ABSTRACT
Periodontal disease is caused by specific pathogens which results in inflammation of the tooth-supporting structures and subsequently causes the continued breakdown of alveolar bone and periodontal ligament. Licorice (Glycyrrhiza glabra) is a perennial herb with substantial medicinal value. Licorice extract is derived from dried, unpeeled stolons and roots of Glycyrrhiza uralensis and G. glabra. The bioactive ingredients in licorice extract such as glycyrrhizin, licoricidin, glabridin, licochalcone A, and licorisoflavan A have anti-inflammatory, antimicrobial, and anti-adherence effects that are beneficial against periodontal disease. Since periodontal disease has a complex etiology that includes the host response and microorganisms, licorice phytochemicals offer a therapeutic advantage due to their dual functionality. The aim of this review was to enumerate the bioactive compounds present in herbal licorice extract and to elucidate the beneficial effects of licorice and its derivatives in periodontal therapy. Literature review and clinical trials evaluating the effect of licorice on periodontopathogens and periodontal disease are included in this article.
Keywords: Gingivitis, Glycyrrhiza glabra, Herbal therapy, Periodontitis
Introduction
Periodontal disease is caused by specific pathogens which results in inflammation of the tooth-supporting structures and subsequently causes the continued breakdown of alveolar bone and periodontal ligament. The pathogenesis of periodontal diseases involves two major causative factors. The first is the microbial factor, that is, the presence of increased levels of periodontopathogenic bacteria in subgingival tissues, which causes periodontal destruction by producing proteinases and toxins (1,2). The pathogens associated with periodontitis are Porphyromonas gingivalis, Treponema denticola, Aggregatibacter actinomycetemcomitans, and Tannerella forsythia. The other factor is the immune response of the host to the periodontal pathogens, which is the over-production of mediators of inflammation such as cytokines, prostaglandins, and matrix metalloproteinases (MMPs), which regulate the continuance of periodontal disease (3).
Periodontal therapy primarily includes scaling and root planing to eliminate the local factors, that is, plaque and calculus, and to maintain satisfactory oral hygiene. Since periodontal disease is known to be an inflammatory condition with a microbial etiology, the adjunctive use of locally applied or systemic administration of antimicrobials and/or host response‐modulating medications has been suggested.
Conventional synthetic agents such as chlorhexidine products which are used therapeutically and prophylactically in dentistry have some disadvantages such as altered taste sensation, tooth staining, and resistance to bacteria, which limit their usage over a long term (4). Therefore, innovative strategies need to be developed against periodontal diseases, such as exploring the extensively available medicinal plants. The active ingredients in medicinal plants restore health, with maximum efficiency and minimal side effects. Herbal extracts incorporated into medications have been found to be safe and efficacious for the treatment of several oral health conditions such as gingival bleeding, dental caries, halitosis, and mouth ulcers. The extracts obtained from aloe vera, green tea plant, neem, tulsi, propolis, rosemary, meswak, turmeric, chamomile, tea tree oil, peppermint oil, cranberry, clove, ginger, etc. have been used commonly for the prevention and treatment of different oral diseases. Herbal extracts contain phytochemicals that are responsible for the desired anti-inflammatory and antimicrobial effects (5). Herbal formulations are gaining widespread attention as they do not contain artificial preservatives, alcohol, colors, or flavors, which are commonly found in other drugstore products.
One such herb with medicinal properties is licorice (Glycyrrhiza glabra). Licorice, synonym being sweet wood, is found in the Mediterranean region and in a few regions of Asia. Licorice is a perennial herb that holds a sweet taste and has widespread pharmacological effects on human beings. Licorice extract is derived from the dried, unpeeled stolons and roots of Glycyrrhiza uralensis and G. glabra.
However, limited information is available on the use of this herb in periodontal therapy. The mechanism of action by which licorice works against periodontal diseases has not been elucidated in previous studies. The aim of this review was to enumerate the bioactive compounds present in herbal licorice extract and to elucidate the beneficial effects of licorice and its derivatives in periodontal therapy.
Methodology
The literature search was performed using three databases which included PubMed, Google Scholar, and Cochrane, in addition to searching reference lists of original and review articles. The combination of the following keywords was used to search for relevant articles: “licorice,” “Glycyrrhiza glabra,” “periodontal therapy,” and “periodontal disease.” Relevant studies published between 1985 and 2022 were selected. Only articles in English language were considered, and unpublished data were not sought. Two reviewers obtained information on the quality and characteristics of the included studies.
Components in licorice extract
Licorice is a potential source of natural anti-inflammatory agents. Its major active component is glycyrrhetinic acid (GA) that is derived from licorice root extract. Major phytochemicals found in licorice are shown in Table I (Fig. 1) (6).
TABLE I -.
