Significance
This work provides unique insight into the evolution and function of two highly unusual transcription factors, WhiA and WhiB, which control sporulation septation in the antibiotic-producing bacteria Streptomyces. Furthermore, in the closely related pathogen Mycobacterium tuberculosis (Mtb), WhiA and WhiB are essential because they are required for cell division, and mutations in whiA and whiB are positively selected in clinical isolates of Mtb, implicating WhiA/B in virulence and/or resistance to drug treatment. Our work will thus provide functional insight into the molecular mechanism of a new class of transcription factors in an important bacterial clade that contains the major antibiotic producers while informing on the adaptive nature of WhiA/B mutations in virulence and antibiotic resistance in a deadly pathogen.
Keywords: transcription, RNA polymerase, WhiA, WhiB, cryo-EM
Abstract
Studies of transcriptional initiation in different bacterial clades reveal diverse molecular mechanisms regulating this first step in gene expression. The WhiA and WhiB factors are both required to express cell division genes in Actinobacteria and are essential in notable pathogens such as Mycobacterium tuberculosis. The WhiA/B regulons and binding sites have been elucidated in Streptomyces venezuelae (Sven), where they coordinate to activate sporulation septation. However, how these factors cooperate at the molecular level is not understood. Here we present cryoelectron microscopy structures of Sven transcriptional regulatory complexes comprising RNA polymerase (RNAP) σA-holoenzyme and WhiA and WhiB, in complex with the WhiA/B target promoter sepX. These structures reveal that WhiB binds to domain 4 of σA (σA4) of the σA-holoenzyme, bridging an interaction with WhiA while making non-specific contacts with the DNA upstream of the −35 core promoter element. The N-terminal homing endonuclease-like domain of WhiA interacts with WhiB, while the WhiA C-terminal domain (WhiA-CTD) makes base-specific contacts with the conserved WhiA GACAC motif. Notably, the structure of the WhiA-CTD and its interactions with the WhiA motif are strikingly similar to those observed between σA4 housekeeping σ-factors and the −35 promoter element, suggesting an evolutionary relationship. Structure-guided mutagenesis designed to disrupt these protein–DNA interactions reduces or abolishes developmental cell division in Sven, confirming their significance. Finally, we compare the architecture of the WhiA/B σA-holoenzyme promoter complex with the unrelated but model CAP Class I and Class II complexes, showing that WhiA/WhiB represent a new mechanism in bacterial transcriptional activation.
Gene expression is orchestrated by transcription factors that activate or inhibit initiation by RNA polymerase (RNAP). A classical paradigm of transcription activation was established by studies of the catabolite activator protein (CAP) in Escherichia coli (Eco), the bacterium where much of our understanding of gene expression is rooted (1). However, recent studies have made it apparent that there are new transcriptional mechanisms to be discovered in diverse bacteria, highlighting the importance of expanding this research to include bacteria from all clades (2). Here we focus on WhiA and WhiB, two proteins of interest because they are the founding members of two highly unusual families of transcription factors and how they coordinate to activate transcription is not understood. WhiA and WhiB play critical roles in the developmental biology of the antibiotic-producing bacteria Streptomyces. Notably, WhiA and WhiB are essential in the pathogen Mycobacterium tuberculosis (Mtb) because they are required for cell division, extending the application and significance of these studies to a deadly disease (3, 4). Furthermore, recent bioinformatic analyses of clinical isolates of Mtb have also revealed roles for WhiA/B in virulence and resistance to antibiotics (5).
Streptomyces are filamentous bacteria with a complicated developmental life cycle involving progression from vegetative growth to the production of reproductive aerial hyphae, which differentiate into spores (6–9) (Fig. 1A). The most dramatic transition in the life cycle is a massive cell division event involving the synchronous formation of dozens of sporulation septa that divides each multigenomic reproductive hypha into a long chain of unigenomic spores (Fig. 1A). This event is controlled by the two key developmental regulators, WhiA and WhiB, and deletion of either whiA or whiB leads to an identical phenotype in which developmental cell division and chromosome segregation are not initiated. These identical phenotypes arise because WhiA and WhiB function cooperatively to co-control the expression of a common set of WhiA/B target genes, including the critical cell division genes ftsZ, ftsW, ftsK, sepH, and sepX (10, 11). Although in vitro WhiA can be footprinted on DNA at high concentrations in the absence of other proteins (11, 12), in vivo DNA binding by WhiA depends on WhiB and vice versa (10). However, WhiA and WhiB function as monomers and do not interact in the absence of DNA, representing a unique mechanism of transcriptional activation in bacteria (10).
Fig. 1.
Activation of the developmentally regulated sepX promoter by WhiA and WhiB. (A) Schematic of the developmental life cycle of Streptomyces, highlighting that the initiation of sporulation-specific cell division is co-controlled by the transcription factors WhiA and WhiB. (B) ChIP-seq and microarray transcriptional profiling data show that sepX is directly regulated by WhiA/B. For the microarray panel, the X-axis indicates the age of the culture in hours, and the y-axis indicates the per gene normalized transcript abundance (log2). For the wild type, 10 to 14 h corresponds to vegetative growth, 14 to 16 h corresponds to the onset of sporulation (fragmentation), and 16 h onwards corresponds to sporulation. These data are derived from the ChIP-seq and microarray datasets described in Bush et al. (10, 11), but sepX was not discussed in those papers. (C) DNase I footprinting analysis of WhiA binding to the sepX promoter. A probe, 5′ end-labeled on the template strand (t-strand), was incubated with increasing concentrations of WhiA (indicated in µM above the lanes) and subjected to DNase I footprinting analysis using the method described in ref. 11. A Maxam and Gilbert sequence ladder (GA) is shown on the Left. The sequence of the WhiA-protected region is shown, together with the over-represented motif associated with WhiA target genes (11), identified using the MEME algorithm (13). The height of the letters in the sequence logo, in bits, is proportional to the frequency of the A, C, T, or G nucleotides at each position of the motif. (D) Sequence of the sepX promoter with the position of the transcription start site (+1) indicated below (the sepX transcript is leaderless, and so this also represents the start of the coding sequence). The −35 and −10 sequences are highlighted in blue. The non-template strand (nt-strand) is shown on Top and the t-strand on the bottom. The t-strand sequence protected by WhiA in the footprinting experiment shown in (C) is underlined, and the sequence complementary to the consensus GACAC WhiA-binding sequence is highlighted in red. (E) Coomassie-stained SDS gels showing the purified 1:1:1 σA:RbpA:WhiB complex in which the WhiB [4Fe-4S] cluster is oxygen stable (Left), and the RNAP-σA-RbpA-WhiB holoenzyme complex produced by mixing with Sven core RNA polymerase followed by repurification on a Superdex 200 gel filtration column (Right). (F) Representative transcription assays showing abortive transcripts synthesized from Sven RNAP, Sven RNAP/WhiA, Sven RNAP/WhiB, and Sven RNAP/WhiA/WhiB holoenzymes. The assay was performed in triplicate, and the quantified results are presented in the histogram. The activity of Sven RNAP was normalized to 1, and the values shown are the average of the three experiments. Error bars indicate the mean SE.
