Abstract
Background:
The legal status of kratom in the United States is complex and varies by state. The U.S. Food and Drug Administration (FDA) and the U.S. Drug Enforcement Administration have repeatedly subjected kratom to regulatory review. However, there hasn’t been a systematic review of the public’s perception of kratom. The present study analyzed open-ended responses from the public to an FDA solicitation for information regarding kratom with the goal of providing a comprehensive assessment of motives for kratom use.
Methods:
To guide decisions regarding kratom regulation, the FDA solicited comments regarding kratom abuse potential, medical usefulness, and impact of scheduling changes from July through August 2021 and posted them to the Federal Register website. We analyzed comments posted during the first 6 weeks of comment solicitation (6,353) using an inductive approach via qualitative content analysis.
Results:
Respondents reported 106 independent health-related reasons for kratom use, with most categorized as mental health, pain management, substance use disorder, or miscellaneous purposes that included increasing focus, treating insomnia, and decreasing fatigue. Neurological diseases and digestive disorders were also reported. Relatively few (< 2%) responses reported recreational use, abuse potential, or adverse effects of kratom.
Conclusions:
Although kratom is not approved as a safe and effective therapy for any indication, individuals use kratom for a broad spectrum of health-related purposes. Limitations of this study include potential bias for respondents with perceived positive experiences using kratom, lack of demographics data, and lack of independent verification of claims made by respondents. Regardless, this study reflects perceptions regarding the therapeutic uses of kratom and provides insight into potential individual-level consequences of regulating kratom in the U.S. It is important to study the public’s perception of kratom use, which can aid regulatory purposes and provide clinically important information on individuals’ use and valuation of kratom.
Keywords: Kratom, Mitragyna speciosa, Mitragynine, Emerging drugs of abuse, Food and drug administration, Federal regulation
Introduction
Kratom (Mitragyna speciosa) is a tree-like plant in the coffee family that is native to Southeast Asia. For at least 150 years, people from Malaysia, Thailand, Indonesia, and other Southeast Asian regions have chewed kratom leaves or have consumed teas made from kratom leaves to combat fatigue, increase work productivity, manage pain, alleviate opiate withdrawal, and treat other common health maladies such as cough, fever, inflammation, diarrhea, and intestinal parasites (Adkins et al., 2011; Boyer et al., 2008; Cinosi et al., 2015; Jansen & Prast, 1988, 1988a,b). Despite a long-standing traditional use of kratom for a broad range of ailments, the pharmacology of kratom is complex and poorly understood. At relatively low doses, kratom has cocalike stimulant effects that relieve fatigue and enhance physical performance. These effects are thought to be mediated by the adrenergic system (Foss et al., 2020; Shellard, 1989). At higher doses, kratom has opioid-like effects, including analgesia and euphoria, which are predominantly mediated by mitragynine and mitragynine-like indole alkaloids that bind and activate opioid receptors (Boyer et al., 2008; Hassan et al., 2013; Takayama et al., 2002; Varadi et al., 2016).
In 2019, approximately 2.3 million individuals in the United States (US) used kratom at least once in the previous year (Palamar, 2021), which is comparable to the number of people who use methamphetamine in the US (NSDUH). The US Food and Drug Administration (FDA) issued a warning that kratom “appears to have properties that expose users to the risks of addiction, abuse, and dependence” (FDA, 09/11/2019). The predominant formulations of kratom used in the US (tablets, capsules, “edibles,” concentrated extracts, and finely ground powders) increase its potential for abuse and overdose relative to traditional use of dried unadulterated kratom leaves (Grundmann, 2017; Prozialeck et al., 2019; Prozialeck et al., 2012). Moderate doses of kratom can induce mild adverse effects that include anxiety, nausea, irritability and vomiting (Prozialeck et al., 2012; Swogger et al., 2015). Reports of serious kratom toxicities, such as tachycardia, seizures, and hepatotoxicity, are rare and are most closely associated with use of high doses or concentrated extracts of kratom (Lu et al., 2014; Nelsen et al., 2010; Pantano et al., 2016). The lethality of kratom is controversial. The main alkaloid in kratom, mitragynine, was measured in postmortem tissue samples following 44 overdose deaths (“U.S. Department of Health & Human Services; HHS Kratom Letter”); however, these deaths were attributed primarily to other drugs (e.g., opioids) taken with kratom or present in adulterated kratom products (Eggleston et al., 2019; Gershman et al., 2019; Henningfield et al., 2019; Neerman et al., 2013; “U.S. Department of Health & Human Services; HHS Kratom Letter”). Moreover, there have been no reported cases of overdose in Asia where kratom is used almost exclusively as leaves or teas, suggesting that adverse effects and overdoses were unique to Western use of kratom. In the U.S., six states (Alabama, Arkansas, Indiana, Tennessee, Vermont and Wisconsin) have banned kratom, while five states (Arizona, Georgia, Nevada, Oklahoma and Utah) have incorporated consumer protection legislation to ensure consumer access to kratom with a framework for regulatory oversight. Elsewhere in the US, the kratom market remains largely unregulated.
Although no kratom products or alkaloids are approved by the FDA or by the International Drug Control Conventions for any therapeutic use, individuals in the US and around the world report using kratom for its perceived health benefits (Board, 2020, 2020a,b). One of the most commonly reported uses of kratom is by opioid-dependent individuals to manage opioid withdrawal and sustain abstinence from opioid use (Boyer et al., 2008; Coe et al., 2019). Other reported uses include pain relief (Coe et al., 2019; Grundmann, 2017), decreased fatigue (Grundmann, 2017; Singh et al., 2019), increased focus (Grundmann, 2017; Singh et al., 2019), and alleviation of depression and anxiety (Coe et al., 2019). Although invaluable for providing insight into motives for using kratom, these studies used closed-end survey questions that limit the potential responses provided by participants regarding their perceived uses of kratom. The full scope of perceived therapeutic uses of kratom is unknown but is important for understanding why people use kratom. Understanding the motivations to use kratom will help identify those who are at risk of kratom dependence or at risk of experiencing adverse drug interactions between kratom and medications. Analyzing the motivations behind kratom use may also help identify potential medicinal uses for kratom.
