Abstract
Background
Annually, infections contribute to approximately 25% of the 2.8 million neonatal deaths worldwide. Over 95% of sepsis‐related neonatal deaths occur in low‐ and middle‐income countries. Hand hygiene is an inexpensive and cost‐effective method of preventing infection in neonates, making it an affordable and practicable intervention in low‐ and middle‐income country settings. Therefore, hand hygiene practices may hold strong prospects for reducing the occurrence of infection and infection‐related neonatal death.
Objectives
To determine the effectiveness of different hand hygiene agents for preventing neonatal infection in both community and health facility settings.
Search methods
Searches were conducted without date or language limits in December 2022 in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and Cumulated Index to Nursing and Allied Health Literature (CINAHL), clinicaltrials.gov and International Clinical Trials Registry Platform (ICTRP) trial registries. The reference lists of retrieved studies or related systematic reviews were screened for studies not identified by the searches.
Selection criteria
We included randomized controlled trials (RCTs), cross‐over trials, and cluster trials that included pregnant women, mothers, other caregivers, and healthcare workers who received interventions within either the community setting or in health facility settings, and the neonates in the neonatal care units or community settings.
Data collection and analysis
We used standard methodological procedures expected by Cochrane and the GRADE approach to assess the certainty of evidence. Primary outcomes were incidence of suspected infection (author‐defined in study) within the first 28 days of life, bacteriologically confirmed infection within the first 28 days of life, all‐cause mortality within the first seven days of life (early neonatal death), and all‐cause mortality from the 8th to the 28th day of life (late neonatal death).
Main results
Our review included six studies: two RCTs, one cluster‐RCT, and three cross‐over trials. Three studies involved 3281 neonates; the remaining three did not specify the actual number of neonates included in their study. Three studies involved 279 nurses working in neonatal intensive care units (NICUs). The number of nurses included was not specified by one study. A cluster‐RCT included 103 pregnant women of over 34 weeks gestation from 10 villages in a community setting (sources of data: 103 mother‐neonate pairs) and another community‐based study included 258 married pregnant women at 32 to 34 weeks of gestation (the trial reported adverse events on 258 mothers and 246 neonates). Studies examined the effectiveness of different hand hygiene practices for the incidence of suspected infection (author‐defined in study) within the first 28 days of life. Three studies were rated as having low risk for allocation bias, two studies were rated as unclear risk, and one was rated as having high risk. One study was rated as having a low risk of bias for allocation concealment, one study was rated as unclear risk, and four werw rated as having high risk. Two studies were rated as having low risk for performance bias and two were rated as having low risk for attrition bias.
One class of agent versus another class of agent: 2% chlorhexidine gluconate (CHG) compared to alcohol hand sanitiser (61% alcohol and emollients)
For this comparison, no study assessed the effect of the intervention on the incidence of suspected infection within the first 28 days of life. Two percent chlorhexidine gluconate (CHG) probably reduces the risk of all infection in neonates compared to 61% alcohol hand sanitiser in regard to the incidence of all bacteriologically confirmed infection within the first 28 days of life (RR 0.79, 95% confidence interval (CI) 0.66 to 0.93; 2932 participants, 1 study; moderate‐certainty evidence), number needed to treat for an additional beneficial outcome (NNTB): 385.
The adverse outcome was reported as mean self‐reported skin change and mean observer‐reported skin change. There may be little to no difference between the effects of 2% CHG on nurses’ skin compared to alcohol hand sanitiser, based on very low‐certainty evidence for mean self‐reported skin change (mean difference (MD) ‐0.80, 95% CI ‐1.59 to 0.01; 119 participants, 1 study) and on mean observer reported skin change (MD ‐0.19, CI ‐0.35 to ‐0.03; 119 participants, 1 study), respectively.
We identified no study that reported on all‐cause mortality and other outcomes for this comparison.
None of the included studies assessed all‐cause mortality within the first seven days of life nor the duration of hospital stay.
One class of agent versus two or more other classes of agent: CHG compared to plain liquid soap + hand sanitiser
We identified no studies that reported on our primary and secondary outcomes for this comparison except for author‐defined adverse events. We are very uncertain whether plain soap plus hand sanitiser is better than CHG for nurses’ skin based on very low‐certainty evidence (MD ‐1.87, 95% CI ‐3.74 to ‐0.00; 16 participants, 1 study; very low‐certainty evidence).
One agent versus standard care: alcohol‐based handrub (hand sanitiser) versus usual care
The evidence is very uncertain whether alcohol‐based handrub is better than 'usual care' in the prevention of suspected infections, as reported by mothers (RR 0.98, CI 0.69 to 1.39; 103 participants, 1 study, very low‐certainty evidence). We are uncertain whether alcohol‐based hand sanitiser is better than 'usual care' in reducing the occurrence of early and late neonatal mortality (RR 0.29, 95% CI 0.01 to 7.00; 103 participants, 1 study; very low‐certainty evidence) and (RR 0.29, CI 0.01 to 7.00; 103 participants, 1 study; very low‐certainty evidence), respectively. We identified no studies that reported on other outcomes for this comparison.
Authors' conclusions
We found a paucity of data that would allow us to reach meaningful conclusions pertaining to the superiority of one form of antiseptic hand hygiene agent over another for the prevention of neonatal infection. Also, the sparse available data were of moderate‐ to very low‐certainty. We are uncertain as to the superiority of one hand hygiene agent over another because this review included very few studies with very serious study limitations.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Anti-Infective Agents, Local; Anti-Infective Agents, Local/therapeutic use; Ethanol; Hand Hygiene; Perinatal Death; Soaps
Plain language summary
Can hand hygiene prevent infection in newborn babies?
Review question
Can hand hygiene prevent infections in newborn babies?
Key messages:
1. Two percent chlorhexidine gluconate ((CHG) antiseptic detergent) probably reduces the risk of bacterial infections in neonates compared to alcohol hand sanitiser within the first 28 days of life.
2. There was not much difference in the undesirable effects of various hand hygiene interventions on the skin of caregivers.
3. We are not sure which type of hand hygiene is best for preventing infection in newborn babies.
Why is hand hygiene important?
Every year, about 500,000 newborn babies die as a result of an infection caused by bacteria. Most of these deaths occur in poor countries. The hands of mothers and other caregivers harbour a lot of germs that are acquired during contact secretions and diaper changes; they have been linked to infections in newborns. These infections may be prevented when caregivers of these babies practice good hand hygiene.
What is hand hygiene?
Hand hygiene refers to any form of hand cleansing. Another word for hand hygiene is handwashing, which implies washing hands with plain or antiseptic soap and water.
How is hand hygiene expected to work?
Frequent and good hand hygiene by mothers, caregivers and healthcare workers may reduce infections of the newborn by reducing dirt, and germs on their hands, thereby reducing their ability to infect babies.
What did we want to find out?
We wanted to find out which antiseptic, soap or alcohol is better for hand hygiene to prevent infection in newborns in the community and healthcare centres.
We also wanted to find out if any of the hand hygiene products will cause harm to mothers and healthcare workers.
What did we do?
We searched for studies carried out in the communities or healthcare centres that compared the benefits and risks of any form of hand hygiene products (like soap, antiseptic, alcohol, hand sanitisers, or handrubs) against another type or against no hand hygiene products for prevention of infection in newborns. We searched for relevant studies up to July 2021. We compared and summarised the results of the studies and rated our confidence in the evidence, based on the quality of the studies
What did we find?
We included six studies that involved nurses working in intensive care units of hospitals, all neonates on admission, and pregnant women in community settings. Three of the studies involved 279 nurses, and one study did not clearly report how many nurses were recruited into the study; two other studies included 361 pregnant women from community settings. Studies compared 'antiseptic detergent' versus alcohol hand rub (sanitiser); 'antiseptic detergent' versus plain soap; alcohol hand sanitiser versus 'usual care'; antiseptic detergent versus 'usual care' and antiseptic that contained iodine versus another (prepodyne versus betadine).
Two percent antiseptic detergent may reduce the risk of bacteria infections in neonates compared to alcohol hand sanitiser within the first 28 days of life. Overall, our review provides no strong evidence to support better effectiveness of one hand hygiene intervention compared to another for preventing infection in newborns. None of the five included studies examined other important issues such as the duration of hospital stay. There was not much difference in the undesirable effects of various hand hygiene interventions on the skin of caregivers.
In conclusion, we are not sure of the hand hygiene intervention that is better for preventing infection in newborn babies. We assessed only a few studies that involved small numbers of nurses and babies. In addition, most of the assessed studies had high risk of bias. Larger studies with low risk of bias are needed so reliable conclusions can be reached.
What are the limitations of the evidence?
We do not have sufficient information that would allow us to reach meaningful conclusions pertaining to which hand hygiene product is better for the prevention of newborn infection as many of the included studies had issues with how they were carried out. We have no confidence in the available evidence to draw conclusions about the effectiveness of these hand hygiene interventions for preventing infection in newborns.
Study funding sources
Sources of funding were declared by four of the included studies, but two studies did not report how they were funded.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to 12 December 2022.
Summary of findings
Summary of findings 1. Two per cent CHG compared to alcohol hand sanitiser (61% alcohol and emollients) for the prevention of infections in neonates.
| Two per cent CHG compared to alcohol hand sanitiser (61% alcohol and emollients) for the prevention of infections in neonates | ||||||
| Patient or population: Neonates and caregivers Setting: Neonatal intensive care unit Intervention: 2% CHG Comparison: Alcohol hand sanitiser (61% alcohol and emollients) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with alcohol hand sanitiser (61% alcohol and emollients) | Risk with 2% CHG | |||||
| Incidence of (author‐defined) suspected infections within the first 28 days of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Incidence of bacteriologically confirmed infections (types of infection as specified by authors) within the first 28 days of life |
Study population | RR 0.79 (0.66 to 0.93) NNTB = 385 | 2932 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | The evidence suggests 2% CHG probably results in a slight reduction in incidence of bacteriologically confirmed infections (types of infection as specified by authors) within the first 28 days of life ‐ all infections (however, rates of participant contact differed significantly in the two groups and this is likely to have affected the outcomes). |
|
| 134 per 1000 | 106 per 1000 (89 to 125) | |||||
| All‐cause mortality within the first seven days of life (early neonatal death) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| All‐cause mortality from the 8th to 28th day of life (late neonatal death) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded by one level for serious risk of bias due to study limitations.
Summary of findings 2. Alcohol‐based handrub compared to usual care for the prevention of infections in neonates.
| Alcohol‐based handrub compared to usual care for the prevention of infections in neonates | ||||||
| Patient or population: Neonates and caregivers Setting: Community setting Intervention: Alcohol‐based handrub Comparison: Usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with alcohol‐based handrub | |||||
| Incidence of suspected infections (author‐defined) within the first 28 days of life | Study population | RR 0.98 (0.69 to 1.39) | 103 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | The evidence is very uncertain as to whether alcohol‐based handrub is better than usual care in the prevention of suspected infection. The effect includes harm and benefit due to a wide confidence interval. | |
| 563 per 1000 | 551 per 1000 (388 to 782) | |||||
| Incidence of bacteriologically confirmed infections within the first 28 days of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| All‐cause mortality within the first seven days of life (early neonatal death) |
Study population | RR 0.29 (0.01 to 7.00) | 103 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | The evidence is very uncertain about the effect of alcohol‐based handrub on early neonatal death. | |
| 21 per 1000 | 6 per 1000 (0 to 146) | |||||
| All‐cause mortality from the 8th to 28th day of life (late neonatal death) | Study population | RR 0.29 (0.01 to 7.00) | 103 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | The evidence is very uncertain about the effect of alcohol‐based handrub on early neonatal death. | |
| 21 per 1000 | 6 per 1000 (0 to 146) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded by one level for serious indirectness because this was a small study from one setting. 2We downgraded by two levels for very serious imprecision due to a wide confidence interval.
Summary of findings 3. Four per cent chlorhexidine + hand hygiene promotion compared to usual care for prevention of infections in neonates.
| Four per cent chlorhexidine + hand hygiene promotion compared to usual care for prevention of infections in neonates | ||||||
|
Patient or population: Neonates and caregivers Setting: Community Intervention: 4% chlorhexidine + hand hygiene promotion Comparison: Usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with [usual care] | Risk with [4% chlorhexidine + hand hygiene promotion] | |||||
| Authors reported adverse outcomes (events without hospitalisation) follow‐up: mean 6 weeks | Study population | RR 0.57 (0.28 to 1.15) | 246 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | ||
| 156 per 1000 | 89 per 1000 (44 to 179) | |||||
| Adverse events requiring hospitalisation (SAE) follow‐up: mean 6 weeks | Study population | RR 1.83 (0.75 to 4.42) | 246 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | ||
| 57 per 1000 | 105 per 1000 (43 to 254) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded by two levels for very serious study limitations.
2 We downgraded by one level for serious imprecision due to wide confidence intervals involving harm and benefit.
Background
Annually, infection contributes to approximately 25% of the 2.8 million neonatal deaths worldwide. Over 95% of sepsis‐related neonatal deaths occur in low‐ and middle‐income countries (Liu 2015). Neonatal infection may be acquired through exposure to contaminated secretions of the birth canal, or through contact with the contaminated environment (Chan 2013; Gebremedhin 2016; Schuchat 2000). Important environmental sources of infection for the neonate include the hands of individuals who care for the many needs of the baby, including mothers and healthcare workers (HCWs) (Ram 2017; Rhee 2008). Contaminated hands play a major role in community‐acquired and hospital‐acquired neonatal infection, particularly amongst preterm infants, who are most susceptible. Community‐based and health facility‐based studies have suggested that handwashing may play a preventive role in neonatal infection in low‐, middle‐, and high‐income countries (Janota 2014; Rhee 2008).
Hand hygiene is an inexpensive and cost‐effective way of preventing neonatal infection, making it a practicable intervention in low‐ and middle‐income settings (WHO 2009). Therefore, hand hygiene practices may hold strong prospects for reducing the occurrence of infection and infection‐related neonatal death.
Description of the condition
The International Paediatric Sepsis Consensus Conference of 2005 defined neonatal sepsis as systemic inflammatory response syndrome in the presence of, or as a result of, suspected or proven infection in a neonate (Goldstein 2005). Neonatal sepsis is caused by a variety of micro‐organisms of bacterial, viral, fungal, or rickettsial origin. Neonatal sepsis can be classified as an early‐onset (mainly acquired before or during delivery, or both) or late‐onset condition (often acquired from exposure to a contaminated environment). Neonates are particularly susceptible to infection because of poor cutaneous and mucosal barrier mechanisms, poor macrophage function, poor opsonisation, and low levels of serum immunoglobulins and complement (Cortese 2016; Wynn 2010). Susceptibility to neonatal infection is inversely related to gestational age, with preterm neonates at higher risk of infection compared to term neonates (Afonso 2017).
