Abstract
Purpose:
Functional outcomes have been proposed for assessing quality of pediatric trauma care. Outcomes assessments often rely on Abbreviated Injury Scale (AIS) severity scores to adjust for injury characteristics, but the relationship between AIS severity and functional impairment is unknown. This study’s primary aim was to quantify functional impairment associated with increasing AIS severity scores within body regions. The secondary aim was to assess differences in impairment between body regions based on AIS severity.
Methods:
Children with serious (AIS≥ 3) isolated body region injuries enrolled in a multicenter prospective study were analyzed. The primary outcome was functional status at discharge measured using the Functional Status Scale (FSS). Discharge FSS was compared (1) within each body region across increasing AIS severity scores, and (2) between body regions for injuries with matching AIS scores.
Results:
The study included 266 children, with 16% having abnormal FSS at discharge. Worse FSS was associated with increasing AIS severity only for spine injuries. Abnormal FSS was observed in a greater proportion of head injury patients with a severely impaired initial Glasgow Coma Scale (GCS) (GCS< 9) compared to those with a higher GCS score (43% versus 9%; p < 0.01). Patients with AIS 3 extremity and severe head injuries had a higher proportion of abnormal FSS at discharge than AIS 3 abdomen or non-severe head injuries.
Conclusions:
AIS severity does not account for variability in discharge functional impairment within or between body regions. Benchmarking based on functional status assessment requires clinical factors in addition to AIS severity for appropriate risk adjustment.
Level of evidence:
1 (Prognostic and Epidemiological).
Keywords: Pediatrics, Injuries and wounds, Trauma severity indices, Activities of daily living, Outcomes assessment, Quality of life
1. Introduction
Although pediatric trauma care has evolved over the past four decades, the metrics used to assess outcomes and quality of pediatric trauma care have not changed. Mortality and inpatient complications have remained the primary metrics used for outcomes and quality assessment [1–3]. Mortality has become uncommon after pediatric injury (< 2%) [4] and now has limited utility as a single metric for benchmarking trauma center performance [5,6]. Functional limitations after injury are estimated to occur ten times more often than mortality and may serve as a more discriminative indicator for outcomes assessment [7–9]. Evaluation of functional outcomes is especially relevant for injured children due to loss of potential future development. For example, injury during critical developmental periods of childhood can arrest or impair growth and maturation and lead to more substantial long-term disability [10].
Traumatic brain injuries (TBI), spinal cord injuries (SCI), and extremity fractures are associated with substantial long-term functional limitation [11–14]. In contrast to abdominal injuries, these injuries contribute independently to functional outcome after injury [15]. supporting an association between body region injured and functional impairment. We have shown differences in the functional impairment risk for children sustaining injuries to different body regions, with a more than 1000-fold difference in impairment between the lowest-risk and highest-risk body region injury types [16]. Current trauma mortality models incorporate measures of injury severity for each body region to adjust for the variability in predicted mortality across body regions [17,18]. Integrating functional metrics into trauma center assessment will require similar adjustment for baseline risk across body regions.
The Abbreviated Injury Scale (AIS) was first proposed in 1969 to quantify tissue damage and fatality risk after automobile crashes [19]. This classification of injury and its associated severity coding is commonly used to adjust for injury profiles in outcomes studies and risk-adjusted mortality models [20]. Body region-specific AIS severity scales are included as covariates in the mortality and complication models used by the American College of Surgeons (ACS) Trauma Quality Improvement Program (TQIP) – the largest program for benchmarking trauma performance worldwide [21]. The anatomically-based AIS system classifies individual injuries into nine body regions based on physical exam, initial imaging, or operative findings. Each injury is also assigned a severity score using a six-point ordinal scale that classifies injuries by increasing severity and threat to life [19]. The risk of mortality associated with each ordinal value of AIS severity, however, is highly variable between different body regions. It is not known whether similar variability occurs between AIS severity scores and functional impairment across body regions. Establishing this variability is needed before using AIS severity scores as covariates to quantify risk for functional impairment in trauma center benchmarking models.
The purpose of this study was to quantify the risk of functional impairment associated with AIS severity scores. Our primary aim was to assess the relationship between functional impairment and ordinal AIS severity scores within each individual body region. Our secondary aim was to assess for differences in functional impairment risk between body regions with matched AIS severity. We hypothesized that functional impairment would be more frequent for injuries with higher AIS severity scores within each individual body region. Our secondary hypothesis was that functional impairment would not differ between body region injuries with equal AIS severity scores.
2. Materials and methods
2.1. Participating centers and patient selection
This study was a planned secondary analysis of the “Assessment of Functional Outcomes and Health-Related Quality of Life after Pediatric Trauma” study, a prospective observational cohort of 427 seriously injured children (< 15 years old) treated at seven US pediatric trauma centers [16]. This subgroup analysis included patients with an isolated serious (defined by AIS post-dot severity score of 3 or greater) injury to a single body region, as defined by site-specific research coordinator review and trauma registry injury codes. Informed consent was obtained from the primary caregiver and assent from the child when appropriate. Patients were incentivized with gift cards at enrollment. All study protocols were approved locally at each site and centrally through the University of Utah Institutional Review Board.
2.2. Outcome measures
The study’s primary outcome was functional status at discharge measured by the Functional Status Scale (FSS). The FSS is a validated tool for rapidly assessing functional status in six domains (mental status, sensory, communication, motor, feeding, and respiratory). Each domain is scored from normal (1) to severe dysfunction (5) with total FSS scores ranging from 6 to 30.[22–24] Site-specific research coordinators administered the FSS at the time of discharge using chart review or caregiver interview. FSS total scores were dichotomized to ‘normal’ (6 or 7) or ‘abnormal’ (> 7) [25].
