $ | 70 | 3 (4.3) | 1 (1.4) | Acute cyclophosphamide cardiotoxicity (d60) | Cyclophosphamide-induced cardiac dysfunction (n = 4) | Pericarditis Fatal cardiac insufficiency (n = 2) |
| Henes et al. 8 | 2021 | CYC 1–4 g/m2 + G-CSF | CYC 50–240 mg/kg + ATG (n = 76) CYC 100 mg/kg + thiotepa 10 mg/kg + ATG (n = 4) |
80 | 5 (6.3) | 4 (5.0) | Acute cyclophosphamide cardiotoxicity (n = 3) (d1, d9, d61), AHSCT associated cardiac toxicity (d1) | Pericardial effusion Arrhythmias Myocardial infarction |
|
| Burt et al. 28 | 2021 | CYC 2 g/m2 + G-CSF | Fludarabine 120 mg/m2 + cyclophosphamide 60 mg/kg + ATG (±rituximab) | 42 | 1 (2.4) | 1 (2.4) | Myocardial infarction (during transplant hospitalisation) | Pericardial effusion with tamponade (n = 1) Atrial fibrillation (n = 2) |
Peripheral blood stem cells were used in all studies unless stated otherwise. *Melphalan 140 mg/m2 in n = 1 due to LVEF < 40%. #CYC 100 mg/kg + thiopeta in one patient with cardiac disease. $Fludarabine 120 mg/m2 and melphalan 120/m2 + ATG if cardiac disease. †Studies drawn from the same registry. Acute cardiovascular events were extracted from events reported during the transplant period or up to day + 100. BM bone marrow; BEAM carmustine, cytarabine, melphalan, etoposide; CYC cyclophosphamide; ATG anti-thymocyte globulin; TBI total body irradiation; LVEF left ventricular ejection fraction; PAH pulmonary arterial hypertension; TRM treatment-related mortality.
Pre-existing cardiac disease such as decreased left ventricular (LV) function increases the risk of TRM. 9 Notably, smoking may be an additional risk factor for treatment-associated death, as 7/8 of deaths in the ASTIS (Autologous Stem Cell Transplantion International Scleroderma) trial were those with a current or former smoking history; 3 however, this risk was not confirmed in a subsequent multi-variate analysis inclusive of the ASTIS cohort. 9 Other independent risk factors of TRM include older age and male gender. 9
Aside from death, studies of AHSCT for SSc demonstrate cardiac morbidity (Tables 1 and 2). Volume overload is reported in up to 30% of patients; baseline PAH and constrictive pericarditis are thought to increase the susceptibility to fluid overload during the conditioning period. 23 Other reported events include arrhythmias, transient LV dysfunction, cardiac failure and elevations in cardiac enzymes.
Long-term cardiovascular outcomes following AHSCT
Conversely, there is evidence that AHSCT has long-term beneficial outcomes for SSc-HI or PAH, as it does for skin and lung disease. Early studies including patients with mild PAH reported stability or improvement of PAH; however, the conditioning regimen and concurrent treatments used in these patients are unknown. 14 In one reported case of a SSc patient with myocarditis, AHSCT using a high-dose CYC (200 mg/kg) regimen resulted in resolution of this manifestation. 29
LV function post-AHSCT appears largely stable over the long term,14,16,19,24 although a small decline was reported up to 8 years post-AHSCT in one study. 18 In ASTIS, there was no difference in LV ejection fraction (LVEF) between the AHSCT and CYC arms at 2 years. 3 One small study using thiotepa conditioning reported stable troponin and NTproBNP levels post-AHSCT. 24 Disease progression involving the heart appears to be uncommon post-AHSCT10,21,25 and in one study with a median follow-up of 4.6 years, there was only one case of cardiac failure in a cohort of 82 (excluding patients with TRM). 9
In the controlled studies, there are more reports of non-acute adverse cardiovascular outcomes in the IV CYC (control) arms compared to the AHSCT arms, including PAH, organ failure and cardiovascular death, although these data either do not reach significance, or significance is not reported (Table 2).2,3 Cardiac deaths during long-term (5.8–6 years) follow-up were seen in the CYC arms of ASTIS and SCOT, whereas there were none in the AHSCT arm of either trial.2,3
Potential cardiotoxic mechanisms in AHSCT
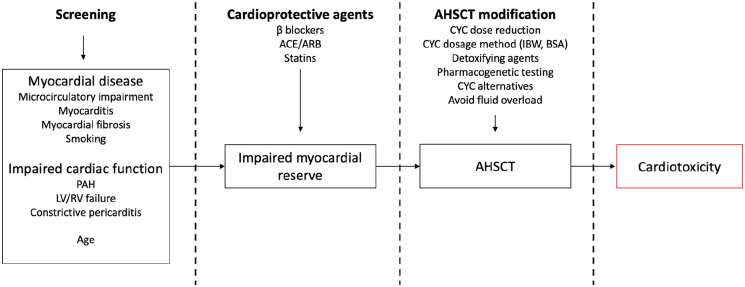
AHSCT may give rise to physiological stressors that interact with highly-prevalent, pre-existing cardiopulmonary disease, resulting in cardiotoxicity (Figure 1).
Figure 1.
Putative mechanisms of (in boxes), and mitigating strategies for preventing cardiotoxicity in SSc patients receiving AHSCT with cyclophosphamide conditioning. SSc-HI leads to impaired myocardial reserve that increases the risk of cardiotoxicity. PAH pulmonary artery hypertension; LV left ventricular; RV right ventricular; ACEi angiotensin converting enzyme inhibitor; ARB angiotensin II receptor blocker; AHSCT autologous haematopoietic stem cell transplantation; CYC cyclophosphamide; IBW ideal body weight; BSA body surface area.
