In the crystal, the molecules are connected by N—H⋯N hydrogen bonds into dimers with an
 (12) motif, forming chains along the b-axis direction. These chains are linked to each other by N—H⋯N hydrogen bonds, N–H⋯π and π–π interactions, forming a three-dimensional network.
(12) motif, forming chains along the b-axis direction. These chains are linked to each other by N—H⋯N hydrogen bonds, N–H⋯π and π–π interactions, forming a three-dimensional network.
Keywords: crystal structure, pyridine ring, thiophene ring, disorder, Hirshfeld surface analysis
Abstract
In the title compound, C19H15N5S, the thiophene ring is disordered in a 0.6:0.4 ratio by an approximate 180° rotation of the ring around the C—C bond linking it to the pyridine ring. In the crystal, the molecules are linked by N—H⋯N hydrogen bonds into dimers with an R 2 2(12) motif, forming chains along the b-axis direction. These chains are connected to each other by further N—H⋯N hydrogen bonds, forming a three-dimensional network. Furthermore, N—H⋯π and π–π [centroid–centroid separations = 3.899 (8) and 3.7938 (12) Å] interactions also contribute to the crystal cohesion. A Hirshfeld surface analysis indicated that the most important contributions to the surface contacts are from H⋯H (46.1%), N⋯H/H⋯N (20.4%) and C⋯H/H⋯C (17.4%) interactions.
1. Chemical context
Diverse C—C, C—N and C—O bond-formation methods play important roles in organic synthesis. The reaction scopes have also been greatly expanded, employing these methods in different fields of chemistry, in both academia and industry (Çelik et al., 2023 ▸; Chalkha et al., 2023 ▸; Tapera et al., 2022 ▸; Gurbanov et al., 2020 ▸; Zubkov et al., 2018 ▸). The pyridine moiety is a widespread structural motif that can be found in various natural products and pharmacologically active compounds. 3,5-Dicyanopyridines have been reported as intermediates in the synthesis of pyrido[2,3-d]pyrimidines, pyridothienotriazines, azabenzanthracenes and pyrimidine S-nucleoside derivatives with a broad spectrum of biological activity (Cocco et al., 2005 ▸; Zhang et al., 2022 ▸; Poustforoosh et al., 2022 ▸). The design of new 3,5-dicyanopyridine derivatives is thus of great interest.
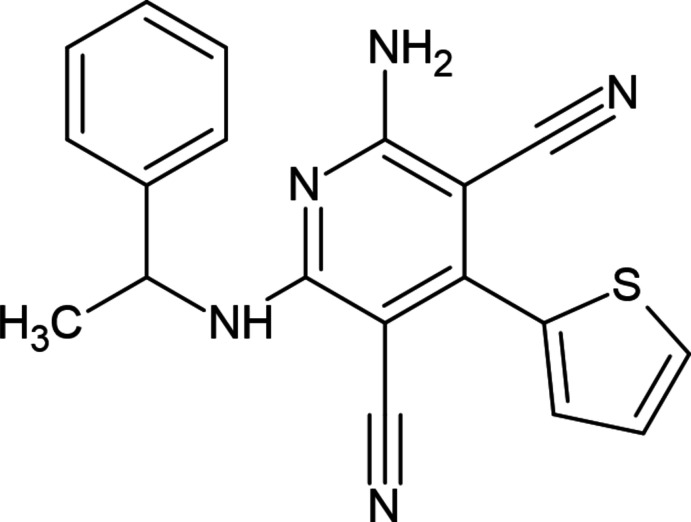
Continuing our studies of pyridine derivatives exhibiting biological activity, we designed and synthesized a novel 3,5-dicyanopyridine in this series. Thus, in the framework of our ongoing structural studies (Naghiyev et al., 2020 ▸, 2021 ▸, 2022 ▸), we report the crystal structure and Hirshfeld surface analysis of the title compound, 2-amino-6-[(1-phenylethyl)amino]-4-(thiophen-2-yl)pyridine-3,5-dicarbonitrile.
2. Structural commentary
The pyridine ring (N1/C2–C6) of the title compound (Fig. 1 ▸) is largely planar [maximum deviation = 0.015 (2) Å for C5]. The thiophene and 1-phenylethan-1-amine groups are linked to the central pyridine ring in an equatorial arrangement. The major and minor parts (S1/C15–C18 and S1A/C15A–C18A) of the disordered thiophene ring make dihedral angles of 44.8 (5) and 48.9 (6)°, respectively, with the pyridine ring. The dihedral angle between the phenyl (C7–C12) and pyridine (N1/C2–C6) rings is 64.42 (11) °.
Figure 1.
The molecular structure of the title compound, showing the atom labelling and displacement ellipsoids drawn at the 30% probability level. For clarity, the minor disorder component is not shown.
3. Supramolecular features and Hirshfeld surface analysis
In the crystal, the molecules are linked by N—H⋯N hydrogen bonds into dimers with an
 (12) motif (Bernstein et al., 1995 ▸; Table 1 ▸, Fig. 2 ▸), forming chains along the b-axis direction. These chains are connected to each other by further N—H⋯N hydrogen bonds, forming a three-dimensional network (Tables 1 ▸ and 2 ▸, Figs. 3 ▸ and 4 ▸). Furthermore, N—H⋯π and π–π interactions [Cg1⋯Cg1i = 3.899 (8) Å; slippage = 1.899 Å; Cg3⋯Cg3ii = 3.7938 (12) Å; slippage = 1.383 Å; symmetry codes: (i) −x, 1 − y, z; (ii) 1 − x, 1 − y, z; Cg1 and Cg3 are the centroids of the major component of the disordered thiophene ring and of the pyridine ring, respectively] also contribute to crystal cohesion (Figs. 5 ▸ and 6 ▸).
