Malaria is one of the most ancient infectious diseases in human history. Eradication of malaria has long been a main global health initiative (1–3). To accelerate progress toward malaria elimination, the Global Technical Strategy for Malaria 2016 to 2030 (GTS) was adopted in 2015, with the aim of reducing the malaria burdens of both death and disease by at least 90% by 2030 (4). Although tremendous efforts have been made to control malaria over the past century, the disease remains one of the leading causes of morbidity and mortality worldwide (5). In particular, a rebound of malaria in sub-Saharan Africa accounted for 95% of all malaria cases and 96% of all malaria deaths worldwide in 2020 (6). Under these circumstances, the most worrying future scenario of malaria risk is the appearance of the invasive malaria vector, Anopheles stephensi, which was recently detected in Africa. Since the identification of the parasites that cause malaria, research has historically been focused on their biology as well as on the population ecology of mosquitoes that transmit malaria parasites (7). Short-term seasonal cycles and long-term trends of climate and land-use change can alter not only biological traits but also the contact between mosquitoes and humans, and hence, the transmission trajectories. In PNAS, Whittaker et al. (8) moved one step closer to understanding the uncertainty in future malaria risk by characterizing the impact of ecological factors on the invasion and establishment of An. stephensi across the Horn of Africa. The authors summarized the existing literature and presented evidence that variation in An. stephensi abundance is associated with temperature and patterns of land-use, with seasonality frequently differing between rural and urban settings. That important study sheds new light on a number of discussions in the environmental sciences related to global change and public health and provides a framework for invasive vector surveillance strategies in malaria control.
Accelerating Urbanization and Land-Use Change
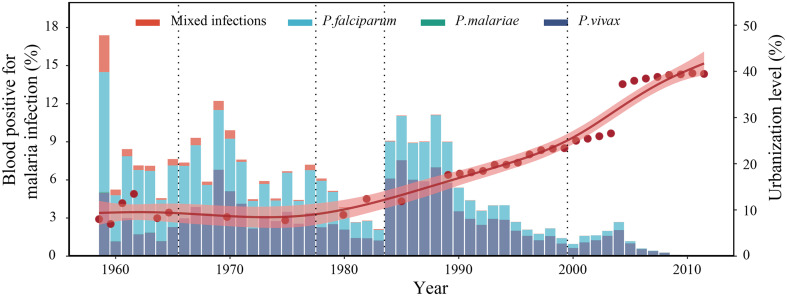
Over 50% of the global population currently lives in urban areas, with the fastest urbanization rates seen in Africa. As a result, malaria-affected countries are expected to see more people moving into their urban areas. Concomitant land-use changes and population immigration, driven by the urbanization process, can have complex effects on shifting the risk of infectious diseases (9), such as rural urbanization, deforestation, and agricultural expansion and irrigation have been linked to the altered patterns of malaria (10). Improvements in housing conditions, which usually accompanies urbanization, have been shown to be an important contributing factor to reducing the malaria risk (11–13). Over the past century, rapid urban development has largely accelerated malaria elimination in Hainan, a tropical island province of southern China, with fluctuations in the malaria prevalence with rapid urbanization and endemic turning points (Fig. 1). However, unplanned urbanization and the presence of invasive mosquito species that adapt to urban settings in malaria-affected countries may heighten the risk of malaria (14). Reports of rapid land-use change and species that amplify pathogens thrive in human-dominated landscapes have been accumulating for decades. An. stephensi, which is known to thrive in urban settings and mostly breeds in man-made water containers, could put population at new risk due to inadequate water supplies, waste management, dense population, and insufficient capacity of vector control and surveillance, especially in unplanned urban areas. Most recently, An. stephensi has been identified in multiple African countries as an invasive vector (15) incriminated in the resurgence of malaria outbreaks in Djibouti (16). Perhaps most intriguing, the meta-analysis of the population dynamics and adaptation of An. stephensi presented by Whittaker et al. (8) suggests the existence of a diverse range of temporal patterns between rural and urban settings, highlighting the urgent actions required to prevent malaria resurgence in sub-Saharan Africa, which previously occurred during the 1980s.
Fig. 1.
Malaria epidemics in Hainan between 1959 and the elimination of endemic malaria in 2011. Hainan is a tropical island province in Southern China. Bars represent the annual malaria prevalence in the population measured using blood tests. Red dots correspond to the urbanization rate. Urbanization is defined as the percentage of the total population living in urban areas and is adjusted by the percentage of the nonagricultural population owing to changes in the official statistical category of “urban population” in 2005. The thick red line shows the fit of urbanization; 95% prediction ranges of regression are shown as red shaded areas. The vertical dashed lines indicate the timing of four major insecticides being successfully introduced since the 1950s: organochlorines, organophosphates, carbamates, and pyrethroids.
