Abstract
Objective.
To assess phonatory function and wound healing of a tissue-engineered vocal fold mucosa (TE-VFM) in rabbits. An “artificial” vocal fold would be valuable for reconstructing refractory scars and resection defects, particularly one that uses readily available autologous cells and scaffold. This work implants a candidate TE-VFM after resecting native epithelium and lamina propria in rabbits.
Study Design.
Prospective animal study.
Setting.
Research laboratory.
Subjects and Methods.
Rabbit adipose-derived stem cells were isolated and cultured in three-dimensional fibrin scaffolds to form TE-VFM. Eight rabbits underwent laryngofissure, unilateral European Laryngologic Society type 2 cordectomy, and immediate reconstruction with TE-VFM. After 4 weeks, larynges were excised, phonated, and examined by histology.
Results.
Uniform TE-VFM implants were created, with rabbit mesenchymal cells populated throughout fibrin hydrogels. Rabbits recovered uneventfully after implantation. Phonation was achieved in all, with mucosal waves evident at the implant site. Histology after 4 weeks showed resorbed fibrin matrix, continuous epithelium, and mildly increased collagen relative to contralateral unoperated vocal folds. Elastic fiber appearance was highly variable. Inflammatory cell infiltrate was limited to animals receiving sex-mismatched implants.
Conclusion.
TE-VFMs were successfully implanted into 8 rabbits, with minor evidence of scar formation and immune reaction. Vibration was preserved 4 weeks after resecting and reconstructing the complete vocal fold cover layer. Further studies will investigate the mechanism and durability of improvement. TE-VFM with autologous cells is a promising new approach for vocal fold reconstruction.
Keywords: vocal fold, mucosa, larynx, cordectomy, rabbit, phonation, tissue engineering
Injury or loss of the vibratory vocal fold lamina propria and epithelium (together known as the vocal fold cover layer [VFCL]) has been inadequately treated. The lamina propria’s unique extracellular matrix (ECM) of aligned collagen fibers, transverse elastic fibers, and interspersed hyaluronic acid is thus far irreplaceable. Rehabilitation after scarring has therefore attempted to minimize glottic insufficiency to compensate for the ECM disruption.1 Numerous surgical techniques have been proposed to lessen the scar’s impact, including scar-releasing incisions, angiolytic laser therapy,2 and subepithelial fascia implants.3,4 While some patients have modest vocal improvement with these techniques, voice normalization is rare. Accordingly, clinical emphasis on scar prevention has prevailed.5 Investigational cell injection therapy holds promise for reducing the impact of scarring and is the subject of ongoing clinical trials worldwide.6,7 A cell injection approach is attractively simple but may not be sufficient to repair severe scars. An implantable cell-based therapy to restore complete VFCL structure at the time of disease extirpation remains desirable. While the initial envisioned treatment scenario for such an implant would be after complete scar resection, it could eventually be found safe and effective for reconstruction of cancer defects as well. However, implantation of a cell-based tissue-engineered vocal fold mucosa (TE-VFM) has not yet been reported in an animal model.
We have developed a 3-dimensional implantable structure containing adipose-derived mesenchymal stem cells (ASCs) within a fibrin scaffold.8,9 This method creates a 3-dimensional autologous implant with mechanical characteristics similar to the native vocal fold mucosa.10 Here we report the implantation of this vocal fold mucosa replacement in a series of rabbits. Unlike prior studies of cell injections within the lamina propria,11–20 tissue transplants,21,22 or acellular matrix implants,23–25 we have implanted a completely tissue-engineered cell-based construct to replace the lamina propria and epithelial layers of the vocal fold. Our previous work developed a surgical technique for implanting rabbit vocal folds, where an autologous graft was applied on top of the thyroarytenoid muscle after resection of the entire membranous vocal fold epithelium and lamina propria21; satisfactory wound healing was found after orthotopic grafting of the native vocal fold mucosa. We hypothesize here that grafting a TE-VFM is similarly feasible and will also result in satisfactory wound healing to produce phonatory vibration. Our objective is to investigate the degrees of scar formation, ECM remodeling, and phonatory impairment after TE-VFM implantation in rabbits.
Materials and Methods
Adipose-Derived Stem Cells and TE-VFM Constructs
Rabbit adipose-derived stem cells (rASCs) were isolated from male rabbits and tissue-engineered constructs created as previously described.8,9,26 Briefly, rASCs were harvested from inguinal fat by collagenase digestion and expanded in culture. Mesenchymal differentiation potential was confirmed by chemical induction to mineral forming and adipogenic phenotypes.8 Undifferentiated rASCs were embedded within polymerized rabbit fibrinogen in Transwell inserts. Resultant neotissue constructs were cylindrical, 12 mm in diameter, and 3 to 4 mm thick. They were cultured with an air interface and were bottom-fed DMEM culture medium containing 10% fetal bovine serum and 100 ng/mL of epidermal growth factor. After 2 weeks, they were harvested for implantation or histology.
Vocal Fold Implant Surgery
The Institutional Animal Care and Use Committee approved this study. Eight New Zealand white rabbits underwent survival surgery for VFCL removal and implantation with TE-VFM. Four male rabbits (Nos. 1–4) and 4 female rabbits (Nos. 5–8) each weighing 3 to 3.5 kg were used. The vocal fold excision procedure was performed as previously described.21 Briefly, rabbits were anesthetized, and the larynx exposed through a neck incision. Tracheotomy was performed, followed by midline laryngofissure. The membranous cover layer was resected from the left inferior vocal fold division with a Beaver blade. Dissection began at the anterior commissure, and the entire cover layer was elevated posteriorly to the vocal process of the arytenoid cartilage where it was transected and removed, leaving exposed thyroarytenoid muscle. The resected cover was saved for histology. Next the TE-VFM was cut to fit the defect and secured in position with 4 sutures of 6–0 plain gut. The laryngofissure and tracheotomy were closed with 4–0 Prolene and the rabbit converted back to mask anesthesia for neck wound closure. Dexamethasone and antibiotics were administered for 3 days.
Endoscopy and In Vivo Phonation
Experimental animals 1 and 2 underwent endoscopy under anesthesia at 2 weeks postimplantation to assess the gross wound healing at that early time point. Animals were anesthetized with inhaled isoflurane delivered by mask, and a pediatric straight laryngoscope and 0° endoscope with CCD camera were inserted to visualize the vocal folds. Because no major abnormality was identified in the first 2 subjects and the procedure was invasive and time-consuming, endoscopy was not performed on subsequent animals.