(12) motif (Bernstein et al., 1995 ▸; Table 1 ▸, Fig. 2 ▸), forming chains along the b-axis direction. These chains are connected to each other by further N—H⋯N hydrogen bonds, forming a three-dimensional network (Tables 1 ▸ and 2 ▸, Figs. 3 ▸ and 4 ▸). Furthermore, N—H⋯π and π–π interactions [Cg1⋯Cg1i = 3.899 (8) Å; slippage = 1.899 Å; Cg3⋯Cg3ii = 3.7938 (12) Å; slippage = 1.383 Å; symmetry codes: (i) −x, 1 − y, z; (ii) 1 − x, 1 − y, z; Cg1 and Cg3 are the centroids of the major component of the disordered thiophene ring and of the pyridine ring, respectively] also contribute to crystal cohesion (Figs. 5 ▸ and 6 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
Cg4 is the centroid of the C7–C12 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯N19i | 0.91 (3) | 2.28 (3) | 3.152 (3) | 163 (3) |
| N6—H6B⋯N14ii | 0.89 (3) | 2.17 (4) | 3.033 (3) | 164 (3) |
| N6—H6A⋯Cg4iii | 0.91 (3) | 2.62 (4) | 3.405 (2) | 145 (3) |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 .
.
Figure 2.
View of the molecular packing along the a axis. N—H⋯N hydrogen bonds are shown as dashed lines. For clarity, the minor disorder component is not shown.
Table 2. Summary of short interatomic contacts (Å) in the title compound.
| Contact | Distance | Symmetry operation |
|---|---|---|
| H13A⋯H6A | 2.36 | −x, 1 − y, z |
| H6B⋯N14 | 2.18 |
 − x,
− x,
 + y, 1 − z
+ y, 1 − z
|
| H16⋯N19 | 2.56 | 1 − x, 1 − y, z |
| C17⋯H9 | 2.86 | −
 + x,
+ x,
 − y, 1 − z
− y, 1 − z
|
| C10⋯C13 | 3.58 | 1 + x, y, z |
| H12⋯H18A | 2.31 | x, y, 1 + z |
| H18⋯H11 | 2.34 | 1 − x, 1 − y, −1 + z |
Figure 3.
View of the molecular packing along the b axis. Hydrogen bonds are shown as dashed lines.
Figure 4.
View of the molecular packing along the c axis. Hydrogen bonds are shown as dashed lines.
Figure 5.
View of the molecular packing along the b axis. N—H⋯π interactions and π–π stacking interactions are shown as dashed lines.
Figure 6.
View of the molecular packing along the c axis. N—H⋯π interactions and π–π stacking interactions are shown as dashed lines.
Crystal Explorer 17.5 (Spackman et al., 2021 ▸) was used to generate Hirshfeld surfaces and two-dimensional fingerprint plots in order to quantify the intermolecular interactions in the crystal. The intermolecular interactions are depicted as red spots, which denotes the N—H⋯N hydrogen bonds, on the Hirshfeld surface mapped over d norm in the range −0.4485 to +1.5784 a.u. (Fig. 7 ▸ a,b). Fig. 8 ▸ shows the two-dimensional fingerprint plots. The H⋯H contacts comprise 46.1% of the total interactions. Besides this contact, N⋯H/H⋯N (20.4%) and C⋯H/H⋯C (17.4%) interactions make significant contributions to the total Hirshfeld surface. The percentage contributions of the C⋯C, N⋯C/C⋯N, N⋯N, S⋯C/C⋯S, S⋯H/H⋯S and S⋯S contacts are 6.9, 3.8, 2.7, 1.5, 0.6 and 0.6%, respectively.
Figure 7.
(a) Front and (b) back sides of the three-dimensional Hirshfeld surface of the title compound mapped over d norm, with a fixed colour scale of −0.4485 to +1.5784 a.u. N—H⋯N hydrogen bonds are shown as dashed lines.
Figure 8.
The two-dimensional fingerprint plots of the title compound, showing (a) all interactions, and delineated into (b) H⋯H, (c) N⋯H/H⋯N and (d) C⋯H/H⋯C interactions. [d e and d i represent the distances from a point on the Hirshfeld surface to the nearest atoms outside (external) and inside (internal) the surface, respectively].
4. Database survey
The four related compounds found as a result of the search for ‘2,6-diamino-4-(thiophen-2-yl)pyridine-3,5-dicarbonitrile’ in the Cambridge Structure Database (CSD, Version 5.42, update of September 2021; Groom et al., 2016 ▸) are MUCLAA (Vu Quoc et al., 2019 ▸), WOJCIJ (Vishnupriya et al., 2014a ▸), WOPLAQ (Vishnupriya et al., 2014b ▸) and DOPWOW (Vishnupriya et al., 2014c ▸).
