Abstract
Background
Advanced chronic liver disease is characterised by a long compensated phase followed by a rapidly progressive 'decompensated' phase, which is marked by the development of complications of portal hypertension and liver dysfunction. Advanced chronic liver disease is considered responsible for more than one million deaths annually worldwide. No treatment is available to specifically target fibrosis and cirrhosis; liver transplantation remains the only curative option. Researchers are investigating strategies to restore liver functionality to avoid or slow progression towards end‐stage liver disease. Cytokine mobilisation of stem cells from the bone marrow to the liver could improve liver function. Granulocyte colony‐stimulating factor (G‐CSF) is a 175‐amino‐acid protein currently available for mobilisation of haematopoietic stem cells from the bone marrow. Multiple courses of G‐CSF, with or without stem or progenitor cell or growth factors (erythropoietin or growth hormone) infusion, might be associated with accelerated hepatic regeneration, improved liver function, and survival.
Objectives
To evaluate the benefits and harms of G‐CSF with or without stem or progenitor cell or growth factors (erythropoietin or growth hormone) infusion, compared with no intervention or placebo in people with compensated or decompensated advanced chronic liver disease.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, three other databases, and two trial registers (October 2022) together with reference‐checking and web‐searching to identify additional studies. We applied no restrictions on language and document type.
Selection criteria
We only included randomised clinical trials comparing G‐CSF, independent of the schedule of administration, as a single treatment or combined with stem or progenitor cell infusion, or with other medical co‐interventions, with no intervention or placebo, in adults with chronic compensated or decompensated advanced chronic liver disease or acute‐on‐chronic liver failure. We included trials irrespective of publication type, publication status, outcomes reported, or language.
Data collection and analysis
We followed standard Cochrane procedures. All‐cause mortality, serious adverse events, and health‐related quality of life were our primary outcomes, and liver disease‐related morbidity, non‐serious adverse events, and no improvement of liver function scores were our secondary outcomes. We undertook meta‐analyses, based on intention‐to‐treat, and presented results using risk ratios (RR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes, with 95% confidence intervals (CI) and I2 statistic values as a marker of heterogeneity. We assessed all outcomes at maximum follow‐up. We determined the certainty of evidence using GRADE, evaluated the risk of small‐study effects in regression analyses, and conducted subgroup and sensitivity analyses.
Main results
We included 20 trials (1419 participants; sample size ranged from 28 to 259), which lasted between 11 and 57 months. Nineteen trials included only participants with decompensated cirrhosis; in one trial, 30% had compensated cirrhosis. The included trials were conducted in Asia (15), Europe (four), and the USA (one). Not all trials provided data for our outcomes. All trials reported data allowing intention‐to‐treat analyses. The experimental intervention consisted of G‐CSF alone or G‐CSF plus any of the following: growth hormone, erythropoietin, N‐acetyl cysteine, infusion of CD133‐positive haemopoietic stem cells, or infusion of autologous bone marrow mononuclear cells. The control group consisted of no intervention in 15 trials and placebo (normal saline) in five trials. Standard medical therapy (antivirals, alcohol abstinence, nutrition, diuretics, β‐blockers, selective intestinal decontamination, pentoxifylline, prednisolone, and other supportive measures depending on the clinical status and requirement) was administered equally to the trial groups.
Very low‐certainty evidence suggested a decrease in mortality with G‐CSF, administered alone or in combination with any of the above, versus placebo (RR 0.53, 95% CI 0.38 to 0.72; I2 = 75%; 1419 participants; 20 trials). Very low‐certainty evidence suggested no difference in serious adverse events (G‐CSF alone or in combination versus placebo: RR 1.03, 95% CI 0.66 to 1.61; I2 = 66%; 315 participants; three trials). Eight trials, with 518 participants, reported no serious adverse events. Two trials, with 165 participants, used two components of the quality of life score for assessment, with ranges from 0 to 100, where higher scores indicate better quality of life, with a mean increase from baseline of the physical component summary of 20.7 (95% CI 17.4 to 24.0; very low‐certainty evidence) and a mean increase from baseline of the mental component summary of 27.8 (95% CI 12.3 to 43.3; very low‐certainty evidence).
G‐CSF, alone or in combination, suggested a beneficial effect on the proportion of participants who developed one or more liver disease‐related complications (RR 0.40, 95% CI 0.17 to 0.92; I2 = 62%; 195 participants; four trials; very low‐certainty evidence).
When we analysed the occurrences of single complications, there was no suggestion of a difference between G‐CSF, alone or in combination, versus control, in participants in need of liver transplantation (RR 0.85, 95% CI 0.39 to 1.85; 692 participants; five trials), in the development of hepatorenal syndrome (RR 0.65, 95% CI 0.33 to 1.30; 520 participants; six trials), in the occurrence of variceal bleeding (RR 0.68, 95% CI 0.37 to 1.23; 614 participants; eight trials), and in the development of encephalopathy (RR 0.56, 95% CI 0.31 to 1.01; 605 participants; seven trials) (very low‐certainty evidence). The same comparison suggested that G‐CSF reduces the development of infections (including sepsis) (RR 0.50, 95% CI 0.29 to 0.84; 583 participants; eight trials) and does not improve liver function scores (RR 0.67, 95% CI 0.53 to 0.86; 319 participants; two trials) (very low‐certainty evidence).
Authors' conclusions
G‐CSF, alone or in combination, seems to decrease mortality in people with decompensated advanced chronic liver disease of whatever aetiology and with or without acute‐on‐chronic liver failure, but the certainty of evidence is very low because of high risk of bias, inconsistency, and imprecision. The results of trials conducted in Asia and Europe were discrepant; this could not be explained by differences in participant selection, intervention, and outcome measurement. Data on serious adverse events and health‐related quality of life were few and inconsistently reported. The evidence is also very uncertain regarding the occurrence of one or more liver disease‐related complications. We lack high‐quality, global randomised clinical trials assessing the effect of G‐CSF on clinically relevant outcomes.
Keywords: Adult, Humans, Acute-On-Chronic Liver Failure, Acute-On-Chronic Liver Failure/complications, Erythropoietin, Esophageal and Gastric Varices, Esophageal and Gastric Varices/complications, Gastrointestinal Hemorrhage, Granulocyte Colony-Stimulating Factor, Granulocyte Colony-Stimulating Factor/therapeutic use, Growth Hormone, Intercellular Signaling Peptides and Proteins, Liver Cirrhosis, Liver Cirrhosis/complications, Quality of Life, Stem Cells
Plain language summary
Granulocyte colony‐stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease
Key messages
In people with chronic liver disease with diffuse scarring (called cirrhosis) and impairment of liver function, the infusion of granulocyte colony‐stimulating factor (G‐CSF) may reduce the risk of death in comparison with standard treatment. Unexpected effects were either poorly reported, or the information was unclear. G‐CSF is a protein that stimulates the growth and spread of undifferentiated cells (immature cells that do not have specialised structures or functions) or partially differentiated (incompletely specialised) cells into the bloodstream and organs, such as the liver. G‐CSF may be administered alone or in combination with another drug. As we are not confident in the evidence provided by the available studies that assessed G‐CSF, the results of this review are likely untrustworthy. Also, the included studies in the review were too different from each other to allow us to draw firm conclusions based on the evidence.
Why is it important to treat people with advanced chronic liver disease?
A wide range of diseases can cause continuous and repeated damage to the liver, which leads to progressive scarring and impairment of the liver function. When damage to the liver is irreversible, it is defined as chronic. Globally, advanced chronic liver disease is considered responsible for more than one million deaths every year. No treatment is available to specifically target liver scarring, and liver transplantation remains the only curative option. Many researchers are investigating strategies to restore the functions of the liver to avoid or slow progression towards end‐stage liver disease (that is, the final stage of a progressive liver condition, such as cirrhosis of the liver, progressive hepatitis (for example, viral hepatitis type C), or liver cancer), which ultimately requires a rescue liver transplantation.
What is granulocyte colony‐stimulating factor?
Granulocyte colony‐stimulating factor is a protein that stimulates the bone marrow to produce white blood cells and immature cells and release them into the bloodstream. This protein can be produced with recombinant DNA technology (DNA molecules formed by laboratory methods) and is currently used to preserve a safe level of white blood cells in people on chemotherapy for cancer. Also, G‐CSF might regulate inflammation and improve the capacity of the liver to replace lost cells and survival in people with advanced chronic liver disease.
What did wewant to find out?
We wanted to find out if G‐CSF, administered alone or in combination with other drugs to people with advanced chronic liver disease, compared with sham treatment or no treatment, is able to improve survival. We were also interested in assessing unwanted or harmful effects of this treatment, complications due to the liver disease, and the treatment effect on well‐being.
What did we do?
We searched for randomised clinical trials that assessed the effect of multiple courses of G‐CSF alone or in combination with other drugs in people with advanced chronic liver disease. In randomised controlled trials, study participants are assigned to groups that receive different treatments by chance (that is, at random).
What did we find?
We included a total of 20 studies with 1419 participants. A total of 188 out of 738 (25.4%) participants randomised to the G‐CSF group, compared with 302 out of 681 (44.3%) participants in the control group, died. (The control group received standard medical therapy and other supportive measures.) The follow‐up in the studies varied between 2 and 12 months. The studies were conducted from 2008 to 2022: 15 in Asia, 4 in Europe, and 1 in the USA. Eight studies included only people with alcoholic liver disease, and the other studies included people with different causes of liver disease, mainly chronic hepatitis B or C. Only few studies reported data on unwanted or harmful effects of the treatment and well‐being. G‐CSF seemed to reduce the proportion of participants with liver‐related complications that may increase the risk of dying. We could not draw any firm conclusions for any of the studied outcomes because of the poor study designs, as this resulted in no confidence in the evidence. Therefore, we cannot be sure if there is a beneficial, harmful, or neutral effect of G‐CSF compared with no treatment or sham treatment on the risk of death, unwanted or harmful effects of the treatment, and complications due to liver disease.
What are the limitations of the evidence?
Our confidence in the evidence is very low because the studies show many limitations, which can potentially lead to prejudiced results. There are not enough studies to be certain about the result estimates. Therefore, we need further randomised clinical studies of high quality.
How up to date is this evidence?
The evidence is up to date to 4 October 2022.
Summary of findings
Summary of findings 1. Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion for people with compensated or decompensated advanced chronic liver disease.
| G‐CSF, administered alone or in combination, compared with placebo or no intervention for advanced chronic liver disease | ||||||
|
Patient or population: adults with compensated or decompensated advanced chronic liver disease Settings: tertiary centres, in Asia (15 trials), in Europe (4 trials), and in the USA (1 trial) Intervention: G‐CSF, independent of the route or schedule of administration, as a single treatment or combined with stem or progenitor cell infusion or with other medical co‐interventions Comparison: placebo or no intervention | ||||||
|
Outcomes |
Illustrative comparative risks* |
Relative effect
(95% CI) |
Number of participants (trials) |
Certainty of the evidence
(GRADE) |
Comments | |
| Risk with control | Risk with G‐CSF (95% CI) | |||||
| All‐cause mortality at maximum follow‐up: mean 5.2 months (range: 2 to 12) | 443 per 1000 | 235 per 1000 (168 to 319) | RR 0.53 (95% CI 0.38 to 0.72) |
1419 (20) | Very low ⊕⊝⊝⊝a
|
‐ |
| Proportion of participants with 1 or more serious adverse events at maximum follow‐up: mean 4 months (range: 3 to 6) | 503 per 1000 | 518 per 1000 (332 to 810) | RR 1.03 (95% CI 0.66 to 1.61) | 315 (3) | Very low ⊕⊝⊝⊝b |
‐ |
|
Health‐related quality of life ‐ PCS physical component summary ‐ MCS mental component summary at maximum follow‐up: 12 months |
Change from baseline: mean increase 20.7 (95% CI 17.4 to 24.0) Change from baseline, mean increase 27.8 (95% CI 12.3 to 43.3) |
165 (2) | Very low ⊕⊝⊝⊝c | PCS and MCS are 2 components of the health‐related quality of life score that ranges from 0 to 100, with higher scores indicating better quality of life. | ||
| Proportion of participants with liver disease‐related morbidity at maximum follow‐up: mean 7.2 months (range: 2 to 12) | 493 per 1000 | 197 per 1000 (84 to 454) |
RR 0.40 (95% CI 0.17 to 0.92) | 195 (4) | Very low ⊕⊝⊝⊝d | ‐ |
| CI: Confidence interval; RR: relative risk; RCT: randomised controlled trial; G‐CSF: granulocyte colony‐stimulating factor; PCS: physical component summary; MCS: mental component summary | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). aDowngraded by 3 levels because of risk of bias (mainly overall high risk of bias), heterogeneity (I2 = 75%), and imprecision (the optimal information size criterion was not met) bDowngraded by 3 levels because of risk of bias, heterogeneity (I2 = 66%), and imprecision (very small sample size, 315 participants) cDowngraded by 3 levels because of risk of bias, heterogeneity (I2 = 62%), and imprecision (very small sample size, 165 participants) dDowngraded by 3 levels because of risk of bias (1 level) and imprecision (2 levels) (very small sample size, 195 participants)
Background
Description of the condition
Different aetiologies of liver disease, such as viral infection; toxin exposure; alcohol abuse; and metabolic, immunological, or genetic diseases, cause continuous and repeated damage to the liver. Persistent injury leads to inflammation, progressive fibrosis, and compensatory hepatocyte hyperplasia, usually culminating in cirrhosis that is characterised by distortion of the hepatic architecture and the formation of regenerative nodules. The histological pattern is generally considered to be irreversible, and the disease is usually asymptomatic until complications develop (Garcia‐Tsao 2010; Tsochatzis 2014). The identification of cirrhosis, based only on clinical and laboratory findings, is imperfect and requires a liver biopsy and histology. Liver stiffness measurement (LSM) by transient elastography and use of biomarkers are non‐invasive accurate tests for the diagnosis of severe fibrosis and cirrhosis (Foucher 2006; Pavlov 2015). It has been proposed that the term 'advanced chronic liver disease' should be used as an alternative to 'cirrhosis' (de Franchis 2015).
Advanced chronic liver disease is characterised by a long compensated phase, with a median survival from diagnosis of around 12 years (D'Amico 2006). This asymptomatic phase, termed 'compensated', is followed by a rapidly progressive phase, termed 'decompensated', which is marked by the development of complications of portal hypertension or liver dysfunction, or both. In the compensated phase, portal pressure may be normal. As the disease progresses, portal pressure increases and liver function decreases, resulting in the development of ascites, portal hypertensive gastrointestinal bleeding, encephalopathy, and jaundice. The development of these complications marks the transition from a compensated to a decompensated phase. Progression may be accelerated by the development of other complications, such as (re)bleeding, renal impairment (refractory ascites, hepato‐renal syndrome), hepato‐pulmonary syndrome, and sepsis (spontaneous bacterial peritonitis). The worst expression of acute decompensation is acute‐on‐chronic liver failure (ACLF), characterised by the development of organ failures amongst liver, kidney, brain, circulation, coagulation, and lung, with a high risk of death (Arroyo 2020). The development of hepatocellular carcinoma (HCC) may accelerate the course of the disease at any stage. When decompensation occurs, the expected median survival is around two years (D'Amico 2006; D'Amico 2014).
Advanced chronic liver disease is considered responsible for more than one million annual deaths worldwide (Rowe 2017). The geographical distribution of liver disease is non‐uniform and reflects the different prevalence of risk factors, including alcohol consumption, hepatitis C virus (HCV) infection, hepatitis B virus infection, obesity, and metabolic syndrome. In 2010, advanced chronic liver disease accounted for approximately 49,500 deaths and was the eighth leading cause of death in the United States (Murray 2013). A recent European Association for the Study of Liver Disease (EASL) report from 35 European countries estimated a median age‐adjusted prevalence of chronic liver disease of 833 people per 100,000. Following data from 2017, the prevalence ranged from a minimum of 447 people per 100,000 in Iceland to a maximum of 1100 people per 100,000 in Romania, with a total of 151,513 deaths from liver disease in European countries (Pimpin 2018). In 2012, in England, people with liver disease admitted to a hospital were more likely to die compared to people classified as all‐cause admissions (8.8% versus 1.4%) (NICE 2016).
Description of the intervention
No treatment is available to specifically target fibrosis and cirrhosis, and liver transplantation remains the only curative option (Rossi 2007). Many researchers are investigating strategies to restore liver functionality to avoid or slow progression towards end‐stage liver disease, ultimately requiring a rescue liver transplantation.
Cell therapy is an emerging strategy that aims to restore liver functionality; in particular, bone marrow‐derived stem cells (BMSCs) seem to be able to contribute to liver regeneration and to differentiate into hepatocyte‐like cells (Forbes 2012; Thomas 2011). These stem cells can be infused, can reach the liver, and can become hepatocytes, improving liver function (Forbes 2016). Furthermore, cytokine mobilisation of BMSCs from the bone marrow to the liver could improve liver function (Alison 2000). Granulocyte colony‐stimulating factor (G‐CSF) is a 175‐amino‐acid protein, obtained through recombinant DNA technology and currently available for mobilisation of haematopoietic stem cells (HSCs) from the bone marrow (Lanthier 2018; Moore 2014). The minimum recommended dosage needed to obtain peripheral cell mobilisation is 5 μg/kg daily for at least five consecutive days (Alison 2000; Duong 2014). Furthermore, based on promising experimental data, growth factors, such as erythropoietin and growth hormone, are sometimes associated with G‐CSF to stimulate hepatic regeneration and consequently improve hepatic function (Lewis 2004; Krupczak‐Hollis 2003).
How the intervention might work
Multiple courses of G‐CSF have been shown to modulate inflammation, mobilise HSCs, increase hepatocyte growth factor, and induce hepatic progenitor cells to proliferate within seven days of administration (Gaia 2013; Gilchrist 2010; Spahr 2008). In compensated or decompensated cirrhosis, multiple courses of G‐CSF, with or without stem or progenitor cell infusion or with growth factors (erythropoietin or growth hormone), might be associated with accelerated hepatic regeneration and improved liver function and survival (Kedarisetty 2015; Verma 2018a; Verma 2018b).
