Abstract
Background
Epidemics of chronic kidney disease of undetermined causes (CKDu) among young male agricultural workers have been observed in many tropical regions. Western Kenya has similar climatic and occupational characteristics as many of those areas. The study objectives were to characterize prevalence and predictors of CKDu, such as, HIV, a known cause of CKD, in a sugarcane growing region of Kenya; and to estimate prevalence of CKDu across occupational categories and evaluate if physically demanding work or sugarcane work are associated with reduced eGFR.
Methods
The Disadvantaged Populations eGFR Epidemiology Study (DEGREE) protocol was followed in a cross-sectional study conducted in Kisumu County, Western Kenya. Multivariate logistic regression was performed to identify predictors of reduced eGFR.
Results
Among 782 adults the prevalence of eGFR < 90 was 9.85%. Among the 612 participants without diabetes, hypertension, and heavy proteinuria the prevalence of eGFR < 90 was 8.99% (95%CI 6.8%, 11.5%) and 0.33% (95%CI 0.04%, 1.2%) had eGFR < 60. Among the 508 participants without known risk factors for reduced eGFR (including HIV), the prevalence of eGFR < 90 was 5.12% (95%CI 3.4%, 7.4%); none had eGFR < 60. Significant risk factors for reduced eGFR were sublocation, age, body mass index, and HIV. No association was found between reduced eGFR and work in the sugarcane industry, as a cane cutter, or in physically demanding occupations.
Conclusion
CKDu is not a common public health problem in this population, and possibly this region. We recommend that future studies should consider HIV to be a known cause of reduced eGFR. Factors other than equatorial climate and work in agriculture may be important determinants of CKDu epidemics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-023-03213-2.
Keywords: Chronic kidney disease of undetermined causes, HIV, Occupational health, Environmental epidemiology, Global health
Background
In the 1990s, cases of end-stage renal disease of young, male agricultural workers with no history of hypertension, diabetes, or glomerular disease, were reported by clinicians in El Salvador [1]. Since then, an epidemic of chronic kidney disease of undetermined causes (CKDu) has been identified among mostly young men in agricultural areas of Central America [2–5]. Sometimes referred to as Mesoamerican nephropathy [6], chronic interstitial nephritis in agricultural communities (CINAC) [7, 8], Uddanam nephropathy, and Sri Lanka nephropathy, cases have also been described in Sri Lanka [9–13] and Andhra Pradesh, India [14–16].
Pesticides, informally produced alcohol, dietary sugar, heavy metals in drinking water, infectious agents, and nonsteroidal anti-inflammatory drugs [17–21], have been evaluated as etiologic factors, but none have been definitively established as cause(s) of CKDu. Presently, recurrent dehydration in the context of physically demanding work in hot climates is a focus of CKDu etiologic research [18, 22–28]. The hot climate of Western Kenya, as well as the widespread prevalence of manual labor in agriculture and other industries, are not unlike climatic conditions and occupational hazards in Central America, Sri Lanka, and India. Thus, if physically demanding work in a hot climate is a risk for CKDu, the condition may be found in Kenya, particularly in sugar growing regions. Only a few studies have addressed CKDu in sub-Saharan Africa. Ekiti et al. [29] described a 3.4% CKDu prevalence among sugarcane workers in Cameroon. A study conducted in Malawi utilized the Disadvantaged Populations eGFR Epidemiology (DEGREE) Study protocol, a standardized approach to evaluate CKDu [30, 31] and reported a 0.2% prevalence [32].
CKD is a common complication of human immunodeficiency virus (HIV) infection, affecting 3.5%–48.5% of people with HIV in sub-Saharan Africa [33–35]. In Western Kenya, among 373 HIV-infected, retrovirally-naive participants without diabetes or hypertension, 11.5% were found to have a creatinine clearance < 60 ml/min indicating possible CKD [36]. The prevalence of HIV infection among adult Kenyans is 4.9% and in Kisumu County it is 17.5% [37]. Thus, HIV should be considered in assessing the prevalence of CKDu in Kenya.
Numerous prevalence studies of CKDu have recruited participants from specific occupational groups, rather than being population based. Associations between CKDu and occupation have been addressed in population-based studies, though the methods for defining and categorizing occupation has varied [5, 27, 38]. The use of standardized methods of occupational classification should help advance our understanding of work-related risk factors for CKDu.
We utilized the DEGREE protocol to characterize the prevalence and risk factors for CKDu in Kisumu County, a sugar-growing region of Western Kenya. The DEGREE definition of CKDu excludes cases with diabetes, hypertension, or heavy proteinuria. Given that HIV accounts for some of the CKD cases, we evaluated renal function among those with and without HIV. Additionally, we used a standardized job classification system [39] and an empirically-based categorization of physical demand at work [40] to evaluate associations between occupation and CKDu.
Methods
Study design and study setting
A cross-sectional, population-based household survey was completed following the DEGREE protocol [30, 31] to allow for regional and international comparisons of CKDu prevalence. The study took place in Western Kenya’s “sugar belt” in a rural area of the Muhoroni Sub-County (population: 154,116, land area: 657.5 km2, population density: 234 persons/km2) located within Kisumu County (Fig. 1). Within Muhoroni Sub-County, three sublocations – Owaga, Muhoroni East and Tonde, which represent the catchment area of Muhoroni County Hospital (the local government hospital), and each consist of ten villages – comprised the sampling frame. Owaga (Latitude: -0.153378, Longitude: 35.221355) and Tonde (Latitude: -0.130709, Longitude: 35.198380) are rural while Muhoroni East (Latitude: -0.145457, Longitude: 35.206700) includes a more urbanized town center.
Fig. 1.
Kisumu County, Kenya
The DEGREE protocol calls for an enrollment goal of 1,000 so that if the true prevalence of eGFR < 90 ml/min is 5%, the resulting 95% confidence interval would be 3.6%-6.4% [30]. Four villages were randomly selected from each of the sublocations, totaling twelve villages and 1,268 households. Recruitment and enrollment were at the household level with a household defined as a structure in which the family sleeps at night. All the households from each village were approached for recruitment of one individual per household. Male and non-pregnant female adults between 18 to 59 years who resided in one of the three sublocations for at least six months prior to enrollment were eligible to participate. To ensure that men and women were equally represented in the sample, the sex of the participant to be targeted for enrollment at each household was predetermined by randomization.
Participant recruitment
Between 27th July and 30th November in 2020, five pairs of research staff, each pair consisting of an interviewer and a licensed phlebotomist, went house to house in the daytime and early evenings to recruit participants Monday through Friday. Additionally, teams recruited participants on one weekend per sublocation. The questionnaire was administered by the interviewer in Dholuo or Kiswahili (based on the participant preference), and responses were entered directly into password-protected electronic tablet devices using SurveyCTO software. The questionnaire included the DEGREE core protocol questionnaire and the DEGREE optional renal protocol (e.g., previous disease diagnoses and medications). Questions regarding occupational history, health habits, and drinking water source originated from a community-based CKDu study [41].
Anthropometry and health metrics
Height and weight were measured in duplicate (and in triplicate if the difference between the first two measurements was more than 0.5 cm or 0.2 kg) using the Road Rod Portable Stadiometer (Hopkins Medical Products) and four calibrated Seca digital floor scales (Seca 813) and one calibrated Tanita digital floor scale (HD-314). Blood pressure was measured with a digital Upper Arm Blood Pressure Monitor (Omron model BP7350) taken three times, five minutes apart while the participant was seated.