In the crystal of MUCLAA (space group P21/c), chains running along the b-axis direction are formed through N—H⋯O interactions between the 1,4-dihydropyridine N atom and one of the O atoms of the ester groups. Neighbouring chains are linked by C—H⋯O and C—H⋯π interactions. In the crystal of WOJCIJ (space group P21/c), inversion dimers linked by pairs of N—H⋯N hydrogen bonds generate
 (16) loops and the dimers are linked by C—H⋯π and aromatic π–π stacking interactions into a three-dimensional network. In WOPLAQ (space group P21/n), inversion dimers linked by pairs of N—H⋯Nc (c = cyanide) hydrogen bonds generate
(16) loops and the dimers are linked by C—H⋯π and aromatic π–π stacking interactions into a three-dimensional network. In WOPLAQ (space group P21/n), inversion dimers linked by pairs of N—H⋯Nc (c = cyanide) hydrogen bonds generate
 (16) loops. In DOPWOW (space group Pbca), inversion dimers linked by pairs of N—H⋯Nn (n = nitrile) hydrogen bonds generate
(16) loops. In DOPWOW (space group Pbca), inversion dimers linked by pairs of N—H⋯Nn (n = nitrile) hydrogen bonds generate
 (16) loops. Aromatic π–π stacking and very weak C—H⋯π interactions are also observed.
(16) loops. Aromatic π–π stacking and very weak C—H⋯π interactions are also observed.
5. Synthesis and crystallization
To a solution of 2-(thiophen-2-ylmethylene)malononitrile (0.82 g; 5.1 mmol) and malononitrile (0.34 g; 5.2 mmol) in methanol (25 mL), phenylethylamine (0.63 g; 5.2 mmol) was added and the mixture was stirred at room temperature for 48 h. Then 15 mL of methanol were removed from the reaction mixture, which was left overnight. The precipitated crystals were separated by filtration and recrystallized from ethanol/water (1:1) solution (yield 94%; m.p. 460–461 K).
1H NMR (300 MHz, DMSO-d 6, ppm): 1.55 (d, 3H, CH3, 3 J H–H = 7 MHz); 5.45 (k, 1H, CH—Ar, 3 J H–H =7,1 MHz); 7.21–7.88 (m, 11H, 5CHarom + 3CHthienyl + NH2 + NH); 13C NMR (75 MHz, DMSO-d 6, ppm): 21.69 (CH3), 50.00 (CH—Ar), 79.77 (=Ctert), 80.92 (=Ctert), 116.85 (CN), 116.97 (CN), 127.14 (2CHarom), 127.22 (CHarom), 128.11 (CHthienyl), 128.63 (2CHarom), 130.14 (CHthienyl), 130.75 (CHthienyl), 134.53 (Car), 144.53 (Cthienyl), 152.30 (=Ctert), 158.70 (N=Ctert), 161.38 (=Ctert).
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The thiophene ring in the title compound was modelled as disordered over two sets of sites related by an approximate rotation of 180° about the C4—C15 bond in a 0.6:0.4 ratio. EADP commands in SHELXL were used for the U ij values of equivalent atom pairs (e.g., C16 and C16A) and DFIX commands were used to restrain the nearest-neighbour and next-nearest-neighbour bond distances in the two disorder components to be equal with a standard deviation of 0.03 Å. All C-bound H atoms were placed in calculated positions (0.95–1.00 Å) and refined as riding with U iso(H) = 1.2 or 1.5U eq(C). The N-bound H atoms were located in a difference map and refined with U iso(H) = 1.2U eq(N) [N2—H2 = 0.91 (3) Å, N6—H6A = 0.91 (3) Å, N6—H6B = 0.89 (3) Å].
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | C19H15N5S |
| M r | 345.42 |
| Crystal system, space group | Orthorhombic, P21212 |
| Temperature (K) | 100 |
| a, b, c (Å) | 7.89079 (13), 16.4990 (3), 13.1394 (3) |
| V (Å3) | 1710.62 (6) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 1.77 |
| Crystal size (mm) | 0.40 × 0.04 × 0.03 |
| Data collection | |
| Diffractometer | XtaLAB Synergy, Dualflex, HyPix |
| Absorption correction | Gaussian (CrysAlis PRO; Rigaku OD, 2022 ▸) |
| T min, T max | 0.532, 0.939 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 26907, 3713, 3612 |
| R int | 0.044 |
| (sin θ/λ)max (Å−1) | 0.638 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.036, 0.096, 1.05 |
| No. of reflections | 3713 |
| No. of parameters | 276 |
| No. of restraints | 12 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.15, −0.25 |
| Absolute structure | Refined as an inversion twin |
| Absolute structure parameter | 0.13 (3) |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989023003845/vm2282sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023003845/vm2282Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989023003845/vm2282Isup3.cml
CCDC reference: 2260011
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Authors’ contributions are as follows. Conceptualization, ANK and IGM; methodology, ANK, FNN and IGM; investigation, ANK, MA and KAA; writing (original draft), MA and ANK; writing (review and editing of the manuscript), MA and ANK; visualization, MA, ANK and IGM; funding acquisition, VNK, AB and ANK; resources, AB, VNK and KAA; supervision, ANK and MA.