Why it is important to do this review
Studies on the effects of multiple courses of G‐CSF on hepatic regeneration and function reported conflicting results (Kedarisetty 2015; Lanthier 2018; Newsome 2018; Verma 2018a; Verma 2018b). A 2014 systematic review, which included studies up to July 2013, concluded that "further robust clinical trials and collaborative protocols are required" (Moore 2014). We consider it important to summarise the results of trials assessing the benefits and harms of G‐CSF, with or without stem or progenitor cell infusion or with growth factors, in people with advanced chronic liver disease.
Objectives
To evaluate the benefits and harms of granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell or growth factors (erythropoietin or growth hormone) infusion, compared with no intervention or placebo in people with compensated or decompensated advanced chronic liver disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials, irrespective of trial design, publication type, publication status, outcomes reported, or language.
We excluded quasi‐randomised studies (i.e. pseudo‐randomised studies in which allocation sequence generation can be anticipated by alternation, date of birth, or day of admission) and other observational studies.
Types of participants
Adults (18 years of age and older) with the diagnosis of advanced chronic liver disease (as defined by trialists), either compensated (i.e. without complications, such as gastro‐oesophageal varices, ascites, jaundice, encephalopathy) or decompensated, with one or more of the above listed complications, or with acute‐on‐chronic liver failure, as defined according to European Association for the Study of the Liver (EASL)‐Chronic Liver Failure (CLIF) Consortium criteria. The EASL‐CLIF definition is "an acute deterioration of pre‐existing chronic liver disease, usually related to a precipitating event and associated with increased mortality at three months due to multisystem organ failure" (Arroyo 2017).
Types of interventions
Experimental intervention
Granulocyte colony‐stimulating factor (G‐CSF), independent of the route or schedule of administration, as a single treatment or combined with stem or progenitor cell infusion, or with other medical interventions.
Control intervention
No intervention or placebo.
Co‐interventions: we also considered for inclusion trials with co‐interventions administered in an equal way to all trial groups of relevance to our review.
Types of outcome measures
Primary outcomes
All‐cause mortality
Proportion of participants with one or more serious adverse events. We considered an event as a serious adverse event if trial authors clearly state that it was due to the experimental or control intervention, and if it fulfils the definition of serious adverse events of the International Conference on Harmonization (ICH) Guidelines (ICH‐GCP 2016), that is, any event that leads to death; is life‐threatening; requires in‐patient hospitalisation or prolongation of existing hospitalisation; or results in persistent or significant disability, congenital birth, or anomaly; and any important medical event that may have jeopardised the patient or required intervention to prevent it. We considered all other adverse events as non‐serious. If an included study reported only a short list of serious adverse events that the trialists deemed important, we used the highest reported number. If trialists clearly stated that a death was due to the experimental or control intervention, we considered this event as a serious adverse event.
Health‐related quality of life (any validated continuous outcome scale used by trialists)
Secondary outcomes
Proportion of participants with liver disease‐related morbidity (i.e. proportion of participants who developed one or more complications, such as ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, jaundice, portal thrombosis, or hepatocellular carcinoma, or who underwent liver transplantation)
Proportion of participants with adverse events considered to be non‐serious
Proportion of participants without improvement of liver function scores, such as Child‐Turcotte‐Pugh (CTP) or Model for End‐Stage Liver Disease (MELD) scores as defined by trialists
We extracted data and assessed the above outcomes only at maximum follow‐up. If the length of follow‐up differed significantly between the included trials, we performed a subgroup analysis to assess whether the different lengths of follow‐up (e.g. more or less than three months) would affect our results.
We did not use the above‐listed outcomes as criteria for including trials.
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Hepato‐Biliary Group (CHBG) Controlled Trials Register (searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web);
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 10) in the Cochrane Library (searched 4 October 2022);
MEDLINE Ovid (1946 to 4 October 2022);
Embase Ovid (1974 to 4 October 2022);
BIOSIS (Web of Science) (1969 to 4 October 2022);
LILACS (Bireme) (Latin American and Caribbean Health Science Information Database; 1982 to 4 October 2022);
Science Citation Index Expanded (Web of Science; 1900 to 4 October 2022); and
Conference Proceedings Citation Index (Web of Science; 1990 to 4 October 2022).
The latter two were searched simultaneously through Web of Science. We applied no language, date, or document type restrictions to the searches.
Appendix 1 presents the search strategies.
Searching other resources
We also searched the bibliographic references of the included randomised clinical trials and of relevant review articles to find randomised clinical trials not identified by the electronic searches. We searched Google Scholar (www.scholar.google.com); the Turning Research into Practice database (www.tripdatabase.com); and the following online trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 4 October 2022);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 4 October 2022);
European Medicines Agency (www.ema.europa.eu/ema; searched 4 October 2022); and
U.S. Food and Drug Administration (www.fda.gov; searched 4 October 2022).
We used the following as search terms: chronic liver disease OR Liver disease OR cirrhosis; granulocyte colony‐stimulating factor OR G‐CSF.
Data collection and analysis
We prepared the review by following recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a). We performed analyses using Review Manager 5.4 (Review Manager).
Selection of studies
Two review authors (AC and DP) independently selected publications reporting randomised clinical trials relevant to the review. If one review author identified a trial as relevant but the other another did not, the two review authors discussed the reasoning behind their decision to obtain an agreement.
AC and DP also scanned any observational studies retrieved through searches for reports on adverse events because of the experimental intervention in this review. None of these studies were eligible for inclusion in the review.
We did not plan to specifically search for observational studies for inclusion in this review, which is a known limitation of the study in terms of adverse events. We are aware that the decision to not search systematically for all observational studies and to extract data on harm only from quasi‐randomised and controlled clinical studies might bias our review towards assessment of benefits and might overlook certain harms, such as late or rare harms (Storebø 2018).
Data extraction and management
Two review authors (AC and DP) independently extracted and validated data. We used data extraction forms that we designed for this purpose. The two review authors resolved disagreements in data extraction by discussion or by asking the review arbitrator (GC) for advice. We judged the reported data to be sufficiently informative; therefore, we did not contact trial investigators about missing information.
Assessment of risk of bias in included studies
We assessed the risk of bias for each primary and secondary outcome of the included trials (Higgins 2011a; Higgins 2011b). Two review authors (AC and MF) independently assessed the risk of bias of each included trial according to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and according to methodological studies (Kjaergard 2001; Moher 1998; Rücker 2008; Savović 2012a; Savović 2012b; Savović 2018; Schultz 1995; Wood 2008). We used the following definitions in our assessment of the risk of bias.
Allocation sequence generation
Low risk of bias: study authors performed sequence generation using computer random number generation or a random numbers table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the study. In general, we classified risk of bias as low if the method used for allocation concealment suggested that it was extremely likely that the sequence was generated randomly (e.g. use of interactive voice response system).
Unclear risk of bias: study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. Investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: study authors did not describe the method used to conceal the allocation, so intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that investigators who assigned participants knew the allocation sequence. We excluded such quasi‐randomised studies.
Blinding of participants and personnel (performance bias)
Low risk of bias: blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken, or rarely, no blinding or incomplete blinding, but review authors judged that the outcome was not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk', or the trial did not address this outcome.
High risk of bias: no blinding or incomplete blinding and the outcome was likely to be influenced by lack of blinding, or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment (detection bias)
Low risk of bias: blinding of outcome assessment ensured, and it was unlikely that the blinding could have been broken, or rarely, no blinding of outcome assessment, but review authors judged that the outcome measurement was not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk', or the trial did not address this outcome.
High risk of bias: no blinding of outcome assessment and the outcome measurement was likely to be influenced by lack of blinding, or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: information was insufficient to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: results were likely to be biased due to missing data.
Selective outcome reporting
Low risk: the trial reported as a primary outcome all‐cause mortality, which is the main reason for treatment with G‐CSF for people with advanced chronic liver disease. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time the trial was begun. If the trial protocol was registered after the trial was begun, we did not consider those outcomes reliable.
Unclear risk of bias: not all pre‐defined or clinically relevant and reasonably expected outcomes were reported fully, or it was unclear whether data on these outcomes were recorded.
High risk of bias: all‐cause mortality or one or more pre‐defined outcomes were not reported, despite the fact that data on these outcomes should have been available and even recorded.
Other bias
Low risk of bias: the trial appeared to be free of other bias domains that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other bias domains that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall risk of bias assessment
We judged a trial to be at overall low risk of bias if we assessed the trial as at low risk of bias for all of the above domains. We judged a trial to be at high risk of bias if we assessed the trial as having an unclear risk of bias or at high risk of bias in one or more risk of bias domains. For the primary outcome, all‐cause mortality, we considered the risk of bias related to the blinding of outcome assessors as low because death was not likely to be influenced by lack of blinding.
Measures of treatment effect
We presented risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. For continuous outcomes, we used the mean difference (MD) between experimental and control groups with their 95% CI. If trials reported quality of life measurements using different tools, we planned to calculate the standardised mean difference (SMD) with 95% CI, rather than the MD. For interpreting SMDs, we planned to use the Cohen’s effect sizes rule, which considers 0.2 as a small effect, 0.5 as a moderate effect, and 0.8 as a large effect (Cohen 1988).
Unit of analysis issues
Our unit of analysis was the trial and the individual participants as randomised within the trial (Li 2019). For adverse events, serious or non‐serious, and for liver disease complications, we used the proportion of participants with one or more events. If trials had more than two intervention groups, we selected those of interest to our review. We did not expect to find, and we did not find, cross‐over or cluster‐randomised trials. For the secondary outcome 'Proportion of participants with liver disease‐related morbidity (i.e. proportion of participants who developed one or more complications, such as ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, jaundice, portal thrombosis, or hepatocellular carcinoma, or who underwent liver transplantation)', we also performed analyses using a single complication (ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, jaundice, portal thrombosis, or liver transplantation) as the unit of analysis, as most studies reported only the occurrence of a single complication, preventing the per‐patient analysis of the composite outcome (Differences between protocol and review).
Dealing with missing data
We planned to perform our analyses according to the intention‐to‐treat method, that is, by analysing participants in the groups to which they were randomised, regardless of whether they had received or adhered to the allocated intervention. If data were not available, we used the data as reported, i.e. a modified intention‐to‐treat analysis, based on the study authors' data. We considered censored participants as no event.
For our dichotomous outcome 'All‐cause mortality', in case of participants with missing or incomplete data (dropouts), we conducted the sensitivity analyses described below.
'Extreme‐case' analysis favouring the experimental intervention, i.e. 'best‐worst case scenario': none of the participants who dropped out from the experimental group experienced the outcome, but all participants who dropped out from the control group experienced the outcome, including all randomised participants in the denominator.
'Extreme‐case' analysis favouring the control, i.e. 'worst‐best case scenario': all participants who dropped out from the experimental group, but none from the control group, experienced the outcome, including all randomised participants in the denominator.
For the continuous outcome 'Health‐related quality of life', if the trial did not report standard deviations, we planned to impute standard deviations according to Chapter 6 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b).
Assessment of heterogeneity
We explored clinical and methodological heterogeneity using extracted information, reported also in the 'Characteristics of included studies'. We compared the characteristics of participants, interventions, outcome measures, follow‐up, and trial design.
We explored the presence of statistical heterogeneity by using the Chi² test, with significance set at P value less than 0.10. In addition, we used the I² statistic to quantify heterogeneity according to the following classification: from 0% to 40%, heterogeneity may not be important; from 30% to 60%, heterogeneity may be moderate; from 50% to 90%, heterogeneity may be substantial; and from 75% to 100%, heterogeneity may be considerable (Deeks 2019).
Assessment of reporting biases
For any outcome with at least 10 trials included in the meta‐analysis, we drew a funnel plot to assess reporting bias from the individual trials by plotting on the x‐axis the RR on a logarithmic scale against its standard error (Page 2022). In order to assess if there were signs of reporting bias, we visually inspected funnel plots and tested for asymmetry using the Harbord test in case Tau2 was less than 0.1 (Harbord 2006), or the Rücker test in case Tau2 was more than 0.1 (Rücker 2008).
To determine whether there was selective reporting of results, we had planned to compare trial protocols and registration data against published reports. However, we did not do this as all trials reported the most important clinical outcome, all‐cause mortality.
Data synthesis
Meta‐analysis
We performed meta‐analyses following recommendations in the Cochrane Handbook for Systematic Reviews of Intervention (Deeks 2019). We used the statistical software Review Manager 5.4, provided by Cochrane, to analyse data (Review Manager). We applied both fixed‐effect and random‐effects models of meta‐analysis (DeMets 1987; DerSimonian 1986). We considered the fixed‐effect model only as a sensitivity analysis.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses for the outcome of all‐cause mortality. Posthoc, we decided to conduct two additional analyses: trials including or excluding participants with acute‐on‐chronic liver failure, and trial conductance based on region (see Differences between protocol and review).
Following overall risk of bias
Trials at low risk of bias compared to trials at high risk of bias as trials at high risk of bias may overestimate or underestimate intervention effects (Kjaergard 2001; Moher 1998; Savović 2012a; Savović 2012b; Savović 2018; Schultz 1995; Wood 2008).
Following funding
Trials without vested interest compared to trials at risk of vested interest, as trials with no vested interest may overestimate or underestimate intervention effects (Lundh 2017)
Following participants' disease
Trials including only decompensated chronic liver disease compared to trials including compensated cirrhosis (because effects of treatment might vary according to the severity of liver dysfunction)
Trials including only or mainly participants with alcoholic liver disease compared to trials including only or mainly participants with other liver diseases of other origins (because effects of treatment might vary according to the cause)
Trials including only participants with acute‐on‐chronic liver failure compared to trials excluding participants with acute‐on‐chronic liver failure (posthoc analysis)
Following trial regimen/dose
Trials using the recommended dosage of G‐CSF for peripheral cell mobilisation (5 μg/kg daily for at least five consecutive days) compared to trials using lower dosages (lower dosages are expected to be ineffective) (Duong 2014)
Trials using a daily dosage of G‐CSF > 10 μg/kg daily compared to trials using a lower dosage (because below this dose, the effects of treatment might be impaired) (Duong 2014)
Following treatment duration
Trials using short‐term treatment schedules (shorter than seven days) compared to trials using longer treatment schedules (long‐term), as shorter treatment might reduce the effects of treatment (Duong 2014)
Following the experimental intervention
Trials using only G‐CSF compared to trials combining G‐CSF with stem or progenitor cell infusion, as infusion of stem or progenitor cells may modify treatment effects (Lanthier 2018)
Trials using only G‐CSF compared to trials combining G‐CSF with other medical intervention, as any added intervention might influence treatment effects (Engelmann 2021b)
Following length of follow‐up
Trials with a length of follow‐up equal to or less than three months compared to trials with a length of follow‐up of more than three months, as the estimated effect of treatment might vary according to the length of the follow‐up
Following trial conductance
Trial location hypothesising population differences: trials conducted in Asia compared to trials conducted in Europe, America, and Africa (posthoc analysis)
Sensitivity analysis
We performed the following sensitivity analyses.
Excluding trials assessed at high risk of bias
Excluding trials published only in abstract or letter form (posthoc analysis, see Differences between protocol and review)
Conducting the analysis with the fixed‐effect model only in order to assess the influence of small‐study effects on the results of our meta‐analysis
'Best‐worst case scenario' and 'worst‐best case scenario' to assess the potential impact of the missing data for the outcome 'All‐cause mortality' (see Dealing with missing data)
Assessment of imprecision with the Trial Sequential Analysis in order to explore differences in assessment of imprecision with GRADE (see below) (Castellini 2018; Gartlehner 2019)
Trial Sequential Analysis
We performed Trial Sequential Analysis on the primary outcomes to calculate the cumulative sample size of the meta‐analysis (information size) and to reduce the risk of random errors because of sparse data and repetitive testing of accumulating data (Thorlund 2017; Wetterslev 2008). We calculated the information size adjusted for heterogeneity (diversity, D²) between trials using the following parameters (Wetterslev 2009): proportion of events in the control group estimated from the included trials (overall mean value); anticipated intervention effect (relative risk reduction, RRR) of 15%; risk of type I error, alpha, of 2.5%, as we use three primary outcomes; and risk of type II error, beta, of 10% (Jakobsen 2014; Wetterslev 2017). We added trials to the analysis according to the year of publication, irrespective of their overall bias risk. If more than one trial was published in a year, we added the trials in alphabetical order, according to the name of the first author. On the basis of the required information size, we constructed the trial sequential monitoring boundaries for benefits and futility using the O'Brien‐Fleming‐Lan‐DeMets alpha spending (for benefit) and beta‐spending (for futility) functions. We used the random‐effects model meta‐analysis.
The boundaries for the benefit are used for meta‐analyses that have not reached the required information size to conclude when statistical significance is reached. If the trial sequential monitoring boundary is crossed before the required information size is reached, a sufficient level of evidence is reached, results of the meta‐analysis can be considered conclusive if bias can be excluded, and no additional trials may be needed. Conversely, if the boundary is not crossed, the meta‐analysis is inconclusive, and more trials may be needed to detect or reject a certain intervention effect. When the cumulative Z‐curve crosses the futility boundaries, a sufficient level of evidence is reached so that the two treatments do not differ by more than 15% (anticipated intervention effect used in information size estimation), and no additional trials may be needed. In all situations where no trial sequential monitoring boundaries are reached, further studies may be needed until the information size is reached or until monitoring boundaries are crossed.
In Trial Sequential Analysis, we downgraded our assessment of imprecision by two levels if the accrued number of participants was below 50% of the diversity‐adjusted required information size (DARIS), and one level if between 50% and 100% of the DARIS. We did not downgrade for imprecision if the cumulative Z‐value reached or crossed benefit, harm, futility, or DARIS. We performed the Trial Sequential Analysis with Trial Sequential Analysis software, version 0.9.5.10 beta (Thorlund 2017; TSA).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach and GRADE software to assess the certainty of evidence (GRADEpro GDT). GRADE uses five domains (i.e. risk of bias (we used the overall risk of bias judgement), heterogeneity, imprecision, indirectness, and publication bias) to assess the certainty of evidence that relates to the trials that contribute data for prespecified outcomes. The outcomes we presented are all‐cause mortality; proportion of participants with one or more serious adverse events; health‐related quality of life; and proportion of participants with liver disease‐related morbidity. We reported the range of follow‐up for each outcome and its mean or the longest follow‐up.