During the household visit, a 5 mL venous blood sample was collected in a red top vacutainer (BD) and a 50 mL urine sample was collected. Samples were transported in coolers with ice packs to the Muhoroni County Hospital laboratory where urine samples were analyzed within 20 min of arrival using the Optical Clarity Urocheck 120 Urine Analyzer. Serum tubes were centrifuged for 10 min at 2400 revolutions per minute (RPM) and then aliquoted into 1.8 mL microcentrifuge tubes (Jiangsu Kangjie Medical Devices Co. Ltd) and frozen at -20 °C in a freezer with a backup generator. Frozen serum samples were transported on ice approximately once per week to the Kenya Medical Research Institute (KEMRI) HIV-R Laboratory in Kisumu City for analysis. There, creatinine and cystatin C were measured using the Roche Cobas Integra 400 plus. The instrument was calibrated for creatinine analysis using isotope dilution mass spectrometry reference standards.
Reduced eGFR and CKDu: definitions
The DEGREE definition of CKDu is an eGFR of < 60 mL/min/1.73m2 based on a single serum creatinine measurement in individuals without diabetes, hypertension, or heavy proteinuria [30, 31]. We followed the convention of prior studies that utilized the DEGREE protocol and also assessed eGFR < 90 as an endpoint because of low observed prevalence of eGFR < 60 [32, 42]. Table 1 defines the terms used for renal function categories. The DEGREE definition excludes those with diabetes, hypertension, heavy proteinuria, or underlying kidney disease. The DEGREE-Kenya definition also excludes those diagnosed with HIV. Diabetes was defined by a self-report of physician-diagnosed diabetes or taking medication for diabetes. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg and/or a self-report of taking medication for hypertension. Heavy proteinuria was defined as a urine dipstick of ≥ 2 + (approximately equivalent to albumin-to-creatinine ratio > 300 mg/g). HIV status was determined by asking whether the participant has ever been told they have one of six listed health conditions, one being HIV.
Table 1.
Definitions for kidney function categories with and without risk factors and heavy proteinuria
| eGFR categories (ml/min/1.73m2) | Participant subsets | ||
|---|---|---|---|
| All participants | Subset 1: no DM, HTN, or heavy proteinuria | Subset 2: no DM, HTN, heavy proteinuria, or HIV | |
| ≥ 90 (normal kidney function) | Normal eGFR | Normal eGFRDEGREE | Normal eGFRKenya |
| ≥ 60 to < 90 (mild kidney dysfunction) | Reduced eGFR | Reduced eGFRDEGREE | Reduced eGFRKenya |
| < 60 (moderate or established kidney dysfunction) | Proxy of CKDa | CKDuDEGREE | CKDuKenya |
aNo case would be considered CKD because serum creatinine was measured only on one occasion
Occupation and physical demands of work
Participants were asked about their current main occupation, and they described their job duties. Based on the participants’ responses, occupation was coded using the International Standard Classification of Occupations 2008 (ISCO-08) [39]. ISCO-08 coding includes the Major Group level (broadest, 1-digit code), Sub-Major Group (2-digit), Minor Group (3-digit), or Unit Group (most specific, 4-digit). Participants who were unemployed, a student, a homemaker, or unclassifiable – which are not ISCO-08 categories – were assigned codes accordingly. Classification was done independently by two coders; discrepancies in coding were adjudicated by consensus. ISCO-08 Major Groups were classified by occupational physical intensity into low-, moderate-, and high-intensity groups using a method validated through the use of energy expenditure and metabolic equivalents metrics [40]. In addition to using the ISCO-08, sugarcane cutting was examined separately for associations with low eGFR because of the association found with CKDu in earlier studies. A “current sugarcane cutter” was defined as someone who reported currently working as a manual sugarcane cutter for at least three consecutive months and a “former sugarcane cutter” was someone who reported having previously cut sugarcane for at least three consecutive months during the past ten years but not working as a cutter at the time of enrollment.
Statistical analysis
Normality, central tendency, and variability were summarized for continuous variables, and frequencies were summarized for categorical variables. eGFR was calculated using the Chronic Kidney Disease – Epidemiology Collaboration (CKD-EPI) equation based on serum creatinine and race was not used in the equation [43–45].
Logistic regression was used to identify potential associations between predictor variables and eGFR < 90 mL/min/1.73m2 (which combines the mild- and established kidney dysfunction categories). Predictors of eGFR < 90 mL/min/1.73m2 that were significant with a p-value of < 0.15 were added to multivariate logistic regression models (p-value < 0.05 with 95% confidence intervals). Based on a priori research questions about occupation, sugarcane work, and occupational physical exertion, these terms were forced into models, regardless of their statistical significance as predictors of eGFR < 90 and a backward selection process was used to arrive at a final multivariate model. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Human subjects research protections
The University of Illinois Chicago (IRB Protocol #: 2019–1236) and Maseno University Ethics Review Committee in Kisumu, Kenya (IRB Protocol #: MSU/DRPI/MUERC/00798/19) reviewed and approved study protocols. Participants provided written informed consent in their preferred language.
Results
Participant enrollment and demographic characteristics
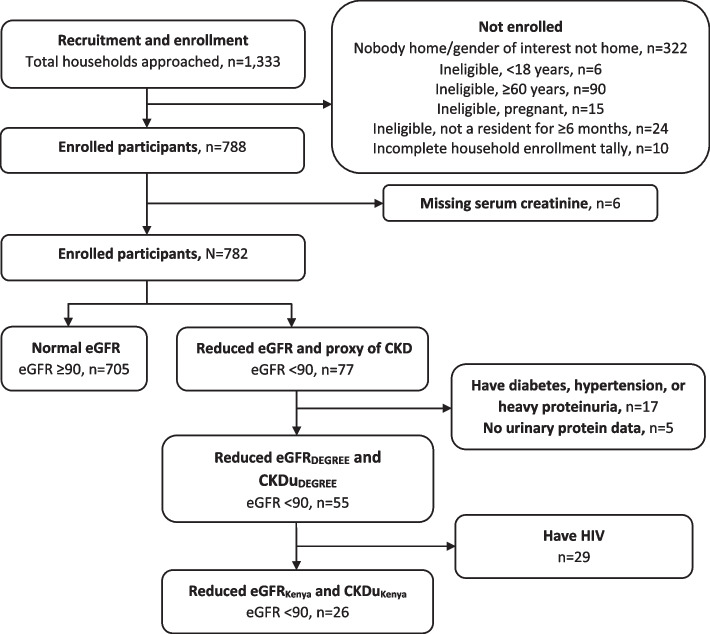
Participant enrollment is summarized in Fig. 2. The 322 “nobody home” households include those in which nobody was home and those in which nobody of the gender to be enrolled (determined in advance) was at home.
Fig. 2.
Participant enrollment and classification by eGFR categories
Table 2 summarizes the sociodemographics, health habits, and anthropometrics of study participants. Of the 782 individuals for whom serum creatinine data were available the median (5th, 95th percentile) age was 35.0 years (20.0, 56.0), the median BMI was 22.8 kg/m2 (18.3, 33.1), the median systolic blood pressure was 120.2 (101.7, 148.7) and the median diastolic blood pressure was 77.0 (63.3, 97.0). Twelve participants (1.5%) met the definition of a current sugarcane cutter for at least three consecutive months. Overall, 84 (10.7%) participants have ever cut sugarcane in their lifetime for income or have done a job related to sugarcane. One-hundred and twenty-five (16.0%) participants self-reported having HIV.
Table 2.