The largely unregulated kratom market in the US has enabled many vendors to market kratom products with unsupported claims for therapeutic use (e.g., “kratom treats depression”) (FDA, 04/03/2019, 06/25/2019, 09/11/2018). Easy accessibility to kratom products and absence of quality control regulations in most states, along with growing concern regarding abuse liability, addiction, and serious health consequences including death, have motivated regulatory agencies like the FDA and the Drug Enforcement Agency to take actions towards criminalizing kratom as a controlled substance. To guide decisions regarding the regulation of kratom and its active alkaloids mitragynine and 7-hydroxymitragynine, the FDA began soliciting comments from the public in July 2021 about the abuse potential of these substances, their trafficking, their medical usefulness, and the impact of scheduling changes. The open-ended format of the comment solicitation allowed respondents to provide a rich source of respondent-driven information regarding their perceptions of kratom use. In the present study, we analyzed comments published on the Federal Register website during the first 6 weeks of the comment solicitation period with the goal of finding previously unreported perceived therapeutic uses of kratom.
Methods
Data acquisition
In July of 2021, the FDA began soliciting comments “concerning abuse potential, actual abuse, medical usefulness, trafficking, and impact of scheduling changes on availability for medical use” of 7 substances: 4F-MDMB-BICA, brorphine, metonitazene, eutylone, BMDP, kratom (including mitragynine and 7-hydroxymitragynine), and phenibut (Federal Register). Comment solicitation was announced on the US Federal Register website, news websites (e.g., Scientific American, Politico, Statnews), and other online resources (e.g., Reddit, American Kratom Association) and social media platforms (e.g., Twitter, Facebook). Respondents were allowed to submit comments by mail or electronically (Federal Register). All comments analyzed in this study were publicly available and obtained by the investigators from the Federal Register website between July 23 and September 2, 2021—the first 6 weeks of the comment solicitation period (Federal Register). Because the data analyzed in this study was obtained from a public source, the UAMS Institutional Review Board (IRF) determined that this study is not human subject research and does not fall under the jurisdiction of the IRB.
The FDA assigned each comment a unique ID number (e.g., FDA-2021-N-0739-0150) when it was posted on the Federal Register website. The last 4 digits of the ID number identified the order the comment was uploaded to the Federal Register website (Federal Register). A total of 6,353 consecutive comments were posted to the Federal Register website and were the basis for the current study (comment numbers: FDA-2021-N-0739-0001 to FDA-2021-N-0739-6353). All comments are available directly from the corresponding author. Acquired data was reviewed for accuracy. Duplicate comments, comments not discussing kratom, and comments not related to the subject matter were excluded from further consideration (Fig. 1). It is uncertain whether the Federal Register website collected IP addresses to ensure the same person does not respond repeatedly. No such information was available on the Federal Register website.
Fig. 1. Comprehensive summary and framework illustrating the screening and categorization process.
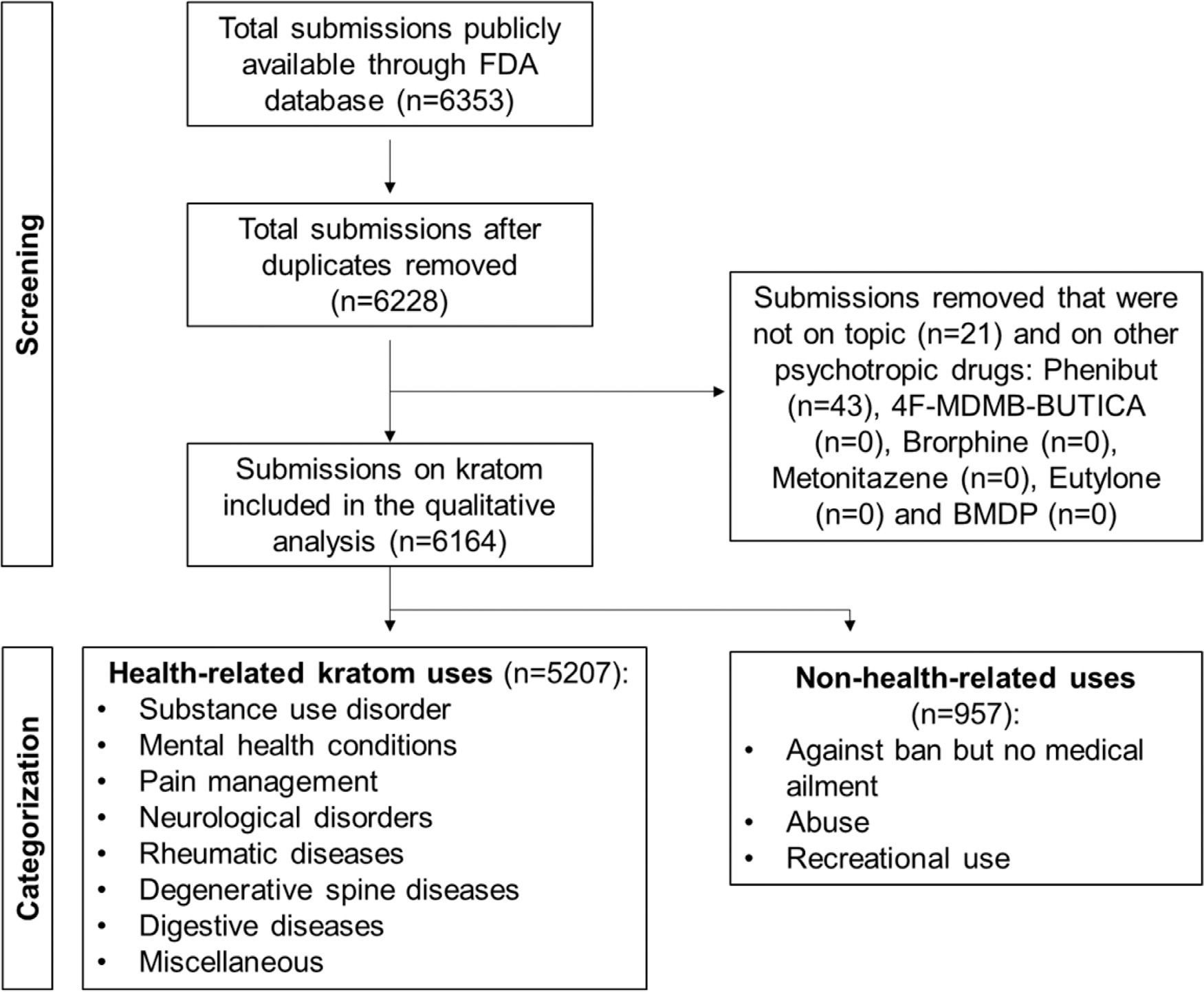
A total of 6,353 submissions were screened. Duplicates and comments on other psychotropic drugs or not on topic were removed. Submissions on kratom were classified into health-related kratom uses (categories 1–8) and non-health-related uses (categories 9–11). FDA: Food and Drug Administration.