Important phytochemicals in licorice root
| Group | Bioactive compounds |
|---|---|
| Aurones | Licoagroaurone |
| Benzofurans | Licocoumarone |
| Chalcones | Isoliquiritigenin, licochalcone A |
| Coumarins | Glycerol, glabrocoumarone, glycocoumarin, licofuranocoumarin, glabrocoumarin |
| Flavonoids | Glabrol, liquiritigenin |
| Isoflavonoids | Glabridin, glabrone, licoricidin, licoisoflavones A and B, licorisoflavan A |
| Pterocarpenes | Glycyrrhizol A |
| Saponins | Glycyrrhizin, glycyrrhizic acid, 18β-glycyrrhetinic acid, liquiritic acid, glabrolide |
| Stilbenes | Gancaonin G |
Fig. 1 -.
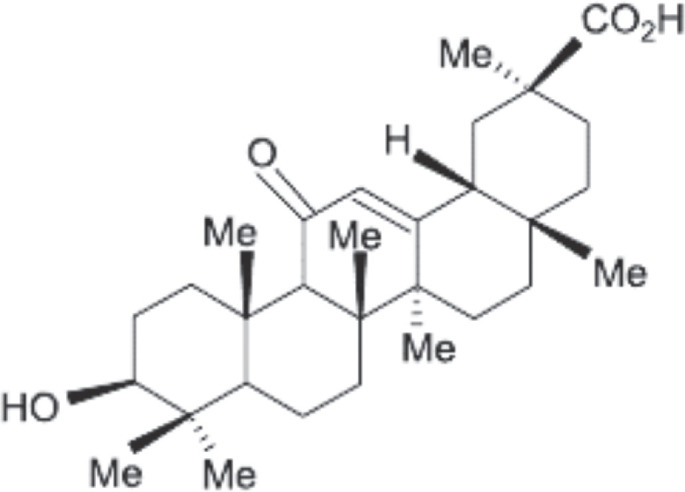
Chemical structure of glycyrrhetinic acid.
Safe usage of licorice
The Food and Drug Administration (FDA) has labeled licorice as “Generally Recognized as Safe.” It has been suggested to be safe when used in minimal quantities by people who are not allergic to glycyrrhizin (7,8). Intake of excessive quantity, that is, over 200 mg, of licorice may cause hypertension, hypokalemia, rhabdomyolysis, respiratory impairment, muscle paralysis, hyperparathyroidism, acute renal failure, and encephalopathy (9). According to the World Health Organization (WHO), 100 mg/day of licorice can be used safely without adverse effects. A potential risk of excessive bleeding may be seen in patients using medications for anti-clotting for cerebrovascular or cardiovascular diseases in conjunction with licorice-containing herbal medications due to its antiplatelet and anticoagulant effects (10).
Effects of licorice
Licorice constituents have shown antimicrobial (11), antiviral (12), anti-inflammatory (13), antidiabetic, antitumor, immunoregulatory (14), sedative (15), antidepressive (16), estrogenic (17,18), antioxidant (19), hepatoprotective (20), neuroprotective activities (21), and skin effects (22). The active constituents of licorice extract have a potential role on the oral microorganisms as well as the host response involved in orodental diseases like periodontitis, dental caries, recurrent aphthous ulcers, and candidiasis.
Antiviral effects – Licorice extracts inhibit the growth of viruses such as herpes simplex, influenza virus, and vesicular stomatitis virus. Glycyrrhizin prevents the replication of viruses and interferes with viral binding (12).
Antidiabetic activity – Glycycoumarin, glycerin, etc., present in G. glabra extracts lower blood glucose level by binding to peroxisome proliferator-activated receptor (PPAR) gamma. Glabridin helps in efficient glucose utilization and prevents glucose intolerance by translocation of GLUT-4 (14).
Antitumor activity – 18-β-GA and glycyrrhizic acids induce mitochondrial permeability transition causing tumor cell apoptosis (14).
Immunoregulatory effect – Glycyrrhiza extracts stimulate the immune system by production of macrophages and lymphocytes, and increasing the phagocytic capacity of neutrophils. It prevented the accumulation of immune complexes involved in autoimmune diseases such as systemic lupus erythematosus (14).
Sedative effect – Glabridin shows sedative and hypotonic effects by positively modulating the gamma-aminobutyric acid (GABA) receptors (15).
Antidepressive effect – Licorice shows antidepressive effects by inhibition of monoamine oxidase and increasing epinephrine and dopamine levels in the brain (16).
Estrogenic effect – Licorice extracts show estrogenic activity through uterine retention and vaginal opening. Isoflavones present in licorice can influence sexual development, impair estrus cycling, and alter the proper functioning of the ovarian, hypothalamus, and pituitary glands. Glabridin can be used as a treatment for menopausal symptoms (17,18).
Hepatoprotective activities – Glycyrrhizin has shown improved liver histology and reduced serum aminotransferases. It shows hepatoprotective effect against CCl4-induced oxidative stress, prevents oxidative and hepatic damage caused due to aflatoxin, and improves liver function (20).