Neonatal infection may lead to life‐threatening multi‐systemic morbidities such as shock, disseminated intravascular coagulopathies, cardiac failure, adrenal insufficiency, renal insufficiency, and metabolic derangements (Cortese 2016; Goldstein 2005). Therefore, in spite of the availability of antibiotics and other adjunctive treatments, neonatal infection still leads to mortality and accounts for about a quarter of global neonatal deaths (Liu 2015), as well as prolonged hospital stay, early complications (Chu 2014), late complications (Adams‐Chapman 2006), and huge economic burden (Ranjeva 2018).
The hands of mothers, other caregivers, and HCWs harbour significant microbial pathogens acquired during contact with patients or environmental surfaces (Aiello 2003). Contact of caregivers' and HCWs' hands with respiratory secretions, diaper changes, and direct skin are often associated with transmission of infection to the newborn (Pessoa‐Silva 2004). Average bacterial loads on the hands of caregivers (usually mothers) and neonatal intensive care unit (NICU) nurses may consist of up to hundreds of thousands of bacteria (Aiello 2003). This pattern of bacterial load may vary amongst caregivers, but it is relatively constant for any individual (Aiello 2003; Larson 1998).
The World Health Organization (WHO) has described five steps of transmission of infection from person to person through the hands of HCWs. These steps include the following.
Organisms present in the skin of HCWs or on objects close to the patient.
Organisms transferred to the hands of HCWs.
Organisms surviving on the hands of HCWs for several minutes.
Handwashing or hand antisepsis by HCWs being inadequate or completely omitted, or use of inappropriate agents by HCWs for hand hygiene.
Contaminated hands of HCWs coming in contact with a baby or with an object that will come in contact with a baby (WHO 2009).
Organisms often found to contaminate the hands of caregivers, which are capable of causing infection in newborns, include Staphylococcus aureus, Klebsiella spp., Proteus mirabilis, and Actinobacter spp (Cortese 2016).
Description of the intervention
Hand hygiene refers to any form of hand cleansing. It is often used interchangeably with handwashing, which implies washing hands with plain or antimicrobial soap and water (WHO 2009). Hand hygiene also includes the use of various alcohol‐based hand rubs, wipes, scrubs, and antiseptic agents such as 0.5% chlorhexidine gluconate (CHG) (CADTH 2014), chlorine derivatives, chloroxylenol (PCMX), quaternary ammonium compounds, and triclosan (WHO 2009). It is recommended that caregivers should perform hand hygiene before touching hospital equipment and instruments, before touching neonates, and between cleaning and caring for neonates (Loveday 2014; WHO 2009).
How the intervention might work
Frequent and adequate hand hygiene by caregivers and HCWs may reduce neonatal infection by reducing dirt, organic materials, and microbial contamination on the hands of these personnel, thereby reducing the risk of contamination of babies and objects that come in contact with babies (Janota 2014; Won 2004).
Handwashing with water alone washes away dirt but may not remove fat and oil on contaminated hands. This necessitates the use of soaps and detergents that have the capacity to dissolve fatty and hydrophobic materials and to facilitate their subsequent removal with water (WHO 2009). Rotter 1999 reported that washing hands for 30 seconds reduced bacterial count to a greater extent than washing hands for 15 seconds.
Alcohol‐based hand antiseptics and rubs have the ability to denature protein (Ali 2001). Alcohol‐based preparations containing 60% to 80% alcohol have been reported to be most effective and safe (Ali 2001). Alcohol has been found to have excellent in vitro germicidal activity against both drug‐susceptible and drug‐resistant bacteria, Mycobacterium tuberculosis, some viruses, and fungi (Ali 2001). Frequent use of appropriate alcohol‐based hand rubs limits the spread of infection from the hands of HCWs to neonates (Janota 2014).
Chlorhexidine solution attaches to and disrupts cytoplasmic membranes of pathogenic bacteria on the hands of HCWs, thereby precipitating their cellular contents and resulting in cellular death (Rotter 1999). This action is similar to that of other hand antiseptic agents. Mortimer 1962 demonstrated that frequent hand hygiene with hexachlorophene antiseptic agents significantly reduced the risk of transmission of Staphylococcus aureus pathogens from nurses to babies admitted to the NICU compared to the risk of transmission from nurses who did no handwashing or hand rubbing with the antiseptic agent. Hand hygiene has also been reported by several study investigators to reduce the rate and cross‐transmission of pathogenic microbial agents, including methicillin‐resistant Staphylococcus aureus strain (MRSA), in neonatal care units (Webster 1994; Zafar 1995).
As effective as hand hygiene may be, compliance on the part of HCWs may present a challenge to the overall benefits to be accrued from the practice. A study of healthcare providers in neonatal and paediatric intensive care units shows overall hand hygiene compliance of 37%, with differential compliance of 41.4% for nurses and 31.9% for doctors and, for both cadres, compliance was best immediately after patients or patients' environments were touched (Karaaslan 2014 [Karaaslan 2014]). Lack of motivation and a heavy workload were proffered as reasons for poor compliance, but it is plausible that the type of hand hygiene agent available may also influence the frequency of hand hygiene practices. Preference for specific hand hygiene agents may be determined by the types of adverse events related to the use of such agents.
Adverse events in the form of skin irritation and allergic skin reaction may occur following the use of hand hygiene agents. Some liquid soaps, hand lotions, creams, and ointments contain ingredients such as iodine, iodophors, triclosan, chlorhexidine, and chloroxylenol, which may act as irritants or allergens. Frequent exposure of the skin to some of these allergens leads to progressive depletion of surface lipids in the superficial layers of the skin, thereby exposing deeper layers of the skin to the effects of allergens (WHO 2009 [WHO 2009]). Adverse events that may follow hand hygiene procedures include skin dryness, burning, erythematic scaling, fissuring, a sensation of roughness, and irritation such as eczema, as described in Abd El‐AAl 2013 [Abd El‐AAl 2013]. In this study, dryness, burning/irritation, and eczema were reported by 61%, 30%, and 1% of nurses, respectively. Indeed, healthcare providers have been reported to have a higher prevalence of skin irritation than is observed in the general population; this was ascribed to frequent hand hygiene during patient care (Larson 2006 [Larson 2006]).
Why it is important to do this review
Stringent hand hygiene practices in communities and health facilities may reduce the risk and incidence of neonatal infection and ultimately may contribute to the desired reduction in infection‐related neonatal death and the economic burden of associated morbidities (Adams‐Chapman 2006; Chu 2014; Ranjeva 2018). A conservative estimate of the economic impact of neonatal sepsis in sub‐Saharan Africa (SSA) revealed that 5.29 to 8.73 million disability‐adjusted life‐years (DALYs) are lost annually in the region to neonatal sepsis. This corresponds to an annual economic burden ranging from United States dollars (USD) 10 billion to USD 469 billion in SSA alone (Ranjeva 2018). This huge economic cost may be reduced substantially through meticulous hand hygiene practices.
Effective handwashing practices may be a more efficient and cost‐effective intervention aimed at reducing neonatal death for developing economies, as the cost of procuring the required materials (soap and water and/or alcohol rubs) may be negligible compared to the direct and indirect costs of taking care of morbidities associated with neonatal infection (WHO 2009). Hand hygiene may also be more psychologically satisfying and thus more acceptable for families compared to more technologically advanced preventive measures (Greenland 2013; WHO 2009). A priority‐setting exercise that involved stakeholders from Anglophone West African countries identified this review question as very important (Effa 2017). However, no systematic reviews have examined the effectiveness of different hand hygiene agents for prevention of neonatal infection and associated morbidities and death.
The United Nations, through global goals termed “Sustainable Development Goals” (SDGs), aims to end preventable death of newborns and children under five years of age by 2030, amongst other lofty goals (UN 2017). The third goal of the 17 SDGs cannot be achieved without reduced neonatal mortality. One way this goal might be achieved is to substantially reduce infection‐related neonatal mortality in low‐ and middle‐income countries (UN 2017). Meticulous hand hygiene practices are potential interventions for reducing these preventable deaths of newborns.
Objectives
To determine the effectiveness of different hand hygiene agents for preventing neonatal infection in community and health facility settings.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT), cross‐over trials, cluster trials, and quasi‐RCTs.
Types of participants
We included individuals providing care to newborn infants within the community or in health facility settings. For the purpose of this Cochrane Review, we defined the community setting as any setting other than a healthcare facility.
Source of outcome data: neonates (from birth to 28 days of life) for the primary outcomes, neonates and individuals providing care to them for secondary outcomes.
Types of interventions
Our criteria allowed comparison of any hand hygiene agent versus another type or standard practice (defined as the current practice adopted by a healthcare centre which could vary from centre to centre depending on the clinical protocol in use).
We included studies that compared any of the following interventions given singly or in combination with any of the comparisons.
Interventions
Handwashing with soap and water
Alcohol‐based hand sanitiser (e.g. rubs, wipes, scrubs)
Antiseptic hand sanitiser (e.g. chlorhexidine gluconate (CHG), chlorine derivatives, parachlorometaxylenol (PCMX), quaternary ammonium compounds, triclosan)
Comparisons
One class of agent versus another class of agent
One class of agent versus two or more other classes of agents
One single agent versus standard practice (usual care)
One single agent versus another agent
One single agent versus two or more other agents
Types of outcome measures
Primary outcomes
Incidence of suspected infection (author‐defined in study) within the first 28 days of life. We defined suspected infection “as defined by the individual provider OR evidenced by parent bringing child to medical care for chief complaint of infection, either parasitic, viral, or bacterial".
Incidence of bacteriologically confirmed infection (types of infection as specified by study authors) within the first 28 days of life (bacteriologically confirmed infection defined as “bacteria isolated from the blood, urine, or cerebrospinal fluid (CSF), or any other infected site on the neonates")
All‐cause mortality within the first seven days of life (early neonatal death)
All‐cause mortality from the 8th to the 28th day of life (late neonatal death)
Secondary outcomes
Duration of hospital stay
Any hospitalisation for neonates managed in the community setting
Incidence of community‐acquired infection and hospital‐acquired infection (hospital‐acquired infection defined as “an infection of bacteria isolated from the blood, urine, or CSF, that were not present or suspected on hospital admission, but presented while the patient was hospitalised")
Author‐reported adverse events, such as skin changes and reactions to handwash and rubs.
Search methods for identification of studies
The Neonatal Group Information Specialist developed search strategies in consultation with the authors. The MEDLINE strategy was translated, using appropriate syntax, for other databases. Search strategies combine intervention terms with standard terms for the neonatal population (developed by the Cochrane Neonatal Group). Methodological filters were used to limit retrieval to randomised controlled trials and systematic reviews. Searches were conducted without date, language, publication type or publication status limits. Clinical trial registries were also searched.
Electronic searches
The following databases were searched without date, language, publication type or publication status limits in December 2022:
Cochrane Library, Cochrane Central Register of Controlled Trials (via CRS Web), Issue 12, 2022 (December 12, 2022)
Ovid MEDLINE and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations and Daily (1946 to December 9, 2022)
Embase (1974 to December 9, 2022) (via OVID)
CINAHL (1981 to December 12, 2022) (via EbscoHost)
Search strategies are available in: Appendix 1; Appendix 2; Appendix 3; Appendix 4.
Searching other resources
We searched the following clinical trial registries for ongoing and recently completed trials in 20 January 2022:
WHO ICTRP database (www.who.int/ictrp/search/en/)
US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov)
Search strategies are available in: Appendix 5.
We searched the reference lists of related studies and systematic reviews to identify studies not found by database and trial registry searches. We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (BPK and EEU) independently assessed the eligibility of results of the literature search for potentially relevant trials using Covidence 2019. These two review authors assessed the full reports of potentially relevant trials and independently determined whether they met the inclusion criteria, using a pre‐tested eligibility form. When there were disagreements on study eligibility, a third review author (DH) resolved these. We listed all studies excluded after full‐text assessment, along with reasons for excluding them, in the Characteristics of excluded studies table. We ensured that trials with multiple publications were included only once, and when multiple publications included different but relevant outcomes, we included all publications on the same trial as one study in the review.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (CO and OO) independently extracted data from the included studies using Covidence (Covidence 2019). One review author (OO) entered the extracted data into Review Manager 5 (RevMan 5) (Review Manager 2020), and two review authors (SB, TAO) cross‐checked the data for completeness and accuracy. We extracted data on the number of participants randomised and the number analysed in each group for each reported outcome.
For continuous outcomes, we extracted the number of participants for each treatment arm, using arithmetic means (mean differenced (MDs)) and calculated standard errors (SEs). When data were presented as rates, we extracted rates in person‐time of follow‐up for person‐time outcomes and calculated the incidence rate ratio. Also, for events presented as counts, we calculated the risk ratio using the formula described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We obtained the standard error of the natural log of the rate ratio by using the formula described in Bello 2020. We used generic inverse variance to calculate the risk ratio for the person‐time outcome, and we calculated the standard error. We extracted data on reported adverse events as dichotomous outcomes (Higgins 2022). For cross‐over studies, when a sufficient washout period before switching interventions for participants was reported, we extracted the final post‐intervention data for our analysis using generic inverse variance (Larson 2005).
We attempted to contact the trial authors to request additional information on missing or unclear data.
Assessment of risk of bias in included studies
Two review authors (CO and OO) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane Risk of bias tool for the following domains (Higgins 2022).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 6 for a more detailed description of the risk of bias for each domain.
Measures of treatment effect
The type of treatment effect used in describing each of the listed outcomes was dependent on the type of data extracted for the specific outcome. For continuous data, we reported the mean difference (MD) for continuous outcomes. We presented all measures of effect along with their corresponding 95% confidence intervaIs (CIs). We extracted post‐intervention values and utilised mean and SD values for the analysis. For binary data, we analysed binary outcomes by calculating the risk ratio (RR) and risk difference (RD) with 95% CIs.
Unit of analysis issues
For cluster‐RCTs, we extracted results that had been adjusted for clustering (Higgins 2022). To avoid unit of analysis errors due to meta‐analysis of results from several time points, we selected a maximum of three most clinically important time points (as reported by the authors of included studies) for each outcome.
For cross‐over trials without carry‐over effects (studies that allowed time for the intervention to wash out before crossing over) that met our inclusion criteria, we presented trial results as for a parallel‐group trial (Larson 2005), and we used generic inverse variance to analyse continuous data. We used data collected at the end of the intervention and downgraded the certainty of evidence as for non‐randomised studies. We did not combine the included cross‐over trials in meta‐analyses because they were too heterogeneous. The effect of chlorhexidine against micro‐organisms lasts for 48 hours on the skin; Larson 2005 reported a washout period of one month.
One included cross‐over trial did not report a 'washout' period but prepared appropriate data analyses (i.e. paired analyses) for 'umbilical cord positivity' ‐ an important outcome that was not one of the outcomes listed in this review (Amortegui 1978). We extracted data from the first phase of the cross‐over trial and analysed them using generic inverse variance for data analysis as if the trial had followed a parallel‐group design (Deeks 2022); we presented the results as 'other outcomes not prespecified'. The study compared betadine (povidone‐iodine) and prepodyne ‐ two iodophor hand hygiene agents. One study reported that povidone‐iodine (betadine) can last for up to two days on the skin (Bigliardi 2017); however another study indicated that the residual effect ranged between 30 minutes and one hour (Gottardi 2001). We are not sure what the carry‐over effect of the intervention was on the outcomes reported. We rated the study as having high risk for other bias and downgraded the certainty of evidence to very low certainty.