Code development and application
Comments were reviewed using an inductive approach via qualitative content analysis as described by Burnard et al. (2008). The codebook was developed by screening the comment section and identifying themes (domain 1), categories (domain 2) and subcategories (domain 3) related to kratom (Supplemental data). Using this data-driven approach, we identified 11 categories of responses regarding kratom in our codebook: 1) mental health conditions (adapted from the National Institute of Mental Health, NIH (“National Institue of Mental Health, NIHa”)); 2) pain defined as distressing experience associated with actual or potential tissue damage with sensory, emotional, cognitive, and social components (Williams & Craig, 2016); 3) substance use disorders (adapted from SAMSHA (SAMSHA, 2016)); 4) rheumatic diseases (adapted from Stony Brook University Hospital, Division of Rheumatology, Allergy, and Immunology (Stony Brook University Hospital)), 5) degenerative spine diseases (adapted from Ascension Seton Medical Center (“Ascension Seton Medical Center”)); 6) digestive diseases (adapted from NIDDK, NIH (“National Institute of Diabetes and Digestive and Kidney Diseases “)); 7) neurological diseases (adapted from Johns Hopkins University (“Johns Hopkins University”)); 8) miscellaneous, defined as other ailments that are difficult to put in a particular category; 9) abuse potential, abuse, and reported adverse effects; 10) recreational use; and 11) against scheduling kratom. Each ailment/response type was assigned to a single category. More detailed information on definitions, categories, subcategories, and keywords were included in our codebook (Supplementary Material). The total number of mentions of each ailment and/or symptom was counted within each subcategory (domain 3), which was summed within each category (domain 2). Julia Tobacyk (JT), Brian Parks (BP) and Lisa Brents (LB) developed the codebook and code application. Furthermore, the codebook was revised and discussed multiple times between investigators. Discussions lead to final consensus on the codebook, upon which one analyst (JT) applied final codes to all comment data.
Interpretation process and examples
Both counts and percentage of total counts within each category and subcategory were calculated. Interpretation included discussing the data after the coding was performed between JT, BP, LB, and NL. The majority of comments reported multiple health-related purposes for kratom use, and all the ailments were counted individually. For example, comment FDA-2021-N-0739-0891 stated, “I suffer from depression, insomnia, anxiety and migraine headaches. From the very start, kratom has helped me greatly manage these symptoms.” Thus, “depression,” “insomnia,” “anxiety,” and “migraines” were reported as 1 count per ailment for 4 ailments in 1 comment. Therefore, the total counts of all ailments and other health-related uses (8,214) exceeded the number of analyzed comments (6,353). Both counts and percentage of total counts within each category and subcategory were calculated. All graphs were plotted as bar graphs in GraphPad Prism (version 9.3.1).
There is overlap between the “pain management” category and some of the other categories that are associated with pain, including rheumatic diseases and degenerative spine diseases. The main factor determining categorization in these cases was whether the respondent attributed their pain to a specific diagnosis (e.g., sciatica or arthritis). If the respondent did, it was counted as an endorsement for that specific diagnosis. If the respondent described general pain without any underlying diagnosis, then it was categorized as “pain.”
Comments regarding family members’ or friends’ experiences using kratom were also included in the analysis. For example, comment FDA-2021-N-0739-0006 stated, “My husband was hurt in a workplace accident in 2000, after therapy, surgery and prescription opioids he began to feel better but unfortunately became addicted to pills. In 2014, he found Kratom and was able to quit the pills.” This comment was reported as 1 count under “opioid use disorder.”
Quality assurance checks
In order to further establish qualitative rigor, quality assurance checks were performed by a second analyst (BP). One hundred comments were randomly picked using google random number generator and coded by the second analyst. There was 83% agreement between coders (BP and JT). Excel was used as an electronic organizer for both quality assurance checks and categorizing data. Additionally, our qualitative methods were guided by a qualitative research expert, Dr. Nakita Lovelady.
Results
During the first 6 weeks of the FDA comment solicitation period (07/23/2021 – 09/02/2021), a total of 6,353 comments were posted to the Federal Register website. These included 125 duplicate comments, 43 comments about phenibut (i.e., not kratom), and 21 comments that were off-topic (i.e., not about the seven substances). These 189 comments were excluded, and the remaining 6,164 comments pertaining to kratom and its alkaloids were analyzed (Fig. 1).
Categories 1 through 8 were health-related categories (mental health conditions, pain, substance use disorder, miscellaneous, rheumatic diseases, degenerative spine diseases, neurological diseases, and digestive diseases) and constituted a total of 8,214 counts (Fig. 2). The remaining non–health-related categories were against a kratom ban but stated no health-related ailments, abuse, or recreational use.
Fig. 2. Health-related categories for which kratom was endorsed.