The large N-terminal domain of WhiA (WhiA-NTD) is homologous to eukaryotic homing endonucleases, but lacks the critical residues found in these endonucleases that are required for enzymatic function and DNA binding (14, 15). Instead, WhiA binds to its asymmetric 5′-GACAC-3′ consensus motif via a small C-terminal helix-turn-helix (HTH) domain (WhiA-CTD) that is absent from homing endonucleases (11, 12, 14), and the function of the N-terminal homing endonuclease-like domain of WhiA is unknown. WhiB is the founding member of the Actinobacteria-specific WhiB-like family of transcription factors that carry a [4Fe-4S] iron-sulfur cluster and play key roles in diverse aspects of Actinobacterial biology, including pathogenesis, antibiotic resistance, and the control of development (16). A few WhiB family members, such as WhiB7, have a recognized DNA-binding motif, the AT-hook (17, 18), but most do not. Consequently, how the remaining family members, including WhiB, bind DNA is not understood.
Here we show that the sepX promoter is activated in vitro by the two activators WhiA and WhiB. We also present the structures of a Streptomyces venezuelae (Sven) RNAP initiation complex composed of the RNAP holoenzyme, EσA (five core subunits; α2ββ'ω, the general transcription factor RbpA, and the initiation factor, σA, also known as σHrdB) (19–23) bound to the sepX promoter in the presence of WhiA and WhiB. We validate the significance of the observed interactions by employing structure-guided mutagenesis with phenotypic assays. The structures reveal how the factors interact with RNAP and DNA to stimulate transcription. We find that WhiB binds both to WhiA and to σA, thereby forming a bridge between WhiA and RNAP. Both WhiA and WhiB interact with the DNA, and we compare the overall architecture to the classical, well-studied type I and II CAP activation complexes (24–26). In addition, the interactions of WhiA with the GACAC motif mimic those of region 4 of σ factors (σA4) with the −35 promoter element (27), revealing how bacteria can repurpose functional domains between the basal transcription machinery and specialized transcription factors.
Results
Dual Activation of the Developmentally Regulated sepX Promoter by WhiA and WhiB.
In Sven, the initiation of sporulation-specific cell division is co-controlled by the WhiA and WhiB transcription factors (Fig. 1A). For structural studies, we chose the promoter of the sepX gene, which is a target of WhiA/B-mediated activation (Fig. 1 B and C). The sequence of the sepX promoter is shown in Fig. 1D. SepX is a membrane protein that contributes to the stabilization of the divisome and is crucial for developmental cell division in Sven (28).The WhiA-binding site in the sepX promoter was determined using DNase I footprinting on the template strand (t-strand) (Fig. 1C). We found that WhiA protected a 17-nucleotide region of the sepX promoter (Fig. 1D) that encompasses the consensus 5′-GACAC-3′ sequence that binds WhiA in the RNAP-WhiA-WhiB-sepX complex (see below).
The WhiB family proteins have been problematic to study in vitro because, when overexpressed in E. coli, their [4Fe-4S] clusters are both poorly loaded and oxygen-sensitive (16, 29). It has previously been shown that WhiB family proteins can form a 1:1 complex with σA and that complex formation depends on the presence of the cluster (30, 31). It has also been shown that σA can form a 1:1 complex with the general transcription factor RbpA (23). A key breakthrough came when we co-overexpressed WhiB with σA and RbpA and found that the three proteins formed a stable 1:1:1 complex (Fig. 1E) indicating that the [4Fe-4S] cluster was present, and the cluster was oxygen-insensitive. We were also able to purify a 1:1 WhiB:σA complex with a fully loaded, oxygen-insensitive cluster, similarly to WhiB1 and WhiB7 from Mtb (17, 18, 30). The reasons why σA protects the WhiB cluster from oxygen became apparent from structural studies (see below). In addition, we could also purify a stable 1:1 σA:RbpA complex.
To assess whether WhiA and WhiB could directly activate transcription from the sepX promoter, we purified endogenous Sven core RNAP and used it in combination with WhiA, the σA:RbpA complex, and the WhiB:σA:RbpA complex in biochemical assays. Consistent with the mutual dependence for DNA binding and transcription activation seen in vivo (10), the assays show that although WhiA activates transcription slightly, both WhiA and WhiB are required for maximal activation of the sepX promoter in vitro (Fig. 1F).
Cryo-EM Analysis of Sven RNAP Initiation Complexes with WhiA and WhiB on the sepX Promoter.
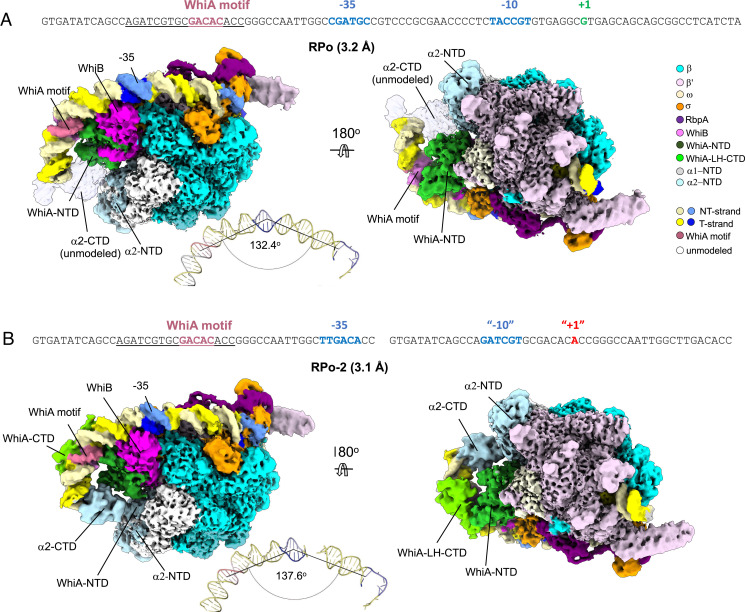
Core RNAP was mixed with WhiB:σA:RbpA and WhiA and then incubated with the WhiA/B-regulated sepX promoter (Fig. 2A) to capture de novo initiation complexes for cryo-EM analysis. Data were curated and classified (SI Appendix, Fig. S1A and Table S1). Processing of the cryo-EM data from the complex yielded a single class at a nominal resolution of 3.2 Å (Fig. 2A and SI Appendix, Figs. S1A and S2A). This class comprised the Sven RNAP initiation complex engaged with the promoter DNA as seen in previously determined RNAP-promoter open complex (RPo) structures from the Actinobacteria Mtb and M. smegmatis (21, 22, 32). However, although the density for the holoenzyme, RbpA, WhiB and WhiA-NTD were clear with side-chain details, the density corresponding to WhiA-CTD and the α-CTD was not well resolved, and neither domain could be modeled with confidence (Fig. 2A). We noted that the angle between the −10, −35, and WhiA motifs on the DNA was 132°, translating to a 48° bend at the −35 motif (Fig. 2A). We hypothesized that if the strain in the DNA was relieved at the vertex (the −35 motif), it might result in better resolution upstream, where the WhiA-CTD and α-CTD are located. Therefore, we repeated the analysis using a sepX promoter DNA with a canonical −35 to facilitate binding, but lacking DNA downstream of the −35, reasoning we would mitigate the strain by removing the DNA downstream of the bend. This second structure was resolved to 3.1 Å (Fig. 2B and SI Appendix, Figs. S1B and S2B). Unexpectedly, a second copy of the DNA was found in the downstream channel and was unwound, as might be expected for a -10 promoter complex (33, 34). Modeling revealed the DNA contained a fortuitous “−10” region with consensus −11A and −7T bases (Fig. 2B) (35). We called this second structure RPo-2. As anticipated, the angle between the −10 motif of the second DNA and the upstream DNA containing the −35 and WhiA motifs was shallower for RPo-2 than RPo (138° versus 132°; Fig. 2B), and this was indeed associated with an increased resolution of the upstream DNA, WhiA-CTD, and α-CTD. We, therefore, focused our analysis on RPo-2.