Dealing with missing data
We analysed according to the intention‐to‐treat principle (all randomised participants were analysed in the groups to which they were originally assigned) when the authors of included studies accounted for all included participants. We would have assumed that data were missing at random when there was no difference in the proportion of missing data between intervention and control groups. If there were too many missing data for one treatment group compared to another group, we would have performed an ‘as‐treated analysis’, using data for those participants who completed the study and an ‘intention‐to‐treat analysis’ by analysing participants in the group to which they were randomised, and we would have assumed that the missing data had a poor outcome, irrespective of whether participants completed the study. We would have compared the two results and used the result that was most representative of the true effect.
We planned to contact trial authors for missing or incomplete data. When this was not feasible, we employed a complete‐case analysis, such that participants for whom no outcome was reported were excluded from the analysis, if we judged the study to be at low risk of bias regarding allocation sequence generation and allocation concealment. This analysis assumes that participants for whom an outcome is available are representative of the original randomised participants (Higgins 2022).
Assessment of heterogeneity
We would have assessed statistical heterogeneity between subgroups by visually inspecting the forest plots for overlapping confidence intervals (CIs), by applying the Chi² test (when P < 0.10 was considered statistically significant), and by using the I² statistic (statistic with values < 25% representing no heterogeneity; 25% to 49% low; 50% to 74% moderate; and ≥ 75% substantially high heterogeneity) if there were sufficient studies for meta‐analysis.
Assessment of reporting biases
We did not explore publication biases by constructing a funnel plot due to an insufficient number of included trials.
Data synthesis
Our analysis compared the effect of each hand hygiene product versus another in a head‐to‐head comparison. We analysed data using Review Manager 5 (RevMan 5) (Review Manager 2014 [Review Manager 2014]). We used generic inverse variance to calculate the risk ratio for the person‐time outcome and the mean difference for adverse events reported as a mean visual score. We extracted data as numbers of events and participants for each group for adverse events reported as dichotomous outcomes and obtained their relative risk. We also calculated risk ratios and standard errors (SEs) for count data using the formula described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022) and applied generic inverse variance for the analysis. We planned to meta‐analyse the data using RevMan when it was feasible to do so. We identified insufficient studies for a meta‐analysis for each of the prespecified comparisons. We presented the results in narrative form and downgraded the certainty of evidence for imprecision and serious risk of bias and displayed results in Summary of findings tables and in additional tables.
We presented the main results of the review alongside a GRADE appraisal of the certainty of evidence in Summary of findings tables and in additional tables.
Subgroup analysis and investigation of heterogeneity
We could not investigate heterogeneity. It was not feasible to do so because we did not meta‐analyse our included studies.
We would have performed a subgroup analysis of community‐based study versus hospital‐based study if we had included a community‐based study.
Sensitivity analysis
We did not conduct a sensitivity analysis to investigate the robustness of study results. Few studies were included in our review.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes:
incidence of suspected infections (author‐defined) within the first 28 days of life;
incidence of bacteriologically confirmed infections (types of infection as specified by authors) within the first 28 days of life;
all‐cause mortality within the first seven days of life (early neonatal death);
all‐cause mortality from the 8th to 28th day of life (late neonatal death).
Two review authors (OO and DH) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We considered evidence from non‐RCTs as very low‐certainty evidence because of very serious risk of bias
We used the GRADEpro GDT Guideline Development Tool to create three Summary of findings tables to report the certainty of the evidence (GRADEpro GDT), for the following comparisons:
one class of agent versus another class of agent (Table 1);
one single agent versus standard practice (usual care): Alcohol‐based handrub versus usual care (Table 2);
one single agent versus standard practice (usual care): Four per cent chlorhexidine + hand hygiene promotion versus standard practice (usual care) (Table 3).
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
Results of the search
Searches identified 1340 records. After removing 290 duplicates, 1050 records were available for screening. We excluded 1030 records based on title/abstract. We assessed 19 full texts and one trial registry record; we included six studies (Amortegui 1978; Ditai 2019; Larson 2000; Larson 2005; Ram 2020; Sharma 2013; Characteristics of included studies); classified one as ongoing (Characteristics of ongoing studies); and excluded 13 (Characteristics of excluded studies). We did not classify any studies as awaiting assessment. For details see Figure 1 .
1.
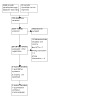
PRISMA Flow Diagram
Included studies
Our review included two RCTs with parallel groups (Larson 2000; Ram 2020), one cluster‐RCT (Ditai 2019), and three cross‐over clinical trials (Amortegui 1978; Larson 2005; Sharma 2013). Three studies (Ditai 2019; Larson 2005; Ram 2020) involved 3281 neonates; the remaining three (Amortegui 1978; Larson 2000; Sharma 2013), were not specific about the actual number of neonates included in their study. Three studies involved 279 nurses working in NICUs (Larson 2000; Larson 2005; Sharma 2013). The number of nurses included in one study (Amortegui 1978) was not clearly reported. Ditai 2019 included 103 pregnant women of over 34 weeks gestation from 10 villages in a community setting (sources of data: 103 mother‐neonate pairs) and Ram 2020 included “258 pregnant women who at the time of the data collector’s visit, were married, at 32 to 34 weeks of gestation” and intending to reside in the location in which they were residing at the time of recruitment into the study through the remainder of the antenatal period and the first four weeks after birth (the trial reported adverse events on 258 mothers and 246 neonates).
Study populations
Amortegui 1978 was conducted between October 1974 and March 1975 in two well‐baby nurseries at Magee Women's Hospital, in Pittsburgh, Pennsylvania, USA. The study included all nurses working in the nursery and all neonates on admission. The actual number of nurses and neonates recruited was unclear because it was not mentioned; however, "a total of 1806 cultures were evaluated and data were reported as counts. The number of nurses involved fluctuated between four and six in each nursery, according to the baby census". This study did not contribute any data to our outcomes of interest.
Ditai 2019 was a pilot cluster‐randomised study that included 103 pregnant women over 34 weeks gestation who met the eligibility criteria in a community setting involving 10 villages (sources of data: 103 mother‐neonate pairs).
Larson 2000 selected 16 full‐time neonatal intensive care unit (NICU) nurses for this study because this design provided sufficient statistical power for researchers to determine moderate‐to‐large effect sizes for the two primary outcome variables: changes in numbers of colony‐forming units (CFUs) on hands, and clinical changes in skin condition. The number of neonates was not mentioned.
Larson 2005 included 119 nurses plus 2932 neonates. Only neonates hospitalised for longer than 24 hours on the study units were eligible for inclusion in the study. Study authors reported that bloodstream infections, pneumonia, conjunctivitis, skin and soft tissue infections, and central nervous system infections were monitored because these represent more than 80% of all healthcare‐associated infections (HIs) in neonates. Surveillance was conducted prospectively by a study nurse epidemiologist who visited the units at least three times weekly. Sources of data included laboratory, radiology, and pharmacy records; patient records; information from physicians and nursing staff; and direct observation of neonates. All microbiological testing was performed by the clinical Microbiology Service of the Columbia University Medical Center, in New York. Standardised definitions from the National Nosocomial Infections Surveillance system (NNIS) adapted for use in neonates were used. The definition of conjunctivitis was broadened from the NNIS definition to include eye drainage with empirical antibiotic treatment. Inter‐rater reliability was first established in pilot work and was confirmed during year one by infection data collected simultaneously and independently by the study nurse epidemiologist and by each hospital’s nurse epidemiologist. Reliability was monitored throughout the study during meetings between the nurse epidemiologist and the physician co‐investigators (a paediatric infectious disease specialist and a neonatologist). Cases with equivocal data and those that did not fulfil NNIS criteria were reviewed, and discrepancies were resolved by consensus.
Ram 2020 was a randomised controlled trial of 258 pregnant women at 32 to 34 weeks gestation (the trial reported adverse events on 258 mothers and 246 neonates).
Sharma 2013 was a randomised cross‐over trial involving 35 female nurses aged 25 to 48 years working in a NICU and all babies in NICU (the actual number of neonates not reported). None of our outcomes of interest were reported by this study.
Study settings
Amortegui 1978 was conducted in two well‐baby nurseries at Magee Women's Hospital, in Pittsburgh, Pennsylvania, USA.
Ditai 2019 was conducted in 10 villages in the Mbale district of Uganda from August 2015 to May 2016.
Larson 2000 was conducted in a 47‐bed NICU at Babies’ and Children’s Hospital of the New York Presbyterian Medical Center, in New York, USA.
Larson 2005 was conducted in two NICUs in Manhattan, New York, USA.
Ram 2020 was conducted in Mirzapur, Bangladesh, a rural area approximately 60 km northwest of Dhaka that has one tertiary‐level private hospital.
Sharma 2013 was conducted at a level III NICU of a tertiary care institute in Northern India.
Most of the included studies recruited participants from NICUs. In most cases, the number of neonates included was not clearly specified.
For our primary outcomes, only one study provided data for the incidence of suspected infection (study author‐defined) within the first 28 days of life (Ditai 2019). One study provided data on the incidence of bacteriologically confirmed infection (types of infection as specified by study authors) within the first 28 days of life (Larson 2005). One study (Ditai 2019) provided data on the following outcomes: all‐cause mortality within the first seven days of life (early neonatal death) and all‐cause mortality from the 8th to the 28th day of life (late neonatal death). For our secondary outcomes, one study (Ditai 2019) provided data on 'any hospitalisation for neonates managed at the community setting'. For the 'incidence of community‐acquired and hospital‐acquired infection' outcome, only Larson 2005 provided data. Lastly, Larson 2000 and Larson 2005 provided data on 'study author‐reported adverse events such as skin changes and reactions to handwashing and rubs.' Two studies did not provide data for any of the listed primary and secondary outcomes (Amortegui 1978; Sharma 2013). However, we presented data on umbilical cord colonisation of neonates as reported by Amortegui 1978 under 'other important outcomes not prespecified' because we agreed that this was an important outcome. Ram 2020 reported only one of our secondary outcomes, 'Study author‐reported adverse events'.
Interventions versus comparators
One class of agent versus another class of agent: 2% CHG compared to alcohol hand sanitiser (61% alcohol and emollients) (Larson 2005)
One class of agent versus two or more other classes of agent: chlorhexidine gluconate (CHG) compared to non‐antimicrobial liquid detergent soap plus ten‐second application of a 60% isopropanol preparation containing emollients (Larson 2000)
One agent versus standard care (Ditai 2019; Ram 2020)
One agent versus another agent: betadine versus prepodyne (Amortegui 1978)
Head‐to‐head comparison of plain soap versus alcohol hand rub versus povidone‐iodine hand scrub (Sharma 2013). No data were provided for our outcomes of interest.
Study funding sources
All included studies declared no conflicts of interest.
Larson 2005 was funded by grant 5 RO1 NR05197 from the National Institute of Health, National Institute for Nursing Research, in Bethesda, Maryland, USA. "The funding organization had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article".
Ditai 2019 was funded by Medical Research Council/WellcomeTrust/DfID (Global Health Trials Scheme.
Ram 2020 had support from the Saving Lives at Birth partners: the U.S. Agency for International Development (USAID), the government of Norway, the Bill & Melinda Gates Foundation, Grand Challenges Canada, and the United Kingdom government. It was executed, and this article was prepared, by the named authors, and did not necessarily reflect the views of the Saving Lives at Birth partners.
Larson 2000 was supported in part by Steris Corporation, St Louis, MO.
The remaining two studies did not declare their funding sources (Amortegui 1978; Sharma 2013).
Excluded studies
We excluded 13 studies because they did not meet our inclusion criteria (Asare 2009; Azor‐Martinez 2018; Azor‐Martinez 2020; CTRI/2016/05/006963; Darmstadt 2005; Herruzo‐Cabrera 2001; Janota 2014; Kaufman 2014; Ng 2004;NCT03078335; Ram 2017; Webster 1989; Webster 1991). Asare 2009, Herruzo‐Cabrera 2001; Janota 2014, Ng 2004, Webster 1989 and Webster 1991 were excluded because they were not RCTs; Azor‐Martinez 2020 and Azor‐Martinez 2018 were excluded due to the wrong participant population. Four studies were excluded because of wrong interventions (CTRI/2016/05/006963; Darmstadt 2005; Kaufman 2014; Ram 2017).
Risk of bias in included studies
Two review authors independently assessed the methodological quality of all included studies according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Figure 2; Figure 3) (Higgins 2022).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated three studies as having low risk of bias for sequence generation (Ditai 2019; Larson 2000; Ram 2020), one study as having high risk of bias (Amortegui 1978), and two studies as having an unclear risk of bias (Larson 2005. Sharma 2013) One study was rated as having low risk of bias for allocation concealment (Sharma 2013), one study (Ditai 2019) was rated as having unclear risk of bias, and the remaining four studies were rated as having high risk of bias for allocation concealment (Amortegui 1978; Larson 2000; Larson 2005; Ram 2020).
Blinding
We rated two studies as low risk (Ditai 2019; Sharma 2013), three studies as high risk (Larson 2000; Larson 2005; Ram 2020), and one study as unclear risk (Amortegui 1978), respectively, for performance bias.
We rated Ditai 2019 and Sharma 2013 as low risk for detection bias, three studies as unclear risk for this domain (Amortegui 1978; Larson 2000; Larson 2005) and one study (Ram 2020) as high risk.
Incomplete outcome data
All six included studies (Amortegui 1978; Ditai 2019; Larson 2000; Larson 2005; Sharma 2013; Ram 2020) were rated as having low risk of attrition bias.
Selective reporting
We rated three studies as low risk of bias (Ditai 2019; Larson 2000; Ram 2020), two studies as unclear risk of bias (Amortegui 1978; Larson 2005), and one study as high risk of bias (Sharma 2013), respectively, for selective reporting.
Other potential sources of bias
Ditai 2019, Larson 2000 and Ram 2020 were rated as low risk of bias and two studies as high risk of bias for suspected publication bias (Amortegui 1978; Larson 2005). Amortegui 1978 did not report whether there was a washout period before participants crossed to a new intervention group. Larson 2005 allowed a four‐week washout period before switching interventions; however, rates of gloving used and rates of participant contact differed significantly between the two groups, and this was likely to have affected outcomes. We rated Sharma 2013 as unclear risk of bias for this domain, the trial did not report any usable data and we had no access to the study protocol for verification.
Effects of interventions
See: Table 1; Table 2; Table 3
st1. One class of agent versus another class of agent
2% CHG compared to alcohol hand sanitiser (61% alcohol and emollients)
See Table 1.
We found one study that compared one class of agent versus another class of agent (Larson 2005)
Primary outcomes
Incidence of suspected infection (study author‐defined) within the first 28 days of life: This study did not assess the incidence of suspected infection within the first 28 days of life.