Notably, AHSCT protocols may include ‘hyperhydration’ intravenous fluid protocols during conditioning and as a result exacerbate an already compromised cardiac state that is unable to endure an excess volume load. This was observed in patients with underlying PAH and constrictive pericarditis and shaped the subsequent screening guidelines.6,23 Patients with myocardial fibrosis may also have an impaired response to a fluid challenge. 23
Myocarditis, myocardial fibrosis or microcirculatory disease may decrease cardiac reserve in response to metabolic, toxic or physiological challenges, such as infection, fever or cytokine release encountered in the conditioning period. Untreated coronary artery disease increases the risk of ischaemic events in the presence of these exogenous factors. 28 Smoking may also increase the susceptibility to cardiotoxicity, accounting for the excess TRM seen in smokers.
The cardiotoxic potential of high-dose CYC is well-known, with consensus that toxicity correlates with dose.11,30,31 While there is no general agreement on stratification of CYC dosing in AHSCT, some have defined CYC < 100 mg/kg as low-dose, 100–200mg/kg as reduced-dose and > 200 mg/kg as high dose. 32 In the non-SSc AHSCT setting, LV dysfunction is estimated to occur in 7–28% of patients undergoing CYC therapy 33 and subclinical cardiac toxicity may be seen in up to 90%. 11 In older studies of haematological malignancies using CYC 180 mg/kg–200 mg/kg, fatality from CYC-cardiotoxicity was 12–19%;11,30 modern data show clear improvement over time (fatality rate of 1.5–2%).34,35
CYC cardiotoxicity classically manifests within 48 h to 10 days30,34 and is characterised by acute decompensated cardiac failure with or without pericardial effusion (including tamponade). 11 Autopsy cases indicate characteristic pathological findings of acute myopericarditis with endothelial damage, haemorrhages and oedema.11,13,36 Predictors of CYC-cardiotoxicity in non-SSc cohorts include pre-existing cardiac disease, 31 age35,37 and previous exposure to CYC. 35
CYC is a prodrug and metabolised by cytochrome P450 enzymes to hydroxycyclophosphamide and subsequently its active forms phosphoramide and acrolein. Hydroxycyclophosphamide and acrolein are thought to be the key mediators of cardiotoxicity, with acrolein resulting in mitochondrial dysfunction and failure of adenosine triphosphate production; an impaired myocardium, as may arise in SSc, may be unable to withstand this metabolic insult.38–40
Other AHSCT conditioning agents may exert cardiotoxic effects. For instance, anti-thymocyte globulin (ATG) is a horse or rabbit-derived polyclonal antibody product directed against human T cells. A reaction analogous to serum sickness is the most commonly reported side effect. Adverse effects on the cardiovascular system are rare and may be due to cytokine release rather than direct toxicity.41–43 It is plausible that the effects of ATG on cardiovascular stability may have a deleterious interaction with pre-existing disease, high-dose CYC and hyperhydration. Although thiotepa is used instead of CYC to mitigate cardiac toxicity in AHSCT for SSc, one report suggests that it also has cardiotoxic potential. 44 Rituximab-induced cardiotoxicity has been reported but remains a controversial association. 45
Comparison with studies of AHSCT for other autoimmune diseases suggests that risk of TRM and particularly cardiotoxicity is elevated in SSc. When comparing outcomes using the same high-dose CYC conditioning protocol, TRM is 0–4% for multiple sclerosis (MS), with no cardiac deaths seen across the three MS studies.46–48 This implicates disease-specific factors in SSc as a cause of poor cardiac outcomes.
Potential measures to reduce cardiac toxicity during AHSCT
Addressing treatment-related cardiotoxicity is essential to improve safety of AHSCT for SSc. Screening for cardiac disease, modifications to conditioning chemotherapy and adjunctive cardioprotective agents may have a role in reducing cardiotoxicity (Figure 1).
Rationale for screening for cardiac disease and PAH prior to AHSCT
The high prevalence, varied manifestations and often subclinical course of cardiopulmonary disease in SSc, combined with the excess risk of acute, adverse cardiac outcomes in SSc patients undergoing AHSCT using the standard (high-dose) CYC protocol, has prompted the development of screening guidelines with specific cardiac exclusion criteria (Tables 3 and 4).6,49 The exclusion criteria from the major studies are outlined in Table 3.
Table 3.
Cardiac exclusion criteria from recent major AHSCT studies in SSc and exclusion criteria recommendations from the EBMT.
| EBMT Recommendations 6 | ASSIST 1 | ASTIS 3 | SCOT 2 | Henrique-Neto et al. 10 | Henes et al. 8 | |
|---|---|---|---|---|---|---|
| PAH assessment | PASP > 40 mmHg at rest or > 45 mmHg post-fluid challenge (RHC) mPAP > 25 mmHg at rest or > 30 mmHg after fluid challenge (RHC) Decrease or lack of augmentation of CO after fluid challenge Pulmonary vascular resistance > 3 wood units |
PASP > 40 mmHg (RHC) mPAP > 25 mmHg (RHC) |
mPAP > 50 mmHg | PA peak systolic pressure > 55 mmHg (echo) PA peak systolic pressure 45-55 mmHg (echo) and mPAP by RHC > 25 mmHg at rest or 30 mmHg with exercise WHO Class III or IV symptoms Taking medications that lower pulmonary artery pressure |
Systolic pulmonary artery pressure > 40 mmHg by echocardiography or mPAP > 27 mmHg by right heart catheter |
Pulmonary arterial hypertension with PASP > 50 mmHg |
| LV function | LVEF < 45% | LVEF < 40% | Clinical evidence of significant CHF (NYHA Class III or IV) LVEF < 50% by echocardiogram |
LVEF < 40% Moderate or severe diastolic dysfunction (echocardiography, CMR or RHC) |
Severe heart failure LVEF < 40% by cardiac echo | |
| CMR abnormalities | D-sign or septal bounce | |||||
| Coronary artery disease | Unrevascularised severe coronary artery disease | |||||
| Arrhythmias | Untreated severe arrhythmia | Uncontrolled clinically significant arrhythmias | Ventricular arrhythmias | |||
| Pericardial abnormalities | Cardiac tamponade Constrictive pericarditis |
Constrictive pericarditis | ||||
| Other | Symptomatic cardiac disease Tricuspid annular plane systolic excursion < 1.8 cm |
Severe major organ involvement | Prior insertion of a pacemaker or cardioverter-defibrillator |
PAH pulmonary arterial hypertension; LV left ventricular; CMR cardiovascular magnetic resonance imaging; EBMT European Society for Blood and Marrow Transplantation; PASP pulmonary artery systolic pressure; mPAP mean pulmonary artery pressure; RHC right heart catheterisation; CO cardiac output; LVEF left ventricular ejection fraction; PA pulmonary artery; WHO World Health Organisation; CHF congestive heart failure; NYHA New York Heart Association; ASSIST Autologous Stem Cell Systemic Sclerosis Immune Suppression Trial; ASTIS Autologous Stem Cell Transplantion International Scleroderma; SCOT Scleroderma: Cyclophosphamide or Transplantation.