Two review authors (AC and MF) independently performed the GRADE assessments following the recommendations in the GRADE Handbook (Schünemann 2013) and related publications (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Guyatt 2017; Mustafa 2013). The two review authors (AC and MF) resolved disagreements through discussions, or if required, they consulted a third review author (GC).
We justified all decisions to downgrade the certainty of evidence using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
We defined the levels of evidence as 'high', 'moderate', 'low', or 'very low':
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
We created a summary of findings table with the comparison 'G‐CSF, administered alone or in combination, compared with placebo or no intervention for advanced chronic liver disease'.
We conducted the review according to our published protocol and reported any deviations from the protocol in the 'Differences between protocol and review' section.
Results
Description of studies
Results of the search
We ran the electronic searches on 4 October 2022. As shown in Figure 1, we identified 4635 references by searching the following databases: Cochrane Hepato‐Biliary Group Controlled Trials Register (via the Cochrane Central Register of Studies Web) (n = 66), Cochrane Central Register of Controlled Trials in the Cochrane Library (n = 852), MEDLINE Ovid (n = 322), Embase Ovid (n = 2811), Latin American and Caribbean Health Science Information Database (Bireme) (n = 13), Science Citation Index Expanded, and Conference Proceedings Citation Index (n = 571).
1.
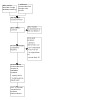
Study flow diagram. Date of search: 4 October 2022
We found two additional records by searching other sources. After the exclusion of 765 duplicates, 3872 references remained for possible eligibility. After reading the title and the abstract of these references, we excluded 3831, as they did not meet the inclusion criteria. We had to evaluate 41 full texts; however, we retrieved full texts of 39 references; for the remaining two we are awaiting the translation of the text from the Chinese language. After reading the 39 full texts available, we excluded 18 studies for various reasons (see Characteristics of excluded studies). Finally, we included in our qualitative and quantitative synthesis 20 references reporting data on 20 trials (De 2021; Duan 2013; Engelmann 2021a; Garg 2012; Haque 2020; Kedarisetty 2015; Morgan 2022; Newsome 2018; Prajapati 2017; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Spahr 2008; Spahr 2013; Tong 2022; Venkitaraman 2022; Verma 2018a), which included a total of 1419 participants. One record was an ongoing randomised clinical trial that included participants with severe alcoholic hepatitis with partial or null response to glucocorticosteroids (Cho 2018). The estimated date of completion of this study was December 2022 (Cho 2018). However, the enrolment of participants was terminated in July 2022 due to the failure to recruit eligible participants, and there are no published results yet.
Included studies
We reported in the Characteristics of included studies tables the main characteristics of the 20 included trials. Three trials are reported only as abstracts (Morgan 2022; Sharma 2017; Singh 2021). The trials were conducted from 2008 to 2022; 15 trials were conducted in Asia (11 in India: De 2021; Garg 2012; Kedarisetty 2015; Prajapati 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Venkitaraman 2022; Verma 2018a; two in Bangladesh: Saha 2017; Haque 2020; and two in China: Duan 2013; Tong 2022), four in Europe (two in Switzerland: Spahr 2008; Spahr 2013; one in the UK: Newsome 2018; and one in Germany: Engelmann 2021a), and one in the USA (Morgan 2022).
All trials reported data allowing intention‐to‐treat analysis. Only one used a "modified intention‐to‐treat analysis" (Newsome 2018), different from what was planned in the protocol (King 2015), and reported that one participant randomly assigned to treatment with granulocyte colony‐stimulating factor (G‐CSF) plus CD133‐positive cell infusion died before receiving any treatment; we attributed the event to the intervention group according to the intention‐to‐treat analysis.
Four trials were interrupted early due to futility (Engelmann 2021a) or lower‐than‐expected participant recruitment (Morgan 2022; Shasthry 2019; Singh 2021), and the results of an interim analysis were reported.
Six trials reported no information about trial registration (Duan 2013; Haque 2020; Saha 2017; Sharma 2017; Singh 2014; Singh 2021).
Funding and conflicts of interest
Nine trials were funded by neutral organisations without vested interests in the trial result (De 2021; Duan 2013; Engelmann 2021a; Newsome 2018; Singh 2018b; Spahr 2008; Spahr 2013; Tong 2022; Verma 2018a), four trials reported no funding (Haque 2020; Kedarisetty 2015; Singh 2014; Venkitaraman 2022), and seven trials reported no information on funding (Garg 2012; Morgan 2022; Prajapati 2017; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2021). None of the trials reported funding by organisations with vested interests in the results of the trial.
Sixteen trials reported no possible conflicts of interest pertaining to the authors (De 2021; Garg 2012; Haque 2020; Kedarisetty 2015; Newsome 2018; Prajapati 2017; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Spahr 2008; Spahr 2013; Tong 2022; Venkitaraman 2022; Verma 2018a), one trial reported in detail possible conflicts of interest (Engelmann 2021a), and three trials reported no information related to conflicts of interest (Duan 2013; Morgan 2022; Singh 2021).
Of those trials reporting on funding, we judged no trial to be of "notable concern about conflicts of interest" (Higgins 2019a).
Participants
A total of 1419 participants were randomised in the 20 trials: the number of participants ranged from 28 in Shasthry 2019 to 259 in Prajapati 2017. The proportion of males ranged from 53% in Kedarisetty 2015 to 100% in Spahr 2013, and the median age ranged from 40 years in Shasthry 2019 to 56 years in Engelmann 2021a and Spahr 2013. In one trial, 30% of the participants had compensated cirrhosis (Newsome 2018), while the other 19 trials included only participants with decompensated cirrhosis, that is, cirrhosis with at least one of the following complications: ascites, bleeding, encephalopathy, and jaundice (EASL 2018). Twelve trials included only participants with acute‐on‐chronic liver failure (Duan 2013; Engelmann 2021a; Garg 2012; Haque 2020; Morgan 2022; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Tong 2022), that is, acutely decompensated cirrhosis (acute development or worsening of one of the following complications: ascites, hepatic encephalopathy, gastrointestinal haemorrhage, or bacterial infection) combined with one or more organ failure(s) (Arroyo 2020). Eight trials included only participants with alcoholic liver disease (Morgan 2022; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Spahr 2008; Spahr 2013), seven trials included different proportions of participants with different aetiologies (mainly alcoholic and viral) (De 2021; Garg 2012; Kedarisetty 2015; Newsome 2018; Prajapati 2017; Venkitaraman 2022; Verma 2018a), and four trials included mainly participants with chronic hepatitis B and zero participants with alcoholic liver disease (Duan 2013; Haque 2020; Saha 2017; Tong 2022). One trial reported no details on the aetiology of cirrhosis in the included participants (Engelmann 2021a).
Experimental interventions
In all 20 trials, the experimental intervention was granulocyte colony‐stimulating factor (G‐CSF) alone or in combination. The dosage in the 20 trials varied from 5 μg/kg to 10 μg/kg daily and was administered for five to six days. Five trials continued the administration of G‐CSF with the same dose for a further four weeks (Engelmann 2021a; Garg 2012; Kedarisetty 2015; Shasthry 2019; Verma 2018a), and one other trial continued with four cycles every three months (De 2021). One trial used a higher dosage of G‐CSF (15 μg/kg daily for five days) (Newsome 2018), and one trial reported no details on the dosage (Singh 2021). One trial used pegfilgrastim, a long‐acting recombinant G‐CSF, 0.6 mg subcutaneously on day one and day eight (Morgan 2022).
In 13 trials, the experimental intervention was G‐CSF alone (De 2021; Duan 2013; Engelmann 2021a; Garg 2012; Morgan 2022; Prajapati 2017; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Spahr 2008; Tong 2022; Venkitaraman 2022), and the remaining seven trials combined G‐CSF with other treatments. Two trials combined G‐CSF with erythropoietin (40 μg per week) (Haque 2020; Kedarisetty 2015); one trial combined G‐CSF with growth hormone (GH) 1 U/day for 12 months (Verma 2018a); two trials combined G‐CSF with intravenous N‐acetyl cysteine (NAC) (Singh 2018b; Singh 2021); one with the infusion of CD133‐positive haemopoietic stem cells on day 5, 30, and 60 after randomisation (Newsome 2018); and another one with an infusion of autologous bone marrow mononuclear cell into the proper hepatic artery (Spahr 2013). Of the seven trials combining G‐CSF with other treatments, three were two‐group trials (Haque 2020; Kedarisetty 2015; Spahr 2013), and four were three‐group trials (Newsome 2018; Singh 2018b; Singh 2021; Verma 2018a). We combined the experimental groups in Newsome 2018 and Verma 2018a to create single pairwise comparisons. In the other two trials with three groups (Singh 2018b; Singh 2021), we considered only the G‐CSF and the control groups, as the third group received multiple mixed co‐interventions, that is, N‐acetyl cysteine or pentoxifylline.
Control interventions
In four trials, the control group received placebo (i.e. prefilled identical syringes with normal saline) (Garg 2012; Kedarisetty 2015; Shasthry 2019; Venkitaraman 2022). In the remaining 16 trials, the control group received no intervention.
Co‐interventions
In all 20 trials, both the experimental group and the control group received standard medical therapy (SMT), including antivirals, abstinence from alcohol, nutrition, diuretics, β‐blockers, selective intestinal decontamination, pentoxifylline, prednisolone, and other supportive measures depending on the clinical status and requirement.
Duration of follow‐up
In one trial, participants were followed for two months (Garg 2012); in three trials, participants were followed for six months (Prajapati 2017; Engelmann 2021a; Tong 2022); in 11 trials, participants were followed for up to three months (Duan 2013; Haque 2020; Morgan 2022; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Spahr 2008; Spahr 2013); and in five trials, participants were followed for 12 months (De 2021; Kedarisetty 2015; Newsome 2018; Venkitaraman 2022; Verma 2018a). The median follow‐up was 4.5 months.
Excluded studies
We excluded 18 studies (see Characteristics of excluded studies); the interventions in 11 studies were different from our experimental intervention, G‐CSF (Amer 2011; Anand 2019; El‐Ansary 2012; Esmaeilzadeh 2019; Kharaziha 2009; Lyra 2010; Mohamadnejad 2016; Ranjan 2022; Salama 2010; Singh 2018a; Terai 2006); five studies were not randomised clinical trials (Fiuza 2002; Gaia 2013; Philips 2020; Sharma 2016; Xing 2013); one study was conducted in animals (Yannaki 2005); and one was a narrative review without any original data (Sakaida 2005).
Risk of bias in included studies
We summarise the risk of bias in Figure 2 and Figure 3. All trials were at unclear or high risk of bias in at least one of the domains, and we considered them to be at overall high risk of bias considering all outcomes. Considering only the primary outcome all‐cause mortality, we judged two trials at low risk of bias (Garg 2012; Shasthry 2019).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of allocation sequence
Fifteen trials were at low risk of bias for the generation of allocation sequence (De 2021; Duan 2013; Engelmann 2021a; Garg 2012; Kedarisetty 2015; Newsome 2018; Prajapati 2017; Shasthry 2019; Singh 2014; Singh 2018b; Spahr 2008; Spahr 2013; Tong 2022; Venkitaraman 2022; Verma 2018a), and the remaining five trials, which did not provide sufficient information, were at unclear risk of sequence generation bias (Haque 2020; Morgan 2022; Saha 2017; Sharma 2017; Singh 2021).
Allocation concealment
Thirteen trials were at low risk of allocation concealment bias (Engelmann 2021a; Garg 2012; Kedarisetty 2015; Newsome 2018; Prajapati 2017; Shasthry 2019; Singh 2014; Singh 2018b; Spahr 2008; Spahr 2013; Tong 2022; Venkitaraman 2022; Verma 2018a), and the remaining seven trials, which did not provide sufficient information, were at unclear risk of allocation concealment bias (De 2021; Duan 2013; Haque 2020; Morgan 2022; Saha 2017; Sharma 2017; Singh 2021).
Blinding
Performance bias
Five trials were at low risk of performance bias as the participants and the healthcare providers were blinded (Duan 2013; Garg 2012; Kedarisetty 2015; Venkitaraman 2022; Verma 2018a). Five trial reports did not provide information, so we judged them at unclear risk of bias (Haque 2020; Saha 2017; Sharma 2017; Singh 2021; Tong 2022). The remaining 10 trial reports stated that the participants and the healthcare providers were not blinded and were at high risk of performance bias (De 2021; Engelmann 2021a; Morgan 2022; Newsome 2018; Prajapati 2017; Singh 2014; Singh 2018b; Spahr 2008; Spahr 2013; Verma 2018a).
Detection bias
We judged all the trials to be at low risk of detection bias for the outcome all‐cause mortality, as knowledge of the assigned intervention does not impact on such an objective outcome. Meanwhile, for the other outcomes, we judged two trials at low risk of bias (De 2021; Verma 2018a). The remaining 18 trials, which did not provide sufficient information, were at unclear risk of detection bias (Duan 2013; Engelmann 2021a; Garg 2012; Haque 2020; Kedarisetty 2015; Morgan 2022; Newsome 2018; Prajapati 2017; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Spahr 2008; Spahr 2013; Tong 2022; Venkitaraman 2022).
Incomplete outcome data
We judged 14 trials as at low risk of attrition bias, because there were no participants with missing data nor postrandomisation dropouts (De 2021; Duan 2013; Garg 2012; Haque 2020; Morgan 2022; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Singh 2021; Spahr 2008; Spahr 2013; Verma 2018a), and six at high risk of bias, as there were postrandomisation dropouts probably related to the outcomes (Engelmann 2021a; Kedarisetty 2015; Newsome 2018; Prajapati 2017; Tong 2022; Venkitaraman 2022).
Selective reporting
We judged all the trials as at low risk of selective reporting bias because they reported the most important clinical outcome, all‐cause mortality, which is expected to be reported in such trials.
Other potential sources of bias
We judged all 20 included trials as at low risk of other potential sources of bias as we did not identify any issues of possible concern.
Effects of interventions
See: Table 1
Primary outcomes
See Table 2.
1. Outcomes.
| Study | Participants with SAE | QOL: PCS, change from baseline | QOL: MCS, change from baseline | Participants with complications | Participants with NSAE | CTP score: change from baseline | MELD score: change from baseline |
| De 2021 | 0% | G‐CSF 20.9 (SD 9.7) Control 1.1 (SD 9.7) |
G‐CSF 31.9 (SD 7.9) Control 11.5 (SD 7.6) |
NR | G‐CSF 37/50 (74%) Control NR |
G‐CSF −2 (95% CI −1 to −4) Control +1 (95% CI 0 to 4) |
G‐CSF −3 (95% CI −9 to 5) Control +1 (95% CI −7 to 5) |
| Duan 2013 | NR | NR | NR | NR | NR | G‐CSF −1 Control +0.61 |
G‐CSF −1.81 Control +3.5 |
| Engelmann 2021a | G‐CSF 54/88 (61.4%) Control 47/88 (53.4%) |
NR | NR | NR | G‐CSF 26/88 (29.5%) Control 31/88 (5.2%) |
NR | NR |
| Garg 2012 | 0% | NR | NR | G‐CSF 4/23 (17.4%) Control 17/24 (70.8%) |
3/23 | G‐CSF −4 Control 0 |
G‐CSF −4.4 Control +3.7 |
| Haque 2020 | NR | NR | NR | NR | NR | NR | G‐CSF −13.3 Control −14.3 |
| Kedarisetty 2015 | 0% | NR | NR | G‐CSF 8/29 (27.6%) Control 10/26 (38.5%) |
G‐CSF 11/29 (37%) Control NR |
G‐CSF −5.3 Control −4.3 |
NR |
| Morgan 2022 | NR | NR | NR | "Similar in both arms" | NR | NR | NR |
| Newsome 2018 | G‐CSF 11/54 (20.4%) Control 2/27 (7.4%) |
Overall CLDQ score, change from baseline: Control 0.2 (95% CI –0.1 to 0.6) G‐CSF only –0.1 (95% CI –0.4 to 0.3) G‐CSF plus CD133‐positive cell infusion: 0.0 (95% CI −0.2 to +0.2) |
NR | NR | NR | G‐CSF −0.5 Control −0.5 |
|
| Prajapati 2017 | NR | NR | NR | NR | NR | Participants without improvement G‐CSF 43/126 (34.1%) Control 62/127 (48.8%) |
NR |
| Saha 2017 | NR | NR | NR | NR | NR | G‐CSF −4.3 Control −2.8 |
G‐CSF −11.7 Control −9.8 |
| Sharma 2017 | NR | NR | NR | NR | NR | G‐CSF CTP −41.97% control −8.84%) |
G‐CSF −50.89% control −10.09% |
| Shasthry 2019 | 0% | NR | NR | NR | G‐CSF 1/14 (7.1%) Control 0% |
G‐CSF −0.23 Control +0.3 |
G‐CSF −0.1 Control +2.3 |
| Singh 2014 | 0% | NR | NR | NR | G‐CSF 5/23 (21.7%) Control NR |
G‐CSF −3.1 Control ‐0.91 |
G‐CSF −11.7 Control ‐10.5 |
| Singh 2018b | NR | NR | NR | NR | G‐CSF 4/18 (22%) control NR |
G‐CSF −1 Control +1 |
G‐CSF −1.5 Control 0 |
| Singh 2021 | NR | NR | NR | NR | NR | No difference | No difference |
| Spahr 2008 | NR | NR | NR | G‐CSF 1/13 (7.7%) Control 1/11 (9.1%) |
NR | G‐CSF −2 Control −4 |
G‐CSF −2 Control −5 |
| Spahr 2013 | G‐CSF arm 17/28 (60.7%) Control arm 24/30 (80.0%) |
NR | NR | NR | NR | NR | G‐CSF arm −7 Control arm −7 |
| Venkitaraman 2022 | 0% | NR | NR | NR | G‐CSF 23/33 (69.7%) Control arm NR | G‐CSF −12.5% (−33.3 to 40) control arm 0 (−20 to 44.4) |
G‐CSF 0 (−35.3 to 90.9) control arm 0 (−37.50 to 58.3) |
| Tong 2022 | 0% | NR | NR | NR | G‐CSF 1/56 (1.8%) | NR | NR |
| Verma 2018a | 0% | G‐CSF + GH +27.1 (95% CI +25.0 to +29.2) G‐CSF +26.6 (95% CI +22.4 to +30.7) Control +3.7 (95% CI −2.3 to +9.8) |
G‐CSF + GH +37.1 (95% CI +32.7 to +41.4) G‐CSF +33.4 (95% CI from +28.4 to +38.4) control –0.96 (95% CI –9.0 to +7.1) |
G‐CSF 2/43 (4.7%) Control 2/21 (9.5%) |
G‐CSF 33/44 (75%) Control NR |
G‐CSF −2 Control +1 |
G‐CSF −30% Control 0 |
CI: confidence interval; CTP: Child‐Turcotte‐Pugh score (scores range from 5 to 15, with higher scores indicating worse prognosis); CLDQL: Chronic Liver Disease Questionnaire; G‐CSF: granulocyte colony‐stimulating factor; GH: growing hormone; MCS: mental component summary of QOL; MELD: Model for End‐Stage Liver Disease score (scores range from 6 to 40, with higher scores indicating worse prognosis); NR: not reported; NSAE: non‐severe adverse event; PCS: physical component summary of QOL; QOL: quality of life score (scores range from 0 to 100, with higher scores indicating better quality of life); SAE: severe adverse event; SD: standard deviation
All‐cause mortality
Meta‐analysis
All included randomised clinical trials reported on all‐cause mortality. A total of 188/738 (25.4%) participants in the G‐CSF group compared with 302/681 (44.3%) in the control group died (follow‐up: 5.2 months (mean), range 2 to 12). The random‐effects meta‐analysis showed that G‐CSF seemed to decrease the risk of all‐cause mortality (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.38 to 0.72; I2 = 75%; 1419 participants; 20 trials; very low‐certainty evidence) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 1: Mortality
GRADE
We downgraded the certainty of the evidence for all‐cause mortality by three levels: one level for risk of bias, one level for heterogeneity, and one level for imprecision. We rated the certainty of the evidence as very low (Table 1).