Fig. 2.
Cryo-EM analysis of Sven WhiA/WhiB σA-RbpA-holoenzyme sepX promoter complex. (A) Top: Sven sepX promoter sequence used for cryo-EM. Only the nt-DNA strand is shown. The promoter elements from upstream to downstream (WhiA-binding motif; rose, −35 element and −10 elements, blue) and the transcription start site (+1, green) are denoted. The nt-strand sequence complementary to the t-strand sequence protected by WhiA in the DNase I footprinting experiment is underlined. The colors of the various subunits and components are annotated in the key to the Right. The Top (non-template) strand and Bottom (template) strand are colored pale and bright yellow, respectively. Bottom: Two views of the locally filtered cryo-EM density map derived from the Sven WhiA/WhiB σA-RbpA-holoenzyme sepX promoter complex (RPo). The angle of the promoter DNA was calculated by PyMOL (The PyMOL Molecular Graphics System, Version 2.23, Schrödinger, LLC). (B) Top: Sven modified sepX upstream promoter sequence used for cryo-EM. The nt-strand sequence complementary to the t-strand sequence protected by WhiA in the DNase I footprinting experiment is underlined. A canonical −35 element was used to replace the native non-canonical −35 to facilitate binding. The fortuitously bound downstream DNA is shown with the sequences that served as “−10” and “+1” for DNA unwinding. Only the non-template (Top) DNA strand is shown. The promoter elements are colored as in (A). Bottom: Two views of the locally filtered cryo-EM density map derived from the “broken” RPo (RPo-2) Sven WhiA/WhiB σA-RbpA-holoenzyme sepX promoter complex. The angle between the two pieces of DNA was calculated by PyMOL as in (A).
WhiB Bridges RNAP and WhiA Interactions on the sepX Promoter.
The cryo-EM structure of the WhiA:WhiB:σA-holoenzyme:sepX initiation complex reveals that one face of WhiB interacts with σA, another interacts with WhiA-NTD, and a third interacts with the DNA (Fig. 3, and SI Appendix, Fig. S3 A and B). The interactions between σA and WhiB are similar to those observed between Mtb σA and WhiB7 or WhiB1 (17, 18, 30). In the case of WhiB and σA4, the WhiB [4Fe-4S] cluster is protected by a substantial interface area of 749 Å2 or a buried surface area of 1,480 Å2 (Fig. 3D), explaining why the cluster is oxygen-insensitive in the WhiB:σA:RbpA complexes. Detailed interactions and density maps of the interfaces are illustrated in Dataset S1 and SI Appendix, Figs. S3A and S4A. Alignments of Actinobacterial WhiA and WhiB homologs demonstrate that 13/14 WhiB residues interacting with WhiA and 14/15 WhiA residues interacting with WhiB are conserved, suggesting this interface is critical for activity (SI Appendix, Fig. S4).
Fig. 3.
Protein–protein interactions of the WhiA/WhiB/RNAP initiation complex. (A) Overall structure of the Sven WhiA/WhiB σA-RbpA-holoenzyme sepX RPo-2 complex demonstrating the spatial configuration of σA4, WhiB, WhiA, α2-CTD, the β′-dock, ω, and the DNA. Proteins discussed in the text and the DNA motifs are colored according to the key on the right. The rest of the σA-RbpA-holoenzyme is shown as a white transparent surface. (B) Zoomed in view of WhiA, WhiB, and σA4 and the upstream DNA from (A) rendered in cartoon. Each feature is colored as labeled in (A). The boundaries of the domain architecture of WhiA are annotated. (C) Top: Close-up view of WhiA and WhiB interaction. Bottom: The complex is “split” and rotated to reveal the interface between each protein, which is highlighted, and the interface area noted. (D) Top: Close-up view of WhiB and σA4 interaction. Bottom: The complex is “split” and rotated to reveal the interface between each protein, which is highlighted, and the interface area noted.
Interaction with WhiB Is Specific to σA.
Consistent with the contacts between WhiB and domain 4 of σA seen in the structure, WhiB interacted directly with σA in an E. coli bacterial adenylate cyclase two-hybrid (BACTH) assay (SI Appendix, Fig. S5A). Removing the [Fe-4S] cluster from WhiB by mutating the cysteine residues that coordinate it (WhiB 4Cys→4Ala) abolished this interaction (SI Appendix, Fig. S5A).
Streptomycetes are unusual in that, in addition to their primary, essential σ factor, σA, (also known as σHrdB), they typically have two or three other Group I σ factors that are non-essential (36–38), and Sven has two of these: σHrdA and σHrdD. Further BACTH analysis revealed that while WhiB interacts with σA, it does not interact with σHrdA or σHrdD (SI Appendix, Fig. S5B). Alignment of the sequences of σA, σHrdA, and σHrdD showed that, although these three Group I σ factors are highly similar, the His-Pro-Ser motif of σA (aa 503 to 505) that interacts with the WhiB [4Fe-4S] cluster is not conserved in σHrdA or σHrdD (SI Appendix, Fig. S5C), providing a molecular explanation for the selective interaction of WhiB with σA.
Structural Architecture of WhiA.
Whereas members of the WhiB family are restricted to the actinomycetes, WhiA is found throughout the Gram-positive bacteria, including non-actinomycetes and bacteria that do not sporulate. The only non-actinomycete in which WhiA has been studied is Bacillus subtilis. In this organism, WhiA binding to DNA facilitates its localization to the nucleoid, where it is proposed to have a direct cell biological role in regulating cell division (39). This suggests that not all WhiA-family members function as transcription factors.
The structure of the WhiA homolog (WhiATM) from Thermotoga maritima (Tma) was previously solved by crystallography (14). However, its biological role has not been investigated, and, like all non-actinomycetes, Tma lacks members of the WhiB family. WhiATM is divided into two structural domains, an NTD and a CTD (14). WhiA from Sven also consists of an NTD and a CTD, but here we delineate an additional feature in the CTD, which we call the long helix (WhiA-LH), to separate its function from the WhiA-CTD HTH motif (Fig. 3B). The WhiA-LH links the NTD to the CTD HTH and, along with the WhiA-NTD and WhiA-CTD HTH, makes DNA contacts (Fig. 3A and discussed in the next section).
WhiA Contacts WhiB and the Core Subunits of RNAP.