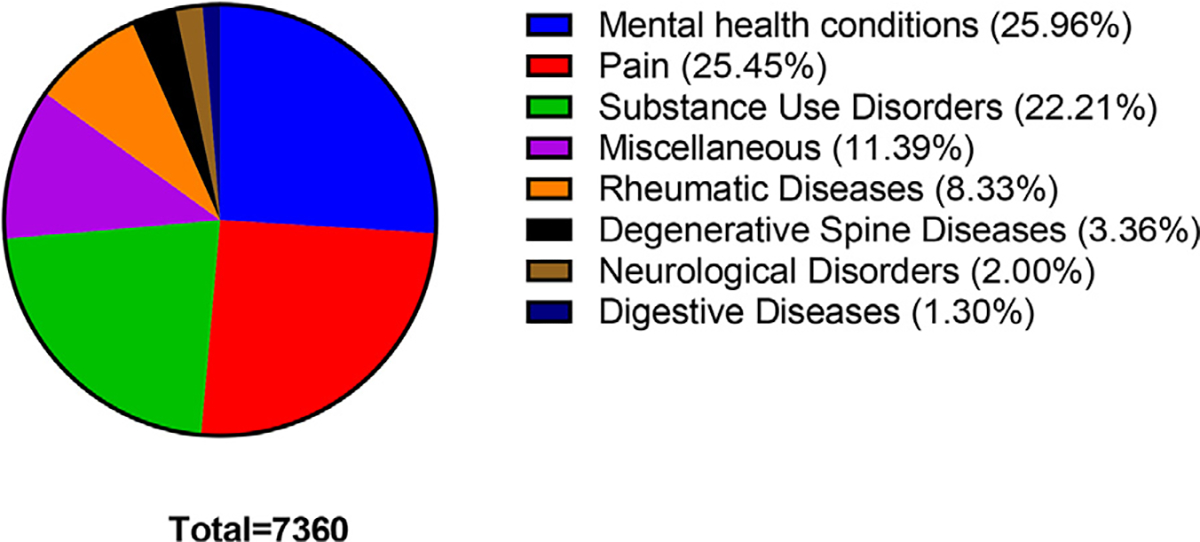
The total counts of all ailments is 7,360 and the number of comment submissions is 5,207.
The vast majority of the counts (7,257; 88.3%) endorsed the medical usefulness of kratom (categories 1–8). In the remaining counts (957; 11.6%), the respondents reported abuse (123 counts), recreational use (2 counts), or were against a kratom ban but did not state any medical usefulness (832 counts) (categories 9–11; Table 1). Respondents reported a diverse array of over 106 medical uses for kratom. The majority of responses on health-related motives for using kratom endorsed its use for mental health conditions (26.3%), pain management (25.8%), or substance use disorders (22.5%; Table 1). Over half (54.9%) of comments endorsing the use of kratom for mental health conditions cited anxiety as the reason, followed by depression (39.2%). Other mental health conditions cited included attention deficit hyperactivity disorder, post-traumatic stress disorder, bipolar disorder, and obsessive-compulsive disorder (Table 1).
Table 1.
Detailed summary of health-related and non-health related categories for kratom use.
| 1st Domain | 2nd Domain Conditions | 3rd Domain |
||
|---|---|---|---|---|
| Conditions | Counts | Ailments | Counts | |
|
| ||||
| Health-related kratom uses | Mental health conditions | 1911 | Anxiety | 941 |
| Depression | 673 | |||
| PTSD | 43 | |||
| ADHD | 35 | |||
| Bipolar disorder | 15 | |||
| OCD | 8 | |||
| Pain | 1873 | Chronic pain | 848 | |
| Unspecified pain | 791 | |||
| Back pain | 529 | |||
| Neuropathic pain | 317 | |||
| Accident-related pain | 208 | |||
| Cancer pain | 55 | |||
| Knee pain | 53 | |||
| Pain related to combat injury | 37 | |||
| Joint pain | 31 | |||
| Menstrual pain | 27 | |||
| CRPS | 24 | |||
| Trigeminal neuralgia | 20 | |||
| Diabetic neuropathy | 13 | |||
| Neck pain | 12 | |||
| Foot pain | 12 | |||
| Hip pain | 11 | |||
| Facet joint syndrome | 10 | |||
| Amputation pain | 3 | |||
| Tendonitis | 3 | |||
| Muscle pain | 2 | |||
| Myofascial pain | 1 | |||
| Burn pain | 1 | |||
| Substance use disorder | 1635 | Opiate/opioid | 1121 | |
| Alcohol | 348 | |||
| Unspecified | 123 | |||
| Stimulant | 17 | |||
| Benzodiazepine | 12 | |||
| Nicotine | 10 | |||
| Cannabis | 4 | |||
| Miscellaneous | 838 | Focus | 272 | |
| Well-being | 196 | |||
| Insomnia | 127 | |||
| Improved quality of life | 113 | |||
| Fatigue | 99 | |||
| Endometriosis | 19 | |||
| Lyme disease | 17 | |||
| Pancreatitis | 14 | |||
| Tinnitus | 13 | |||
| Hypertension | 10 | |||
| Interstitial cystitis | 9 | |||
| Thoracic outlet syndrome | 7 | |||
| Osteomyelitis | 5 | |||
| Ehlers-danlos syndrome | 5 | |||
| Anemia | 4 | |||
| COPD | 4 | |||
| Diabetes | 3 | |||
| Appetite control | 3 | |||
| PCOS | 3 | |||
| Others | 1 | |||
| Rheumatic diseases | 613 | Arthritis | 256 | |
| Fibromyalgia | 151 | |||
| Osteoarthritis | 61 | |||
| Rheumatoid arthritis | 53 | |||
| Lupus | 22 | |||
| Psoriatic arthritis | 22 | |||
| Ankylosing spondylitis | 14 | |||
| Carpal tunnel | 9 | |||
| Osteoporosis | 7 | |||
| Gout | 5 | |||
| Sjögren Syndrome | 4 | |||
| Osteogenesis | 4 | |||
| Ankylosis | 1 | |||
| Spondyloarthritis | 1 | |||
| Polymyalgia | 1 | |||
| Degenerative spine diseases | 247 | Degenerative disc disease | 91 | |
| Herniated disc | 74 | |||
| Stenosis | 44 | |||
| Sciatica | 38 | |||
| Neurological diseases | 147 | Migraines | 65 | |
| Restless leg syndrome | 38 | |||
| Multiple sclerosis | 14 | |||
| Narcolepsy | 12 | |||
| Myelitis | 8 | |||
| Encephalitis | 3 | |||
| Epilepsy/seizures | 2 | |||
| Cerebral palsy | 2 | |||
| Guillain-barre syndrome | 1 | |||
| Raynaud’s syndrome | 1 | |||
| Parkinson disease | 1 | |||
| Digestive diseases | 96 | IBS | 28 | |
| Crohn’s disease | 21 | |||
| Digestive issues | 16 | |||
| Diverticulitis | 9 | |||
| Colitis | 7 | |||
| Gastroparesis | 5 | |||
| Short bowel syndrome | 3 | |||
| Indigestion | 2 | |||
| Heartburn | 1 | |||
| IBD | 1 | |||
| Non-health-related uses | Against ban but no medical ailment stated | 832 | N/A | N/A |
| Abuse | 123 | N/A | N/A | |
| Recreational use | 2 | N/A | N/A | |
Other means that there was one count of each of the following ailments: hidradenitis suppurativa, angina, pelvic floor disorder, non-allergic rhinitis, autoimmune thyroid disease, Bell’s palsy, Legg-Calve-Perthes disease, avascular necrosis, esophageal necrosis, vasculitis, benign fasciculation syndrome, and tardive dyskinesia.