Incidence of bacteriologically confirmed infection (types of infection as specified by study authors) within the first 28 days of life: 2% chlorhexidine gluconate probably reduces the overall risk of infections amongst neonates compared to 61% alcohol hand sanitiser within the first 28 days of life (RR 0.79, 95% confidence interval (CI) 0.66 to 0.93; 2932 participants, 1 study; moderate‐certainty evidence), number needed to treat for an additional beneficial outcome (NNTB) 385. However, the evidence was very uncertain in regard to the following types of infection: pneumonia (RR 0.77, 95% CI 0.29 to 2.03; 2932 participants, 1 study; very low‐certainty evidence), central nervous system (CNS) infection (RR 0.94, 95% CI 0.35 to 2.53; 2932 participants, 1 study; very low‐certainty evidence), conjunctivitis (RR 1.16, 95% CI 0.87 to 1.55; 2932 participants, 1 study; very low‐certainty evidence), skin sepsis (RR 0.56, 95% CI 0.30 to 1.04; 2932 participants, 1 study; very low‐certainty evidence) and bloodstream infection (RR 0.81, 95% CI 0.65 to 1.02; 2932 participants, 1 study; very low‐certainty evidence; Analysis 1.1). Larson 2005 used standardised definitions from the NNIS for the diagnosis of neonatal infection adapted for use in neonates.
All‐cause mortality within the first seven days of life (early neonatal death): Not reported for this comparison
All‐cause mortality from the 8th to the 28th day of life (late neonatal death): Not reported for this comparison
1.1. Analysis.

Comparison 1: One class of agent versus another class of agent, Outcome 1: Incidence rate of infection
Secondary outcomes
Duration of hospital stay: Not reported for this comparison
Any hospitalisation for neonates managed in the community setting: Not reported for this comparison
Incidence of community‐acquired and hospital‐acquired infection: None of our included studies reported this outcome; our intention was to disaggregate this outcome into community‐acquired infection and hospital‐acquired infection. However, Larson 2005 reported seven clusters of bacterial infection during this study. Four clusters were in the 2% CHG group, and three were in the alcohol hand sanitiser group. Study authors indicated that they were not sure if the outbreaks were related to the interventions and stated that "because of these uncertainties, we could not control for these clusters in the analysis".
Study author‐reported adverse events such as skin changes and reactions to handwashing and rubs: The adverse outcome was reported as mean self‐reported skin change and mean observer‐reported skin change. There may be little to no difference between the effects of 2% CHG on nurses’ skin compared to alcohol hand sanitiser based on very low‐certainty evidence for self‐reported skin change by the nurses (MD ‐0.80, 95% CI ‐1.59 to 0.01; 119 participants, 1 study) and on observer‐reported skin change (MD ‐0.19, CI ‐0.35 to ‐0.03; 119 participants, 1 study; Analysis 1.2), respectively.
1.2. Analysis.

Comparison 1: One class of agent versus another class of agent, Outcome 2: Adverse events (higher score is better)
2. One class of agent versus two or more other classes of agent
Chlorhexidine gluconate (CHG) compared to plain liquid soap + 60% isopropanol
We found one study that assessed one class of agent vs. two or more other classes of agent (Larson 2000).
Primary outcomes
Incidence of suspected infection (author‐defined in study) within the first 28 days of life: Not reported for this comparison
Incidence of bacteriologically confirmed infection (types of infection as specified by study authors) within the first 28 days of life: Not reported for this comparison
All‐cause mortality within the first seven days of life (early neonatal death): Not reported for this comparison
All‐cause mortality from the 8th to the 28th day of life (late neonatal death): Not reported for this comparison
Secondary outcomes
Duration of hospital stay: Not reported for this comparison
Any hospitalisation for neonates managed in the community setting: Not reported for this comparison
Incidence of community‐acquired and hospital‐acquired infection: Not reported for this comparison
Study author‐reported adverse events such as skin changes and reactions to handwashing and rubs: Larson 2000 reported that the skin condition in the plain soap was rated as better than in the CHG group for mean 'visual score of skin condition' (MD ‐1.87, 95% CI ‐3.74 to ‐0.00; 16 participants, 1 study; very low‐certainty evidence) and mean 'hand self‐assessment score' (MD ‐9.25, 95% CI ‐12.29 to ‐6.21; 16 participants, 1 study; very low‐certainty evidence; Analysis 2.1); we are uncertain whether plain soap has a better effect on the skin compared to 4% CHG.
2.1. Analysis.

Comparison 2: One class of agent versus two or more other classes of agents, Outcome 1: Adverse outcome (higher score is better)
3. One single agent versus standard practice (usual care)
We found two studies that assessed one agent versus standard care (usual care) (Ditai 2019; Ram 2020).
Alcohol‐based handrub versus usual care
See Table 2.
Primary outcomes
Incidence of suspected infection (author‐defined in study) within the first 28 days of life: reported by Ditai 2019 as total non‐malaria infection. The evidence was very uncertain whether alcohol‐based handrub is better than usual care in the prevention of suspected infection reported by mothers (RR 0.98, CI 0.69 to 1.39; 103 participants, 1 study; very low‐certainty evidence; Analysis 3.1).
Incidence of bacteriologically confirmed infection: Not reported for this comparison
All‐cause mortality within the first seven days of life (early neonatal death): We are uncertain about the comparative occurrence of early neonatal death in the alcohol‐handrub group compared to usual care (RR 0.29, CI 0.01 to 7.00; 103 participants, 1 study; very low‐certainty evidence; Analysis 3.2).
All‐cause mortality from the 8th to the 28th day of life (late neonatal death): We are uncertain about the comparative occurrence of late neonatal death in the alcohol‐handrub group compared to usual care (RR 0.29, CI 0.01 to 7.00; 103 participants, 1 study; very low‐certainty evidence; Analysis 3.3).
3.1. Analysis.

Comparison 3: One agent versus standard care:, Outcome 1: Total suspected infection
3.2. Analysis.

Comparison 3: One agent versus standard care:, Outcome 2: Early neonatal death
3.3. Analysis.

Comparison 3: One agent versus standard care:, Outcome 3: Late neonatal death
Secondary outcomes
Duration of hospital stay: Not reported for this comparison
Any hospitalisation for neonates managed in the community setting: We are uncertain if the alcohol‐based handrub group was better than the 'usual care' group in reducing the rate of hospitalisation in neonates managed in the community (RR 1.75, CI 0.46 to 6.60; 103 participants, 1 study; very low‐certainty evidence; Analysis 3.5).
Incidence of community‐acquired and hospital‐acquired infection: Not reported for this comparison
Study author‐reported adverse events such as skin changes and reactions to handwashing and rubs: Not reported for this comparison
3.5. Analysis.

Comparison 3: One agent versus standard care:, Outcome 5: Hospitalisation
Four per cent chlorhexidine + hand hygiene promotion versus standard practice (usual care)
See Table 3.
Primary outcomes
Incidence of suspected infection (author‐defined in study) within the first 28 days of life: Not reported for this comparison
Incidence of bacteriologically confirmed infection: Not reported for this comparison
All‐cause mortality within the first seven days of life (early neonatal death): Not reported for this comparison
All‐cause mortality from the 8th to the 28th day of life (late neonatal death): Not reported for this comparison
Secondary outcomes
Duration of hospital stay: Not reported for this comparison
Any hospitalisation for neonates managed in the community setting: Not reported for this comparison
Incidence of community‐acquired and hospital‐acquired infection: Not reported for this comparison
Study author‐reported adverse events such as skin changes and reactions to handwashing and rubs: Ram 2020 assessed potential adverse events amongst all participating households and whether hospitalisation followed the event. Authors reported this as events requiring hospitalisation (serious adverse events)(neonates (RR 1.83, 95% CI 0.75 to 4.42; 246 participants, 1 study; very low‐certainty evidence) and mothers (RR 1.97, 95% CI 0.18 to 21.45; 258 participants; 1 study; very low‐certainty evidence) Analysis 3.6), and events not requiring hospitalisation [neonates (RR 0.57, 95% CI 0.28 to 1.15, 246 participants, 1 study; very low‐certainty evidence) and mothers (RR 0.98, 95% CI 0.06 to 15.57; 258 participants, 1 study; very low‐certainty evidence) Analysis 3.7].
3.6. Analysis.

Comparison 3: One agent versus standard care:, Outcome 6: SAE
3.7. Analysis.

Comparison 3: One agent versus standard care:, Outcome 7: Adverse outcomes
4. One single agent versus another agent
Betadine versus prepodyne
None of our outcomes of interest were reported for this comparison. However, Amortegui 1978 assessed the effects of two iodophor products (betadine versus prepodyne) on umbilical cord swab culture positivity.
5. One single agent versus two or more other agents
Plain soap handwashing, alcohol handrub and povidone‐iodine hand scrub
None of our outcomes of interest were reported for this comparison. However, Sharma 2013 assessed the effects of plain soap handwashing, alcohol handrub and povidone‐iodine hand scrub on post‐hygiene colony forming unit count.
Discussion
Summary of main results
The main objective of this review was to ascertain the effectiveness of different hand hygiene agents for the prevention of neonatal infection in community and health facility settings. Infections including sepsis in newborns are major causes of death (Liu 2015), prolonged hospital stay, and early and long‐term complications, as well as huge economic burdens, particularly in low‐ and middle‐income countries (LMICs) (Ranjeva 2018). Contaminated hands of mothers, other caregivers, and healthcare workers (HCWs) are major sources of these infections; therefore, it is plausible that good hand hygiene interventions, which are relatively cheap and easy to implement, may reduce the incidence and burden of infection amongst newborns. We conducted this review with the aim of generating evidence‐based statements with regard to the effectiveness of different hand hygiene agents for preventing infection in the newborn.
In summary, we found six studies, four of which provided data for our prespecified outcomes (Ditai 2019; Larson 2000; Larson 2005; Ram 2020); unfortunately, all provided evidence of low to very low certainty. Pertaining to the primary outcomes, none of the retrieved studies compared any form of hand hygiene practice versus no intervention, possibly because hand hygiene is a universally recommended aspect of hospital care (WHO 2009); hence, it would be unlikely to have studies with the outright exclusion of some forms of hand hygiene for ethical reasons. However, one of the studies compared alcohol handrub to 'usual care' (Ditai 2019). Four studies assessed the superiority or otherwise of the use of one form of hand hygiene agent over another as regards the prevention of non‐invasive infection and invasive infection in the newborn, however, only two of the studies provided data for some of our listed outcomes of interest (Larson 2000; Larson 2005). Unfortunately, the heterogeneity of assessed hand hygiene agents and the high risk of bias of most of the included studies did not permit any meaningful conclusions.
One class of agent versus another class of agent: 2% CHG compared to alcohol hand sanitiser (61% alcohol and emollients)
Two per cent chlorhexidine gluconate probably reduces the overall Incidence of bacteriologically confirmed infection within the first 28 days of life compared to 61% alcohol hand sanitiser. Further, there may be little to no difference between the effects of 2% CHG on nurses’ skin compared to alcohol hand sanitiser for self‐reported skin change by the nurses and on observer‐reported skin change based on very low‐certainty evidence.
One class of agent versus two or more other classes of agent: chlorhexidine gluconate (CHG) compared to plain liquid soap + 60% isopropanol
For this comparison, we found no study that reported our primary outcomes. One study reported adverse events of the intervention. However, we are uncertain whether plain soap plus 60% isopropanol has a better effect on the skin of nurses compared to 4% CHG due to very low‐certainty evidence.
One agent versus no intervention: alcohol‐based handrub versus standard care (usual care)
For this comparison, we are uncertain of the effect of alcohol‐based handrub compared to usual care in the prevention of suspected infection reported by mothers. Also, we do not have sufficient evidence to conclude that alcohol‐based hand rub was better than 'usual care' for preventing all‐cause early or late neonatal death, duration of hospital stay, or the need for in‐hospital care for neonates studied in the community. Futhermore, we are uncertain of the adverse effects of 4% chlorhexidine gluconate on neonates compared to usual care.
Overall completeness and applicability of evidence
The certainty of evidence from this review was from moderate certainty to very low certainty.The identified studies fell short in addressing all objectives set out in the protocol for this study based on low to very low‐certainty evidence. Only one community‐based trial (Ditai 2019), assessed the effects of hand hygiene practice on important outcomes such as all‐cause mortality, the incidence of suspected infection, and need for in‐hospital care. The trials included in this review assessed different hand antiseptic agents, making meta‐analysis not feasible. Additionally, only two (Ditai 2019; Sharma 2013) of the included studies were conducted in a low‐ or middle‐income country (LMIC); however, one of these studies was not usable as it did not provide data on any of the prespecified outcomes of interest (Sharma 2013). It is also important to note that most trials included in this study were conducted in the hospital setting, specifically in neonatal intensive care units. Caregivers in the trials were mostly nurses; however, Ditai 2019, a community‐based trial reported parental involvement.
In view of the paucity of reliable clinical trials on this topic, no conclusive statement can be made for now on the effectiveness of any hand hygiene agent for preventing infection in newborns. The challenge is the diverse nature of agents deployed for the various trials. It probably would have been a lot better if most of the studies had used the same or similar hand hygiene agents. It is important to note that only one study measured a clinically important infection rate in terms of hospital‐acquired infection, but this finding is of limited value as the study authors could not confidently ascribe the observed clustering of infection outbreaks to the interventions (Larson 2005). Chlorhexidine, in varying concentrations and alcohol hand sanitisers are currently recommended for hand hygiene practice in patient care. Three studies provided data on the adverse effects of these interventions, and fewer adverse events were reported for plain soap Larson 2000, and disinfectant detergents only (Larson 2005; Ram 2020). Ram 2020 reported adverse events as events requiring hospitalisation and those without hospitalisation of mothers and neonates. However, patterns and grading of severity of these adverse events with respect to each type of intervention were not described in detail.
Thus, the applicability of findings in this review is limited by the fact that most of the assessed trials were conducted in high‐income countries (HICs), and within hospital settings, amongst nurses without non‐healthcare worker involvement. However, antiseptic soaps, chlorhexidine, and alcohol hand sanitisers continue to be recommended for use during patient care.
Quality of the evidence
This review included data from two randomised controlled trials (RCTs), three cross‐over trials, and one cluster‐randomised controlled trial with low to high risk of bias across all domains (Figure 2). Two studies were rated low risk, two studies were rated high risk, and one study was rated unclear risk for selection bias. One study was rated low risk and four studies were rated high risk for allocation bias. Only one of the studies was rated low risk for performance bias. Using the GRADE approach (Schünemann 2013), we rated the certainty of evidence for the primary outcomes of incidence of study author‐defined infection as moderate for 2% CHG compared to alcohol hand sanitiser; we downgraded by one level for serious risk of bias (Table 1).