Neuroprotective activities – Licorice has an antioxidant activity that can reduce brain damage by eliminating or utilizing the free radicals and improving neural function and memory (21).
Skin effects – Licorice is popular in treating dermatitis, pruritus, cysts, and eczema. It is also used for cosmetic formulation as a depigmenting agent to inhibit the tyrosinase enzyme (22).
Mechanism of action
The beneficial effects of licorice can be due to various mechanisms.
Antimicrobial activity
Microbial growth is selectively inhibited by the isoprenoid phenols present in G. glabra. The presence of secondary metabolites, such as alkaloids, saponins, flavonoids, and alkaloids, is responsible for the antibacterial activity (23,24). The reduction in bacterial gene expression, decrease in growth of bacteria, and inhibition of production of bacterial toxins are suggested as the mechanism behind this (24,25).
Licorice extract showed antimicrobial effects against P. gingivalis with minimum inhibitory concentration (MBC) and minimum bactericidal concentration (MIC) of 25 and 62.5 μg/mL, respectively (26). Glycyrrhizol A showed a strong antibacterial effect against Streptococcus mutans with MIC of 1 μg/mL. GA at an appropriate concentration has good efficacy against isolated periodontopathogenic and capnophilic bacteria (27). The MICs of GA were 8, 16, and 8 mg/L for A. actinomycetemcomitans, Eikenella corrodens, and Capnocytophaga, respectively, and the MBC was 16 mg/L for all species (28).
Antioxidant activity
Licorice phytochemicals exhibit significant antioxidant activity. Licorice hinders the synthesis of reactive oxygen species (ROS) by neutrophils at the site of inflammation. G. glabra contains licochalcones B and D, which show powerful scavenging activity on DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) radical and also have the ability to prevent the peroxidation of microsomal lipids (29,30).
Anti‐inflammatory activity
GA activates signaling of glucocorticoid receptors since its chemical structure resembles the glucocorticoids, and it also inhibits the classical complement pathway, both of which are responsible for its anti-inflammatory properties.
Preclinical studies have also shown that licorice inhibits synthesis of prostaglandins and cyclooxygenase activity, and also indirectly inhibits aggregation of platelets as well as the components of the inflammatory cascade (31). Licorice extract prevents the phosphorylation of proteins involved in intracellular signaling of macrophages, such as the transcription factors, nuclear factor-kappa B, and activator protein (AP) 1, which play an important role in the pathways of inflammatory signaling (32).
Licorice in periodontal disease
The bioactive ingredients in licorice like glycyrrhizin, glabridin, licorisoflavan A, licochalcone A, and licoricidin are effective against periodontal disease. Table II summarizes the studies which suggest the potential use of licorice in periodontal therapy.
TABLE II -.
List of studies suggesting the potential use of licorice in periodontal therapy
| Authors | Study design | Findings |
|---|---|---|
| Sharma et al 2022 (33) | Randomized controlled trial | Both licorice and chlorhexidine mouthwash inhibited the accumulation of plaque and inflammation of the gingiva. The herbal mouthwash was shown to be effective as a self-care treatment since chemical formulations are associated with adverse effects with long-term usage. |
| Madan et al 2019 (34) | Randomized clinical trial | Bleeding of the gingiva, probing pocket depth, and attachment loss were significantly decreased in patients using Glycyrrhiza glabra gum paint in 10% concentration. It can be used for longer periods to prevent and treat periodontal disease as they do not have any side effects, and thus, it is also effective as an alternative for synthetic agents. |
| Takamori et al 2018 (35) | Animal study | Loss of attachment, immune complex formation, and inflammatory cell infiltration were greater in the lipopolysaccharide (LPS) group than in the control, and were completely reduced in the glycyrrhetinic acid (GA) groups. Increased alveolar bone destruction was seen in the LPS group than in the GA or control groups. Hence, in the experimentally induced periodontitis model in rats, GA had the ability to reduce periodontal destruction. |
| Suwannakul and Chaibenjawong 2017 (26) | In vitro study | Licorice extract showed antimicrobial effects against Porphyromonas gingivalis with MBC and MIC of 25 and 62.5 μg/mL respectively. It also reduced the quantity of biofilm and the activities of Arg- and Kgp-proteases. |
| Salehi et al 2017 (36) | Double-blind clinical trial | Mucoadhesive tablets containing licorice extract can relieve pain, decrease the diameter of the ulcer and the inflammation around it, and improve the recovery in aphthous stomatitis. |
| Jain et al 2017 (37) | Randomized clinical trial | Licorice mouthwash reduced the accumulation of plaque and inflammation of gingiva, without any tooth discoloration or unpleasant taste sensation. |
| Shivprasad et al 2017 (38) | Randomized controlled trial | Subgingivally delivered licorice as an adjunctive treatment modality to scaling and root planing showed clinical and microbiological benefits in periodontal therapy, with a reduction in the prevalence of P. gingivalis. |