Abbreviations: ADHD: attention-deficit hyperactive disorder, COPD: chronic obstructive pulmonary disease, CRPS: complex regional pain syndrome, IBS: irritable bowel syndrome, IBD: inflammatory bowel disease, OCD: obsessive-compulsive disorder, PCOS: polycystic ovarian syndrome, PTSD: post-traumatic stress disorder.
Responses that endorsed the use of kratom to treat pain that was not associated with a diagnosis of rheumatic disease, degenerative spine disease, or neurological disorder were categorized as “pain management” (Table 1). Within this category, the majority of responses endorsed the use of kratom for chronic pain (37.7%) and unspecified pain (27.4%). Back pain, neuropathic pain, and accident-related pain were also commonly reported motivators of kratom use, comprising 11.8%, 7.2%, and 5.6% of the “pain management” category, respectively. There were 17 occurrences of other pain-related uses that were cited at least once (Table 1). Within the “substance use disorders” category, opioid use disorder was clearly the most commonly reported motivator of kratom use (68.6%), followed by alcohol use disorder (21.3%; Table 1). There were 123 endorsements of kratom to treat or prevent relapse of unspecified substance use disorders (7.5%). Kratom was endorsed for treating problematic use of stimulants, benzodiazepines, nicotine, or cannabis in less than 2.5% of the “substance use disorders” reports.
In addition to mental health, pain management, and substance use disorders, respondents treated several categories of conditions with kratom (Table 1). Because of the diversity of conditions, the fourth largest category was “miscellaneous” (935 counts; 11.5% of responses), which included the reported use of kratom to increase focus (29.1%), improve wellbeing (21.0%) to treat insomnia (13.6%), to decrease fatigue (10.6%), and 27 additional conditions with fewer than 20 endorsements each (Table 1).
Rheumatic diseases comprised 8.45% of the total health-related responses. Within the rheumatic diseases category, arthritis was the most commonly reported motivator of kratom use (41.8%), followed by fibromyalgia (24.6%) and osteoarthritis (10.0%). Other conditions included rheumatoid arthritis (8.6%), lupus (3.6%), psoriatic arthritis (3.6%), and 9 other conditions, each comprising less than 7.8% of the category (Table 1).
The remaining categories each comprised less than 4% of the total health-related motives reported by respondents (Table 1). Degenerative spine diseases reportedly treated with kratom by respondents include degenerative disc disease, herniated disc disease, spinal stenosis, and sciatica (Table 1). Eleven neurological diseases were reportedly treated with kratom, primarily migraines (44.2%) and restless leg syndrome (25.9%) (Table 1). Ten gastrointestinal diseases or disorders were reported (Table 1), primarily irritable bowel syndrome (IBS; 30.1%), Crohn’s disease (22.6%), and vaguely stated “digestive issues” (17.2%).
Discussion
This study is the first to categorize and quantify self-reported health-related motives for kratom use in an open-ended response format. An advantage of this format is that it enabled the respondents to supply a rich body of information beyond what can be gathered with close-ended survey questions. Researchers are more frequently mining such sources for information on perceptions and opinions in the general public (Bunting et al., 2021; Krawczyk et al., 2021; Spadaro et al., 2022). The respondents in the present study are a sample of over 6,000 individuals who were motivated to describe their uses of and opinions about kratom to the US FDA in order to influence federal regulation of kratom. These individuals overwhelmingly endorsed kratom for a remarkably diverse assortment of medical uses, and relatively few respondents reported adverse effects or recommended restricting kratom. Consistent with previous survey studies (Coe et al., 2019; Garcia-Romeu et al., 2020; Prozialeck et al., 2019; Saref et al., 2019), the most frequently endorsed uses for kratom were to treat pain, followed by opioid use disorder, anxiety, and depression. Many different types of pain and pain-related conditions were represented in the responses, including emotional (e.g., anxiety and mood disorders), musculoskeletal, cancer, menstrual, migraine, joint, neuropathic, and rheumatic pain, with almost all of these conditions being chronic or recurrent in nature and often difficult to manage. The endorsement of kratom to treat pain and painful conditions is unsurprising; however, this study is the first to show the diversity of self-reported pain-related conditions treated with kratom. To date, Vicknasingam et al., has conducted the only completed clinical trial to test kratom as an analgesia (Vicknasingam et al., 2020). They recruited Malaysian men who used kratom daily into a placebo-controlled study that measured pain tolerance using the cold pressor task immediately before (t = 0), one hour after, and two hours after consumption of a kratom tea or a placebo drink designed to taste like the kratom tea. Relative to placebo and the t = 0 time point, the kratom tea increased pain tolerance and decreased ratings of unpleasantness of the cold pressor task at the one-hour time point. Another study that used a rat model of neuropathic pain showed that mitragynine dose-dependently reduced mechanical allodynia in an 𝛼-adrenoceptor- and opioid-receptor dependent manner (Foss et al., 2020). Although more studies are needed, these studies suggest that kratom and kratom alkaloids hold promise for treating pain, including neuropathic pain that is often difficult to manage with current therapies (Bates et al., 2019).