Sociodemographics and anthropometrics of study participants, n = 782
| Variable | n | % |
|---|---|---|
| Sex | ||
| Female | 392 | 50.1 |
| Male | 390 | 49.9 |
| Sublocation | ||
| Tonde | 233 | 29.8 |
| Muhoroni East | 260 | 33.2 |
| Owaga | 289 | 37.0 |
| Age | ||
| 18 – 29 years | 243 | 31.1 |
| 30 – 39 years | 252 | 32.2 |
| 40 – 49 years | 170 | 21.7 |
| 50 – 59 years | 117 | 15.0 |
| Level of education | ||
| Did not attend school | 10 | 1.3 |
| Some primary school | 175 | 22.4 |
| Completed primary school | 207 | 26.5 |
| Some secondary school | 117 | 15.0 |
| Completed secondary school | 158 | 20.2 |
| Some undergraduate school | 80 | 10.2 |
| Completed undergraduate school | 29 | 3.7 |
| Postgraduate education | 6 | 0.8 |
| Body Mass Index (BMI) (kg/m2) | ||
| Underweight (≤ 18.5) | 46 | 5.9 |
| Normal (> 18.5 to ≤ 25) | 475 | 60.7 |
| Overweight (> 25 to ≤ 30) | 178 | 22.8 |
| Obese (> 30) | 83 | 10.6 |
| Health habits | ||
| Smoker and/or alcohol drinker | 167 | 21.4 |
| Non-smoker and non-alcohol drinker | 615 | 78.6 |
| Self-reported HIV | ||
| Yes | 125 | 16.0 |
| No | 657 | 84.0 |
| Blood pressure and antihypertensive medications | ||
| ≥ 140 or ≥ 90 or on antihypertensive medications | 111 | 14.2 |
| < 140 and < 90 and not on antihypertensive medications | 671 | 85.8 |
| Physician diagnosed diabetes or on medication for diabetes | ||
| Yes | 8 | 1.0 |
| No | 774 | 99.0 |
| Proteinuria | ||
| Negative, Trace or 1 + | 717 | 91.7 |
| 2 + or 3 + | 5 | 0.6 |
| Missing | 60 | 7.7 |
eGFR and prevalence of eGFR < 90 with and without known causes of CKD
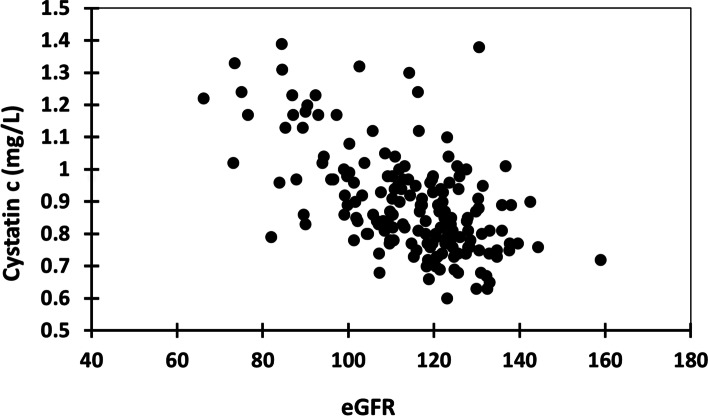
The median (5th, 95th percentile) serum creatinine was 0.71 (0.49, 1.02) mg/dL and the median eGFR was 115.3 (82.2, 137.6) mL/min/1.73m2. The prevalence of eGFR < 90 was 9.85%. Cystatin c data were available for 173 participants and completed on a random subset of samples throughout the data collection phase. A single cystatin c testing kit had 225 tests, including those used for controls. The median (5th, 95th percentile) of cystatin c was 0.86 (0.68, 1.23) mg/L. eGFR and cystatin c were moderately correlated (r = -0.52, Spearman p-value < 0.0001) (Fig. 3). Among Subset 1 participants (n = 612), the median (5th, 95th percentile) eGFR was 117.0 (83.7, 137.7) mL/min/1.73m2 and the prevalence of eGFR < 90 was 8.99% (95%CI 6.8%, 11.5%) (n = 55) with 0.33% (95%CI 0.04%, 1.2%) (n = 2) having an eGFR < 60. Among Subset 2 participants (n = 508), the observed prevalence of eGFR < 90 was 5.12% (95%CI 3.4%, 7.4%) (n = 26); none had an eGFR < 60.
Fig. 3.
Scatterplot of eGFR and Cystatin c correlation
Occupational and physical intensity classifications and prevalence of reduced eGFR
Table 3 displays the distribution of overall median eGFR (5th, 95th percentile) by ISCO-08 Major Groups 1–9 and other work status categories (e.g., homemaker). Skilled Agricultural, Forestry and Fishery Workers and Elementary Occupations, which includes agriculture and non-agriculture manual jobs, together accounted for 41.6% of participants. The prevalence of eGFR < 90 was about 12% across the major groups and other work status categories except for Major Group 1: Managers, who had a 33.3% prevalence. Among agricultural workers, which included almost 35% of all participants, the prevalence of eGFR < 90 was 12.5% (n = 34). Among those without diabetes, hypertension, and heavy proteinuria, the prevalence of reduced eGFRDEGREE was 12.9% (n = 27) and 8.6% (n = 14) for reduced eGFRKenya. Among participants with an ISCO-08 classification and eGFR (n = 604), there was no statistically significant association between the high-intensity group and an eGFR < 90 (OR: 1.29 [95%CI 0.75, 2.22], p = 0.36). Among Subset 1 participants there was a suggestion of an association between eGFR < 90 and the high-intensity group (OR: 1.73 [95%CI 0.87, 3.4], p = 0.12), however, it was not statistically significant. Lastly, an association between the high-intensity group and an eGFR < 90 was not present for reduced eGFRKenya (p = 0.48).
Table 3.