| Ali and Mohammed 2016 (39) | Comparative human study | Licorice extract based mouthwash inhibits plaque formation and inflammation of gingiva without any adverse effects. Therefore, it can be used as an adjunctive to scaling and root planing in periodontal treatment. |
| Hamdon et al 2014 (40) | Human study | Licorice extract showed antibacterial effects against Aggregatibacter actinomycetemcomitans, based on antimicrobial sensitivity tests. The antibacterial effect was greater against planktonic cells as compared to the cells within the biofilm. It produced an inhibition zone similar to tetracyclines with a concentration of 250 μg. |
| Kim et al 2013 (41) | Invitro study | 18α-GA is effective in the treatment of vascular diseases caused by P. gingivalis. It reduces vascular permeability induced by LPS by inhibiting IL-8 production from the endothelium. |
| Farhad et al 2013 (42) | Experimental human study | A significant reduction of MMP-8 concentration was seen in both licorice and doxycycline groups than in the placebo group. The licorice group showed better reduction of MMP-8 concentration than doxycycline group, which was not statistically significant. Hence, licorice extract can be as potent as antibiotics such as doxycycline to treat periodontal diseases by preventing the MMP production by host cells. |
| Kim et al 2012 (43) | In vitro study | Glabridin inhibits activation of signaling molecules induced by RANKL and other transcription factors of osteoclast precursors, and so it can be used to inhibit osteoclastogenesis. |
| Zhu et al 2012 (44) | Animal study | Isoliquiritigenin (ISL) inhibits osteoclastogenesis induced by RANKL and bone loss by various signaling pathways. Hence, it has the potential to be used as a therapeutic or preventive agent for the treatment of lytic bone diseases. |
| Feldman et al 2012 (45) | In vitro study | Licochalcone A inhibits the two primary causative factors of periodontitis, i.e., formation of biofilm with P. gingivalis and the immune response of the host. |
| La et al 2011 (46) | In vitro study | Licoricidin (LC) and licorisoflavan A (LIA) inhibited the production of IL-6, MMP-7, -8, and -9 in macrophages. They can be used to treat MMP and cytokine-mediated conditions such as periodontal disease, and are potent host-modulating agents. |
| Bodet et al 2008 (32) | In vitro study | Licorice extract showed anti-inflammatory effects by reducing the IL-1b, -6, -8 and TNF-α responses of macrophages induced by LPS. It is a potential therapeutic agent to prevent or treat the tissue destruction caused due to periodontal disease. |
| Wittschier et al 2006 (47) | In vitro study | The polysaccharides in G. glabra inhibit bacterial adhesion and thus can be potential therapeutic agents against bacterial infection. |
| He et al 2006 (27) | In vitro study | Glycyrrhizol B and gancaonin G showed moderate antibacterial effect against Streptococcus mutans, while Glycyrrhizol A showed strong antibacterial effect with MIC of 1 μg/mL. Hence, the roots of Glycyrrhiza uralensis contain isoflavones which exhibit antibacterial effects. |
| Choi 2005 (48) | Animal study | Glabridin, an estrogenic plant product, stimulates the in vitro formation of bone in cultured osteoblasts. Thus, glabridin can be a potent agent in the management of osteoporosis. |
| Salari et al 2003 (49) | Human study | Enoxolone with the mentioned concentrations is effective against isolated periodontopathogenic and capnophilic bacteria. Its MICs were 8 µg/mL for A. actinomycetemcomitans and Capnocytophaga species, and 16 µg/mL for Eikenella corrodens. The MBC was also 16 µg/mL for all the microorganisms. |
| Salari and Kadkhoda 2003 (28) | In vitro study | GA at an appropriate concentration has good efficacy against isolated periodontopathogenic and capnophilic bacteria. The MICs of GA were 8, 16, and 8 mg/L for A. actinomycetemcomitans, Eikenella corrodens and Capnocytophaga, respectively, and the MBC was 16 mg/L for all species. |
| Saeedi et al 2003 (50) | Randomized, controlled trial | Edema, erythema, and itching were more effectively reduced with 2% licorice topical gel than with 1% gel in 2 weeks. |
IL = interleukin; MBC = minimum inhibitory concentration; MIC = minimum bactericidal concentration; MMP = matrix metalloproteinase; TNF = tumor necrosis factor.
These ingredients exhibit antimicrobial, anti-inflammatory, and anti-adherence effects (Fig. 2). Since the etiology of periodontal diseases is complex involving the periodontal pathogens and host immune response, dual functionality compounds such as the phytochemicals in licorice offer therapeutic superiority. Phytochemicals are structurally distinct from the conventional microbial-derived antibiotics, and so are advantageous as antimicrobials. The phytochemicals act against different bacterial strains by inhibiting the efflux pumps, inhibiting the cell wall biosynthesis by interacting with the cell membrane, and by inhibition of enzymes such as dihydrofolate reductase, urease, and sortase A (51). Thus, their mechanisms of action are different from classic substances and against which microbial resistance does not develop.