In PNAS, Whittaker et al. moved one step closer to understanding the uncertainty in future malaria risk by characterizing the impact of ecological factors on the invasion and establishment of An. stephensi across the Horn of Africa.
Climate Change and Global Warming
As a climate-sensitive disease, the impact of climate change on malaria is of major public health interest (17, 18). Seasonal cycles are fundamental to malaria epidemiology. Variations mainly in temperature and rainfall can affect malaria incidence and transmissibility through the population dynamics of mosquito vectors and parasites or indirectly via other pathways (19). By analyzing environmental factors related to mosquito population dynamics, Whittaker et al. (8) in PNAS offer a fresh perspective that seasonal abundance of An. stephensi is associated with temperature but poorly predicted by rainfall. Owing to clear differences with the results obtained from analysis of dominant vectors across Africa, this finding again prompts the long-studied question of how future climate change will affect malaria transmission and geographic distribution. In light of previous theory, by estimating the optimal temperature range for malaria transmission, thermal response models (20) can help to explain the dependency between varying patterns of temperature and the changes in malaria incidence along altitudinal gradients across continents (21, 22). However, great uncertainties and controversies remain. Biological experiments, field surveillance, and population studies mainly analyze the currently dominant species. As such, mechanistic models linking climate variability to the biological response and ecological dynamics of mosquito vectors remain by far the most important tools for predicting malaria risk (23–25). Taken together, the disparate climate drivers identified for An. stephensi versus other dominant species in Africa imply a potential caveat regarding the scenarios of malaria risk predicted using previous climate–biological models.
Implications for Malaria Control
The patterns detected by Whittaker et al. (8) in their analyses are striking. The distinct patterns of An. stephensi abundance in rural and urban settings also raise interesting questions about the feasibility of and requirements for the effective control of malaria in the near future. Approaches to reduce the malaria burden in rural areas may not work or may be unsuitable in urban settings. Other challenges include the high population density and high levels of human mobility in urban settings (26) as well as rural-to-urban migration with malaria infection. Although some of the poorest countries have substantially reduced malaria transmission or even achieved malaria elimination, with some developing countries close to malaria elimination, the emergence and dissemination of invasive vectors that adapt to urban environments increase the risk of malaria resurgence and threaten the ultimate goal of malaria eradication.
Another key contribution of the novel study by Whittaker et al. (8) is to demonstrate the unanticipated challenges in combining knowledge about the temporal profiles of An. stephensi abundance with environmental data to predict future malaria risk with current entomological surveillance and vector control conducted in a stronger and more parsimonious manner. Most previous work has shown the importance of mosquito vector control in eliminating malaria, particularly the timing of vector control campaigns. For example, over the past century, indoor residual spraying has usually been implemented before the start of malaria epidemic season in Hainan (Fig. 1). However, several types of data are critical to test the proposed mechanisms (27–29). Causality has not been established from the inferred statistical framework between environmental conditions and the seasonal abundance of An. stephensi. Insecticide resistance profiles in malaria vectors are another important issue, and laboratory capacity remains scarce in many tropical countries (30). Going forward, such limitations suggest an urgent need for longitudinal entomological monitoring of invasive vectors in their new environments, given the above threats.
An. stephensi may not be the first and will not be the last invasive malaria vector in the 21st century. Owing to the rapid geographic expansion of An. stephensi and the potential replacement of efficient malaria vectors, particularly in Africa, it has proved challenging to anticipate malaria scenarios as targeted in the GTS (31). Changing epidemiological, environmental, and sociological conditions largely increase the complexity of malaria eradication. More powerful statistical and machine learning frameworks that can incorporate information of multiple driving factors, including the changing landscape, climate, and wide-ranging potential vectors, will be useful in optimizing the assessment of future malaria risk.
Acknowledgments
This work was supported by National Key Research and Development Program of China (2022YFC2303803, 2021YFC0863400), Beijing Science and Technology Planning Project (Z221100007922019), National Natural Science Foundation of China (82073616), Beijing Advanced Innovation Program for Land Surface Science (110631111), Research on Key Technologies of Plague Prevention and Control in Inner Mongolia Autonomous Region (2021ZD0006), and Fundamental Research Funds for the Central Universities. I would like to thank Ziyan Liu for collecting the data in Hainan that have helped improve this Article.