Distribution of eGFR by ISCO-08 category and occupational physical intensity group for all participants
| eGFR ( n = 782) | eGFR categories, n (%) | |||||
|---|---|---|---|---|---|---|
| n (%) | Median | 5th Pctl | 95th Pctl | < 90, n = 77 | ≥ 90, n = 705 | |
| ISCO-08 Major Groups 1–9 | ||||||
| Major Group 1: Managers | 6 (0.8) | 102.9 | 84.1 | 125.9 | 2 (33.3) | 4 (66.7) |
| Major Group 2: Professionals | 47 (6.0) | 111.8 | 87 | 135.7 | 3 (6.4) | 44 (93.6) |
| Major Group 3: Technicians and Associate Professionals | 18 (2.3) | 112.8 | 62.6 | 143.6 | 2 (11.1) | 16 (88.9) |
| Major Group 4: Clerical Support Workers | 2 (0.3) | 112.5 | 106.9 | 118 | 0 (0) | 2 (100) |
| Major Group 5: Services and Sales Workers | 120 (15.4) | 118.1 | 81.4 | 135.3 | 11 (9.2) | 109 (90.8) |
| Major Group 6: Skilled Agricultural, Forestry and Fishery Workers | 163 (20.8) | 109 | 79.6 | 132.3 | 20 (12.3) | 143 (87.7) |
| Major Group 7: Craft and Related Trades Workers | 64 (8.2) | 110.8 | 85.5 | 132.4 | 7 (10.9) | 57 (89.1) |
| Major Group 8: Plant and Machine Operators and Assemblers | 21 (2.7) | 110.3 | 80.5 | 130.4 | 3 (14.3) | 18 (85.7) |
| Major Group 9: Elementary Occupations | 163 (20.8) | 115.8 | 82.2 | 132.9 | 21 (12.9) | 142 (87.1) |
| Total ISCO-08 Major Groups | 604 (77.2) | 113.0 | 81.1 | 133.9 | 69 (11.4) | 535 (88.6) |
| Other work status categories | ||||||
| Student | 53 (6.8) | 130.4 | 105.8 | 158.8 | 0 (0) | 53 (100) |
| Homemaker | 40 (5.1) | 113 | 73.6 | 144.2 | 6 (15.0) | 34 (85.0) |
| Unemployed | 63 (8.1) | 123.4 | 94.8 | 138.6 | 1 (1.6) | 62 (98.4) |
| Not classifiable | 22 (2.8) | 119.9 | 93.1 | 136.7 | 1 (4.6) | 21 (95.4) |
| Occupational physical intensity group | ||||||
| Low-intensity occupational group | 55 (7.0) | 111.6 | 84.9 | 135.7 | 5 (9.1) | 50 (90.9) |
| Moderate-intensity occupational group | 159 (20.3) | 115.8 | 76.3 | 136.2 | 16 (10.1) | 143 (89.9) |
| High-intensity occupational group | 390 (49.9) | 112.1 | 81.1 | 132.8 | 48 (12.3) | 342 (87.7) |
| Not classifiable | 178 (22.8) | 124.7 | 90.3 | 143.8 | 8 (4.5) | 170 (95.5) |
Of the ten sugarcane occupation definitions evaluated, we found that only work in the sugarcane industry (but not work as a current or former sugarcane cutter) was, in unadjusted models associated with reduced eGFR (OR: 2.84 [95%CI 1.18, 6.82], p: 0.02), reduced eGFRDEGREE (OR: 2.99 [95%CI 1.07, 8.41], p = 0.04), and reduced eGFRKenya (OR: 5.59 [95%CI 1.46, 21.40], p = 0.01). However, none of these associations approached statistical significance in multivariate models.
Risk factors for reduced eGFR
Table 4 displays the bivariate predictors associated with low eGFR (p < 0.15 level of significance, which were then entered into multivariate regression models). Three categories of low eGFR were evaluated: reduced eGFR, reduced eGFRDEGREE (eGFR < 90 in the absence of diabetes, hypertension, or heavy proteinuria), and reduced eGFRKenya (which added to the DEGREE definition the exclusion of those with HIV). Covariates that, after model selection were found to be significant predictors (p < 0.05) in multivariate models, are shown in Table 5. The association that remained significant in models of reduced eGFR and reduced eGFRDEGREE, was sublocation. The odds of having eGFR < 90 was nearly three times greater among those living in Owaga or Muhoroni East sublocations relative to those living in Tonde after adjustment for covariates. The age distribution and prevalence of BMI, diabetes, hypertension, heavy proteinuria, and HIV were comparable in Tonde relative to the other two sublocations.
Table 4.
Bivariate logistic regression of characteristics associated with reduced eGFR, reduced eGFRDEGREE, and reduced eGFRKenya
| Variable | Reduced eGFR | Reduced eGFRDEGREE | Reduced eGFRKenya | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR (95%CI) | p -value | n | OR (95%CI) | p -value | n | OR (95%CI) | p -value | |
| Sex | |||||||||
| Female | 392 | 1.64 (1.01, 2.65) | 0.04 | 312 | 1.77 (1.00, 3.14) | 0.05 | 244 | 2.12 (0.93, 4.85) | 0.07 |
| Male | 390 | Ref | 300 | Ref | 264 | Ref | |||
| Sublocation | |||||||||
| Muhoroni East and Owaga | 549 | 2.23 (1.21, 4.14) | 0.01 | 414 | 2.02 (1.02, 4.01) | 0.04 | 341 | 2.12 (0.78, 5.74) | 0.13 |
| Tonde | 233 | Ref | 198 | Ref | 167 | Ref | |||
| Age | |||||||||
| Per 1-year increase | 782 | 1.11 (1.08, 1.14) | < .0001 | 612 | 1.12 (1.09, 1.16) | < .0001 | 508 | 1.14 (1.09, 1.19) | < .0001 |
| Body Mass Index (BMI) (kg/m2) | |||||||||
| Underweight (≤ 18.5), overweight (> 25 to ≤ 30), or obese (> 30) | 307 | 2.11 (1.31, 3.40) | 0.002 | 222 | 2.48 (1.41, 4.34) | 0.0015 | 187 | 5.07 (2.09, 12.31) | 0.0003 |
| Normal (> 18.5 to ≤ 25) | 475 | Ref | 390 | Ref | 321 | Ref | |||
| Blood pressure | |||||||||
| ≥ 140 or ≥ 90 or on hypertensive medication | 111 | 1.54 (0.84, 2.81) | 0.16 | - | - | - | - | - | - |
| < 140 and < 90 and not on hypertensive medication | 671 | Ref | - | Ref | - | - | Ref | - | |
| Previous diabetes diagnosis by a physician | |||||||||
| Yes | 8 | 5.68 (1.33, 24.23) | 0.01 | - | - | - | - | - | - |
| No | 774 | Ref | - | Ref | - | - | Ref | - | |
| Self-reported HIV | |||||||||
| Yes | 125 | 4.68 (2.83, 7.74) | < .0001 | 104 | 7.17 (4.00, 12.84) | < .0001 | - | - | - |
| No | 657 | Ref | 508 | Ref | - | Ref | - | ||
| Medication against HIV | |||||||||
| Yes | 122 | 4.25 (2.56, 7.06) | < .0001 | 102 | 6.18 (3.46, 11.07) | < .0001 | - | - | - |
| No | 659 | Ref | 509 | Ref | - | Ref | - | ||
| Missing | 1 | 1 | - | - | - | ||||
| Tuberculosis | |||||||||
| Yes | 30 | 1.89 (0.70, 5.09) | 0.20 | 25 | 2.69 (0.97, 7.46) | 0.05 | 11 | a | |
| No | 752 | Ref | 587 | Ref | 497 | Ref | |||
| Prescribed medications | |||||||||
| Yes | 248 | 3.48 (2.15, 5.63) | < .0001 | 189 | 3.86 (2.18, 6.82) | < .0001 | 89 | 0.84 (0.28, 2.52) | 0.76 |
| No | 534 | Ref | 423 | Ref | 419 | Ref | |||
| Antibiotic medication for infection | |||||||||
| Yes | 466 | 1.46 (0.89, 2.41) | 0.13 | 364 | 1.58 (0.87, 2.88) | 0.13 | 287 | 1.78 (0.76, 4.17) | 0.18 |
| No | 316 | Ref | 248 | Ref | 221 | Ref | |||
| Painkillers most days | |||||||||
| Yes | 347 | 1.24 (0.78, 2.00) | 0.35 | 271 | 1.45 (0.83, 2.53) | 0.18 | 221 | 2.16 (0.96, 4.86) | 0.06 |
| No | 435 | Ref | 341 | Ref | 287 | Ref | |||
| Herbal or traditional remedies | |||||||||
| Yes | 269 | 0.85 (0.51, 1.41) | 0.53 | 212 | 0.62 (0.33, 1.16) | 0.13 | 191 | 0.72 (0.31, 1.70) | 0.46 |
| No | 513 | Ref | 400 | Ref | 317 | Ref | |||
| Consumption of sugary drinks in past 7 days | |||||||||
| 5 or more drinks | 57 | 2.09 (1.01, 4.33) | 0.04 | 44 | 2.05 (0.87, 4.85) | 0.10 | 32 | 1.25 (0.28, 5.56) | 0.76 |
| None to 4 drinks | 725 | Ref | 568 | Ref | 476 | Ref | |||
| Currently smoke tobacco | |||||||||
| Yes | 39 | 0.23 (0.03, 1.70) | 0.15 | 33 | 0.30 (0.04, 2.26) | 0.24 | 28 | a | |
| No | 743 | Ref | 579 | Ref | 480 | Ref | |||
| Occupational physical intensity group | |||||||||
| High-intensity | 390 | 1.29 (0.75, 2.22) | 0.36 | 298 | 1.73 (0.87, 3.41) | 0.12 | 231 | 1.39 (0.55, 3.47) | 0.48 |
| Low- and moderate-intensity | 214 | Ref | 158 | Ref | 138 | Ref | |||
| Missing/Unclassifiable | 178 | 156 | 139 | ||||||
aQuasi-complete separation of data points detected. The odds ratio (OR) could not be calculated
Reduced kidney function was defined as eGFR < 90 mL/min/1.73m2
Ref reference category for odds ratio (OR) calculation
Table 5.