Animals 1 and 2 underwent in vivo phonation at 4 weeks postimplantation as previously described.21 After a neck incision and tracheotomy, the vocal folds were exposed superiorly via thyrohyoid pharyngotomy. The epiglottis and superior thyroid cartilage were resected for clear visualization. A second endotracheal tube supplied air upward through the glottis, and manual pressure on the thyroid cartilage adducted the vocal folds. Vibration was recorded with a high-speed digital video camera (Phantom v210; Vision Research Inc, Wayne, New Jersey) at 8000 frames per second. After phonation, the rabbit was euthanized and the larynx frozen at −80°C before excised phonation. Animals 3 to 8 did not undergo in vivo phonation, because better experimental control and visualization were achieved with immediate excised larynx phonation, without disruption of the microstructure from freezing.
Excised Phonation and Kymography
Animals 3 to 8 were euthanized and larynges excised for phonation. Larynges from animals 1 and 2 were thawed. The epiglottis and superior edges of the thyroid cartilage were removed for visualization, and a single adduction suture was placed through both vocal processes. A large-animal larynx phonation setup has been previously described.10 A custom adapter fabricated to fit the rabbit larynx was mounted on the tracheal pipe. Airflow was supplied through the glottis at 500 mL/s, and subglottic pressure was measured with a digital manometer. A high-speed digital video camera captured vibration at 10,000 frames per second. A 2-second segment of each larynx’s vibration was converted to kymogram via a Matlab algorithm performed at the midpoint of the membranous vocal folds.
Histology and Immunohistochemistry
After excised phonation, larynges were split in the posterior midline to separate the vocal folds, trimmed to fit the vocal folds in specimen cassettes, and positioned for axial sectioning. Specimens were formalin fixed and paraffin embedded. Larynges 1 and 2 had been frozen prior to excised phonation. Their histology demonstrated artifact that distorted the microstructure, so larynges 3 to 8 were never frozen. Stains included hematoxylin and eosin, elastic van Gieson (EVG), and Masson’s trichrome for collagen. Extra TE-VFM and the resected rabbit VFCL were also processed for microscopy. Immunohistochemistry used mouse monoclonal primary antibodies against vimentin (clone AMF17B, Developmental Studies Hybridoma Bank, University of Iowa) and pancytokeratin (Abcam ab961).
Results
VFCL Resection and TE-VFM
Resected VFCL and TE-VFM samples were examined histologically (Figure 1). Resection specimens demonstrated complete excision of epithelium and lamina propria and a small amount of thyroarytenoid muscle equivalent to a European Laryngological Society type II-III cordectomy or Imaizumi transmucosal-transmuscular injury.27 The normal appearance of rabbit lamina propria elastin and collagen is demonstrated in Figure 1A and 1B. TE-VFM at the time of implantation shows a bland fibrin microstructure without appreciable elastin or collagen (Figure 1C).
Figure 1.

Histology. Excised rabbit vocal fold cover layer at 4× (column A) and 40× (column B); tissue-engineered vocal fold mucosa at implantation, 40× (column C). Hematoxylin and eosin (row 1), Verhoeff’s elastic van Gieson (row 2), and Masson’s trichrome (row 3).
Immunofluorescence demonstrated the mesenchymal marker vimentin in all TE-VFM cells, including the superficial layer, in contrast to the excised VFCL, which does not express vimentin in the epithelium (Figure 2). Cells in the TE-VFM did not demonstrate pan-cytokeratin as an epithelial marker (data not shown). Together, these findings indicate that the rASCs in the construct did not differentiate to an epithelial phenotype in vitro.
Figure 2.
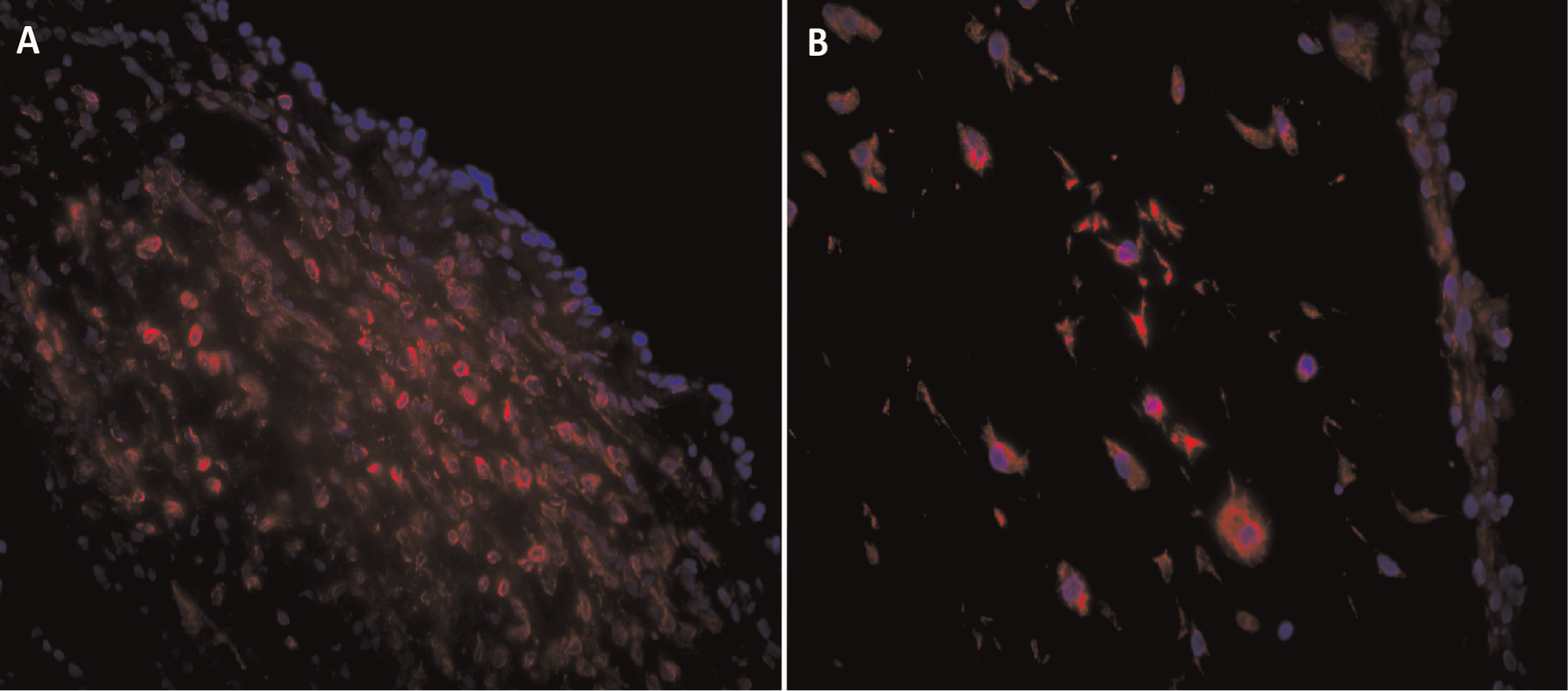
Immunofluorescent labeling: (A) vocal fold cover layer showing vimentin expression throughout the lamina propria but not the epithelium; (B) tissue-engineered vocal fold mucosa showing vimentin expression in all cells. Vimentin, red; nuclei, blue; 40×.