We assessed most trials (18/20) at high risk of bias, particularly the three trials with the highest weight (De 2021; Engelmann 2021a; Prajapati 2017). We found substantial heterogeneity (I2 = 75%), and we were unable to explain it. The number of included participants (1419) was lower than the optimal information size of 1544 participants (777 per trial arm), calculated assuming a relative risk reduction (RRR) of 15%; thus, we downgraded for imprecision. Visual inspection of the funnel plot seemed to suggest the presence of publication bias arising from data missing from the bottom left of the distribution (Figure 4). When we performed a formal analysis using the Rücker test (Rücker 2008), we found no asymmetry (P = 0.0964).
4.

Funnel plot of G‐CSF compared with no intervention or placebo; outcome: 1.1 All‐cause mortality. Visual inspection seemed to suggest presence of publication bias arising from data missing from the bottom left of the distribution. When performing a formal analysis with the Rücker test (Rücker 2008), we found no statistically significant asymmetry (P = 0.0964)
Subgroup analysis and investigation of heterogeneity
Our results showed substantial heterogeneity in mortality (I2 = 75%). None of the planned and conducted subgroup analyses of mortality found subgroup differences in effect (Table 3).
2. Subgroup comparisons and sensitivity analyses.
| Subgroup comparisons | ||||
| Trials | Number of trials (participants) | Risk ratio (95% confidence interval) | P value | |
| All | 20 (1419) | 0.53 (0.38 to 0.72) | ‐ | |
| Treatment duration | > 7 days | 8 (640) | 0.50 (0.29 to 0.87) | P = 0.74 |
| < 7 days | 12 (779) | 0.56 (0.40 to 0.78) | ||
| Aetiology | Alcoholic | 8 (341) | 0.46 (0.29 to 0.74) | P = 0.56 |
| Mixed | 12 (1078) | 0.56 (0.38 to 0.82) | ||
| Intervention | Only granulocyte colony‐stimulating factor | 14 (1075) | 0.55 (0.38 to 0.81) | P = 0.53 |
| Staminal cells infusion | 2 (113) | 1.43 (0.12 to 17.23) | ||
| Other | 4 (205) | 0.50 (0.29 to 0.86) | ||
| Geographic location* (continent) | Asia | 15 (1036) | 0.47 (0.38 to 0.59) | P < 0.00001 |
| Europe | 4 (349) | 1.43 (1.11 to 1.84) | ||
| Risk of bias | Low | 2 (75) | 0.46 (0.28 to 0.76) | P = 0.52 |
| High | 18 (1344) | 0.56 (0.40 to 0.78) | ||
| Follow‐up length | Short (≤ 3 months) | 13 (595) | 0.56 (0.40 to 0.77) | P = 0.76 |
| Long (> 3 months) | 7 (824) | 0.50 (0.27 to 0.) | ||
| Acute‐on‐chronic liver failure (ACLF) | ACLF | 12 (693) | 0.59 (0.40 to 0.87) | P = 0.28 |
| No ACLF | 8 (726) | 0.45 (0.33 to 0.61) | ||
| Sensitivity analyses | ||||
| Only trials at low risk of bias | 2 (75) | 0.46 (0.28 to 0.76) | ‐ | |
| Only full text | 17 (1290) | 0.50 (0.35 to 0.72) | ‐ | |
| Meta‐analysis model: fixed‐effect | 20 (1419) | 0.62 (0.54 to 0.71) | ‐ | |
| Missing data: "best‐worst case scenario" | 20 (14019) | 0.49 (0.36 to 0.65) | ‐ | |
| Missing data: "worst‐best case scenario" | 20 (1419) | 0.61 (0.45 to 0.84) | ‐ | |
*this subgroup comparison was planned posthoc at the review stage.
We could not perform subgroup analyses of the following.
Overall risk of bias: we assessed all trials to be at high risk of bias. However, we performed a subgroup analysis with risk of bias at an outcome level (Analysis 1.2).
Funding: none of the trials that reported on funding mentioned that the trial was sponsored by investors with vested interests.
Trials including only decompensated chronic liver disease compared to trials including compensated chronic liver disease: only one trial included 30% (24/81) of participants with decompensated cirrhosis (Newsome 2018).
Trials using the recommended dosage of G‐CSF for peripheral cell mobilisation (5 μg/kg daily for at least five consecutive days) compared to trials using lower dosages: no trial used lower dosages.
Trials using a daily dosage of G‐CSF > 10 μg/kg daily compared to trials using a lower dosage: only one trial used a higher dosage (15 μg/kg per five days) (Newsome 2018).
1.2. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 2: Mortality (subgroup analysis): risk of bias
We could perform subgroup analyses of the following.
Risk of bias: at an outcome level, we assessed two trials as being at low risk of bias for all cause‐mortality (Garg 2012; Shasthry 2019), and the remaining 18 trials as at high risk of bias. The subgroup analysis showed no subgroup differences (Analysis 1.2).
Trials using only G‐CSF compared to trials combining G‐CSF with other medical interventions: the subgroup analysis showed no subgroup differences (Analysis 1.3).
Trials using only G‐CSF compared to trials combining G‐CSF with stem or progenitor cell infusion; the subgroup analysis showed no subgroup differences (Analysis 1.3).
Trials including only or mainly participants with alcoholic liver disease compared to trials including only or mainly participants with other liver diseases: the subgroup analysis showed no subgroup differences (Analysis 1.4).
Trials including only participants with acute‐on‐chronic liver failure compared to trials excluding participants with acute‐on‐chronic liver failure: the subgroup analysis showed no subgroup differences (Analysis 1.5).
Trials using short‐term treatment schedules, shorter than seven days (short‐term), compared to trials using longer treatment schedules (long‐term): the subgroup analysis showed no subgroup differences (Analysis 1.6).
Trials with a length of follow‐up equal to or less than three months compared to trials with a length of follow‐up of more than three months: the subgroup showed no subgroup differences (Analysis 1.7).
Trial location hypothesising population differences: trials conducted in Asia compared to trials conducted in Europe, America, and Africa (posthoc analysis). The subgroup analysis showed different results between subgroups defined according to trial location (15 trials, with 1036 participants, conducted in Asia: RR 0.47, 95% CI 0.38 to 0.59; four trials, with 349 participants, conducted in Europe: RR 1.43, 95% CI 1.11 to 1.84) (Analysis 1.8).
1.3. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 3: Mortality (subgroup analysis) based on G‐CSF alone or with other treatments
1.4. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 4: Mortality (subgroup analysis): ALD and other liver diseases
1.5. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 5: Mortality (subgroup analysis): ACLF and no ACLF
1.6. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 6: Mortality (subgroup analysis): short and long course
1.7. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 7: Mortality (subgroup analysis): long and short follow‐up
1.8. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 8: Mortality (subgroup analysis): based on continents
Sensitivity analyses
Excluding trials assessed at high risk of bias: considering only the two trials at low risk of bias, a total of 12/37 participants died in the G‐CSF group versus 27/38 in the control group, and the RR was 0.46 (95% CI 0.28 to 0.76) (Garg 2012; Shasthry 2019).
Excluding trials published only in abstract or letter form: considering only trials reported in full text (i.e. excluding the three trials published as abstracts (Morgan 2022; Sharma 2017; Singh 2021), we found a RR of 0.50 (95% CI 0.35 to 0.72).
Repeating the analysis with the fixed‐effect model: the results obtained with the fixed‐effect meta‐analysis (RR 0.62, 95% CI 0.54 to 0.71; I2 = 75%) confirmed the results obtained with the random‐effects model.
'Extreme‐case scenario' analyses: in the 'best‐worst‐case scenario' analysis, i.e. favouring the experimental intervention, we found a RR of 0.49 (95% CI 0.36 to 0.65) (Analysis 1.10). In the 'extreme‐case worst‐best‐case scenario' analysis, i.e. favouring the control intervention, we found a RR of 0.61 (95% CI 0.45 to 0.84) (Analysis 1.11). The two 'extreme‐case scenario' analyses showed that incomplete outcome data did not influence our results.
Assessment of imprecision with Trial Sequential Analysis: Trial Sequential Analysis showed that the required information size was 11,216 participants (details in Figure 5). The cumulative number of participants enroled in the trials included in this meta‐analysis was 1419, corresponding to 12.7% of the required information size. The cumulative Z‐curve did not cross the trial sequential monitoring boundaries (Figure 5). The results of the Trial Sequential Analysis do not support the finding of the conventional meta‐analysis, and we downgraded for imprecision by two levels.
1.10. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 10: Mortality (sensitivity analysis): best/worst case scenario
1.11. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 11: Mortality (sensitivity analysis): worst/best case scenario
5.

All‐cause mortality. Twenty trials (1419 participants) provided data. The diversity‐adjusted required information size (DARIS) was calculated based on all‐cause mortality of 44.3% in the control group; relative risk reduction (RRR) in the G‐CSF group of 15%; type I error of 2.5%; type II error of 10% (90% power). Trial diversity was 75.79%. The required information size was 11,216 participants. The blue line represents the cumulative Z‐score of the cumulative meta‐analysis. The green dotted lines show the conventional boundaries of the nominal alpha level of 5%. The two pieces of red inward‐sloping lines represent the trial sequential monitoring boundaries.
We did not perform a sensitivity analysis on excluding trials not reporting intention‐to‐treat analyses, as all included trials reported intention‐to‐treat analysis results.
Proportion of participants with one or more serious adverse events
Serious adverse events were inconsistently reported. Eight trials, with 518 participants, reported no serious adverse events (De 2021; Garg 2012; Kedarisetty 2015; Shasthry 2019; Singh 2014; Tong 2022; Venkitaraman 2022; Verma 2018a). Three trials, with 315 participants, reported a total of 82/170 (48.2%) participants with one or more serious adverse events in the G‐CSF group versus 73/145 (50.3%) participants with serious adverse events in the control group; the meta‐analysis showed no difference between the G‐CSF group and the control group at follow up (4 months (mean), range 3 to 6) (RR 1.03, 95% CI 0.66 to 1.61; I2 = 66%; very low‐certainty evidence) (Engelmann 2021a; Newsome 2018; Spahr 2013) (Analysis 1.12). The remaining nine trials reported no information on serious adverse events (Duan 2013; Haque 2020; Morgan 2022; Prajapati 2017; Saha 2017; Sharma 2017; Singh 2018b; Singh 2021; Spahr 2008).
1.12. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 12: Serious adverse events
Risk of bias
We assessed the three trials that reported this outcome result as at high risk of bias (Engelmann 2021a; Newsome 2018; Spahr 2013).
GRADE
We downgraded the certainty of the evidence by three levels: one level for risk of bias, one level for heterogeneity (I2 = 66%), and one level for imprecision (because of the very small sample size: 315 participants), and we graded the certainty of evidence as very low (Table 1).
Assessment of heterogeneity and sensitivity analyses
We could not perform neither assessment of heterogeneity nor sensitivity analyses because of the paucity of data in the included trials.
Health‐related quality of life
Health‐related quality of life was inconsistently reported. Two trials, with 165 participants, reported data on the change of the physical component summary (from baseline to 12 months of follow‐up) and of the mental component summary of the quality of life score (which ranges from 0 to 100, with higher scores indicating better quality of life) (De 2021; Verma 2018a). The meta‐analysis showed that G‐CSF seemed to improve health‐related quality of life in both components. Concerning the physical component summary, the mean increase from baseline was 20.7 (95% CI 17.4 to 24.0; 165 participants; two trials; very low‐certainty evidence; Analysis 1.13); concerning the mental component summary, the mean increase from baseline was 27.8 (95% CI 12.3 to 43.3; 165 participants; two trials; very low‐certainty evidence; Analysis 1.13).
1.13. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 13: Quality of life: PCS or MCS ‐ change from baseline
Risk of bias
We assessed both trials reporting on this outcome as at high risk of bias (De 2021; Verma 2018a).
GRADE
We downgraded the certainty of the evidence by three levels: one level for risk of bias and two levels for imprecision (because of the very small sample size: 165 participants), and we graded the certainty of evidence as very low (Table 1).
Assessment of heterogeneity and sensitivity analyses
We could not perform neither assessment of heterogeneity nor sensitivity analyses because of the paucity of data in the included trials.
Secondary outcomes
Proportion of participants with liver disease‐related morbidity (i.e. who developed one or more liver‐related complications)
Four trials, with 195 participants, reported the proportion of participants with liver disease‐related morbidity (i.e. proportion of participants who underwent liver transplantation or developed one or more complications, such as ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, jaundice, or portal thrombosis) at follow‐up (7.2 months (mean), range 2 to 12) (Garg 2012; Kedarisetty 2015; Spahr 2008; Verma 2018a) (Table 2). These four trials reported a total of 18 events in the 108 (16.7%) participants in the G‐CSF group versus 41 events in the 87 (47.1%) participants in the control group. The meta‐analysis showed that G‐CSF seemed to reduce the proportion of participants with liver‐related morbidity (RR 0.40, 95% CI 0.17 to 0.92; I2 = 62%; very low‐certainty evidence) (Analysis 1.14).
1.14. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 14: Proportion of participants who developed one or more liver disease‐related complications
The remaining trials did not report the proportion of participants with complications but only the occurrence of a single complication, which prevented the per‐patient analysis of the composite outcome. We then performed analyses using the occurrence of a single complication as the unit of analysis (Differences between protocol and review).
Five trials, with 692 participants, reported the number of participants who underwent liver transplantation during the follow‐up period: a total of 15 transplants in 369 (4.1%) participants in the G‐CSF group versus 13 transplants in 323 (4.0%) participants in the control group; the meta‐analysis suggested no difference in effect between the experimental and control groups (RR 0.85, 95% CI 0.39 to 1.85) (Engelmann 2021a; Prajapati 2017; Newsome 2018; Tong 2022; Verma 2018a) (Analysis 1.15).
1.15. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 15: Othotopic liver transplantation
Five trials, with 520 participants, reported the development of hepatorenal syndrome in 90 out of 273 (32.0%) participants in the G‐CSF group versus 109 out of 247 (44.1%) participants in the control group; the meta‐analysis suggested no difference in effect between the experimental and control groups (RR 0.65, 95% CI 0.33 to 1.30) (Engelmann 2021a; Garg 2012; Newsome 2018; Sharma 2017; Tong 2022) (Analysis 1.16). Eight trials, with 614 participants, reported variceal bleeding in 18 out of 334 (5.4%) participants in the G‐CSF group versus 24 out of 280 (8.6%) participants in the control group; the meta‐analysis suggested no difference in effect between the experimental and control groups (RR 0.68, 95% CI 0.37 to 1.23) (De 2021; Engelmann 2021a; Garg 2012; Kedarisetty 2015; Newsome 2018; Spahr 2008; Venkitaraman 2022; Verma 2018a) (Analysis 1.17). Seven trials, with 605 participants, reported the development of encephalopathy in 87 out of 302 (28.8%) participants in the G‐CSF group versus 119 out of 303 (39.3%) participants in the control group; the meta‐analysis suggested no difference in effect between the experimental and control groups (RR 0.56, 95% CI 0.31 to 1.01) (De 2021; Engelmann 2021a; Garg 2012; Kedarisetty 2015; Sharma 2017; Tong 2022; Venkitaraman 2022) (Analysis 1.18). Eight trials, with 583 participants, reported the development of infections (including sepsis) in 121 out of 305 (39.7%) participants in the G‐CSF group versus 169 out of 278 (60.8%) participants in the control group; the meta‐analysis suggested benefit of the experimental treatment on sepsis (RR 0.50, 95% CI 0.29 to 0.84) (De 2021; Engelmann 2021a; Garg 2012; Kedarisetty 2015; Sharma 2017; Spahr 2008; Venkitaraman 2022; Verma 2018a) (Analysis 1.19). Two trials reported the development of hepatocellular carcinoma (HCC) during follow‐up: Kedarisetty 2015 found one HCC in 29 participants (3.4%) in the G‐CSF group and no HCC in 26 participants in the control group; Spahr 2013 found no HCC in neither the 28 participants in the G‐CSF group nor in the 26 participants in the control group.