For generations, indigenous people of Southeast Asia have used kratom for various health conditions such as cough, fever, diarrhea, hypertension, treatment of pain as well as to enhance work performance, decrease fatigue and manage opioid dependence. In the US, studies show that individuals use kratom to treat conditions such as chronic pain, depression, anxiety and opioid withdrawals, and self-reporting strong effectiveness for these ailments (Garcia-Romeu et al., 2020). Individuals report using kratom for similar health maladies in the U.S. and Southeast Asia though more studies are needed to address the broad list of therapeutic uses seen in the U.S. compared to Southeast Asia. While kratom overdoses have been reported in the US, there have been zero kratom overdoses reported in Southeast Asia. This could possibly be due to unregulated kratom-based products being sold in the U.S including phenylethylamine (Nacca et al., 2020) and artificially elevated concentrations of 7-hydroxymitragynine (Lydecker et al., 2016). Many studies suggest that kratom has a relatively low risk profile, with relatively few respondents endorse adverse effects. In the Garcia-Romeu study, conducted in the U.S., the kratom adverse effects were reported as mild and short-lasting, with less than 1% of the participants experienced adverse effects severe enough to seek medical treatment (Garcia-Romeu et al., 2020). In contrast to products sold in Southeast Asia, unregulated kratom-based products sold in the U.S. may contain adulterants that are harmful to the public.
The results of this study should be interpreted with caution. The goal of this study is to understand beliefs of people who use kratom in context of its medical usefulness. Neither the stated diagnoses nor the effects claimed by respondents were verified by investigators. Many factors can contribute to beliefs in the medical usefulness of kratom, including relief due to a direct effect of kratom, a placebo effect, and social effects (e.g., endorsements by friends or family, or social use of kratom). Randomized placebo (or standard of care)-controlled clinical trials are needed to determine whether kratom has therapeutic efficacy for a given indication.
The present study is likely biased towards reports of positive experiences using kratom for a few reasons. First, people who had negative effects or no positive effect from their first use(s) of kratom would have likely stopped using kratom and would therefore have no interest in writing about their experiences to the FDA. People who use kratom on regular basis would have more frequent contact with vendors and consumer advocacy organizations (e.g., the American Kratom Association) that encouraged their patrons and members to write to the FDA. Second, people who perceive benefit from kratom use have a vested interest in the legal status of kratom and are motivated to write to the FDA. For these reasons, results of the present study should not be used to judge whether kratom has therapeutic effects, and whether any benefits out-weigh potential risks of toxicities, dependence, or other adverse effects. Third, we cannot eliminate the possibility that respondents practiced deception. A respondent who uses kratom for recreational purposes may have reported using it for pain and may have submitted multiple entries. Accuracy in self-reporting is a challenge that is not unique to this study. Although respondents had the option to respond anonymously, most (~70%, as seen on the website) provided a first and last name, and most comments were highly detailed, lowering the possibility of significant organized “brigading” of the Federal Register website by individuals or groups trying to manipulate the overall perceptions of kratom use. Fourth, the submitted FDA comments never asked respondents to rate kratom efficacy regarding disease management. Depending on what form of kratom is ingested (brewed kratom decoction, kratom extracts, etc.), the reported health benefits may differ from person to person. Establishing standardized manufacturing practices for kratom production, product labeling, and safety and efficacy verification would be beneficial to the public from a safety perspective. Lastly, many vendors sell kratom-based products that may contain adulterants. Since there has been no laboratory analysis conducted to verify the chemical content of kratom, other adulterants may be contributing to the perceived therapeutic effectiveness of individuals self-medicating with kratom.
The lack of demographic information on respondents is a limitation of the present study. A previous online survey study of 8094 respondents that was conducted in 2016 reported that people who use kratom in the US tend to be White (89%), middle income (>$35,000/year; 63%) males (57%), ages 21–50 (80%), and self-employed (15%) or employed for wages (57%) (Grundmann, 2017). Garcia-Romeu et al. collected data on 2,789 people in the U.S. who use kratom. These data included demographics, use patterns, and perceived therapeutic and adverse effects. The majority of participants were white (90%), women (61%), college-educated (83.9%), and employed (68.4%). These studies, like the present study, may be biased for younger more affluent populations because of the reliance on respondent accessibility to and fluency with the internet. However, several of the conditions endorsed by people who use kratom are diagnosed more frequently in older individuals (e.g., degenerative spine diseases, arthritis) (Chhatre et al., 2017; Sarbacher & Halper, 2019), suggesting that our sample likely includes older individuals.
In summary, this study provides novel information on the perceptions of people with a personal stake in the legal status of kratom in the US. It is important to note that this is a study of a biased sample of people motivated to report positive effects of kratom to the FDA. Thus, the adverse effects and abuse liability of kratom use may be under-reported and the perceived therapeutic benefits overestimated. In our study, Americans endorsed using kratom to for a range of health conditions with the intention of improved health and well-being. Harm associated with kratom use is controversial. Moderate to high acute doses of kratom can cause adverse effects and chronic use can cause dependence. Adulterants and contaminants may also cause harm, but consumer protection regulation of kratom can be enacted, as done in five states, to combat this problem. Kratom may also interact with other substances, such as opioids, with additive or synergistic effects and thus contribute to harm caused by opioid abuse. However, more research is needed to better understand the potential harm and benefits of kratom use, and potential effectiveness and safety of kratom for the treatment of several indications.
Supplementary Material
Funding sources
This research received funding from the following sources.
The project described was supported by the Translational Research Institute (TRI), grants UL1 TR003107 and TL1 TR003109 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Efforts were also supported by the UAMS NIDA T32 Addiction Research Training Program, grant T32 DA022981; and the NCATS funded UAMS Translational Research Institute, grant KL2 TR003108.
Abbreviations:
- ADHD
attention deficit hyperactivity disorde
- BMDP
Methylenedioxypyrovalerone
- COPD
chronic obstructive pulmonary disease
- FDA
food and drug administration
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- MDMB-BUTICA
methyl 2-({[1-(4-fluorobutyl)-1H-indol3-yl]carbonyl} amino)-3,3-dimethylbutanoate
- OCD
obsessive-compulsive disorder
- PCOS
polycystic ovarian syndrome
- PTSD
post-traumatic stress disorder
Footnotes
Declarations of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
The authors declare that the work reported herein did not require ethics approval because it did not involve animal or human participation.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.drugpo.2022.103817.