Surgical Recovery
All 8 rabbits undergoing vocal fold implant surgery survived the 1-month postoperative period. Rabbits 1 and 2 underwent endoscopy at 2 weeks postoperatively to assess early wound healing. This showed grossly intact wound sites (Figure 3). Edema and edge irregularity relative to the contralateral unoperated vocal fold were noted, but no hematoma, exudate, or ulcer was seen. Because of these reassuring findings, subsequent animals did not undergo the invasive procedure.
Figure 3.
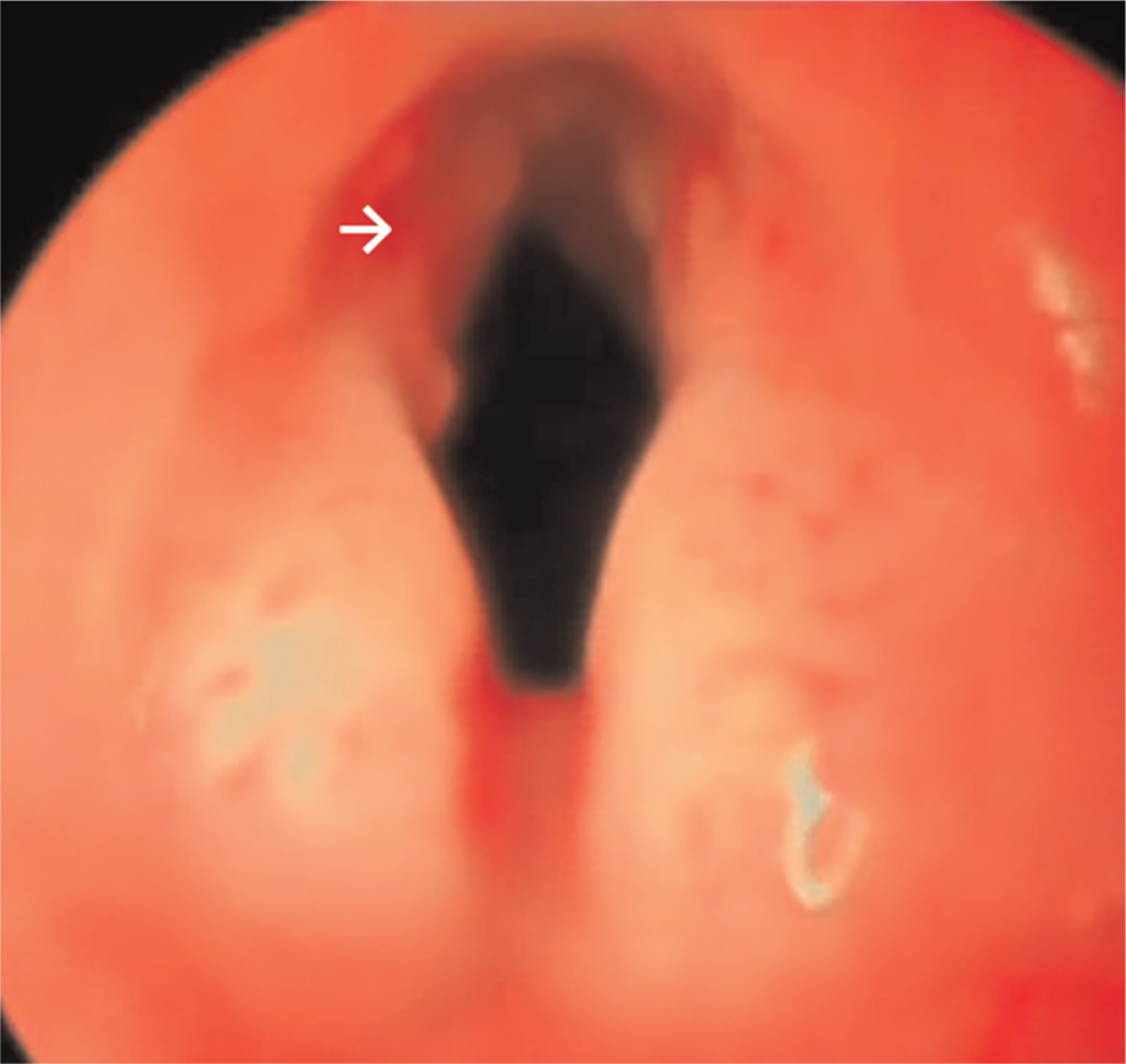
Endoscopy at 2 weeks postimplant. The white arrow indicates the implant edge on the left vocal fold. Surface appears intact.
Phonation
Rabbits underwent laryngeal harvest and phonation 1 month after implantation. Rabbits 1 and 2 were successfully phonated in vivo prior to larynx harvest. Excised larynges 1 to 8 all produced sound at airflow of 500 mL/s; subglottic pressure ranged from 2.5 to 3.8 kPA. Vocal fold vibration was observed on high-speed video, with mucosal waves proceeding inferior to superior in all larynges (Figure 4). Kymography revealed good left-right symmetry (Figure 5).
Figure 4.