1.16. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 16: Hepatorenal syndrome
1.17. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 17: Variceal bleeding
1.18. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 18: Encephalopathy
1.19. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 19: Infection (including sepsis)
The certainty of evidence for all the presented results is very low.
Risk of bias
We judged all four trials reporting the proportion of participants with liver disease‐related morbidity as at high risk of bias (Garg 2012; Kedarisetty 2015; Spahr 2008; Verma 2018a).
GRADE
We downgraded the certainty of the evidence by three levels: one level for risk of bias, one level for heterogeneity (I2 = 62%), and one level for imprecision (because of the very small sample size, 195 participants). We graded the certainty of evidence as very low (Table 1).
Proportion of participants with adverse events considered to be non‐serious
One trial reported this outcome in 26 out of 88 participants (29.5%) in the G‐CSF group and in 31 out of 88 participants (35.2%) in the control group (RR 0.84, 95% CI 0.55 to 1.29) (Engelmann 2021a) (Analysis 1.20). Five trials reported data on this outcome only in the G‐CSF group; the following proportions of participants had adverse events: De 2021, 37/50; Kedarisetty 2015, 11/29; Tong 2022, 1/56; Venkitaraman 2022, 23/33; Verma 2018a, 33/44.
1.20. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 20: Proportion of participants with adverse events considered to be non‐serious
Risk of bias
We judged all trials reporting data on non‐serious adverse events as at high risk of bias (De 2021; Engelmann 2021a; Kedarisetty 2015; Tong 2022; Venkitaraman 2022; Verma 2018a).
Proportion of participants without improvement in liver function scores
Two trials, with 319 participants, reported the number of participants without improvement in liver function, as judged by the worsening of the Child‐Turcotte‐Pugh score: the score worsened in 58 out of 159 participants (36.4%) in the G‐CSF group compared with 86 out of 160 participants (53.8%) in the control group; the meta‐analysis showed RR 0.67, 95% CI 0.53 to 0.86 (Analysis 1.21) (Prajapati 2017; Venkitaraman 2022).
1.21. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 21: Proportion of participants without improvement in liver function scores
Nine trials, with 452 participants, reported only the difference in the mean Child‐Turcotte‐Pugh score (De 2021; Duan 2013; Garg 2012; Kedarisetty 2015; Saha 2017; Shasthry 2019; Singh 2014; Spahr 2008; Verma 2018a), and 12 trials, with 628 participants, reported only the difference in the mean Model for End‐Stage Liver Disease (MELD) score, without any further information on standard error, which prevented us calculating a summary result (De 2021; Duan 2013; Garg 2012; Saha 2017; Sharma 2017; Shasthry 2019; Singh 2014; Singh 2018b; Spahr 2008; Spahr 2013; Venkitaraman 2022; Verma 2018a). We showed the estimated mean difference in every single study in Table 2.
One trial, available in abstract form, reported no difference in both Child‐Turcotte‐Pugh score and MELD score, without any further specification (Singh 2021).
Risk of bias
We assessed the two trials reporting data on this outcome as at high risk of bias (Prajapati 2017; Venkitaraman 2022).
Discussion
Summary of main results
We included 20 trials, which randomised a total of 1419 participants (739 to granulocyte colony‐stimulating factor (G‐CSF) alone or in combination with growth hormone, erythropoietin, N‐acetyl cysteine, infusion of CD133‐positive haemopoietic stem cells, or infusion of autologous bone marrow mononuclear cells, and 681 participants to no intervention or placebo). All included trials reported data on mortality, whereas there were limited data on the remaining clinical outcomes, i.e. quality of life, serious and non‐serious adverse events, liver disease‐related morbidity, and absence of improvement of liver function. For the outcome 'All‐cause mortality', 18 of 20 trials were at high risk of bias, whereas for all the other outcomes, all 20 trials were at high risk of bias. The evidence is very uncertain about the effect of G‐CSF administered alone or in combination with growth factors (erythropoietin or growth hormone) showing a beneficial effect with a reduction of all‐cause mortality (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.38 to 0.72). Serious adverse events were either poorly reported or the information was unclear. Nine trials reported no information on adverse events, eight trials reported no serious adverse events, and three trials showed a similar proportion of participants with serious adverse events in the two groups (RR 1.03, 95% CI 0.66 to 1.61; very low‐certainty evidence). Data on the other outcomes, such as health‐related quality of life, disease‐related morbidity, non‐serious adverse events, and improvement in liver function, were inconsistently reported in few trials, which prevented a summary of the results.
We summarise our main results in Table 1.
Overall completeness and applicability of evidence
We searched for published and unpublished trials irrespective of trial design, publication type, publication status, publication date, and language. We also searched bibliographies of systematic and non‐systematic reviews for any trials we could have missed. We are confident that the search strategy resulted in the detection of most eligible studies, with a low probability of undetected relevant studies. We included 20 trials published between 2008 and 2022. Most trials (15/20) were conducted in Asia (11 in India), Europe (four), and North America (one). No trials were conducted in Africa, Australia, and South America. The visual inspection of the funnel plot for all‐cause mortality suggests possible asymmetry arising from data missing from the bottom left of the distribution (Figure 4). However, on formal analysis, we have not found evidence of asymmetry. Although in our analysis data from smaller trials (presumably showing beneficial effects of G‐CSF) may be missing, the impact of missing data on the results is negligible. Similar results obtained with the random‐effects model (RR 0.53, 95% CI 0.38 to 0.72) and fixed‐effect model (RR 0.62, 95% CI 0.54 to 0.71) do not indicate that small‐study effects exaggerate results of the primary analysis.
There were intertrial differences in the severity and aetiology of the liver disease. Nineteen trials included only participants with decompensated cirrhosis, and in one trial, 30% of the participants had compensated cirrhosis. Eight trials excluded participants with acute‐on‐chronic liver failure, and 12 trials included only participants with acute‐on‐chronic liver failure. Concerning the aetiology, some trials included only participants with alcoholic liver disease, others only participants with chronic hepatitis B, and others with a mix of different aetiologies. Therefore, the findings of this review are applicable only to adults with decompensated cirrhosis, independent of different aetiologies, and those with acute‐on‐chronic liver failure.
Quality of the evidence
We have assessed the quality of the evidence, i.e. certainty of evidence, for the results of four outcomes: all‐cause mortality, proportion of participants with one or more serious adverse events, health‐related quality of life, and proportion of participants with liver disease‐related morbidity (Table 1).
The GRADE assessments showed that the certainty of evidence was very low for mortality (due to risk of bias, heterogeneity, and imprecision). Even if we consider mortality, but not the other outcomes, as an outcome that is robust to detection bias (Savović 2012a; Savović 2012b; Savović 2018), potential biases are revealed in most included trials, particularly the three trials with the highest weight (De 2021; Engelmann 2021a; Prajapati 2017). Some trials reported no information on allocation concealment, and even in the absence of differences between intervention and control groups, we cannot exclude the possibility of selection bias. In addition, some trials were open trials, without blinding; hence, we judged them at high risk of bias for this domain. Most trials performed intention‐to‐treat analyses, and only one trial used a "modified intention‐to‐treat analysis" (Newsome 2018). However, the trial reported complete data, allowing the intention‐to‐treat analysis. Lastly, we judged six trials at high risk for attrition bias as there were postrandomisation dropouts probably related to the outcomes.
We found substantial heterogeneity (I2 =75%), and the planned subgroup analyses showed no differences (Table 3). Considering the possible role of different trial locations and the inclusion of more severely diseased participants, we performed two unplanned (posthoc) comparisons. We found no difference between trials including only participants with acute‐on‐chronic liver failure compared to trials excluding participants with acute‐on‐chronic liver failure. Conversely, we found conflicting results in trials conducted in Europe when compared to trials conducted in Asia. The latter showed a clear benefit, contrary to trials conducted in Europe that showed a possible harm of G‐CSF. However, we were unable to further characterise these discrepancies. The characteristics of participants seemed to be similar concerning the severity of liver disease when assessed at trial level. The Model for End‐Stage Liver Disease (MELD) score, the Child‐Turcotte‐Pugh score, and also the mortality in the control groups were equivalent. The intervention and the definition of the outcomes were completely equivalent; therefore, we downgraded the certainty of evidence by one level for heterogeneity. Also, we downgraded imprecision by one level as the optimal information size criterion was not met. Interestingly, even the Trial Sequential Analysis showed that the cumulative number of participants enroled in the trials corresponds to only 12.7% of the diversity‐adjusted required information size (DARIS), and the cumulative Z‐curve did not cross the trial sequential monitoring boundaries.
We did not downgrade for indirectness as, considering the review's clinical question, we found no relevant differences in population, intervention, comparator, or outcomes.
The other outcomes (i.e. proportion of participants with one or more serious adverse events, health‐related quality of life, proportion of participants with liver disease‐related morbidity, proportion of participants with adverse events considered to be non‐serious, and proportion of participants without improvement of liver function scores) were reported in a few trials, and we judged the certainty of evidence as very low. In addition to the above detailed risks of selection, performance, and attrition bias, most trials did not document the blinding assessment of these outcomes, which clearly involves a judgement. Therefore, we downgraded the certainty of evidence for risk of bias because of blinding. Furthermore, we downgraded the certainty of evidence for heterogeneity and also for imprecision, considering the paucity of data, their substantial heterogeneity, and the width of the CIs.
Potential biases in the review process
Strengths
We searched for and included trials regardless of trial design, publication type, publication status, language, and choice of outcomes; therefore, we consider it unlikely that we missed any published trials that could have been included in our review. We included trials with a wide inclusion criteria for participants, encompassing all aetiologies and different severity of liver disease, in order to assess the effect of the intervention in all clinical conditions of interest. We chose all‐cause mortality as the primary outcome in our review; all included trials reported data on this outcome. The mortality outcome is considered the most optimal patient‐relevant outcome with the fewest methodological limitations amongst the remaining clinically relevant outcomes (Garattini 2016). We tested the robustness of our results with sensitivity analyses ('best‐worst case scenario' and 'worst‐best case scenario') and used Trial Sequential Analysis; we adjusted our thresholds for significance to control the risks of random errors (Jakobsen 2014; Wetterslev 2017).
Limitations
Our systematic review has several limitations. Despite an important reduction of all‐cause mortality with G‐CSF, the certainty in this result is impaired by the overall high risk of bias, substantial heterogeneity, and imprecision. Our risk of bias assessment showed that the majority of trials, i.e. 18 out of 20, were at high risk of bias. It is, therefore, probable that our results are also biased, that is, there is a great risk that our results overestimate benefits and underestimate harms (Jakobsen 2014; Savović 2012a; Savović 2012b). We found a substantial heterogeneity that remains unexplained by our planned subgroup analyses. Most trials were conducted in Asia, with those trial results opposing the results of the few trials conducted in Europe. This discrepancy could depend on participant selection, as both the intervention and the outcomes were consistent between trials. However, we were unable to find any other disparities in the included participants. To note, we investigated only characteristics that could be assessed by aggregate statistics at a study level, with inherent risk of ecological bias. If participants' individual data were available, we could potentially have made our exploration more informative.
Another limitation is that only a few trials assessed the remaining two primary outcomes, serious adverse events and health‐related quality of life, as well as the secondary outcomes liver disease‐related morbidity, non‐serious adverse events, and failure to improve liver function scores, and all of these outcomes were inconsistently reported.
We did not plan to specifically search for observational studies for inclusion in this review, which is a known limitation of the study in terms of adverse events. We are aware that the decision to not search systematically for all observational studies and to extract data on harm only from quasi‐randomised and controlled clinical studies might bias our review towards the assessment of benefits and might overlook certain harms, such as late or rare harms (Storebø 2018).
Finally, the total number of included participants was lower than the GRADE optimal information size, which limits our conclusions as we do not have enough information to confirm or refute our anticipated clinical intervention effects. The Trial Sequential Analysis confirmed and extended this limitation and showed that the cumulative number of participants enroled in the trials corresponded to only 12.7% of the diversity‐adjusted required information size (DARIS), and the cumulative Z‐curve did not cross the trial sequential monitoring boundaries.
Agreements and disagreements with other studies or reviews
A 2022 systematic review found 17 trials (15 included in our review and two available only in Chinese and awaiting classification) and reports results comparable to ours: an improved survival with substantial heterogeneity (RR 1.46, 95% CI 1.21 to 1.76; I2 =79%) (Shi 2022). By excluding the trials conducted in Europe (Engelmann 2021a), sensitivity analyses showed that "the heterogeneity amongst the remaining 16 Asian trials was eliminated".
Some reviews included only trials in people with acute‐on‐chronic liver failure. We found four systematic reviews with meta‐analysis assessing G‐CSF in people with acute‐on‐chronic liver failure (Chavez‐Tapia 2015; Hou 2021; Huang 2021; Yang 2016). These studies included few studies (two to seven) and reported conflicting results.
Another review included only trials in people with alcoholic hepatitis (Marot 2020). This review with meta‐analysis included seven trials, with 396 participants with alcoholic hepatitis, and showed a reduced risk of death at 90 days (odds ratio (OR) 0.28, 95% CI 0.09 to 0.88) with substantial heterogeneity (I2 = 80%). Different study locations were given as a possible reason for the substantial heterogeneity: the trials conducted in Asia showed a reduced risk of death (OR 0.15, 95% CI 0.08 to 0.28), whereas the trials conducted in Europe showed that G‐CSF tended to increase mortality (OR 1.89, 95% CI 0.90 to 3.98) (Marot 2020). These results are in complete agreement with those we obtained in the subgroup of trials including participants with alcoholic liver disease. We also included trials that included participants with other aetiologies of decompensated cirrhosis, and the results were again quite similar, suggesting that the aetiology is not a determinant of treatment response. The Engelmann 2021b review, a narrative review conducted by the author of one of the most weighted trials included in our meta‐analysis (Engelmann 2021a), was unable to explain the discrepancy in the results between Asian studies and European studies, because there was a great overlap regarding disease aetiology, severity of the liver disease, G‐CSF dosage, and timing of G‐CSF administration. This review reported and discussed the results obtained by the GRAFT study (Engelmann 2021a) and seven other trials, of which we have included five (De 2021; Kedarisetty 2015; Newsome 2018; Prajapati 2017; Verma 2018a). We excluded the other two, as one was not a randomised trial (Philips 2020), and one compared G‐CSF plus erythropoietin to G‐CSF alone (Anand 2019).
Authors' conclusions
Implications for practice.
Granulocyte colony‐stimulating factor (G‐CSF), alone or in combination, seems to decrease mortality in people with decompensated advanced chronic liver disease of whatever aetiology and with or without acute‐on‐chronic liver failure. However, the certainty of evidence is very low because of high risk of bias, inconsistency, and imprecision. The results of trials conducted in Asia and Europe were discrepant; this could not be explained by differences in the selection of participants, interventions, and outcome measurement. Data on serious adverse events and health‐related quality of life were few and inconsistently reported. The evidence is also very uncertain about the occurrence of one or more liver disease‐related complications. We lack high‐quality, global randomised clinical trials assessing the effect of G‐CSF on clinically relevant outcomes.
Implications for research.
As there could be some people with decompensated chronic liver disease, severe alcoholic hepatitis, and acute‐on‐chronic liver failure who might benefit from G‐CSF, further randomised clinical trials assessing the effect of G‐CSF on short‐term all‐cause mortality are needed for a higher level of certainty. Future randomised clinical trials, hopefully to be conducted in countries all over the world, ought to report individual participant data, so that proper individual participant data meta‐analyses of the effects of G‐CSF in people with different aetiologies of decompensated or compensated disease can be conducted. Future randomised clinical trials ought to report outcome data on all participants and be designed according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement and reported according to the CONSORT (Consolidated Standards of Reporting Trials) statement.
History
Protocol first published: Issue 2, 2020
Acknowledgements
The following people from the Editorial Team Office of the Cochrane Hepato‐Biliary Group conducted the editorial process for this article.
Contact Editor (provided comments, checked the review): Christian Gluud, Denmark
Managing Editor (selected peer reviewers, provided comments, provided editorial guidance to authors, edited the article): Dimitrinka Nikolova, Cochrane Hepato‐Biliary Group, Denmark
Information Specialist (developed search strategies and trial search): Sarah Louise Klingenberg, Cochrane Hepato‐Biliary Group, Denmark
Peer reviewers: Robin M Featherstone, Canada (peer reviewer of the search review); Goran Bjelakovic, Serbia; Mario Masarone, Italy; Christian Gluud, Denmark (provided expert comments)
The following people from the Cochrane Central Editorial Service supported the production of his review.