2.3. Baseline patient and injury characteristics
Trauma registry data from each site were obtained and linked to enrolled patients. Demographic data abstracted included patient age and sex. Age was categorized as < 1 year, 1 to 4 years, 5 to 9 years, and 10 to 14 years. Mechanism of injury was classified as blunt or penetrating based on International Classification of Diseases 10th edition (ICD-10) external cause codes. Initial emergency department systolic blood pressure and heart rate were dichotomized to normal or abnormal using age-normalized z-scores [26–28]. AIS post-dot severity scores for each injury were abstracted according to AIS-05 definitions. Injuries in the upper and lower extremity body regions were grouped as ‘extremity’ injuries. Patients with multiple injuries to a single body region were classified based on the injury with the highest AIS severity score. TBI patients were stratified based on initial Glasgow Coma Scale (GCS) scores into ‘not severe’ (GCS total ≥ 9) or ‘severe’ (GCS total < 9 or GCS motor < 5) subgroups. Patients with missing GCS scores were assigned to the ‘severe’ category based on the associations between missing GCS scores and moderate to severe TBI [25].
2.4. Statistical analyses
Data were summarized by body region and AIS level using frequencies and percentages. Categorical variables were compared between groups using Fisher’s exact test. Inverse probability weighting and multiple imputation for missing data were implemented using techniques described in the parent study [16]. Logistic regression was first used to predict abnormal functional status at discharge between body regions with AIS 3 injuries, controlling for age, GCS, abnormal initial systolic blood pressure, and abnormal initial heart rate. A second logistic model evaluated the stratification of TBI into ‘severe’ and ‘non-severe’ on functional status at discharge between body regions with AIS 3 injuries. This model stratified head injuries based on GCS while controlling for age, abnormal initial systolic blood pressure, and abnormal initial heart rate. We defined significance at p < 0.05. All analyses were analyzed using SAS V9.4 (Cary, NC).
3. Results
3.1. Study sample characteristics
The initial cohort of 427 patients included 266 patients with isolated single-body region injuries (Fig. 1). The distribution of injuries by AIS severity included 65% (N = 174) AIS 3 injuries, 25% (N = 67) AIS 4 injuries, and 9% (N = 25) AIS 5 injuries. Specific isolated body region injuries included 31% (N = 82) head, 7% (N = 18) thorax, 25% (N = 66) abdomen, 5% (N = 12) spine, and 33% (N = 88) extremity injuries. Based on admission GCS, 83% (N = 68) had non-severe TBI and 17% (N = 14) severe TBI. Head injuries were more frequent in younger patients, and chest, abdomen, and spine injuries in older patients. Patients were more commonly male (62%, N = 166) and less commonly infants (15%, N = 41). Pre-existing chronic conditions were present in 14% (N = 38) of patients. Most patients were injured by a blunt injury mechanism (91%, N = 243). Initial physiological derangement was uncommon, with 4% (N = 10) having an abnormal initial systolic blood pressure, 32% (N = 85) having an abnormal initial heart rate, and 7% (N = 19) having an abnormal initial GCS motor score. Physiological derangement on presentation was most common among TBI patients (Table 1).
Fig. 1.
STROBE diagram showing patient selection.
Table 1.
Baseline demographic, injury, and physiologic characteristics for individual body region injury types and AIS severity scores.
| Isolated injury region |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head (Severeb) | Head (Not Severeb) | Thorax | Abdomen | Spine | Extremity | ||||||||||
|
|
|
|
|
|
|||||||||||
| AIS Score (n) | 3 (n = 6) | 4 (n = 3) | 5 (n = 5) | 3 (n = 33) | 4 (n = 27) | 5 (n = 8) | 3 (n = 12) | 4 (n = 6) | 3 (N = 27) | 4 (n = 28) | 5 (n = 11) | 3 (n = 8) | 4 (n = 3) | 5 (n = 1) | 3 (n = 88) |
|
| |||||||||||||||
| Age Group | |||||||||||||||
| <1 | 2 (33.3) | 1 (33.3) | 1 (20.0) | 10 (30.3) | 13 (48.1) | 2 (25.0) | 1 (8.3) | 0 (0.0) | 1 (3.7) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (10.2) |