For alcohol‐based handrub versus standard care (usual care), we downgraded the certainty of the evidence by two levels for study author‐defined infection for very serious imprecision due to wide confidence intervals and we downgraded by another level for serious indirectness because the trial was small and from a single setting (Table 2). For the outcomes of late and early all‐cause mortality, comparing alcohol‐based handrub versus standard care (usual care), we downgraded the certainty of the evidence by two levels for very serious imprecision due to very wide confidence intervals because this was a small study. We also downgraded the certainty of the evidence for serious indirectness because this was a study from one setting (Table 2).
The outcome, adverse skin reactions or skin changes following the use of various hand hygiene agents, was rated as very low certainty; we downgraded the certainty of the evidence for this outcome for the comparison of 2% CHG compared to alcohol hand sanitiser (61% alcohol and emollients) by one level for serious risk of bias due to study limitations, we downgraded by one level for serious imprecision due to wide confidence intervals and we downgraded by one level for serious indirectness because the study was carried out in one setting; for 4% + 2% chlorhexidine gluconate (CHG) versus plain liquid soap + hand sanitiser, we downgraded the certainty of the evidence by one level for serious risk of bias due to study limitations, we downgraded by two for very serious imprecision and we downgraded by one for serious indirectness because the study was from a single setting. For adverse events requiring hospitalisation and adverse events not requiring hospitalisation for one trial comparing 4% CHG plus promotion of hand hygiene versus 'usual care', we downgraded the certainty of the evidence by two levels for very serious risk of bias due to study limitations, and we downgraded by one level for serious imprecision due to wide confidence intervals involving harm and benefits (Table 3).
Potential biases in the review process
This review clearly highlights the paucity of high‐quality clinical trials on the effectiveness of hand hygiene for the prevention of infection amongst newborns. This is regarded as a limitation of this review. An extensive search of standard databases was conducted, and this search has been lately updated with no additional significantly useful findings. We are not oblivious to the likelihood of a few relevant publications in some hard‐to‐reach databases currently not being accessed. If there were studies in the databases comparing interventions with no form of hand hygiene, such studies would have been found due to their associated ethical importance.
One of the included trials reported on umbilical cord colonisation rather than suspected or confirmed neonatal infection as a prespecified outcome (Amortegui 1978). Inclusion of the study with a suspected non‐validated scale for measuring skin conditions complicating the use of hand hygiene agents was considered helpful, as this study provided insight into the spectrum of skin conditions that may occur following the use of hand hygiene agents. The prespecified outcome was author‐reported skin changes rather than grades of severity of skin conditions. To resolve the problem of missing data in the included study with unusable data (Sharma 2013), we contacted one of the study authors but received no response. We had initially excluded Ram 2020 because we felt that the trial did not meet our inclusion criteria: this trial assessed 4% chlorhexidine gluconate plus hand hygiene promotion. However, after discussion with Cochrane Neonatal's Editors, it was decided to include this study in the qualitative analysis but not the quantitative analysis.
Agreements and disagreements with other studies or reviews
Tanner 2016 conducted a systematic review which included only randomised controlled trials comparing surgical hand antiseptic. The review found no conclusive evidence "that one type of hand antisepsis was better than another in reducing surgical site infections".
Bello 2020 conducted a systematic review which compared the effectiveness of handwash versus handrub techniques for preventing nosocomial infections in hospital intensive care units. The study included RCTs, before‐and‐after designs, cluster‐cross‐over and observational designs. Based on low‐certainty evidence, the authors concluded that handrub techniques appeared more effective compared to handwash techniques in hospital intensive care units.
Authors' conclusions
Implications for practice.
RCTs have provided insufficient data for review authors to determine the most effective agent for hand hygiene for prevention of neonatal infection.
In addition, we found a paucity of data that would allow us to reach meaningful conclusions pertaining to the superiority of one form of antiseptic hand hygiene agent over another for prevention of neonatal infection. Also, the sparse available data were of moderate to very low certainty evidence for reported outcomes.
Other critical aspects of hand hygiene such as the actual technique of hand hygiene, particularly the seven steps and five moments of handwashing, as well as the duration of the hand hygiene procedure, were not measured in the included trials. These are also critical in influencing the effectiveness of hand hygiene for preventing neonatal infection or the superiority of one antiseptic agent over another. We received insufficient data from the included trials to determine the best hand hygiene agents, resulting in no confidence in the effect estimates due to very serious study limitations and imprecision.
Implications for research.
The findings of this review, derived from trials conducted mostly in high‐income countries (HICs), clearly call for more carefully designed randomised controlled trials (RCTs) that will be conducted in both HICs and low‐ and middle‐income countries (LMICs) to assess the effects of hand hygiene practices on prevention of non‐invasive and invasive infection amongst newborns and infection‐related mortality and morbidity, including length of hospital stay, in order to provide information of more widespread applicability. Future studies should measure outcomes of invasive and non‐invasive infection, as well as associated mortality. This implies that subsequent trials should specify accepted definitions of suspected and confirmed infection as measurable outcomes. These future studies should adopt validated scales by which to grade the severity of skin conditions occurring as adverse effects of hand hygiene agents. This, along with the ease and duration of application, may be used to assess the acceptability of hand hygiene agents. In addition, such trials should specifically adopt a universally recommended technique for hand hygiene such as the seven steps and five moments of hand hygiene. It is also important for researchers to assess compliance with hand hygiene needs.
The use or otherwise of these hand hygiene agents depends on their acceptability by caregivers and healthcare workers (HCWs) which, in turn, depends on whether the agent is skin‐friendly. Conducting additional clinical trials on the effects of these hand hygiene agents on skin reactions and skin changes amongst caregivers and HCWs will be worthwhile.
What's new
| Date | Event | Description |
|---|---|---|
| 2 June 2023 | New citation required but conclusions have not changed | Segun Bello and Delia Horn joined the author team |
| 2 June 2023 | New search has been performed | Search updated December 2022 |
History
Protocol first published: Issue 5, 2019 Review first published: Issue 1, 2021
| Date | Event | Description |
|---|---|---|
| 27 May 2021 | Amended | Minor edits to forest plots. |
Notes
A previous version of this Cochrane Review had been withdrawn from publication due to errors identified in the data extraction process and in the reporting of results and, as such, the findings of the review may not have been reliable (Kuti 2021b).
This current review represents a new version of the review which has addressed previous concerns.
Acknowledgements
We would like to thank Cochrane Neonatal ‐ Colleen Ovelman former Managing Editor and Michelle Fiander and Jane Cracknell, Managing Editors; Roger Soll, Co‐coordinating Editor; and Bill McGuire, Co‐coordinating Editor ‐ who provided editorial and administrative support. Michelle Fiander, Information Specialist, designed and ran the literature searches. Jeffrey Horbar and Jacqueline Ho peer‐reviewed and offered feedback on this review.
This Cochrane Review is a product of the priority setting exercise conducted by Cochrane Nigeria for the Cochrane African Network Project. We acknowledge the contributions of the staff and associates of Cochrane Nigeria and all stakeholders including consumer representatives who participated in the priority setting exercise. We thank Yemisi Pius, a member of the Cochrane Consumer Network, for her comments and suggestions.
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
We thank the following two peer reviewers: Matteo Bruschettini, Lund University, Skåne University Hospital, Lund, Sweden; and Thangaraj Abiramalatha, Associate Professor, Neonatology, KMCH Institute of Health Sciences and Research (KMCHIHSR), Coimbatore, Tamil Nadu, India.
Appendices
Appendix 1. Cochrane CRS strategy
| Cochrane CRS | |||
| December‐12‐2022 | |||
| 1 | (infant or infants or infant's or infantile or infancy or newborn* or new born or new borns or newly born or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or pre term or premies or preemie or preemies or low birth weight or low birthweight or VLBW or LBW or ELBW or NICU) AND CENTRAL:TARGET | 100164 | |
| 2 | MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 18097 | |
| 3 | #2 OR #1 | 100164 | |
| 4 | MESH DESCRIPTOR Hand Hygiene EXPLODE ALL AND CENTRAL:TARGET | 526 | |
| 5 | MESH DESCRIPTOR Hand Disinfection EXPLODE ALL AND CENTRAL:TARGET | 451 | |
| 6 | MESH DESCRIPTOR Hand Sanitizers EXPLODE ALL AND CENTRAL:TARGET | 43 | |
| 7 | (handwash* or handrub* or hand‐wash* or hand‐rub* or handgel or handgels or hand‐gel or hand‐gel):ti,ab,kw AND CENTRAL:TARGET | 1056 | |
| 8 | ((hand or hands) NEAR3 (alcohol* or antiinfect* or anti‐infect* or antisep* or aseps* or aseptic* or chlorhexidin* or clean* or decontaminat* or disinfect* or ethanol* or hygiene or hygienic* or propanol* or rub or rubs or rubbing or saniti* or scrub* or soap? or soaping or sterili* or triclosan* or wash* or wipe* or wiping)):ti,ab,kw AND CENTRAL:TARGET | 1624 | |
| 9 | (hand* NEAR2 chlorine adj2 derivative*):ti,ab,kw AND CENTRAL:TARGET | 0 | |
| 10 | (hand* NEAR2 iodine chloroxylenol):ti,ab,kw AND CENTRAL:TARGET | 0 | |
| 11 | (hand* NEAR2 quaternary ammonium compound*):ti,ab,kw AND CENTRAL:TARGET | 0 | |
| 12 | (scrub* NEAR2 surgical):ti,ab,kw AND CENTRAL:TARGET | 106 | |
| 13 | #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 | 1984 | |
| 14 | MESH DESCRIPTOR Hand EXPLODE ALL AND CENTRAL:TARGET | 2568 | |
| 15 | (hand or hands):ti,ab,kw AND CENTRAL:TARGET | 38089 | |
| 16 | #14 OR #15 | 38950 | |
| 17 | MESH DESCRIPTOR Bacterial Infections EXPLODE ALL WITH QUALIFIER PC AND CENTRAL:TARGET | 42 | |
| 18 | MESH DESCRIPTOR Cross Infection EXPLODE ALL AND CENTRAL:TARGET | 1417 | |
| 19 | MESH DESCRIPTOR Hygiene EXPLODE ALL AND CENTRAL:TARGET | 2736 | |
| 20 | MESH DESCRIPTOR Infection Control EXPLODE ALL AND CENTRAL:TARGET | 1346 | |
| 21 | MESH DESCRIPTOR Disinfectants EXPLODE ALL AND CENTRAL:TARGET | 3864 | |
| 22 | MESH DESCRIPTOR Solutions EXPLODE ALL AND CENTRAL:TARGET | 10857 | |
| 23 | MESH DESCRIPTOR Alcohols EXPLODE ALL AND CENTRAL:TARGET | 40097 | |
| 24 | MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND CENTRAL:TARGET | 9575 | |
| 25 | MESH DESCRIPTOR Soaps EXPLODE ALL AND CENTRAL:TARGET | 257 | |
| 26 | MESH DESCRIPTOR Antisepsis EXPLODE ALL AND CENTRAL:TARGET | 130 | |
| 27 | MESH DESCRIPTOR Disinfection EXPLODE ALL AND CENTRAL:TARGET | 404 | |
| 28 | MESH DESCRIPTOR Sterilization EXPLODE ALL AND CENTRAL:TARGET | 551 | |
| 29 | MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND CENTRAL:TARGET | 2557 | |
| 30 | MESH DESCRIPTOR Triclosan EXPLODE ALL AND CENTRAL:TARGET | 434 | |
| 31 | MESH DESCRIPTOR Quaternary Ammonium Compounds EXPLODE ALL AND CENTRAL:TARGET | 5126 | |
| 32 | #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 | 63104 | |
| 33 | #3 AND #13 | 323 | |
| 34 | #3 AND #16 AND #32 | 115 | |
| 35 | #33 OR #34 | 362 |
Appendix 2. MEDLINE strategy
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions 1946 to December 09, 2022 | ||
| # | Searches | Results |
| 1 | exp Hand Hygiene/ | 8023 |
| 2 | exp Hand Disinfection/ | 6303 |
| 3 | Hand sanitizers/ | 348 |
| 4 | (handwash* or handrub* or hand‐wash* or hand‐rub* or handgel? or hand‐gel?).ti,ab,kw,kf. | 7203 |
| 5 | ((hand or hands) adj3 (alcohol* or antiinfect* or anti‐infect* or antisep* or aseps* or aseptic* or chlorhexidin* or clean* or decontaminat* or disinfect* or ethanol* or hygiene or hygienic* or propanol* or rub or rubs or rubbing or saniti* or scrub* or soap? or soaping or sterili* or triclosan* or wash* or wipe* or wiping)).ti,ab,kw,kf. | 13807 |
| 6 | (hand* adj2 chlorine adj2 derivative*).ti,ab,kw,kf. | 0 |
| 7 | (hand* adj2 iodine chloroxylenol).ti,ab,kw,kf. | 0 |
| 8 | (hand* adj2 quaternary ammonium compound*).ti,ab,kw,kf. | 4 |
| 9 | (scrub* adj2 surgical).ti,ab,kw,kf. | 421 |
| 10 | or/1‐9 [Hand Hygiene] | 18126 |
| 11 | exp Hand/ | 86364 |
| 12 | hand?.ti,ab,kw,kf. | 487892 |
| 13 | or/11‐12 [Hands] | 530818 |
| 14 | exp Bacterial Infections/pc | 100498 |
| 15 | exp Cross Infection/pc | 25747 |
| 16 | exp Hygiene/ | 45117 |
| 17 | exp Infection Control/ | 69871 |
| 18 | exp Disinfectants/ | 74478 |
| 19 | exp Solutions/ | 135506 |
| 20 | exp Alcohols/ | 674334 |
| 21 | exp soaps/ | 2718 |
| 22 | exp antisepsis/ | 4592 |
| 23 | exp disinfection/ | 17071 |