References
- Adkins JE, Boyer EW, & McCurdy CR (2011). Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Current Topics in Medicinal Chemistry, 11(9), 1165–1175. 10.2174/156802611795371305. [DOI] [PubMed] [Google Scholar]
- Ascension Seton Medical Center. Retrieved from https://www.seton.net/brain-and-spine-care/2016/12/08/4-types-degenerative-spine-disease/.
- Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, . . . Hunter CW (2019). A comprehensive algorithm for management of neuropathic pain. Pain Medicine, 20(suppl 1), S2–S12. 10.1093/pm/pnz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board INC (2020a). List of narcotic drugs under international control: Yellow list. Vienna, Austria: International Narcotics Control Board; Retrieved from https://www.incb.org/documents/Publications/AnnualReports/AR2020/Annual_Report/E_INCB_2020_1_eng.pdf. [Google Scholar]
- Board INC (2020b). List of psychotropic substances under international control: Green list. Retrieved from Vienna, Austria: International Narcotics Control Board. [Google Scholar]
- Boyer EW, Babu KM, Adkins JE, McCurdy CR, & Halpern JH (2008). Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction, 103(6), 1048–1050. 10.1111/j.1360-0443.2008.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting AM, Frank D, Arshonsky J, Bragg MA, Friedman SR, & Krawczyk N (2021). Socially-supportive norms and mutual aid of people who use opioids: An analysis of Reddit during the initial COVID-19 pandemic. Drug and Alcohol Dependence, 222, Article 108672. 10.1016/j.drugalcdep.2021.108672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnard P, Gill P, Stewart K, Treasure E, & Chadwick B (2008). Analysing and presenting qualitative data. British Dental Journal, 204(8), 429–432. 10.1038/sj.bdj.2008.292. [DOI] [PubMed] [Google Scholar]
- Chhatre S, Cook R, Mallik E, & Jayadevappa R (2017). Trends in substance use admissions among older adults. BMC Health Services Research, 17(1), 584. 10.1186/s12913-017-2538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, . . . Corazza O (2015). Following “the Roots” of kratom (Mitragyna speciosa): The evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in western countries. BioMed Research International, 2015, Article 968786. 10.1155/2015/968786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe MA, Pillitteri JL, Sembower MA, Gerlach KK, & Henningfield JE (2019). Kratom as a substitute for opioids: Results from an online survey. Drug and Alcohol Dependence, 202, 24–32. 10.1016/j.drugalcdep.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Eggleston W, Stoppacher R, Suen K, Marraffa JM, & Nelson LS (2019). Kratom use and toxicities in the United States. Pharmacotherapy, 39(7), 775–777. 10.1002/phar.2280. [DOI] [PubMed] [Google Scholar]
- FDA. (April/03/2019). Laboratory analysis of kratom products for heavy metals. Retrieved from https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals.
- FDA. (June/25/2019). FDA issues warnings to companies selling illegal, unapproved kratom drug products marketed for opioid cessation, pain treatment and other medical uses. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-issues-warnings-companies-selling-illegal-unapproved-kratom-drug-products-marketed-opioid.
- FDA. (September/11/2018). Statement from FDA Commissioner Scott Gottlieb, M.D., on new warning letters FDA is issuing to companies marketing kratom with unproven medical claims; and the agency’s ongoing concerns about kratom. Retrieved from https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-new-warning-letters-fda-issuing-companies-marketing.
- FDA. (September/11/2019). FDA and kratom. Retrieved from https://www.fda.gov/news-events/public-health-focus/fda-and-kratom.
- Federal Register, F. Retrieved from https://www.federalregister.gov/documents/2021/07/23/2021-15685/international-drug-scheduling-convention-on-psychotropic-substances-single-convention-on-narcotic.
- Foss JD, Nayak SU, Tallarida CS, Farkas DJ, Ward SJ, & Rawls SM (2020). Mitragynine, bioactive alkaloid of kratom, reduces chemotherapy-induced neuropathic pain in rats through alpha-adrenoceptor mechanism. Drug and Alcohol Dependence, 209, Article 107946. 10.1016/j.drugalcdep.2020.107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A, Cox DJ, Smith KE, Dunn KE, & Griffiths RR (2020). Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug and Alcohol Dependence, 208, Article 107849. 10.1016/j.drugalcdep.2020.107849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman K, Timm K, Frank M, Lampi L, Melamed J, Gerona R, & Monte AA (2019). Deaths in Colorado attributed to kratom. New England Journal of Medicine, 380(1), 97–98. 10.1056/NEJMc1811055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann O (2017). Patterns of kratom use and health impact in the US-Results from an online survey. Drug and Alcohol Dependence, 176, 63–70. 10.1016/j.drugalcdep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R,... Muller CP (2013). From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neuroscience and Biobe-havioral Reviews, 37(2), 138–151. 10.1016/j.neubiorev.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Grundmann O, Babin JK, Fant RV, Wang DW, & Cone EJ (2019). Risk of death associated with kratom use compared to opioids. Preventive Medicine, 128, Article 105851. 10.1016/j.ypmed.2019.105851. [DOI] [PubMed] [Google Scholar]
- Jansen KL, & Prast CJ (1988a). Ethnopharmacology of kratom and the Mitragyna alkaloids. Journal of Ethnopharmacology, 23(1), 115–119. 10.1016/0378-8741(88)90121-3. [DOI] [PubMed] [Google Scholar]
- Jansen KL, & Prast CJ (1988b). Psychoactive properties of mitragynine (kratom). Journal of Psychoactive Drugs, 20(4), 455–457. 10.1080/02791072.1988.10472519. [DOI] [PubMed] [Google Scholar]
- Johns Hopkins University. Retrieved from https://www.hopkinsmedicine.org/health/conditions-and-diseases/neurological-disorders.