Evidence Synthesis Development Editor: Leslie Choi, Evidence Production and Methods Department, Cochrane, UK
Sign‐off Editor (provided final approval for publication): Ronald Koretz, Cochrane Hepato‐Biliary Group, USA
Copy‐Editor (copy‐editing and production): Laura Prescott, Central Production Service, Cochrane, UK
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, the Capital Region of Denmark, Rigshospitalet, Copenhagen, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register (via the Cochrane Register of Studies Web) | 4 October 2022 | (colony‐stimulating factor or G‐CSF or GCSF or CSF3 OR CSF 3 or filgrastim or lenograstim) and (liver or hepat* or cirrho* or fibrosis) |
| Cochrane Central Register of Controlled Trials in the Cochrane Library | 2022, Issue 10 | #1 MeSH descriptor: [Granulocyte Colony‐Stimulating Factor] explode all trees #2 colony‐stimulating factor or G‐CSF or GCSF or CSF3 OR CSF 3 or filgrastim or lenograstim #3 #1 or #2 #4 MeSH descriptor: [Liver Diseases] explode all trees #5 (liver or hepat* or cirrho* or fibrosis) #6 #4 or #5 #7 #3 and #6 |
| MEDLINE Ovid |
1946 to 4 October 2022 | 1. exp Granulocyte Colony‐Stimulating Factor/ 2. (colony‐stimulating factor or G‐CSF or GCSF or CSF3 or CSF 3 or filgrastim or lenograstim).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 3. 1 or 2 4. exp Liver Diseases/ 5. (liver or hepat* or cirrho* or fibrosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6. 4 or 5 7. 3 and 6 8. (randomized controlled trial or controlled clinical trial).pt. or clinical trials as topic.sh. or trial.ti. 9. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 10. 7 and (8 or 9) |
| Embase Ovid |
1974 to 4 October 2022 | 1. exp granulocyte colony stimulating factor/ 2. (colony‐stimulating factor or G‐CSF or GCSF or CSF3 or CSF 3 or filgrastim or lenograstim).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] 3. 1 or 2 4. exp liver disease/ 5. (liver or hepat* or cirrho* or fibrosis).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] 6. 4 or 5 7. 3 and 6 8. Randomized controlled trial/ or Controlled clinical study/ or randomization/ or intermethod comparison/ or double blind procedure/ or human experiment/ or retracted article/ 9. (random$ or placebo or parallel group$1 or crossover or cross over or assigned or allocated or volunteer or volunteers).ti,ab. 10. (compare or compared or comparison or trial).ti. 11. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 12. (open adj label).ti,ab. 13. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (controlled adj7 (study or design or trial)).ti,ab. 16. (erratum or tombstone).pt. or yes.ne. 17. or/8‐16 18. (random$ adj sampl$ adj7 ('cross section$' or questionnaire$ or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 19. Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 20. (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 21. (Systematic review not (trial or study)).ti. 22. (nonrandom$ not random$).ti,ab. 23. 'Random field$'.ti,ab. 24. (random cluster adj3 sampl$).ti,ab. 25. (review.ab. and review.pt.) not trial.ti. 26. 'we searched'.ab. and (review.ti. or review.pt.) 27. 'update review'.ab. 28. (databases adj4 searched).ab. 29. (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 30. Animal experiment/ not (human experiment/ or human/) 31. or/18‐30 32. 17 not 31 33. 7 and 32 |
| LILACS (Bireme) |
1982 to 4 October 2022 | (colony‐stimulating factor or G‐CSF or GCSF or CSF3 OR CSF 3 or filgrastim or lenograstim) [Words] and (liver or hepat$ or cirrho$ or fibrosis) [Words] |
| BIOSIS (Web of Science) | 1969 to 4 October | #3 #2 AND #1 #2 TS=(advanc* and chronic and (liver* or hepat*)) #1 TS=(colony‐stimulating factor or G‐CSF or GCSF or CSF3 OR CSF 3 or filgrastim or lenograstim) |
| Science Citation Index Expanded (Web of Science) | 1900 to 4 October 2022 | #5 #4 AND #3 #4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(liver or hepat* or cirrho* or fibrosis) #1 TS=(colony‐stimulating factor or G‐CSF or GCSF or CSF3 or CSF 3 or filgrastim or lenograstim) |
| Conference Proceedings Citation Index (Web of Science) | 1990 to 4 October 2022 | #5 #4 AND #3 #4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(liver or hepat* or cirrho* or fibrosis) #1 TS=(colony‐stimulating factor or G‐CSF or GCSF or CSF3 or CSF 3 or filgrastim or lenograstim) |
Data and analyses
Comparison 1. Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.38, 0.72] |
| 1.2 Mortality (subgroup analysis): risk of bias | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.40, 0.75] |
| 1.2.1 Low risk of bias | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.28, 0.76] |
| 1.2.2 High risk of bias | 18 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.78] |
| 1.3 Mortality (subgroup analysis) based on G‐CSF alone or with other treatments | 20 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.38, 0.73] |
| 1.3.1 Only G‐CSF | 14 | 1075 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.38, 0.81] |
| 1.3.2 G‐CSF plus stem or progenitor cell infusion | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.12, 17.23] |
| 1.3.3 G‐CSF plus additional treatment | 4 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.17, 0.87] |
| 1.4 Mortality (subgroup analysis): ALD and other liver diseases | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.38, 0.72] |
| 1.4.1 ALD | 8 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.74] |
| 1.4.2 Other liver diseases | 12 | 1078 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.38, 0.82] |
| 1.5 Mortality (subgroup analysis): ACLF and no ACLF | 20 | 1434 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 1.5.1 ACLF | 12 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 1.5.2 No ACLF | 8 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.32, 0.63] |
| 1.6 Mortality (subgroup analysis): short and long course | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.73] |
| 1.6.1 Short course < 7 days | 12 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.78] |
| 1.6.2 Long course > 7 days | 8 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.29, 0.87] |
| 1.7 Mortality (subgroup analysis): long and short follow‐up | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.74] |
| 1.7.1 Follow‐up: less than 3 months | 13 | 595 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.77] |
| 1.7.2 Follow‐up: more than 3 months | 7 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.91] |
| 1.8 Mortality (subgroup analysis): based on continents | 19 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.36, 0.69] |
| 1.8.1 Asia | 15 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.38, 0.59] |
| 1.8.2 Europe | 4 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.11, 1.84] |
| 1.9 Mortality (sensitivity analysis): only full‐text publications | 17 | 1290 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.35, 0.72] |
| 1.10 Mortality (sensitivity analysis): best/worst case scenario | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.36, 0.65] |
| 1.11 Mortality (sensitivity analysis): worst/best case scenario | 20 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.84] |
| 1.12 Serious adverse events | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.66, 1.61] |
| 1.13 Quality of life: PCS or MCS ‐ change from baseline | 2 | 330 | Mean Difference (IV, Random, 95% CI) | 23.56 [18.55, 28.58] |
| 1.13.1 PCS | 2 | 165 | Mean Difference (IV, Random, 95% CI) | 20.69 [17.42, 23.96] |
| 1.13.2 MCS | 2 | 165 | Mean Difference (IV, Random, 95% CI) | 27.79 [12.28, 43.29] |
| 1.14 Proportion of participants who developed one or more liver disease‐related complications | 4 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.17, 0.92] |
| 1.15 Othotopic liver transplantation | 5 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.39, 1.85] |
| 1.16 Hepatorenal syndrome | 6 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.33, 1.30] |
| 1.17 Variceal bleeding | 8 | 614 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.37, 1.23] |
| 1.18 Encephalopathy | 7 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.01] |
| 1.19 Infection (including sepsis) | 8 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.29, 0.84] |
| 1.20 Proportion of participants with adverse events considered to be non‐serious | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.29] |
| 1.21 Proportion of participants without improvement in liver function scores | 2 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.53, 0.86] |
1.9. Analysis.

Comparison 1: Granulocyte colony‐stimulating factor (G‐CSF) with or without stem or progenitor cell infusion versus placebo or no intervention for people with compensated or decompensated advanced chronic liver disease, Outcome 9: Mortality (sensitivity analysis): only full‐text publications
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De 2021.
| Study characteristics | ||
| Methods | Open‐label randomised controlled trial, conducted at a tertiary centre | |
| Participants | Country: India Period of recruitment: from July 2016 to June 2018 Number randomised: 100 Postrandomisation dropouts: 0 Revised sample size: 100 Average age (years): 49 Males: 85% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 0% Alcohol‐related cirrhosis: 48% Viral‐related cirrhosis: 21% Autoimmune disease‐related cirrhosis: 4% Other causes of cirrhosis: 27% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included nutritional support, rifaximin, lactulose or lactitol, albumin, diuretics, prophylaxis for spontaneous bacterial peritonitis and variceal bleed, multivitamins, calcium, and vitamin D supplementation. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: the authors disclosed no conflicts. Funding: supported in part by the Society for the Study of Liver Diseases ClincialTrials.gov number: NCT03415698 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were numbered sequentially and allocated randomly (1:1) using computer‐generated random number tables provided by an independent statistician. |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The questionnaire was administered in English or Hindi by a Research Fellow (AK) who was blinded to the patients’ group allotment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 participants were randomised and 92 were analysed. 4 participants, 2 in both groups, were lost to follow up: the other 4 dropouts were withdrawn for HCC or other cancer. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Duan 2013.
| Study characteristics | ||
| Methods | Double‐blind randomised controlled trial | |
| Participants | Country: China Period of recruitment: from June 2009 to May 2011 Number randomised: 55 Postrandomisation dropouts: 0 Revised sample size: 55 Average age (years): 45 Males: 80% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 0% Viral‐related cirrhosis: 100% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy: entecavir (0.5 mg/d, Squibb Pharmaceuticals Ltd., Shang Hai, China) and standard therapy (including reduced glutathione, glycyrrhizin, ademetionine, polyene phosphatidylcholine, alprostadil, and human serum albumin) on the day of admission |
|
| Outcomes |
|
|
| Notes | Conflicts of interest: no information provided Funding: "supported by National Natural Science Foundation of China, No. 81171641; the Army Medical and Health Scientific Research Fund of China, No. 06H057" No information on registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation number code was generated for each participant. Based on this code, each participant was assigned to receive G‐CSF therapy plus standard medical therapy (G‐CSF group), or standard medical therapy alone (control group). |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the study investigators and the participants were blinded to the study treatment through identical vials coded as A and B in the 2 groups, respectively. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fifty‐five participants were randomised and analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as the primary outcome. |
| Other bias | Low risk | We found no other bias. |
Engelmann 2021a.
| Study characteristics | ||
| Methods | Multicentre, prospective, open‐label, phase 2 randomised controlled trial | |
| Participants | Country: Germany Period of recruitment: from March 2016 to April 2019 Number randomised: 176 Postrandomisation dropouts: 28 Revised sample size: 176 Average age (years): 56 Males: 63% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: not reported (alcohol‐related precipitating events in 54% of participants, as reported in Table 2) Viral‐related cirrhosis: not reported Autoimmune disease‐related cirrhosis: not reported Other causes of cirrhosis: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. No details were provided on standard medical therapy. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: present and detailed Funding: The German Research Foundation (DFG) – EN 1100/1‐1, funded the study. Cornelius Engelmann is a participant in the BIH‐Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. Moritz Schmelzle and Katrin Splith received grants for ancillary studies funded by the German Research Foundation (DFG) (SCHM2661/ 3‐1, SCHM2661/3‐2). Jonel Trebicka is supported by grants from the German Research Foundation (DFG) (SFB TRR57 to P18), European Union’s Horizon 2020 Research and Innovation Programme (Galaxy, No. 668031 and MICROB‐PREDICT, No. 825694), and Societal Challenges ‐ Health, Demographic Change and Wellbeing (No. 731875), and Cellex Foundation (PREDICT). ClinicalTrials.gov number: NCT02669680 "The trial was prematurely terminated due to futility after the reported results of the planned interim analysis and conditional power calculation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1 ratio to receive either standard medical therapy (SMT) (SMT group) or recombinant G‐CSF (G‐CSF, Ratiograstim®) plus SMT (G‐CSF+SMT group). Participants were centrally randomised with a Web‐based system by using a minimisation algorithm with a factor for centre and a probability of 0.2 for random allocation, to avoid potential loss of allocation concealment within centres. |
| Allocation concealment (selection bias) | Low risk | Participants were centrally randomised with a Web‐based system by using a minimisation algorithm with a factor for centre and a probability of 0.2 for random allocation, to avoid potential loss of allocation concealment within centres. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "A placebo controlled trial was not considered feasible in this population." |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One hundred and seventy‐six participants were included and 148 were analysed. In the intervention arm: 10 dropouts (5 withdrawal of consent, 3 lost to follow‐up, and 2 for other reasons); in the control arm: 18 dropouts (14 withdrawal of consent, 4 lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Garg 2012.
| Study characteristics | ||
| Methods | Randomised clinical trial, monocentric | |
| Participants | Country: India Period of recruitment: between December 2008 and August 2010 Number randomised: 47 Postrandomisation dropouts: 0 Revised sample size: 47 Average age (years): 40 Males: 87% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 62% Viral‐related cirrhosis: 23% Other causes of cirrhosis: 15% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included as per requirement: lactulose, bowel wash, albumin, fresh frozen plasma, terlipressin, and antibiotics. Whenever needed, participants were supported with mechanical ventilation, vasopressors, and renal replacement therapy. Participants with reactivation of hepatitis B and alcoholic hepatitis were treated with tenofovir and pentoxifylline, respectively. Liver transplant and liver support devices could not be offered to the participants because they were not available at the centre. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: the authors disclosed no conflicts.of interest. Funding: no information ClincalTrials.gov number: NCT01036932 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within 48 hours of admission, the participants were enroled and randomised into 2 groups. A randomisation code was generated. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed with sequentially numbered envelopes to either standard medical therapy with or without G‐CSF, and the investigators as well as the participants were blinded to the treatment allotted. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants in group A received G‐CSF at a dose of 5 g/kg subcutaneously, 12 doses over a period of 1 month (daily doses for the first 5 days and then every third day), along with the standard medical therapy, and participants in group B received placebo (1 mL saline subcutaneously each time) along with the standard medical therapy. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Forty‐seven participants were included and analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Haque 2020.
| Study characteristics | ||
| Methods | Randomised clinical trial, monocentric | |
| Participants | Country: Bangladesh Period of recruitment: not reported Number randomised: 39 Postrandomisation dropouts: 0 Revised sample size: 39 Average age (years): 42 Males: 79% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 0% Viral‐related cirrhosis: 82% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 18% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical care included antiviral agents, intravenous antibiotics, supervised diet, lactulose, and close monitoring, as needed. Nasogastric feeding or parenteral nutrition was provided as and when required. |
|
| Outcomes |
|
|
| Notes | Conflicts of interest: none Funding: "Source of support: nil" No information on trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was given. Consecutive participants with ACLF were randomly assigned into group A and group B. |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no blinding. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants were analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as the primary outcome. |
| Other bias | Low risk | We found no other bias. |
Kedarisetty 2015.
| Study characteristics | ||
| Methods | Randomised clinical trial, monocentric | |
| Participants | Country: India Period of recruitment: May 2011 to June 2012 Number randomised: 55 Postrandomisation dropouts: 3 Revised sample size: 55 Average age (years): 45 Males: 53% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 0% Alcohol‐related cirrhosis: 64% Viral‐related cirrhosis: 6% Autoimmune disease‐related cirrhosis: excluded Other causes of cirrhosis: 25% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included albumin, diuretics, nutritional rehabilitation, b‐blockers and treatment based on aetiology, such as antivirals for hepatitis B. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: the authors disclosed no conflicts. Funding: Dr. Reddys’ Laboratories, Hyderabad, India, provided the generic drugs G‐CSF, DPO, and placebos; they provided no financial assistance of any kind. Clinicaltrials.gov number: NCT01384565 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The block randomisation was done with a block size of 10. An independent statistician, using a computer‐generated random number table, performed sequence generation. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done using a sequentially numbered, opaque, sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the study investigators and the participants were blinded to the study treatment through identical vials coded as A and B in the 2 groups, respectively. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three participants were lost at follow up in 1 group, but none were lost in the other group. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as the primary outcome. |
| Other bias | Low risk | We found no other bias. |
Morgan 2022.
| Study characteristics | ||
| Methods | Phase II, multicentre, open‐label randomised trial | |
| Participants | Country: USA Period of recruitment: not reported Number randomised: 34 Postrandomisation dropouts: 0 Revised sample size: 34 Average age (years): not reported Males: not reported Decompensated cirrhosis:100% Alcohol‐related cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy: prednisolone *Pegfilgrastim: a long‐acting recombinant G‐CSF |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: no information provided Funding: no information provided ClinicalTrials.gov number: NCT02776059 Published only in abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This open‐label trial randomised participants. No further information was given. |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All included participants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Newsome 2018.
| Study characteristics | ||
| Methods | Multicentre, open‐label, phase 2 randomised controlled trial; "protocol updated in March, 2015, to include the second coprimary outcome" | |
| Participants | Country: UK (3 hospitals) Period of recruitment: "Between May 18, 2010, and Feb 6, 2015, 153 patients with liver cirrhosis were screened, and 81 underwent randomisation (figure 1)." Number randomised: 81 Postrandomisation dropouts: 1 Revised sample size: 80 Average age (years): 55 Males: 65% Decompensated cirrhosis: 70% Acute‐on‐chronic liver failure: 0% Alcohol‐related cirrhosis: 47% Viral‐related cirrhosis: 12% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 41% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy for compensated cirrhosis Standard medical therapy could include disease‐specific medications and treatments for the complications of cirrhosis. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Conflicts of interest: "We declare no competing interests." Funding: National Institute of Health Research, The Sir Jules Thorn Charitable Trust Sample size performed, however, "The trial was powered to compare each treatment to control with respect to the first coprimary outcome, but was not powered to detect differences between the two treatment groups." Authors reported that 1 participant, randomly assigned to treatment with G‐CSF plus CD133‐positive cell infusion, died before receiving any treatment; we attributed the event to the intervention group according to the intention to treat analysis, differently from authors who classified it according to "as treated" analysis. "After randomisation, all patients returned for study visits at days 30, 60, 90, 180, and 360 (end of study)." Registered on Current Controlled Trials on November 18, 2009, ISRCTN (number 91288089), and the European Clinical Trials Database (number 2009‐010335‐41) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centre‐delegated staff telephoned a randomisation office at the Cancer Research UK Clinical Trials Unit (CRCTU; Birmingham, UK), who used a computer‐generated, centrally administered procedure to randomly assign eligible participants (1:1:1) to 1 of 3 treatments. |
| Allocation concealment (selection bias) | Low risk | Each participant was then allocated a unique patient trial number and scheduled for treatment and follow‐up visits. The local site staff could not predetermine treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 4 dropouts in 1 group, and none in the other groups. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Prajapati 2017.
| Study characteristics | ||
| Methods | Open‐label randomised controlled trial, single centre | |
| Participants | Country: India Period of recruitment: June 2014 and February 2016 Number randomised: 259 Postrandomisation dropouts: 6 Revised sample size: 253 Average age (years): 54 Males: 83% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure 0% Alcohol‐related cirrhosis 25% Viral‐related cirrhosis: 13% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 50% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included antivirals, abstinence from alcohol, nutrition, diuretics, β‐blockers, selective intestinal decontamination, and other supportive measures depending on clinical status and requirement. |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Conflicts of interest: "There are no conflicts of interest." Funding: no information ClinicalTrials.gov number: NCT02642003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence remained with the statistician. |
| Allocation concealment (selection bias) | Low risk | The sequence remained concealed from the investigators until the intervention was assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twenty participants were lost at follow up (treatment arm: 9, control arm: 11). |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Saha 2017.