Multivariate logistic regression (adjusted full model) of characteristics associated with reduced eGFR, reduced eGFRDEGREE, and reduced eGFRKenya
| Variable | Reduced eGFR | Reduced eGFRDEGREE | Reduced eGFRKenya | ||||||
|---|---|---|---|---|---|---|---|---|---|
| na | OR (95%CI) | p -value | na | OR (95%CI) | p -value | n | OR (95%CI) | p -value | |
| Sublocation | |||||||||
| Muhoroni East and Owaga | 548 | 2.97 (1.52, 5.83) | 0.002 | 413 | 2.95 (1.34, 6.50) | 0.007 | - | - | - |
| Tonde | 233 | Ref | 198 | Ref | - | Ref | - | ||
| Age | |||||||||
| Per 1-year increase | 781 | 1.10 (1.07, 1.14) | < .0001 | 611 | 1.12 (1.08, 1.16) | < .0001 | 508 | 1.14 (1.09, 1.19) | < .0001 |
| Body Mass Index (BMI) (kg/m2) | |||||||||
| Underweight (≤ 18.5), overweight (> 25 to ≤ 30), or obese (> 30) | 307 | 1.84 (1.08, 3.13) | 0.024 | 222 | 2.44 (1.26, 4.72) | 0.008 | 187 | 4.26 (1.65, 10.97) | 0.003 |
| Normal BMI (> 18.5 to ≤ 25) | 474 | Ref | 389 | Ref | 321 | Ref | |||
| Self-reported HIV | |||||||||
| Yes | 125 | 4.11 (2.36, 7.15) | < .0001 | 104 | 6.05 (3.12, 11.73) | < .0001 | - | - | - |
| No | 656 | Ref | 507 | Ref | - | Ref | - | ||
aThe total n for reduced eGFR and reduced eGFRDEGREE is missing 1 observation because of a missing response to taking HIV medication
Ref reference category for odds ratio (OR) calculation
Discussion
This population-based study in an agricultural region of Kenya found that chronic kidney disease of unknown origin was uncommon. Among the 782 participants with eGFR data, 9.85% had eGFR < 90, and of that, 0.51% had an eGFR < 60. However, among the 508 participants without DM, HTN, heavy proteinuria, or HIV, the observed prevalence of eGFR < 90 was 5.12% (95%CI 3.4%, 7.4%); none had an eGFR < 60. Self-reported HIV was common (16.0% of participants, which is similar to the reported 17.5% prevalence for Kisumu County) [37] and after adjusting for covariates, the odds (95%CI) of having eGFR < 90 among those with HIV was 4.11 (2.36, 7.15) times greater than among those without HIV. Prior studies have reported a high prevalence of CKDu among workers who engage in physically demanding work in hot climates, including sugarcane workers [2, 5, 17, 27, 46]. We found no association between physically demanding work and reduced eGFR.
The significant risk factors identified in the multivariate model associated with reduced eGFR and reduced eGFRDEGREE were sublocation, increasing age, having a non-normal BMI, and HIV positivity. Increasing age and a non-normal BMI were significant predictors of reduced eGFRKenya. Table 6 summarizes other study findings that utilized DEGREE methods [32, 42, 47, 48]. The estimated prevalence of reduced eGFRDEGREE appears to be greater in Sri Lanka and the Southern region of India compared to Peru, Malawi, and the present study [32, 42, 47, 48]. This suggests that local factors not present in Kenya, Malawi, or Peru may be present in locations with epidemics of CKDu.
Table 6.
Comparison of reduced eGFR, reduced eGFRDEGREE, and CKDuDEGREE prevalence and risk factors across DEGREE protocol studies
| First author and country/region | Total sample size | Reduced eGFR < 90 | Proxy for CKD eGFR < 60 | Reduced eGFRDEGREE < 90 | CKDuDEGREE eGFR < 60 | Associated risk factors with eGFR < 90 and < 60 |
|---|---|---|---|---|---|---|
| Hamilton, et al. (2020). Malawi, southeast Africa | 821 | Did not report | Did not report | 4.6% (95%CI 3.2, 6.3); n = 38 | 0.2% (95%CI 0.1, 0.9); n = 2 | Increasing age, BMI |
| Ruiz-Alejos, et al. (2021). Northern Peru, Tumbes region | 1,514 total sample; 1,272 after excluding those with DM, HTN, and HP | eGFR ≥ 60 to < 90: 16.6% (95%CI 14.8, 18.6) | 1.7% (95%CI 1.1, 2.5); n = 26 | eGFR ≥ 60 to < 90: 13%; n = 165 | 0.9% (95%CI 0.4, 1.5); n = 11 | Low physical activity levels, kidney stones; sugarcane work was protective |
| Ruwanpathirana, et al. (2019). Anuradhapura, Sri Lanka | 4,803 total sample; 3,351 after excluding those with DM, HTN, and HP | Did not report | 12%; n = 576 | Did not report | 6.0% (95%CI 5.2, 6.8); n = 202 | Advanced age, history of CKD among parents or siblings, living in areas classified as moderate and high CKDu-endemicity; agricultural work was not significant |
| O’Callaghan-Gordo, et al. (2019). Northern and Southern Indiaa | 12,500 | Did not report | Did not report | eGFR ≥ 60 to < 90: 17.0% (95%CI 16.0, 17.0); n = 2,125 | Overall: 1.6% (95%CI 1.4, 1.9). Varied from 1.4% in northern urban areas to 4.8% southern rural | Older age, residence in a rural area, being male, and less formal education for each 5 years of school |
| Western Kenya, Muhoroni Sub-County | 782 | 9.85% (95%CI 7.9, 12.2); n = 77 | 0.51% (95%CI 0.14, 1.3); n = 4 | 8.99% (95%CI 6.8, 11.5); n = 55 | 0.33% (95%CI 0.04, 1.2); n = 2 | Sublocation, increasing age, non-normal BMI, HIV positivity |
DM diabetes mellitus, HTN hypertension, HP heavy proteinuria, BMI Body Mass Index, HIV Human Immunodeficiency Virus
aData collected prospectively for other purposes but analyzed per DEGREE protocol
We observed that among participants residing in Owaga or Muhoroni East, after adjustment for confounders, the odds of having eGFR < 90 was three times higher than among those living in Tonde. The two sublocations at elevated risk differ from one another in terms of urbanization. Owaga is rural, like the low-risk sublocation of Tonde, while Muhoroni East is more urban. An explanation for this association is not obvious, as the age distribution and prevalence of BMI, diabetes, hypertension, and HIV were similar across the sublocations. Possible explanations might include contaminants in local drinking water sources and the use of fertilizers and pesticides on crops, though data needed to investigate those possibilities are not available. Focused investigations into the apparent sublocation clustering of cases are warranted to further evaluate environmental risk factors.