WhiA makes minor contacts with RNAP via the ω subunit and the dock domain of the β′ subunit (Fig. 3A and SI Appendix, Figs. S4B and S6 A–C) and forms a more substantial interface of 637 Å2 (or a buried surface area of 1,274 Å2) with WhiB (Fig. 3C). The dock domain helps form the RNA exit channel. The dock interacts with the antitermination protein N (40), presumably affecting RNA conformation during exit. Recently, we found the WhiB7 initiation transcription factor also interacts with the dock (17). WhiA is now the second transcription initiation factor shown to interact with the dock, suggesting this domain serves as a target for regulatory factors. We tested the in vivo contribution of the interface between the WhiA-NTD and core RNAP by mutating three residues in the WhiA-NTD that sit at the interface with the β′-dock domain (K21E, Q98A, and Q115A). This triple mutant, which expressed similarly to the wild-type WhiA (SI Appendix, Fig. S6E), abolished developmental cell division (SI Appendix, Fig. S6D), suggesting the interaction between the β′-dock domain and the WhiA-NTD is critical for activation of the WhiA/B regulon. Therefore, it seems that the extensive interfaces between WhiB:σA4 and WhiB:WhiA-NTD and the minor interface between WhiA and the β′-dock domain provide a scaffold to assemble the entire complex on DNA that results in the activation of transcription (Fig. 1F).
An α-CTD Binds Upstream of the WhiA-Binding Site.
We also observed density for the C-terminal domain of one of the α subunits of RNAP (the one which interacts more with the β′ subunit and is called α2), upstream of the WhiA-binding site (Fig. 2B). The α subunit comprises two domains (41) and forms a dimer via its N-terminal domain (α-NTD) that is required for RNAP assembly (42). The α-NTD is connected to the C-terminal domain (α-CTD) by a flexible linker. The role of the α-CTD in Eco has been well documented: The α-CTD binds to A/T-rich regions upstream of the −35 element (UP-elements), activating transcription at such promoters (43). The Eco α-CTD is also a major target for transcription factors (1, 44), stimulating transcription. The α-CTD interaction with UP-elements in Actinobacteria is less well understood but has been shown to occur in mycobacterial in vitro transcription assays (45). Here we observe density for α2-CTD and the linker to its cognate α2-NTD, with the α2-CTD bound to DNA directly upstream of the WhiA motif (Fig. 3A and SI Appendix, Fig. S6C). Although the Sven α2-CTD binds to the upstream DNA in a way that is reminiscent of the structures of the Eco α-CTD bound to DNA (46), the Sven DNA sequence (CCAGAT) bound by the α2-CTD is not A/T-rich in the same way as the Eco UP-elements. In Eco, E261 in the α-CTD binds to σA4 and is important for activation at UP-elements (25, 47). By contrast, the equivalent residue in Sven α2-CTD (E255) lies 4 Å away from WhiA-CTD, and indeed the entire α2-CTD does not contact the WhiA:WhiB:σA-holoenzyme initiation complex (SI Appendix, Fig. S6C). It, therefore, seems likely that the α-CTDs play a relatively minor role in activating the sepX promoter.
Protein–DNA Interactions Between WhiB and the Promoter.
Both WhiB and WhiA contact the DNA (Figs. 3–5 and 6A and SI Appendix, Figs. S3 and S4). The only other resolved structures of a WhiB family protein in a complex with a target promoter DNA are of Mtb WhiB7; one a cryo-EM structure with holo-RNAP (17) and the other a crystal structure with σA (18). Both structures showed that the DNA-binding motif of WhiB7 was a C-terminal AT-hook that was nestled into the minor groove of an AT-rich motif. Comparing WhiB and WhiB7 interactions with the DNA reveals parallels and differences (Fig. 4A). The main DNA-binding structural motif of WhiB is a conserved loop (residues K37 to K45), which, while not an AT-hook, inserts into the minor groove of the DNA just upstream of the −35 promoter motif. Three conserved C-terminal residues (S75, R77, and E78) make additional interactions with the minor groove via the phosphate backbone (Fig. 4A). The WhiB-interacting minor groove DNA sequence (GCCAATT) is not as AT-rich as the WhiB7 binding motif (AGAAAAT) (Fig. 6A).
Fig. 4.
Protein–DNA interactions of the WhiA/WhiB/RNAP initiation complex. (A) Left: WhiB is magnified from the view shown in (3A), and side chains contacting the DNA in both the major and minor groove are shown in stick and labeled. The [4Fe-4S] cluster is shown as spheres. WhiA and σA4 are removed for clarity. Details of the interactions are included in Dataset S1 and in Fig. 6A. Right: WhiB7 from PDBID:7KIF (17) is shown, highlighting the differences in how each factor interacts with DNA as well as the change in the trajectory of the DNA upstream of the −35 element. 7KIF was aligned to the WhiA/WhiB/RNAP initiation complex by the RNAP (rmsd of 0.810 Å over 2,757 atoms), but only WhiB7 and the DNA are shown. Faint gray lines show that although the beginning of the −35 and the iron cluster align, the contacts between WhiB and the DNA are more intimate, and the DNA curves toward WhiB instead of away as in WhiB7. (B) Interactions between WhiA-NTD and the DNA (WhiA motif; nt-strand is pink and t-strand is deep rose) are shown. The view is rotated 135° around the X-axis from (A). All interactions between the WhiA-NTD involve R177 and R185 and the DNA backbone and are not base-specific. (C) Interactions between WhiA-LH and WhiA-CTD and the promoter DNA. The view is rotated 240° around the X-axis from (A). The recognition helix of WhiA-CTD makes all the base-specific interactions with the WhiA motif [colored as in (B) and bolded GACAC]. Interacting residues are drawn in sticks and labeled. Residues making backbone contacts to the DNA but modeled as Ala due to poor side-chain density are bracketed. (D) View as in (B) showing the surface electrostatic potential of WhiA calculated in PyMOL, demonstrating that the positively charged surface of WhiA clasps the WhiA motif via the NTD, CTD, and the bridging LH. A cartoon view above is shown for reference.
Fig. 5.
WhiA-CTD Interactions with the WhiA Motif mimic σ4/−35 Contacts. (A) Structural alignment of σA4 from Taq (gray) bound to a canonical −35 promoter sequence (TTGACA; blue) (27) to WhiA-CTD (green) bound to the WhiA motif (salmon; GACAC) drawn in cartoon. (B) Comparison of contacts between σA4 with the major groove of the −35 promoter and WhiA-CTD with the major groove of the WhiA motif. WhiA-CTD residues colored red and italicized were chosen for subsequent mutagenesis studies (Fig. 6B). The corresponding residues in σA4 are also colored red. (C) Contacts illustrated in (B) are schematized. Contacts between Taq σA4 (gray) and the −35 promoter sequence (blue) (27) are color-coded as denoted in the key. Homologous residues in WhiA-CTD interacting with the WhiA motif similarly to Taq σA4 with the −35 promoter are boxed in blue. Residues making base-specific interactions are underlined, and the bases are highlighted in pink borders.
Fig. 6.