supplementary crystallographic information
Crystal data
| C19H15N5S | Dx = 1.341 Mg m−3 |
| Mr = 345.42 | Cu Kα radiation, λ = 1.54184 Å |
| Orthorhombic, P21212 | Cell parameters from 17132 reflections |
| a = 7.89079 (13) Å | θ = 3.4–79.2° |
| b = 16.4990 (3) Å | µ = 1.77 mm−1 |
| c = 13.1394 (3) Å | T = 100 K |
| V = 1710.62 (6) Å3 | Needle, colourless |
| Z = 4 | 0.40 × 0.04 × 0.03 mm |
| F(000) = 720 |
Data collection
| XtaLAB Synergy, Dualflex, HyPix diffractometer | 3612 reflections with I > 2σ(I) |
| Radiation source: micro-focus sealed X-ray tube | Rint = 0.044 |
| φ and ω scans | θmax = 79.7°, θmin = 3.4° |
| Absorption correction: gaussian (CrysAlisPro; Rigaku OD, 2022) | h = −10→9 |
| Tmin = 0.532, Tmax = 0.939 | k = −21→20 |
| 26907 measured reflections | l = −16→16 |
| 3713 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.036 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.096 | w = 1/[σ2(Fo2) + (0.0507P)2 + 0.5356P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 3713 reflections | Δρmax = 0.15 e Å−3 |
| 276 parameters | Δρmin = −0.25 e Å−3 |
| 12 restraints | Absolute structure: Refined as an inversion twin |
| Primary atom site location: difference Fourier map | Absolute structure parameter: 0.13 (3) |
Special details
| Experimental. CrysAlisPro 1.171.41.123a (Rigaku OD, 2022); Numerical absorption correction based on Gaussian integration over a multifaceted crystal model; Empirical absorption correction using spherical harmonics implemented in SCALE3 ABSPACK scaling algorithm. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S1 | 0.1793 (3) | 0.56636 (9) | 0.24471 (14) | 0.0306 (3) | 0.6 |
| S1A | 0.3236 (6) | 0.42012 (16) | 0.2521 (3) | 0.0254 (6) | 0.4 |
| N1 | 0.2686 (2) | 0.50614 (12) | 0.64994 (15) | 0.0250 (4) | |
| C1 | 0.1399 (3) | 0.38542 (14) | 0.77369 (17) | 0.0270 (5) | |
| H1 | 0.1288 | 0.4439 | 0.7928 | 0.032* | |
| C2 | 0.2054 (3) | 0.44038 (13) | 0.60337 (17) | 0.0239 (4) | |
| N2 | 0.1451 (2) | 0.38039 (12) | 0.66239 (15) | 0.0263 (4) | |
| H2 | 0.112 (4) | 0.332 (2) | 0.636 (2) | 0.032* | |
| C3 | 0.1980 (3) | 0.43395 (13) | 0.49462 (17) | 0.0237 (4) | |
| C4 | 0.2528 (2) | 0.49903 (13) | 0.43479 (15) | 0.0236 (4) | |
| C5 | 0.3127 (3) | 0.56866 (13) | 0.48450 (17) | 0.0244 (4) | |
| C6 | 0.3206 (3) | 0.56878 (12) | 0.59318 (17) | 0.0242 (4) | |
| N6 | 0.3851 (3) | 0.63253 (13) | 0.64322 (17) | 0.0288 (4) | |
| H6A | 0.392 (4) | 0.6297 (19) | 0.712 (3) | 0.035* | |
| H6B | 0.413 (4) | 0.679 (2) | 0.615 (2) | 0.035* | |
| C7 | 0.2992 (3) | 0.35278 (14) | 0.82352 (17) | 0.0255 (4) | |
| C8 | 0.3550 (3) | 0.27466 (15) | 0.80460 (19) | 0.0313 (5) | |
| H8 | 0.2958 | 0.2417 | 0.7571 | 0.038* | |
| C9 | 0.4974 (3) | 0.24370 (16) | 0.8545 (2) | 0.0352 (5) | |
| H9 | 0.5354 | 0.1903 | 0.8402 | 0.042* | |
| C10 | 0.5827 (3) | 0.29053 (17) | 0.9245 (2) | 0.0352 (5) | |
| H10 | 0.6777 | 0.2691 | 0.9599 | 0.042* | |
| C11 | 0.5292 (3) | 0.3690 (2) | 0.9429 (2) | 0.0429 (6) | |
| H11 | 0.5883 | 0.4019 | 0.9904 | 0.051* | |
| C12 | 0.3887 (3) | 0.39989 (17) | 0.8918 (2) | 0.0361 (6) | |
| H12 | 0.3540 | 0.4541 | 0.9041 | 0.043* | |