- Krawczyk N, Bunting AM, Frank D, Arshonsky J, Gu Y, Friedman SR, & Bragg MA (2021). “How will I get my next week’s script?” Reactions of Reddit opioid forum users to changes in treatment access in the early months of the coron-avirus pandemic. International Journal of Drug Policy, 92, Article 103140. 10.1016/j.drugpo.2021.103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wei H, Wu J, Jamil MF, Tan ML, Adenan MI,... Shim W (2014). Evaluation of the cardiotoxicity of mitragynine and its analogues using human in-duced pluripotent stem cell-derived cardiomyocytes. Plos One, 9(12), Article e115648. 10.1371/journal.pone.0115648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydecker AG, Sharma A, McCurdy CR, Avery BA, Babu KM, & Boyer EW (2016). Suspected adulteration of commercial kratom products with 7-hydroxymitragynine. Journal of Medical Toxicology, 12(4), 341–349. 10.1007/s13181-016-0588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacca N, Schult RF, Li L, Spink DC, Ginsberg G, Navarette K, & Marraffa J (2020). Kratom adulterated with phenylethylamine and associated in-tracerebral hemorrhage: Linking toxicologists and public health officials to identify dangerous adulterants. Journal of Medical Toxicology, 16(1), 71–74. 10.1007/s13181-019-00741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institue of Mental Health, NIH. Retrieved from https://www.nimh.nih.gov/health/topics.
- National Institute of Diabetes and Digestive and Kidney Diseases Retrieved from https://www.niddk.nih.gov/health-information/digestive-diseases.
- Neerman MF, Frost RE, & Deking J (2013). A drug fatality involving Kratom. Journal of Forensic Science, 58(suppl 1), S278–S279. 10.1111/1556-4029.12009. [DOI] [PubMed] [Google Scholar]
- Nelsen JL, Lapoint J, Hodgman MJ, & Aldous KM (2010). Seizure and coma following Kratom (Mitragynina speciosa Korth) exposure. Journal of Medical Toxicology, 6(4), 424–426. 10.1007/s13181-010-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSDUH, S. a. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Retrieved from https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/019NSDUHFFRPDFWHTML/2019NSDUHFFR090120.htm}illi5.
- Palamar JJ (2021). Past-year kratom use in the U.S.: Estimates from a nationally repre-sentative sample. American Journal of Preventive Medicine, 61(2), 240–245. 10.1016/j.amepre.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano F, Tittarelli R, Mannocchi G, Zaami S, Ricci S, Giorgetti R,... Marinelli E (2016). Hepatotoxicity Induced by “the 3Ks”: Kava, kratom and khat. International Journal of Molecular Sciences, 17(4), 580. 10.3390/ijms17040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Avery BA, Boyer EW, Grundmann O, Henningfield JE, Kruegel AC,... Singh D (2019). Kratom policy: The challenge of balancing therapeutic potential with public safety. International Journal of Drug Policy, 70, 70–77. 10.1016/j.drugpo.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Jivan JK, & Andurkar SV (2012). Pharmacology of kratom: An emerging botanical agent with stimulant, analgesic and opioid-like effects. Journal of the American Osteopathic Association, 112(12), 792–799. [PubMed] [Google Scholar]
- SAMSHA. (2016). Impact of the DSM-IV to DSM-5 changes on the National Survey on Drug Use and Health Rockville (MD). [PubMed]
- Sarbacher CA, & Halper JT (2019). Connective tissue and age-related diseases. Sub-Cellular Biochemistry, 91, 281–310. 10.1007/978-981-13-3681-2_11. [DOI] [PubMed] [Google Scholar]
- Saref A, Suraya S, Singh D, Grundmann O, Narayanan S, Swogger MT,... Balasingam V (2019). Self-reported prevalence and severity of opioid and kratom (Mitragyna speciosa korth.) side effects. Journal of Ethnopharmacology, 238, Article 111876. 10.1016/j.jep.2019.111876. [DOI] [PubMed] [Google Scholar]
- Shellard EJ (1989). Ethnopharmacology of kratom and the Mitragyna alkaloids. Journal of Ethnopharmacology, 25(1), 123–124. 10.1016/0378-8741(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Singh D, Narayanan S, Muller CP, Swogger MT, Chear NJY, Dzulkapli EB,... Vicknasingam B (2019). Motives for using kratom (Mitragyna speciosa Korth.) among regular users in Malaysia. Journal of Ethnopharmacology, 233, 34–40. 10.1016/j.jep.2018.12.038. [DOI] [PubMed] [Google Scholar]
- Spadaro A, Sarker A, Hogg-Bremer W, Love JS, O’Donnell N, Nelson LS, & Perrone J (2022). Reddit discussions about buprenorphine associated precipitated withdrawal in the era of fentanyl. Clinical Toxicology, 1–8. 10.1080/15563650.2022.2032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stony Brook University Hospital, D. o. R., Allergy and Immunology. Retrieved from https://www.stonybrookmedicine.edu/patientcare/rheumatology.
- Swogger MT, Hart E, Erowid F, Erowid E, Trabold N, Yee K, & Walsh Z (2015). Experiences of kratom users: A qualitative analysis. Journal of Psychoactive Drugs, 47(5), 360–367. 10.1080/02791072.2015.1096434. [DOI] [PubMed] [Google Scholar]
- Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D,... Horie S (2002). Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: Discovery of opioid agonists structurally different from other opioid ligands. Journal of Medicinal Chemistry, 45(9), 1949–1956. 10.1021/jm010576e. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services; HHS Kratom Letter. Retrieved from https://www.documentcloud.org/documents/5031552-HHS-kratom-letter.html.
- Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V,... Ma-jumdar S (2016). Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with Mu agonism and delta antagonism, which do not recruit beta-arrestin-2. Journal of Medicinal Chemistry, 59(18), 8381–8397. 10.1021/acs.jmedchem.6b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicknasingam B, Chooi WT, Rahim AA, Ramachandram D, Singh D, Ra-manathan S,... Chawarski MC (2020). Kratom and pain tolerance: A randomized, placebo-controlled, double-blind study. Yale Journal of Biology and Medicine, 93(2), 229–238. [PMC free article] [PubMed] [Google Scholar]
- Williams ACC, & Craig KD (2016). Updating the definition of pain. Pain, 157(11), 2420–2423. 10.1097/j.pain.0000000000000613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