Fig. 2 -.
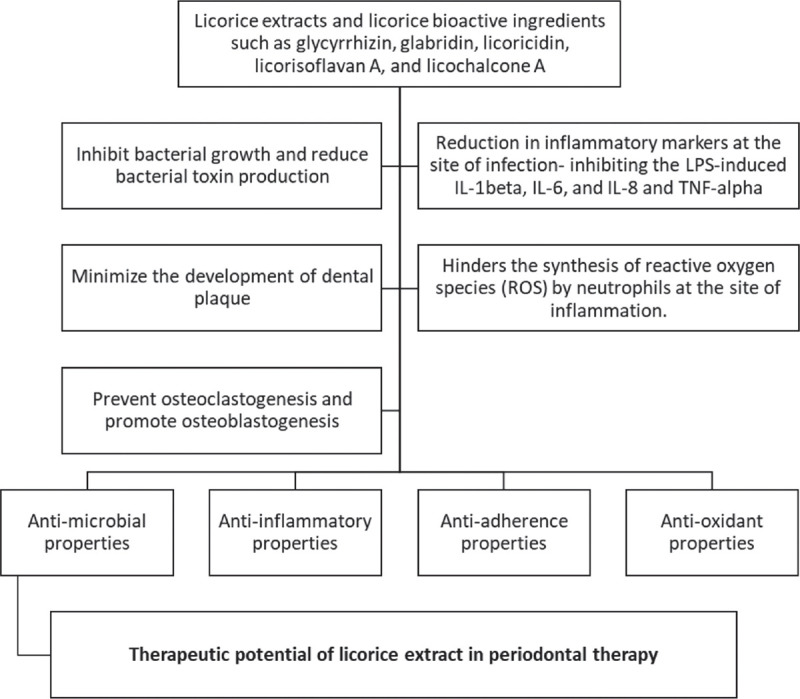
Mechanism of action of licorice in periodontal therapy.
The ability of licorice to inhibit the formation of dental plaque enhances its significance in the treatment of periodontal disease. At high concentrations of licorice extract (5%-10%), there was a slight reduction in bacterial growth and formation of plaque was completely inhibited. No effect was seen on either adherence or growth with lower concentrations. At high concentrations (0.5%-1%) of its pure active component, that is, glycyrrhizin, there was a complete inhibition in the adherence, whereas partial inhibition was observed at lower concentrations. The surface activity of glycyrrhizin could be responsible for its inhibitory effect, as bacterial adherence and growth are known to be affected by surfactants. Bacterial adherence can also be inhibited by the adsorption of glycyrrhizin onto smooth surfaces. Licorice extract shows minimal antibacterial activity along with its effect on inhibition of plaque. Glycyrrhizin as a vehicle can be effective for topical agents used orally due to its sweetness, good dispersing properties, and the ability to remain stable in the form of aqueous gels. This suggests that the balance of the oral microbial flora will not be affected on using glycyrrhizin as an oral medication (52).
Phytochemicals of G. glabra reduce bacterial growth and inhibit the mediators of inflammation at the infection site. It also inhibits the activity of osteoclasts responsible for destruction of alveolar bone in periodontal disease and promotes the formation of bone by stimulating osteoblastogenesis. High amounts of inflammatory markers like interleukin (IL)-1β, IL-2, IL-6, IL-8, tumor necrosis factor (TNF)-α, and RANKL are present in patients with periodontal disease. Licorice extract showed potent anti-inflammatory properties by inhibiting these proinflammatory mediators stimulated by lipopolysaccharide (LPS) from A. actinomycetemcomitans and P. gingivalis (32).
Resorption of alveolar bone is an important feature of periodontitis. The differentiation, activation, and survival of osteoclasts are regulated by RANKL leading to bone resorption. Glabridin can be used to inhibit osteoclastogenesis by preventing the activation of signaling molecules induced by RANKL and subsequent transcription factors for osteoclast precursors, suggesting its therapeutic potential (34).
Recommendations for future research
Though the use of herbals for medicinal purpose is traced back to several centuries, it is only in recent evidence-based era that methodical and systematic approaches to study their properties have been reinstituted. This has sparked a wide interest for their application in all healthcare specialties including periodontal therapy. The beneficial phytochemicals in licorice must be studied so as to incorporate these herbal extracts in oral care products that may be useful in dental therapy.
In vitro studies have shown the capability of licorice and its bioactive components in periodontal therapy; however, most of the clinical studies have limitations pertaining to the design of the study and the number of participants included in the study, which makes them statistically insignificant. Thus, further clinical studies need to be carried out to investigate the oral care products containing licorice extracts, in the forms of toothpaste, mouthwash chewing gum, and gel to be able to validate its beneficial effects. The local application of these bioactive compounds would be more suitable. For example, the local application of a licorice-based gel into sites with periodontal disease permits the bioactive ingredients to be released slowly, which will act locally on the periodontal pathogens and the host immune response, the two contributory factors in the destruction of periodontal tissues.