| Study characteristics | ||
| Methods | Randomised open‐label trial | |
| Participants | Country: Bangladesh Period of recruitment: not reported Number randomised: 32 Postrandomisation dropouts: 0 Revised sample size: 32 Average age (years): 43 Males: 87.5% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 0% Viral‐related cirrhosis: 91% Autoimmune disease‐related cirrhosis: 3% Other causes of cirrhosis: 6% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical treatment included symptomatic treatment and general management of emergency patients. |
|
| Outcomes |
|
|
| Notes | Conflicts of interest: "There are no conflicts of interest." Funding: no information Trial registration: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was given. |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants were analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Sharma 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Country: India Period of recruitment: not reported Number randomised: 50 Postrandomisation dropouts: 0 Revised sample size: 50 Average age (years): not reported Males: not reported Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. No detail on standard medical therapy |
|
| Outcomes |
|
|
| Notes | Conflicts of interest: all authors declared no conflicts of interest. Funding: no information Trial registration: no information Published only in abstract format |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was given. |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All included participants were analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Shasthry 2019.
| Study characteristics | ||
| Methods | Double‐blind randomised controlled trial, single centre | |
| Participants | Country: India Period of recruitment: from March 2013 to June 2016 Number randomised: 28 Postrandomisation dropouts: 0 Revised sample size: 28 Average age (years): 40 Males: 96% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy: all participants also received conservative management in the form of diuretics, antibiotics, protein, and nutritional supplements per clinical requirements. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflict of interest: "nothing to report" Funding: no information ClinicalTrials.gov number: NCT01820208 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in 1:1 ratio. Sequence was generated using computer‐generated random numbers, which was kept with the trial co‐ordinator. |
| Allocation concealment (selection bias) | Low risk | The allocation was concealed in sealed opaque envelopes. The participants and the investigators were both blinded to the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patient and the investigators were both blinded to the allocation. The trial coordinator provided the prefilled syringes (0.5 mL) (stored in refrigerator at 2℃‐8 ℃) with either the drug (G‐CSF) or placebo (normal saline), labelled as drug A or B, to the patients. After discharge, patients collected the prefilled syringes (labelled drug A/B) from the coordinator on an outpatient basis." |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost at follow up. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Singh 2014.
| Study characteristics | ||
| Methods | Open‐label randomised pilot study | |
| Participants | Country: India Period of recruitment: from July 2010 to June 2012 Number randomised: 46 Postrandomisation dropouts: 0 Revised sample size: 46 Average age: 43 Males: 100% Decompensated cirrhosis: not reported Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy: diuretics, sodium restriction, and albumin for the treatment of ascites, or fresh frozen plasma for coagulopathy, antibiotics for any focus of infection, and also primary treatment with pentoxifylline at a dose of 400 mg three times a day and normal hospital nutrition (1800 to 2000 kcal per day). |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: none Funding: none Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After admission, the participants were randomised into 2 groups. A randomisation code was generated. Randomisation was performed using sequentially numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants were analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Singh 2018b.
| Study characteristics | ||
| Methods | Prospective, open‐label, single‐centre, pilot randomised study | |
| Participants | Country: India Period of recruitment: from October 2014 and March 2017 Number randomised: 57 Postrandomisation dropouts: 0 Revised sample size: 57 Average age: 45 Males: 100% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria Patients with severe AH admitted to the Liver Intensive Care Unit were eligible for inclusion in the study if they fulfilled the following criteria.
Exclusion criteria Patients with any of the following were excluded.
|
|
| Interventions |
Experimental
Control Standard medical treatment alone.
The standard medical treatment arm involved treatment with pentoxifylline (Sanofi Pharma, Mumbai, India) at a dose of 400 mg 3 times a day for 28 days and normal hospital nutrition (1800–2000 kcal/day). Diuretics, sodium restriction and albumin for the treatment of ascites, fresh frozen plasma for coagulopathy, and antibiotics for any focus of infection such as spontaneous bacterial peritonitis (SBP), pneumonia, cellulitis, and urinary tract infection were administered as indicated. All participants were admitted for at least 6 days following randomisation, and longer, if necessary, and were abstinent from alcohol throughout the study. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: the authors declare no conflicts. Funding: "The authors who have taken part in this study declare that partial funding was done for conducting this trial by the society for the study of liver diseases." ClincialTrials.gov number: NCT02971306 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After obtaining informed written consent and confirming inclusion and exclusion criteria, participants were randomised to 1 of the 3 treatment groups. The randomisation was computer generated. |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was placed in opaque sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants were lost at follow up. |
| Selective reporting (reporting bias) | Unclear risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Unclear risk | We found no other bias. |
Singh 2021.
| Study characteristics | ||
| Methods | Open‐label randomised controlled study | |
| Participants | Country: India Period of recruitment: from October 2017 to February 2021 Number randomised: 45 Postrandomisation dropouts: 0 Revised sample size: not reported Average age: not reported Males: not reported Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. No detail on standard medical therapy |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: no information reported Funding: no information reported Trials register: not reported Published only as abstract "...recruitment of patients for the study was slow and was hampered by the COVID‐19 pandemic. Therefore, we proceeded with an interim analysis of the patients who have already been enroled." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was given. |
| Allocation concealment (selection bias) | Unclear risk | No information was given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed 90 days of follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Spahr 2008.
| Study characteristics | ||
| Methods | Single‐centre, open‐label randomised controlled trial, conducted at a tertiary centre | |
| Participants | Country: Switzerland Period of recruitment: September 2005 to August 2006 Number randomised: 43 Postrandomisation dropouts: 0 Revised sample size: 43 Average age (years): 54 Males: 71% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 0% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included a 28‐day course of prednisone, 40 mg daily in case of severe alcoholic steatohepatitis. |
|
| Outcomes |
|
|
| Notes | Conflicts of interest: nothing to report Funding: "Supported by an unrestricted grant from the Foundation for Liver and Gut Studies (Geneva, Switzerland)" Clinical trial registration number: ISRCTN 86571875 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation code was generated in blocks of 4. Randomisation was done with sequentially numbered envelopes, with allocation to either standard medical therapy with or without G‐CSF. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done with sequentially numbered envelopes, with allocation to either standard medical therapy with or without G‐CSF. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information but low risk of bias for mortality outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants were analysed. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Spahr 2013.
| Study characteristics | ||
| Methods | Single‐centre, open‐label randomised controlled trial | |
| Participants | Country: Switzerland Period of recruitment: from February 2008 to March 2011 Number randomised: 58 Postrandomisation dropouts: 1 Revised sample size: 57 Average age (years): 56 Males: 76% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 81% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 0% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria "Patients admitted to our hospital for decompensated ALD were considered eligible if they met the following criteria."
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical treatment included vitamin B supplements, stimulation of calorie intake, specialised support regarding alcohol abstinence but no pharmacological intervention, and a 4‐week course of prednisone 40 mg/day in case of severe AH, as defined by a Maddrey’s score > 32). |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Conflicts of interests: the authors declared that no competing interests exist. Funding: this study was financially supported by the Clinical Research Center, University Hospital and Faculty of Medicine, Geneva, and the Louis‐Jeantet Foundation, and FLAGS (Foundation for Liver and Gut Studies in Geneva), and la Loterie Romande. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Trial registration number: ISRCTN83972743 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation to each treatment group was performed using a computer generated randomization code inserted in sequentially numbered opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | "Allocation to each treatment group was performed using a computer generated randomization code inserted in sequentially numbered opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant (1/58) was lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial reported the outcome all‐cause mortality. |
| Other bias | Low risk | We found no other bias. |
Tong 2022.
| Study characteristics | ||
| Methods | Open‐label randomised controlled trial | |
| Participants | Country: China Period of recruitment: from June 2014 to September 2016 Number randomised: 114 Postrandomisation dropouts: 3 Revised sample size: 111 Average age (years): 44 Males: 82% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 100% Alcohol‐related cirrhosis: 100% Viral‐related cirrhosis: 100% HBV Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 0% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included intensive care monitoring, antiviral therapy, supportive therapy, and prevention and treatment for complications. All participants with infection were treated with antibiotics. Participants received albumin terlipressin, and so on if required. |
|
| Outcomes |
|
|
| Notes | Conflict of Interest: the authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Funding: no funding ClinicalTrials.gov number: NCT02331745 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation number code was prepared for each participant. |
| Allocation concealment (selection bias) | Low risk | The participants were randomly allocated to the G‐CSF group (receiving G‐CSF therapy plus standard medical therapy) or control group (only receiving standard medical therapy). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three participants were excluded from analyses (2 participants in the G‐CSF arm: 1 underwent liver transplantation, 1 discontinued treatment; in the control arm, 1 participant underwent liver transplantation). |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Unclear risk | We found no other bias. |
Venkitaraman 2022.
| Study characteristics | ||
| Methods | Single‐centre, double‐blind randomised placebo‐controlled trial | |
| Participants | Country: India Period of recruitment: May 2019 to June 2020 Number randomised: 70 Postrandomisation dropouts: 4 Revised sample size: 66 Average age (years): 49 (25 to74) Males: 86% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 0% Alcohol‐related cirrhosis: 61% Viral‐related cirrhosis: 12% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 25% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy: salt restriction, calories 35 to 40 kcal/kg/day, protein 1.5 g/kg/day, and alcohol abstinence), rifaximin and lactulose for hepatic encephalopathy, diuretics and large‐volume paracentesis for ascites, prophylaxis with norfloxacin for past spontaneous bacterial peritonitis (SBP) and beta‐blockers for variceal bleed prophylaxis. All participants with hepatitis B were on treatment with tenofovir disoproxil fumarate. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: Aswath Venkitaraman, Arka De, Nipun Verma, Sunita Kumari, Bidyalaxmi Leishangthem, Ratti Ram Sharma, Naveen Kalra, Sandeep Grover, Virendra Singh declare no conflicting interests. Funding: no financial disclosures ClinicalTrials.gov number: NCT03911037 Out of the 70 participants who were initially included in the study, 3 participants (2 in group A and 1 in group B) developed variceal bleed and 1 participants in group B developed acute‐on‐chronic liver failure, after randomisation but before treatment initiation. These 4 participants (2 each in groups A and B) were neither given G‐CSF nor placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables randomised participants into group A (G‐CSF group) or group B (placebo group). |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done using serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study: placebo was normal saline in vials identical to G‐CSF. An independent research fellow (BL), who was not involved in participant care, dispensed drugs according to code generation. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | No information was given, but for the mortality outcome, low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three participants were lost at follow up (2 in the control arm). |
| Selective reporting (reporting bias) | Unclear risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
Verma 2018a.
| Study characteristics | ||
| Methods | Single‐centre, open‐label randomised controlled trial, conducted at a tertiary centre | |
| Participants | Country: India Period of recruitment: May 2015 to June 2016 Number randomised: 65 Postrandomisation dropouts: 4 Revised sample size: 65 Average age (years): 51 Males: 80% Decompensated cirrhosis: 100% Acute‐on‐chronic liver failure: 0% Alcohol‐related cirrhosis: 68.5% Viral‐related cirrhosis: 18.5% Autoimmune disease‐related cirrhosis: 0% Other causes of cirrhosis: 13% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Experimental
Control
All participants received standard medical therapy. Standard medical therapy included (as per requirement) nutrition with salt‐restricted high‐protein diet with target calories 35 to 40 kcal/kg/day, protein 1.5 g/kg/day, and periodic nutrition counselling and reassessment at every visit. Oral rifaximin, lactulose, or lactitol for participants with past or current hepatic encephalopathy, intravenous albumin (20%) during large‐volume paracentesis and diuretics for control of ascites, oral norfloxacin prophylaxis for participants with past spontaneous bacterial peritonitis were given. Beta‐blockers were given as primary or secondary prophylaxis when indicated and withheld in participants with refractory ascites. Multivitamins, oral calcium, and vitamin D and antibiotics were given as and when indicated. Antivirals were given for hepatitis B virus and hepatitis C virus. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Conflicts of interest: nothing to report Funding: "Partially funded by Society for the Study of Liver Diseases (SSLD)" ClinicalTrials.gov number: NCT02451033 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study used a computer‐generated random number table provided by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done using sequentially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study. |
| Blinding of outcome assessment (detection bias) overall mortality | Low risk | A research scholar (AK) who was blinded to the group allocated to the participant assessed the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A research scholar (AK) who was blinded to the group allocated to the participant assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants were lost at follow‐up: 2 of 21 in the control arm and 2 of 44 in the intervention arm. |
| Selective reporting (reporting bias) | Low risk | The trial reported all‐cause mortality as a primary outcome. |
| Other bias | Low risk | We found no other bias. |
ACLF: acute‐on‐chronic liver failure; AH: alcoholic hepatitis; ALD: alcoholic liver disease; ALT: alanine aminotransferase; APASL criteria: the Asian Pacific Association for the Study of the Liver; ASH: alcoholic steatohepatitis; CANONIC study: European Association for the Study of the Liver (EASL)–Chronic Liver Failure (CLIF) Consortium acute‐on‐chronic liver failure in cirrhosis; CLDQ: Chronic Liver Disease Questionnaire; CTP: Child‐Turcotte‐Pugh; DC: decompensated cirrhosis; ELF test: Enhanced liver fibrosis test; G‐CSF: granulocyte colony‐stimulating factor; GH: growing hormone; HBV: hepatitis B virus; HCV: hepatitis C virus; HE: hepatic encephalopathy; HRS: hepatorenal syndrome; HSCs: haematopoietic stem cells; INR: international normalised ratio; LSM: liver stiffness measurement; LT: liver transplantation; mDF score: Maddrey's discriminant function score; MELD: Model for End‐Stage Liver Disease; NAC: n‐acetyl cysteine; QOL: quality of life; SOFA score: Sequential Organ Failure Assessment score; UKELD score: UK End‐Stage Liver Disease score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amer 2011 | No treatment with G‐CSF |
| Anand 2019 | This study was designed to evaluate potential clinical benefit of adding erythropoietin (EPO) in G‐CSF‐mobilised stem cell therapy. 60 consecutive decompensated cirrhosis patients received either G‐CSF with EPO (group A; n = 30) or G‐CSF and placebo (group B; n = 30) for 2 months. |
| El‐Ansary 2012 | No treatment with G‐CSF |
| Esmaeilzadeh 2019 | No treatment with G‐CSF |
| Fiuza 2002 | It is a case‐control study that included participants with liver cirrhosis and gender‐matched healthy volunteers, not a randomised clinical trial. |
| Gaia 2013 | This was not a randomised clinical trial. |
| Kharaziha 2009 | This was a case series; there was no treatment with G‐CSF. |
| Lyra 2010 | No treatment with G‐CSF |
| Mohamadnejad 2016 | No treatment with G‐CSF |
| Philips 2020 | This was a non‐randomised study (historical controls). |
| Ranjan 2022 | Use of granulocyte‐macrophage colony‐stimulating factor (instead of G‐CSF) |
| Sakaida 2005 | This was a narrative review without any original data. |
| Salama 2010 | No treatment with G‐CSF |
| Sharma 2016 | This was not a randomised clinical trial. |
| Singh 2018a | This was not a randomised clinical trial. It was a retrospective cohort study in oncologic patients after autologous peripheral blood stem cell transplantation. |
| Terai 2006 | This was a case series (no treatment with G‐CSF). |
| Xing 2013 | This was not a randomised clinical trial. |
| Yannaki 2005 | This study was conducted in animals. |
G‐CSF: granulocyte colony‐stimulating factor
Characteristics of studies awaiting classification [ordered by study ID]
Xu 2016.
| Methods | We are still waiting for a requested translation of the paper. |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Notes | ‐ |
Zhou 2020.
| Methods | We are still waiting for a requested translation of the paper. |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Notes | ‐ |
Characteristics of ongoing studies [ordered by study ID]
Cho 2018.
| Study name | Efficacy of granulocyte colony stimulating factor in patients with severe alcoholic hepatitis with partial or null response to steroid (GRACIAH trial): study protocol for a randomised controlled trial |
| Methods | Prospective, double‐blind, multicentre (15 centres), randomised placebo‐controlled trial, with 2 parallel subgroups |
| Participants | Patients with severe alcoholic hepatitis, with high mortality, despite corticosteroid treatment. Inclusion criteria
|
| Interventions |
Experimental
Control
|
| Outcomes | The primary aim was to evaluate whether G‐CSF treatment prolongs 6‐month overall survival (OS) of participants with a partial response (PR) to steroids and 2‐month OS of participants with a null response (NR) to steroids. Partial responders (0.16 < Lille score < 0.56) or null responders (Lille score ≥ 0.56) are identified based on the Lille score after 1 week of corticosteroid treatment (40 mg daily prednisolone dose). The secondary aims were to identify the risk factors in relation to mortality and the predictive factors associated with responses to standard corticosteroid treatment or rescue G‐CSF therapy. |
| Starting date | Recruitment started on 13 May 2015. The estimated primary completion date was April 2022 (estimated study completion: December 2022). The enrolment was terminated (failure to recruit eligible participants) in July 2022, but results are still not available. |
| Contact information | ‐ |
| Notes | ‐ |
G‐CSF: granulocyte colony‐stimulating factor; MDF: Maddrey's discriminant function score
Differences between protocol and review
For the secondary outcome 'Proportion of participants with liver disease‐related morbidity (i.e. proportion of participants who developed one or more complications, such as ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, jaundice, or portal thrombosis, or who underwent liver transplantation)', we added hepatocellular carcinoma to the list of possible complications. We also performed meta‐analyses using a single complication (ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, jaundice, portal thrombosis, or liver transplantation), because most trials reported only the occurrence of a single complication, which prevented per‐patient analysis of the composite outcome.
We did not perform the sensitivity analysis including only the trials that reported intention‐to‐treat analyses, as all included trials reported intention‐to‐treat analyses.
We performed an additional sensitivity analysis excluding trials published only in abstract or letter format.
We performed two additional (posthoc) subgroup analyses, assessing the following:
trial location, in which we compared trials conducted in Asia to trials conducted in Europe, America, and Africa, hypothesising population differences; and
participants' disease, in which we compared participants with acute‐on‐chronic liver failure (ACLF) to trials excluding participants with ACLF, as the effect of G‐CSF may vary according to the presence ACLF (i.e. associated with the worst prognosis).