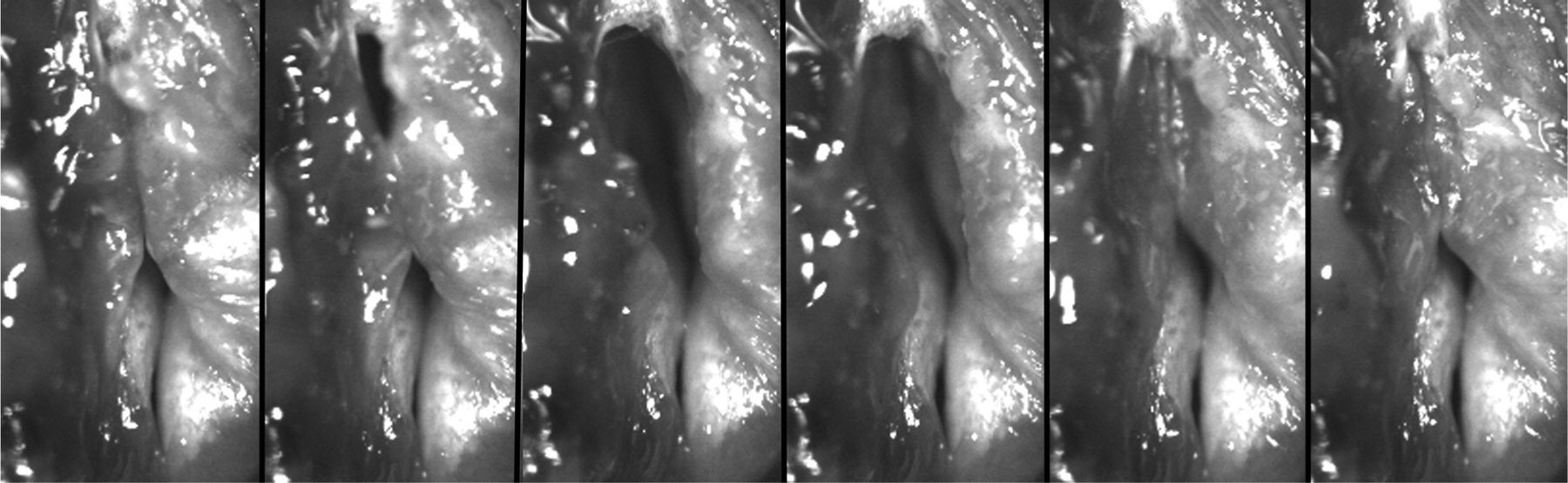
High-speed images of rabbit vocal folds during excised larynx phonation 1 month after tissue-engineered vocal fold mucosa implant. Superior thyroarytenoid folds have been excised, and a stitch adducts the vocal processes (bottom of the image).
Figure 5.
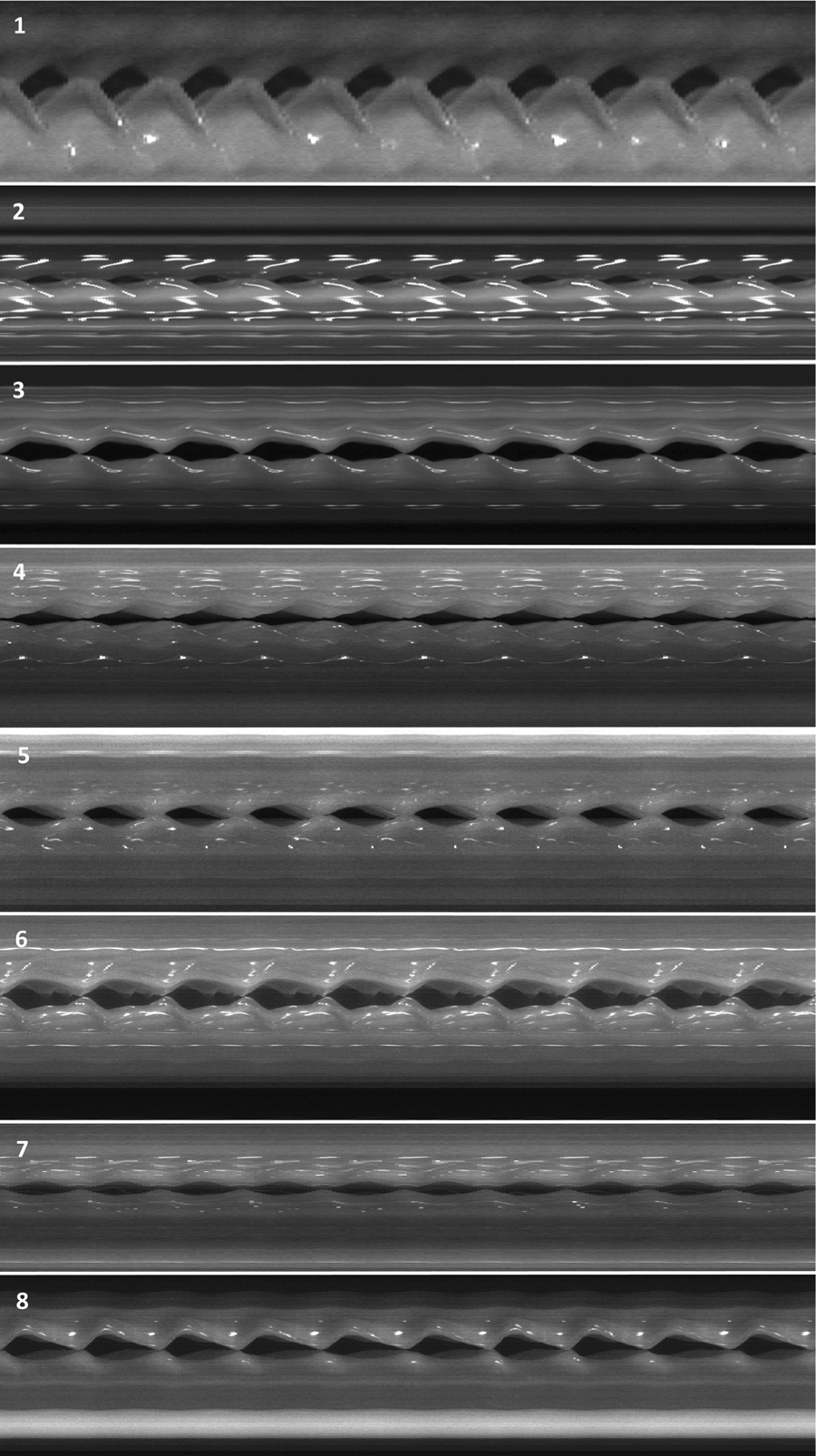
Kymography of phonation at 1 month after tissue-engineered vocal fold mucosa implant. The implanted left vocal fold movement appears at the bottom of each image and the unoperated right vocal fold at the top. Ten glottic cycles are shown for each of 8 larynges.