Further research focusing on the in vivo anti-inflammatory/antimicrobial effects of licorice is required on larger sample sizes to better understand its specific role in the management of periodontitis. Clinical trials evaluating the effect of licorice extract on periodontopathogens and inflammatory cytokines would be recommended.
Conclusion
The usage of herbal agents for the treatment of periodontal disease is considered as an intriguing alternative to conventional antibiotics due to their lesser negative effects and to overcome drug resistance during treatment. Licorice extracts exhibit a wide range of biological effects such as anti-inflammatory, antioxidant, and antimicrobial activities. It has the ability to prevent the release of proinflammatory mediators and MMPs from host cells, and hence it is a potent agent for periodontal therapy. The bioactive ingredients present in this herb help in reducing loss of alveolar bone, which is commonly associated with periodontal disease. It should also be emphasized that no adverse effects have been seen with the use of licorice extracts. Hence, local application of licorice-containing agents into the diseased periodontal sites can be beneficial, which would act locally on periodontopathogens and the host inflammatory response.
Disclosures
Conflict of interest: The authors declare no conflict of interest. Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.O’Brien‐Simpson NM, Veith PD, Dashper SG, Reynolds EC. Antigens of bacteria associated with periodontitis. Perio 2000. 2004;35(1):101–134. doi: 10.1111/j.0906-6713.2004.003559.x. PubMed [DOI] [PubMed] [Google Scholar]
- 2.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Perio 2000. 2006;40(1):50–76. doi: 10.1111/j.1600-0757.2005.00148.x. PubMed [DOI] [PubMed] [Google Scholar]
- 3.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402. https://www.ncbi.nlm.nih.gov/pubmed/20739705 [DOI] [PubMed] [Google Scholar]
- 4.Poppolo Deus F. Ouanounou A. Chlorhexidine in dentistry: pharmacology, uses, and adverse effects. Int Dent J. 2022;72(3):269–277. doi: 10.1016/j.identj.2022.01.005. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Şener B, Kiliç M. Herbal extracts used in dental disorders. Biomed J Sci Tech Res. 2019;19(1):14107–14111. doi: 10.26717/BJSTR.2019.19.003254. [DOI] [Google Scholar]
- 6.Sidhu P, Shankargouda S, Rath A, Hesarghatta Ramamurthy P, Fernandes B, Kumar Singh A. Therapeutic benefits of liquorice in dentistry. J Ayurveda Integr Med. 2020;11(1):82–88. doi: 10.1016/j.jaim.2017.12.004. https://www.ncbi.nlm.nih.gov/pubmed/30391123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. PubMed [DOI] [PubMed] [Google Scholar]
- 8.Messier C, Epifano F, Genovese S, Grenier D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012;18(1):32–39. doi: 10.1111/j.1601-0825.2011.01842.x. PubMed [DOI] [PubMed] [Google Scholar]
- 9.Yasue H, Itoh T, Mizuno Y, Harada E. Severe hypokalemia, rhabdomyolysis, muscle paralysis, and respiratory impairment in a hypertensive patient taking herbal medicines containing licorice. Intern Med. 2007;46(9):575–578. doi: 10.2169/internalmedicine.46.6316. PubMed [DOI] [PubMed] [Google Scholar]
- 10.Tsai HH, Lin HW, Lu YH, Chen YL, Mahady GB. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One. 2013;8(5):e64255. doi: 10.1371/journal.pone.0064255. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long DR, Mead J, Hendricks JM, Hardy ME, Voyich JM. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob Agents Chemother. 2013;57(1):241–247. doi: 10.1128/AAC.01023-12. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Yeh C, Wang KC, Chiang LC, Shieh DE, Yen MH, San Chang J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 2013;148(2):466–473. doi: 10.1016/j.jep.2013.04.040. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekaran CV, Deepak HB, Thiyagarajan P et al. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine. 2011;18(4):278–284. doi: 10.1016/j.phymed.2010.08.001. PubMed [DOI] [PubMed] [Google Scholar]
- 14.Li S, Zhu JH, Cao LP et al. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci. 2014;35(7):1115–1120. doi: 10.1007/s10072-014-1661-4. PubMed [DOI] [PubMed] [Google Scholar]