| C13 | −0.0172 (3) | 0.34112 (18) | 0.8124 (2) | 0.0361 (6) | |
| H13A | −0.1190 | 0.3670 | 0.7847 | 0.054* | |
| H13B | −0.0203 | 0.3435 | 0.8869 | 0.054* | |
| H13C | −0.0133 | 0.2844 | 0.7904 | 0.054* | |
| C14 | 0.1206 (3) | 0.36403 (14) | 0.45060 (17) | 0.0257 (4) | |
| N14 | 0.0540 (3) | 0.30718 (12) | 0.41871 (16) | 0.0310 (4) | |
| C15 | 0.251 (3) | 0.4906 (6) | 0.3229 (3) | 0.029 (3) | 0.6 |
| C16 | 0.3006 (16) | 0.4236 (4) | 0.2681 (5) | 0.025 (2) | 0.6 |
| H16 | 0.3422 | 0.3748 | 0.2974 | 0.030* | 0.6 |
| C17 | 0.2816 (10) | 0.4371 (4) | 0.1618 (5) | 0.0346 (15) | 0.6 |
| H17 | 0.3135 | 0.3981 | 0.1121 | 0.042* | 0.6 |
| C18 | 0.2137 (10) | 0.5107 (4) | 0.1371 (5) | 0.0366 (17) | 0.6 |
| H18 | 0.1893 | 0.5284 | 0.0698 | 0.044* | 0.6 |
| C15A | 0.246 (4) | 0.4989 (10) | 0.3229 (3) | 0.024 (4) | 0.4 |
| C16A | 0.180 (2) | 0.5595 (5) | 0.2643 (5) | 0.025 (2) | 0.4 |
| H16A | 0.1294 | 0.6078 | 0.2899 | 0.030* | 0.4 |
| C17A | 0.1963 (15) | 0.5398 (6) | 0.1596 (6) | 0.029 (2) | 0.4 |
| H17A | 0.1595 | 0.5750 | 0.1069 | 0.035* | 0.4 |
| C18A | 0.2696 (14) | 0.4661 (7) | 0.1404 (6) | 0.032 (2) | 0.4 |
| H18A | 0.2877 | 0.4439 | 0.0746 | 0.038* | 0.4 |
| C19 | 0.3707 (3) | 0.63853 (14) | 0.43145 (18) | 0.0256 (4) | |
| N19 | 0.4186 (3) | 0.69712 (13) | 0.39337 (16) | 0.0298 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0317 (6) | 0.0288 (5) | 0.0314 (6) | −0.0050 (5) | −0.0049 (7) | 0.0067 (5) |
| S1A | 0.0253 (13) | 0.0248 (10) | 0.0261 (9) | 0.0020 (6) | 0.0027 (10) | −0.0027 (8) |
| N1 | 0.0238 (8) | 0.0243 (9) | 0.0269 (9) | −0.0001 (7) | −0.0007 (7) | −0.0006 (7) |
| C1 | 0.0261 (10) | 0.0271 (10) | 0.0279 (11) | 0.0024 (8) | 0.0027 (9) | −0.0004 (8) |
| C2 | 0.0183 (9) | 0.0223 (9) | 0.0311 (11) | 0.0022 (8) | 0.0001 (8) | 0.0025 (8) |
| N2 | 0.0276 (9) | 0.0248 (9) | 0.0266 (9) | −0.0007 (7) | 0.0000 (7) | 0.0024 (7) |
| C3 | 0.0199 (9) | 0.0236 (10) | 0.0276 (10) | 0.0018 (9) | −0.0015 (8) | 0.0009 (8) |
| C4 | 0.0157 (9) | 0.0249 (11) | 0.0303 (11) | 0.0022 (8) | −0.0005 (7) | 0.0013 (9) |
| C5 | 0.0190 (9) | 0.0246 (10) | 0.0298 (10) | 0.0012 (9) | 0.0002 (8) | 0.0012 (8) |
| C6 | 0.0190 (9) | 0.0221 (10) | 0.0314 (11) | 0.0014 (8) | −0.0005 (8) | −0.0016 (8) |
| N6 | 0.0314 (9) | 0.0257 (10) | 0.0293 (10) | −0.0028 (8) | −0.0022 (8) | −0.0013 (8) |
| C7 | 0.0234 (10) | 0.0289 (11) | 0.0242 (9) | −0.0018 (8) | 0.0052 (8) | 0.0007 (8) |
| C8 | 0.0296 (11) | 0.0290 (11) | 0.0355 (12) | −0.0022 (9) | −0.0070 (9) | −0.0009 (9) |
| C9 | 0.0301 (11) | 0.0311 (12) | 0.0443 (13) | 0.0014 (10) | −0.0047 (11) | 0.0011 (11) |
| C10 | 0.0246 (10) | 0.0491 (15) | 0.0319 (11) | −0.0009 (10) | −0.0018 (9) | 0.0025 (11) |
| C11 | 0.0339 (13) | 0.0532 (16) | 0.0415 (14) | −0.0033 (12) | −0.0083 (11) | −0.0148 (13) |
| C12 | 0.0335 (12) | 0.0384 (14) | 0.0364 (12) | 0.0008 (10) | −0.0007 (10) | −0.0108 (11) |
| C13 | 0.0260 (11) | 0.0479 (15) | 0.0344 (12) | 0.0015 (11) | 0.0037 (10) | 0.0083 (11) |
| C14 | 0.0245 (9) | 0.0252 (10) | 0.0273 (10) | 0.0023 (8) | −0.0004 (8) | 0.0028 (9) |
| N14 | 0.0334 (10) | 0.0264 (10) | 0.0331 (10) | −0.0023 (8) | −0.0034 (8) | 0.0012 (8) |