| 24 | exp sterilization/ | 33595 |
| 25 | exp chlorhexidine/ | 9270 |
| 26 | exp triclosan/ | 3253 |
| 27 | exp Anti‐Infective Agents, Local/ | 255655 |
| 28 | or/14‐27 [infection prevention; antiinfective agents; hygiene] | 1178745 |
| 29 | exp infant, newborn/ | 663647 |
| 30 | (infant or infants or infant? or infantile or infancy or newborn* or new born or new borns or newly born or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or pre term or premies or low birth weight or low birthweight or VLBW or LBW or ELBW or NICU).ti,ab,kw,kf. | 994149 |
| 31 | or/29‐30 [Filter: Neonatal Population 2021‐‐MEDLINE] | 1277026 |
| 32 | randomized controlled trial.pt. | 582235 |
| 33 | controlled clinical trial.pt. | 95125 |
| 34 | (randomized or randomised).ti,ab. | 755923 |
| 35 | placebo.ab. | 233827 |
| 36 | drug therapy.fs. | 2553906 |
| 37 | randomly.ab. | 397137 |
| 38 | trial.ab. | 626605 |
| 39 | groups.ab. | 2444627 |
| 40 | (quasirandom* or quasi‐random*).ti,ab. | 5464 |
| 41 | exp animals/ not humans.sh. | 5071725 |
| 42 | (or/32‐40) not 41 [RCT Filter‐Sensitivity Max ] | 4853141 |
| 43 | systematic review.pt. | 214130 |
| 44 | (systematic adj2 review).ti. | 206655 |
| 45 | meta analysis/ | 171945 |
| 46 | (meta‐analysis or metaanalysis).ti,ab,kw. | 221763 |
| 47 | (cochrane or systematic review?).jw. | 19587 |
| 48 | overview of reviews.ti. | 109 |
| 49 | or/43‐48 [SR filter] | 386925 |
| 50 | 10 and 31 and 42 [Hand Hygiene & Neonate & RCT] | 355 |
| 51 | 13 and 28 and 31 and 42 [Hands & Hygiene/Infection Control etc & Neonate & RCT] | 261 |
| 52 | or/50‐51 [RCT Results MEDLINE] | 457 |
| 53 | 10 and 31 and 49 [Hand Hygiene & Neonate & SR] | 39 |
| 54 | 13 and 28 and 31 and 49 [Hands & Hygiene/Infection Control etc & Neonate & SR] | 41 |
| 55 | 52 or 54 [Results] | 469 |
Appendix 3. Embase strategy
| Embase 1974 to 2022 December 09 | ||
| # | Searches | Results |
| 1 | hand washing/ or hand disinfection/ or hand sanitizer/ | 20444 |
| 2 | (handwash* or handrub* or hand‐wash* or hand‐rub* or handgel? or hand‐gel?).ti,ab,kw. | 9381 |
| 3 | ((hand or hands) adj3 (alcohol* or antiinfect* or anti‐infect* or antisep* or aseps* or aseptic* or chlorhexidin* or clean* or decontaminat* or disinfect* or ethanol* or hygiene or hygienic* or propanol* or rub or rubs or rubbing or saniti* or scrub* or soap? or soaping or sterili* or triclosan* or wash* or wipe* or wiping)).ti,ab,kw. | 18795 |
| 4 | (hand* adj2 chlorine adj2 derivative*).ti,ab,kw. | 0 |
| 5 | (hand* adj2 iodine chloroxylenol).ti,ab,kw. | 0 |
| 6 | (hand* adj2 quaternary ammonium compound*).ti,ab,kw. | 6 |
| 7 | (scrub* adj2 surgical).ti,ab,kw. | 473 |
| 8 | or/1‐7 [Hand Hygiene] | 29789 |
| 9 | exp Hand/ | 91951 |
| 10 | hand?.ti,ab,kw. | 650441 |
| 11 | or/9‐10 [Hands] | 696645 |
| 12 | exp bacterial infection/pc [Prevention] | 83591 |
| 13 | exp cross infection/pc [Prevention] | 10339 |
| 14 | exp hygiene/ | 88086 |
| 15 | exp infection control/ | 119034 |
| 16 | exp disinfectant agent/ | 544106 |
| 17 | exp "solution and solubility"/ | 276760 |
| 18 | exp alcohol derivative/ | 523770 |
| 19 | exp alcohol/ | 286815 |
| 20 | exp topical antiinfective agent/ | 678358 |
| 21 | exp soap/ | 4926 |
| 22 | exp antisepsis/ | 3773 |
| 23 | exp disinfection/ | 31698 |
| 24 | exp instrument sterilization/ | 21468 |
| 25 | exp chlorhexidine/ | 20871 |
| 26 | exp triclosan/ | 6220 |
| 27 | exp quaternary ammonium derivative/ | 87743 |
| 28 | or/12‐27 [DIsinfection, Hygiene etc] | 1487294 |
| 29 | Newborn/ | 584101 |
| 30 | Prematurity/ | 120171 |
| 31 | (infant or infants or infant? or infantile or infancy or newborn* or new born or new borns or newly born or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or pre term or premies or preemie or preemies or low birth weight or low birthweight or VLBW or LBW or ELBW or NICU).ti,ab,kw. | 1164340 |
| 32 | or/29‐31 [Cochrane Neonatal standard search terms‐EMBASE] | 1385093 |
| 33 | Randomized controlled trial/ or Controlled clinical study/ | 931563 |
| 34 | random$.ti,ab,kw. | 1871160 |
| 35 | Randomization/ | 95675 |
| 36 | placebo.ti,ab,kw. | 350891 |
| 37 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 263673 |
| 38 | double blind procedure/ | 201486 |
| 39 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 425629 |
| 40 | parallel group$1.ti,ab. | 30519 |
| 41 | (crossover or cross over).ti,ab. | 119558 |
| 42 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 394379 |
| 43 | (open adj label).ti,ab. | 102224 |
| 44 | or/33‐43 [ RCT‐EMBASE; terms based on Cochrane Central strategy] | 2659853 |
| 45 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 24435743 |
| 46 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 31477763 |
| 47 | 46 not 45 [Animal exclusion‐] | 7042020 |
| 48 | 44 not 47 [Filter: RCT‐EMBASE] | 2379119 |
| 49 | (systematic adj2 review).ti. | 243564 |
| 50 | meta analysis/ | 264492 |
| 51 | (meta‐analysis or metaanalysis).ti,ab,kw. | 286383 |
| 52 | (cochrane or systematic review?).jx. | 30842 |
| 53 | overview of reviews.ti. | 115 |
| 54 | or/49‐53 [SR filter‐EMBASE] | 485720 |
| 55 | 8 and 32 and 48 [Hand Hygiene & Neonate & RCT] | 242 |
| 56 | 11 and 28 and 32 and 48 [Hands and Disinfection & Neonate & RCT] | 174 |
| 57 | or/55‐56 [RCT results EMBASE] | 313 |
| 58 | 8 and 32 and 54 [Hand Hygiene & Neonate & SR] | 43 |
| 59 | 11 and 28 and 32 and 54 [Hands and Disinfection & Neonate & SR] | 40 |
| 60 | or/58‐59 [SR Results] | 71 |
| 61 | or/57,60 [Embase results] | 349 |
Appendix 4. CINAHL search strategy
| CINAHL Complete (Ebscohost) | ||
| December‐12‐2022 | ||
| Search screen: Advanced Search | ||
| # | Query | Results |
| 1 | hand hygiene | 4,279 |
| 2 | (MH "Handwashing") OR (MH "Surgical Scrubbing") | 9,822 |
| 3 | TI ( (handwash* or handrub* or hand‐wash* or hand‐rub*) ) OR AB ( (handwash* or handrub* or hand‐wash* or hand‐rub*) ) OR SU ( (handwash* or handrub* or hand‐wash* or hand‐rub*) ) | 10,920 |
| 4 | TI ( ((hand or hands) N3 (alcohol* or antiinfect* or anti‐infect* or antisep* or aseps* or aseptic* or chlorhexidin* or clean* or decontaminat* or disinfect* or ethanol* or hygiene or hygienic* or propanol* or rub or rubs or rubbing or saniti* or scrub* or soap? or soaping or sterili* or triclosan* or wash* or wipe* or wiping)) ) OR AB ( ((hand or hands) N3 (alcohol* or antiinfect* or anti‐infect* or antisep* or aseps* or aseptic* or chlorhexidin* or clean* or decontaminat* or disinfect* or ethanol* or hygiene or hygienic* or propanol* or rub or rubs or rubbing or saniti* or scrub* or soap? or soaping or sterili* or triclosan* or wash* or wipe* or wiping)) ) | 7,687 |
| 5 | TI (surgical N2 scrub*) OR AB (surgical N2 scrub*) OR SU (surgical N2 scrub*) | 344 |
| 6 | S1 OR S2 OR S3 OR S4 OR S5 | 13,190 |
| 7 | ( TI (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) ) OR ( AB (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) ) | 255,167 |
| 8 | (MH "Infant, Large for Gestational Age") OR (MH "Infant, Low Birth Weight+") OR (MH "Infant, Postmature") OR (MH "Infant, Premature") | 38,675 |
| 9 | S7 OR S8 | 260,427 |
| 10 | (MH "Randomized Controlled Trials+") OR (MH "Triple‐Blind Studies") OR (MH "Single‐Blind Studies") | 145,118 |
| 11 | TI ( randomized or randomised or randomly OR placebo ) OR AB ( randomized or randomised or randomly OR placebo ) | 388,529 |
| 12 | TI (control* N2 trial) OR AB (control* N2 trial) | 163,977 |
| 13 | PT randomized controlled trial | 148,888 |
| 14 | TI (quasirandom* or quasi‐random*) OR AB (quasirandom* or quasi‐random*) | 2,221 |
| 15 | S10 OR S11 OR S12 OR S13 OR S14 | 434,223 |
| 16 | S6 AND S9 AND S15 | 55 |
| 17 | PT (systematic review) | 136,882 |
| 18 | (MH "Meta Analysis") | 67,300 |
| 19 | (MH "Systematic Review") | 116,411 |
| 20 | TI (systematic N2 review) | 105,511 |
| 21 | TI (meta‐analysis or metaanalysis) OR AB (meta‐analysis or metaanalysis) | 99,900 |
| 22 | TI (overview N2 (review or reviews)) OR AB (overview N2 (review or reviews)) | 2,692 |
| 23 | S17 OR S18 OR S19 OR S20 OR S21 OR S22 | 228,929 |
| 24 | S6 AND S9 AND S23 | 33 |
| 25 | S24 OR S16 | 80 |
Appendix 5. Trial registry search terms
| Date | |||
| 12‐12‐2022 | clinicaltrials gov | hand hygiene AND neonate [Other terms] | 19 |
| 12‐12‐2022 | clinicaltrials gov | handwash AND neonate [other terms] | 20 |
| 12‐12‐2022 | clinicaltrials gov | handrub AND neonate [other terms] | 5 |
| 12‐12‐2022 | clinicaltrials gov | hand disinfection AND neonate [other terms] | 1 |
| 12‐12‐2022 | clinicaltrials gov | hand sanitizer AND neonate [other terms] | 2 |
| 12‐12‐2022 | clinicaltrials gov | hand sanitzing AND neonate [other terms] | 0 |
| 12‐12‐2022 | clinicaltrials gov | hand sanitization AND neonate [other terms] | 1 |
| 12‐12‐2022 | hand hygiene neonatal | 12 | |
| 12‐12‐2022 | ICTRP | handwash neonate [homepage search box] | 0 |
| 12‐12‐2022 | ICTRP | handwash neonatal [homepage search box] | 0 |
| 12‐12‐2022 | ICTRP | handwashing AND neonate [homepage search box] | 1 |
| 12‐12‐2022 | ICTRP | handwashing neonatal [homepage search box] | 4 |
| 12‐12‐2022 | ICTRP | handrub neonate / handrub neonatal | 2 |
| 12‐12‐2022 | ICTRP | hand disinfection AND neonate [homepage search box] | 0 |
| 12‐12‐2022 | ICTRP | hand sanitizer AND neonate | 0 |
| 12‐12‐2022 | ICTRP | hand sanitzing AND neonate | 0 |
| 12‐12‐2022 | ICTRP | hand sanitization AND neonate | 0 |
| 12‐12‐2022 | ICTRP | hand disinfection neonatal | 0 |
| 12‐12‐2022 | ICTRP | hand hygiene neonatal | 12 |
| 12‐12‐2022 | ICTRP | hand sanitizer neonatal | 1 |
| 80 |
Appendix 6. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. One class of agent versus another class of agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence rate of infection | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Pneumonia | 1 | 2932 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.29, 2.03] |
| 1.1.2 CNS | 1 | 2932 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.35, 2.53] |
| 1.1.3 Conjunctivitis | 1 | 2932 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [0.87, 1.55] |
| 1.1.4 Skin | 1 | 2932 | Risk Ratio (IV, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 1.1.5 Bloodstream | 1 | 2932 | Risk Ratio (IV, Fixed, 95% CI) | 0.81 [0.65, 1.02] |
| 1.1.6 All infections | 1 | 2932 | Risk Ratio (IV, Fixed, 95% CI) | 0.79 [0.66, 0.93] |
| 1.2 Adverse events (higher score is better) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Mean self‐reported skin change of nurses (mean score) | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.59, ‐0.01] | |
| 1.2.2 Mean observer‐reported skin changes of nurses (mean score) | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.35, ‐0.03] |
Comparison 2. One class of agent versus two or more other classes of agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adverse outcome (higher score is better) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Mean visual scoring of skin (mean score) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐3.74, ‐0.00] |
| 2.1.2 Mean hand self‐assessment (mean score) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐9.25 [‐12.29, ‐6.21] |
Comparison 3. One agent versus standard care:
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total suspected infection | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.30] |
| 3.2 Early neonatal death | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.00] |
| 3.3 Late neonatal death | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.00] |
| 3.4 Infant mortality | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.56] |
| 3.5 Hospitalisation | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.46, 6.60] |
| 3.6 SAE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.6.1 Neonates | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.75, 4.42] |
| 3.6.2 Mothers | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.45] |
| 3.7 Adverse outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 Neonates | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.30, 1.15] |
| 3.7.2 Mothers | 1 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.57] |
3.4. Analysis.

Comparison 3: One agent versus standard care:, Outcome 4: Infant mortality
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amortegui 1978.
| Study characteristics | ||
| Methods | Cross‐over clinical trial | |
| Participants | The actual number of nurses and neonates recruited was unclear because it was not mentioned; however, "a total of 1806 cultures were evaluated and data were reported as counts. The number of nurses involved fluctuated between 4 and 6 in each nursery, according to the baby census". This study did not contribute any data to our outcomes of interest. Inclusion criteria
Exclusion criteria Not reported |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | A total of 1806 cultures were evaluated. The study was single‐blind to the nurses. Authors did not declare their funding source. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation was applied. Quote: "both products were used alternatively for handwash in each of the two study nurseries in an effort to minimise nursery variation". |
| Allocation concealment (selection bias) | High risk | Not reported; probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was unclear what the study authors meant by (quote) "The study was single blinded to the nurses". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the cultures were interpreted by one of the technologists in the microbiology laboratory. This person was also involved in culture collection". It was not stated whether this person was blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no report of attrition. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol; it was unclear. |
| Other bias | High risk | The numbers of nurses and neonates involved in the study were not clearly stated. Also the study did not report if there was a washout period before participants crossed to a new intervention group. |
Ditai 2019.