- 15.Jin Z, Kim S, Cho S, Kim IH, Han D, Jin YH. Potentiating effect of glabridin on GABAA receptor-mediated responses in dorsal raphe neurons. Planta Med. 2013;79(15):1408–1412. doi: 10.1055/s-0033-1350698. PubMed [DOI] [PubMed] [Google Scholar]
- 16.Dhingra D, Sharma A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(3):449–454. doi: 10.1016/j.pnpbp.2005.11.019. PubMed [DOI] [PubMed] [Google Scholar]
- 17.Sharma G, Kar S, Palit S, Das PK. 18β-glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell Physiol. 2012;227(5):1923–1931. doi: 10.1002/jcp.22920. PubMed [DOI] [PubMed] [Google Scholar]
- 18.Su Wei, Poh M, Voon Chen Yong P, Viseswaran N, Chia YY. Estrogenicity of glabridin in Ishikawa cells. PLoS One. 2015;10(3):e0121382. doi: 10.1371/journal.pone.0121382. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh V, Pal A, Darokar MP. A polyphenolic flavonoid glabridin: oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic Biol Med. 2015;87:48–57. doi: 10.1016/j.freeradbiomed.2015.06.016. PubMed [DOI] [PubMed] [Google Scholar]
- 20.Sharifzadeh M, Shamsa F, Shiran S et al. A time course analysis of systemic administration of aqueous licorice extract on spatial memory retention in rats. Planta Med. 2008;74(5):485–490. doi: 10.1055/s-2008-1074494. PubMed [DOI] [PubMed] [Google Scholar]
- 21.Michel HE, Tadros MG, Abdel-Naim AB, Khalifa AE. Prepulse inhibition (PPI) disrupting effects of Glycyrrhiza glabra extract in mice: a possible role of monoamines. Neurosci Lett. 2013;544:110–114. doi: 10.1016/j.neulet.2013.03.055. PubMed [DOI] [PubMed] [Google Scholar]
- 22.Halder RM, Richards GM. Topical agents used in the management of hyperpigmentation. Skin Therapy Lett. 2004;9(6):1–3. PubMed [PubMed] [Google Scholar]
- 23.Fukui H, Goto K, Tabata M. Two antimicrobial flavanones from the leaves of Glycyrrhiza glabra. Chem Pharm Bull (Tokyo). 1988;36(10):4174–4176. doi: 10.1248/cpb.36.4174. PubMed [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Yang R, Yuan B, Liu Y, Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. 2015;5(4):310–315. doi: 10.1016/j.apsb.2015.05.005. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta VK, Fatima A, Faridi U et al. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 2008;116(2):377–380. doi: 10.1016/j.jep.2007.11.037. PubMed [DOI] [PubMed] [Google Scholar]
- 26.Suwannakul S, Chaibenjawong P. Antibacterial activities of Glycyrrhiza glabra Linn. (Licorice) root extract against Porphyromonas gingivalis and its inhibitory effects. J Dent Indones. 2017;24(3):85–92. doi: 10.14693/jdi.v24i3.1075. [DOI] [Google Scholar]
- 27.He J, Chen L, Heber D, Shi W, Lu QY. Antibacterial compounds from Glycyrrhiza uralensis. J Nat Prod. 2006;69(1):121–124. doi: 10.1021/np058069d. PubMed [DOI] [PubMed] [Google Scholar]
- 28.Salari MH, Kadkhoda Z. In vitro antibacterial effects of glycyrrhetinic acid on periodontopathogenic and capnophilic bacteria isolated from adult periodontitis. Clin Microbiol Infect. 2003;9(9):987–988. doi: 10.1046/j.1469-0691.2003.00721.x. PubMed [DOI] [PubMed] [Google Scholar]
- 29.Biondi DM, Rocco C, Ruberto G. New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. J Nat Prod. 2003;66(4):477–480. doi: 10.1021/np020365s. PubMed [DOI] [PubMed] [Google Scholar]
- 30.Sharma V, Katiyar A, Agrawal RC. Glycyrrhiza glabra: chemistry and pharmacological activity. Sweeteners; 2018;87 doi: 10.1007/978-3-319-27027-2_21. [DOI] [Google Scholar]
- 31.Hasan MK, Ara I, Mondal MSA, Kabir Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon. 2021;7(6):e07240. doi: 10.1016/j.heliyon.2021.e07240. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodet C, La VD, Gafner S, Bergeron C, Grenier D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J Periodontol. 2008;79(9):1752–1761. doi: 10.1902/jop.2008.080052. PubMed [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Sogi GM, Saini V, Chakraborty T, Sudan J. Effect of liquorice (root extract) mouth rinse on dental plaque and gingivitis - a randomized controlled clinical trial. J Indian Soc Periodontol. 2022;26(1):51–57. doi: 10.4103/jisp.jisp_517_20. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madan S, Kashyap S, Mathur G. Glycyrrhiza glabra: an efficient medicinal plant for control of periodontitis – a randomized clinical trial. J Int Clin Dent Res Organ. 2019;11(1):32–35. doi: 10.4103/jicdro.jicdro_7_19. [DOI] [Google Scholar]