It is well-established that HIV is associated with impaired renal function [34–36, 49, 50]. We found that positive HIV status was strongly associated with reduced renal function. In single-predictor models, reported use of HIV medication was predictive of reduced eGFR and reduced eGFRDEGREE categories, however, this association did not remain significant in the final multivariate model whereas HIV status did. Prior studies of CKDu have not addressed HIV or antiretroviral therapy (ART) medications as known causes of kidney dysfunction. Future studies of CKDu should evaluate HIV and ART medication history among participants and consider low eGFR among those with HIV to be of known cause. Consideration should be given to the standardization of asking sensitive questions like HIV status and collecting ART medication history to determine nephrotoxicity when in regions with high rates of HIV, and/or by including serologic testing for HIV in the study protocol. It is also possible that HIV accounted for some of the cases of CKDu in prior studies that did not address this known cause of impaired renal function.
The observed lack of association between occupational factors and reduced eGFR and CKDu is somewhat surprising. Studies conducted in Central America have found that workers who engage in physically demanding work in high temperatures, such as, sugarcane cutting, corn production, mining, and brickmaking, had a CKDu prevalence as high as 14.0% in Nicaragua and almost 19.0% in El Salvador [2, 5]. In our study participants who did sugarcane-related work but not specifically cane cutting, were more likely to have eGFR < 90 in unadjusted models. This association disappeared when evaluated in multivariate models. When we estimated the significance between eGFR < 90 and sugarcane cutting as an occupation (including both current and former cane cutters), we observed no association. This could mean that methods for cane cutting, approaches to paying workers (such as per weight of cane cut vs. per hour), and pace of sugarcane work differ in Kenya and Central America. Opportunities for water, rest, and shade (WRS) may differ as well, and WRS intervention studies in Central America appear to limit the impact of work on biomarkers of kidney function [25, 51].
Physically demanding workload was not found to be significantly associated with eGFR < 90 in multivariate models. Taken together, we found that neither work in the sugarcane industry, as a cane cutter, or in physically demanding occupations to be independent predictors of reduced eGFR in this population. These findings are in contrast to what Schlader et al. [28] reviewed, describing the potential mechanisms supporting how physical workload in the heat may lead to heat strain and dehydration, which then may increase one’s risk for acute kidney injury (AKI). Hansson et al. [26] built on this concept, hypothesizing that sugarcane cutters may have repeated exposures to hypoxia, fructose, uric acid, and the release of pro-inflammatory cytokines in tubuli due to physically demanding work in heat and rehydrating with sugary drinks. The results in the present study are consistent with arguments made by others that strenuous exertion in heat does not clearly lead to CKDu after recurrences of AKI [52]. Interestingly, in an occupational-based cohort study of sugarcane workers in Nicaragua, those who had the highest workload (sugarcane cutters) also had the greatest eGFR increase across the harvest season compared to those with lesser workloads [27].
Distinct from the issue of what sugarcane cutters do, the classification of job titles in population-based studies has not been standardized across CKDu studies. As a result, it is difficult to know what job duties workers have, how physically strenuous the work is, under what conditions, and for what duration. This limits our ability to interpret the observed prevalence of CKDu across studies and even within studies, across occupational groups. One study in India classified six categories of occupations, including homemaker, student, and unemployed; however, it was not clear the methods used to classify [53]. The current study sought to use a standardized set of occupational categories defined in ISCO-08 [39]. Nevertheless, this approach has its limitations. We asked participants about their “current” or “main” occupation, yet individuals may have multiple jobs at the same time or at different times of the year. The ISCO-08 was also not designed to ask follow-up questions about the working conditions, such as duration engaged in physically intense work. Improving CKDu surveillance should include improved methods to describe one’s occupation, including those who are not part of the formal workforce (e.g., homemakers).
The findings of this study are subject to several limitations. First, because the study design is cross-sectional reported exposures (e.g., sublocation, occupation, BMI, diet) and disease outcome (e.g., low eGFR) are measured at the same time for each participant, making it challenging to infer causality in the observed associations. Second, the generalizability of the study findings to other populations in East Africa and beyond is limited as only one sub-county of one county was studied. Third, the study enrolled only 12 participants who, at the time of the study, worked for the past three months cutting cane, limiting the statistical power of analyses of occupation and eGFR < 90. Additionally, the CKD-EPI equation has not been validated in African populations, which may lead to an underestimation of CKDu prevalence [44, 54–56]. However, this would not impact our assessment of associations between reduced eGFR and other factors, as the same equation was used for all participants. The study also had numerous strengths such as utilizing the standardized DEGREE protocol to estimate the distribution of eGFR in a population, a high enrollment rate (90.0%), and a representative population sample by using random selection of villages that included participants from both urban and rural settings.
Conclusion
We found that in one sugarcane producing region of Western Kenya, CKDu prevalence was low, and that HIV was common among those with reduced eGFR. Thus, future studies of CKDu should account for HIV as a known cause of reduced renal function. If physically demanding work in a hot climate were the sole cause of CKDu, one might expect a high CKDu prevalence in the study area, given the equatorial setting in which sugarcane is grown and cut; however, this was not observed. The current findings suggest that in CKDu-endemic regions, heat stress and physically demanding work may interact with other exposures to put populations at risk for CKDu.
Supplementary Information
Acknowledgements
Many thanks to Drs. Ineke Wesseling, Linda Forst, and Lee Friedman for their guidance and support on my dissertation research; the staff at Safe Water and AIDS Project; the interviewers and phlebotomists; Julius Okuku and staff at the Muhoroni County Hospital Laboratory; and Boaz Oyaro, Valarie Opollo, and Janet Adhiambo at the KEMRI HIV-R Laboratory.
Abbreviations
- CKD
Chronic kidney disease
- CKDu
Chronic kidney disease of undetermined causes
- eGFR
Estimated glomerular filtration rate
- DEGREE
The Disadvantaged Populations eGFR Epidemiology Study
- DM
Diabetes mellitus
- HTN
Hypertension
- HP
Heavy proteinuria
- HIV
Human immunodeficiency virus
- BMI
Body Mass Index
- EPI
Epidemiology
- ISCO-08
International Standard Classification of Occupations 2008
- AKI
Acute kidney injury
- WRS
Water, rest, shade
- ART
Antiretroviral therapy
- OR
Odds ratio
- Pctl
Percentile
- KEMRI
Kenya Medical Research Institute
- IRB
Institutional Review Board
- SAS
Statistical Analysis Software
- CINAC
Chronic interstitial nephritis in agricultural communities
- RPM
Revolutions per minute
Authors’ contributions
M.H. was the principal investigator and led and co-led all aspects of the study, including the design of the study and overseeing data collection, conducting analysis and interpretation of data, and writing the first draft of the manuscript. S.D. co-led all aspects of the study, including the conceptualization and design of the study, development of the data collection protocols, supporting data analysis and interpretation of data, and co-writing, editing, and reviewing the manuscript. C.P. contributed to study design and planning the implementation of the study. A.O. assisted with study protocol submission to the Kenyan ethics review committee and secured permits to conduct research in Kenya, assisted with field training, oversaw all management of electronic survey data, including adherence to privacy and confidentiality requirements, and tracked and managed participants’ informed consents. D.O. contributed to the development of the study and provided input from the Kisumu County Department of Health. All authors read and approved the final manuscript.