WhiA Interactions with the WhiA motif are Critical for Cell Division. (A) Schematic of contacts illustrating the interactions between WhiB and WhiA and the DNA. For greater detail, see Dataset S1. Residues making base-specific interactions are underlined, and the bases making base-specific interactions are highlighted in blue borders. Residues bordered in red cause a defect in sporulation septation when mutated, suggesting their interactions are critical for transcriptional activation. R174 and R181 do not make direct contact with the DNA but are noted because they contribute to the positive surface surrounding the DNA and abolish septation when mutated with R177 and R185. (B) Scanning electron micrographs showing the effects of mutating WhiA residues on sporulation septation. whiA mutant alleles driven by the native promoter were cloned into the single-copy integrative vector pIJ10770 and introduced into the S. venezuelae whiA mutant SV11. Mutations that abolish sporulation septation are marked with two yellow asterisks and those that cause a partial defect are marked with one. Representative sporulation septa are indicated by orange arrows. (Scale bar, 2 μm.) WhiA Western blots of the mutants are shown in SI Appendix, Fig. S6E.
Unlike WhiB7, WhiB has a second DNA-interacting motif formed by residue R80 from the C-terminal helix and residue R67 from a loop buttressing the [4Fe-4S] cluster (Fig. 4A). Both residues are conserved (SI Appendix, Fig. S4A) and interact with the phosphate backbone of the −35 major groove (Fig. 4A). It is worth noting that genome-wide ChIP-seq analysis did not identify a consensus WhiB-binding motif (10), suggesting that, unlike WhiB7, WhiB may not interact with any specific sequences.
The interactions between WhiB and the DNA are more extensive than that of WhiB7, and the DNA follows a different trajectory (Fig. 4A). The light gray lines in Fig. 4A demonstrate that although the [4Fe-4S] cluster of WhiB7 and WhiB and the beginning of the −35 align well, the DNA upstream diverges, likely due to the additional interactions afforded by WhiB having two DNA-interacting regions, as well as those mediated by WhiA (discussed in the next section) and α2-CTD.
WhiA Clasps the DNA via its NTD, LH, and CTD.
WhiA interacts with the DNA via three regions. First, the homing endonuclease-like NTD contains two conserved positively charged residues (R177 and R185), which make ionic interactions with the backbone phosphates of the downstream C of the WhiA GACAC motif and the adjacent downstream bases, as well as non-polar interactions with the ribose carbons of the downstream A of the WhiA motif (Fig. 4B and SI Appendix, Figs. S4B and S6A).
The LH of WhiA also provides multiple contacts to the minor groove downstream of the WhiA motif (Fig. 4C and 6A), all with the DNA backbone. These observations suggest that, like the NTD, the LH does not contribute to sequence-specific contacts. Instead, the base-specific contacts with the WhiA motif are mediated by conserved amino acids in the recognition helix of the HTH-fold of the WhiA-CTD (Fig. 4C). Together, the WhiA-NTD, LH, and CTD encircle the WhiA motif and flanking DNA via a positively charged surface (Fig. 4D). WhiA thus forms an unusual clamp that interacts with the DNA via several interfaces, including the major and minor groove of the WhiA motif and the downstream neighboring minor groove.
Overall, the protection observed in the DNase I footprint closely matches the WhiA-DNA structural observations. Most of the WhiA-DNA contacts seen in the structure fall within the DNase I footprint (Fig. 1C), but a few (F225, N229, R221, and L222; Fig. 6A) from the LH domain that contact the non-template strand (which was not footprinted) fall on the edge of the footprint determined on the template strand. One explanation is certain contacts from the NTD, and the LH domain of WhiA may only form in the presence of WhiB and RNAP.
WhiA-CTD Interactions With the WhiA Motif Are Similar to σA4 Interactions With a Consensus −35 Promoter Element.
The WhiA-CTD contains an HTH, a protein structural motif commonly used for DNA binding. It was previously noted that the WhiA-CTD from Tma bears similarity to other HTHs (14), including that of σA4 (27). However, a structure of the WhiA-CTD bound to DNA was not available. A superposition of Taq σA4 bound to a consensus −35 element (27) with the WhiA-CTD results in a close alignment of the bound DNAs (Fig. 5A). Notably, the last four nucleotides of the t-strand of the consensus −35 element “TTGACA” are identical in sequence and position to the first four nucleotides of the t-strand of the WhiA motif “GACAC” (Fig. 5). These four bases make most of the base-specific interactions from the t-strand of the −35 motif (Fig. 5C). Remarkably, WhiA-CTD uses homologous or identical residues to make similar base-specific and backbone interactions with the t-strand of the GACA motif as σA4 makes with the corresponding bases of the −35 motif (Fig. 5 B and C). Thus, although sequence alignments did not pick up similarity at the primary amino acid level, WhiA-CTD and σA4 not only share a similar tertiary structure, but they also share a similar cognate DNA recognition motif and the structural determinants to recognize that motif.
WhiA-NTD Zinc Binding Residues Are Not Required For WhiA Function.
The WhiA-NTD coordinates a Zn2+-ion via C18, C19, and C122 (SI Appendix, Fig. S6B). We tested if Zn2+ coordination was critical for WhiA activity by substituting the three Cys residues with Ala. The triple Cys > Ala mutant restored wild-type sporulation to the whiA null mutant (Fig. 6B) despite lower expression levels (SI Appendix, Fig. S6E). We conclude that the Zn2+ coordination is not required for WhiA function. We note that the Cys residues are not conserved among WhiA homologs (SI Appendix, Fig. S4B) and that a Zn2+-ion is not present in the structure of the WhiA homolog from Tma (14).
WhiA-NTD Interactions With the DNA Are Critical For Sporulation-Specific Cell Division.
We then probed the interaction between the positively charged surface of the homing endonuclease-like WhiA-NTD and the DNA, including residues R174, R177, R181, and R185, which contact or approach the DNA phosphate backbone in the WhiA motif and downstream. Substituting these residues with Glu prevented septation, suggesting that this positively charged interface encircling the DNA is essential for WhiA activation function (Fig. 6B).
WhiA-CTD Interactions With the WhiA Motif Are Essential for Sporulation-Specific Cell Division.
We then tested the in vivo contribution of the interface between the WhiA-CTD and DNA by mutating selected residues, focusing on those that contact the WhiA GACAC motif. Substitutions R266E, L274A, and K287E abolished developmental cell division, while the R293E and R295E substitutions caused a partial defect (Fig. 6B). We conclude that the WhiA-CTD contacts with the WhiA GACAC motif are also essential for WhiA function. The single substitution mutations with the most severe defects in sporulation septation were R266E, which makes backbone interactions, L274A, and K287E. K287 was chosen because, like the equivalent residue in Taq σA4 (R409), which makes base-specific contacts with the −35 element (Fig. 5 B and C), K287 is positioned to make a base-specific interaction with N7 of the G opposite the underlined C in the “GACAC” WhiA motif. The K287-WhiA motif contact is the only base-specific hydrogen bond we observed. The K287E substitution abolished developmental cell division (Fig. 6B), demonstrating that this base-specific interaction is critical for activation of the WhiA/B regulon. Although L274 does not contact the backbone or base of the WhiA motif, it interacts with K287 and positions it to interact with the DNA.
In sum, the structure-guided mutagenesis/phenotypic studies confirm that both the DNA backbone-interacting residues of the WhiA-NTD and CTD and the base-specific interacting residues, K287 and R295, plus the supporting L274 residue, are critical for activation of the sporulation septation genes. Furthermore, we note that all the substitutions which led to defects in developmental septation phenotypes are conserved in WhiA homologs (SI Appendix, Fig. S4B). Importantly, because the local resolution around WhiA-CTD is around 4 to 5 Å, the mutagenesis validates the interactions proposed by the model.