Table 4.
Cardiac screening tests prior to AHSCT (adapted from Farge et al. 6 ) CMR cardiovascular magnetic resonance imaging.
| BNP or NTproBNP |
| Electrocardiography (ECG) |
| Holter monitor if history of palpitations or abnormal ECG |
| Echocardiogram with Doppler and tissue Doppler plus dobutamine stress |
| CMR with contrast |
| Right heart catheterisation with fluid challenge |
AHSCT Autologous haematopoietic stem cell transplantation; CMR cardiovascular magnetic resonance.
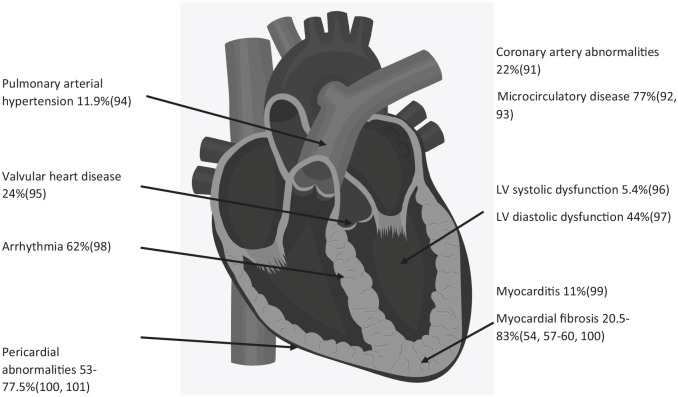
Primary or secondary SSc-HI 50 has been described in all anatomic sites of the heart (Figure 2), is associated with a significantly increased risk of death.51,52 Those with rapidly progressive skin thickening or diffuse disease are at highest risk of SSc-HI; these same disease characteristics are indications for AHSCT and hence the AHSCT population may be enriched for SSc-HI.53,54
Figure 2.
Manifestations and prevalence of cardiac disease and PAH in systemic sclerosis.
PAH pulmonary arterial hypertension.
Early trials, without strict exclusion criteria for PAH, reported PAH in 19–31% undergoing AHSCT.14,17 An abnormal ECG is reported in up to 80% undergoing AHSCT, 1 an abnormal ambulatory ECG in 20% 8 and a pericardial effusion in 6.3%. 8 CMR abnormalities are reported in up to 50% of consecutive patients screened for AHSCT. 23
The introduction of CMR and right heart catheterisation (RHC) into screening coincides with the reduction of TRM in one study. 9 Adherence to these guidelines is variable, however, with Henes et al. 8 reporting that 26% of patients (including the four deaths) transplanted between 2012 and 2016 did not have either a RHC or CMR, despite the publication of updated guidelines in 2012. 49
Critically, despite exhaustive screening, cardiac death and morbidity still occur in a small percentage of Sac patients receiving AHSCT, with CYC-induced cardiotoxicity listed as a key cause in recent studies.8,10,13,26 Henes et al reported CYC-cardiotoxicity in three patients, all of whom had a normal baseline CMR; hence, there are likely to be other contributory patient and disease-specific factors for carditoxicity not captured by current screening modalities.
Screening tests
Echocardiogram
Echocardiogram is an important initial test in the assessment of candidates for SSc and specific findings alone (e.g. significant LV systolic dysfunction) may identify patients at too high a risk for conventional AHSCT treatment. 9 Echocardiogram may show incidental findings that would warrant further evaluation prior to transplant, for example severe valvular disease. However, it is well recognised that echocardiogram alone does not have the required sensitivity to detect all clinically relevant disease; for example, echocardiogram may underestimate pulmonary arterial pressure and cannot be used to diagnose PAH. 55 Addition of dobutamine stress testing to echocardiography can assess cardiac reserve and potential myocardial ischaemia due to coronary artery disease, and is recommended. 6
Right heart catheterisation
RHC is the gold standard test to confirm PAH and is an essential test to investigate constrictive pericarditis. Guidelines suggest that all SSc patients undergo RHC prior to AHSCT. The thresholds for exclusion are listed in Table 3 and current guidelines essentially exclude any patient with pulmonary hypertension (PH). Importantly, it is suggested that patients undergo a fluid challenge (if RA pressure < 12 mmHg and pulmonary capillary wedge pressure < 20 mmHg), as the response to fluid challenge may identify those at higher risk of complications from AHSCT.6,23 PH was defined as a mean pulmonary artery pressure (mPAP) ⩾ 25 mmHg when the cardiac screening guidelines were published; however, it is now proposed that this threshold be reduced to mPAP > 20 mmHg and to also include the pulmonary vascular resistance of ⩾ 3 Wood Units in the definition. 56 It is yet to be determined if this change of definition should prompt change to the screening guidelines.
Cardiovascular magnetic resonance
CMR provides excellent anatomical, perfusion and functional data with superior accuracy compared to echocardiography and hence it is a critical screening investigation. Cine images accurately define ventricular volumes, mass and function. Flattening of the interventricular septum (D-sign) is an important sign of RV pressure and volume overload and its presence is a contraindication to high-dose CYC AHSCT. 6 Focal myocardial fibrosis can be accurately assessed by late-gadolinium enhanced CMR, while advanced tissue mapping techniques (e.g. assessing extracellular volume (ECV) via T1 mapping) can identify diffuse myocardial fibrosis, present in 20.5–83% of SSc patients.54,57–64 Advanced tissue mapping techniques may also identify myocarditis.62,63,65 However, it is unknown if myocardial fibrosis or myocarditis associates with poor outcomes in AHSCT, and presence of these CMR findings does not currently exclude SSc patients from AHSCT. First-pass perfusion on CMR provides sensitive assessment of myocardial microcirculatory disease. Addition of adenosine to assess myocardial perfusion during stress increases the sensitivity of detecting microcirculatory disease, as recently reported in SSc patients. 66 Again, it is unknown if microcirculatory disease increases the risk of poor cardiac outcomes during AHSCT.