| C15 | 0.022 (6) | 0.027 (4) | 0.036 (6) | −0.011 (3) | −0.004 (4) | 0.009 (3) |
| C16 | 0.023 (3) | 0.033 (3) | 0.020 (3) | 0.0021 (19) | 0.003 (2) | −0.0031 (17) |
| C17 | 0.027 (3) | 0.049 (4) | 0.028 (3) | −0.006 (3) | −0.001 (2) | −0.003 (3) |
| C18 | 0.029 (3) | 0.061 (6) | 0.020 (3) | −0.016 (4) | 0.001 (3) | 0.004 (3) |
| C15A | 0.017 (7) | 0.036 (6) | 0.020 (6) | 0.001 (6) | 0.004 (6) | −0.005 (5) |
| C16A | 0.023 (3) | 0.033 (3) | 0.020 (3) | 0.0021 (19) | 0.003 (2) | −0.0031 (17) |
| C17A | 0.027 (3) | 0.048 (6) | 0.012 (4) | 0.000 (4) | 0.003 (3) | −0.001 (3) |
| C18A | 0.022 (4) | 0.053 (8) | 0.020 (4) | 0.011 (5) | −0.001 (3) | 0.005 (5) |
| C19 | 0.0224 (9) | 0.0251 (10) | 0.0293 (10) | 0.0005 (8) | −0.0009 (9) | −0.0030 (9) |
| N19 | 0.0300 (9) | 0.0265 (10) | 0.0331 (10) | −0.0034 (8) | 0.0004 (8) | −0.0006 (8) |
Geometric parameters (Å, º)
| S1—C18 | 1.708 (4) | C8—H8 | 0.9500 |
| S1—C15 | 1.713 (4) | C9—C10 | 1.377 (4) |
| S1A—C18A | 1.707 (4) | C9—H9 | 0.9500 |
| S1A—C15A | 1.711 (4) | C10—C11 | 1.384 (4) |
| N1—C6 | 1.339 (3) | C10—H10 | 0.9500 |
| N1—C2 | 1.342 (3) | C11—C12 | 1.392 (4) |
| C1—N2 | 1.465 (3) | C11—H11 | 0.9500 |
| C1—C7 | 1.516 (3) | C12—H12 | 0.9500 |
| C1—C13 | 1.526 (3) | C13—H13A | 0.9800 |
| C1—H1 | 1.0000 | C13—H13B | 0.9800 |
| C2—N2 | 1.344 (3) | C13—H13C | 0.9800 |
| C2—C3 | 1.434 (3) | C14—N14 | 1.154 (3) |
| N2—H2 | 0.91 (3) | C15—C16 | 1.377 (4) |
| C3—C4 | 1.399 (3) | C16—C17 | 1.423 (4) |
| C3—C14 | 1.428 (3) | C16—H16 | 0.9500 |
| C4—C5 | 1.404 (3) | C17—C18 | 1.368 (9) |
| C4—C15A | 1.472 (4) | C17—H17 | 0.9500 |
| C4—C15 | 1.477 (3) | C18—H18 | 0.9500 |
| C5—C19 | 1.423 (3) | C15A—C16A | 1.368 (4) |
| C5—C6 | 1.429 (3) | C16A—C17A | 1.420 (4) |
| C6—N6 | 1.341 (3) | C16A—H16A | 0.9500 |
| N6—H6A | 0.91 (3) | C17A—C18A | 1.369 (13) |
| N6—H6B | 0.89 (3) | C17A—H17A | 0.9500 |
| C7—C12 | 1.382 (3) | C18A—H18A | 0.9500 |
| C7—C8 | 1.384 (3) | C19—N19 | 1.152 (3) |
| C8—C9 | 1.398 (3) | ||
| C18—S1—C15 | 93.0 (4) | C9—C10—C11 | 119.5 (2) |
| C18A—S1A—C15A | 92.3 (5) | C9—C10—H10 | 120.2 |
| C6—N1—C2 | 118.95 (19) | C11—C10—H10 | 120.2 |
| N2—C1—C7 | 112.82 (18) | C10—C11—C12 | 120.1 (2) |
| N2—C1—C13 | 109.13 (19) | C10—C11—H11 | 119.9 |
| C7—C1—C13 | 111.07 (19) | C12—C11—H11 | 119.9 |
| N2—C1—H1 | 107.9 | C7—C12—C11 | 121.0 (3) |
| C7—C1—H1 | 107.9 | C7—C12—H12 | 119.5 |
| C13—C1—H1 | 107.9 | C11—C12—H12 | 119.5 |
| N1—C2—N2 | 117.6 (2) | C1—C13—H13A | 109.5 |
| N1—C2—C3 | 122.0 (2) | C1—C13—H13B | 109.5 |
| N2—C2—C3 | 120.4 (2) | H13A—C13—H13B | 109.5 |
| C2—N2—C1 | 122.9 (2) | C1—C13—H13C | 109.5 |
| C2—N2—H2 | 122 (2) | H13A—C13—H13C | 109.5 |
| C1—N2—H2 | 115 (2) | H13B—C13—H13C | 109.5 |
| C4—C3—C14 | 121.65 (19) | N14—C14—C3 | 177.1 (2) |
| C4—C3—C2 | 119.4 (2) | C16—C15—C4 | 126.3 (4) |
| C14—C3—C2 | 118.7 (2) | C16—C15—S1 | 111.5 (3) |
| C3—C4—C5 | 118.08 (19) | C4—C15—S1 | 122.2 (3) |
| C3—C4—C15A | 123.3 (11) | C15—C16—C17 | 110.9 (5) |
| C5—C4—C15A | 118.6 (11) | C15—C16—H16 | 124.5 |
| C3—C4—C15 | 118.9 (7) | C17—C16—H16 | 124.5 |
| C5—C4—C15 | 123.0 (7) | C18—C17—C16 | 114.4 (6) |
| C4—C5—C19 | 122.9 (2) | C18—C17—H17 | 122.8 |