Discussion
In conclusion, we present the structure of a Sven RNAP initiation complex composed of the RNAP holoenzyme bound to the sepX promoter with the two activators WhiA and WhiB. This study presents the first structure of RNAP from Streptomyces, a genus that serves as our most abundant source of clinically important antibiotics. We find that WhiB bridges WhiA and RNAP by binding to WhiA-NTD and σA, as well as to the DNA. The WhiA-NTD is related to homing endonucleases, but WhiA-NTD does not bind DNA via the same interface found in the homing endonucleases (48). Indeed, the two amino acids in the WhiA-NTD that contact the DNA, R177, and R185 are not conserved in I-DmoI (14), a homing endonuclease with a similar fold. Instead, the homing endonuclease domain serves to link WhiB with the WhiA-CTD—the domain that makes the base-specific interactions.
WhiA-CTD was predicted to interact with DNA in a manner similar to other HTHs, based on the crystal structure of WhiA from Tma (14). However, it was only recently demonstrated that WhiA is a transcription factor and that it functions cooperatively with WhiB to co-control the expression of a common set of WhiA/B target genes that trigger developmental cell division (10). Although WhiA is widespread in Gram-positive bacteria, WhiB family proteins are confined to the Actinobacteria, and the function of WhiA in other clades is unknown. The similarity between the interactions of WhiA-CTD with the WhiA motif and those of σA4 with the −35 element was unexpected. We show by structure-guided mutagenesis that these conserved interactions are functionally important.
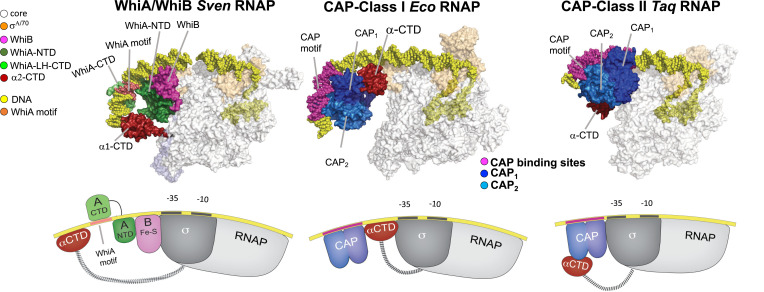
The WhiA/WhiB architectures represent a new class of transcriptional activators with notable parallels and differences from structures of the CAP Class I and II activator complexes in Eco and Taq (Fig. 7) (24–26). The different activation paradigms established in Eco are defined by the position of the CAP dimer relative to the promoter and RNAP, as well as its mechanism of activation (1, 49–51). In Class I, the α-CTD binds immediately upstream of the −35, between σA4 and a homodimer of CAP bound to the CAP DNA motif, as elucidated by biochemical, genetic, and structural studies. Protein–protein contacts between the neighboring CAP monomer and the α-CTD are essential for activation (52, 53) (Fig. 7), and the Class I activation mechanism is by recruitment of RNAP to the promoter (1). In Class II, CAP binds directly upstream of the −35 element, contacts σA4, and an α-CTD binds upstream of the CAP dimer (Fig. 7), except the α-CTD was not observed in its anticipated position and is illustrated as seen in the crystal structure (24). Protein–protein contacts between the downstream CAP monomer and RNAP and between the upstream CAP monomer and the bound α-CTD are important for transcription activation (53). The Eco CAP Class II activation mechanism is described as a combination of RNAP recruitment and isomerization of the promoter to the open complex state (1).
Fig. 7.
Comparison of the structures of the WhiA/WhiB, CAP Class I, and CAP Class II initiation complexes. Top: Cryo-EM structure of the WhiA/WhiB Sven RNAP initiation structure, cryo-EM the CAP Class I Eco RNAP initiation complex (25), and the crystal structure of CAP (TAP) -Class II Taq initiation complex (24). Bottom: Schematics illustrating the position of the α-CTD relative to σ and the transcription activators (CAP or WhiA/B) for each of these structures.
While the trajectory of the DNA in the WhiA/B activation complex is more similar to the Class I complex architecture, the protein organization more closely resembles the Class II activation complex, where WhiA/B serves as a surrogate for the CAP dimer: An α-CTD is also situated upstream. In the Class II system, the evidence for the α-CTD binding DNA upstream of the activators is biochemical and genetic (1); however, this placement of the α-CTD was not observed in the two Class II structures (Fig. 7). It should be noted that a structure of RNAP from the pathogenic γ-proteobacterium, Francisella tularensis, in complex with three virulence transcription factors (PigR, MglA, and SspA) on a virulence promoter revealed the placement of both α-CTDs (54). Unlike the CAP, WhiA, and WhiB factors, PigR, MglA, and SspA bind between the −10 and −35 (similarly to RbpA), and the α-CTDs overlap with WhiB and WhiA-NTD of the WhiA/B activation complex. Therefore, the observed position of WhiA-CTD and the α-CTD is thus far unique. In summary, the WhiA/B activation structure presented here establishes a distinct mechanism in bacterial transcription activation and provides the first view of an α-CTD bound upstream of activators that bind upstream of the −35. The structure of the WhiA/WhiB activator complex and the structures from F. tularensis highlight the need to look at transcriptional mechanisms in organisms beyond Eco and other classic model systems.
The asymmetric GACAC WhiA-binding sequence occurs on the non-template strand in some WhiAB target promoters, like sepX, but on the template strand in others (11). In addition, the spacing relative to the −35 is also variable (11). It seems likely that the N-terminal homing endonuclease-like domain of WhiA packs against WhiB and RNAP at all target promoters in the manner seen in the current structure on the sepX promoter, and that the long linker between the N-terminal and C-terminal domains of WhiA provides the flexibility to allow the C-terminal HTH domain to bind the GACAC motif in either orientation and varying distance from the −35.
Transcription of the whiB gene is tightly regulated, being subject to direct repression by the master repressor of development BldD (55) and by a dedicated whiB-specific repressor, BldO (56), and to direct activation by BldM (57). By contrast, Westerns show that WhiA is constitutively expressed across the life cycle, but ChIP-seq reveals that WhiA is only bound to its target promoters at the onset of sporulation (11). This is because WhiA and WhiB function cooperatively, and so WhiA only binds its target promoters when WhiB is also present in the cell (10). Consequently, the regulation of whiB expression is key in controlling the switch between hyphal growth and sporulation. It is also notable that the [4Fe-4S] clusters of WhiB family proteins are generally reactive toward O2 and reactive oxygen species, and extremely reactive to nitric oxide (58), leading to suggestions that WhiB-like proteins might function as sensors of oxidative and/or nitrosative stress (16).