CMR is an excellent modality to assess pericardial disease, with accuracy similar to catheterisation to diagnose constrictive pericarditis. 67 Constrictive pericarditis is an exclusion criterion for AHSCT. CMR can also be used to assess diastolic dysfunction, 68 which although not part of the current consensus guidelines, has been used as an exclusion criterion. 10
Electrophysiological studies
Twelve-lead ECG and 24 h ECG (Holter) monitoring are both recommended investigations. Current guidelines advocate for Holter monitoring only if clinically indicated, while older guidelines recommended this for all patients. 7 Uncontrolled arrhythmia is listed as contraindication for AHSCT. Baseline ECG changes may offer prognostic information: for example, older data suggest that QTc dispersion may predict acute cardiac failure during autologous transplantation for haematological malignancies. 69
Circulating biomarkers
Measurement of natriuretic peptides or troponin may have role in identifying some causes of SSc-HI. 70 NTproBNP may assist identification of SSc-PAH. 71 Previous studies have noted that NT-proBNP and troponin are higher in SSc patients compared to controls, that higher values correlate with poorer ventricular function and ECG abnormalities, and portend worse outcomes. 72 Elevated BNP was reported in 21% of SSc patients pre-AHSCT; however, the association with clinical outcome was not reported. 28 The role of NT-proBNP and troponin in risk stratification of patients prior to AHSCT has not been established, although measurement of NT-proBNP is suggested.6,26
Other
Unrevascularised coronary artery disease is a contraindication to AHSCT. So far, no routine direct assessment (coronary artery CT or coronary angiography) is recommended as part of screening. At our institution, coronary artery CT is used to screen patients deemed high risk during clinical assessment. Cardiopulmonary exercise testing is another possible means of patient stratification, given its prognostic value in the general SSc population. 73 Although beyond the scope of this review, pulmonary function testing is a critical assessment that may inform patient eligibility.
Mitigating cardiotoxicity with changes to AHSCT regimens
The first reported autologous HSCT for SSc used a high-dose CYC conditioning protocol (200 mg/kg) as was historically used for aplastic anaemia given the hypothesis that SSc, like aplastic anaemia, was a T-cell driven disease.74,75 CYC 200 mg/kg in conjunction with anti-thymocyte globulin (ATG) has been used in most published studies of AHSCT for SSc (Table 2).
Given that toxicity from CYC is proportional to dose, modification to CYC dosing or using CYC alternatives are clear strategies to improve outcomes. Recently published pilot studies have attempted to mitigate cardiac risk by reducing the conditioning dose of CYC while introducing more ‘cardiac-safe’ chemotherapeutic agents. Henes et al. 24 used a thiotepa and CYC regimen in six patients with biopsy-proven cardiac fibrosis, reporting no treatment-related deaths. Henrique-Neto et al. 10 used a fludarabine 120 mg/m2 and melphalan 120 mg/m2 regimen in those with cardiac involvement (n = 5); no cardiac adverse events were seen in this small group, compared to acute cardiotoxcity in four patients and one cardiac death in the CYC 200 mg/kg group (n = 65). Farge et al. 6 reported data on 42 patients unable to have CYC 200 mg/kg due to cardiac disease (excluded using the European Society for Blood and Marrow Transplantation guidelines); these patients received fludarabine 120 mg/m2, CYC 60 mg/kg and ATG with a subgroup receiving rituximab or rituximab and intravenous immunoglobulin (IVIg). 28 TRM was 2.4% (one death due to myocardial infarction in a patient with known coronary artery disease), lower than that seen in randomised trials but at a cost of decreased efficacy and higher rates of secondary autoimmune disease in patients who did not receive rituximab. More secondary autoimmune disease was also seen in the small study using the thiotepa-based protocol. 24 While these initial data are encouraging, currently, there are no data to compare efficacy of these regimens to myeloablative or CYC 200 mg/kg lymphoablative AHSCT.