| C4—C5—C6 | 118.7 (2) | C16—C17—H17 | 122.8 |
| C19—C5—C6 | 118.3 (2) | C17—C18—S1 | 110.1 (6) |
| N1—C6—N6 | 116.7 (2) | C17—C18—H18 | 125.0 |
| N1—C6—C5 | 122.8 (2) | S1—C18—H18 | 125.0 |
| N6—C6—C5 | 120.5 (2) | C16A—C15A—C4 | 125.1 (5) |
| C6—N6—H6A | 118 (2) | C16A—C15A—S1A | 112.8 (3) |
| C6—N6—H6B | 125 (2) | C4—C15A—S1A | 122.1 (4) |
| H6A—N6—H6B | 117 (3) | C15A—C16A—C17A | 109.9 (6) |
| C12—C7—C8 | 118.5 (2) | C15A—C16A—H16A | 125.0 |
| C12—C7—C1 | 120.3 (2) | C17A—C16A—H16A | 125.0 |
| C8—C7—C1 | 121.1 (2) | C18A—C17A—C16A | 115.0 (9) |
| C7—C8—C9 | 120.8 (2) | C18A—C17A—H17A | 122.5 |
| C7—C8—H8 | 119.6 | C16A—C17A—H17A | 122.5 |
| C9—C8—H8 | 119.6 | C17A—C18A—S1A | 110.0 (8) |
| C10—C9—C8 | 120.1 (2) | C17A—C18A—H18A | 125.0 |
| C10—C9—H9 | 120.0 | S1A—C18A—H18A | 125.0 |
| C8—C9—H9 | 120.0 | N19—C19—C5 | 176.4 (2) |
| C6—N1—C2—N2 | −176.22 (19) | C1—C7—C8—C9 | −176.9 (2) |
| C6—N1—C2—C3 | 2.5 (3) | C7—C8—C9—C10 | 0.9 (4) |
| N1—C2—N2—C1 | 2.4 (3) | C8—C9—C10—C11 | −1.7 (4) |
| C3—C2—N2—C1 | −176.30 (19) | C9—C10—C11—C12 | 0.8 (4) |
| C7—C1—N2—C2 | −90.5 (2) | C8—C7—C12—C11 | −1.8 (4) |
| C13—C1—N2—C2 | 145.6 (2) | C1—C7—C12—C11 | 176.0 (2) |
| N1—C2—C3—C4 | −2.2 (3) | C10—C11—C12—C7 | 1.0 (4) |
| N2—C2—C3—C4 | 176.47 (18) | C3—C4—C15—C16 | −42 (2) |
| N1—C2—C3—C14 | −176.64 (19) | C5—C4—C15—C16 | 135.4 (17) |
| N2—C2—C3—C14 | 2.0 (3) | C15A—C4—C15—C16 | 173 (27) |
| C14—C3—C4—C5 | 174.0 (2) | C3—C4—C15—S1 | 136.5 (12) |
| C2—C3—C4—C5 | −0.3 (3) | C5—C4—C15—S1 | −46 (2) |
| C14—C3—C4—C15A | −4.1 (12) | C15A—C4—C15—S1 | −8 (23) |
| C2—C3—C4—C15A | −178.4 (11) | C18—S1—C15—C16 | 0.1 (16) |
| C14—C3—C4—C15 | −8.0 (9) | C18—S1—C15—C4 | −179.0 (17) |
| C2—C3—C4—C15 | 177.7 (8) | C4—C15—C16—C17 | −179.8 (17) |
| C3—C4—C5—C19 | −179.6 (2) | S1—C15—C16—C17 | 1 (2) |
| C15A—C4—C5—C19 | −1.4 (11) | C15—C16—C17—C18 | −2.2 (17) |
| C15—C4—C5—C19 | 2.4 (9) | C16—C17—C18—S1 | 2.3 (10) |
| C3—C4—C5—C6 | 2.2 (3) | C15—S1—C18—C17 | −1.3 (10) |
| C15A—C4—C5—C6 | −179.5 (11) | C3—C4—C15A—C16A | 130 (2) |
| C15—C4—C5—C6 | −175.7 (8) | C5—C4—C15A—C16A | −48 (4) |
| C2—N1—C6—N6 | −179.01 (19) | C15—C4—C15A—C16A | 168 (28) |
| C2—N1—C6—C5 | −0.4 (3) | C3—C4—C15A—S1A | −50 (3) |
| C4—C5—C6—N1 | −2.0 (3) | C5—C4—C15A—S1A | 132.2 (18) |
| C19—C5—C6—N1 | 179.74 (19) | C15—C4—C15A—S1A | −12 (23) |
| C4—C5—C6—N6 | 176.58 (19) | C18A—S1A—C15A—C16A | 0 (2) |
| C19—C5—C6—N6 | −1.6 (3) | C18A—S1A—C15A—C4 | −180 (2) |
| N2—C1—C7—C12 | 126.2 (2) | C4—C15A—C16A—C17A | 179 (2) |
| C13—C1—C7—C12 | −110.9 (3) | S1A—C15A—C16A—C17A | −1 (3) |
| N2—C1—C7—C8 | −56.0 (3) | C15A—C16A—C17A—C18A | 1 (2) |
| C13—C1—C7—C8 | 66.9 (3) | C16A—C17A—C18A—S1A | −1.2 (15) |
| C12—C7—C8—C9 | 0.9 (4) | C15A—S1A—C18A—C17A | 0.6 (15) |
Hydrogen-bond geometry (Å, º)
Cg4 is the centroid of the C7–C12 ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···N19i | 0.91 (3) | 2.28 (3) | 3.152 (3) | 163 (3) |
| N6—H6B···N14ii | 0.89 (3) | 2.17 (4) | 3.033 (3) | 164 (3) |
| N6—H6A···Cg4iii | 0.91 (3) | 2.62 (4) | 3.405 (2) | 145 (3) |
Symmetry codes: (i) −x+1/2, y−1/2, −z+1; (ii) −x+1/2, y+1/2, −z+1; (iii) −x+1, −y+1, z.