WhiB can associate with holoenzyme via complex formation with σA and RbpA, but neither WhiA nor WhiB binds their regulons in vivo without each other (10). This suggests that WhiA likely provides the specificity to the WhiB-bound holoenzyme via the WhiA motif. Conversely, the interaction between WhiB holoenzyme and DNA would also likely stabilize WhiA contacts with its motif. This mechanism explains the mutual dependence of the factors for binding and activation (Fig. 1). The coordination of these two factors allows for an increased layer of regulation compared to a homodimeric complex like CAP. This exquisite regulation controls a key step in the sophisticated life cycle of Sven and is reminiscent of transcriptional activation in eukaryotes, which often requires multiple transcription factors. Lastly, WhiA and WhiB are individually essential in Mtb because they are required for cell division in this major pathogen. However, adaptive mutations in the coding sequences and promoters of whiA and whiB are positively selected in clinical isolates of Mtb, suggesting roles in virulence and/or resistance to drug treatment (5). Our work will now allow the mapping of these mutations onto the structure, providing functional insight into the adaptive nature of these mutations in both virulence and antibiotic resistance.
Materials and Methods
Detailed descriptions of the following are provided in SI Appendix: Constructions of strains and plasmids for expression and purification of Sven RNAP, σA, RbpA, WhiA, and WhiB proteins; protein purification methods; preparation of RNAP σA-holoenzyme complexes with RbpA, WhiA, WhiB, and the sepX promoter for transcription assays and cryo-EM grid preparation; in vitro transcription assays; cryo-EM data acquisition and processing; model building and refinement of structures; generation of whiA mutants and Western analysis in Sven; scanning electron microscopy of whiA mutants; bacterial two-hybrid assays. A complete list of oligonucleotides, strains, and plasmids used is provided in SI Appendix, Table S2.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (TXT)
Dataset S02 (TXT)
Acknowledgments
We thank members of the Darst-Campbell and Buttner Laboratories for helpful discussions and Susan Schlimpert for help with the figures. We thank Seth A. Darst for critical reading of and feedback on the manuscript. We thank M. Ebrahim, J. Sotiris, and H. Ng at The Rockefeller University, and Carolina Hernandez at NYSBC, Evelyn Gruss Lipper Cryo-electron Microscopy Resource Center for help with cryo-EM data collection. Some of the work was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy, located at the New York Structural Biology Center, supported by grants from the Simons Foundation (SF349247), New York State Office of Science, Technology and Academic Research, and the NIH National Institute of General Medical Sciences (GM103310). This work was supported by NIH grant R01 GM114450 to E.A.C. and by BBSRC Institute Strategic Program Grant BB/J004561/1 to the John Innes Centre.
Author contributions
M.L., M.J. Buttner, and E.A.C. designed research; M.L., N.A.H., M.J. Bush, A.K.M., D.A.W., K.C.F., Y.J.C., and E.A.C. performed research; M.L., N.A.H., M.J. Bush, R.F., S.K., M.J. Buttner, and E.A.C. analyzed data; and M.L., N.A.H., M.J. Bush, M.J. Buttner, and E.A.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Mark J. Buttner, Email: mark.buttner@jic.ac.uk.
Elizabeth A. Campbell, Email: campbee@rockefeller.edu.
Data, Materials, and Software Availability
The cryo-EM density maps have been deposited in the Electron Microscopy Data Bank under accession codes EMD-27777 (RPo) (59) and EMD-27778 (RPo-2) (60). The atomic coordinates have been deposited in the Protein Data Bank under accession codes 8DY7 (RPo) (61) and 8DY9 (RPo-2) (62). The source code used to identify amino acid contacts is publicly available online on GitHub (https://github.com/darst-campbell-lab/residue-contacts-app). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request (campbee@rockefeller.edu).
Supporting Information
References
- 1.Busby S., Ebright R. H., Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293, 199–213 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Chen J., Boyaci H., Campbell E. A., Diverse and unified mechanisms of transcription initiation in bacteria. Nat. Rev. Microbiol. 19, 95–109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch B., et al. , Genome-wide gene expression tuning reveals diverse vulnerabilities of M. tuberculosis. Cell 184, 4579–4592.e24 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeJesus M. A., et al. , Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8, e02133–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q., et al. , Tuberculosis treatment failure associated with evolution of antibiotic resilience. Science 378, 1111–1118 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush M. J., Tschowri N., Schlimpert S., Flärdh K., Buttner M. J., c-di-GMP signalling and the regulation of developmental transitions in streptomycetes. Nat. Rev. Microbiol. 13, 749–760 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Flärdh K., Buttner M. J., Streptomyces morphogenetics: Dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 7, 36–49 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Flärdh K., Richards D. M., Hempel A. M., Howard M., Buttner M. J., Regulation of apical growth and hyphal branching in Streptomyces. Curr. Opin. Microbiol. 15, 737–743 (2012). [DOI] [PubMed] [Google Scholar]
- 9.McCormick J. R., Flärdh K., Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 36, 206–231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush M. J., Chandra G., Bibb M. J., Findlay K. C., Buttner M. J., Genome-wide chromatin immunoprecipitation sequencing analysis shows that WhiB is a transcription factor that cocontrols its regulon with WhiA to initiate developmental cell division in Streptomyces. mBio 7, e00523–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush M. J., Bibb M. J., Chandra G., Findlay K. C., Buttner M. J., Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae. mBio 4, e00684–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser B. K., Stoddard B. L., DNA recognition and transcriptional regulation by the WhiA sporulation factor. Sci. Rep. 1, 156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey T. L., Elkan C., Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2, 28–36 (1994). [PubMed] [Google Scholar]
- 14.Kaiser B. K., Clifton M. C., Shen B. W., Stoddard B. L., The structure of a bacterial DUF199/WhiA protein: Domestication of an invasive endonuclease. Structure 17, 1368–1376 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knizewski L., Ginalski K., Bacterial DUF199/COG1481 proteins including sporulation regulator WhiA are distant homologs of LAGLIDADG homing endonucleases that retained only DNA binding. Cell Cycle 6, 1666–1670 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Bush M. J., The actinobacterial WhiB-like (Wbl) family of transcription factors. Mol. Microbiol. 110, 663–676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilic M., Darst S. A., Campbell E. A., Structural basis of transcriptional activation by the Mycobacterium tuberculosis intrinsic antibiotic-resistance transcription factor WhiB7. Mol. Cell 81, 2875–2886.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan T., et al. , Structural insights into the functional divergence of WhiB-like proteins in Mycobacterium tuberculosis. Mol. Cell 81, 2887–2900.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess R. R., Separation and characterization of the subunits of ribonucleic acid polymerase. J. Biol. Chem. 244, 6168–6176 (1969). [PubMed] [Google Scholar]
- 20.Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. F., Factor stimulating transcription by RNA polymerase. Nature 221, 43–46 (1969). [DOI] [PubMed] [Google Scholar]