| 1–4 years | 2 (33.3) | 1 (33.3) | 2 (40.0) | 14 (42.4) | 5 (18.5) | 2 (25.0) | 3 (25.0) | 0 (0.0) | 4 (14.8) | 3 (10.7) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 30 (34.1) |
| 5–9 years | 2 (33.3) | 1 (33.3) | 2 (40.0) | 5 (15.2) | 5 (18.5) | 1 (12.5) | 4 (33.3) | 1 (16.7) | 10 (37.0) | 12 (42.9) | 2 (18.2) | 4 (50.0) | 0 (0.0) | 0 (0.0) | 23 (26.1) |
| 10–14 years | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (12.1) | 4 (14.8) | 3 (37.5) | 4 (33.3) | 5 (83.3) | 12 (44.4) | 12 (42.9) | 5 (45.5) | 4 (50.0) | 3 (100.0) | 1 (100.0) | 26 (29.5) |
| Sex N (%) | |||||||||||||||
| Male | 4 (66.7) | 1 (33.3) | 2 (40.0) | 15 (45.5) | 17 (63.0) | 5 (62.5) | 9 (75.0) | 4 (66.7) | 19 (70.4) | 16 (57.1) | 9 (81.8) | 7 (87.5) | 2 (66.7) | 1 (100.0) | 55 (62.5) |
| Female | 2 (33.3) | 2 (66.7) | 3 (60.0) | 18 (54.5) | 10 (37.0) | 3 (37.5) | 3 (25.0) | 2 (33.3) | 8 (29.6) | 12 (42.9) | 2 (18.2) | 1 (12.5) | 1 (33.3) | 0 (0.0) | 33 (37.5) |
| Injury Type | |||||||||||||||
| Blunt | 6 (100.0) | 2 (66.7) | 5 (100.0) | 30 (90.9) | 22 (81.5) | 8 (100.0) | 12 (100.0) | 3 (50.0) | 23 (85.2) | 28 (100.0) | 11 (100.0) | 7 (87.5) | 3 (100.0) | 1 (100.0) | 82 (93.2) |
| Penetrating | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (9.1) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 3 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) |
| Missing | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 4 (14.8) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 5 (5.7) |
| Initial Blood Pressure a | |||||||||||||||
| Normal | 6 (100.0) | 3 (100.0) | 5 (100.0) | 29 (87.9) | 19 (70.4) | 7 (87.5) | 12 (100.0) | 5 (83.3) | 25 (92.6) | 27 (96.4) | 10 (90.9) | 8 (100.0) | 3 (100.0) | 1 (100.0) | 78 (88.6) |
| Not normal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.0) | 4 (14.8) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 2 (7.4) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (9.1) | 4 (14.8) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (10.2) |
| Initial Heart Rate a | |||||||||||||||
| Normal | 2 (33.3) | 2 (66.7) | 3 (60.0) | 14 (42.4) | 11 (40.7) | 6 (75.0) | 8 (66.7) | 5 (83.3) | 24 (88.9) | 22 (78.6) | 8 (72.7) | 8 (100.0) | 3 (100.0) | 1 (100.0) | 58 (65.9) |
| Not normal | 4 (66.7) | 1 (33.3) | 2 (40.0) | 18 (54.5) | 16 (59.3) | 2 (25.0) | 4 (33.3) | 1 (16.7) | 3 (11.1) | 5 (17.9) | 3 (27.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 26 (29.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.5) |
| GCS Motor Score | |||||||||||||||
| Normal (6) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 32 (97.0) | 26 (96.3) | 6 (75.0) | 8 (66.7) | 5 (83.3) | 24 (88.9) | 26 (92.9) | 10 (90.9) | 8 (100.0) | 2 (66.7) | 1 (100.0) | 79 (89.8) |
| Not normal (<6) | 3 (50.0) | 2 (66.7) | 4 (80.0) | 0 (0.0) | 1 (3.7) | 2 (25.0) | 2 (16.7) | 1 (16.7) | 0 (0.0) | 1 (3.6) | 1 (9.1) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (1.1) |
| Missing | 3 (50.0) | 0 (0.0) | 1 (20.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 3 (11.1) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (9.1) |
All data expressed as N (%).GCS: Glasgow Coma Scale.
Adjusted for age using z-scores, normal = −1.96 to 1.96, not normal = < −1.96 OR > 1.96.
Severe head injury defined as initial GCS total <.
3.2. Association between functional impairment and AIS severity within body regions
Functional impairment at discharge was observed in 16% (N = 42) of patients. Abnormal functional status was not correlated with ordinal AIS severity scores within any body region except the spine (Fig. 2). The only patients with abnormal discharge FSS for isolated abdominal or isolated thoracic injuries were in the AIS 3 categories. In contrast, abnormal FSS scores after spine injury were observed in only the AIS 4 and 5 categories. An association between head AIS and functional impairment was not observed. Functional impairment was higher in the severe TBI group (6/14, 43%) compared to 9% (6/68) in the not severe TBI group (p < 0.01). No AIS 4 or 5 severity extremity severity patients were in the study cohort, precluding comparisons within this body region.
Fig. 2.
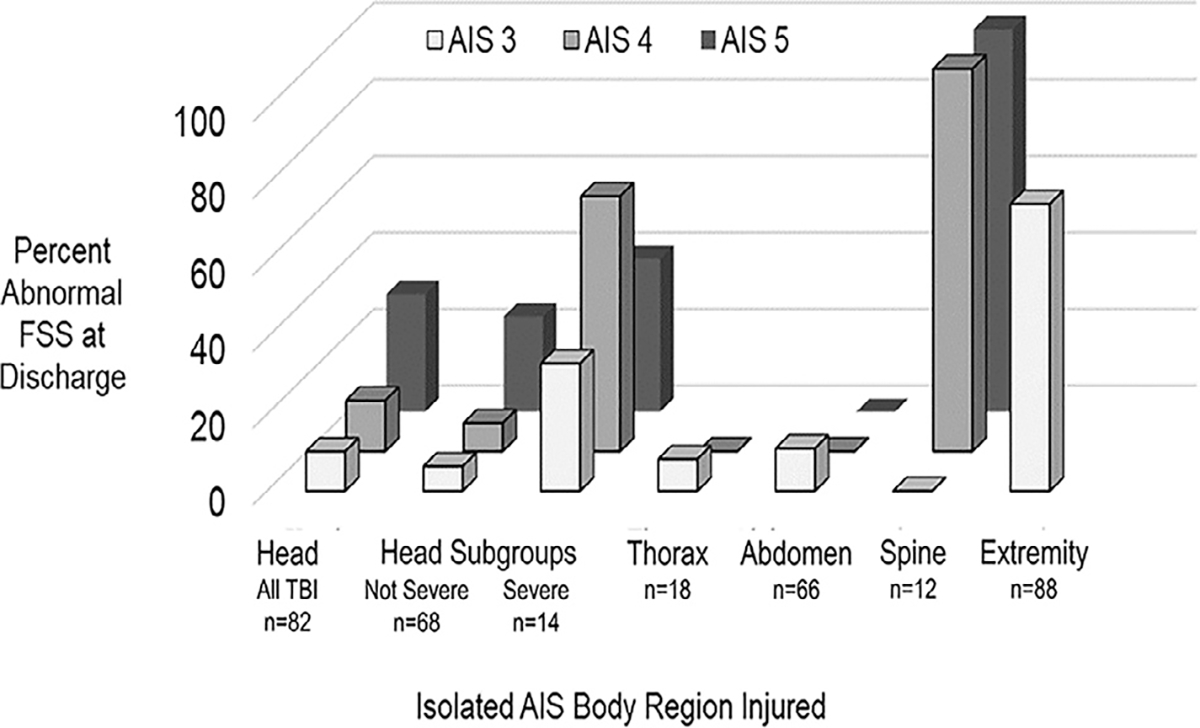
Percentage of abnormal functional status by body region and AIS post-dot severity scores for injured children at discharge. †Severe head injury defined as initial GCS total < 9 or GCS motor < 5.