In the randomised studies, ASSIST (Autologous Stem Cell Systemic Sclerosis Immune Suppression Trial) utilised a CYC mobilisation dose of 2 g/m2 whereas ASTIS used a higher dose of 4 g/m2.1,3 It is possible the cardiac deaths seen in ASTIS could result from a higher mobilisation dose of CYC (there were no cardiac deaths in ASSIST); however, a subsequent study using the ASSIST protocol did report cardiac deaths, including one during mobilisation (Table 2). Both ASSIST and ASTIS were lymphoablative, using CYC 200 mg/kg in conjunction with rabbit ATG whereas the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial was myeloablative and used a lower dose of CYC (120 mg/kg over two days) with total body irradiation (TBI) and equine ATG. The apparent reduced cardiotoxicity in SCOT and other studies using this protocol may be due to lower doses of CYC conditioning; however, this is offset by the concern over increased risk of treatment-related malignancies due to TBI (noting that direct comparisons between trials on this latter point are contentious).76,77
Despite the widespread adoption of the CYC 200 mg/kg protocol, the optimal conditioning dose of CYC in AHSCT for SSc has not been established in a clinical trial. At our centre, one small dose-finding study was performed in AHSCT for rheumatoid arthritis which confirmed CYC 200 mg/kg conditioning as more efficacious than CYC 100 mg/kg in context of that disease; however, this study did not include ATG. 78 Registry studies have included patients who received lower doses of CYC conditioning, with or without ATG; however, no study has yet compared response to CYC conditioning dose.8,14,17,20 A case report of two SSc patients treated with lower doses of conditioning CYC described long-term improvement of skin and pulmonary outcomes. 79
Risk may also be reduced by modifying CYC dosage according to ideal body weight or body surface area; for example, CYC < 1.55 g/m2/day is associated with a lower risk of cardiotoxicity. 30 Guidelines suggest using the lesser of total body weight or ideal body weight when using CYC 200 mg/kg. 80
Although no data exist in the setting of high-dose CYC conditioning, there is a theoretical role for cardiac drugs (such as β-blockers) for primary prevention of cardiac toxicity. For example, in anthracycline-based chemotherapy regimens, beta-blockers confer a protective effect. 81 In CYC, doxorubicin, vincristine and prednisolone-based chemotherapy for non-Hodgkin’s lymphoma, the addition of valsartan prevents development of acute cardiotoxicity. 82 The Canadian Cardiovascular Society recommends the use of cardiovascular agents for primary prevention in patients at high risk of chemotherapeutic agent-driven cardiac dysfunction. 83
Careful attention should be given to prevention of fluid overload and electrolyte disturbances. Observation of fluid overload in SSc patients undergoing AHSCT has resulted in modification to hyperhydration protocols. 23
Preclinical studies suggest N-acetylcysteine abrogates CYC-induced toxicity.40,84 Others have investigated the protective roles of allopurinol and febuxostat 85 or nicorandil. 86 One challenge for both clinical and pre-clinical studies is the unpredictable pharmacokinetics of high dose CYC, with substantial intra-individual variation. 87 Pharmacogenetic factors (e.g. CYP2C19, CYP2B6 and ALDH1A1) may influence CYC pharmacokinetics and subsequently drug toxicity. 88
The future: improving the safety of AHSCT in SSc
Future AHSCT-based treatments for SSc must continue to improve safety while providing at least equal efficacy compared to current regimens. Current screening recommendations are a critical component in mitigating risk, particularly to identify patients at risk of fluid overload. Nonetheless, screening fails to completely obviate the risk of cardiac death, particularly acute cardiotoxicity associated with high-dose CYC. One critical aim of future research should be to determine the optimal dose of CYC in lymphoablative AHSCT without the confounding effect of introducing other therapeutic agents. At our centre, a dose-finding study is currently underway to determine the safety and efficacy of lower doses of CYC in patients with pre-existing cardiopulmonary risk factors. 89 Other potential methods to reduce cardiotoxicity include refinement of screening guidelines (e.g. through investigation of circulating biomarkers, or sensitive imaging findings), use of cardiovascular drugs such as β-blockers and use of agents that counteract toxic chemotherapeutic metabolites. Future studies could advance our understanding of cardiac function during AHSCT by including echocardiography, cardiac enzyme and CMR data from the periconditioning and post-AHSCT periods.
In the meantime, adopting further precautions for patients undergoing high-dose CYC AHSCT may reduce risk. Experience at our centre suggests that current or former smokers have poor outcomes, confirming the findings of others. 3 As such, we treat active smoking as a contraindication to AHSCT. Patients considered to be higher risk at baseline clinical assessment are also admitted to our coronary care unit for continuous cardiac monitoring and daily input from the cardiology service. Assessment of and treatment for significant coronary artery disease should be completed prior to AHSCT.
Data from studies using alternatives to CYC 200 mg/kg are encouraging and should inform the design of a future randomised controlled trial to assess safety and non-inferiority of a ‘cardiac-safe’ lymphoablative regimen versus ‘standard dose’ CYC. Mechanistic studies examining immune reconstitution post-AHSCT will also help to design targeted interventions to improve or individualise AHSCT.
Finally, in the rightful pursuit of safety, ever more stringent criteria have resulted in exclusion of SSc patients with severe cardiopulmonary disease from accessing AHSCT. This leaves a significant proportion of patients unable to receive a treatment with clear disease-modifying and mortality benefits, which is lamentable given that AHSCT-ineligible SSc patients have the poorest prognosis. 90 Addressing the safety of current AHSCT regimens is critical to rectify this shortcoming.
Footnotes
Author contributions: RP and JM conceived the review. RP drafted the manuscript. RP, LG, HE, XB, AJ, EK, DM and JM edited the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Penglase’s studies are supported by a University of New South Wales University Postgraduate Award and the Vera Glazebrook Trust Fund for Arthritis.
ORCID iD: Ross Penglase  https://orcid.org/0000-0002-4061-4301
https://orcid.org/0000-0002-4061-4301
References