| Study characteristics | ||
| Methods | Cluster‐RCT | |
| Participants | Participants were 103 pregnant women of over 34 weeks gestation who met the eligibility criteria in a community setting involving 10 villages (sources of data: 103 mother‐neonate pairs). Inclusion criteria: "The study village was selected if it had recorded at least ten births within the past 3 months and had one or more active VHWs and the village leaders committed to study participation and implementation. Meanwhile, the health centres were included if they had participated previously in community trials or research. Pregnant women of over 34 weeks gestation were recruited over a 3‐month period and followed up for 3 months postnatally". Exclusion criteria: "The exclusion criteria included temporary visitors" (defined as any pregnant mother found as a visitor in the home who did not stay afterward). Other clinical criteria (e.g. malaria in pregnancy, previous caesarean section) were not used as exclusion criteria in this pilot study due to the nature of the intervention. |
|
| Interventions | Intervention: "Pregnant women in intervention villages were provided with ABHR (Alsoft V, Saraya East Africa Co. Ltd.) at recruitment. The recruiting research midwives provided the ABHR free of charge to each woman in a 1‐l bottle for use while at home, along with a refillable 100‐mL bottle for use while travelling. The recruiting midwives trained each woman in the intervention villages on the use of ABHR, the basic hand rub steps, and the ‘three moments for community neonatal hand hygiene’, developed by the study team for the pilot trial. This was adopted from the WHO ‘5 Moments for Hand Hygiene’. The three moments for community neonatal hand hygiene instructions were printed on a poster with a pictorial illustration, which was given to the participants as instructions to display in a visible area and follow in their homes. The poster was available in both English and the local language (Lumasaba). Participants were also given Maama Kits which consisted of basic supplies, i.e. sterile gloves, plastic sheets, cord ligature, razor blades, tetracycline, cotton, gauze, soap, and sanitary pads (usual care)". Control: Control villages: current standard care practice. Women in the control villages received the current standard care of Maama Kits for delivery and the usual antenatal education. At the time of recruitment, the recruiting midwives advised the women to deliver at health facilities. The midwives encouraged women to attend postnatal checks and immunisation clinics at 6–24 h, 1–2 weeks, 6 weeks, 10 weeks, and 14 weeks at the nearby health facilities in line with the local guidelines. The Maama Kit consisted of basic supplies, i.e. sterile gloves, plastic sheets, cord ligature, razor blades, tetracycline, cotton, gauze, soap, and sanitary pads. | |
| Outcomes | 1) infant sepsis (physician‐defined and microbiological) 2) infant mortality 3) rate of positive infections using the WHO’s IMCI screening criteria for infection 4) early neonatal death 5) late neonatal death 6) mother‐reported infant infection 7) hospitalisation |
|
| Notes | Sponsor: Medical Research Council/WellcomeTrust/DfID (Global Health Trials Scheme) Country: Uganda Settting: "The study was conducted in ten clusters (villages) around two community health centres (health centre three (HCIII) and health centre four (HCIV)). The HCIII was surrounded by three control villages, while the HCIV was surrounded by five intervention and three control villages. The clusters were rural villages located in Mbale region, Eastern Uganda. A map of the villages surrounding the targeted community health centres in Mbale district was reviewed by the research team in collaboration with the village health team workers (VHWs). Ten villages were selected from a map of 28 villages if they were within the catchment area of the participating community health centres, were not served by another community health centre, and had a village health worker." The study also adjusted for clustering effects in the analysis. Contact: Ditai J, INSTITUTION Sanyu Africa Research Institute (SAfRI), Mbale, Uganda EMAIL: J.Ditai@safri.ac.ug ADDRESS: 1 Sanyu Africa Research Institute (SAfRI), Mbale Regional Referral Hospital, Pallisa‐Kumi Road Junction, P.O Box 2190, Mbale, Uganda 2 Sanyu Research Unit, Department of Women’s and Children’s Health, Liverpool Women’s Hospital, University of Liverpool This study was funded by Medical Research Council/WellcomeTrust/DfID (Global Health Trials Scheme). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 10 villages were assigned to either intervention or control by simple random sampling in a central office in Mbale in a 1:1 ratio by a person who did not draw the map. This represented a variety of distances of villages from each other, from market areas, from the health centres, and from the control villages, thus allowing contamination to be effectively assessed. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment
|
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was not possible to blind women, health workers, local council leaders, or researchers, due to the nature of the intervention. This was unlikely to influence microbiological outcomes and mortality.
|
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not possible to blind women, health workers, local council leaders, or researchers, due to the nature of the intervention. This was unlikely to influence microbiological outcomes and mortality. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants and clusters were accounted for and the attrition rate was similar across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in the protocol and method section were reported. |
| Other bias | Low risk | Not suspected |
Larson 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 16 full‐time neonatal intensive care unit (NICU) nurses. Number of neonates not mentioned Inclusion criteria: nurses were selected for inclusion in the study because they represented stable and consistent staff and because approximately 70% of direct patient contacts on that unit were provided by nurses.
"Nurses were eligible to participate if they worked full time on day or night shift in the NICU, had no dermatologic conditions such as psoriasis or latex hypersensitivity, were not receiving topical or systemic steroids or antibiotics, were willing to be randomly assigned to one of 2 treatment groups and follow all study regimens, and had no holiday scheduled during the 4‐week study period". Exclusion criteria Not reported |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | This study was supported in part by Steris Corporation, St Louis, MO. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 16 nurses (8 per group) were randomly assigned by coin toss to 1 of 2 hand care regimens. |
| Allocation concealment (selection bias) | High risk | It was not reported if allocation was concealed; probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and 1 of the outcomes (self‐rating hand skin assessment) was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | All important outcomes were reported. |
| Other bias | Low risk | None was suspected. |
Larson 2005.
| Study characteristics | ||
| Methods | Controlled cross‐over trial | |
| Participants | 119 nurses plus 2932 neonates. Only neonates hospitalised for longer than 24 hours on the study units were eligible for inclusion in the study. Inclusion criteria
Exclusion criteria "Part‐time and agency nurses and nurses from other units who occasionally worked in the NICU were not eligible". |
|
| Interventions | Antiseptic detergent handwash (2% chlorhexidine gluconate) vs alcohol hand sanitiser (61% alcohol and emollients) (3M Avagard D Instant Antiseptic Hand Sanitizer with Moisturizers; 3M HealthCare, St Paul, MN, USA) "During the alcohol phase, a non‐antimicrobial liquid soap (Kindest Kare Body Wash and Shampoo; Steris Corporation) was also provided for use when hands were physically soiled. Hand lotion (Soft Skin Conditioner; Steris Corporation) was provided throughout the study in both NICUs. Other hand hygiene policies and products were standardized across both study units". |
|
| Outcomes |
|
|
| Notes | Data collection periods were 1 March 2001 to 31 January 2002 (year 1) and 1 March 2002 to 31 January 2003 (year 2) with a month hiatus (February 2002), during which the product cross‐over occurred and the staff became accustomed to the change. During year 1, NICU 1 used CHG and NICU 2 used ALC, and products were reversed in year 2. All staff and visitors to each NICU used the designated product. "Prior to the study, a CHG product was used in both study units for traditional hand hygiene, and alcohol hand products were not in use".
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the clinical trial used a sequential cross‐over design in which each hand hygiene product was used by each study NICU in random order for 11 consecutive months". Randomisation process not described |
| Allocation concealment (selection bias) | High risk | Allocation to intervention arms was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and 1 of the outcomes (self‐rating hand skin assessment) was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | It was unclear, as we did not have access to the study protocol. |
| Other bias | High risk | Rates of gloving used and rates of participant contact differed significantly in the 2 groups; this was likely to affect outcomes. |
Ram 2020.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 258 pregnant women, who at the time of the data collector’s visit were married, between 32 and 34 weeks of gestation (the trial reported adverse events on 258 mothers and 246 neonates) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Treatment group
Participants were informed that chlorhexidine would be replenished as needed. Health promoters recommended using one pump, which, as designed, dispensed approximately 3 mL of the chlorhexidine product. "In the intervention group, soap was neither encouraged nor discouraged during the course of the study. Amongst mothers and secondary caregivers (typically other adult women, adolescent girls, fathers, or others who might provide direct care for the neonate), health promoters promoted chlorhexidine hand cleansing before umbilical cord care and after contact with respiratory secretions. Amongst all others who might come into contact with the neonate, including children in the household and adult or child visitors from outside the household, health promoters promoted chlorhexidine hand cleansing before touching the neonate and after contact with respiratory secretions. These roles [had] specific times for hand cleansing [which] will hereafter be referred to as “recommended times.” Health promoters also recommended hand cleansing with chlorhexidine amongst mothers at three fixed times of day (morning, noon, and night) to assure at least a minimum number of hand cleanses per day". Control group Standard practices (this arm will receive maternal and neonatal health counselling which includes discussion and education about antenatal care, safe and clean delivery, recognition of danger signs for the mother and neonate, immediate newborn care, and essential newborn care. Each mother will receive a clean delivery kit and pictorial cue cards for danger sign recognition). |
|
| Outcomes |
Adverse events. Data collectors assessed potential adverse events amongst all participating households, recording the nature of the event and whether hospitalisation followed the event. Skin complaints, such as rash, have been cited elsewhere as the most common adverse event resulting from chlorhexidine use. |
|
| Notes | Health promoters, who were distinct from data collectors, delivered all intervention components pertinent to each treatment arm in a total of three visits to each participating household. Health promoters provided maternal and neonatal health counselling to participants and interested family members in both arms. Both treatment arms received a clean delivery kit, which included sufficient chlorhexidine for one‐time application to the umbilical stump. No additional communication regarding hand hygiene was addressed to control participants until the completion of all data collection; in postnatal week 6, participants in both intervention and control arms were counselled on handwashing with soap to prevent diarrhoea and respiratory infections in the infant. The goal of the chlorhexidine hand cleansing intervention was to increase hand cleansing amongst mothers and others who were likely to serve as secondary caregivers, or who were likely to come into direct contact with the neonate; secondary caregivers were typically adult women, other than the mother, or older girls in the home who might provide support to the mother in caring for the newborn. This study received support from the Saving Lives at Birth partners: the U.S. Agency for International Development (USAID), the government of Norway, the Bill & Melinda Gates Foundation, Grand Challenges Canada, and the United Kingdom government. It was executed, and this article was prepared, by the named authors, and does not necessarily reflect the views of the Saving Lives at Birth partners. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomization, using blocks of four, to randomly assign participants to either the chlorhexidine intervention or to standard practices (control). The block randomization approach was used to promote balanced sample sizes across the treatment arms over the enrollment period". |
| Allocation concealment (selection bias) | High risk | Probably not done. Although, (quote:) "an investigator with no access to study participants constructed the assignment table. A field supervisor consulted the assignment table to assign the participant to intervention or control". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not done. Quote: "Health promoters, who were distinct from data collectors, delivered all intervention components". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Probably not done. Quote: "the data collector used a structured questionnaire to inquire about the ease of chlorhexidine use, scent, skin comfort, and the occurrence of skin reactions". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We used an intent‐to‐treat approach for the quantitative analyses. Quote: "Because the primary objective was to evaluate the effect of the intervention on the mother’s complete handwashing behavior during the neonatal period, in our intent‐to‐treat analysis, we excluded those mothers who had fetal loss, stillbirths, or whose newborns died within the first 24 hours postpartum". |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Not suspected |
Sharma 2013.
| Study characteristics | ||
| Methods | Randomised cross‐over study | |
| Participants | 35 female nursing staff and all babies in NICU (actual number not reported) Inclusion criteria
Exclusion criteria: nurses with a history of iodine sensitivity were excluded. |
|
| Interventions | Alcohol hand rub (45% 2‐propanol, 30% 1‐propanol, and 0.2% ethyl‐hexadecyl‐dimethyl‐ammonium‐ethylsulphate) vs povidone‐iodine hand scrub with 0.5% w/v available iodine vs plain (non‐antimicrobial) soap (head‐to‐head comparison) Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
"The scores range from 6 (normal, no observable scale or irritation) to 1 (extensive cracking of skin surface, widespread reddening or occasional bleeding)". "In previous studies, including validation with dermatologist ratings, an interrater agreement of > 95% within a score ± 1 was consistently obtained over a large spectrum of damaged and undamaged hands of various skin types".
"Subjects gave themselves a score from 1 to 7 in 4 dimensions: appearance, intactness, moisture content, and sensation. The possible range of scores is 4 to 28, with 28 indicating totally healthy skin. In previous studies, scores correlated significantly with other physiologic measures of skin damage".
This study did not declare funding source. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The random sequences were generated online". Randomisation was done at the end of the first neutral period. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque and sealed envelopes were opened as nurses were enrolled. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded but outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the microbiologist was blinded to the hand hygiene method used and the identity of the subjects". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no attrition. |
| Selective reporting (reporting bias) | High risk | Neonatal infection at baseline was reported; however, the result post‐intervention was not available for this important outcome. |
| Other bias | Unclear risk | No access to study protocol |
ABHR: alcohol based hand rub ALC: alcohol CFU: colony‐forming unit CFU‐C: colony‐forming unit count CHG: chlorhexidine gluconate F: female HAI: Healthcare associated infection HSAF: Hand Skin Assessment Form IMCI: Integrated Management of Childhood Illness IQR: Interquartile range M: male MRSA: methicillin‐resistant Staphylococcus aureus NICU: neonatal intensive care unit NNIS: National Nosocomial Infections Surveillance System RCT: randomised controlled trial SCBU: special baby care unit SD: standard deviation VHW: village health workers vs: versus VSS: Visual Scoring of Skin Condition w/v: weight per volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asare 2009 | Not an RCT |
| Azor‐Martinez 2018 | Wrong patient population |
| Azor‐Martinez 2020 | Wrong patient population |
| CTRI/2016/05/006963 | Wrong intervention |
| Darmstadt 2005 | Wrong intervention |
| Herruzo‐Cabrera 2001 | Not an RCT |
| Janota 2014 | Not an RCT |
| Kaufman 2014 | Not the intervention of interest; study authors assessed hand hygiene and non‐sterile hand gloves vs hand hygiene alone. |
| NCT03078335 | Wrong intervention |
| Ng 2004 | Not an RCT |
| Ram 2017 | Wrong intervention |
| Webster 1989 | Not an RCT |
| Webster 1991 | Not an RCT |
Characteristics of ongoing studies [ordered by study ID]
NCT03755635.
| Study name | Neonatal sepsis at Neonatal Intensive Care Units in Ghana |
| Methods | Non‐randomised intervention model: parallel assignment, open‐label |
| Participants | |
| Interventions | WHO multimodal hand hygiene strategy Age: up to 48 hours (child) |
| Outcomes | Prevention |
| Starting date | 1 October 2018 |
| Contact information | Korle Bu Teaching Hospital, Accra, Ghana |
| Notes | Completed 1 May 2020 |
WHO: World Health Organization
Differences between protocol and review
Search strategies were revised to increase the sensitivity of hand hygiene terms and now include relevant phrases such as handgel and handrub in addition to original terms.
Contributions of authors
BPK and EEU screened title/abstracts and full texts for inclusion.
DH resolved conflicts.
CO and OO extracted data.
OO entered data into RevMan.
SB and TAO cross‐checked data for completeness and accuracy.
All authors contributed to the concept and the design of the review and appraised and approved the final draft.