Explant Histology
Larynges were harvested and examined after 1 month of implantation. Two representative examples in Figures 6 and 7 show the implanted left vocal folds and the unoperated right vocal folds in male and female rabbits. Implant sites were difficult to distinguish from surrounding normal tissue. Epithelium was continuous, and graft borders were contiguous with surrounding soft tissue without clear demarcation. Lamina propria cellularity appeared similar on operated and control sides. Inflammatory cell infiltrate was identified at the deep interface, primarily in female rabbits 5 to 8 that received male cell implants. Based on trichrome staining, collagen content was normal to mildly increased in male rabbits and more notably increased in female rabbits when compared with the unoperated side. Status of elastic fibers was difficult to interpret, due to variability within animals, nonspecificity of EVG staining, and the uncertainty of the graft borders. EVG staining consistent with elastic fibers was noted in some operated vocal folds at the presumed implant site. However, definitive presence, origin, and functionality of elastic fibers cannot be ascertained by these histologic examinations alone.
Figure 6.

Representative vocal fold micrographs of male rabbit. Left column: operated vocal fold at the site of implantation. Right column: contralateral unoperated vocal fold. (A, B) Hematoxylin and eosin. (C, D) Masson’s trichrome. (E, F) Verhoeff’s elastic van Gieson. All at 20× magnification.
Figure 7.
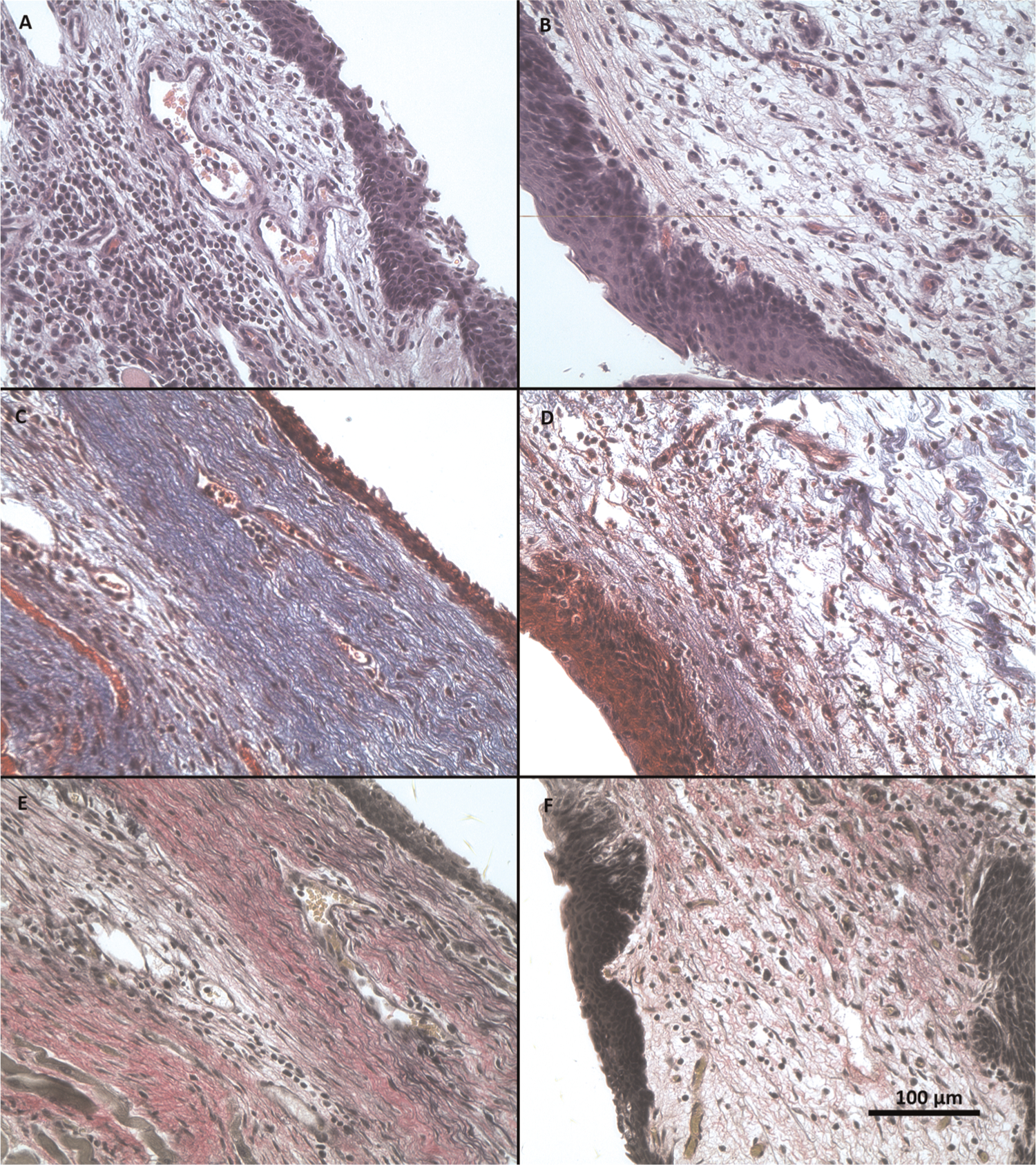
Representative vocal fold micrographs of female rabbit. Left column: operated vocal fold at the site of implantation. Right column: contralateral unoperated vocal fold. (A, B) Hematoxylin and eosin. (C, D) Masson’s trichrome. (E, F): Verhoeff’s elastic van Gieson. All at 40× magnification.
Discussion
The vocal fold epithelium and lamina propria form a functional vibrating VFCL28,29 that is susceptible to scarring from injury or radiation.30 Collagenous scar tissue disrupts the normal mucosal wave and leads to dysphonia and voice fatigue31,32 with few options for improvement; the deranged ECM viscoelasticity remains altered with speech therapy, medialization, or augmentation.1,6,33 A regenerative medicine approach to the scarred tissue could revolutionize treatment and mitigate the permanent dysphonia of oncologic treatment and trauma.