- 1.Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 2011; 378(9790): 498–506. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med 2018; 378(1): 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014; 311(24): 2490–2498. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan KM, Majhail NS, Bredeson C, et al. Systemic sclerosis as an indication for autologous hematopoietic cell transplantation: position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2018; 24(10): 1961–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashitani K, Takase-Minegishi K, Yoshimi R, et al. Benefits and risks of hematopoietic stem cell transplantation for systemic sclerosis: a systematic review and meta-analysis. Mod Rheumatol 2022; 2022: roac026. [DOI] [PubMed] [Google Scholar]
- 6.Farge D, Burt RK, Oliveira MC, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transplant 2017; 52(11): 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saccardi R, Tyndall A, Coghlan G, et al. Consensus statement concerning cardiotoxicity occurring during haematopoietic stem cell transplantation in the treatment of autoimmune diseases, with special reference to systemic sclerosis and multiple sclerosis. Bone Marrow Transplant 2004; 34(10): 877–881. [DOI] [PubMed] [Google Scholar]
- 8.Henes J, Oliveira MC, Labopin M, et al. Autologous stem cell transplantation for progressive systemic sclerosis: a prospective non-interventional study from the European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. Haematologica 2021; 106(2): 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Bijnen S, de Vries-Bouwstra J, van den Ende CH, et al. Predictive factors for treatment-related mortality and major adverse events after autologous haematopoietic stem cell transplantation for systemic sclerosis: results of a long-term follow-up multicentre study. Ann Rheum Dis 2020; 79(8): 1084–1089. [DOI] [PubMed] [Google Scholar]
- 10.Henrique-Neto A, Vasconcelos MYK, Dias JBE, et al. Hematopoietic stem cell transplantation for systemic sclerosis: Brazilian experience. Adv Rheumatol 2021; 61(1): 9. [DOI] [PubMed] [Google Scholar]
- 11.Gottdiener JS, Appelbaum FR, Ferrans VJ, et al. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med 1981; 141(6): 758–763. [PubMed] [Google Scholar]
- 12.Tiersten A, Wo J, Jacobson C, et al. Cardiac toxicity observed in association with high-dose cyclophosphamide-based chemotherapy for metastatic breast cancer. Breast 2004; 13(4): 341–346. [DOI] [PubMed] [Google Scholar]
- 13.Martin M, Fornecker LM, Marcellin L, et al. Acute and fatal cardiotoxicity following high-dose cyclophosphamide in a patient undergoing autologous stem cell transplantation for systemic sclerosis despite satisfactory cardiopulmonary screening. Bone Marrow Transplant 2017; 52(12): 1674–1677. [DOI] [PubMed] [Google Scholar]
- 14.Binks M, Passweg JR, Furst D, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis 2001; 60(6): 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farge D, Marolleau JP, Zohar S, et al. Autologous bone marrow transplantation in the treatment of refractory systemic sclerosis: early results from a French multicentre phase I-II study. Br J Haematol 2002; 119(3): 726–739. [DOI] [PubMed] [Google Scholar]
- 16.McSweeney PA, Nash RA, Sullivan KM, et al. High-dose immunosuppressive therapy for severe systemic sclerosis: initial outcomes. Blood 2002; 100(5): 1602–1610. [PMC free article] [PubMed] [Google Scholar]
- 17.Farge D, Passweg J, van Laar JM, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis 2004; 63(8): 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood 2007; 110(4): 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyama Y, Barr WG, Statkute L, et al. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone Marrow Transplant 2007; 40(6): 549–555. [DOI] [PubMed] [Google Scholar]
- 20.Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica 2010; 95(2): 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henes JC, Schmalzing M, Vogel W, et al. Optimization of autologous stem cell transplantation for systemic sclerosis – a single-center longterm experience in 26 patients with severe organ manifestations. J Rheumatol 2012; 39(2): 269–275. [DOI] [PubMed] [Google Scholar]
- 22.Moore J, Englert H, Furlong T, et al. Auto-HSCT induces sustained responses in severe systemic sclerosis patients failing pulse cyclophosphamide. Bone Marrow Transplant 2012; 47(11): 1486–1487. [DOI] [PubMed] [Google Scholar]
- 23.Burt RK, Oliveira MC, Shah SJ, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet 2013; 381(9872): 1116–1124. [DOI] [PubMed] [Google Scholar]
- 24.Henes JC, Koetter I, Horger M, et al. Autologous stem cell transplantation with thiotepa-based conditioning in patients with systemic sclerosis and cardiac manifestations. Rheumatology 2014; 53(5): 919–922. [DOI] [PubMed] [Google Scholar]
- 25.Del Papa N, Onida F, Zaccara E, et al. Autologous hematopoietic stem cell transplantation has better outcomes than conventional therapies in patients with rapidly progressive systemic sclerosis. Bone Marrow Transplant 2017; 52(1): 53–58. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Odani T, Yasuda S, et al. Autologous haematopoietic stem cell transplantation for Japanese patients with systemic sclerosis: long-term follow-up on a phase II trial and treatment-related fatal cardiomyopathy. Mod Rheumatol 2018; 28(5): 879–884. [DOI] [PubMed] [Google Scholar]
- 27.Nair V, Vasdev V, Kumar A, et al. Stem cell transplant in systemic sclerosis: an Indian experience. Int J Rheum Dis 2018; 21(4): 859–865. [DOI] [PubMed] [Google Scholar]
- 28.Burt RK, Han X, Quigley K, et al. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: a pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transplant 2021; 56(1): 50–59. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mashaleh M, Bak H, Moore J, et al. Resolution of sclerodermatous myocarditis after autologous stem cell transplantation. Ann Rheum Dis 2006; 65(9): 1247–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg MA, Antin JH, Guinan EC, et al. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood 1986; 68(5): 1114–1118. [PubMed] [Google Scholar]
- 31.Braverman AC, Antin JH, Plappert MT, et al. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 1991; 9(7): 1215–1223. [DOI] [PubMed] [Google Scholar]
- 32.Mori T, Koh H, Onishi Y, et al. Impact of cyclophosphamide dose of conditioning on the outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia from human leukocyte antigen-identical sibling. Int J Hematol 2016; 103(4): 461–468. [DOI] [PubMed] [Google Scholar]