Author contributions
H.T. wrote the paper.
Competing interests
The author declares no competing interest.
Footnotes
See companion article, “Seasonal dynamics of Anopheles stephensi and its implications for mosquito detection and emergent malaria control in the Horn of Africa,” 10.1073/pnas.2216142120.
References
- 1.Gething P. W., et al. , Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. New Eng J. Med. 375, 2435–2445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray C. J., et al. , Global malaria mortality between 1980 and 2010: A systematic analysis. Lancet 379, 413–431 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Cox F. E. G., History of the discovery of the malaria parasites and their vectors. Parasit Vectors 3, 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Global Malaria Programme, Global Technical Strategy for Malaria 2016–2030 (World Health Organization, 2015). [Google Scholar]
- 5.Ferguson N. M., Challenges and opportunities in controlling mosquito-borne infections. Nature 559, 490–497 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Feachem R. G., et al. , Malaria eradication within a generation: Ambitious, achievable, and necessary. Lancet 394, 1056–1112 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Ross R., On some peculiar pigmented cells found in two mosquitos fed on malarial blood. BMJ 2, 1786 (1897). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittaker C., et al. , Seasonal dynamics of Anopheles stephensi and its implications for mosquito detection and emergent malaria control in the Horn of Africa. Proc. Natl. Acad. Sci. U.S.A. 120, e2216142120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian H., et al. , Urbanization prolongs hantavirus epidemics in cities. Proc. Natl. Acad. Sci. U.S.A. 115, 4707–4712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeza A., et al. , Long-lasting transition toward sustainable elimination of desert malaria under irrigation development. Proc. Natl. Acad. Sci. U.S.A. 110, 15157–15162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt S., et al. , The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallup J., Sachs J., The economic burden of malaria. Am. J. Trop. Med. Hyg. 64, 85–96 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Tusting L. S., et al. , Socioeconomic development as an intervention against malaria: A systematic review and meta-analysis. Lancet 382, 963–972 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Wilson M. L., et al. , Urban malaria: Understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am. J. Trop Med. Hyg. 93, 110–123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinka M., et al. , A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. U.S.A. 117, 24900–24908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Santi V. P., et al. , Role of Anopheles stephensi mosquitoes in malaria outbreak, Djibouti, 2019. Emerg. Infect. Dis. 27, 1697–1700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gething P. W., et al. , Climate change and the global malaria recession. Nature 465, 342–345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cash B., et al. , Malaria epidemics and the influence of the tropical South Atlantic on the Indian monsoon. Nat. Clim. Change 3, 502–507 (2013). [Google Scholar]
- 19.Altizer S., et al. , Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Mordecai E. A., et al. , Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 16, 22–30 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Siraj A., et al. , Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 343, 1154–1158 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., et al. , The relationship between rising temperatures and malaria incidence in Hainan, China, from 1984 to 2010: A longitudinal cohort study. Lancet Planet. Health 6, e350–e358 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Paaijmans K. P., et al. , Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. U.S.A. 107, 15135–15139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual M., Ahumada J. A., Chaves L. F., Rodo X., Bouma M., Malaria resurgence in the East African highlands: Temperature trends revisited. Proc. Natl. Acad. Sci. U.S.A. 103, 5829–5834 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paaijmans K. P., Read A. F., Thomas M. B., Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. U.S.A. 106, 13844–13849 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wesolowski A., et al. , Quantifying the impact of human mobility on malaria. Science 338, 267–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin J. T., et al. , Reducing Plasmodium falciparum malaria transmission in Africa: A model-based evaluation of intervention strategies. PLoS Med. 7, e1000324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady O. J., et al. , Role of mass drug administration in elimination of Plasmodium falciparum malaria: A consensus modelling study. Lancet Glob Health 5, e680–e687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker P. G., Griffin J. T., Ferguson N. M., Ghani A. C., Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: A modelling study. Lancet Glob. Health 4, e474–e484 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A., Abubakr M., Ali Y., Siddig E. E., Mohamed N. S., Vector control strategy for Anopheles stephensi in Africa. Lancet Microbe. 3, e403 (2022). [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization Strategic Advisory Group on Malaria Eradication, Malaria Eradication: Benefits, Future Scenarios and Feasibility (World Health Organization, Geneva, Switzerland, 2020). [Google Scholar]