Among regenerative medicine techniques, cell injections into the superficial lamina propria are furthest along the clinical development pipeline.5,7 Severe scarring, however, results in mucosal epithelium tethering to underlying muscle or ligament. The entire cover layer is thus involved and may not respond to injection therapy.6 In those situations, replacing the entire cover layer would remove the stiff non-vibratory tissue completely. This laboratory previously described the TE-VFM, intended for en bloc replacement of the lamina propria and epithelium.5,8 The concept of a 3-dimensional VFCL replacement was demonstrated in a previous rabbit experiment that reimplanted native VFCL after an European Laryngological Society type II-III cordectomy.21 However, native VFCL is not available for clinical implantation. Therefore, the TE-VFM is now tested in that orthotopic implant model.
The TE-VFM implanted here is based on rASCs. ASCs are capable of self-renewal and multipotent differentiation; they are easily harvested and maintained in culture; and they have immune-modulating and wound-healing properties.26,34 As a result, they have been proposed for regenerative medicine of nearly all tissue types, with many human clinical trials underway.35,36 There is, however, some variability in ASC phenotype and behavior among species.36 In this system, rabbit ASCs were cultured within a fibrin hydrogel for 2 weeks in vitro with an air interface and epidermal growth factor supplementation. Those conditions produced bilayered differentiation of human ASCs to epithelial and mesenchymal cells but not with rASCs.8,9 The reason for this difference is unknown. Regardless, the rASCs implanted here are considered to be uniformly mesenchymal in phenotype based on their vimentin expression without ectodermal or endodermal markers. This rabbit TE-VFM thus represents a simpler construct than the bilayered structure that was previously described with human ASCs.9
Despite the lack of an epithelial layer in this 3-dimensional construct, implantation outcomes were equivalent or superior to our prior implantation of the native cover layer. Histologic results show excellent similarity with the unoperated vocal fold after 4 weeks, and symmetric vibration was achieved. Defining the fibrin-ASC implant characteristics that generated these benefits is an area of ongoing investigation. Potential explanations are that the fibrin matrix provides an improved temporary “biologic dressing” on the wound, that the ASCs directly engraft into the vocal fold to improve function, and that ASCs modulate wound healing via cytokine actions. Our prior findings—taken with the literature on ASC treatment of mature or acute vocal fold scars, described below—suggest that ASCs and the 3-dimensional scaffold both contribute. This implant series included a subpopulation of sex-mismatched implants, with male rASCs implanted into female rabbits. Interestingly, that group demonstrated increased inflammatory cells as well as increased collagen deposition. We hypothesize that the greater immune response toward implanted male cells in female rabbits contributed to worsening scar formation. Alternatively, sex-mismatched ASCs might less effectively downregulate the baseline host inflammatory response. The significance of immune modulation by the implanted rASCs is an area of further interest.
Other research has found weak immunogenicity and immune-modulating properties of mesenchymal stem cells via inhibition of proliferating T cells and activated B cells, as well as suppression of antigen-presenting cells.34,37,38 Injected stem cells have improved histologic and viscoelastic properties in the vocal folds in numerous animal models, whether injected before or after injury.11–16 In vivo injections of ASCs or bone marrow–derived stem cells led to decreased deposition of dense or disordered collagen, improved vocal fold mobility and smoothness, and increased elastin and hyaluronic acid, as compared with untreated scar or injury.12,18 Using a transmuscular injury in rabbits followed by immediate injection therapy, Xu et al found that gradual normalization of collagen, hyaluronic acid, and fibronectin occurred after 12 months.19 Whether the improvement in scarring from injected cells occurs because of immune modulation, direct ECM influence, or regulation of fibroblast activity is only beginning to be established through in vitro and animal models.20,39,40
Additional studies have shown benefit by coinjecting ASCs with a scaffold that may further support ASC survival, engraftment, and differentiation and provide a template for ECM deposition.40–42 Compared with stem cell or scaffold injections by themselves, combined injections have been shown to reduce collagen and improve viscoelastic, histologic, and endoscopic properties.40,42 Persistence of the injected stem cell population in recipient tissue is highly variable and of unclear significance.11–14,17–19,42 Physical size and protein characteristics of the scaffold also influence its resorption, cell adhesion, and immunogenicity.43,44 Fibrin was selected as the scaffold in this work because it, like the ASCs, can be prepared in autologous or immune-tolerated form from human cryoprecipitate.45 Fibrin has also been shown to support elastoneogenesis in other systems.46
The status of elastic fibers in this system is not yet completely determined, which parallels the vocal fold wound-healing literature. Complete recovery with synthesis of new, organized elastic fibers would be unlikely to occur at the early 4-week time point, based on other experimental models. In injured rabbits, elastin was decreased with shortened fibers at 2 months, disorganized or tangled at 3 months, and near normal density but disordered at 6 months.31,47,48 Yet, full-thickness laser injury in rabbits increased elastin at 4 weeks.49 It is therefore still unclear how the type and depth of vocal fold injury affects elastin recovery; variability among species, ages, and individual animal genetics also likely contributes. Elastogenesis generally is rare in adult wounds, but treating with ASCs may promote it both acutely47 and over a period of months.41 In this TE-VFM study, some rabbits exhibited EVG staining suggestive of elastic fibers in or near the implant site. Longer implantation periods and more specific elastin detection methods are required before drawing definitive conclusions. Also, rabbit elastic fibers are concentrated in the deep lamina propria, in contrast to humans, who have a trilayered lamina propria with densest elastic fibers in the middle layer.
The significance of species differences in vocal fold microstructure on translating therapies to humans remains to be determined. Rabbits lack a vocal ligament of dense collagen; their deep lamina propria layer comprises both elastic and collagenous fibers. It is conceivable that this predilection for reduced collagen production would improve wound-healing results in rabbits relative to humans. Another key difference is the local regenerative cell population. Vocal fold stellate cells residing in the macula flava in humans are hypothesized to drive ECM synthesis and repair and maintain viscoelasticity.50–52 Yet recent studies were unable to demonstrate macula flava or vitamin A–storing stellate cells in rabbits, leading to the conclusion that these cell types are not critical to rabbit vocal fold development or differ significantly from their human counterparts.53 Finally, anatomically, rabbits have a laryngeal ventricle, but the thyroarytenoid muscle spans it, to underlie what is termed the superior and inferior divisions of the thyroarytenoid fold.54 We implanted the inferior division for consistency with our prior work and for anatomic similarity with human larynges.