- 35.Takamori A, Yoshinaga Y, Ukai T et al. Topical application of glycyrrhetinic acid in the gingival sulcus inhibits attachment loss in lipopolysaccharide-induced experimental periodontitis in rats. J Periodontal Res. 2018;53(3):422–429. doi: 10.1111/jre.12529. PubMed [DOI] [PubMed] [Google Scholar]
- 36.Salehi M, Saeedi M, Ehsani H et al. Analyzing Glycyrrhiza glabra (Licorice) extract efficacy in recurrent aphthous stomatitis recovery. J Res Med Dent Sci. 2018;6(1):68–75. Online [Google Scholar]
- 37.Jain P, Sontakke P, Walia S, Yadav P, Biswas G, Kaur D. Assessment of the efficacy of licorice versus 0.2% chlorhexidine oral rinse on plaque-induced gingivitis: a randomized clinical trial. Indian J Oral Health Res. 2017;3(1):15–18. doi: 10.4103/ijohr.ijohr_18_17. [DOI] [Google Scholar]
- 38.Shivprasad BM, Sonali C, Navnita S, Shilpa S, Sruthi KN, Savita S. Liquorice as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a clinico-microbiological study. Int J Sci Res. 2017;6(7):73–76. doi: 10.36106/ijsr. [DOI] [Google Scholar]
- 39.Ali A, Mohammed R. The Iraqi method of natural liquorice as a mouth rinse and its effect in patient with chronic periodontitis. Iraqi Dent J. 2016;38(1):43–47. doi: 10.26477/idj.v38i1.72. [DOI] [Google Scholar]
- 40.Hamdon SM, Ghada Y, Rahman A. Glycyrrhiza glabara as antibacterial agent on biofilm and planktonic cell of Aggregatibacter actinomycetemcomitans. Int J Dent Sci Res. 2014;2:42–46. doi: 10.12691/ijdsr-2-2-4. [DOI] [Google Scholar]
- 41.Kim SR, Jeon HJ, Park HJ et al. Glycyrrhetinic acid inhibits Porphyromonas gingivalis lipopolysaccharide-induced vascular permeability via the suppression of interleukin-8. Inflamm Res. 2013;62(2):145–154. doi: 10.1007/s00011-012-0560-5. PubMed [DOI] [PubMed] [Google Scholar]
- 42.Farhad SZ, Aminzadeh A, Mafi M, Barekatain M, Naghney M, Ghafari MR. The effect of adjunctive low-dose doxycycline and licorice therapy on gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. Dent Res J (Isfahan). 2013;10(5):624–629. PubMed [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HS, Suh KS, Sul D, Kim BJ, Lee SK, Jung WW. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29(2):169–177. doi: 10.3892/ijmm.2011.822. PubMed [DOI] [PubMed] [Google Scholar]
- 44.Zhu L, Wei H, Wu Y et al. Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Int J Biochem Cell Biol. 2012;44(7):1139–1152. doi: 10.1016/j.biocel.2012.04.003. PubMed [DOI] [PubMed] [Google Scholar]
- 45.Feldman M, Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol. 2012;113(2):438–447. doi: 10.1111/j.1365-2672.2012.05329.x. PubMed [DOI] [PubMed] [Google Scholar]
- 46.La VD, Tanabe S, Bergeron C, Gafner S, Grenier D. Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: potential therapeutic approach for periodontitis. J Periodontol. 2011;82(1):122–128. doi: 10.1902/jop.2010.100342. PubMed [DOI] [PubMed] [Google Scholar]
- 47.Wittschier N, Faller G, Beikler T, Stratmann U, Hensel A. Polysaccharides from Glycyrrhiza glabra L. exert significant anti-adhesive effects against Helicobacter pylori and Porphyromonas gingivalis. Planta Med. 2006;72(11):238. doi: 10.1055/s-2006-950038. [DOI] [Google Scholar]
- 48.Choi EM. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem Pharmacol. 2005;70(3):363–368. doi: 10.1016/j.bcp.2005.04.019. PubMed [DOI] [PubMed] [Google Scholar]
- 49.Salari MH, Sohrabi N, Kadkhoda Z, Khalili MB. Antibacterial effects of enoxolone on periodontopathogenic and capnophilic bacteria isolated from specimens of periodontitis patients. Iran Biomed J. 2003;7(1):39–42. [Google Scholar]
- 50.Saeedi M, Morteza-Semnani K, Ghoreishi MR. The treatment of atopic dermatitis with licorice gel. J Dermatolog Treat. 2003;14(3):153–157. doi: 10.1080/09546630310014369. PubMed [DOI] [PubMed] [Google Scholar]
- 51.Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8(118):118. doi: 10.1186/s13756-019-0559-6. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segal R, Pisanty S, Wormser R, Azaz E, Sela MN. Anticariogenic activity of licorice and glycyrrhizine I: inhibition of in vitro plaque formation by Streptococcus mutans. J Pharm Sci. 1985;74(1):79–81. doi: 10.1002/jps.2600740121. PubMed [DOI] [PubMed] [Google Scholar]