3.3. Differences in functional impairment between body regions matched for AIS severity
Only 67 patients had an AIS 4 injury and only 25 had AIS 5 injury, with no AIS 4 or 5 injuries in the extremity region. Only 12 children had an isolated spine injury and only 18 had an isolated thorax injury. For these reasons, adjusted comparison of impairment between patients with injuries of equal AIS severity in different body regions was limited to AIS 3 injuries for head, abdomen, and extremity. Overall, there was a relationship between injury group and new domain morbidity in patients with AIS 3 (p = 0.057). Univariate comparisons of discharge FSS for patients with AIS 3 injuries showed a greater frequency of abnormal status at discharge for extremity injuries (25% abnormal FSS) compared to non-severe TBI (6.1% abnormal, p = 0.02). Differences in functional impairment were not observed among patients with AIS 3 abdomen (11% abnormal FSS), thorax (8% abnormal FSS), and spine (no abnormal FSS) injuries. The patients with AIS 3 severe TBI (N = 6) had the highest frequency of abnormal FSS (33%).
Multivariable analysis comparing AIS 3 injuries showed unstratified head injuries to have a lower risk for functional impairment at discharge than abdomen or extremity injuries (Table 2a). After stratification of AIS 3 head injuries into severe and not severe based on GCS category, a second multivariable analysis showed severe head and extremity injuries to have the highest level of impairment (Table 2b). Abdomen injuries were associated with a lower risk for functional impairment than either severe head injury or extremity injury, and non-severe TBI had a lower risk of impairment than any other region (Table 2b).
Table 2a.
Adjusted odds ratios for risk of abnormal functional status at discharge by body region for all patients with serious (AIS Severity 3) Injuries.
|
Reference Category
|
|||
|---|---|---|---|
| Abdomen | Extremity | ||
|
| |||
| Comparison Category | Head (All) Abdomen |
0.32 (0.13, 0.77) | 0.17 (0.11, 0.28) 0.54 (0.25, 1.17) |
Data presented as odds ratio (95% confidence interval) after adjusting for age, Glasgow Coma Scale, abnormal initial systolic blood pressure, and abnormal initial heart rate.
Table 2b.
Adjusted odds ratios for risk of abnormal functional status at discharge by body region for all patients with serious (AIS Severity 3) injuries, after stratification of head injuries by Glasgow coma scale.
|
Reference category
|
||||
|---|---|---|---|---|
| Head (Not Severe) | Abdomen | Extremity | ||
|
| ||||
| Comparison Category | Head (Severe a) | 8.03 (4.03, 16.00) | 2.78 (1.09, 7.08) | 1.29 (0.73, 2.27) |
| Head (Not Severe) | 0.35 (0.14, 0.83) | 0.16 (0.10, 0.26) | ||
| Abdomen | 0.46 (0.22, 0.99) | |||
Data presented as odds ratio (95% confidence interval) after adjusting for age, abnormal initial systolic blood pressure, and abnormal initial heart rate.
Severe head injury defined as initial GCS total < 9 or GCS motor <5.
3.4. Assessment of abdominal injury characteristics and associations with outcome metrics
We performed a post hoc analysis to assess the relationship between abdominal injury AIS severity and additional clinical features, including the number of organs injured, type of organ injured, and need for laparotomy. The distribution of AIS 3, 4, and 5 injuries was similar between patients with single abdominal organ injury and those with multiple injured intra-abdominal organs. Two of the three patients with abnormal FSS at discharge had multiple intra-abdominal injuries. The only patient with a single abdominal injury with functional impairment at discharge had a hollow viscus injury (Table 3). In the non-operatively managed group, 58% (N = 28) of children had a high-grade AIS 4 or 5 injury. Five of the seven (71%) patients who underwent laparotomy had a lower-grade AIS 3 injury. Patients undergoing laparotomy (N = 7) had a higher rate of functional impairment at discharge (29%) than those who did not require laparotomy (2%, p = 0.04).
Table 3.