Funding Statement
This paper was supported by Baku State University and the RUDN University Strategic Academic Leadership Program.
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Çelik, M. S., Çetinus, A., Yenidünya, A. F., Çetinkaya, S. & Tüzün, B. (2023). J. Mol. Struct. 1272, 134158.
- Chalkha, M., Ameziane el Hassani, A., Nakkabi, A., Tüzün, B., Bakhouch, M., Benjelloun, A. T., Sfaira, M., Saadi, M., Ammari, L. E. & Yazidi, M. E. (2023). J. Mol. Struct. 1273, 134255.
- Cocco, M. T., Congiu, C., Lilliu, V. & Onnis, V. (2005). Eur. J. Med. Chem. 40, 1365–1372. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Gurbanov, A. V., Kuznetsov, M. L., Mahmudov, K. T., Pombeiro, A. J. L. & Resnati, G. (2020). Chem. Eur. J. 26, 14833–14837. [DOI] [PubMed]
- Naghiyev, F. N., Akkurt, M., Askerov, R. K., Mamedov, I. G., Rzayev, R. M., Chyrka, T. & Maharramov, A. M. (2020). Acta Cryst. E76, 720–723. [DOI] [PMC free article] [PubMed]
- Naghiyev, F. N., Khrustalev, V. N., Novikov, A. P., Akkurt, M., Rzayev, R. M., Akobirshoeva, A. A. & Mamedov, I. G. (2022). Acta Cryst. E78, 554–558. [DOI] [PMC free article] [PubMed]
- Naghiyev, F. N., Tereshina, T. A., Khrustalev, V. N., Akkurt, M., Rzayev, R. M., Akobirshoeva, A. A. & Mamedov, İ. G. (2021). Acta Cryst. E77, 516–521. [DOI] [PMC free article] [PubMed]
- Poustforoosh, A., Hashemipour, H., Tüzün, B., Azadpour, M., Faramarz, S., Pardakhty, A., Mehrabani, M. & Nematollahi, M. H. (2022). Curr. Microbiol. 79, 241. [DOI] [PMC free article] [PubMed]
- Rigaku OD (2022). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. & Spackman, M. A. (2021). J. Appl. Cryst. 54, 1006–1011. [DOI] [PMC free article] [PubMed]
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Tapera, M., Kekeçmuhammed, H., Tüzün, B., Sarıpınar, E., Koçyiğit, M., Yıldırım, E., Doğan, M. & Zorlu, Y. (2022). J. Mol. Struct. 1269, 133816.
- Vishnupriya, R., Suresh, J., Bharkavi, S., Perumal, S. & Lakshman, P. L. N. (2014a). Acta Cryst. E70, o968–o969. [DOI] [PMC free article] [PubMed]
- Vishnupriya, R., Suresh, J., Gunasekaran, P., Perumal, S. & Lakshman, P. L. N. (2014b). Acta Cryst. E70, o978. [DOI] [PMC free article] [PubMed]
- Vishnupriya, R., Suresh, J., Sakthi, M., Perumal, S. & Lakshman, P. L. N. (2014c). Acta Cryst. E70, o1120–o1121. [DOI] [PMC free article] [PubMed]
- Vu Quoc, T., Tran Thi Thuy, D., Phung Ngoc, T., Vu Quoc, M., Nguyen, H., Duong Khanh, L., Tu Quang, A. & Van Meervelt, L. (2019). Acta Cryst. E75, 1861–1865. [DOI] [PMC free article] [PubMed]
- Zhang, X., Tao, F., Cui, T., Luo, C., Zhou, Z., Huang, Y., Tan, L., Peng, W. & Wu, C. (2022). Molecules, 27, 7187. [DOI] [PMC free article] [PubMed]
- Zubkov, F. I., Mertsalov, D. F., Zaytsev, V. P., Varlamov, A. V., Gurbanov, A. V., Dorovatovskii, P. V., Timofeeva, T. V., Khrustalev, V. N. & Mahmudov, K. T. (2018). J. Mol. Liq. 249, 949–952.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989023003845/vm2282sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023003845/vm2282Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989023003845/vm2282Isup3.cml
CCDC reference: 2260011
Additional supporting information: crystallographic information; 3D view; checkCIF report