- 33.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2012; 23(Suppl. 7): vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 34.Ishida S, Doki N, Shingai N, et al. The clinical features of fatal cyclophosphamide-induced cardiotoxicity in a conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Ann Hematol 2016; 95(7): 1145–1150. [DOI] [PubMed] [Google Scholar]
- 35.Duléry R, Mohty R, Labopin M, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC Cardiooncol 2021; 3(2): 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appelbaum F, Strauchen JA, Graw RG, et al. Acute lethal carditis caused by high-dose combination chemotherapy. A unique clinical and pathological entity. Lancet 1976; 1(7950): 58–62. [DOI] [PubMed] [Google Scholar]
- 37.Brockstein BE, Smiley C, Al-Sadir J, et al. Cardiac and pulmonary toxicity in patients undergoing high-dose chemotherapy for lymphoma and breast cancer: prognostic factors. Bone Marrow Transplant 2000; 25(8): 885–894. [DOI] [PubMed] [Google Scholar]
- 38.Kurauchi K, Nishikawa T, Miyahara E, et al. Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res Notes 2017; 10(1): 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mythili Y, Sudharsan PT, Varalakshmi P.Dl-alpha-lipoic acid ameliorates cyclophosphamide induced cardiac mitochondrial injury. Toxicology 2005; 215(1–2): 108–114. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa T, Miyahara E, Kurauchi K, et al. Mechanisms of fatal cardiotoxicity following high-dose cyclophosphamide therapy and a method for its prevention. PLoS ONE 2015; 10(6): e0131394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godown J, Deal AM, Riley K, et al. Worsening bradycardia following antithymocyte globulin treatment of severe aplastic anemia. J Pediatr Pharmacol Ther 2011; 16(3): 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pihusch R, Holler E, Mühlbayer D, et al. The impact of antithymocyte globulin on short-term toxicity after allogeneic stem cell transplantation. Bone Marrow Transplant 2002; 30(6): 347–354. [DOI] [PubMed] [Google Scholar]
- 43.Marotta S, Ricci P, Marano L, et al. Acute immune toxicity during anti-thymocyte globulin: that’s CARPA! Am J Hematol 2018; 93(1): E22–E24. [DOI] [PubMed] [Google Scholar]
- 44.Alidina A, Lawrence D, Ford LA, et al. Thiotepa-associated cardiomyopathy during blood or marrow transplantation: association with the female sex and cardiac risk factors. Biol Blood Marrow Transplant 1999; 5(5): 322–327. [DOI] [PubMed] [Google Scholar]
- 45.Shah C, Gong Y, Szady A, et al. Unanticipated cardiotoxicity associated with targeted anticancer therapy in patients with hematologic malignancies patients: natural history and risk factors. Cardiovasc Toxicol 2018; 18(2): 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burt RK, Loh Y, Cohen B, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol 2009; 8(3): 244–253. [DOI] [PubMed] [Google Scholar]
- 47.Burt RK, Balabanov R, Han X, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 2015; 313(3): 275–284. [DOI] [PubMed] [Google Scholar]
- 48.Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 2016; 388(10044): 576–585. [DOI] [PubMed] [Google Scholar]
- 49.Snowden JA, Saccardi R, Allez M, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2012; 47(6): 770–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruni C, Buch MH, Furst DE, et al. Primary systemic sclerosis heart involvement: a systematic literature review and preliminary data-driven, consensus-based WSF/HFA definition. J Scleroderma Relat Disord 2022; 7(1): 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komócsi A, Vorobcsuk A, Faludi R, et al. The impact of cardiopulmonary manifestations on the mortality of SSc: a systematic review and meta-analysis of observational studies. Rheumatology 2012; 51(6): 1027–1036. [DOI] [PubMed] [Google Scholar]
- 52.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med 2005; 118(1): 2–10. [DOI] [PubMed] [Google Scholar]
- 53.Perera A, Fertig N, Lucas M, et al. Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis Rheum 2007; 56(8): 2740–2746. [DOI] [PubMed] [Google Scholar]
- 54.Rodríguez-Reyna TS, Morelos-Guzman M, Hernández-Reyes P, et al. Assessment of myocardial fibrosis and microvascular damage in systemic sclerosis by magnetic resonance imaging and coronary angiotomography. Rheumatology 2015; 54(4): 647–654. [DOI] [PubMed] [Google Scholar]
- 55.Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139(5): 988–993. [DOI] [PubMed] [Google Scholar]
- 56.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1): 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muresan L, Oancea I, Mada RO, et al. Relationship between ventricular arrhythmias, conduction disorders, and myocardial fibrosis in patients with systemic sclerosis. J Clin Rheumatol 2018; 24(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 58.Ntusi NA, Piechnik SK, Francis JM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis–a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014; 16: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Cesare E, Battisti S, Di Sibio A, et al. Early assessment of sub-clinical cardiac involvement in systemic sclerosis (SSc) using delayed enhancement cardiac magnetic resonance (CE-MRI). Eur J Radiol 2013; 82(6): e268–e273. [DOI] [PubMed] [Google Scholar]
- 60.Dumitru RB, Bissell LA, Erhayiem B, et al. Predictors of subclinical systemic sclerosis primary heart involvement characterised by microvasculopathy and myocardial fibrosis. Rheumatology 2021; 60(6): 2934–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214: 465–468. [DOI] [PubMed] [Google Scholar]
- 62.Bing R, Dweck MR.Myocardial fibrosis: why image, how to image and clinical implications. Heart 2019; 105(23): 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020; 22(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross L, Costello B, Brown Z, et al. Myocardial fibrosis and arrhythmic burden in systemic sclerosis. Rheumatology 2022; 61(11):4497–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lurz P, Luecke C, Eitel I, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-trial. J Am Coll Cardiol 2016; 67(15): 1800–1811. [DOI] [PubMed] [Google Scholar]
- 66.Gyllenhammar T, Kanski M, Engblom H, et al. Decreased global myocardial perfusion at adenosine stress as a potential new biomarker for microvascular disease in systemic sclerosis: a magnetic resonance study. BMC Cardiovasc Disord 2018; 18(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vidalakis E, Kolentinis M, Gawor M, et al. CMR in pericardial diseases-an update. Curr Cardiovasc Imag 2020; 13(4): 14. [Google Scholar]
- 68.Chamsi-Pasha MA, Zhan Y, Debs D, et al. CMR in the evaluation of diastolic dysfunction and phenotyping of HFpEF: current role and future perspectives. JACC Cardiovasc Imaging 2020; 13(1 Pt. 2): 283–296. [DOI] [PubMed] [Google Scholar]