Characteristics of abdominal injuries by abbreviated injury scale (AIS) severity score and associations with discharge functional status.
| Intra-abdominal organs injured | Single intra-abdominal organ injuries | Laparotomy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Single Organ Injury (n = 45) | Multi-organ Injured (n = 21) | p-value | Liver, Spleen, or Kidney (n = 42) |
Pancreas (n = 1) | Hollow Viscus ( n = 2) | p-value | No (n = 48) | Yes (n = 7) | p-value | |
|
| ||||||||||
| AIS Severity | 0.50 | 0.09 | 0.33 | |||||||
| Serious | 20 (44.4) | 7 (33.3) | 18 (42.9) | 0 (0.0) | 2 (100.0) | 20 (41.7) | 5 (71.4) | |||
| Severe | 19 (42.2) | 9 (42.9) | 19 (45.2) | 0 (0.0) | 0 (0.0) | 19 (39.6) | 1 (14.3) | |||
| Critical | 6 (13.3) | 5 (23.8) | 5 (11.9) | 1 (100.0) | 0 (0.0) | 9 (18.8) | 1 (14.3) | |||
| FSS Total at Discharge | 0.24 | 0.07 | 0.04 | |||||||
| Normal (6,7) | 44 (97.8) | 19 (90.5) | 42 (100.0) | 1 (100.0) | 1 (50.0) | 47 (97.9) | 5 (71.4) | |||
| Not Normal (> 7) | 1 (2.2) | 2 (9.5) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (2.1) | 2 (28.6) | |||
All data expressed as N (%). AIS: Abbreviated Injury Scale, FSS: Functional Status Scale. All comparisons using Fisher’s exact test.
4. Discussion
This analysis showed that the AIS injury severity score alone does not reliably quantify the risk of functional impairment at hospital discharge. We observed that body region-specific clinical qualifiers, such as the GCS score for TBI and the need for laparotomy for abdominal injuries, need to be considered as modifiers of risk for functional impairment. Differences in mortality associated with ordinal AIS severity scores have previously been shown to differ by body region [17,29–33]. This finding may be explained by deriving these scores using expert opinion rather than a data-driven approach. Our findings are consistent with previous studies reported in the adult literature [34] and support that AIS severity scores alone are insufficient for risk-adjustment. The approach to severity adjustment should account for injuries on a level more granular than body region. The calculation and derivation of specific risk ratios for impairment associated with individual injuries is an example of this type of approach.
We observed that the association between functional impairment and AIS severity within individual body regions was heterogeneous. Our finding that increasing frequency of impairment with greater AIS severity among patients with spine injuries is expected. AIS 3 spine injuries by definition have no neurological impairment, and more severe injuries (AIS 4 or 5) are defined by the presence of a SCI. In this way, the level of functional impairment is embedded into the AIS spine severity score lexicon [35]. In other body regions, AIS severity scores do not have an ordinal association with impairment at discharge, showing that injuries of higher AIS severity (AIS 4 or 5) do not predict abnormal FSS at discharge or worse functional outcomes.
We examined clinical factors within body regions that may account for differences in functional impairment not accounted for by AIS severity. Severely abnormal GCS was a predictor of functional impairment independent of AIS severity within the head region, consistent with previous evidence supporting GCS and other features as measures of head injury severity [25,36]. Although we were not able to evaluate AIS 4 or 5 extremity injuries, others have shown that the presence of vascular injury in the extremity is associated with mortality independent of AIS severity score [37]. Our post hoc analysis of abdominal injuries suggests that operative intervention is a stronger predictor of abnormal functional outcomes at discharge than AIS severity scores. Among 48 children with abdominal injuries who did not undergo laparotomy, all but one (98%) had a normal FSS at discharge, despite over half having severe (AIS 4) or critical (AIS 5) injuries. About 1/3 of the children that did require laparotomy had an abnormal FSS at discharge, despite 70% having less severe injuries. Most hollow viscus injuries are designated AIS 3 severity but often needed operative repair. Laparotomy is associated with postoperative pain and gastrointestinal symptoms that impact functional status at discharge. Patients with non-operatively managed high-grade solid organ injuries do not have postoperative pain and are less likely to have gastrointestinal symptoms at discharge despite having AIS 4 and 5 injuries. These clinical factors such as GCS, the need for laparotomy, and presence of associated vascular injury suggest that injury-specific factors may be more strongly associated with functional impairment than AIS severity alone.
We found differences in impairment risk between isolated AIS 3 injuries in the head, abdomen, and extremity body regions. Similar to within-region comparisons, the inclusion of stratification based on admission GCS for head injuries impacted our findings. Without this stratification, head injuries have a lower risk of impairment at discharge than extremity or abdominal injury. After GCS-based stratification, AIS 3 severe TBI has the highest risk for functional impairment, while non-severe TBI has the lowest. In unadjusted analyses, patients with TBI had only a 15% rate of observed functional impairment at discharge – similar to AIS 3 thorax and abdomen patients. Patients with AIS 3 ‘severe’ (based on GCS) TBI, however, had a 33% rate of functional impairment. This observation shows the limitations of AIS severity for accounting for impairment risk across body regions.