Sources of support
Internal sources
-
None, Other
None
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
BPK has no conflicts to declare. TAO has no conflicts to declare. OO has no conflicts to declare. CO has no conflicts to declare. EU has no conflicts to declare. SB has no conflicts to declare. DH has no conflicts to declare. MM has no conflicts to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amortegui 1978 {published data only}
- Amortegui AJ, Buffenmeyer C. Comparison of the antiseptic effect of two Iodophor preparations on hand washing in a well-baby nursery. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1978;7(6):35-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ditai 2019 {published data only}
- Ditai J, Abeso J, Odeke NM, Mobbs N, Dusabe-Richards J, Mudoola M, et al. BabyGel pilot: a pilot cluster randomised trial of the provision of alcohol handgel to postpartum mothers to prevent neonatal and young infant infection-related morbidity in the community. Pilot and Feasibility Studies 2019;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2000 {published data only}
- Larson E, Silberger M, Jakob K, Whittier S, Lai L, Latta PD, et al. Assessment of alternative hand hygiene regimens to improve skin health among neonatal intensive care unit nurses. Heart and Lung: Journal of Critical Care 2000;29(2):136-42. [PMID: ] [PubMed] [Google Scholar]
Larson 2005 {published data only}
- Cook HA, Cimiotti JP, Della-Latta P, Saiman L, Larson EL. Antimicriobial resistance patterns of colonizing flora on nurses' hands in the neonatal intensive care unit. American Journal of Infection Control 2007;35(4):231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EL, Cimiotti J, Haas J, Parides M, Nesin M, Della-Latta P, et al. Effect of antiseptic handwashing vs alcohol sanitizer on health care-associated Infections in neonatal intensive care units. Archives of Pediatrics and Adolescent Medicine 2005;159(4):377-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ram 2020 {published data only}
- Ram PK, Begum F, Crabtree-Ide C, Uddin MR, Weaver AM, Dostogir Harun MG, et al. Waterless hand cleansing with chlorhexidine during the neonatal period by mothers and other household members: findings from a randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2020;103(5):2116-26. [DOI: 10.4269/ajtmh.19-0773] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma VS, Dutta S, Taneja N, Narang A. Comparing hand hygiene measures in a neonatal ICU: a randomized crossover trial. Indian Pediatrics 2013;50(10):917-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asare 2009 {published data only}
- Asare A, Enweronu-Laryea CC, Newman MJ. Hand hygiene practices in a neonatal intensive care unit in Ghana. Journal of Infection in Developing Countries 2009;3(5):352-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Azor‐Martinez 2018 {published data only}
- Azor-Martinez E, Yui-Hifume R, Muñoz-Vico FJ, Jimenez-Noguera E, Strizzi JM, Martinez-Martinez I, et al. Effectiveness of a hand hygiene program at child care centers: a cluster randomized trial. Pediatrics 2018;142(5):e20181245. [PMID: ] [DOI] [PubMed] [Google Scholar]
Azor‐Martinez 2020 {published data only}
- Azor-Martinez E, Garcia-Fernandez L, Strizzi JM, Cantarero-Vallejo MD, Jimenez-Lorente CP, Balaguer-Martinez JV, et al. Effectiveness of a hand hygiene program to reduce acute gastroenteritis at child care centers: a cluster randomized trial. American Journal of Infection Control 2020;48(11):1315-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
CTRI/2016/05/006963 {published data only}
- CTRI/2016/05/006963. Improving the quality of healthcare at the time of childbirth in primary health centres in two districts of Haryana, India. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2016/05/006963 (first received 25 May 2016).
Darmstadt 2005 {published data only}
- Darmstadt GL, Nawshad Uddin Ahmed AS, Saha SK, Azad Chowdhury MA, Alam MA, Khatun M, et al. Infection control practices reduce nosocomial infections and mortality in preterm infants in Bangladesh . Journal of Perinatology 2005;25(5):331-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Herruzo‐Cabrera 2001 {published data only}
- Herruzo-Cabrera R, Garcia-Caballero J, Martin-Moreno JM, Graciani-Perez-Regadera MA, Perez-Rodriguez J. Clinical assay of N-duopropenide alcohol solution on hand application in newborn and pediatric intensive care units: control of an outbreak of multiresistant Klebsiella pneumoniae in a newborn intensive care unit with this measure. American Journal of Infection Control 2001;29(3):162-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Janota 2014 {published data only}
- Janota J, Šebková S, Višňovská M, Kudláčková J, Hamplová D, Zach J. Hand hygiene with alcohol hand rub and gloves reduces the incidence of late onset sepsis in preterm neonates. Acta Paediatrica 2014;103(10):1053-6. [DOI: 10.1111/apa.12731] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaufman 2014 {published data only}
- Kaufman DA, Blackman A, Conaway MR, Sinkin RA. Nonsterile glove use in addition to hand hygiene to prevent late-onset infection in preterm infants: randomized clinical trial. JAMA Pediatrics 2014;168(10):909-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT03078335 {unpublished data only}
- NCT03078335 . Glove-based care in the NICU to prevent late onset sepsis. clinicaltrials.gov/show/NCT03078335 (first received 13 March 2017).
Ng 2004 {published data only}
- Ng PC, Wong HL, Lyon DJ, So KW, Liu F, Lam RK, et al. Combined use of alcohol hand rub and gloves reduces the incidence of late onset infection in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(4):F336-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ram 2017 {published data only}
- Ram PK, Nasreen S, Kamm K, Allen J, Kumar S, Rahman MA, et al. Impact of an intensive perinatal handwashing promotion intervention on maternal handwashing behavior in the neonatal period: findings from a randomized controlled trial in rural Bangladesh. BioMed Research International. 2017;2017:60814702017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 1989 {published data only}
- Webster J, Faoagali JL. An in-use comparison of chlorhexidine gluconate 4% w/v, glycol-poly-siloxane plus methylcellulose and a liquid soap in a special care baby unit. Journal of Hospital Infection 1989;14:141-51. [DOI] [PubMed] [Google Scholar]
Webster 1991 {published data only}
- Webster J, Faoagali JL, Cartwright D. Elimination of methicillin-resistant Siaphylococcus aureus from a neonatal intensive care unit after hand washing with triclosan. Journal of Paediatric Child Health 1994;30:59-64. [DOI] [PubMed] [Google Scholar]
- Webster J. Hand-washing in a neonatal Intensive care unit: comparative effectiveness of chlorhexidine gluconate 4% w/v and triclosan 1% w/v. Australian College of Midwives Incorporated Journal 1991;4(2):25-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Webster J. Handwashing in a neonatal intensive care nursery: product acceptability and effectiveness of chlorhexidine gluconate 4% and triclosan 1%. Journal of Hospital Infection 1992;21:137-41. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03755635 {published data only}
- NCT03755635. Neonatal sepsis at neonatal intensive care units in Ghana. clinicalTrials.gov/show/NCT03755635 (first received 28 November 2018). [DOI: ]
Additional references
Adams‐Chapman 2006
- Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290-7. [DOI: 10.1097/01.qco.0000224825.57976.87] [PMID: ] [DOI] [PubMed] [Google Scholar]
Afonso 2017
- Afonso ED, Blot S. Effect of gestational age on the epidemiology of late-onset sepsis in neonatal intensive care units - a review. Expert Review of Anti-infective Therapy 2017;15(10):917-24. [DOI: 10.1080/14787210.2017.1379394] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aiello 2003
- Aiello AE, Cimiotti J, Della-Latta P, Larson EL. A comparison of the bacteria found on the hands of ‘homemakers' and neonatal intensive care unit nurses. Journal of Hospital Infection 2003;54(4):310-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2001
- Ali Y, Dolan MJ, Fendler EJ, Larson EL. Alcohols. In: Block SS, editors(s). Disinfection, Sterilization, and Preservation. 5th edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2001:229-54. [Google Scholar]
Bello 2020
- Bello S, Bamgboye EA, Ajayi DT, Ossai EN, Aniwada EC, Salawu MM, et al. Handwash versus hand-rub practices for preventing nosocomial infection in hospital intensive care units: a systematic review and meta-analysis. Canadian Journal of Infection Control 2020;35(2):82-90. [Google Scholar]
Bigliardi 2017
- Bigliardi PL, Alsagoff SA, El-Kafrawi HY, Pyon JK, Wa CT, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. International Journal of Surgery 2017;1(44):260-8. [DOI] [PubMed] [Google Scholar]
CADTH 2014
- Canadian Agency for Drugs and Technologies in Health. Alcohol with chlorhexidine hand sanitizers: clinical effectiveness and guidelines. cadth.ca/alcohol-chlorhexidine-hand-sanitizers-clinical-effectiveness-and-guidelines (accessed 19 March 2019).
Chan 2013
- Chan GJ, Lee AC, Baqui AH, Tan J, Black RE. Risk of early-onset neonatal infection with maternal infection or colonization: a global systematic review and meta-analysis. PLoS Medicine 2013;10(8):e1001502. [DOI: 10.1371/journal.pmed.1001502] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2014
- Chu SM, Hsu JF, Lee CW, Lien R, Huang HR, Chiang MC, et al. Neurological complications after neonatal bacteremia: the clinical characteristics, risk factors, and outcomes. PLOS One 2014;9(11):e105294. [DOI: 10.1371/journal.pone.0105294] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cortese 2016
- Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late infections in newborns: where do we stand? A review. Pediatrics and Neonatology 2016;57(4):265-73. [DOI: 10.1016/j.pedneo.2015.09.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Covidence. Covidence. Melbourne, Australia: Veritas Health Innovation, 2019. Available at covidence.org.
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 10: Analysing data and undertaking meta-analyses.In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Effa 2017
- Effa E, Durao S, Kredo T, Mbuagbaw L, Meremikwu M, Ongolo-Zogo P, et al. Process and lessons learned during priority setting in three countries in Africa. In: Abstracts of the Global Evidence Summit. Vol. 9 Suppl 1. Cape Town, South Africa: Cochrane Database of Systematic Reviews, 2017:107. [DOI: 10.1002/14651858.CD201702] [DOI]
Gebremedhin 2016
- Gebremedhin D, Berhe H, Gebrekirstos K. Risk factors for neonatal sepsis in public hospitals of Mekelle City, North Ethiopia, 2015: unmatched case control study. PLOS One 2016;11(5):e0154798. [DOI: 10.1371/journal.pone.0154798] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 2005
- Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Medicine 2005;6(1):2-8. [DOI: 10.1097/01.PCC.0000149131.72248.E6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gottardi 2001
- Gottardi W. Iodine and iodine compounds. In: Block SS, editors(s). Disinfection, sterilization, and preservation. 5th edition. Vol. 4. Gainesville, FL: Lippincott Williams & Wilkins, 2001:159-83. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 2 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Greenland 2013
- Greenland K, Iradati E, Ati A, Maskoen YY, Aunger R. The context and practice of handwashing among new mothers in Serang, Indonesia: a formative research study. BMC Public Health 2013;13:830. [DOI: 10.1186/1471-2458-13-830] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Larson 1998
- Larson EL, Hughes CA, Pyrek JD, Sparks SM, Cagatay EU, Bartkus JM. Changes in bacterial flora associated with skin damage on hands of health care personnel. American Journal of Infection Control 1998;26(5):513-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2015
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385(9966):430-40; Erratum in: Lancet; 2015; 31;385(9966):420. [DOI: 10.1016/S0140-6736(14)61698-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Loveday 2014
- Loveday HP, Wilson JA, Pratt RJ, Golsorki M, Tingle A, Bak A, et al. Epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. Journal of Hospital Infection 2014;86(Suppl 1):S1-70. [DOI: 10.1016/S0195-6701(13)60012-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mortimer 1962
- Mortimer EA Jr, Lipsitz PJ, Wolinsky E, Gonzaga AJ, Rammelkamp CH Jr. Transmission of staphylococci between newborns. Importance of the hands to personnel. American Journal of Diseases of Children 1962;104:289-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pessoa‐Silva 2004
- Pessoa-Silva CL, Dharan S, Hugonnet S, Touveneau S, Posfay-Barbe K, Pfister R, et al. Dynamics of bacterial hand contamination during routine neonatal care. Infection Control and Hospital Epidemiology 2004;25(3):192-7. [DOI: 10.1086/502376] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ranjeva 2018
- Ranjeva SL, Warf BC, Schiff SJ. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ Global Health 2018;3(1):e000347. [DOI: 10.1136/bmjgh-2017-000347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Cochrane Collaboration, 2020.
Rhee 2008
- Rhee V, Mullany LC, Khatry SK, Katz J, LeClerq SC, Darmstadt GL, et al. Maternal and birth attendant hand washing and neonatal mortality in southern Nepal. Archives of Pediatrics & Adolescent Medicine 2008;162(7):603-8. [DOI: 10.1001/archpedi.162.7.603] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotter 1999
- Rotter M. Hand washing and hand disinfection. In: Mayhall CG, editors(s). Hospital Epidemiology and Infection Control. 2nd edition. Philadelphia (PA): Lippincott Williams & Wilkins, 1999:1339-55. [Google Scholar]
Schuchat 2000
- Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O'Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics 2000;105(1 Pt 1):21-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Tanner 2016
- Tanner J, Dumville JC, Norman G, Fortnam M. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No: CD004288. [DOI: 10.1002/14651858.CD004288.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2017
- United Nations Economic and Social Council. Progress towards the sustainable development goals: report of the Secretary-General. 2017. unstats.un.org/sdgs/files/report/2017/secretary-general-sdg-report-2017--EN.pdf (accessed 28 June 2018).
Webster 1994
- Webster J, Faoagali JL, Cartwright D. Elimination of methicillin-resistant Staphylococcus aureus from a neonatal intensive care unit after hand washing with triclosan. Journal of Paediatrics and Child Health 1994;30(1):59-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. WHO guidelines on hand hygiene in health care: a summary. apps.who.int/iris/bitstream/10665/70126/1/WHO_IER_PSP_2009.07_eng.pdf (accessed 2 April 2018).
Won 2004
- Won SP, Chou HC, Hsieh WS, Chen CY, Huang SM, Tsou KI, et al. Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit. Infection Control and Hospital Epidemiology 2004;25(9):742-6. [DOI: 10.1086/502470] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wynn 2010
- Wynn JL, Levy O. Role of innate host defences in susceptibility to early-onset neonatal sepsis. Clinics in Perinatology 2010;37(2):307-37. [DOI: 10.1016/j.clp.2010.04.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zafar 1995
- Zafar AB, Butler RC, Reese DJ, Gaydos LA, Mennonna PA. Use of 0.3% triclosan (Bacti–Stat) to eradicate an outbreak of methicillin-resistant Staphylococcus aureus in a neonatal nursery. American Journal of Infection Control 1995;23(3):200-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kuti 2019
- Kuti BP, Ogunlesi TA, Oduwole O, Oringanje C, Udoh EE, Meremikwu MM. Hand hygiene for the prevention of infections in neonates. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013326. [DOI: 10.1002/14651858.CD013326] [DOI] [Google Scholar]
Kuti 2021a
- Kuti BP, Ogunlesi TA, Oduwole O, Oringanje C, Udoh EE, Meremikwu MM. Hand hygiene for the prevention of infections in neonates. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013326. [DOI: 10.1002/14651858.CD013326.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Kuti 2021b
- Kuti BP, Ogunlesi TA, Oduwole O, Oringanje C, Udoh EE, Meremikwu MM. Hand hygiene for the prevention of infections in neonates. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD013326. [DOI: 10.1002/14651858.CD013326.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