At this stage in clinical voice medicine, the promising findings from cell injections in animals still await wide-spread translation into human therapies for existing scars. An injectable carrier with cells may be ideal for minimally invasive treatment of scarring that is limited to the lamina propria,55 but more severe scarring that tethers epithelium to muscle is unlikely to be reversed by injection alone.6 Rather, resection and replacement of the scarred tissue may be required in those severe cases. We present the first animal implantation of a potential tissue-engineered vocal fold replacement. The good results noted with 3-dimensional implants in this study are likely due to combined benefits of adipose-derived stem cells, immediate biologic protection of the wound, and presence of a scaffold to support ASC survival and template new ECM deposition. Based on immunohistochemistry, the implanted fibrin scaffold was resorbed by 1 month. The fate of implanted ASCs was not investigated but is the subject of ongoing study that will also shed light on the mechanisms of improvement.
Conclusions
The difficult problem of vocal fold scarring has been addressed in numerous clinical studies and animal models. Here we demonstrate an essential step in developing new treatments for severe vocal fold scarring. Tissue-engineered 3-dimensional vocal fold mucosa implants that were formed with adipose-derived stem cells healed well and preserved function after implantation in rabbits. We found good incorporation into the vocal fold with limited scarring, which suggests that either the surgical method or the implanted construct itself has advantageous effects on the local wound environment. Ongoing studies will distinguish these possibilities. This model has potential as an autologous tissue replacement for human vocal fold application.
Acknowledgments
Vimentin antibody was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biology, University of Iowa (Iowa City, Iowa).
Funding source:
Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (Jennifer L. Long and Jordan Hardy), VA Career Development Award IK2BX001944 (Jennifer L. Long), and the AAO-HNSF Resident Research Award (Travis L. Shiba). Additional institutional support was provided by the Jonsson Comprehensive Cancer Center of the University of California, Los Angeles. None of these funding sources played any role in study design, conduct, data collection, or writing of the manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Competing interests: None.
References
- 1.Welham NV, Choi SH, Dailey SH, Ford CN, Jiang JJ, Bless DM. Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. Laryngoscope. 2011;121:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prufer N, Woo P, Altman KW. Pulse dye and other laser treatments for vocal scar. Curr Opin Otolaryngol Head Neck Surg. 2010;18:492–497. [DOI] [PubMed] [Google Scholar]
- 3.Tsunoda K, Kondou K, Kaga K, et al. Autologous transplantation of fascia into the vocal fold: long-term result of type-1 transplantation and the future. Laryngoscope. 2005;115:1–10. [DOI] [PubMed] [Google Scholar]
- 4.Pitman MJ, Rubino SM, Cooper AL. Temporalis fascia transplant for vocal fold scar and sulcus vocalis. Laryngoscope. 2014;124:1653–1658. [DOI] [PubMed] [Google Scholar]
- 5.Long J Tissue engineering for the treatment of vocal fold scar. Curr Opin Otolaryn Head Neck Surg. 2010;18:521–525. [DOI] [PubMed] [Google Scholar]
- 6.Chhetri D, Berke G. Injection of cultured autologous fibroblasts for human vocal fold scars. Laryngoscope. 2011;121:785–792. [DOI] [PubMed] [Google Scholar]
- 7.Hertegard S, Karolinska University Hospital, Swedish Research Council, Laryngfonden. Karolinska Institutet pilot study of 8 patients with severe hoarseness and vocal fold scarring treated with mesenchymal stem cells with and without hyaluronan gel. https://clinicaltrials.gov/ct2/show/NCT01981330. Accessed March 30, 2015.
- 8.Shiba TL, Hardy J, Long J. Laryngeal tissue engineering using rabbit adipose derived stem cells in fibrin: a pre-clinical model. J Oto Advances. 2015;1:28–40. [Google Scholar]
- 9.Long J, Zuk P, Berke G, Chhetri D. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope. 2010;120:125–131. [DOI] [PubMed] [Google Scholar]
- 10.Long JL, Neubauer J, Zhang Z, et al. Functional testing of a tissue-engineered vocal fold cover replacement. Otolaryngol Head Neck Surg. 2010;142:438–440. [DOI] [PubMed] [Google Scholar]
- 11.Hertegard S, Cedervall J, Svensson B, et al. Viscoelastic and histological properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope. 2006;116:1248–1254. [DOI] [PubMed] [Google Scholar]
- 12.Cedervall J, Ährlund-Richter L, Svensson B, et al. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: Short-term results. Laryngoscope. 2007;117:2075–2081. [DOI] [PubMed] [Google Scholar]
- 13.Svensson B, Nagubothu SR, Cedervall J, et al. Injection of human mesenchymal stem cells improves healing of vocal folds after scar excision: a xenograft analysis. Laryngoscope. 2011;121:2185–2190. [DOI] [PubMed] [Google Scholar]
- 14.Hong SJ, Lee SH, Jin SM, et al. Vocal fold wound healing after injection of human adipose-derived stem cells in a rabbit model. Acta Otolaryngol. 2011;131:1198–1204. [DOI] [PubMed] [Google Scholar]
- 15.Thibeault S, Klemuk S, Smith M, et al. In vivo comparison of biomimetic approaches for tissue regeneration of the scarred vocal fold. Tissue Eng: Part A. 2009;15:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanemaru S, Nakamura T, Yamashita M, et al. Destiny of autologous bone marrow-derived stromal cells implanted in the vocal fold. Ann Otol Rhinol Laryngol. 2005;114:907–912. [DOI] [PubMed] [Google Scholar]
- 17.Kanemaru S, Nakamura T, Omori K. Regeneration of vocal fold scarring using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915–920. [DOI] [PubMed] [Google Scholar]
- 18.Hiwatashi N, Hirano S, Mizuta M, et al. Adipose derived stem cells versus bone marrow derived stem cells for vocal fold regeneration. Laryngoscope. 2014;124:E461–E469. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Hu R, Fan R, Han D. Adipose-derived mesenchymal stem cells in collagen-hyaluronic acid gel composite scaffolds for vocal fold regeneration. Ann Otol Rhin Laryngol. 2011;120:123–130. [DOI] [PubMed] [Google Scholar]