Our findings that AIS severity scores do not consistently correlate with functional outcomes align with studies that show a weak association between mortality and AIS severity [29,38–40]. Incorporation of clinical qualifiers in addition to AIS severity score have been proposed for addressing variability of AIS severity score when used as a predictor of mortality [41–46]. Recognizing that not all injuries of the same AIS severity score confer the same risk of mortality, the ACS pediatric TQIP program has incorporated survival risk ratios for each specific AIS injury code into risk-adjusted benchmarking models [20]. These risk ratios are calculated for each combination of pre-dot anatomic injury code and post-dot severity code to incorporate the specific anatomic organ injured and the severity of each injury into the models. Recent TQIP models now include survival risk ratios from the three worst injuries – even if from the same body region [47]. A similar difference in risk for disability has been associated with injury groupings in adults using ICD-10 diagnosis codes. It is unclear if ICD-10-based injury groupings are sufficiently granular or if the specific injury and severity coding associated with the AIS system are adequate for risk adjustment [48]. Functional impairment risk ratios based on more granular injury groupings rather than body-region severity scores alone will likely improve the quality of risk-adjusted discharge functional status.
Our study has several limitations. First, the inclusion of injuries isolated to a single body region limited our analysis to a small cohort of patients. We also did not quantify the utility of AIS severity scores in the context of the multiple injuries. Second, the sample size for injuries with higher AIS severity scores and the event rate for abnormal FSS at discharge limited the statistical power of several aspects of our analyses. Third, while FSS is a validated metric for morbidity [24,49,50] assessments across multiple centers may be biased. We attempted to mediate any effect related to interrater reliability with standardized training for research coordinators. Our results may not be valid outside of a controlled research study in the absence of standardized rater training. The validity and reliability of FSS outside of a controlled research study needs to be determined.
In conclusion, AIS severity scores are not sufficient for quantifying the risk for functional impairment within body regions. Injuries of similar AIS severity have different levels of impairment across body regions. Specific injury characteristics (such as admission GCS and injuries associated with the need for laparotomy) may be more reliable predictors of functional outcome than AIS severity alone. Deriving risks ratios for functional impairment associated with specific individual injuries may improve risk stratification. Future studies analyzing a larger cohort with a higher proportion of high-grade injuries are needed to define clinical qualifiers, risk ratios associated with individual injuries, and their effects on functional impairment at discharge.
Supplementary Material
Acknowledgments
We want to acknowledge the contribution of the research coordinators and trauma administrative teams at each site for assisting with the data acquisition.
Funding
The research reported in this article was supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN), National Institutes of Health, Department of Health and Human Services: U01HD049934, UG1HD050096, UG1HD083166, UG1HD049981, UG1HD083171, UG1HD083170, UG1HD063108, and U10HD049983.
The funders had no involvement in any component of the study design, data interpretation, manuscript or publication process.
Abbreviations
- ACS
American College of Surgeons
- AIS
Abbreviated Injury Scale
- FSS
Functional Status Scale
- GCS
Glasgow Coma Scale
- ICD-10
International Classification of Diseases 10th edition
- SCI
spinal cord injury
- TBI
traumatic brain injury
- TQIP
Trauma Quality Improvement Program
Footnotes
Declaration of Competing Interest
None of the authors have any personal or financial conflicts to disclose.
Availability of data and material
The study dataset will be publicly available through the Collaborative Pediatric Critical Care Research Network (CPCCRN) website after manuscript publication. Data requests can be filed at https://www.cpccrn.org/study-datasets/.
Code availability: The code uses proprietary macros and is not going to be shared publicly.
Ethics approval
This prospective observational study was performed in line with the principles of the Declaration of Helsinki. All study protocols were approved locally by each site-specific Institutional Review Board and centrally through the University of Utah Institutional Review Board.
CRediT authorship contribution statement
Lauren L. Evans: Visualization, Investigation, Data curation, Writing – original draft, Writing – review & editing. Aaron R. Jensen: Visualization, Investigation, Data curation, Writing – original draft, Writing – review & editing. Kathleen L. Meert: Visualization, Data curation, Writing – review & editing. John M. VanBuren: Visualization, Formal analysis, Writing – original draft, Writing – review & editing. Rachel Richards: Visualization, Formal analysis, Writing – review & editing. Jessica S. Alvey: Formal analysis, Writing – review & editing. Joseph A. Carcillo: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – review & editing. Patrick S. McQuillen: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – review & editing. Peter M Mourani: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – review & editing. Michael L. Nance: Conceptualization, Data curation, Project administration, Writing – review & editing. Richard Holubkov: Visualization, Formal analysis, Writing – review & editing. Murray M. Pollack: Visualization, Data curation, Writing – original draft, Writing – review & editing. Randall S. Burd: Visualization, Investigation, Data curation, Writing – original draft, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpedsurg.2021.09.052.