- 21.Hubin E. A., Lilic M., Darst S. A., Campbell E. A., Structural insights into the mycobacteria transcription initiation complex from analysis of X-ray crystal structures. Nat. Commun. 8, 16072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubin E. A., et al. , Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA. eLife 6, e22520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubin E. A., et al. , Structural, functional, and genetic analyses of the actinobacterial transcription factor RbpA. Proc. Natl. Acad. Sci. U.S.A. 112, 7171–7176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y., Zhang Y., Ebright R. H., Structural basis of transcription activation. Science 352, 1330–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B., Hong C., Huang R. K., Yu Z., Steitz T. A., Structural basis of bacterial transcription activation. Science 358, 947–951 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Shi W., Jiang Y., Deng Y., Dong Z., Liu B., Visualization of two architectures in class-II CAP-dependent transcription activation. PLOS Biol. 18, e3000706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell E. A., et al. , Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol. Cell 9, 527–539 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Bush M. J., Gallagher K. A., Chandra G., Findlay K. C., Schlimpert S., Hyphal compartmentalization and sporulation in Streptomyces require the conserved cell division protein SepX. Nat. Commun. 13, 71 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crack J. C., et al. , Characterization of [4Fe-4S]-containing and cluster-free forms of Streptomyces WhiD. Biochemistry 48, 12252–12264 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan T., et al. , Structural basis of non-canonical transcriptional regulation by the σA-bound iron-sulfur protein WhiB1 in M. tuberculosis. Nucleic Acids Res. 48, 501–516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart M. Y. Y., Bush M. J., Crack J. C., Buttner M. J., Le Brun N. E., Interaction of the Streptomyces Wbl protein WhiD with the principal sigma factor σHrdB depends on the WhiD [4Fe-4S] cluster. J. Biol. Chem. 295, 9752–9765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyaci H., et al. , Fidaxomicin jams Mycobacterium tuberculosis RNA polymerase motions needed for initiation via RbpA contacts. Elife 7, e34823 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae B., Feklistov A., Lass-Napiorkowska A., Landick R., Darst S. A., Structure of a bacterial RNA polymerase holoenzyme open promoter complex. Elife 4, e08504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyaci H., Chen J., Jansen R., Darst S. A., Campbell E. A., Structures of an RNA polymerase promoter melting intermediate elucidate DNA unwinding. Nature 565, 382–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harley C. B., Reynolds R. P., Analysis of E. coli promoter sequences. Nucleic Acids Res. 15, 2343–2361 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buttner M. J., Chater K. F., Bibb M. J., Cloning, disruption, and transcriptional analysis of three RNA polymerase sigma factor genes of Streptomyces coelicolor A3(2). J. Bacteriol. 172, 3367–3378 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buttner M. J., Lewis C. G., Construction and characterization of Streptomyces coelicolor A3(2) mutants that are multiply deficient in the nonessential hrd-encoded RNA polymerase sigma factors. J. Bacteriol. 174, 5165–5167 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka K., Shiina T., Takahashi H., Multiple principal sigma factor homologs in Eubacteria: Identification of the “rpoD box”. Science 242, 1040–1042 (1988). [DOI] [PubMed] [Google Scholar]
- 39.Surdova K., et al. , The conserved DNA-Binding protein WhiA is involved in cell division in Bacillus subtilis. J. Bacteriol. 195, 5450–5460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krupp F., et al. , Structural basis for the action of an all-purpose transcription anti-termination factor. Mol. Cell 74, 143–157.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Igarashi K., Ishihama A., Bipartite functional map of the E. coli RNA polymerase alpha subunit: Involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell 65, 1015–1022 (1991). [DOI] [PubMed] [Google Scholar]
- 42.Zhang G., Darst S. A., Structure of the Escherichia coli RNA polymerase alpha subunit amino-terminal domain. Science 281, 262–266 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Gourse R. L., Ross W., Gaal T., UPs and downs in bacterial transcription initiation: The role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37, 687–695 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Hochschild A., Dove S. L., Protein–protein contacts that activate and repress prokaryotic transcription. Cell 92, 597–600 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Hubin E. A., Lilic M., Darst S. A., Campbell E. A., Structural insights into the mycobacteria transcription initiation complex from analysis of X-ray crystal structures. Nat. Commun. 8, 16072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benoff B., et al. , Structural basis of transcription activation: The CAP-αCTD-DNA complex. Science 297, 1562–1566 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Ross W., An intersubunit contact stimulating transcription initiation by E. coli RNA polymerase: Interaction of the alpha C-terminal domain and sigma region 4. Genes Dev. 17, 1293–1307 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chevalier B. S., Homing endonucleases: Structural and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Res. 29, 3757–3774 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busby S., et al. , Transcription activation by the Escherichia coli cyclic AMP. J. Mol. Biol. 241, 341–352 (1994). [DOI] [PubMed] [Google Scholar]
- 50.Kolb A., et al. , E. coli RNA polymerase, deleted in the C-terminal part of its α-subunit, interacts differently with the cAMP-CRP complex at the lac P1 and at the gal P1 promoter. Nucleic Acids Res. 21, 319–326 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niu W., Kim Y., Tau G., Heyduk T., Ebright R. H., Transcription activation at class II CAP-dependent promoters: Two Interactions between CAP and RNA polymerase. Cell 87, 1123–1134 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y., Zhang X., Ebright R. H., Identification of the activating region of catabolite gene activator protein (CAP): Isolation and characterization of mutants of CAP specifically defective in transcription activation. Proc. Natl. Acad. Sci. U.S.A. 90, 6081–6085 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y., Merkel T. J., Ebright R. H., Characterization of the activating region of Escherichia coli catabolite gene activator protein (CAP) II. Role at class I and class II CAP-dependent promoters. J. Mol. Biol. 243, 603–610 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Travis B. A., et al. , Structural basis for virulence activation of Francisella tularensis. Mol. Cell 81, 139–152.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tschowri N., et al. , Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell 158, 1136–1147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bush M. J., Chandra G., Findlay K. C., Buttner M. J., Multi-layered inhibition of Streptomyces development: BldO is a dedicated repressor of whiB. Mol. Microbiol. 104, 700–711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Bassam M. M., Bibb M. J., Bush M. J., Chandra G., Buttner M. J., Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet. 10, e1004554 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crack J. C., et al. , Mechanistic insight into the nitrosylation of the [4Fe-4S] cluster of WhiB-like proteins. J. Am. Chem. Soc. 133, 1112–1121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lilic M., et al. , Streptomyces venezuelae RNAP transcription open promoter complex with WhiA and WhiB transcription factors. https://emdataresource.org/EMD-27777. Deposited 8 August 2022.
- 60.Lilic M., et al. , Streptomyces venezuelae RNAP unconstrained open promoter complex with WhiA and WhiB transcription factors. https://emdataresource.org/EMD-27778. Deposited 8 August 2022.
- 61.Lilic M., et al. , Streptomyces venezuelae RNAP transcription open promoter complex with WhiA and WhiB transcription factors. https://www.rcsb.org/structure/8DY7. Deposited 8 August 2022.
- 62.Lilic M., et al. , Streptomyces venezuelae RNAP unconstrained open promoter complex with WhiA and WhiB transcription factors. https://www.rcsb.org/structure/8DY9. Deposited 8 August 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (TXT)
Dataset S02 (TXT)
Data Availability Statement
The cryo-EM density maps have been deposited in the Electron Microscopy Data Bank under accession codes EMD-27777 (RPo) (59) and EMD-27778 (RPo-2) (60). The atomic coordinates have been deposited in the Protein Data Bank under accession codes 8DY7 (RPo) (61) and 8DY9 (RPo-2) (62). The source code used to identify amino acid contacts is publicly available online on GitHub (https://github.com/darst-campbell-lab/residue-contacts-app). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request (campbee@rockefeller.edu).