- 20.Coppoolse JM, Van Kooten TG, Heris HK, et al. An in vivo study of composite microgels based on hyaluronic acid and gelatin for the reconstruction of surgically injured rat vocal folds. J Speech Lang Hear Res. 2014;57:S658–S673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long J, Salinas J, Rafizadeh S, Luegmair G, Zhang Z, Chhetri D. In vivo vocal fold cover layer replacement. Laryngoscope. 2015;125:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Pankratov M, Rebeiz E, et al. Endoscopic diode laser welding of mucosal grafts on the larynx: a new technique. Laryngoscope. 1995;105:49–52. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura M, Hirano S, Kanemaru SI, et al. Glottic regeneration with a tissue-engineering technique, using acellular extracellular matrix scaffold in a canine model [published online January 8, 2014]. J Tissue Eng Regen Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto Y, Welham NV, Hirano S. Implantation of atelocollagen sheet for vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010;18:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duflo S, Thibeault SL, Li W, et al. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. [DOI] [PubMed] [Google Scholar]
- 26.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell based therapies. Tissue Eng. 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 27.Imaizumi M, Thibeault SL, Leydon C. Classification for animal vocal fold surgery: resection margins impact histological outcomes of vocal fold injury. Laryngoscope. 2014;124:E437–E444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berke GS, Gerratt BR. Laryngeal biomechanics: an overview of mucosal wave mechanics. J Voice. 1993;7:123–128. [DOI] [PubMed] [Google Scholar]
- 29.Hirano M Phonosurgery: basic and clinical investigations. Otologia (Fukuoka). 1975;21:239–260. [Google Scholar]
- 30.Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–482. [DOI] [PubMed] [Google Scholar]
- 31.Thibeault S, Gray S, Bless D, et al. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. [DOI] [PubMed] [Google Scholar]
- 32.Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich G, Dikkers FG, Arens C, et al. Vocal fold scars: current concepts and future directions. Consensus report of the Phonosurgery Committee of the European Laryngologic Society. Eur Arch Otorhinolaryngol. 2013;270:2491–2507. [DOI] [PubMed] [Google Scholar]
- 34.Leto Barone AA, Khalifian S, Lee WPA, Brandacher G. Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int. 2013;2013:383685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim MH, Ong WK, Sugii S. The current landscape of adipose-derived stem cells in clinical applications. Expert Rev Mol Med. 2014;16:E8–E50. [DOI] [PubMed] [Google Scholar]
- 36.Baer PC. Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada N, Gronthos S, Bartold PM. Immunomodulatory effects of stem cells. Periodontol2000. 2013;63:198–216. [DOI] [PubMed] [Google Scholar]
- 38.Franquesa M, Mensah FK, Huizinga R, et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells. 2015;33:880–891. [DOI] [PubMed] [Google Scholar]
- 39.Kumai Y, Kobler JB, Park H, Galindo M, Herrera VL, Zeitels SM. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope. 2010;120:330–337. [DOI] [PubMed] [Google Scholar]
- 40.Liang Q, Liu S, Han P, Li X, et al. Micronized acellular dermal matrix as an efficient expansion substrate and delivery vehicle of adipose-derived stem cells for vocal fold regeneration. Laryngoscope. 2012;122:1815–1825. [DOI] [PubMed] [Google Scholar]
- 41.Hu R, Ling W, Xu W, Han D. Fibroblast-like cells differentiated from adipose-derived mesenchymal stem cells for vocal fold wound healing. PLoS ONE. 2014;9:E92676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YM, Oh SH, Choi JS, et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing. Laryngoscope. 2014;124:E64–E72. [DOI] [PubMed] [Google Scholar]
- 43.Park H, Karajanagi S, Wolak K, et al. Three-dimensional hydrogel model using adipose-derived stem cells for vocal fold augmentation. Tissue Eng Part A. 2010;16:535–543. [DOI] [PubMed] [Google Scholar]
- 44.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siedentop KH, Park JJ, Shah AN, Bhattacharyya TK, O’Grady KM. Safety and efficacy of currently available fibrin tissue adhesives. Am J Otolaryngol. 2001;22:230–235. [DOI] [PubMed] [Google Scholar]
- 46.Long J, Tranquillo R. Elastic fiber production in cardiovascular tissue equivalents. Matrix Biol. 2003;22:339–350. [DOI] [PubMed] [Google Scholar]
- 47.Lim JY, Choi BH, Lee S, Jang YH, Choi J-S, Kim YM. Regulation of wound healing by granulocyte-macrophage colony-stimulating factor after vocal fold injury. PLoS ONE. 2013;8:e54256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–124. [DOI] [PubMed] [Google Scholar]
- 49.Mau T, Du M, Xu C. A rabbit vocal fold laser scarring model for testing lamina propria tissue-engineering therapies. Laryngoscope. 2014;124:2321–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato K, Hirano M, Nakashima T. Stellate cells in the human vocal fold. Ann Otol Rhinol Laryngol. 2001;110:319–325. [DOI] [PubMed] [Google Scholar]
- 51.Sato K, Umeno H, Nakashima T. Functional histology of the macula flava in the human vocal fold: part 2. Its role in the growth and development of the vocal fold. Folia Phoniatr Logop. 2010;62:263–270. [DOI] [PubMed] [Google Scholar]
- 52.Sato K, Umeno H, Nakashima T. Vocal fold stem cells and their niche in the human vocal fold. Ann Otol Rhinol Laryngol. 2012;121:798–803. [DOI] [PubMed] [Google Scholar]
- 53.Toya Y, Riabroy N, Davis R, et al. Interspecies comparison of stellate cell-containing macula flavae and vitamin A storage in vocal fold mucosa. J Anat. 2014;225:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negus VE. The Comparative Anatomy and Physiology of the Larynx. New York, NY: Grune & Stratton; 1949. [Google Scholar]
- 55.Bartlett RS, Thibeault SL, Prestwich GD. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed Mater. 2012;7:024103. [DOI] [PMC free article] [PubMed] [Google Scholar]