References
- [1].Stelfox HT, Bobranska-Artiuch B, Nathens A, et al. A systematic review of quality indicators for evaluating pediatric trauma care. Crit Care Med 2010;38:1187–96. doi: 10.1097/CCM.0b013e3181d455fe. [DOI] [PubMed] [Google Scholar]
- [2].Cooper CG, Santana MJ, Stelfox HT. A comparison of quality improvement practices at adult and pediatric trauma centers*. Pediatr Crit Care Med 2013;14:e365–71. doi: 10.1097/PCC.0b013e3182917a4c. [DOI] [PubMed] [Google Scholar]
- [3].McCarthy A, Curtis K, Holland AJ. Paediatric trauma systems and their impact on the health outcomes of severely injured children: an integrative review. Injury 2016;47:574–85. doi: 10.1016/j.injury.2015.12.028. [DOI] [PubMed] [Google Scholar]
- [4].Burd RS, Madigan D. The impact of injury coding schemes on predicting hospital mortality after pediatric injury. Acad Emerg Med 2009;16:639–45. doi: 10.1111/j.1553-2712.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- [5].Hashmi ZG, Schneider EB, Castillo R, et al. Benchmarking trauma centers on mortality alone does not reflect quality of care: implications for pay-for-performance. J Trauma Acute Care Surg 2014;76:1184–91. doi: 10.1097/ta.0000000000000215. [DOI] [PubMed] [Google Scholar]
- [6].Haider AH, Hashmi ZG, Zafar SN, et al. Developing best practices to study trauma outcomes in large databases: an evidence-based approach to determine the best mortality risk adjustment model. J Trauma Acute Care Surg 2014;76:1061–9. doi: 10.1097/ta.0000000000000182. [DOI] [PubMed] [Google Scholar]
- [7].Gabbe BJ, Simpson PM, Sutherland AM, et al. Functional and health-related quality of life outcomes after pediatric trauma. J Trauma 2011;70:1532–8. doi: 10.1097/TA.0b013e31820e8546. [DOI] [PubMed] [Google Scholar]
- [8].Bennett TD, Dixon RR, Kartchner C, et al. Functional status scale in children with traumatic brain injury: a prospective cohort study. Pediatr Crit Care Med 2016;17:1147–56. doi: 10.1097/pcc.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmed OZ, Holubkov R, Dean JM, et al. Change in functional status among children treated in the intensive care unit after injury. J Trauma Acute Care Surg 2019;86:810–16. doi: 10.1097/ta.0000000000002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Doud AN, Weaver AA, Talton JW, et al. Mortality risk in pediatric motor vehicle crash occupants: accounting for developmental stage and challenging abbreviated injury scale metrics. Traffic Inj Prev 2015;16(Suppl 2):S201–8. doi: 10.1080/15389588.2015.1048337. [DOI] [PubMed] [Google Scholar]
- [11].Dang B, Chen W, He W, et al. Rehabilitation treatment and progress of traumatic brain injury dysfunction. Neural Plast 2017;2017:1582182. doi: 10.1155/2017/1582182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khorasanizadeh M, Yousefifard M, Eskian M, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine 2019:1–17. doi: 10.3171/2018.10.Spine18802. [DOI] [PubMed] [Google Scholar]
- [13].Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 2014;95:986–95 e1. 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nota SP, Bot AG, Ring D, et al. Disability and depression after orthopaedic trauma. Injury 2015;46:207–12. doi: 10.1016/j.injury.2014.06.012. [DOI] [PubMed] [Google Scholar]
- [15].Weninger P, Aldrian S, Koenig F, et al. Functional recovery at a minimum of 2 years after multiple injury-development of an outcome score. J Trauma 2008;65:799–808 discussion. doi: 10.1097/TA.0b013e3181820dae. [DOI] [PubMed] [Google Scholar]
- [16].Burd RS, Jensen AR, VanBuren JM, et al. Factors associated with functional impairment after pediatric injury. JAMA Surg 2021:e212058. doi: 10.1001/jamasurg.2021.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Russell R, Halcomb E, Caldwell E, et al. Differences in mortality predictions between injury severity Score triplets: a significant flaw. J Trauma 2004;56:1321–4 00005373–200406000-00024 [pii]. [DOI] [PubMed] [Google Scholar]
- [18].Heaney JB, Schroll R, Turney J, et al. Implications of the trauma quality improvement project inclusion of nonsurvivable injuries in performance benchmarking. J Trauma Acute Care Surg 2017;83:617–21. doi: 10.1097/ta.0000000000001577. [DOI] [PubMed] [Google Scholar]
- [19].Rating the severity of tissue damage. I. The abbreviated scale. JAMA 1971;215:277–80 [doi]. doi: 10.1001/jama.1971.03180150059012. [DOI] [PubMed] [Google Scholar]
- [20].Newgard CD, Fildes JJ, Wu L, et al. Methodology and analytic rationale for the American college of surgeons trauma quality improvement program. J Am Coll Surg 2013;216:147–57. doi: 10.1016/j.jamcollsurg.2012.08.017. [DOI] [PubMed] [Google Scholar]
- [21].Newgard CD, Staudenmayer K, Hsia RY, et al. The cost of overtriage: more than one-third of low-risk injured patients were taken to major trauma centers. Health Aff (Millwood) 2013;32:1591–9. doi: 10.1377/hlthaff.2012.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pollack MM, Holubkov R, Funai T, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med 2014;15:821–7. doi: 10.1097/pcc.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pollack MM, Holubkov R, Glass P, et al. Functional status scale: new pediatric outcome measure. Pediatrics 2009;124:e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pollack MM, Holubkov R, Funai T, et al. Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: a new paradigm for outcomes assessment. Crit Care Med 2015;43:1699–709. doi: 10.1097/ccm.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kochanek PM, Tasker RC, Bell MJ, et al. Management of pediatric severe traumatic brain injury: 2019 consensus and guidelines-based algorithm for first and second tier therapies. Pediatr Crit Care Med 2019;20:269–79. doi: 10.1097/pcc.0000000000001737. [DOI] [PubMed] [Google Scholar]