We improved the title of the review by adding 'or growth factors', so that it reflects the combinations in terms of the studied experimental intervention.
Contributions of authors
AC wrote the protocol, performed searches for references, evaluated references to obtain full reports, evaluated studies for inclusion, extracted data from studies, assessed risk of bias, and wrote the final review. MF commented on the protocol, evaluated studies for inclusion, extracted data from studies, assessed risk of bias, and critically commented on the final review. DP co‐ordinated protocol design, evaluated references to obtain full‐text reports, and critically commented on the final review. GC wrote the protocol, conducted statistical analyses, acted as arbiter if review authors did not reach a consensus, and wrote the final review. All authors approved the review for publication.
Sources of support
Internal sources
-
None, Italy
none
External sources
-
Cochrane Hepato‐Biliary, Denmark
Provided help with the review preparation until publication, including the peer review process
Declarations of interest
AC: has declared that they have no conflict of interest. MF: has declared that they have no conflict of interest. DP: has declared that they have no conflict of interest. GC: has declared that they have no conflict of interest.
New
References
References to studies included in this review
De 2021 {published data only}
- De A, Kumari S, Singh A, Kaur A, Sharma R, Bhalla A, et al. Multiple cycles of granulocyte colony-stimulating factor increase survival times of patients with decompensated cirrhosis in a randomized trial. Clinical Gastroenterology and Hepatology 2021;19:375-83. [DOI] [PubMed] [Google Scholar]
Duan 2013 {published data only}
- Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World Journal of Gastroenterology 2013;19(7):1104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engelmann 2021a {published data only}
- Engelmann C, Herber A, Franke A, Bruns T, Reuken P, Schiefke I, et al. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: a multicenter randomized trial (GRAFT study). Journal of Hepatology 2021;75(6):1346-54. [DOI] [PubMed] [Google Scholar]
Garg 2012 {published data only}
- Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012;142(3):505-12. [DOI] [PubMed] [Google Scholar]
Haque 2020 {published data only}
- Haque MN, Al-Mahtab M, Das DC, Mohammad NS, Mamun AA, Khan MSI, et al. Effect of granulocyte colony-stimulating factor and erythropoietin on patients with acute-on-chronic liver failure. Euroasian Journal of Hepato-Gastroenterology 2020;10(2):64-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kedarisetty 2015 {published data only}
- Kedarisetty CK, Anand L, Bhardwaj A, Bhadoria AS, Kumar G, Vyas AK, et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology 2015;148(7):1362-70. [DOI] [PubMed] [Google Scholar]
Morgan 2022 {published data only}
- Morgan T, Asghar A, Tayek J, Nguyen D, Fleischman M W, Donovan J, et al. A phase II, multicenter, open-label, randomized trial of pegfilgrastim for patients with alcohol-associated hepatitis. Journal of Hepatology 2022;77:S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newsome 2018 {published data only}
- Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet. Gastroenterology & Hepatology 2018;3(1):25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prajapati 2017 {published data only}
- Prajapati R, Arora A, Sharma P, Bansal N, Singla V, Kumar A. Granulocyte colony-stimulating factor improves survival of patients with decompensated cirrhosis: a randomized-controlled trial. European Journal of Gastroenterology & Hepatology 2017;29:448-55. [DOI] [PubMed] [Google Scholar]
Saha 2017 {published data only}
- Saha BK, Mahtab MA, Akbar SMF, Noor-E-Alam SM, Mamun AA, Hossain SM, et al. APASL ACLF working party. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: increased survival and containment of liver damage. Hepatology International 2017;11(6):540-6. [DOI] [PubMed] [Google Scholar]
Sharma 2017 {published data only}
- Sharma A, Setia A, Rai RR. Effect of granulocyte colony-stimulating factor (G-CSF) on mortality and complications viz. sepsis, encephalopathy, hepatorenal syndrome, and gastrointestinal bleed in severe alcoholic hepatitis: a randomized controlled study. United European Gastroenterology Journal 2017;5:A17. [Google Scholar]
Shasthry 2019 {published data only}
- Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of granulocyte colony-stimulating factor in the management of steroid-nonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology 2019;70(3):802-11. [DOI] [PubMed] [Google Scholar]
Singh 2014 {published data only}
- Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R, et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. American Journal of Gastroenterology 2014;109(9):1417-23. [DOI] [PubMed] [Google Scholar]
Singh 2018b {published data only}
- Singh V, Keisham A, Bhalla A, Sharma N, Agarwal R, Sharma R, et al. Efficacy of granulocyte colony-stimulating factor and n-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clinical Gastroenterology and Hepatology 2018;16(10):1650-6. [DOI] [PubMed] [Google Scholar]
Singh 2021 {published data only}
- Singh V, Singh A, Sharma N, De A, Leishangthem B, Sharma N, et al. Granulocyte-colony stimulating factor (G-CSF) with or without four weeks of n-acetyl cysteine (NAC) in severe alcoholic hepatitis. Hepatology 2021;Suppl 1:215A. [Google Scholar]
Spahr 2008 {published data only}
- Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48(1):221-9. [DOI] [PubMed] [Google Scholar]
Spahr 2013 {published data only}
- Spahr L, Chalandon Y, Terraz S, Kindler V, Rubbia-Brandt L, Frossard JL, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLOS One 2013;8(1):e53719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2022 {published data only}
- Tong J, Wang H, Xu X, Wan Z, Fang H, Chen J, et al. Granulocyte colony-stimulating factor accelerates the recovery of hepatitis B virus-related acute-on-chronic liver failure by promoting M2-like transition of monocytes. Frontiers in Immunology 2022;13:885829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkitaraman 2022 {published data only}
- Venkitaraman A, De A, Verma N, Kumari S, Leishangthem B, Sharma RR, et al. Multiple cycles of granulocyte colony-stimulating factor in decompensated cirrhosis: a double-blind RCT. Hepatology International 2022;16(5):1127-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verma 2018a {published data only}
- Verma N, Kaur A, Sharma R, Bhalla A, Sharma N, De A, et al. Outcomes after multiple courses of granulocyte colony-stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology 2018;68(4):1559-73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amer 2011 {published data only}
- Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. European Journal of Gastroenterology & Hepatology 2011;23(10):936-41. [DOI] [PubMed] [Google Scholar]
Anand 2019 {published data only}
- Anand L, Bihari C, Kedarisetty CK, Rooge SB, Kumar D, Shubham S, et al. Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis. Liver International 2019;39(1):115-26. [DOI] [PubMed] [Google Scholar]
El‐Ansary 2012 {published data only}
- El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Reviews and Reports 2012;8(3):972-81. [DOI] [PubMed] [Google Scholar]
Esmaeilzadeh 2019 {published data only}
- Esmaeilzadeh A, Ommati H, Kooshyar MM, Jarahi L, Rezayat KA, Saberi S, et al. Autologous bone marrow stem cell transplantation in liver cirrhosis after correcting nutritional anomalies, a controlled clinical study. Cell Journal 2019;21(3):268-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiuza 2002 {published data only}
- Fiuza C, Salcedo M, Clemente G, Tellado JM. Granulocyte colony-stimulating factor improves deficient in vitro neutrophil transendothelial migration in patients with advanced liver disease. Clinical And Diagnostic Laboratory Immunology 2002;9(2):433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaia 2013 {published data only}
- Gaia S, Olivero A, Smedile A, Ruella M, Abate ML, Fadda M, et al. Multiple courses of G-CSF in patients with decompensated cirrhosis: consistent mobilization of immature cells expressing hepatocyte markers and exploratory clinical evaluation. Hepatology International 2013;7(4):1075-83. [DOI] [PubMed] [Google Scholar]
Kharaziha 2009 {published data only}
- Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. European Journal of Gastroenterology & Hepatology 2009;21(10):1199-205. [DOI] [PubMed] [Google Scholar]
Lyra 2010 {published data only}
- Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, et al. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. European Journal of Gastroenterology & Hepatology 2010;22(1):33-42. [DOI] [PubMed] [Google Scholar]
Mohamadnejad 2016 {published data only}
- Mohamadnejad M, Vosough M, Moossavi S, Nikfam S, Mardpour S, Akhlaghpoor S, et al. Intraportal infusion of bone marrow mononuclear or cd133+ cells in patients with decompensated cirrhosis: a double-blind randomized controlled trial. Stem Cells Translational Medicine 2016;5(1):87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philips 2020 {published data only}
- Philips CA, Augustine P, Rajesh S, Ahamed R, George T, Padsalgi G, et al. Granulocyte colony-stimulating factor use in decompensated cirrhosis: lack of survival benefit. Journal of Clinical and Experimental Hepatology 2020;10:124-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranjan 2022 {published data only}
- Ranjan A, Sarin SK, Maiwall R, Jindal A, Rajan V. Comparison of imipenem and tigecycline versus imipenem, tigecycline combined with GM-CSF in patients with spontaneous bacterial peritonitis and septic shock. Journal of Clinical and Experimental Hepatology 2022;12(Suppl 2):S26. [Google Scholar]
Sakaida 2005 {published data only}
- Sakaida I, Terai S, Nishina H, Okita K. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Medical Molecular Morphology 2005;38(4):197-202. [DOI] [PubMed] [Google Scholar]
Salama 2010 {published data only}
- Salama H, Zekri AR, Zern M, Bahnassy A, Loutfy S, Shalaby S, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplantation 2010;19:1475-86. [DOI] [PubMed] [Google Scholar]
Sharma 2016 {published data only}
- Sharma V, Sharma R, Rao P. Exciting results with injection darbepoietin alfa and pegfilgrastim in patients with decompensated liver cirrhosis. Journal of Liver Research, Disorders & Therapy 2016;2(5):131-2. [Google Scholar]
Singh 2018a {published data only}
- Singh AD, Parmar S, Patel K, Shah S, Shore T, Gergis U, et al. Granulocyte colony-stimulating factor use after autologous peripheral blood stem cell transplantation: comparison of two practices. Biology of Blood and Marrow Transplantation 2018;24(2):288-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terai 2006 {published data only}
- Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006;24(10):2292-8. [DOI] [PubMed] [Google Scholar]
Xing 2013 {published data only}
- Xing TJ, Xu HT, Xian JC, Shen ML, Li H, Ye J, et al. Mechanism and efficacy of mobilization of granulocyte colony-stimulating factor in the treatment of chronic hepatic failure. Hepatogastroenterology 2013;60(121):170-5. [DOI] [PubMed] [Google Scholar]
Yannaki 2005 {published data only}
- Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Experimental Hematology 2005;33(1):108-19. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Xu 2016 {published data only}
- Xu X, Liu XY, Chen J, Xiao L, Tong JJ, Guan CD, et al. Randomised controlled clinical study of granulocyte colony-stimulating factor in the treatment of chronic hepatitis B-related acute liver failure. Infectious Diseases Information 2016;29(5):279-83. [Google Scholar]
Zhou 2020 {published data only}
- Zhou P, Yu Z, Qv SY. The clinical trial of G-CSF for end-stage alcoholic liver disease. Chinese Hepatology 2020;25:521-4. [Google Scholar]
References to ongoing studies
Cho 2018 {published data only}
- Cho Y, Park YS, Kim HY, Kim W, Lee HJ, Kim DJ. Efficacy of granulocyte colony stimulating factor in patients with severe alcoholic hepatitis with partial or null response to steroid (GRACIAH trial): study protocol for a randomized controlled trial. Trials 2018;19(1):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alison 2000
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000;406:257. [DOI] [PubMed] [Google Scholar]
Arroyo 2017
- Arroyo V, Moreau R. Diagnosis and prognosis of acute on chronic liver failure (ACLF) in cirrhosis. Journal of Hepatology 2017;66:451-3. [DOI] [PubMed] [Google Scholar]
Arroyo 2020
- Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. New England Journal of Medicine 2020;382(22):2137-45. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(110):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chavez‐Tapia 2015
- Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Noreña-Herrera C, Vidaña-Perez D, Delgado-Sanchez G, et al. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Annals of Hepatology 2015;14(5):631-41. [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
D'Amico 2006
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44:217-31. [DOI] [PubMed] [Google Scholar]
D'Amico 2014
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39:1180-93. [DOI] [PubMed] [Google Scholar]
de Franchis 2015
- Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63:743-52. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341-50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Duong 2014
- Duong HK, Savani BN, Copelan E, Devine S, Costa LJ, Wingard JR, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation 2014;20:1262-73. [DOI] [PubMed] [Google Scholar]
EASL 2018
- European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 2018;69(2):406-60. [DOI] [PubMed] [Google Scholar]
Engelmann 2021b
- Engelmann C, Martino VD, Kerbert AJ, Weil-Verhoeven D, Aehling NF, Herber A, et al. The current status of granulocyte-colony stimulating factor to treat acute-on-chronic liver failure. Seminars in Liver Disease 2021;41(3):298-307. [DOI] [PubMed] [Google Scholar]
Forbes 2012
- Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. Journal of Hepatology 2012;56:496-9. [DOI] [PubMed] [Google Scholar]
Forbes 2016
- Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nature Reviews. Gastroenterology and Hepatology 2016;13:473-85. [DOI] [PubMed] [Google Scholar]
Foucher 2006
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55(3):403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garattini 2016
- Garattini S, Jakobsen JC, Wetterslev J, Bertele V, Banzi R, Rath A, et al. Evidence-based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internal Medicine 2016;32:13-21. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2010
- Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gartlehner 2019
- Gartlehner G, Nussbaumer-Streit B, Wagner G, Patel S, Swinson-Evans T, Dobrescu A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2019;106:50-9. [DOI] [PubMed] [Google Scholar]
Gilchrist 2010
- Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transplantation 2010;16:118-29. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 4 December 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395-400. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. Journal of Clinical Epidemiology 2011;64(12):1277-82. [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311-6. [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151-7. [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing Summary of Findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables - continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173-83. [DOI] [PubMed] [Google Scholar]
Guyatt 2013d
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719-25. [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated August 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
Higgins 2019b
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Hou 2021
- Hou X, Li Y, Yuan H, Cai J, Liu R, Li J, Zhu C. Therapeutic effect and safety of granulocyte colony-stimulating factor therapy for acute-on-chronic liver failure: a systematic review and meta-analysis of randomized controlled trials. Frontiers in Medicine 2021;8:784240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2021
- Huang W, Ma Y, Du L, Kang S, Liu CH, Bai L, et al. Effectiveness of granulocyte colony-stimulating factor for patients with acute-on-chronic liver failure: a meta-analysis. Annals of Saudi Medicine 2021;41(6):383-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 2016
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 16 June 2022).
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2015
- King A, Barton D, Beard HA, N Than, Moore J, Corbett C, et al. REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): a multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial. BMJ Open 2015;5:e007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(1):982-9. [DOI] [PubMed] [Google Scholar]
Krupczak‐Hollis 2003
- Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology 2003;38(6):1552-62. [DOI] [PubMed] [Google Scholar]
Lanthier 2018
- Lanthier N. Haemopoietic stem cell therapy in cirrhosis: the end of the story? Lancet. Gastroenterology & Hepatology 2018;3(1):3-5. [DOI] [PubMed] [Google Scholar]
Lewis 2004
- Lewis LD. Preclinical and clinical studies: a preview of potential future applications of erythropoietic agents. Seminars in Hematology 2004;41(Suppl 7):17-25. [DOI] [PubMed] [Google Scholar]
Li 2019
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: MR000033. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marot 2020
- Marot A, Singal AK, Moreno C, Deltenre P. Granulocyte colony-stimulating factor for alcoholic hepatitis: a systematic review and meta-analysis of randomised controlled trials. JHEP Reports 2020;2(5):100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore K, Stutchfield BM, Forbes SJ. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Alimentary Pharmacology & Therapeutics 2014;39:673-85. [DOI] [PubMed] [Google Scholar]
Murray 2013
- Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310(6):591-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42; quiz 742.e1-5. [DOI] [PubMed] [Google Scholar]
NICE 2016
- NICE Guideline [NG50]. Cirrhosis in over 16s: assessment and management. www.nice.org.uk/guidance/ng50 (accessed 9 November 2022).
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Pavlov 2015
- Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Burroughs AK, Ivashkin VT, et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD010542. [DOI: 10.1002/14651858.CD010542.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pimpin 2018
- Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. Journal of Hepatology 2018;69(3):718-35. [DOI] [PubMed] [Google Scholar]
Review Manager [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rossi 2007
- Rossi M, Mennini G, Lai Q, Ginanni Corradini S, Drudi FM, Pugliese F, et al. Liver transplantation. Journal of Ultrasound 2007;10(1):28-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2017
- Rowe IA. Lessons from epidemiology: the burden of liver disease. Digestive Diseases 2017;35:304-9. [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine 2008;27:746-63. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1-82. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: the ROBES Meta-Epidemiologic Study. American Journal of Epidemiology 2018;187(5):113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shi 2022
- Shi P, Zhang J, Wu M, Zheng T, Liang A, Wen Z, et al. The effects of granulocyte-colony stimulating factor on chronic liver disease: a meta-analysis. Journal of Infection in Developing Countries 2022;16(3):537-46. [DOI] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2011
- Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration and function. Hepatology 2011;53:2003-15. [DOI] [PubMed] [Google Scholar]
Thorlund 2017
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA); 2nd edition. Copenhagen Trial Unit, 2017. Available from ctu.dk/tsa/learn-more (accessed 16 June 2022).
TSA [Computer program]
- TSA - Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2017. Available at ctu.dk/tsa/downloads/.
Tsochatzis 2014
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. [DOI] [PubMed] [Google Scholar]
Verma 2018b
- Verma N, Singh A, Singh V. Reply. Hepatology 2018;68(1):388. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random-effects meta-analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical Research Ed.) 2008;366:601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2016
- Yang Q, Yang Y, Shi Y, Lv F, He J, Chen Z. Effects of granulocyte colony-stimulating factor on patients with liver failure: a meta-analysis. Journal of Clinical and Translational Hepatology 2016;4(2):90-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Colli 2020
- Colli A, Prati D, Fraquelli M, Casazza G. Granulocyte colony‐stimulating factor with or without stem or progenitor cell infusion for people with compensated or decompensated advanced chronic liver disease. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD013532. [DOI: 10.1002/14651858.CD013532] [DOI] [PMC free article] [PubMed] [Google Scholar]