Funding
National Institute for Occupational Safety and Health (NIOSH), Illinois Education and Research Center (ERC) Pilot Project Research Training Grant #: T42/OH008672; Environmental and Occupational Health unrestricted global health research funds; Michael Bruton Workplace Safety Foundation Scholarship (2019); UIC Graduate College Award for Graduate Research (2019); Paul Brandt-Rauf Scholarship in Global Health (2018–19); and Donna Farley Global Health Scholarship (2020–21).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The University of Illinois Chicago (UIC Institutional Review Board Protocol #: 2019–1236) and Maseno University Ethics Review Committee in Kisumu, Kenya (Maseno University Institutional Review Board Protocol #: MSU/DRPI/MUERC/00798/19) reviewed and approved study protocols. Participants provided written informed consent in their preferred language.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garcia Trabanino R, Aguilar R, Reyes Silva C, Ortiz Mercado M, Leiva Merino R. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador [End-stage renal disease among patients in a referral hospital in El Salvador] Rev Panam Salud Publica. 2002;12:202–206. doi: 10.1590/S1020-49892002000900009. [DOI] [PubMed] [Google Scholar]
- 2.Peraza S, Wesseling C, Aragon A, Leiva R, García-Trabanino RA, Torres C, et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Wesseling C, Van Wendel De Joode B, Crowe J, Rittner R, Sanati NA, Hogstedt C, et al. Mesoamerican nephropathy: Geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Environ Med. 2015;72:714–21. doi: 10.1136/oemed-2014-102799. [DOI] [PubMed] [Google Scholar]
- 4.Wesseling C, Crowe J, Hogstedt C, Jakobsson K, Lucas R, Wegman DH. The epidemic of Chronic Kidney Disease of unknown etiology in mesoamerica: a call for interdisciplinary research and action. Am J Public Health. 2013;103:1927–1930. doi: 10.2105/AJPH.2013.301594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres C, Aragón A, González M, López I, Jakobsson K, Elinder CG, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: the case for a mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayasumana C, Orantes C, Herrera R, Almaguer M, Lopez L, Silva LC, et al. Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational and environmental determinants. Nephrol Dial Transplant. 2017;32:234–241. doi: 10.1093/ndt/gfw346. [DOI] [PubMed] [Google Scholar]
- 8.Vervaet BA, Nast CC, Jayasumana C, Schreurs G, Roels F, Herath C, et al. Chronic interstitial nephritis in agricultural communities is a toxin-induced proximal tubular nephropathy. Kidney Int. 2020;97:350–69. doi: 10.1016/j.kint.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Jayatilake N, Mendis S, Maheepala P, Mehta FR. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayasekara JM, Dissanayake DM, Adhikari SB, Bandara P. Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. Ceylon Med J. 2013;58:6–10. doi: 10.4038/cmj.v58i1.5356. [DOI] [PubMed] [Google Scholar]
- 11.Agampodi SB, Amarasinghe GS, Naotunna PGCR, Jayasumana CS, Siribaddana SH. Early renal damage among children living in the region of highest burden of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. BMC Nephrol BMC Nephrology. 2018;19:1–7. doi: 10.1186/s12882-018-0911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wijkström J, Jayasumana C, Dassanayake R, Priyawardane N, Godakanda N, Siribaddana S, et al. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): Similarities and differences with Mesoamerican Nephropathy. PLoS ONE. 2018;13:1–19. doi: 10.1371/journal.pone.0193056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanayakkara S, Senevirathna STMLD, Harada KH, Chandrajith R, Nanayakkara N, Koizumi A. The Influence of fluoride on chronic kidney disease of uncertain aetiology (CKDu) in Sri Lanka. Chemosphere. Elsevier Ltd; 2020;257:127186. Available from: 10.1016/j.chemosphere.2020.127186. [DOI] [PubMed]
- 14.Reddy DV, Gunasekar A. Chronic kidney disease in two coastal districts of Andhra Pradesh, India: Role of drinking water. Environ Geochem Health. 2013;35:439–454. doi: 10.1007/s10653-012-9506-7. [DOI] [PubMed] [Google Scholar]
- 15.Abraham G, Agarwal SK, Gowrishankar S, Vijayan M. Chronic Kidney Disease of Unknown Etiology: Hotspots in India and Other Asian Countries. Semin Nephrol. 2019;39:272–7. doi: 10.1016/j.semnephrol.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Gummidi B, John O, Ghosh A, Modi GK, Sehgal M, Kalra OP, et al. A Systematic Study of the Prevalence and Risk Factors of CKD in Uddanam. India. Kidney Int Reports. 2020;5:2246–55. doi: 10.1016/j.ekir.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RJ, Wesseling C, Newman LS. Chronic Kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380:1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 18.Glaser J, Lemery J, Rajagopalan B, Diaz HF, García-Trabanino R, Taduri G, et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol. 2016;11:1472–1483. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valcke M, Levasseur ME, Soares Da Silva A, Wesseling C. Pesticide exposures and chronic kidney disease of unknown etiology: an epidemiologic review. Environ Heal A Glob Access Sci Source. Environmental Health; 2017;16:1–20. [DOI] [PMC free article] [PubMed]
- 20.Wesseling C, Glaser J, Rodríguez-Guzmán J, Weiss I, Lucas R, Peraza S, et al. Chronic kidney disease of non-traditional origin in Mesoamerica: A disease primarily driven by occupational heat stress. Rev Panam Salud Publica/Pan Am J Public Heal. Pan American Health Organization; 2020;44:1–12. [DOI] [PMC free article] [PubMed]
- 21.Schaeffer JW, Adgate JL, Reynolds SJ, Butler-Dawson J, Krisher L, Dally M, et al. A pilot study to assess inhalation exposures among sugarcane workers in guatemala: Implications for chronic kidney disease of unknown origin. Int J Environ Res Public Health. 2020;17:1–15. doi: 10.3390/ijerph17165708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodin T, García-Trabanino R, Weiss I, Jarquín E, Glaser J, Jakobsson K, et al. Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: Phase 1. Occup Environ Med. 2016;73:409–416. doi: 10.1136/oemed-2016-103555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nerbass FB, Pecoits-Filho R, Clark WF, Sontrop JM, McIntyre CW, Moist L. Occupational heat stress and kidney health: from farms to factories. Kidney Int Reports. 2017;2:998–1008. doi: 10.1016/j.ekir.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dally M, Butler-Dawson J, Krisher L, Monaghan A, Weitzenkamp D, Sorensen C, et al. The impact of heat and impaired kidney function on productivity of Guatemalan sugarcane workers. PLoS ONE. 2018;13:1–15. doi: 10.1371/journal.pone.0205181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser J, Hansson E, Weiss I, Wesseling C, Jakobsson K, Ekström U, et al. Preventing kidney injury among sugarcane workers: promising evidence from enhanced workplace interventions. Occup Environ Med BMJ Publishing Group. 2020;77:527–534. doi: 10.1136/oemed-2020-106406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansson E, Glaser J, Jakobsson K, Weiss I, Wesseling C, Lucas RAI, et al. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients. 2020;12:1–22. doi: 10.3390/nu12061639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansson E, Glaser J, Weiss I, Ekström U, Apelqvist J, Hogstedt C, et al. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup Environ Med. 2019;76:818–826. doi: 10.1136/oemed-2019-105986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlader ZJ, Hostler D, Parker MD, Pryor RR, Lohr JW, Johnson BD, et al. The potential for renal injury elicited by physical work in the heat. Nutrients. 2019;11:2087–3012. doi: 10.3390/nu11092087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekiti ME, Zambo JB, Assah FK, Agbor VN, Kekay K, Ashuntantang G. Chronic kidney disease in sugarcane workers in Cameroon: a cross-sectional study. BMC Nephrol BioMed Central Ltd. 2018;19:1–8. doi: 10.1186/s12882-017-0798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caplin B, Jakobsson K, Glaser J, Nitsch D, Jha V, Singh A, et al. International Collaboration for the Epidemiology of eGFR in Low and Middle Income Populations - Rationale and core protocol for the Disadvantaged Populations eGFR Epidemiology Study (DEGREE) BMC Nephrol. 2017;18:1–8. doi: 10.1186/s12882-016-0417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caplin B, Yang CW, Anand S, Levin A, Madero M, Saran R, et al. The international society of nephrology’s international consortium of collaborators on chronic kidney disease of unknown etiology: report of the working group on approaches to population-level detection strategies and recommendations for a minimum dataset. Kidney Int. 2019;95:4–10. doi: 10.1016/j.kint.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton SA, Nakanga WP, Prynn JE, Crampin AC, Fecht D, Vineis P, et al. Prevalence and risk factors for chronic kidney disease of unknown cause in Malawi: a cross-sectional analysis in a rural and urban population. BMC Nephrol BMC Nephrology. 2020;21:1–12. doi: 10.1186/s12882-020-02034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swanepoel CR, Atta MG, D’Agati VD, Estrella MM, Fogo AB, Naicker S, et al. Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2018;93:545–559. doi: 10.1016/j.kint.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naicker S, Rahmanian S, Kopp JB. HIV and chronic kidney disease. Clin Nephrol. 2015;83:S32–S38. doi: 10.5414/CNP83S032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabian J, Naicker S. HIV and kidney disease in sub-Saharan Africa. Nat Rev Nephrol Nature Publishing Group. 2009;5:591–598. doi: 10.1038/nrneph.2009.141. [DOI] [PubMed] [Google Scholar]
- 36.Wools-Kaloustian K, Gupta SK, Muloma E, Owino-Ong’or W, Sidle J, Aubrey RW, et al. Renal disease in an antiretroviral-naïve HIV-infected outpatient population in Western Kenya. Nephrol Dial Transplant. 2007;22:2208–2212. doi: 10.1093/ndt/gfm223. [DOI] [PubMed] [Google Scholar]
- 37.National AIDS and STI Control Programme. National AIDS and STI Control Programme (NASCOP), Preliminary KENPHIA 2018 Report. Nairobi; 2020. Available from: http://nascop.or.ke/.
- 38.Navarro CMO, López MA, Galbán PA, Amaya MD, Hernández S, Valdés RH, et al. The chronic kidney disease epidemic in El Salvador: a cross-sectional study. MEDICC Rev. 2019;21:29–37. doi: 10.37757/MR2019.V21.N2-3.7. [DOI] [PubMed] [Google Scholar]
- 39.International Labour Office. International Standard Classification of Occupations: ISCO-08. Geneva; 2012. Available from: http://www.ilo.org/public/english/bureau/stat/isco/index.htm.
- 40.Brighenti-Zogg S, Mundwiler J, Schüpbach U, Dieterle T, Wolfer DP, Leuppi JD, et al. Physical workload and work capacity across occupational groups. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0154073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González-Quiroz M, Camacho A, Faber D, Aragón A, Wesseling C, Glaser J, et al. Rationale, description and baseline findings of a community-based prospective cohort study of kidney function amongst the young rural population of Northwest Nicaragua. BMC Nephrol BioMed Central Ltd. 2017;18:1–8. doi: 10.1186/s12882-016-0422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Alejos A, Caplin B, Miranda JJ, Pearce N, Bernabé-Ortiz A. CKD and CKDu in northern Peru: a cross-sectional analysis under the DEGREE protocol. BMC Nephrol BMC Nephrology. 2021;22:1–12. doi: 10.1186/s12882-021-02239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 44.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–288.e1. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen CJ, Butler-Dawson J, Dally M, Krisher L, Griffin BR, Johnson RJ, et al. Risk factors and mechanisms underlying cross-shift decline in kidney function in Guatemalan sugarcane workers. J Occup Environ Med. 2019;61:239–250. doi: 10.1097/JOM.0000000000001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruwanpathirana T, Senanayake S, Gunawardana N, Munasinghe A, Ginige S, Gamage D, et al. Prevalence and risk factors for impaired kidney function in the district of Anuradhapura, Sri Lanka: a cross-sectional population-representative survey in those at risk of chronic kidney disease of unknown aetiology. BMC Public Health BMC Public Health. 2019;19:1–11. doi: 10.1186/s12889-019-7117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Callaghan-Gordo C, Shivashankar R, Anand S, Ghosh S, Glaser J, Gupta R, et al. Prevalence of and risk factors for chronic kidney disease of unknown aetiology in India: Secondary data analysis of three population-based cross-sectional studies. BMJ Open. 2019;9:1–12. doi: 10.1136/bmjopen-2018-023353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halle MP, Essomba N, Djantio H, Tsele G, Fouda H, Luma NH, et al. Clinical characteristics and outcome of HIV infected patients with chronic kidney disease in Sub Saharan Africa: An example from Cameroon. BMC Nephrol BMC Nephrology. 2019;20:1–11. doi: 10.1186/s12882-019-1446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekrikpo UE, Kengne AP, Bello AK, Effa EE, Noubiap JJ, Salako BL, et al. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS ONE. 2018;13:1–24. doi: 10.1371/journal.pone.0195443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wegman DH, Apelqvist J, Bottai M, Ekström U, García-Trabanino R, Glaser J, et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Heal. 2018;44:16–24. doi: 10.5271/sjweh.3659. [DOI] [PubMed] [Google Scholar]
- 52.Herath C, Jayasumana C, De Silva PMCS, De Silva PHC, Siribaddana S, De Broe ME. Kidney Diseases in Agricultural Communities: a case against heat-stress nephropathy. Kidney Int. Reports. 2018. p. 271–80. [DOI] [PMC free article] [PubMed]
- 53.Parameswaran S, Rinu PK, Kar SS, Harichandrakumar KT, James TD, Priyamvada PSP, et al. A Newly Recognized Endemic Region of CKD of Undetermined Etiology (CKDu) in South India—“Tondaimandalam Nephropathy.” Kidney Int Reports. Elsevier Inc; 2020;5:2066–73. Available from: 10.1016/j.ekir.2020.08.032. [DOI] [PMC free article] [PubMed]
- 54.Wyatt CM, Schwartz GJ, Owino Ong’or W, Abuya J, Abraham AG, Mboku C, et al. Estimating Kidney Function in HIV-Infected Adults in Kenya: Comparison to a Direct Measure of Glomerular Filtration Rate by Iohexol Clearance. PLoS One. 2013;8(8):e69601. doi: 10.1371/journal.pone.0069601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yilma D, Abdissa A, Kæstel P, Tesfaye M, Olsen MF, Girma T, et al. Serum creatinine and estimated glomerular filtration rates in HIV positive and negative adults in Ethiopia. PLoS ONE. 2019;14:1–13. doi: 10.1371/journal.pone.0211630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–62. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.