- [26].Bonafide CP, Brady PW, Keren R, et al. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 2013;131:e1150–7. doi: 10.1542/peds.2012-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Swiet M, Fayers P, Shinebourne EA. Systolic blood pressure in a population of infants in the first year of life: the Brompton study. Pediatrics 1980;65:1028–35. [PubMed] [Google Scholar]
- [28].Rabbia F, Grosso T, Cat Genova G, et al. Assessing resting heart rate in adolescents: determinants and correlates. J Hum Hypertens 2002;16:327–32. doi: 10.1038/sj.jhh.1001398. [DOI] [PubMed] [Google Scholar]
- [29].Rau CS, Wu SC, Kuo PJ, et al. Same abbreviated injury scale values may be associated with different risks to mortality in trauma patients: a cross-sectional retrospective study based on the trauma registry system in a Level I trauma center. Int J Environ Res Public Health 2017;14 [pii]. doi: 10.3390/ijerph14121552.E1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Weaver AA, Barnard RT, Kilgo PD, et al. Mortality-based quantification of injury severity for frequently occurring motor vehicle crash injuries. Ann Adv Automot Med 2013;57:235–46. [PMC free article] [PubMed] [Google Scholar]
- [31].Aharonson-Daniel L, Giveon A, Stein M, et al. Different AIS triplets: different mortality predictions in identical ISS and NISS. J Trauma 2006;61:711–17 [doi]. doi: 10.1097/01.ta.0000235294.32326.e6. [DOI] [PubMed] [Google Scholar]
- [32].Hudak AM, Caesar RR, Frol AB, et al. Functional outcome scales in traumatic brain injury: a comparison of the Glasgow outcome scale (Extended) and the functional status examination. J Neurotrauma 2005;22:1319–26 10.1089/neu.2005.22.1319. [DOI] [PubMed] [Google Scholar]
- [33].Rizoli S, Petersen A, Bulger E, et al. Early prediction of outcome after severe traumatic brain injury: a simple and practical model. BMC Emerg Med 2016;16:32. doi: 10.1186/s12873-016-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koo M, Otero I, Sabate A, et al. Do the severity and the body region of injury correlate with long-term outcome in the severe traumatic patient? Braz J Anesthesiol 2014;64:134–9. doi: 10.1016/j.bjane.2013.03.008. [DOI] [PubMed] [Google Scholar]
- [35].Association for the Advancement of Automotive Medicine. THe Abbreviated IN-jury Scale 2005. Barrington, IL. 2005. [Google Scholar]
- [36].Keenan HT, Clark AE, Holubkov R, et al. Latent class analysis to classify injury severity in pediatric TBI. J Neurotrauma 2020. doi: 10.1089/neu.2019.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Loh SA, Rockman CB, Chung C, et al. Existing trauma and critical care scoring systems underestimate mortality among vascular trauma patients. J Vasc Surg 2011;53:359–66 [doi]. doi: 10.1016/j.jvs.2010.08.074. [DOI] [PubMed] [Google Scholar]
- [38].Rowell SE, Barbosa RR, Diggs BS, et al. Specific abbreviated injury scale values are responsible for the underestimation of mortality in penetrating trauma patients by the injury severity score. J Trauma 2011;71:384 [doi]. doi: 10.1097/TA.0b013e3182287c8d. [DOI] [PubMed] [Google Scholar]
- [39].Wang M, Wu D, Qiu W, et al. An injury mortality prediction based on the anatomic injury scale. Medicine (Baltimore) 2017;96:e7945. doi: 10.1097/md.0000000000007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brown JB, Gestring ML, Leeper CM, et al. Characterizing injury severity in nonaccidental trauma: does injury severity score miss the mark? J Trauma Acute Care Surg 2018;85:668–73. doi: 10.1097/ta.0000000000001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang MD, Fan WH, Qiu WS, et al. The exponential function transforms the abbreviated injury scale, which both improves accuracy and simplifies scoring. Eur J Trauma Emerg Surg 2014;40:287–94. doi: 10.1007/s00068-013-0331-1. [DOI] [PubMed] [Google Scholar]
- [42].Gabbe BJ, Simpson PM, Lyons RA, et al. Association between the number of injuries sustained and 12-month disability outcomes: evidence from the injury-VIBES study. PLoS One 2014;9:e113467. doi: 10.1371/journal.pone.0113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Flynn-O’Brien KT, Fallat ME, Rice TB, et al. Pediatric trauma assessment and management database: leveraging existing data systems to predict mortality and functional status after pediatric injury. J Am Coll Surg 2017;224:933–44 e5. doi: 10.1016/j.jamcollsurg.2017.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brown JB, Gestring ML, Leeper CM, et al. The value of the injury severity score in pediatric trauma: time for a new definition of severe injury? J Trauma Acute Care Surg 2017;82:995–1001. doi: 10.1097/ta.0000000000001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shi J, Shen J, Caupp S, et al. A new weighted injury severity scoring system: better predictive power for pediatric trauma mortality. J Trauma Acute Care Surg 2018;85:334–40. doi: 10.1097/ta.0000000000001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dawes AJ, Sacks GD, Needleman J, et al. Injury-specific variables improve risk adjustment and hospital quality assessment in severe traumatic brain injury. J Trauma Acute Care Surg 2019;87:386–92. doi: 10.1097/ta.0000000000002297. [DOI] [PubMed] [Google Scholar]
- [47].Pediatric TQIP Benchmark Reference Report. Chicago, IL: American College of Surgeons Trauma Quality Improvement Program; 2019. [Google Scholar]
- [48].Gabbe BJ, Lyons RA, Simpson PM, et al. Disability weights based on patient-reported data from a multinational injury cohort. Bull World Health Organ 2016;94:806 16c. doi: 10.2471/blt.16.172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Varni JW, Limbers CA. The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatr Clin North Am 2009;56:843–63. doi: 10.1016/j.pcl.2009.05.016. [DOI] [PubMed] [Google Scholar]
- [50].Desai AD, Zhou C, Stanford S, et al. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr 2014;168:1114–21. doi: 10.1001/jamapediatrics.2014.1600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