- 69.Nakamae H, Hino M, Akahori M, et al. Predictive value of QT dispersion for acute heart failure after autologous and allogeneic hematopoietic stem cell transplantation. Am J Hematol 2004; 76(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 70.Ross L, Moxey J, Nikpour M.Are troponin and B-type natriuretic peptides useful biomarkers for the diagnosis of systemic sclerosis heart involvement? A systematic literature review. Semin Arthritis Rheum 2021; 51(1): 299–309. [DOI] [PubMed] [Google Scholar]
- 71.Thakkar V, Stevens WM, Prior D, et al. N-terminal pro-brain natriuretic peptide in a novel screening algorithm for pulmonary arterial hypertension in systemic sclerosis: a case-control study. Arthritis Res Ther 2012; 14(3): R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosello S, De Luca G, Berardi G, et al. Cardiac troponin T and NT-proBNP as diagnostic and prognostic biomarkers of primary cardiac involvement and disease severity in systemic sclerosis: a prospective study. Eur J Intern Med 2019; 60: 46–53. [DOI] [PubMed] [Google Scholar]
- 73.Ewert R, Ittermann T, Habedank D, et al. Prognostic value of cardiopulmonary exercise testing in patients with systemic sclerosis. BMC Pulm Med 2019; 19(1): 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tyndall A, Black C, Finke J, et al. Treatment of systemic sclerosis with autologous haemopoietic stem cell transplantation. Lancet 1997; 349(9047): 254. [DOI] [PubMed] [Google Scholar]
- 75.Marmont A, Tyndall A, Gratwohl A, et al. Hematopoietic precursor-cell transplants for autoimmune-diseases. Lancet 1995; 345(8955): 978.7715303 [Google Scholar]
- 76.Keyes-Elstein L, Brittain E, Pinckney A, et al. Safety and efficacy of HSCT for systemic sclerosis across clinical trials. Nat Rev Rheumatol 2020; 16(11): 661. [DOI] [PubMed] [Google Scholar]
- 77.Burt RK, Farge D.Systemic sclerosis: autologous HSCT is efficacious, but can we make it safer? Nat Rev Rheumatol 2018; 14(4): 189–191. [DOI] [PubMed] [Google Scholar]
- 78.Snowden JA, Biggs JC, Milliken ST, et al. A phase I/II dose escalation study of intensified cyclophosphamide and autologous blood stem cell rescue in severe, active rheumatoid arthritis. Arthritis Rheum 1999; 42(11): 2286–2292. [DOI] [PubMed] [Google Scholar]
- 79.Penglase R, Englert H, Tymms K, et al. Successful lymphoablative autologous haematopoietic stem cell transplantation in three cases of severe autoimmune disease despite reduced dose cyclophosphamide conditioning-do we need 200 mg/kg cyclophosphamide? Bone Marrow Transplant 2022; 57(7): 1207–1209. [DOI] [PubMed] [Google Scholar]
- 80.Bubalo J, Carpenter PA, Majhail N, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transplant 2014; 20(5): 600–616. [DOI] [PubMed] [Google Scholar]
- 81.Shah P, Garris R, Abboud R, et al. Meta-analysis comparing usefulness of beta blockers to preserve left ventricular function during anthracycline therapy. Am J Cardiol 2019; 124(5): 789–794. [DOI] [PubMed] [Google Scholar]
- 82.Nakamae H, Tsumura K, Terada Y, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005; 104(11): 2492–2498. [DOI] [PubMed] [Google Scholar]
- 83.Virani SA, Dent S, Brezden-Masley C, et al. Canadian Cardiovascular Society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol 2016; 32(7): 831–841. [DOI] [PubMed] [Google Scholar]
- 84.Mansour HH, El Kiki SM, Hasan HF.Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol 2015; 40(2): 417–422. [DOI] [PubMed] [Google Scholar]
- 85.El-Sheikh AA, Abdelzaher WY, Gad AA, et al. Purine versus non-purine xanthine oxidase inhibitors against cyclophosphamide-induced cardiac and bone marrow toxicity in rats. Hum Exp Toxicol 2020; 39(3): 249–261. [DOI] [PubMed] [Google Scholar]
- 86.Refaie MMM, Shehata S, El-Hussieny M, et al. Role of ATP-sensitive potassium channel (KATP) and eNOS in mediating the protective effect of nicorandil in cyclophosphamide-induced cardiotoxicity. Cardiovasc Toxicol 2020; 20(1): 71–81. [DOI] [PubMed] [Google Scholar]
- 87.Nieto Y, Vaughan WP.Pharmacokinetics of high-dose chemotherapy. Bone Marrow Transplant 2004; 33(3): 259–269. [DOI] [PubMed] [Google Scholar]
- 88.Helsby NA, Yong M, van Kan M, et al. The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br J Clin Pharmacol 2019; 85(9): 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Australian New Zealand Clinical Trials Registry (ANZCTR), https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371990
- 90.Spierings J, Nihtyanova SI, Derrett-Smith E, et al. Outcomes linked to eligibility for stem cell transplantation trials in diffuse cutaneous systemic sclerosis. Rheumatology 2022; 61(5): 1948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akram MR, Handler CE, Williams M, et al. Angiographically proven coronary artery disease in scleroderma. Rheumatology 2006; 45(11): 1395–1398. [DOI] [PubMed] [Google Scholar]
- 92.Follansbee WP, Curtiss EI, Medsger TA, et al. Physiologic abnormalities of cardiac function in progressive systemic sclerosis with diffuse scleroderma. N Engl J Med 1984; 310(3): 142–148. [DOI] [PubMed] [Google Scholar]
- 93.Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12): 1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening programme. Arthritis Res Ther 2017; 19(1): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kurmann RD, El-Am EA, Radwan YA, et al. Increased risk of valvular heart disease in systemic sclerosis: an underrecognized cardiac complication. J Rheumatol 2021; 48(7): 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allanore Y, Meune C, Vonk MC, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1): 218–221. [DOI] [PubMed] [Google Scholar]
- 97.Vemulapalli S, Cohen L, Hsu V.Prevalence and risk factors for left ventricular diastolic dysfunction in a scleroderma cohort. Scand J Rheumatol 2017; 46(4): 281–287. [DOI] [PubMed] [Google Scholar]
- 98.Roberts NK, Cabeen WR, Jr, Moss J, et al. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1): 38–40. [DOI] [PubMed] [Google Scholar]
- 99.Mavrogeni S, Koutsogeorgopoulou L, Karabela G, et al. Silent myocarditis in systemic sclerosis detected by cardiovascular magnetic resonance using Lake Louise criteria. BMC Cardiovasc Disord 2017; 17(1): 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Byers RJ, Marshall DA, Freemont AJ.Pericardial involvement in systemic sclerosis. Ann Rheum Dis 1997; 56(6): 393–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D’Angelo WA, Fries JF, Masi AT, et al. Pathologic observations in systemic sclerosis (scleroderma). Am J Med 1969; 46(3): 428–440. [DOI] [PubMed] [Google Scholar]